Abstract
Congenital hearing loss (hearing loss present at birth) is one of the most prevalent chronic conditions in children. In the majority of developed countries, neonatal hearing-screening programmes enable early detection; early intervention will prevent delays in speech and language development and have long-lasting beneficial effects on social and emotional development and quality of life. A hearing loss diagnosis is usually followed by a search for an underlying aetiology. Congenital hearing loss might be attributed to environmental and prenatal factors, which prevail in low-income settings; congenital infections, particularly cytomegalovirus, are also a common risk factor for hearing loss. Genetic causes probably account for the majority of cases in developed countries; mutations can affect any component of the hearing pathway, in particular inner ear homeostasis (endolymph production and maintenance) and mechano-electrical transduction (conversion of a mechanical stimulus into electrochemical activity). Once the underlying cause of hearing loss is established, it might direct therapeutic decision-making and guide prevention and (genetic) counseling. Management options include specific antimicrobial therapies, surgical treatment of cranio-facial abnormalities and hearing aids. An improved understanding of the pathophysiology and molecular mechanisms underlying hearing loss and increased awareness of recent advances in genetic testing will promote the development of new treatment and screening strategies.
Introduction
Congenital hearing loss — hearing loss present at birth—occurs when the ability of the ear to convert the vibratory mechanical energy of sound into the electrical energy of nerve impulses is impaired (Figure 1). Hearing loss is categorized according to the site of the lesion; in conductive hearing loss the outer or middle ear are affected and in sensorineural hearing loss the inner ear, auditory nerve or central auditory pathway are affected. Mixed hearing loss is defined as conductive and sensorineural hearing loss. In conductive hearing loss, sound waves cannot propagate through the ear, either secondary to maldevelopment of the middle ear, the external ear or both, or following transient obstruction of the middle ear caused by effusion (as in the case of otitis media) 1. Sensorineural hearing loss can be further subdivided in sensory hearing loss (when the hair cells are affected), central hearing loss when the cause is located along the central auditory pathway or Auditory Neuropathy Spectrum Disorder. 2 Auditory Neuropathy Spectrum Disorder includes a wide range of clinical conditions characterized by the presence of oto-acoustic emissions and a cochlear microphonic with abnormal or absent auditory brainstem responses, and results in impaired speech discrimination. Auditory Neuropathy Spectrum Disorder may be caused by a primary lesion located in the inner hair cells, in the auditory nerve of intervening synapse and may also include damage to neuronal populations in the auditory pathway. 3, 4
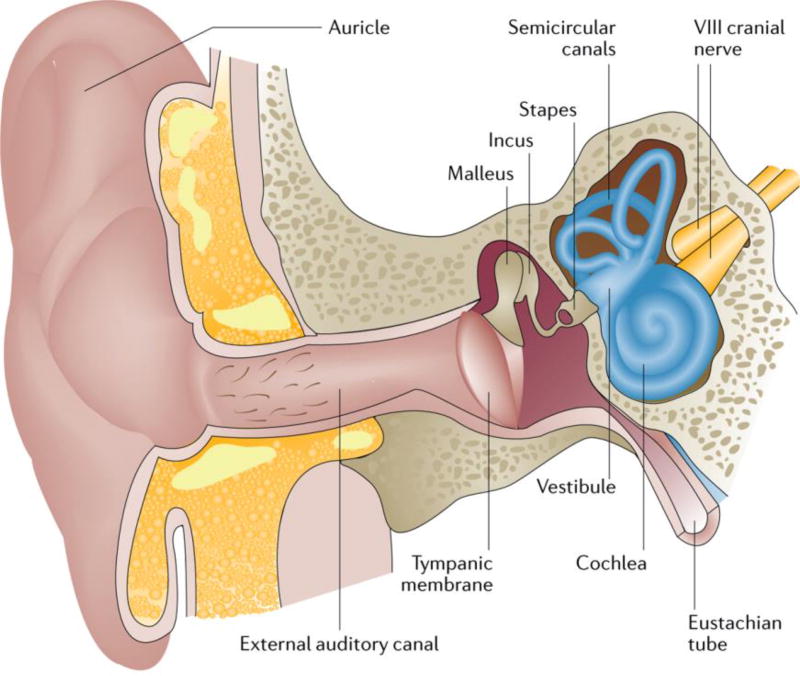
Figure 1. Cross-section of the outer, middle and inner ear.
The ear is composed of three major parts, the outer, middle and inner ear. The outer ear includes the auricle and external auditory canal, and is separated from the middle ear by the tympanic membrane. The middle ear, a mucosal-lined air-filled space, houses three bones (ossicles) — the malleus, incus and stapes — and bridges the external and inner ear. The inner ear is divided into two parts: the vestibular portion, which includes the vestibule and the three semicircular canals, and the cochlear portion, which contains the outer and inner hair cells of the sensory epithelium. The footplate of the stapes covers the oval window of the inner ear. The VIII cranial nerve (the auditory or cochlear nerve) links the inner ear with the brainstem. The Eustachian tube links the cavity of the middle ear to the pharynx, permitting the equalization of pressure on each side of the tympanic membrane. The ear converts the vibratory mechanical energy of sound into the electrical energy of nerve impulses. Sound is transmitted through the external auditory canal to the tympanic membrane and middle ear ossicles. Here, air vibration is translated and amplified to mechanical vibration. At the level of the footplate, these mechanical vibrations are transmitted to the cochlea, resulting in movement of the cochlear fluids. The movement of the cochlear fluids moves and alters the shape of the outer hair cells of the cochlea. This process mediates sound amplification and increases frequency specificity. The movement of the inner hair cells of the cochlea stimulates the adjacent nerve fibres and transmits the electrical signal to the brain.
In most developed countries, neonatal hearing screening programmes are available for this prevalent condition. These programmes aim to screen all newborns within 1 month of birth. Early diagnosis and subsequent early intervention and treatment promote improved developmental outcome later in childhood. 5 Because hearing loss can progress over time, neonatal hearing screening programmes might miss children with progressive hearing loss. Thus, repeated screening at regular intervals is advised for at-risk infants. Medical and supportive treatment of congenital hearing loss depends on aetiology and type of hearing loss. Hearing loss is most often caused by genetic factors (including both non-syndromic forms, in which hearing loss is the only clinical feature, and syndromes such as Usher or Jervell and Lange–Nielsen syndromes), cranio-facial abnormalities and congenital infections.
In this Primer, we focus on unilateral and bilateral permanent congenital hearing loss, defined as hearing loss ≥40 dB in the better hearing ear averaged over the frequency range important for speech recognition (500, 1,000, 2,000 and 4,000 Hz), 6 which is typically assessed by hearing screening programmes. 7, 8 The epidemiology, mechanisms, diagnosis and management are discussed.
Epidemiology
Since the end of the last century, neonatal hearing screening programmes have become available in North America, Europe and most developed countries. Universal neonatal hearing screening (that is, screening all newborns rather than only those with risk factors for hearing loss) is advocated. 7 On the basis of these programmes, the estimated prevalence of permanent bilateral hearing loss is 1.33 per 1,000 live births. 9 In children of primary school age, the prevalence increases to 2.83 per 1,000 children,10, 11 with a further increase to 3.5 per 1,000 in adolescents. 9 This increase over time presumably reflects the cumulative addition of patients with hearing loss owing to progressive, acquired or late-onset genetic causes. For some types of hearing loss, such as Auditory Neuropathy Spectrum Disorder, diagnostic findings are often not conclusive in newborns because language skills are still developing and not aberrant at that time; accordingly, prevalence estimates vary widely. 12
In countries without universal neonatal hearing screening programmes, prevalence estimates vary between 19 per 1,000 newborns in sub-Saharan Africa up to 24 per 1,000 in South Asia. 13 The large difference in the prevalence estimates between high-income and low-income countries is only in part accounted for by the use of different diagnostic methods or criteria for hearing loss thresholds. The presence of risk factors is the most important predictor.
Risk factors
The Joint Committee on Infant Hearing from the American Academy of Pediatrics has identified several risk factors for congenital or late-onset childhood hearing loss (Box 1). 7 A positive family history of permanent congenital hearing loss is suggested as a risk factor, but the body of evidence for its relevance is low, as only 1.43% of children with a positive family history have hearing loss.14 Admission to a neonatal intensive care unit is a relevant risk factor; with the prevalence of hearing loss increasing as gestational age and birth weight decrease (1.2–7.5% in premature babies born at 24–31 weeks and 1.4%–4.8% in babies weighing 750–1500 g).15 Necessary medical interventions (such as assisted ventilation, venous access and aminoglycoside use) while in the neonatal intensive care unit increase the likelihood of hearing loss. Duration of hospitalization of ≥12 days and a history of treatment by high-frequency ventilation have also been identified as independent risk factors for hearing loss in this population.16 In addition, delayed maturation of the auditory system is has been postulated as a concern in infants hospitalized in this setting.17
In the majority of hearing-impaired children, hearing loss is due to genetic factors, most often a single gene defect. 18 These defects can have different modes of inheritance and different prevalences. Hearing loss is classified to reflect the presence (syndromic hearing loss, Table 1) or absence (non-syndromic) of co-inherited physical or laboratory findings. Non-syndromic hearing loss is extremely heterogeneous. Autosomal recessive non-syndromic hearing loss, which accounts for 80% of genetic cases, is typically congenital, whereas autosomal dominant non-syndromic hearing loss, which accounts for the remaining 20% of cases, is often progressive with a later age of onset X-linked or maternal mitochondrial DNA-related modes of inheritance are rare. 19
Table 1.
Common syndromic forms of hearing loss
| Syndrome | Proteins involved (coding genes) | Clinical characteristics* |
|---|---|---|
|
| ||
| Jervell and Lange–Nielsen | Potassium voltage-gated channel subfamily E member 1 (KCNE1) and potassium voltage-gated channel subfamily KQT member 1 (KCNQ1) | Cardiac arrhythmia (long QT interval) |
|
| ||
| Usher | Usher syndrome type 1: Unconventional myosin-VIIa (MYO7A), harmonin (USH1C), cadherin-23 (CDH23), protocadherin-15 (PCDH15), Usher syndrome type-1G protein (USH1G) and calcium and integrin-binding family member 2 (CIB2) | Retinitis pigmentosa |
| Usher syndrome type 2: usherin (USH2A), adhesion G protein-coupled receptor V1 (ADGRV1) and whirlin (WHRN) | ||
| Usher syndrome type 3: clarin-1 (CLRN1) | ||
|
| ||
| Alport | Collagen alpha-3(IV) chain (COL4A3), collagen alpha-4(IV) chain (COL4A4) and collagen alpha-5(IV) chain (COL4A5) | Glomerular kidney disease and eye abnormalities |
|
| ||
| Branchio-oto-renal | Eyes absent homolog 1 (EYA1), homeobox protein SIX1 (SIX1) and homeobox protein SIX5 (SIX5) | Branchial cysts or fistulae, external and middle ear anomalies and renal abnormalities |
|
| ||
| Waardenburg | Paired box protein Pax-3 (PAX3), microphthalmia-associated transcription factor (MITF, endothelin-3 (EDN3), endothelin B receptor (EDNRB), zinc finger protein SNAI2 (SNAI2) and transcription factor SOX-10 (SOX10) | Pigmentary abnormalities of skin, hair and iris |
|
| ||
| Pendred | Pendrin (SLC26A4) | Enlarged vestibular aqueduct and thyroid goiter |
|
| ||
| Stickler | Collagen alpha-1151 chain (COL2A1), collagen alpha-1(IX) chain (COL9A1), collagen alpha-2(IX) chain (COL9A2), collagen alpha-1(XI) chain (COL11A1) and collagen alpha-2(XI) chain (COL11A2) | Skeletal and joint abnormalities, myopia and vitreoretinal degeneration |
|
| ||
| Treacher Collins | Treacle protein (TCOF1), DNA-directed RNA polymerases I and III subunit RPAC1 (POLR1C) and DNA-directed RNA polymerases I and III subunit RPAC2 (POLR1D) | Characteristic facies caused by underdevelopment of facial bones (malar and zygomatic hypoplasia, small jaw), cleft palate, eyelid colobomata and external and middle ear anomalies |
Aside from the manifestations that affect the ear or the auditory system.
