Abstract
Background
Despite massive expenditures in preclinical studies, many breast cancer agents show efficacy in murine models but fail in human trials. In humans, metastatic disease determines survival, but preclinical murine models only evaluate drug efficacy against the primary tumor. We hypothesized that evaluating efficacy against metastatic breast cancer would more efficiently predict efficacy in a murine model than evaluating the primary tumor alone. This study (1) critically evaluated a murine tumor removal model with metastatic tumor burden quantification for breast cancer preclinical trials and (2) validated the model with an agent that previously passed preclinical trials but failed human trials.
Materials and methods
Tumorectomy and Halsted (radical) mastectomy procedures after inoculation of 4T1-luc2 cells were compared. The effect of AZD0530, an oral Src inhibitor that passed preclinical trials but failed human trials, was evaluated using an inoculation model with/without Halsted mastectomy.
Results
Significant amounts of residual disease were confirmed by bioluminescence (p = 0.003) and 100% developed local recurrence after tumorectomy versus 14% (p = 0.005) after Halsted mastectomy. Bioluminescence value at 15min after luciferin injection highly correlated with peak except for 24 hours after injection. AZD0530 significantly suppressed primary tumor burden compared with no treatment (p = 0.002); but not in lung metastases. In a Halsted mastectomy model, AZD0530 had no efficacy against lung metastases or difference in survival.
Conclusions
We critically evaluated and established a murine mastectomy model to evaluate metastatic tumors. It provides a new model for preclinical drug development that mimics the human adjuvant setting.
Keywords: Breast cancer, Mastectomy, Mouse, Model, Drug development, Metastasis
1. Introduction
Breast cancer is the most prevalent and 2nd leading cause of cancer related death in women in the US (1). Although billions of US dollars have been invested in treating breast cancer, large portions of these investments have been lost in efforts that have failed to deliver (2, 3). Given the huge cost required for human clinical trials that a drug needs to pass to prove its efficacy for patients, it is conducted only for drugs that show significant promise in preclinical animal experiments (2). However, despite these efforts, most drugs that passed murine studies still failed in human clinical trials (4), which at least partly can be explained by the use of murine models that do not represent human conditions (2). In order to predict the outcome of human clinical trials, murine models for preclinical study need to reproduce the condition of human cancer (2, 3, 5, 6).
To date, the most commonly used murine breast cancer models evaluate drug efficacy against primary tumors, either in orthotopic (mammary pads) or ectopic sites (subcutaneous tissues) (2, 3, 5, 6). Although drugs are sometimes used in humans as a neoadjuvant therapy to treat the primary breast tumor, the vast majority is used as adjuvant therapy where drugs are given after surgery to reduce the risk of recurrence by treating undetectable remaining or metastatic cancer (2, 3, 5). Recently, our group and others have shown that the genetic profiles of metastatic lung tumors are significantly different from that of their primary tumors, which implicates that their biology is different (2, 3, 7, 8). Therefore, we hypothesized that metastatic tumors may respond to drugs differently from primary tumors. Accordingly, a new drug should be evaluated by its efficacy against metastatic tumors. To our knowledge, there are few reports utilizing murine tumor removal models for preclinical drug development studies (2, 3, 5, 6), although we have previously reported the use of a murine mastectomy model in breast cancer non-drug development research (6, 9).
Because of the disconnect between what determines breast cancer survival in humans and how murine models determine drug efficacy, we hypothesized that aligning murine models more with the human condition would improve the efficiency of breast cancer drug development. Accordingly, we hypothesized that a model that evaluates efficacy against metastatic breast cancer would more efficiently predict efficacy in humans than one that evaluates efficacy against the primary tumor alone. Therefore, this study (1) critically evaluated a murine radical mastectomy (Halsted) model with metastatic tumor burden quantification for breast cancer preclinical trials and (2) validated the model with an agent that previously passed preclinical trials but failed human trials.
2. Material and Methods
2.1. Cell culture
4T1-luc2 cells, a mouse mammary adenocarcinoma cell line derived from BALB/c mice that has been engineered to express luciferase, were used (Caliper Life Sciences/PerkinElmer, Hopkinton, MA). 4T1-luc2 cells were cultured in RPMI Medium 1640 with 10% fetal bovine serum.