Although the frequency of causative genes varies across different populations and ethnicities, the most frequent genetic cause of severe-to-profound autosomal recessive non-syndromic hearing loss is mutation in the gap junction protein beta 2 gene (GJB2). 20 Mutations in this gene account for ≤50% of the autosomal recessive non-syndromic hearing loss cases in the white populations of Europe and the United States. 20
In some patients, clinical examination might point towards a syndromic cause. Physical findings such as pre-auricular pits and tags, branchial cysts or fistulae or dystopia canthorum (the lateral displacement of the inner corners of the eyes, giving the appearance of a widened nasal bridge), heterochromia iridis and pigmentary abnormalities might be associated with syndromes known to cause hearing loss, which must also be considered. 21 Over 400 of these syndromes have been described. The responsible genes for several are known and genetic testing is available for many of these. 22
Congenital infection is also an important risk factor, with congenital cytomegalovirus (CMV) infection standing out as the most common non-genetic cause of sensorineural hearing loss.23 Whereas the prevalence of congenital CMV infection is 0.58% in industrialized countries, this figure increases to 1–6% in developing countries with a high rate of maternal seroprevalence. 24 The virus is shed in bodily fluids, such as urine, saliva and blood, and exposure to CMV is most commonly encountered both through sexual contact or contact with bodily fluids of young children with CMV infection. The risk of congenital hearing loss caused by an infection might largely depend upon socio-economic status (congenital CMV infection), availability of prevention strategies such as vaccination (congenital rubella) or hygienic measures (congenital toxoplasmosis). In countries without a rubella vaccination programme, congenital rubella infection is the leading environmental cause of congenital hearing loss. 25
All the aforementioned risk factors are included in surveys of the aetiology of congenital hearing loss, which divide causality between genetic and environmental factors. However, in most studies, a definitive cause cannot be identified in a considerable proportion of children with hearing loss.26–28
Mechanisms/pathophysiology
Congenital hearing loss can be divided into genetic and acquired forms. The mechanisms and pathophysiology of these two forms differ considerably.
Genetic congenital hearing loss
The study of hearing loss of genetic origin has greatly increased our understanding of normal auditory function and the pathophysiological processes that can disrupt it. Genetic mutations can affect any component of the hearing pathway. Most genes implicated in syndromic hearing loss are associated with an eponymous syndrome, whereas the loci linked to non-syndromic hearing loss are conventionally named using a prefix followed by a suffix integer: DFNA for autosomal dominant loci, DFNB for autosomal recessive loci and DFNX for X-linked loci. A regularly updated overview of all hearing loss-associated genes can be found online (http://hereditaryhearingloss.org). In this Primer, we have concentrated on genes that affect inner ear homeostasis, (particularly endolymph production and maintenance) and mechano-electrical transduction (especially stereociliary bundle formation and function), as these genes are the most extensively studied.
Inner ear homeostasis: stria vascularis and endolymph
At the cornerstone of inner ear homeostasis is the stria vascularis, situated on the lateral wall of the cochlear duct (Figure 2). This highly specialized tissue produces a unique fluid — the endolymph — that bathes the sensory hair cells of the inner ear and is crucial for auditory transduction. The unique ionic composition of the endolymph, high in potassium ions (K+, 150 mM), low in sodium ions (Na+, 1 mM) and with a high positive endocochlear potential (+80–100 mV), reflects the function of a number of channels, pumps and gap junctions. The stria vascularis consists of marginal, intermediate and basal cell layers. The marginal cells face the endolymph, and the intermediate and basal cells below communicate via gap junctions with each other and with the fibrocytes of the underlying spiral ligament (the thick periosteum that forms the outer wall of the cochlear duct) of the lateral wall. This network of gap junctions, whose proteins are encoded by the gap junction protein beta 2 and 6 genes, GJB2 and GJB6, among others, facilitates ion transport between cells and couples the cells electrically.29 Mutations in GJB2 are the commonest cause of severe-to-profound autosomal recessive congenital hearing loss in many populations.
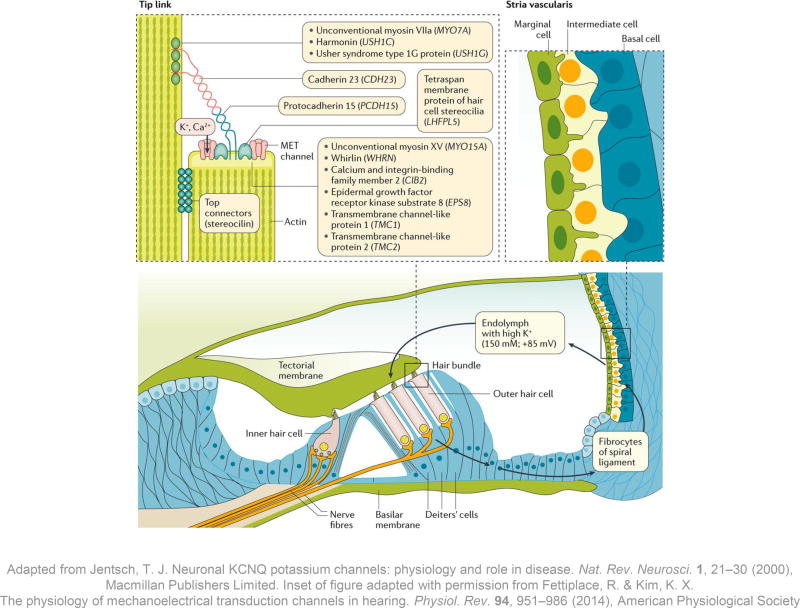
Figure 2. The stria vascularis and sensory hair cells.
The stria vascularis is the highly specialized tissue that produces the endolymph; it is situated on the lateral wall of the endolymphatic duct in the cochlea, 138 and consists of marginal, intermediate and basal cell layers. On the apical membrane of the marginal cells, ion channels secrete K+ into the endolymph against a concentration gradient. Endolymphatic K+ flows into sensory hair cells when mechanotransduction (MET) channels on the apical surface of the stereocilia open. The K+ influx depolarizes the hair cells and triggers electrical activity in the fibres of the auditory nerve. The hair cells subsequently release K+ via channels in the basolateral surface and K+ is recycled through one of several pathways back towards the stria vascularis (arrows). 138, 139
The acellular tectorial membrane overlies the sensory hair cells; mutations in genes coding for its various constituents can all cause hearing loss, although not all of these forms of genetic hearing loss are congenital in onset. 140, 141, 142,143 The tip link connecting two adjacent stereocilia is located between the apical surface of the shorter one and the lateral surface of the taller one, whereas stereocilin connects the sides of the two stereocilia (top left inset). Mutations in the genes encoding components of the tip links and their interacting proteins might cause syndromic and non-syndromic forms of congenital hearing loss. Unconventional myosin-VIIa, a motor protein that moves along the stereociliary actin filaments, interacts with the PDZ-domain-containing protein harmonin, Usher syndrome type-1G protein and cadherin-23. 144 Cadherin-23 is a long transmembrane molecule that homodimerizes, and its large extracellular domains interact with homodimers of protocadherin-15. 145 These five tip link proteins form the ‘Usher interactome’ and mutations in their coding genes are responsible for Usher syndrome type 1 (Table 1), the most common cause of the dual sensory impairment of hearing and vision loss. Protocadherin-15 forms the lower half of the tip link. Its anchor includes a complex of the motor protein unconventional myosin-XV, its cargo whirlin, epidermal growth factor receptor kinase substrate 8 and calcium and integrin-binding family member 2 (which is also associated with Usher syndrome type 1). 146, 147, 148, 149. The MET channel at the lower tip link density might interact directly with protocadherin-15 (reviewed in Fettiplace & Kim). 37
The intrastrial space (the intermediate cell layer and capillaries), is separated from the marginal cell layer and the basal cell layer by two tight junction barriers, which limit the passive movement of ions. Components of tight junctions include claudins, such as Claudin-14, encoded by CLDN14, and MARVEL domain-containing protein 2 (also known as tricellulin), encoded by MARVELD2, and mutations in either gene give rise to autosomal recessive non-syndromic hearing loss in humans. 30, 31 Mutations in a number of other genes expressed in the stria vascularis that are important for ionic homeostasis in the endolymph result in a variety of syndromic forms of hearing loss (Table 1, and also EAST syndrome – causing epilepsy, ataxia, sensorineural hearing loss and renal tubulopathy – and Bartter syndrome – causing renal tubulopathy and hearing loss) and non-syndromic forms (for example, DFNB73).
Maintenance of the correct pH of the endolymph is also crucial for inner ear homeostasis. Mutations in genes coding for ion transporters and pumps that control the pH and ionic composition of the endolymph can cause Pendred syndrome (resulting in hearing loss and goiter), distal renal tubular acidosis with deafness or non–syndromic early-onset severe-to-profound hearing loss, which is associated with enlarged vestibular aqueducts. Enlarged vestibular aqueducts, whether syndromic (eg. Pendred syndrome) or non-syndromic, cause a fluctuating hearing loss in about one-third of patients. 32 These fluctuations might be associated with endolymphatic hydrops (an excessive accumulation of endolymph in the cochlea and the vestibular system), but the exact mechanism causing hearing impairment and sudden drops in hearing is not well known. 33
Mechano-electrical transduction: stereocilia
Several other forms of inherited hearing loss affect the morphology or function of the inner and the stereociliary bundle of inner and outer hair cells, the cells that converts mechanical stimuli into electrical activity (Figure 2). Stereocilia are actin-rich projections on the apical surface of the hair cells that are arranged in a staircase-like fashion and are tethered together by protein links. The tips of the tallest stereocilia of the outer hair cells are embedded in the overlying tectorial membrane, an acellular gel composed of radial collagen fibres embedded in non-collagenous glycoproteins such as alpha-tectorin, beta-tectorin, otogelin, otogelin-like protein, otolin-1 and carcinoembryonic antigen-related cell adhesion molecule 16. Thus, when the basilar membrane moves in response to sound, the stereocilia anchored in the hair cells move against the tectorial membrane causing a shearing motion.
This deflection of the stereocilia physically opens mechano-electrical transduction channels on the apical surface of the stereocilia (Figure 2) and K+ flows into the sensory hair cells under the electrochemical gradient. This K+ influx depolarizes the hair cells leading to a cascade of events that triggers activity in the fibres of the auditory nerve. K+ in the hair cells is subsequently released from the basolateral surface via channels encoded by the potassium voltage-gated channel subfamily Q member 4 gene (KCNQ4). Mutations in KCNQ4 cause a relatively common form of autosomal dominant non-syndromic progressive hearing loss.
Within the stereocilia, there are longitudinal actin fibres cross-linked for strength and rigidity by several proteins, one of which is espin, encoded by the ESPN gene. Mutations in ESPN cause either autosomal recessive non-syndromic hearing loss, with or without balance problems (absence of reflexes) 34, or autosomal dominant hearing loss, which is progressive and associated with normal balance. 35 At the base of the stereocilia, the actin filaments are tightly packed to form rootlets, which extend into the cell body. Mutations in TRIOBP, the gene encoding the cytoskeleton-associated TRIO and F-actin binding protein, prevent actin filaments from organizing into dense bundles and cause a non-syndromic recessive hearing loss (DFNB28). 36 At the other end of the stereocilium, tip links run from the apical surface of the shorter stereocilium to the lateral surface of its adjacent taller neighbouring cell, where there is an electron-dense anchor composed of several interacting proteins important for hearing (Figure 2). 37 At the bottom of inner hair cells lies the ribbon synapse, which is a specialized type of neuronal synapse with thousands of vesicles that contain the neurotransmitter glutamate (released by calcium-dependent exocytosis of the vesicles). This structure allows rapid and sustained release of thousands of vesicles to accurately encode sound intensity and temporal acuity necessary for speech perception. In this exocytosis process, otoferlin (encoded by OTOF) plays an important part, and Otof knockout mice have hearing loss because of the absence of exocytosis from inner hair cells. 38 Mutations of OTOF in humans may give rise to Auditory Neuropathy Spectrum Disorder (see above).
Acquired congenital hearing loss
Several infectious causes of acquired congenital hearing loss have been identified (Table 2). The emergence of congenital Zika virus infection as a major cause of fetal injury and newborn disability has revealed that this virus can also produce congenital hearing loss. An analysis of 70 infants of 0–10 months of age with microcephaly and laboratory evidence of Zika virus infection in Brazil demonstrated that 7% had sensorineural hearing loss. 39
Table 2.