2.2. Animal models
Approval from the Roswell Park Cancer Institute Institutional Animal Care and Use Committee was obtained for all experiments. 9–12 weeks female BALB/c mice were obtained from Jackson Laboratory. 1×105 4T1-luc2 cells in 20 μl Matrigel were inoculated into #2 fat pads under direct vision as described before (4–6, 10). 8 days after inoculation, either tumorectomy or Halsted mastectomy were performed (n = 7, each). Resected tumor weight, bioluminescence on the day after tumor removal, and recurrence rate were compared between these two procedures using previously reported techniques (4, 6, 10–13).
2.3. Tumorectomy
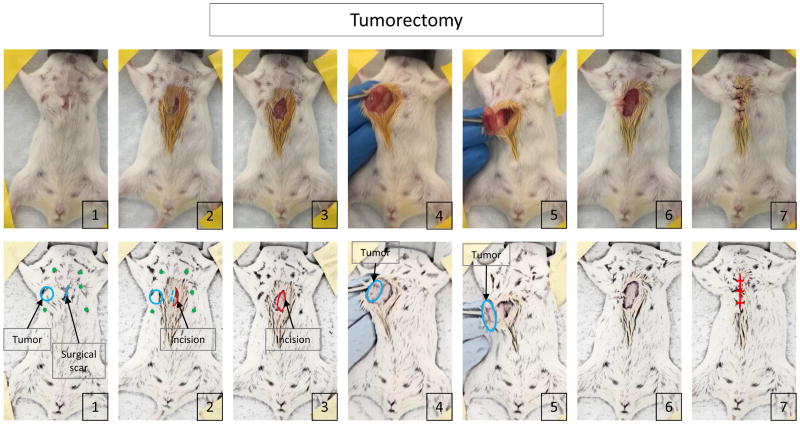
Spindle shaped skin incision was made to remove the surgical scar which was made at inoculation. The skin was then inverted and the tumor was resected off the chest wall. After the tumor was completely freed from the chest wall, the tumor was also resected from the skin and the wound was closed with suture (Figure 1).
Figure 1.
The procedure of tumorectomy.
2.4. Halsted (radical) mastectomy
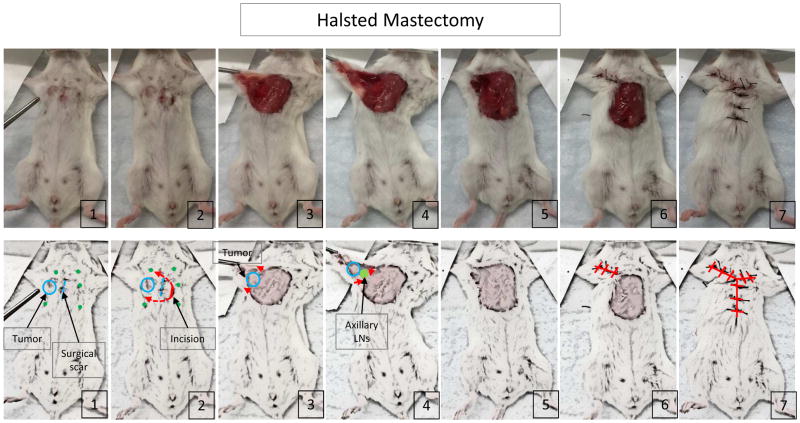
William Stewart Halsted M.D., who established surgical residency training and is often named as the greatest surgeon in American surgery, established the operation radical mastectomy, removal of all the breast tissue with the skin, including the nipple-areola complex, axillary lymph nodes and pectoralis major muscle. Honoring his contribution to surgical research, we named our mouse model Halsted mastectomy, which removes all the structures named above. Briefly, skin incision was made 5 mm from the inoculation surgical scar. Then, it was extended both cephalad and caudad to include both the scar and tumor. The tumor was removed from the chest wall en bloc with the pectoralis muscle and the skin incision was extended to the axilla to anatomically allow for standard axillary lymph node dissection (only swollen lymph nodes were visible). The wound was closed with suture (Figure 2). After removal, only the primary tumor was detached from surrounding tissue including, fat, muscle, lymph nodes and skin, and weighed for tumor weight comparison.