Congenital infections associated with acquired hearing loss
| Infectious agent | Type (mode of acquisition) |
Transmission | Epidemiology of symptomatic congenital infection |
Early clinical manifestations* |
Late clinical manifestations* |
Diagnosis (prenatal in mother or in amniotic fluid |
Diagnosis (postnatal in newborn) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Toxoplasma gondii | Intracellular protozoan (foodborne) | Higher risk of transmission in later gestation. More-severe symptoms in earlier gestation 152 | 1–10 per 100,000 live births153 | Intracranial calcifications, hydrocephalus and chorioretinitis | Retardation, seizures and SNHL | Maternal serology Amniotic fluid PCR | T. gondii specific IgG, IgM and IgA and PCR in blood and CSF |
| Asymptomatic in >60% of cases | |||||||
|
| |||||||
| Rubella virus | RNA virus Togaviridae (inhalation) | Highest in first trimester 42 | 0–598 per 100,000 live births154 | Cataract, SNHL, cardiac defects and microphthalmia | Retardation | Maternal serology | Rubella IgM and PCR in blood, nasopharyngeal swab, urine and CSF 153 |
|
| |||||||
| Cytomegalovirus | DNA-herpes virus (bodily fluids and sexual contact) | Transplacental | 0.65 per 1,000 live births 41 | Retinitis and intra-cranial calcifications 80 | Mental retardation, cerebral palsy and seizure disorders 80 | Maternal serology and amniotic fluid PCR | PCR in blood, urine and saliva |
| Asymptomatic in 85% of cases | Specimens must be obtained <21 days of age to reliably diagnose | ||||||
|
| |||||||
| Herpes simplex virus | DNA-herpes virus (bodily fluids) | Intrapartum | 0.3 per 100,000 live births155 | Microcephaly, intra-cranial calcifications, skin andocular findings155 | Maternal serology | HSV1-2 surface cultures and HSV1-2 PCR in blood and CSF | |
|
| |||||||
| Treponema pallidum | Spirochete (sexual contact) | Highest in third trimester156 | 8.7 per 100,000 live births157 | “The great mimic”. Diverse array of symptoms in any major body system 153 | Interstitial keratitis, SNHL and notched teeth158 | Maternal serology (nontreponemal and treponemal) | Non-treponemal test in blood |
CNS Central Nervous System; CSF Cerebrospinal fluid; PCR polymerase chain reaction; SNHL Sensorineural hearing loss.
Other than those associated with the infection (for example, rash, thrombocytopenia, anaemia, lymphadenopathy and hepato-splenomegaly)
Cytomegalovirus
CMV, a member of the Herpesviridae family, is the most common potentially disabling perinatal infectious agent. 9, 23 Once an individual is infected, viral DNA is detectable in bodily fluids for months (CMV shedding), especially in the saliva and urine of young children, and represents a potential exposure risk for pregnant women.40 The risk of congenital CMV infection is highest following primary infection during pregnancy, with a risk of vertical transmission in this setting of 32%.41 In seropositive mothers, however, the risk of vertical transmission during reactivation or reinfection is only ~1.4 %. Given that seropositivity for CMV in women of childbearing age in developed countries is ~50%, the incidence of congenital CMV infection is roughly 1 in every 100–200 live births.41
The pathophysiologic basis of sensorineural hearing loss following congenital CMV infection is unclear. Studies in the temporal bone have demonstrated inflammation and oedema of the cochlea and spiral ganglion, and viral antigens have been found in the spiral ganglion, organ of Corti, scala media and Reissner’s membrane.42 Evidence from murine models suggests infection and direct cytolysis of components of the labyrinth, including hair cells.43, 44 In another murine model of CMV infection, hearing loss was associated with a loss in spiral ganglion neurons following experimental challenge.45 In addition to the direct cytolytic effect of viral infection, there is also evidence for immune injury, mediated by both the host immune response as well as the expression of viral genes encoding pro-inflammatory chemokines. 46 The virulence of the virus and the immune responses of the mother, fetus and placenta have a crucial role in the outcome.47 Approximately 10% of CMV-infected newborns are symptomatic at birth and the risk of symptoms in the neonate is highest when maternal infection occurs around conception or within the first trimester of pregnancy.48
Rubella
Infants with congenital rubella virus infection are usually born at term, but often have a lower birth weight than non-infected newborns of the same gestational age. The most common complication of congenital rubella infection is hearing loss.49 Other common findings are heart defects, cataracts, hepato-splenomegaly and microcephaly. Hearing loss, cataracts and congenital heart disease represent the classic triad of manifestations of congenital rubella syndrome, 50 although clinical signs can vary depending on the timing of fetal infection. In a prospective study of pregnant women with confirmed rubella infection, a range of rubella-associated complications (including congenital heart disease and hearing loss) were observed in nine infants who were infected by the 11th gestational week. 35% (9 out of 26) of infants infected between 13–16 gestational weeks had only one complication — hearing loss.49 Hearing loss associated with congenital rubella syndrome may not occur until after birth.51 The mechanism of rubella infection-induced hearing loss has not been fully explained, although the virus can cause direct cochlear damage, cell death in the organ of Corti and stria vascularis and alterations in the composition of the endolymph following strial damage. 52, 53, 42
Diagnosis, screening and prevention
In the past century, targeted screening was performed exclusively in babies considered at high risk of hearing loss (that is, infants admitted to the neonatal intensive care unit and those with a family history of hearing loss or craniofacial anomalies). However, given the growing evidence that early detection of hearing loss is beneficial to child development, universal neonatal hearing screening programmes have been introduced. In many developed countries, universal neonatal hearing screening is now well established and typically uses a two-phase screening paradigm (that is, two electrophysiological measurements performed sequentially).
Assessment of hearing status
Screening usually consists of the measurement of oto-acoustic emissions (see below) repeated twice, measurement of oto-acoustic emissions and automated auditory brain stem responses (see below), or measurement of automated auditory brain stem responses repeated twice.54 Infants who do not pass the screening require appropriate audiological and medical evaluation to confirm the presence of hearing loss, ideally before the age of 3 months. 7 However, passing the neonatal hearing test does not exclude progressive, late-onset and less-severe congenital hearing loss (30–40 dB hearing loss), which is not detected in most neonatal hearing screening programmes. Children who pass neonatal hearing screening but have risk factors for hearing loss or whose parents express concern about their child’s hearing abilities need regular follow-up, because hearing loss can develop later in life, depending upon the underlying cause.
Upon referral from neonatal hearing screening, a complete audiometric assessment is required to confirm the presence of hearing loss and to assess its severity and laterality (unilateral or bilateral). The severity of the hearing impairment should be applied to the better hearing ear and averaged over 500, 1,000, 2,000 and 4,000 Hz. Hearing loss is classified based on laterality and severity as mild (20–40 dB hearing loss), moderate (41–70 dB), severe (71–95 dB) and profound (> 95 dB). 55
The audiometric assessment includes electrophysiological (oto-acoustic emissions, which estimate the function of outer hair cells, and auditory brain stem responses, which estimate of the function of inner hair cells and the integrity of the auditory pathways) and behavioural (audiometry) testing. In clinical practice, the functional integrity of the ear is assessed by different tests to cross-check the results of both physiological and behavioural measures.
Oto-acoustic emissions
Oto-acoustic emissions are sounds caused by the motion of the outer hair cells as they energetically respond to auditory stimulation.56 Transient evoked oto-acoustic emissions occur after the application of a click stimulus. The oscillatory sound pressure waveform seen in a transient evoked oto-acoustic emissions response corresponds to the motion of the tympanic membrane (eardrum) being pushed back and forwards by fluid pressure fluctuations generated in the cochlea. Transient evoked oto-acoustic emissions responses can give a frequency-specific indication of cochlear status and can be measured by a small probe in the external auditory canal. As their detection requires adequate sound transmission to and from the cochlea, oto-acoustic emissions should not be used as a stand-alone test for assessing normal hearing status, but rather must be interpreted in the context of otoscopy, tympanometry and auditory brain stem responses testing.
Auditory brain stem responses and auditory steady state responses
Auditory brain stem responses are electrical potentials elicited by auditory stimuli that reflect neural activity at several discrete points along the auditory pathway. The activity is recorded from scalp electrodes using computer-averaging techniques. Click- or tone burst-induced auditory brain stem responses are most commonly used and are considered the gold standard for the objective assessment of hearing in infants and children of all ages. The obtained thresholds are typically within 10 dB of the behavioural auditory thresholds at the higher frequencies (between 2,000–4,000 Hz).
Auditory steady state responses are elicited by AM/FM-modulated tonal stimuli. The stimulus is a continuous signal and can deliver higher average sound pressure levels than click stimuli, making auditory steady state responses useful for obtaining threshold data in children with profound hearing loss (>90 dB). The absence of auditory steady state response thresholds indicates no usable hearing and predicts poor hearing aid performance.57 For all types of hearing loss, there is a close correlation between click auditory brain stem responses and average auditory steady state responses thresholds at 2,000 and 4,000 Hz, with a difference ≤10 dB between the thresholds measured with the two methods in the majority of cases.58 Auditory steady state responses are also useful to estimate the bone conductive hearing thresholds and to distinguish conductive hearing loss from sensorineural hearing loss.
Audiometry
Visual re-enforcement audiometry can be used to test hearing in children between 6–24 months of age. In children with adequate hearing, a new sound source will provoke an orientation reflex towards the sound. Skilled audiologists can obtain reliable results. Play audiometry is used in children 2–4 years of age, by means of conditioning them to respond to an auditory stimulus through play activities.59 After 4 years of age, standard audiometry is typically used, with an air-conduction transducer (for example, an earphone) or a bone-conduction transducer (or both) (Figure 3). The former tests the integrity of the complete auditory system, whereas the latter vibrates the skull, which stimulates the cochlea directly, bypassing the external and middle ear. Air conduction and bone conduction thresholds, mostly obtained at octave frequencies of 250–8,000 Hz, differentiate sensorineural hearing loss and conductive hearing loss.
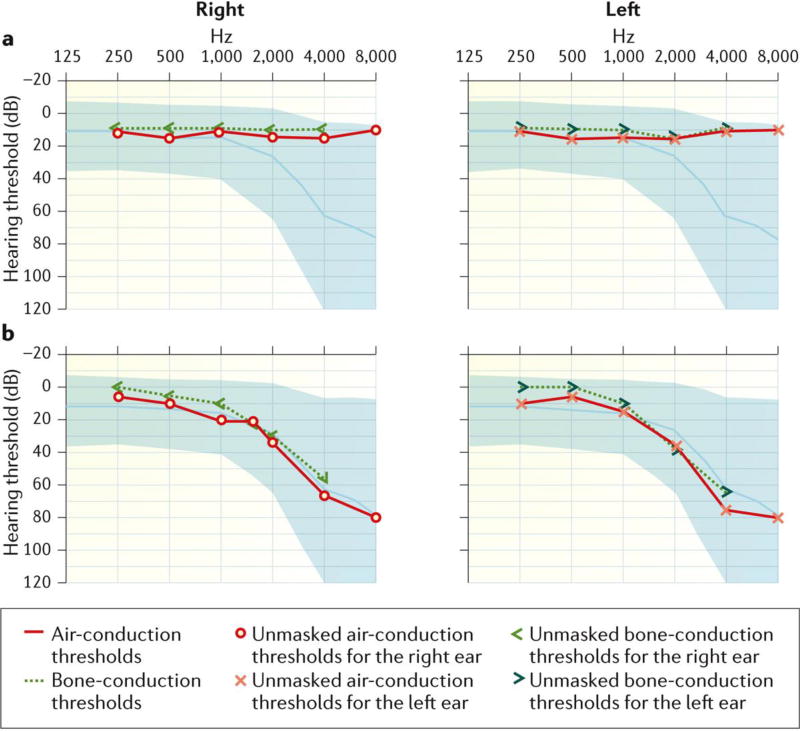
Figure 3. Audiometry assessment.
A. Pure tone audiometry obtained in a child with bilateral normal hearing thresholds across all frequencies. B. Pure tone audiometry obtained in a child with bilateral and symmetric sensorineural hearing loss. Hearing thresholds are normal up to 1,000 Hz. A ski-slope audiometric configuration is recorded at higher frequencies, showing mild hearing loss at 2,000 Hz that increases to severe hearing loss at 8,000 Hz.
Aetiological work up
Once a diagnosis of bilateral permanent congenital hearing loss is established, the search for an underlying aetiological diagnosis is required. The available guidelines include screening for congenital infections, imaging and genetic testing (see below). 28, 60, 61, 62 First line genetic tests are usually limited to screening for mutations in GJB2 and GJB6. The work-up is complemented by ophthalmologic screening to check for ocular signs of congenital infection or syndrome specific details, kidney ultrasonography to check for congenital malformations, electrocardiogram to rule out long-QT syndrome (as is seen in Jervell Lange-Nielsen) and other tests based upon clinical findings. In unilateral hearing loss, the aetiological work-up can be limited to a thorough clinical examination for a syndromic cause of hearing loss, investigation of possible congenital infections and imaging of the inner ear.
Most available guidelines on the aetiological work-up of congenital hearing loss do not incorporate the diagnostic power of next generation DNA sequencing technology and the use of comprehensive genetic testing using gene panels (see below). Comprehensive genetic testing using targeted genomic enrichment with massively parallel DNA sequencing has changed the diagnostic algorithm and in the future, the need for complementary examinations might be guided by the suspected diagnosis and the results of comprehensive genetic testing. A guideline published by the American College of Medical Genetics and Genomics recognizes the value of this new technology and recommends the use of gene panels. 61 Meanwhile, the use of a sequential diagnostic algorithm (Figure 4) based on the degree of hearing loss is advocated for children with bilateral congenital hearing loss 63 and, through a multidisciplinary approach, an aetiological factor can be identified in about half of the patients. 28
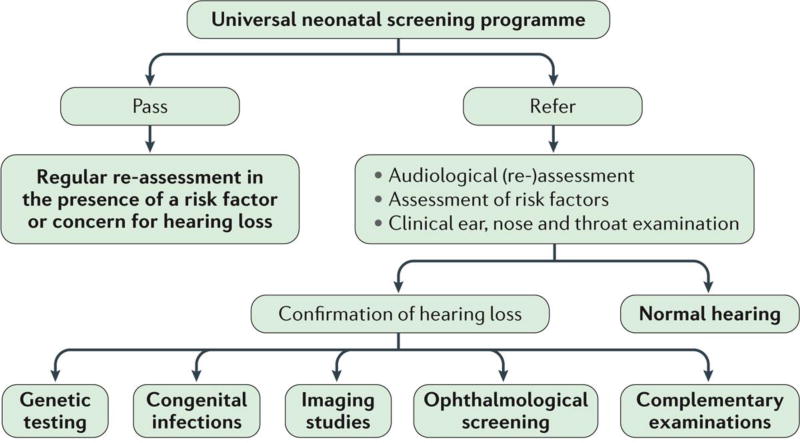
Figure 4. Multidisciplinary algorithm for the assessment of hearing function in infants.
Newborns who pass the neonatal hearing screening should undergo regular follow-up when risk factors for hearing loss (as defined by the Task Force of the American Academy of Pediatrics) are present. 7 If a newborn fails the screening and bilateral congenital hearing loss is suspected, a comprehensive audiological and aetiologic work-up is required. Audiological tests can confirm the presence of hearing loss and determine its type (conductive, sensorineural or auditory neuropathy spectrum disorder), laterality and severity. Genetic testing is an integral part of the aetiological work-up, as are the exploration of perinatal insults and the presence of congenital infections as possible causative agents. In particular, timely investigation for congenital cytomegalovirus (CMV) infection is essential, as it is the most common infectious aetiology of hearing loss. Virological identification of CMV must be made in the first 3 weeks of life to ensure that the infection was truly congenital and not post-natally acquired. Imaging studies are recommended in all cases of bilateral hearing loss ≥60 dB or with craniofacial malformations. 62 Imaging exams can rule out the presence of structural inner ear anomalies, which might occur as an independent entity, be part of a syndrome, or have therapeutic implications. Certain inner ear anomalies might place the child at increased risk for sudden hearing loss (for example, enlarged vestibular aqueducts) or meningitis and require appropriate counselling. Imaging studies are a prerequisite before cochlear implantation to assess cochlear anatomy and confirm the presence of a cochlear nerve. A detailed assessment of the test results by a paediatric ophtalmologist is recommended, given the high prevalence (40–60%) of ophthalmologic problems in hearing impaired children. 150 Other complementary investigations include, for example, electrocardiogram and renal ultrasonography. ENT; ear, nose and throat.
An aetiological work-up for congenital hearing loss should be conducted for many reasons. It can provide parents with an answer as to why their child has hearing loss, allow accurate and personalized genetic counseling, provide relief from guilt in some cases and aid in management. A genetic diagnosis may provide an accurate estimate of risk for hearing loss in future children and may be helpful for parents in terms of family planning. Identifying the aetiology might help to choose appropriate therapeutic or management options (for example, hearing aids, cochlear implantation or adapted educational needs), might identify coexisting medical problems that need to be treated or monitored (especially when a syndromic genetic cause of hearing loss is found in a child referred as non-syndromic), secure preventable risk factors for future hearing deterioration (for example, aminoglycoside use or head trauma) and might also predict the progression of hearing loss to a certain extent.
Genetic diagnostics
Genetic diagnosis always starts with family history and the creation of a pedigree. This information is essential to define the most probable mode of inheritance, which can in turn limit the list of potential causative genes. A patient without other affected family members might represent a case of autosomal recessive inheritance, but environmental causes of hearing loss must also be considered.
DNA diagnostics for non-syndromic hearing loss is challenging, as there are numerous possible responsible genes and generally few diagnostic clues based on the phenotype. Thus, for a long time, diagnostic application of the identification of genes associated with hearing loss has lagged behind scientific progress. From 1998–2010, diagnostic laboratories in different countries typically analyzed a handful of genes for non-syndromic hearing loss, including the most frequently mutated GJB2. This approach typically identified the responsible gene in only 10–20% of patients with non-syndromic hearing loss. Technological developments, such as next generation DNA sequencing, have enabled the simultaneous analysis of large numbers of genes. Using these techniques, several laboratories are now offering genetic testing for large panels of genes linked to syndromic or non-syndromic forms of hearing loss at an affordable price. Comprehensive genetic testing now has the highest diagnostic rate of any test in the evaluation of hearing loss once it is confirmed by audiometry results. 64
At present, genetic diagnosis can also assist in predicting the success of specific clinical treatments. For example, in cases of auditory neuropathy spectrum disorder with OTOF gene mutations, the function of the auditory nerve is expected to be preserved. However, the finding of cochlear nerve hypoplasia in some patients with auditory neuropathy spectrum disorder associated with mutations in the apoptosis inducing factor, mitochondria associated 1 gene (AIFM1) suggests that cochlear implantation in these patients might have limited success. 65 Thus, genetic diagnosis is useful in clinical decision-making.
Diagnostics in acquired congenital hearing loss
Any newborn with signs of congenital infection should be tested for CMV infection, as congenital CMV infection is the leading non-genetic cause of congenital hearing loss in developed countries. This infection should also be considered in children with hearing loss who are otherwise healthy and asymptomatic.66 Signs and symptoms of CMV infection include intrauterine growth retardation, microcephaly and jaundice, with sensorineural hearing loss present in approximately 30% of symptomatic CMV-infected children.67 In these instances, a diagnostic evaluation is indicated.48 Pre-natally, CMV PCR on amniotic fluid can confirm congenital CMV infection (the positive predictive value is close to 100%). 68 After birth, the urine, saliva or throat swab specimens of the newborn should be collected (samples must be collected within 3 weeks of birth, since viral shedding after this time point may reflect postnatally acquired, and not congenital, infection) and analysed. 69 In children undergoing evaluation of the aetiology of sensorineuroal hearing loss beyound three weeks of age, congenital CMV infection can only be confirmed in retrospect, by using stored dried newborn blood spots as the source of template for PCR-based diagnosis. In many developed countries, a blood sample is taken routinely during the first week of life for screening for metabolic, endocrine and other disorders. The remaining blood is stored on dried blood spots. The availability of these samples, however, depends on local storage policies; moreover, these bloodspots have suboptimal diagnostic sensitivity, compared to saliva or urine samples obtained in “real-time”.
If congenital CMV infection is suspected, in addition to laboratory tests, brain imaging (cerebral ultrasonography or MRI), visual function assessment and hearing assessment are required. However, in 90% of newborns congenital CMV infection is virtually asymptomatic. These children generally experience fewer neurodevelopmental problems than those who are symptomatic at birth, but 10% will nonetheless develop substantial sensorineural hearing loss sometime in childhood. 24
A definitive laboratory diagnosis of congenital rubella infection can usually only be made definitively within 12 months from birth. Rubella infection is diagnosed if at least one of four criteria is met 70: a positive anti-rubella IgM titre (possibly measured with enzyme immunoassays), a substantial rise in anti-rubella IgG titer 2–3 weeks after the acute phase of the infection or high titers persisting beyond what can be expected from passive maternal antibody transfer, the isolation of rubella virus in cultures from throat, nasal, blood, urine or cerebrospinal fluid specimens, or the detection of the virus by reverse transcriptase PCR in throat swabs, cerebrospinal fluid or surgical samples (from congenital cataracts, as the virus can be isolated from the lens).
Despite having neutralizing antibodies, a child infected with rubella can be infectious for months, posing a hazard to susceptible individuals. 71 Occasionally, the virus can be isolated even after 12 months, for example from the cataract, where rubella can survive up to 3 years. In late-onset disease, the virus might also be present in the skin and lungs. Additional laboratory test results that can confirm the diagnosis of congenital rubella infection are thrombocytopenia, hyperbilirubinaemia and leukopenia. Although congenital rubella infection has become rare in the developed world owing to the eradication of the virus from the Western hemisphere, cases of imported disease can still be observed. Moreover, congenital rubella infection is still endemic in some low-income countries in the developing world, and, therefore, rubella infection should be considered in the diagnostic work-up of unexplained hearing loss if the infection cannot be excluded on historical or epidemiological grounds. 60
Management
Therapeutic nonsurgical management of pathogen-associated hearing loss currently focuses on two key areas of intervention: specific antimicrobial therapies and anti-inflammatory therapies to mitigate the host’s immune response to the infection and thereby reduce the damage to the cochlea. With a better understanding of infectious disease-related hearing loss, however, novel therapies might emerge, such as the use of free radical scavengers72, anti-oxidants73 and nanoparticle-based systems74. Surgical treatment might be beneficial in select cases in which there is an air-bone gap that is amenable to correction by surgical intervention. Examples of non-medical support are special education and sign language. 75, 76, 77
Nonsurgical treatment
Cytomegalovirus
Clinically manifest congenital CMV infection with central nervous system involvement requires antiviral treatment. Therapy with intravenous ganciclovir or oral valganciclovir for 6 weeks was initially viewed the preferred treatment option, although neutropenia was recognized as a possible adverse effect. 78 More recently, it has been demonstrated that administration of the oral pro-drug of ganciclovir, valganciclovir, for 6 months starting in the first month of life improves neurodevelopmental outcomes and hearing in infants with symptomatic congenital CMV infection. 79 Other therapeutic options currently being explored include prophylactic vaccination, immunoglobulin therapy and prenatal antiviral therapy.68, 69, 80 Children with congenital CMV infection require special follow-up with serial audiometry even if hearing is normal at birth, because of they are at substantial risk for late-onset or progressive hearing loss. 81
Rubella and other infections
Prepubertal vaccination can prevent congenital rubella infection. Live attenuated rubella virus is typically administered in a trivalent formulation, combined with measles and mumps vaccines, or in a quadrivalent formulation that also includes varicella vaccine. Vaccination is recommended for children 12–15 months of age, with a booster at 4–5 years of age, and for women of childbearing age who are not pregnant and have a negative haemagglutination inhibition antibody test.
Only limited data support a role for medical management for other infections that can lead to sensorineural hearing loss (Table 3). Congenital toxoplasmosis, caused by the parasite Toxoplasma gondii, is typically associated with intracranial calcifications, chorioretinitis and hydrocephalus.82 However, the prevalence of hearing loss in congenital toxoplasmosis might be higher than appreciated, because the majority of infected children are asymptomatic at birth.83 Maternal T. gondii infections are treated with spiramycin, and recommended therapies in infants with congenital infection include pyrimethamine and sulfadiazine. Syphilis, caused by Treponema pallidum infection, is causally related to sensorineural hearing loss in both infants who have congenital infection and (albeit more rarely) in children who acquire it post-natally.
Table 3.
Characteristics of infectious congenital hearing loss
| Infectious agent | Pathophysiology (presumed) | Prevalence | Unilateral/bilateral | Severity | Progression | Treatment |
|---|---|---|---|---|---|---|
|
| ||||||
| Toxoplasma gondii | Inflammatory response to the tachyzoite form of T. gondii induces CNS necrosis159 | 0%160 | unknown | unknown | Unknown | The effect of pyrimethamine and sulfadiazine on hearing loss is unknown160, 161 |
|
| ||||||
| Rubella virus | Direct damage and cell death in the organ of Corti and stria vascularis52 | 58%42–66%153 | Bilateral42 | Mild to severe42 | Possible | There is no specific treatment available |
|
| ||||||
| Cytomegalovirus | Viral labyrinthitis and inflammatory injury 43 | 15% (industrialized countries) | Unilateral or bilateral | Mild to profound | Common | Ganciclovir or valganciclovir slow the progression and stabilize hearing loss |
| 33% (developing countries) 162, 163 | Established hearing loss is generally irreversible even with antiviral therapy | |||||
|
| ||||||
| Herpes simplex virus | Only in association with confounding factors associated with SNHL164 | Unknown | Unilateral or bilateral164 | Mild to severe | Absent164 | The effect of acyclovir155 on hearing loss is unknown |
|
| ||||||
| Treponema pallidum | Obliterative endarteritis165 | Unknown | Bilateral | Profound | Possible | The effect of intravenous penicillin on hearing loss is unknown156 |
CNS, central nervous system; SNHL, sensorineural hearing loss
All infants born to women with suspected or confirmed Zika virus infection during pregnancy should undergo newborn hearing screening before hospital discharge. Infants with laboratory evidence of congenital Zika virus infection should be referred for hearing evaluation by auditory brain stem responses in the first month of life; if the test is normal, repeat automated auditory brain stem response testing is recommended at 4–6 months of age. 84
Restoration of hearing
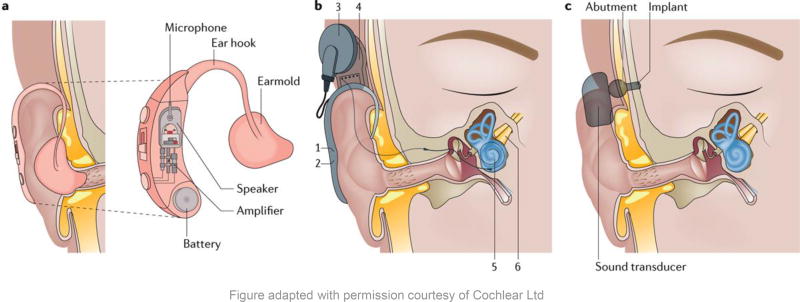
Restoration of hearing is achieved by implantable or non-implantable hearing devices, including conventional hearing aids, cochlear implants and bone-anchored hearing aids; their basic principles and working mechanisms are illustrated in Figure 5.