Figure 2.
The procedure of Halsted mastectomy.
2.5. Treatment with AZD0530 with or without mastectomy
1 × 105 and 1 × 104 4T1-luc2 cells were inoculated for with and without mastectomy in BALB/c mice. A smaller number of cells were inoculated for the orthotopic model because 4T1 primary tumors grow too fast and quickly reach euthanasia criteria, On the other hand, a larger number of cells was inoculated for mastectomy model because mastectomy needs to be conducted after metastatic cells spread. Halsted mastectomy was performed on 8 days after inoculation of cancer cells. AZD0530 was administrated by oral gavage at a dose of 50 mg/kg daily for the whole experimental period. To mimic human chemotherapy for locally advanced tumors, treatment was started when tumors became palpable (5 days after inoculation) in the no mastectomy group, and to mimic human adjuvant therapy, it was started at the day after mastectomy in the mastectomy group. The mice which developed local recurrence were excluded from this experiment. Tumor burden was quantified using bioluminescence imaging. Pathological analysis was performed after formalin fixation of the tumors as previously reported (4–6, 10–15).
2.6. Bioluminescent quantification of tumor burden
IVIS Spectrum and Living Image software (PerkinElmer) were used to quantify the photons emitted by 4T1-luc2 cells. D-Luciferin (150 mg/kg; PerkinElmer) was injected intraperitoneally into mice previously implanted with 4T1-luc2 cells. Bioluminescence was measured and quantified at 5 minute intervals up to photons peak. Bioluminescence was then determined by the peak number of photons calculated over this time frame as we described previously (4, 5). Ex vivo bioluminescence was measured 30 minutes after intraperitoneal injection of D-luciferin following bilateral pneumonectomy 15 minutes after injection.
2.7. Statistical analysis
Statistical analyses were performed with the chi-square test or the Fisher exact test with a single degree of freedom, and the Student’s t test was used to analyze the differences between continuous values. P values less than 0.05 were considered to have statistical significance. All statistical analyses were performed using R software version 3.1.2 (The R Foundation).
3. Results
3.1. Establishing the best model for studying breast cancer metastasis
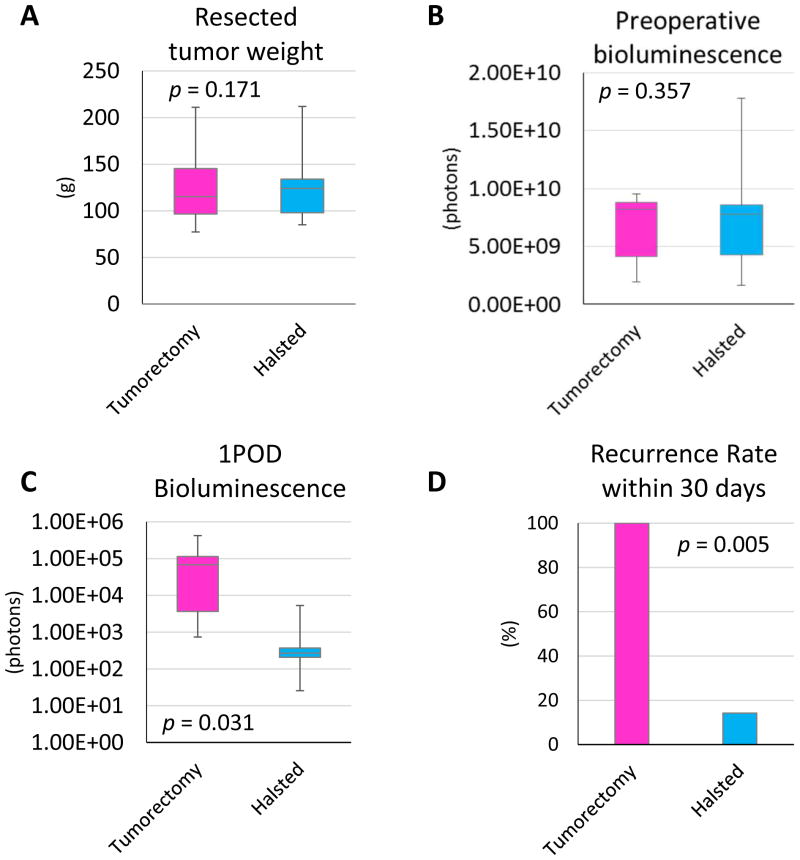