Figure 5. Non-medical treatments for hearing loss.
A conventional hearing aid converts environmental sounds to amplified sounds. A hard case is worn behind the auricle and contains all the electronic parts (microphone, amplifier and battery). A sound is picked up by a microphone and converted to an electrical signal that corresponds to the pressure variation produced by the sound. This signal is then amplified and delivered to a speaker that converts the amplified electrical signal back to sound. The speaker sends the sound signal to the tympanic membrane by a slim tube connecting the hearing aid to an earmold that fits in the external auditory canal. B. A cochlear implant converts sounds to electrical signals and is composed of different parts. A microphone (1) picks up environmental sounds and transmits them to a speech processor. Through a magnetic coil (3), acoustic signals are transmitted from the speech processor to a subcutaneously implanted receiver/stimulator (4) that converts the acoustic signal into electric impulses. An electrode array (5) placed in the scala tympani of the cochlea directly stimulates electrically the auditory nerve (6). C. A bone-anchored hearing aid converts a sound signal to micro-vibrations: it uses the principle of bone conduction to directly stimulate the cochlear fluids by vibrating the skull behind the ear at auditory frequencies. A titanium screw (the implant) is surgically anchored in the bone and becomes fixed through a process called osseo-integration. The implant is connected to a sound transducer by means of an abutment (connector). The sound transducer captures the sound, converts it to vibrations and sends them to the implant. The implant then transmits the vibrations through the bone directly to the inner ear. In the most recent bone-anchored hearing aid systems, the abutment is replaced by a magnetic connection. Copyright permission obtained from Cochlear Limited ©
Conventional hearing aids
In most patients with sensorineural hearing loss, auditory rehabilitation consists of conventional hearing aids. Even in some patients with conductive hearing loss, hearing aids are the principal treatment, especially when medical or surgical options are not feasible. Continued developments in signal processing and increasing miniaturization have increased the performance of hearing aids and their acceptance among patients. 85, 86 For example, the maximum gains for digital in-the-ear, in-the-canal and completely-in-canal aids are about 55–65 dB, 45–55 dB and 35–50 dB, respectively. Nearly all hearing aids are digital and programmable and, therefore, can be customized to the characteristics of the patient’s hearing.86
Despite these advantages, hearing aids have a number of limitations, including lack of sufficient perceived benefit, high expenses, complications (such as occlusion of the external auditory canal) and cosmetic concerns.87–89 As a result, only one in five eligible adults actually uses a hearing aid.90, 91 By contrast, hearing aids use in children is much more widespread, because of the recognized critical importance of early intervention for hearing loss management made possible by universal neonatal hearing screening.92 The limitations of conventional hearing aids have generated enthusiasm for implantable middle ear hearing technologies that attempt to solve many of the difficulties imposed by conventional aids. Implants couple vibration stimuli directly to the inner ear through multiple ways, thereby offering higher gains and reduced sound distortion than conventional aids However, the need for surgery, high costs and lack of insurance reimbursement have limited their widespread implementation.93–95
Cochlear implants
For patients with mild-to-severe sensorineural hearing loss, conventional hearing aids can provide excellent hearing rehabilitation. However, once hearing loss becomes severe-to-profound, these technologies no longer provide adequate clarity of sound for meaningful speech understanding, and cochlear implantation is preferred. Meta-analyses document that unilateral cochlear implants provide improved hearing and quality of life scores in recipients, whereas bilateral cochlear implants lead to substantial improvements in sound localization and hearing in noise. 96 Furthermore, cochlear implantation is no longer limited to patients with severe-to-profound hearing loss and is now suitable for patients with relatively good low-frequency hearing but poor high-frequency hearing. In these patients, ‘hybrid’ or ‘electro-acoustic stimulation’ implants are used, which offer considerably improved sound quality in combination with natural hearing in the low frequencies.97, 98 These implants are very nearly identical to standard cochlear implants, though have smaller diameter electrode arrays which are only inserted into the basal turn of the cochlea.
Cochlear implantation is now the standard of care for children with profound congenital hearing loss whose parents choose to use oral communication. With the availability of screening and early detection, the age at first implantation has progressively declined and many children are now fitted with an implant before their first birthday. 99 Since bilateral cochlear implantation has shown to be superior to unilateral cochlear implantation in terms of vocabulary outcomes, speech perception and sound localisation, a growing number of countries provide reimbursement for a second implant in children. The outcome of cochlear implantation in children might depend upon the underlying aetiology and associated comorbidities, especially in children with syndromic hearing loss or associated disabilities.100, 101 Genetic testing is an important tool for predicting the outcomes of cochlear implantation or electro-acoustic stimulation and is useful for choosing the appropriate treatment. 102, 103, 104, 105
Bone-anchored hearing aids
Improvement or restoration of hearing in conductive hearing loss might be challenging in children with congenital anomalies, such as the spectrum of atresia (abnormal narrowing or absence) of the external auditory canal. Children with atresia of the external auditory canal with minimal or no involvement of the ossicular chain can benefit from microsurgical intervention, though surgery is usually postponed until ≥6 years of age.106 In children with complete bony atresia, conventional air-conduction hearing aids are not an option. When surgical reconstruction is not possible or declined by the family, bone-anchored hearing aids are indicated. 106, 107 Compared with adults, however, children have a higher incidence of complications, including non-osseo integration (as high as 15%).108 A meta-analysis of bone-anchored implant complications has demonstrated skin reactions in 2.4–38.1% of cases, failure of osseo integration up to 18% and revision surgery rates up to 44%.109 These results are consistent across studies. Underlying cochlear function is an important determinant of benefit with bone-anchored implants; in general, children with pure tone bone-conduction averages better than 45 dB receive excellent benefit from bone-anchored hearing implants, whereas those with averages 45–60 dB have intermediate benefit.
An additional important advantage of bone-anchored hearing aids is their rehabilitation of unilateral sensorineural hearing loss.110–112 Placed in the hearing-impaired ear, these devices expand the sound field for the patient and significantly improve speech understanding in noise, similarly to a contralateral routing of offside signal hearing aid or a transcranial system. In these situations, the processor placed on the side of the deaf ear acts as a microphone which then directs sound to the hearing ear directly through the skull bone, whereas a contralateral routing of offside signal aid will transmit that sound wirelessly to a hearing aid worn in the good ear. An increasing body of evidence has clearly demonstrated that the primary benefit is improved hearing in noise, whereas the implants provide little or no objective benefit in sound localization.
Other surgical treatment options
Both implantable and non-implantable hearing devices offer excellent rehabilitative options for patients with hearing loss. Depending on the nature and extent of hearing loss, other surgical options might also be available. For patients who have conductive hearing loss due to abnormalities of the external auditory canal (for example, congenital aural atresia), tympanic membrane (from acute or chronic infections) or ossicles (from congenital or acquired fixation of the ossicles), surgery can be attempted to correct these defects, often with excellent functional results. 113
Quality of life
Hearing-impaired children who do not receive early intervention and rehabilitation will fall behind their normal-hearing peers in reading skills, cognition and socio-emotional development. This gap might in turn result in modest educational achievements and employment levels in adulthood. 114
Speech development is impaired by delayed age-at-diagnosis of hearing loss. Children whose hearing loss is identified by 6 months of age have significantly better receptive and expressive skills than children whose hearing loss is identified later.5 The age of hearing loss identification effect is evident across age, sex, socio-economic status, ethnicity, cognitive status, degree of hearing loss, mode of communication and presence or absence of other disabilities.115 The positive effect on language outcomes of early confirmation of permanent childhood hearing impairment that is observed in children at primary school age is also found in teenagers. Moreover, the gap in reading skills between children with early confirmed permanent childhood hearing impairment and children with later confirmed impairment widens with age. 116
Developmental impairment is measurable as early as at 3 years of age. The DECIBEL study compared development and quality of life for children whose hearing loss had been identified through a neonatal hearing screening programme (that is, before 2 weeks of age) and children whose hearing loss had been diagnosed after a distraction hearing programme performed at 9 months of age. Children in the neonatal screening programme received hearing amplification 13 months earlier than children in the distraction hearing screening. Quality of life scores (Pediatric Quality of Life Inventory) at 3–5 years of age were significantly better in children with permanent hearing impairment identified in the neonatal screening programme. 117, 118
Factors that might contribute to the delay between diagnosis and intervention include: time for reflection requested by the child’s parents, cultural considerations, doubts about the degree of hearing loss and the benefits of hearing amplification, acceptance and wearing of hearing aids and practical and technical considerations.
The introduction of hearing aids before 6 months of age will improve subsequent hearing development and is now considered a standard goal in the management of children with bilateral hearing loss. 7
Outlook
The biggest challenge in the short term in congenital hearing loss is the better prevention of infectious etiologies. In the long term, it is moderating the effects of genetic hearing loss.
Cytomegalovirus-associated hearing loss
The complex correlations among CMV-induced inflammation, local and systemic host response and the development and progression of sensorineural hearing loss are not adequately understood. Ribosomal profiling has shown that the protein-coding capacity of CMV includes up to 751 open-reading frames (including splice variants), most of which are translated into small proteins of unknown function that are predicted to be shorter than 100 amino acids in length. 119 Because CMV depends on inflammation for reactivation, some of these proteins might simultaneously induce local inflammation and protect the reactivated virus from the host’s immune recognition and destruction. 120 CMV genome includes multiple genes associated with immune evasion, which promote persistence of the infection and preclude its clearance. 43, 121 Furthermore, the presence of multiple epitopes across CMV strains means that IgG seropositivity does not completely guarantee protection against re-infection, which increases the challenge of developing a vaccine to completely prevent congenital transmission and its attendant sequelae. 122, 123, 124
Improving genetic diagnoses
A complete panel of diagnostic tests for hearing loss, which includes a number of laboratory studies, serum chemistries and a variety of imaging tests, is collectively very costly and often only of limited value in establishing aetiology. Comprehensive genetic testing is less expensive than any temporal bone imaging technique and has the highest single-test positive diagnostic rate in the evaluation of non-syndromic hearing loss. As the cornerstone of precision medicine for hearing health care, genetic results can lead to major differences in patient diagnosis and management (that is, Jervell and Lange-Nielsen syndrome, Usher syndromes and mitochondrial DNA-related hearing loss) and therefore makes personalized management possible. However, one of the current problems with comprehensive genetic testing is the challenge associated with the interpretation of a large number of genetic variants. Therefore, high quality comprehensive genetic testing requires careful interpretation of genetic results in light of the phenotypic data by a multidisciplinary panel of experts that should include human geneticists with focused expertise in hereditary hearing loss, bio-informaticians, clinicians, genetic counsellors, research scientists and technicians. In addition to interpreting single-nucleotide variants, this panel should also always provide copy number variant interpretation, as copy number variants have been implicated in up to 20% of diagnoses of non-syndromic genetic hearing loss. 125 One of the factors that complicate the evaluation of the pathogenicity of variants is the ethnicity of the patients, as genetic variants often have different frequencies in different ethnicities. Concerted efforts should be made to sample multiple distinct ethnicities, as these data are of great importance for assessing possible pathogenicity of identified variants.
Integration of the patient’s hearing loss phenotype with the underlying genotype is essential. Except for GJB2, no large-scale genotype-phenotype studies have been performed.20 However, these genotype-phenotype correlations are of great importance for the interpretation of the results of genetic testing, as they provide crucial information about the possible pathogenicity of genetic variants. Complex modelling tools like AudioGene (http://audiogene.eng.uiowa.edu/) offer several enhancements to traditional audiometry and might provide a better tool with which to dissect complex genotype–phenotype interrelationships. 126
Hearing preservation and restoration
Emerging therapies for hearing loss can be broadly sub-classified as hearing preservation or hearing restoration strategies. 127, 128 Hearing preservation would probably be easier to achieve than hearing restoration; preservation strategies seek to promote hair cell survival and correct protein defects before complete and irreversible hair cell damage occurs. Once hair cells have died, hearing restoration strategies are required, with options depending on the condition of the remaining supporting cells.