Two methods of tumor removal technique, tumorectomy and Halsted mastectomy, were compared (Figure 1, 2). As expected, there was no significant difference in the preoperative bioluminescence and the weight of resected tumors between these two procedures (p = 0.171 and p = 0.357, respectively) (Figure 3A and 3B). However, the bioluminescence after tumorectomy was significantly higher than Halsted mastectomy at one day after the operation (p = 0.003) (Figure 3C). All (100%) of the mice that underwent tumorectomy developed palpable tumor in the anterior chest wall by 30 days after operation, whereas only 1 out of 7 cases (14%) did after Halsted mastectomy (p = 0.005) (Figure 3D). All of the mice, which expressed more than 1.00E+06E photons in bioluminescence at the day after operation, developed palpable tumors in the anterior chest wall by 10 days after operation (Supplementary figure 1A–C). On the other hand, no mice with less than 1.00E+06E photons in bioluminescence at the day after operation developed palpable tumors during 30 day observation periods, even 10 days after the bioluminescence reached 1.00E+06E photons. Ex vivo results demonstrated that the Halsted mastectomy group mice had lung metastasis (Supplementary figure 1D), therefore, the bioluminescence after Halsted mastectomy reflects tumor burden of lung metastasis. And the mice which showed more than 1.00+06E photons on the day after mastectomy were considered to have remnant disease and were thus excluded from further experiments. Despite the tightened chest due to closure of a wide skin defect by Halsted mastectomy, no mice developed respiratory complications after this procedure. These results demonstrated that Halsted mastectomy is a more suitable procedure to evaluate metastatic lesions in a murine model. We utilized this Halsted mastectomy model for the following experiments.
Figure 3.
Two procedures for mastectomy were compared: (A) Resected tumor weight, (B) bioluminescence 24 hours after implantation and (C) recurrence rate within 30 days after implantation. Data are expressed as means ± SEM.
3.2. Bioluminescence at 15 minutes correlated with the peak in both orthotopic and mastectomy models
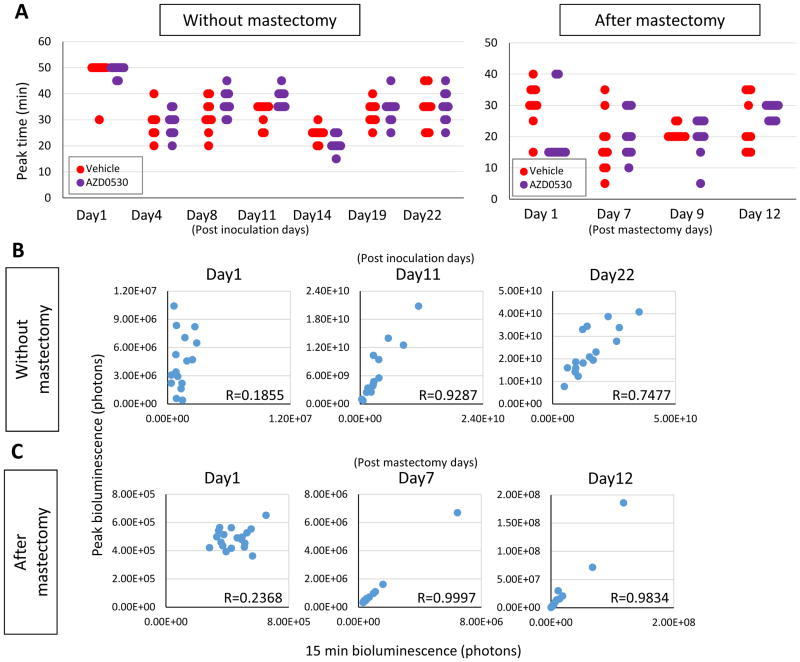
To ask if the bioluminescence of the mastectomy model reflects lung metastasis tumor burden, the bioluminescence in vivo and ex vivo were compared. In vivo bioluminescence was correlated with ex vivo bioluminescence (R2 = 0.6578) (Supplementary Figure 1E). The IVIS imaging system detects and quantifies bioluminescence emitted by luciferase-tagged cells when injected luciferin reaches them. When we began using the bioluminescence system in vivo a decade ago (4–6, 16), we noticed that the time it takes until the value reaches the peak varies by each animal and by each assay (5). Since the objective is to quantify all viable cells we have been measuring bioluminescence every 5 minutes after the intraperitoneal administration of luciferin up to the time that the value peaks out. The time to reach peak value is usually between 15 and 50 minutes (Figure 4A). Time to reach peak was equivocally longest at a day after inoculation and in general short after mastectomy. Otherwise, there was no trend by time course or treatment (Figure 4A). In order to improve the efficiency of the assay, it was of interest to analyze the correlation between peak values and earlier time points because that will significantly shorten the entire assay time. The mouse that developed local recurrence after mastectomy was excluded. We found that except for 24 hours after inoculation of the cells, the bioluminescence value of the peak highly correlated with that at 15 minutes in the orthotopic model (R > 0.7) (Figure 4B). Furthermore, even higher correlation was seen in the mastectomy model, which reflects metastatic tumor burden, except 24 hours after mastectomy (R > 0.9) (Figure 4C).
Figure 4.
The time required to reach peak value and the peak value of bioluminescence in the orthotopic model and mastectomy model. (A) The time required to reach peak value in the orthotopic model and mastectomy model with or without AZD0530 treatment. Correlation of peak value and 15minute bioluminescence in the orthotopic model (B) and mastectomy model (C).
3.3. Validation of the Halsted mastectomy model using AZD0530
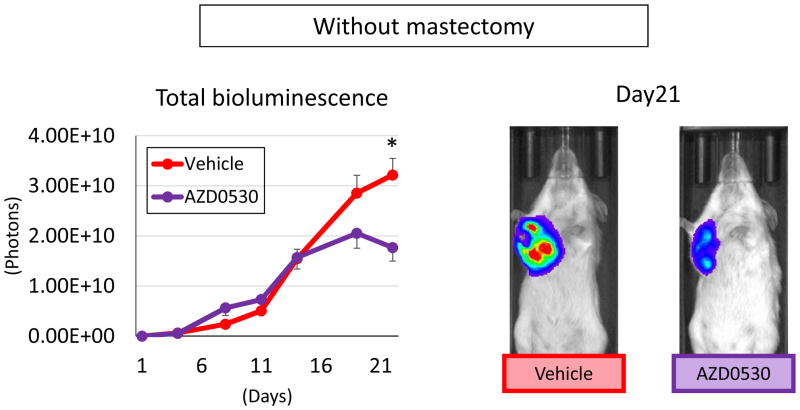
We hypothesized that some drugs failed clinical trials despite their success in preclinical animal studies because the murine model used failed to demonstrate the ineffectiveness of the drug in metastatic tumors. In order to test our hypothesis, we used AZD0530, a Src inhibitor that demonstrated efficacy in murine studies (17, 18), but failed in clinical trials in human breast cancer patients (19). When bioluminescence of total tumor burden without mastectomy was measured, AZD0530 treatment demonstrated significant suppression of tumor burden on Day21 (p = 0.002) (Figure 5). This result is in agreement with the previous report that this drug was effective in a murine breast cancer model (17, 18).
Figure 5.
Whole body bioluminescence of AZD0530 treated orthotopic model. Tumor burden which mainly reflected primary tumor quantified by IVIS. Data are expressed as means ± SEM. *: p < 0.05.