Thanks to progress in genetic testing, it is possible to identify groups of patient with gene-specific mutations. Studies aimed at elucidating the broader effects of mutations at the gene level are needed to identify pathways interactions and crosstalk, which in turn are likely to provide novel insights into hearing preservation strategies.
It might be possible to trans-differentiate healthy supporting cells into hair cells in the cochlea. 129 For trans-differentiated hair cells to function properly, however, they must also integrate into their local microenvironment. Thus, even if the hurdle of trans-differentiation were overcome, other substantial challenges would remain. Although trans-differentiation therapy might be successful, several problems have to be resolved before its clinical application is contemplated 128. For example, trans-differentiated hair cells often are not completely normal and instead exhibit a phenotype intermediate between a hair cell and a supporting cell. With more-severe inner ear damage, the response to trans-differentiation therapy is not very good, suggesting that a better understanding of the mechanism of hair cell death (apoptosis or necrosis, or both) is crucial 130. Once hair cells are lost, the basilar membrane becomes lined with non-sensory cells, making protective therapies, even at this late stage, important to prevent the transition to a flat epithelium. 128
Drug delivery and therapeutic targets
Pharmacological treatment to enhance self-repair of hair cells (hearing preservation) and trans-differentiation of supporting cells (hearing restoration) will require high throughput, rapid screening systems to optimize rational drug design and the development of drug delivery systems for the cochlea. Studies have demonstrated the efficiency of zebra-fish models in drug design 131 and middle ear infused, gelatine-based hydrogels appear promising as a means to deliver drugs to the cochlea. 132 A novel intra-tympanic polymer gel delivery system has been evaluated and tested as a strategy for antiviral drug delivery in a guinea pig model 133 and holds promise for providing antiviral therapy to the CMV-infected cochlea while sparing the patient the substantial toxicities associated with systemic delivery of antiviral agents.
Neonatal hearing screening programmes
Over 50% of cases of permanent childhood hearing impairment can be detected shortly after birth through a programme of neonatal hearing screening. However, passing the neonatal screening does not guarantee normal hearing in childhood and is not a valid reason to disregard parental suspicion of hearing impairment. Progressive or late-onset hearing impairment, as seen with congenital CMV infection or in some genetic conditions, is undetected by neonatal screening programmes. Thus, postnatal identification of childhood hearing loss will remain dependent upon the interaction between parents and professionals. All individuals working with children (for example, teachers and health care providers) should monitor the child’s general development and especially language development.
Other limitations of neonatal hearing screenings are related to the sensitivity and specificity of the screening method, coverage and follow-up after a referral from screening. For example, although universal neonatal hearing screening in Flanders, Belgium, has high sensitivity (94.02%) and specificity (99.96%) 134, false positive test results might cause unnecessary anxiety in parents during the vulnerable first weeks of the their newborn infant.
Coverage might be a concern in low-income countries where hearing screening programmes are not available or access to them is limited. In many universal neonatal hearing screening programmes, the progress from screening to intervention is the weakest point of the health care pathway, with the proportion of children lost to follow-up (and treatment) as high as 52% of those referred.135
An increasing body of evidence shows that universal neonatal hearing screening is not only beneficial for the child’s development and quality of life, but is also cost-effective. The costs of neonatal hearing screenings are comparable with other newborn screening programmes and the benefits are expected to outweigh the costs. 7, 136 An economic analysis has confirmed that both universal and targeted screenings for congenital CMV infection are cost-effective, an observation that should help drive the expansion of screening programmes for this infectious cause of hearing loss in infants. 137
Box 1. Risk factors for permanent congenital, delayed or progressive hearing loss in childhood.
Hearing, speech, language or developmental delay
Family history of hearing loss
Neonatal intensive care unit stay >5 days or receiving any of the following treatments: extra corporal membrane oxygenation, assisted ventilation, ototoxic drugs (for example, gentamycin and tobramycin), loop diuretics or exchange transfusion for hyperbilirubinaemia
In utero infections (toxoplasmosis, rubella, cytomegalovirus, herpes simplex or syphilis)
Craniofacial anomalies, including ear tags (small flaps of skin in front of the ear), ear pits (A tiny opening in the skin usually in front of the ear and above the ear canal, connected to a sinus tract travelling under the skin) and anomalies that involve the outer ear, external auditory canal and temporal bone
Physical findings associated with a syndrome known to cause permanent hearing loss (for example, white forelock, a patch of white hair above the forehead)
Syndromes associated with congenital hearing loss or progressive or late-onset hearing loss
Neurodegenerative disorders or sensorimotor neuropathies
Confirmed bacterial or viral meningitis (in particular if caused by mump, herpes viruses or virus)
Head trauma, especially of the basal skull, or temporal bone fractures, that require hospitalization
Chemotherapy
Source: the American Academy of Pediatrics7
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders RO1s DC003544, DC002842 and DC012049 to R.J.H.S. and National Institute of Child Health and Human Development R01s HD044864 and HD079918 to M.R.S. M B-G is supported by Great Ormond Street Hospital Children’s Charity and the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. All authors thank collegue Ad Snik for his input to earlier versions of this manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Introduction (G.V.C. and A.B.), Epidemiology (A.M.H.K, M.A.K.B.-G. and M.R.S); Mechanisms/pathophysiology (A.M.H.K., M.A.K.B.-G. and M.R.S.); Diagnosis, screening and prevention (G.V.C., S.U., A.M.H.K., M.R.S. and A.B.); Management (M.R.S. and L.R.L.); Quality of life (A.B.); Outlook (R.J.H.S. and S.U.); Overview of the Primer (A.M.H.K. and A.B.).
References
- 1.Boudewyns A, et al. Otitis media with effusion: an underestimated cause of hearing loss in infants. Otol Neurotol. 2011;32:799–804. doi: 10.1097/MAO.0b013e31821b0d07. [DOI] [PubMed] [Google Scholar]
- 2.Rapin I, Gravel JS. Auditory neuropathy: a biologically inappropriate label unless acoustic nerve involvement is documented. J Am Acad Audiol. 2006;17:147–50. doi: 10.3766/jaaa.17.2.7. [DOI] [PubMed] [Google Scholar]
- 3.Cone-Wesson B, R G. Auditory neuropathy: a brief review. Curr Opin Otolaryngol Head Neck Surg. 2000;8:421–425. [Google Scholar]
- 4.Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119(Pt 3):741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–71. doi: 10.1542/peds.102.5.1161. This paper shows the effect of delayed detection and treatment for congenital hearing loss on speech and language development. [DOI] [PubMed] [Google Scholar]
- 6.Fortnum H, Davis A. Epidemiology of permanent childhood hearing impairment in Trent Region, 1985–1993. Br J Audiol. 1997;31:409–46. doi: 10.3109/03005364000000037. [DOI] [PubMed] [Google Scholar]
- 7.Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. This paper presents the guidelines of the American Academy of Pediatrics on the importance of universal neonatal hearing screening, risk factors for congenital hearing loss and management strategies for those who fail the screening test. [DOI] [PubMed] [Google Scholar]
- 8.Norton SJ, et al. Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brain stem response test performance. Ear Hear. 2000;21:508–28. doi: 10.1097/00003446-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354:2151–64. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 10.Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ. 2001;323:536–40. doi: 10.1136/bmj.323.7312.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watkin P, Baldwin M. The longitudinal follow up of a universal neonatal hearing screen: the implications for confirming deafness in childhood. Int J Audiol. 2012;51:519–28. doi: 10.3109/14992027.2012.673237. [DOI] [PubMed] [Google Scholar]
- 12.Nikolopoulos TP. Auditory dyssynchrony or auditory neuropathy: understanding the pathophysiology and exploring methods of treatment. Int J Pediatr Otorhinolaryngol. 2014;78:171–3. doi: 10.1016/j.ijporl.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 13.http://www.who.int/healthinfo/statistics/bod_hearingloss.pdf.
- 14.Driscoll C, Beswick R, Doherty E, D'Silva R, Cross A. The validity of family history as a risk factor in pediatric hearing loss. Int J Pediatr Otorhinolaryngol. 2015 doi: 10.1016/j.ijporl.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 15.van Dommelen P, Verkerk PH, van Straaten HL. Hearing Loss by Week of Gestation and Birth Weight in Very Preterm Neonates. J Pediatr. 2015 doi: 10.1016/j.jpeds.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 16.van Dommelen P, Mohangoo AD, Verkerk PH, van der Ploeg CP, van Straaten HL. Risk indicators for hearing loss in infants treated in different neonatal intensive care units. Acta Paediatr. 2010;99:344–9. doi: 10.1111/j.1651-2227.2009.01614.x. [DOI] [PubMed] [Google Scholar]
- 17.Koenighofer M, Parzefall T, Ramsebner R, Lucas T, Frei K. Delayed auditory pathway maturation and prematurity. Wien Klin Wochenschr. 2014 doi: 10.1007/s00508-014-0653-y. [DOI] [PubMed] [Google Scholar]
- 18.Marazita ML, et al. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet. 1993;46:486–91. doi: 10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- 19.Smith RJ, Bale JF, Jr, White KR. Sensorineural hearing loss in children. Lancet. 2005;365:879–90. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 20.Snoeckx RL, et al. GJB2 mutations and degree of hearing loss: a multicenter study. Am J Hum Genet. 2005;77:945–57. doi: 10.1086/497996. This paper reports a large multicentre study that is unique in establishing a genotype-phenotype correlation for GJB2 based on a very large set of patients, providing detailed information for many common genotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cone-Wesson B, et al. Identification of neonatal hearing impairment: infants with hearing loss. Ear Hear. 2000;21:488–507. doi: 10.1097/00003446-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Cox SKP, De Palma SR, Ebert J, Thompson F, Kenna MA, et al. An integrated approach to molecular diagnostic testing for hearing loss and related syndromes; 6th Molecular Biology of Hearing & Deafness Conference; 2007. p. 80. [Google Scholar]
- 23.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol. 2008;41:57–62. doi: 10.1016/j.jcv.2007.09.004. This is an outstanding overview of the overall economic impact of CMV-associated hearing loss. [DOI] [PubMed] [Google Scholar]
- 24.Goderis J, et al. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134:972–82. doi: 10.1542/peds.2014-1173. [DOI] [PubMed] [Google Scholar]
- 25.Banatvala JE, Brown DW. Rubella. Lancet. 2004;363:1127–37. doi: 10.1016/S0140-6736(04)15897-2. [DOI] [PubMed] [Google Scholar]
- 26.Korver AM, et al. Causes of permanent childhood hearing impairment. Laryngoscope. 2011;121:409–16. doi: 10.1002/lary.21377. [DOI] [PubMed] [Google Scholar]
- 27.Lammens F, Verhaert N, Devriendt K, Debruyne F, Desloovere C. Aetiology of congenital hearing loss: a cohort review of 569 subjects. Int J Pediatr Otorhinolaryngol. 2013;77:1385–91. doi: 10.1016/j.ijporl.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Declau F, Boudewyns A, Van den Ende J, Peeters A, van den Heyning P. Etiologic and audiologic evaluations after universal neonatal hearing screening: analysis of 170 referred neonates. Pediatrics. 2008;121:1119–26. doi: 10.1542/peds.2007-1479. [DOI] [PubMed] [Google Scholar]
- 29.Zelante L, et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet. 1997;6:1605–9. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- 30.Riazuddin S, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–51. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox ER, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–72. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 32.Grimmer JF, Hedlund G. Vestibular symptoms in children with enlarged vestibular aqueduct anomaly. Int J Pediatr Otorhinolaryngol. 2007;71:275–82. doi: 10.1016/j.ijporl.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim BG, et al. Early deterioration of residual hearing in patients with SLC26A4 mutations. Laryngoscope. 2016;126:E286–91. doi: 10.1002/lary.25786. [DOI] [PubMed] [Google Scholar]
- 34.Naz S, et al. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J Med Genet. 2004;41:591–5. doi: 10.1136/jmg.2004.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donaudy F, et al. Espin gene (ESPN) mutations associated with autosomal dominant hearing loss cause defects in microvillar elongation or organisation. J Med Genet. 2006;43:157–61. doi: 10.1136/jmg.2005.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitajiri S, et al. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell. 2010;141:786–98. doi: 10.1016/j.cell.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fettiplace R, Kim KX. The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev. 2014;94:951–86. doi: 10.1152/physrev.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roux I, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–89. doi: 10.1016/j.cell.2006.08.040. This paper demonstrates that otoferlin interacts with SNARE (soluble NSF (N-ethylmaleimide-sensitive factor) molecules at the afferent ribbon synapses in inner hair cells of the cochlea to trigger exocytosis of the neurotransmitter glutamate. [DOI] [PubMed] [Google Scholar]
- 39.Leal MC, et al. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection - Brazil, November 2015-May 2016. MMWR Morb Mortal Wkly Rep. 2016;65:917–9. doi: 10.15585/mmwr.mm6534e3. Zika virus infection is the latest of the ‘TORCH’ infection complex to be demonstrated as a cause of sensorineural hearing loss. Zika virus infection should be added to the list of infectious diseases that are known to induce hearing loss in infants. [DOI] [PubMed] [Google Scholar]
- 40.Cannon MJ, et al. Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect Dis. 2014;14:569. doi: 10.1186/s12879-014-0569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–76. doi: 10.1002/rmv.535. This is a detailed analysis of the epidemiology of congenital CMV infection using available literature and previously published work, and is an outstanding review of the range of disabilities and clinical manifestations of congenital infection. [DOI] [PubMed] [Google Scholar]
- 42.Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18 doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schleiss MR, Choo DI. Mechanisms of congenital cytomegalovirus- induced deafness. Drug Discovery Today: Disease Mechanisms. 2006:105–113. [Google Scholar]
- 44.Schachtele SJ, Mutnal MB, Schleiss MR, Lokensgard JR. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol. 2011;17:201–11. doi: 10.1007/s13365-011-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradford RD, et al. Murine CMV-induced hearing loss is associated with inner ear inflammation and loss of spiral ganglia neurons. PLoS Pathog. 2015;11:e1004774. doi: 10.1371/journal.ppat.1004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schraff SA, et al. Macrophage inflammatory proteins in cytomegalovirus-related inner ear injury. Otolaryngol Head Neck Surg. 2007;137:612–8. doi: 10.1016/j.otohns.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 47.Schleiss MR. Cytomegalovirus in the neonate: immune correlates of infection and protection. Clin Dev Immunol. 2013;2013:501801. doi: 10.1155/2013/501801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enders G, Daiminger A, Bader U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol. 2011;52:244–6. doi: 10.1016/j.jcv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet. 1982;2:781–4. doi: 10.1016/s0140-6736(82)92677-0. [DOI] [PubMed] [Google Scholar]
- 50.Bouthry E, et al. Rubella and pregnancy: diagnosis, management and outcomes. Prenat Diagn. 2014;34:1246–53. doi: 10.1002/pd.4467. This is an outstanding overview of the presentation, epidemiology and management of maternal and fetal rubella virus infection, with an excellent perspective and overview of diagnostic studies. [DOI] [PubMed] [Google Scholar]
- 51.Sever JL, South MA, Shaver KA. Delayed manifestations of congenital rubella. Rev Infect Dis. 1985;7(Suppl 1):S164–9. doi: 10.1093/clinids/7.supplement_1.s164. [DOI] [PubMed] [Google Scholar]
- 52.Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev. 2000;13:571–87. doi: 10.1128/cmr.13.4.571-587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster WS. Teratogen update: congenital rubella. Teratology. 1998;58:13–23. doi: 10.1002/(SICI)1096-9926(199807)58:1<13::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 54.Lin HC, Shu MT, Lee KS, Lin HY, Lin G. reducing false positives in newborn hearing screening program: how and why. Otol Neurotol. 2007;28:788–92. doi: 10.1097/mao.0b013e3180cab754. [DOI] [PubMed] [Google Scholar]
- 55.Stephens D. In: Definitisions, protocols & guidelines in genetic hearing impairment. Martini AMM, Stephens D, Read A, editors. Whurr Publishers; London & Philadelphia: 2001. [Google Scholar]
- 56.Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223–41. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- 57.Rance G, Briggs RJ. Assessment of hearing in infants with moderate to profound impairment: the Melbourne experience with auditory steady-state evoked potential testing. Ann Otol Rhinol Laryngol Suppl. 2002;189:22–8. doi: 10.1177/00034894021110s505. [DOI] [PubMed] [Google Scholar]
- 58.Swanepoel D, Ebrahim S. Auditory steady-state response and auditory brainstem response thresholds in children. Eur Arch Otorhinolaryngol. 2009;266:213–9. doi: 10.1007/s00405-008-0738-1. [DOI] [PubMed] [Google Scholar]
- 59.Harlor AD, Jr, et al. Hearing assessment in infants and children: recommendations beyond neonatal screening. Pediatrics. 2009;124:1252–63. doi: 10.1542/peds.2009-1997. [DOI] [PubMed] [Google Scholar]
- 60.De Leenheer EM, et al. Etiological diagnosis in the hearing impaired newborn: proposal of a flow chart. Int J Pediatr Otorhinolaryngol. 2011;75:27–32. doi: 10.1016/j.ijporl.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 61.Alford RL, et al. American College of Medical Genetics and Genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet Med. 2014;16:347–55. doi: 10.1038/gim.2014.2. This paper summarizes protocols for aetiological work-up of congenital hearing loss and advocates the use of comprehensive genetic testing. [DOI] [PubMed] [Google Scholar]
- 62.Bamiou DE, MacArdle B, Bitner-Glindzicz M, Sirimanna T. Aetiological investigations of hearing loss in childhood: a review. Clin Otolaryngol Allied Sci. 2000;25:98–106. doi: 10.1046/j.1365-2273.2000.00346.x. [DOI] [PubMed] [Google Scholar]
- 63.Hart CK, Choo DI. What is the optimal workup for a child with bilateral sensorineural hearing loss? Laryngoscope. 2013;123:809–10. doi: 10.1002/lary.23425. [DOI] [PubMed] [Google Scholar]
- 64.Sloan-Heggen CM, et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet. 2016;135:441–50. doi: 10.1007/s00439-016-1648-8. This paper demonstrates that comprehensive genetic testing is a foundational diagnostic test, which allows health care providers to make evidence-based decisions in the evaluation of hearing loss, thereby providing better and more cost-effective patient care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zong L, et al. Mutations in apoptosis-inducing factor cause X-linked recessive auditory neuropathy spectrum disorder. J Med Genet. 2015;52:523–31. doi: 10.1136/jmedgenet-2014-102961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park AH, et al. A diagnostic paradigm including cytomegalovirus testing for idiopathic pediatric sensorineural hearing loss. Laryngoscope. 2014;124:2624–9. doi: 10.1002/lary.24752. [DOI] [PubMed] [Google Scholar]
- 67.Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57(Suppl 4):S178–81. doi: 10.1093/cid/cit629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ville Y, Leruez-Ville M. Managing infections in pregnancy. Curr Opin Infect Dis. 2014;27:251–7. doi: 10.1097/QCO.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 69.Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am. 2013;60:335–49. doi: 10.1016/j.pcl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freij BJ, South MA, Sever JL. Maternal rubella and the congenital rubella syndrome. Clin Perinatol. 1988;15:247–57. [PubMed] [Google Scholar]
- 71.Nagasawa K, et al. Congenital Rubella Syndrome: A Case Report on Changes in Viral Load and Rubella Antibody Titers. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3333. [DOI] [PubMed] [Google Scholar]
- 72.Alvarado JC, et al. Synergistic effects of free radical scavengers and cochlear vasodilators: a new otoprotective strategy for age-related hearing loss. Frontiers in aging neuroscience. 2015;7:86. doi: 10.3389/fnagi.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjea D, et al. Early investigational drugs for hearing loss. Expert opinion on investigational drugs. 2015;24:201–17. doi: 10.1517/13543784.2015.960076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen G, Zhang X, Yang F, Mu L. Disposition of nanoparticle-based delivery system via inner ear administration. Current drug metabolism. 2010;11:886–97. doi: 10.2174/138920010794479673. [DOI] [PubMed] [Google Scholar]
- 75.Fitzpatrick EM, et al. Sign Language and Spoken Language for Children With Hearing Loss: A Systematic Review. Pediatrics. 2016;137 doi: 10.1542/peds.2015-1974. [DOI] [PubMed] [Google Scholar]
- 76.Nittrouer S. Beyond Early Intervention: Supporting Children With CIs Through Elementary School. Otol Neurotol. 2016;37:e43–9. doi: 10.1097/MAO.0000000000000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Francois M, Boukhris M, Noel-Petroff N. Schooling of hearing-impaired children and benefit of early diagnosis. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132:251–5. doi: 10.1016/j.anorl.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Kimberlin DW, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 79.Kimberlin DW, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–43. doi: 10.1056/NEJMoa1404599. This landmark paper reports the results of a randomized controlled trial, which demonstrates that therapy with oral valganciclovir improves both hearing and neurodevelopmental outcomes in symptomatic congenital CMV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schleiss MR. Developing a Vaccine against Congenital Cytomegalovirus (CMV) Infection: What Have We Learned from Animal Models? Where Should We Go Next? Future Virol. 2013;8:1161–1182. doi: 10.2217/fvl.13.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Royackers L, Christian D, Frans D, Ermelinde R. Hearing status in children with congenital cytomegalovirus: up-to-6-years audiological follow-up. Int J Pediatr Otorhinolaryngol. 2011;75:376–82. doi: 10.1016/j.ijporl.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 82.Oz HS. Maternal and congenital toxoplasmosis, currently available and novel therapies in horizon. Frontiers in microbiology. 2014;5:385. doi: 10.3389/fmicb.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wright R, et al. Congenital lymphocytic choriomeningitis virus syndrome: a disease that mimics congenital toxoplasmosis or Cytomegalovirus infection. Pediatrics. 1997;100:E9. doi: 10.1542/peds.100.1.e9. [DOI] [PubMed] [Google Scholar]
- 84.Russell K, et al. Update: Interim Guidance for the Evaluation and Management of Infants with Possible Congenital Zika Virus Infection - United States, August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:870–8. doi: 10.15585/mmwr.mm6533e2. [DOI] [PubMed] [Google Scholar]
- 85.McCreery RW, Venediktov RA, Coleman JJ, Leech HM. An evidence-based systematic review of amplitude compression in hearing aids for school-age children with hearing loss. Am J Audiol. 2012;21:269–94. doi: 10.1044/1059-0889(2012/12-0013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu J, Han W. Improvement of Adult BTE Hearing Aid Wearers' Front/Back Localization Performance Using Digital Pinna-Cue Preserving Technologies: An Evidence-Based Review. Korean J Audiol. 2014;18:97–104. doi: 10.7874/kja.2014.18.3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blair B. Audio Engineering Handbook. McGraw-Hill; New York: 1988. [Google Scholar]
- 88.Boothroyd A, Springer N, Smith L, Schulman J. Amplitude compression and profound hearing loss. J Speech Hear Res. 1988;31:362–76. doi: 10.1044/jshr.3103.362. [DOI] [PubMed] [Google Scholar]
- 89.Moore BC. Characterization and simulation of impaired hearing: implications for hearing aid design. Ear Hear. 1991;12:154S–161S. doi: 10.1097/00003446-199112001-00009. [DOI] [PubMed] [Google Scholar]
- 90.Disorders, N.I.o.D.a.O.C. 2010. [Google Scholar]
- 91.McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol. 2013;52:360–8. doi: 10.3109/14992027.2013.769066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spivak L, Sokol H, Auerbach C, Gershkovich S. Newborn hearing screening follow-up: factors affecting hearing aid fitting by 6 months of age. Am J Audiol. 2009;18:24–33. doi: 10.1044/1059-0889(2008/08-0015). [DOI] [PubMed] [Google Scholar]
- 93.Kasic JF, Fredrickson JM. The Otologics MET ossicular stimulator. Otolaryngol Clin North Am. 2001;34:501–13. doi: 10.1016/s0030-6665(05)70345-5. [DOI] [PubMed] [Google Scholar]
- 94.Luers J, Huttenbrink K, Zahnert T, Bornitz M, Beutner D. Vibroplasty for mixed and conductive hearing. Otol Neurotol. 2013;34:1005–12. doi: 10.1097/MAO.0b013e3182990d2b. [DOI] [PubMed] [Google Scholar]
- 95.Mueller H, Hall J. Audiologists Desk Reference. Singular Publishing Group Inc; San Diego: 1998. [Google Scholar]