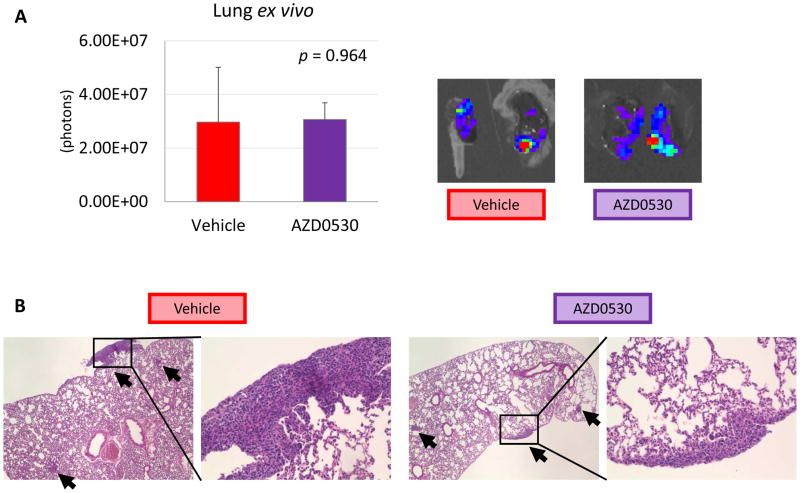
However, ex vivo bioluminescence of the lungs of this experiment, which detect all viable cancer cells in the lungs, demonstrated no significant difference in lung metastasis by AZD0530 treatment (p = 0.964) (Figure 6A). Metastatic lung tumors were assessed by histological analysis as well, which also demonstrated that there was no difference in tumor burden between vehicle and AZD053 treatment (Figure 6B). These results suggest that AZD0530 was effective against the primary tumor, but not metastatic lung tumors.
Figure 6.
Lung metastasis of AZD0530 treated orthotopic model. (A) Lung ex vivo. (B) H&E staining of lung metastases. Data are expressed as means ± SEM. *: p < 0.05.
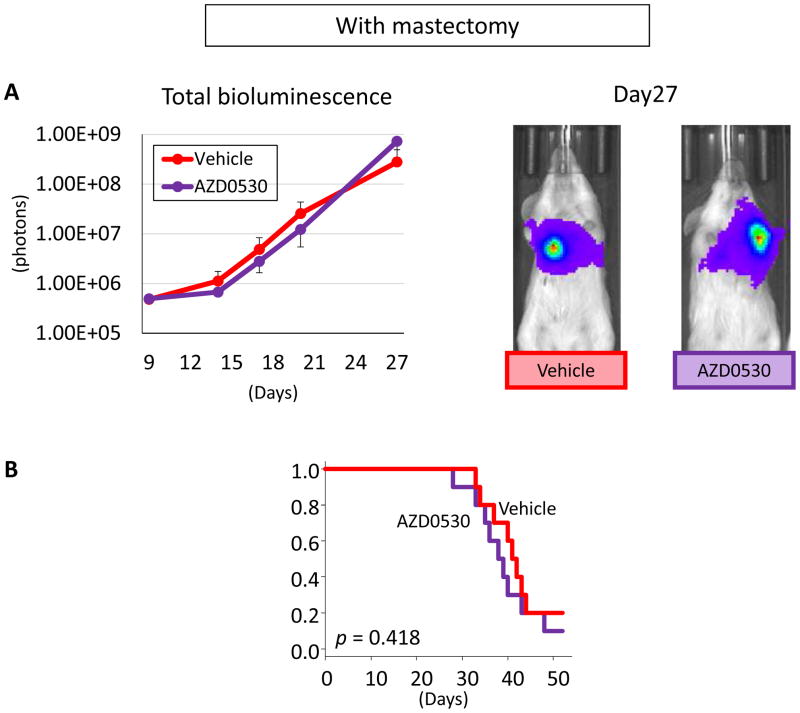
We then tested AZD0530 in our Halsted mastectomy model. Total tumor burden, which reflects lung metastasis in the control group, increased as we described before (4). AZD0530 treatment failed to demonstrate any significant effect on bioluminescence, i.e. tumor burden, after mastectomy (Figure 7A). Since bioluminescence technology allows us to monitor metastatic lesions in living animals, AZD0530 effect on survival was also analyzed. AZD0530 treatment in the mastectomy model showed no survival benefit (p = 0.418) (Figure 7B). Our results suggest that AZD0530 has efficacy against primary but not metastatic breast cancer. Without murine models that mimic human patient conditions, it is not difficult to imagine that the fact that AZD0530 only suppresses the primary and not metastatic tumors was missed prior to the clinical trial.