- 96.Gaylor JM, et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139:265–72. doi: 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 97.Roland JT, Jr, Gantz BJ, Waltzman SB, Parkinson AJ, Multicenter Clinical Trial G. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 2015 doi: 10.1002/lary.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Golub JS, Won JH, Drennan WR, Worman TD, Rubinstein JT. Spectral and temporal measures in hybrid cochlear implant users: on the mechanism of electroacoustic hearing benefits. Otol Neurotol. 2012;33:147–53. doi: 10.1097/MAO.0b013e318241b6d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fitzpatrick EM, Ham J, Whittingham J. Pediatric Cochlear Implantation: Why Do Children Receive Implants Late? Ear Hear. 2015;36:688–94. doi: 10.1097/AUD.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buchman CA, et al. Cochlear implantation in children with congenital inner ear malformations. Laryngoscope. 2004;114:309–16. doi: 10.1097/00005537-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 101.Birman CS, Elliott EJ, Gibson WP. Pediatric cochlear implants: additional disabilities prevalence, risk factors, and effect on language outcomes. Otol Neurotol. 2012;33:1347–52. doi: 10.1097/MAO.0b013e31826939cc. [DOI] [PubMed] [Google Scholar]
- 102.Eppsteiner RW, et al. Prediction of cochlear implant performance by genetic mutation: the spiral ganglion hypothesis. Hear Res. 2012;292:51–8. doi: 10.1016/j.heares.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu CC, Liu TC, Wang SH, Hsu CJ, Wu CM. Genetic characteristics in children with cochlear implants and the corresponding auditory performance. Laryngoscope. 2011;121:1287–93. doi: 10.1002/lary.21751. [DOI] [PubMed] [Google Scholar]
- 104.Usami S, et al. Patients with CDH23 mutations and the 1555A>G mitochondrial mutation are good candidates for electric acoustic stimulation (EAS) Acta Otolaryngol. 2012;132:377–84. doi: 10.3109/00016489.2011.649493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyagawa M, Nishio SY, Usami S. A Comprehensive Study on the Etiology of Patients Receiving Cochlear Implantation With Special Emphasis on Genetic Epidemiology. Otol Neurotol. 2016;37:e126–34. doi: 10.1097/MAO.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cremers CW, Teunissen E, Marres EH. Classification of congenital aural atresia and results of reconstructive surgery. Adv Otorhinolaryngol. 1988;40:9–14. doi: 10.1159/000415666. [DOI] [PubMed] [Google Scholar]
- 107.Doshi J, McDermott AL. Bone anchored hearing aids in children. Expert Rev Med Devices. 2015;12:73–82. doi: 10.1586/17434440.2015.975117. [DOI] [PubMed] [Google Scholar]
- 108.Tjellstrom A, Hakansson B, Granstrom G. Bone-anchored hearing aids: current status in adults and children. Otolaryngol Clin North Am. 2001;34:337–364. doi: 10.1016/s0030-6665(05)70335-2. [DOI] [PubMed] [Google Scholar]
- 109.Kiringoda R, Lustig L. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol. 2013;34:790–794. doi: 10.1097/MAO.0b013e318291c651. [DOI] [PubMed] [Google Scholar]
- 110.Snik AF, et al. Consensus statements on the BAHA system: where do we stand at present? Ann Otol Rhinol Laryngol Suppl. 2005;195:2–12. [Google Scholar]
- 111.Niparko JK, Cox KM, Lustig LR. Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol. 2003;24:73–8. doi: 10.1097/00129492-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 112.Saroul N, Akkari M, Pavier Y, Gilain L, Mom T. Long-term benefit and sound localization in patients with single-sided deafness rehabilitated with an osseointegrated bone-conduction device. Otol Neurotol. 2013;34:111–4. doi: 10.1097/MAO.0b013e31827a2020. [DOI] [PubMed] [Google Scholar]
- 113.Schwager K. Reconstruction of middle ear malformations. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2007;6 Doc01. [PMC free article] [PubMed] [Google Scholar]
- 114.Holden-Pitt L, Albertorio J. Thirty years of the Annual Survey of Deaf and Hard-of-Hearing Children & Youth: a glance over the decades. Am Ann Deaf. 1998;143:72–6. [PubMed] [Google Scholar]
- 115.Lustig LR, Leake PA, Snyder RL, Rebscher SJ. Changes in the cat cochlear nucleus following neonatal deafening and chronic intracochlear electrical stimulation. Hear Res. 1994;74:29–37. doi: 10.1016/0378-5955(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 116.Pimperton H, et al. The impact of universal newborn hearing screening on long-term literacy outcomes: a prospective cohort study. Arch Dis Child. 2016;101:9–15. doi: 10.1136/archdischild-2014-307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 118.Korver AM, et al. Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. 2010;304:1701–8. doi: 10.1001/jama.2010.1501. [DOI] [PubMed] [Google Scholar]
- 119.Stern-Ginossar N, et al. Decoding human cytomegalovirus. Science. 2012;338:1088–93. doi: 10.1126/science.1227919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soderberg-Naucler C. Treatment of cytomegalovirus infections beyond acute disease to improve human health. Expert Rev Anti Infect Ther. 2014;12:211–22. doi: 10.1586/14787210.2014.870472. [DOI] [PubMed] [Google Scholar]
- 121.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics. 1999;104:55–60. doi: 10.1542/peds.104.1.55. [DOI] [PubMed] [Google Scholar]
- 123.Ross SA, et al. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis. 2010;201:386–9. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344:1366–71. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 125.Shearer AE, et al. Copy number variants are a common cause of non-syndromic hearing loss. Genome Med. 2014;6:37. doi: 10.1186/gm554. This paper shows that copy number variants are a common cause of genetic hearing loss. Their involvement in roughly one in five genetic diagnoses mandates their identification in any clinical genetic diagnostic test for hearing loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taylor KR, et al. AudioGene: predicting hearing loss genotypes from phenotypes to guide genetic screening. Hum Mutat. 2013;34:539–45. doi: 10.1002/humu.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakagawa T. Strategies for developing novel therapeutics for sensorineural hearing loss. Front Pharmacol. 2014;5:206. doi: 10.3389/fphar.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shibata SB, Raphael Y. Future approaches for inner ear protection and repair. J Commun Disord. 2010;43:295–310. doi: 10.1016/j.jcomdis.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Werner M, Van De Water TR, Hammarsten P, Arnoldsson G, Berggren D. Morphological and morphometric characterization of direct transdifferentiation of support cells into hair cells in ototoxin-exposed neonatal utricular explants. Hear Res. 2015;321:1–11. doi: 10.1016/j.heares.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 130.Wan G, Corfas G, Stone JS. Inner ear supporting cells: rethinking the silent majority. Semin Cell Dev Biol. 2013;24:448–59. doi: 10.1016/j.semcdb.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Esterberg R, et al. Fish in a Dish: Drug Discovery for Hearing Habilitation. Drug Discov Today Dis Models. 2013;10 doi: 10.1016/j.ddmod.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McCall AA, et al. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 2010;31:156–65. doi: 10.1097/AUD.0b013e3181c351f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sidell D, et al. Combination therapies using an intratympanic polymer gel delivery system in the guinea pig animal model: A safety study. Int J Pediatr Otorhinolaryngol. 2016;84:132–6. doi: 10.1016/j.ijporl.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 134.Van Kerschaver E, Boudewyns AN, Declau F, Van de Heyning PH, Wuyts FL. Sociodemographic determinants of hearing impairment studied in 103 835 term babies. Eur J Public Health. 2012 doi: 10.1093/eurpub/cks010. [DOI] [PubMed] [Google Scholar]
- 135.Rohlfs AK, et al. Interdisciplinary approach to design, performance, and quality management in a multicenter newborn hearing screening project: introduction, methods, and results of the newborn hearing screening in Hamburg (Part I) Eur J Pediatr. 2010;169:1353–60. doi: 10.1007/s00431-010-1228-1. [DOI] [PubMed] [Google Scholar]
- 136.Colgan S, et al. The cost-effectiveness of universal newborn screening for bilateral permanent congenital hearing impairment: systematic review. Acad Pediatr. 2012;12:171–80. doi: 10.1016/j.acap.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gantt S, et al. Cost-effectiveness of Universal and Targeted Newborn Screening for Congenital Cytomegalovirus Infection. JAMA Pediatr. 2016 doi: 10.1001/jamapediatrics.2016.2016. This paper demonstrates the cost-effectiveness of screening for congenital CMV infection, both in the context of universal screening programmes and in a ‘targeted’ screening approach in which infants who fail their neonatal hearing screening are specifically tested for CMV infection. This paper should help to drive the implementation of newborn screening for congenital CMV infection. [DOI] [PubMed] [Google Scholar]
- 138.Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 2009;24:307–16. doi: 10.1152/physiol.00018.2009. This is an excellent overview of inner ear structure and function, particularly with respect to the role of molecular components of the stria vascularis in endolymph production and the recycling of K+ ions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jagger DJ, Forge A. Connexins and gap junctions in the inner ear--it's not just about K(+) recycling. Cell Tissue Res. 2015;360:633–44. doi: 10.1007/s00441-014-2029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verhoeven K, et al. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat Genet. 1998;19:60–2. doi: 10.1038/ng0598-60. [DOI] [PubMed] [Google Scholar]
- 141.Schraders M, et al. Mutations of the gene encoding otogelin are a cause of autosomal-recessive nonsyndromic moderate hearing impairment. Am J Hum Genet. 2012;91:883–9. doi: 10.1016/j.ajhg.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zheng J, et al. Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with alpha-tectorin and is mutated in autosomal dominant hearing loss (DFNA4) Proc Natl Acad Sci U S A. 2011;108:4218–23. doi: 10.1073/pnas.1005842108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yariz KO, et al. Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. Am J Hum Genet. 2012;91:872–82. doi: 10.1016/j.ajhg.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci U S A. 2011;108:11476–81. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 146.Manor U, et al. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol. 2011;21:167–72. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Delprat B, et al. Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum Mol Genet. 2005;14:401–10. doi: 10.1093/hmg/ddi036. [DOI] [PubMed] [Google Scholar]
- 148.Belyantseva IA, et al. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–56. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 149.Riazuddin S, et al. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet. 2012;44:1265–71. doi: 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nikolopoulos TP, Lioumi D, Stamataki S, O'Donoghue GM. Evidence-based overview of ophthalmic disorders in deaf children: a literature update. Otol Neurotol. 2006;27:S1–24. doi: 10.1097/01.mao.0000185150.69704.18. discussion S20. [DOI] [PubMed] [Google Scholar]
- 151.van Gils RF, et al. Effectiveness of prevention programmes for hand dermatitis: a systematic review of the literature. Contact Dermatitis. 2011;64:63–72. doi: 10.1111/j.1600-0536.2010.01825.x. [DOI] [PubMed] [Google Scholar]
- 152.Thiebaut R, Leproust S, Chene G, Gilbert R. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients' data. Lancet. 2007;369:115–22. doi: 10.1016/S0140-6736(07)60072-5. [DOI] [PubMed] [Google Scholar]
- 153.Neu N, Duchon J, Zachariah P. TORCH Infections. Clin Perinatol. 2015;42:77–103. doi: 10.1016/j.clp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 154.Cutts FT, Vynnycky E. Modelling the incidence of congenital rubella syndrome in developing countries. Int J Epidemiol. 1999;28:1176–84. doi: 10.1093/ije/28.6.1176. [DOI] [PubMed] [Google Scholar]
- 155.James SH, Kimberlin DW. Neonatal Herpes Simplex Virus Infection: Epidemiology and Treatment. Clin Perinatol. 2015;42:47–59. doi: 10.1016/j.clp.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 156.Chau J, Atashband S, Chang E, Westerberg BD, Kozak FK. A systematic review of pediatric sensorineural hearing loss in congenital syphilis. Int J Pediatr Otorhinolaryngol. 2009;73:787–92. doi: 10.1016/j.ijporl.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 157.Sexually transmitted disease surveillance 2013. CDC; 2013. http://www.cdc.gov/std/stats13/tables/1.htm. [Google Scholar]
- 158.Pessoa L, Galvao V. Clinical aspects of congenital syphilis with Hutchinson's triad. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.11.2011.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Salviz M, Montoya JG, Nadol JB, Santos F. Otopathology in congenital toxoplasmosis. Otol Neurotol. 2013;34:1165–9. doi: 10.1097/MAO.0b013e31828297b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Austeng ME, et al. Maternal infection with toxoplasma gondii in pregnancy and the risk of hearing loss in the offspring. Int J Audiol. 2010;49:65–8. doi: 10.3109/14992020903214053. [DOI] [PubMed] [Google Scholar]
- 161.McLeod R, et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto AY, et al. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr Infect Dis J. 2011;30:1043–6. doi: 10.1097/INF.0b013e31822d9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Westerberg BD, Atashband S, Kozak FK. A systematic review of the incidence of sensorineural hearing loss in neonates exposed to Herpes simplex virus (HSV) Int J Pediatr Otorhinolaryngol. 2008;72:931–7. doi: 10.1016/j.ijporl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 165.Phillips JS, Gaunt A, Phillips DR. Otosyphilis: a neglected diagnosis? Otol Neurotol. 2014;35:1011–3. doi: 10.1097/MAO.0000000000000361. [DOI] [PubMed] [Google Scholar]