Figure 7.
AZD0530 treatment in mastectomy model. (A) Tumor burden which mainly reflected lung metastasis quantified by IVIS. (B) Survival of AZD0530 or vehicle treated mice. Data are expressed as means ± SEM.
4. Discussion
For the last decade, our laboratory has been studying murine cancer models that mimic human cancer progression for use in preclinical trials (4–6, 10, 11, 13, 20). The purpose of murine models in preclinical trials is to predict whether a novel therapeutic agent will demonstrate efficacy in humans (2–5, 7). Therefore, it is more important for a murine model to reproduce human conditions, rather than to mimic tumorigenesis, in order to produce translatable clinical endpoints to predict efficacy in human clinical trials (2–5, 7). Our group has previously compared the genetic profiles of lung inoculation tumor after tail vein injection of cancer cells vs. lung metastasis developed after orthotopic implantation of the cells (16). Although we found that genetic profiles of tumors between these two models were similar, the morphology of tumors was very different and, together with the fact that the tail vein injection model is known to cause sudden death due pulmonary embolization of cancer cells, we believe mastectomy is more a suitable method to assess drug efficacy of lung metastasis. In this study, we compared two tumor removal models, tumorectomy and Halsted mastectomy, and demonstrated that Halsted mastectomy is the suitable murine model for preclinical study given its low local recurrence rate.]
Bioluminescence imaging with luciferase-tagged cells allowed us to quantify tumor burden and study cancer progression in vivo (21, 22). Indeed, this technology is commonly used for a wide variety of research (21); however, there is still room for improvement. First, we demonstrated that lung ex vivo tumor burden quantification by bioluminescence reflected in vivo bioluminescence. The ex vivo signal of the lung compared with the in vivo signal correlated with the relatively higher intensity observed in vivo peak value. The ex vivo signal was lower than the in vivo signal with a range from 0.9 to 40.
In the vast majority of the published studies, only one time point has been chosen to measure bioluminescence for tumor burden. During our previous study, we found that time to reach peak bioluminescence value differs considerably in each animal for each assay, thus, we suggested in that publication to obtain measurements every 5 minutes until the peak has been obtained in each and every assay (10). Our current study revealed that the 15 minute after injection bioluminescence value correlated highly with peak value, except for 24 hours after inoculation or mastectomy. This result implies that the 15 minute value is acceptable to be used to monitor tumor burden. It can dramatically reduce time-consuming experimental time. On the other hand, for the studies with a small cohort when a few outliers can greatly affect the results, it may be worthwhile to monitor peak time to detect all viable cancer cells because there are a wide variety of peak times in each animal for each assay.
As shown in Figure 4B and C, there is a more than 100 times difference in bioluminescence between the orthotopic model and the mastectomy model. This may reflect the difference in the tumor volume and tumor depth between the primary tumor and lung metastasis. The time to reach peak is affected by vascular density involving angiogenesis (23). In our results, the time to reach peak value in the primary lesion varied, and thus the peak and 15 minutes value had a relatively low correlation. The primary lesion was generated by inoculation of Matrigel suspended cancer cells. Therefore, there is a variety of distance to the nearest vessel and spread of Matrigel. On the other hand, there is less variety of time to reach peak value in lung metastatic lesion. It was formed by a biological process and thus it may not have a variety of hemodynamics affected by luciferin perfusion time in Matrigel. These might be reasons that bioluminescence of 15 minutes value showed higher correlation with peak value in the mastectomy model. The reason of no correlation between peak time and 15 minutes value at 24 hours after inoculation is likely because inoculated cancer cells had not engrafted yet. This is in agreement with the fact that 24 hours after inoculation took the longest time to reach the peak, which may be because luciferin diffusion in Matrigel takes longer compared with at later time points when angiogenesis has occurred to deliver luciferin faster. This notion is in agreement with the result that time to reach the peak was generally faster in lung metastatic tumors that are known to be hypervasular compared to primary inoculated tumors. Our results also suggest that bioluminescence value may not be reliable before 24 hours after inoculation and thus not suitable to be used as criteria for randomization.
We used AZD0530, an oral Src inhibitor, to test our hypothesis that some drugs fail clinical trials despite their success in preclinical animal studies because the murine model used failed to demonstrate the ineffectiveness of the drug against metastatic tumors. Previous reports demonstrated that AZD0530 significantly suppressed primary lesions, including a triple negative breast cancer cell line (MDA-MB-231), during its preclinical studies (17, 18). However, a phase II clinical trial for hormone receptor negative metastatic breast cancer failed because a majority of patients’ cancer progressed even with AZD0530, which means it showed no effect against metastatic cancer (19). AZD0530 suppressed overall tumor burden when the majority of cancer cells is in the primary tumor in an orthotopic model. On the other hand, ex vivo bioluminescence that reflects metastatic lung tumor burden demonstrated that AZD0530 did not suppress metastatic tumors. The limitation of this model for preclinical studies is that the data on metastasis are only available after the animal is euthanized because the bioluminescence from the primary tumor overshadows any signal from the lung, and survival data cannot be obtained. On the other hand, the Halsted mastectomy model allows for monitoring of the metastatic tumor in live animals since the primary tumor is removed, and survival can be assessed. AZD0530 demonstrated no efficacy against metastatic lung tumor progression or any survival benefit in live animals after Halsted mastectomy. We speculate that the difference in response to treatment is due to the fact that metastatic lesions have different biological features from their primary site. We have previously reported that metastatic lesions showed a completely different genetic profile and biological behavior compared to their primary lesion, including primary lesions created by inoculation in orthotopic versus ectopic sites (5, 6, 9, 16). This is in agreement with other studies that demonstrated the difference between primary and metastatic lesions (24–26). In addition, treatment response may be due to multiple factors, including microenvironment and cancer stem cells.
Clearly, the fact that AZD0530 has efficacy against the primary breast tumor but not against metastatic breast cancer was either missed or not considered relevant during preclinical studies of its efficacy against breast cancer. Accordingly, if the efficacy against metastatic lesions had been a prerequisite for entry into human clinical trials, then all the cost, time, effort and lives of human subjects wasted in clinical trials would have been allocated elsewhere. Therefore, our results propose that preclinical animal studies use cancer cells that metastasize and reflect human conditions, such as after mastectomy which mimics the adjuvant setting. Evaluation of the primary tumor alone is clearly is not enough, and may even mislead investigators in preclinical studies. Although there has been recent interest in technological innovations in molecular biology, genomic analysis, and computer simulations to model and predict cancer outcomes, breast cancer drug development still relies on animal models for preclinical trials and we should continue to critically evaluate and improve these models to improve the efficiency of drug development.
5. Conclusions
In conclusion, we critically evaluated and established a murine radical mastectomy model for evaluating drug efficacy against metastatic breast cancer lesions. It provides a new model for preclinical drug development that mimics the human adjuvant setting.
Supplementary Material
Supplementary Figure 1. Bioluminescence of with/without local recurrence and ex vivo. (A & B) Individual bioluminescence of the mice which received tumorectomy (A) or Halsted mastectomy (B). (C) 1POD bioluminescence of the mice which received tumorectomy and Halsted mastectomy. (D) Comparison of bioluminescence between tumorectomy and Halsted mastectomy. (E) The correlation of lung metastasis between in vivo and ex vivo in lung metastasis. Data are expressed as means ± SEM. *: p < 0.05.
Supplementary Figure 2. H&E staining of lung metastasis of AZD0530 treated orthotopic model for all mice. (A) Vehicle control and (B) AZD0530 treated mice.
Acknowledgments
This work was supported by NIH grant R01CA160688 and Susan G. Komen Foundation Investigator Initiated Research grant (IIR12222224) to K.T. The tumor sections and staining were performed by Ellen Karasik, Mouse Tumor Model Shared Resource at Roswell Park Cancer Institute. Mice bioluminescence images were acquired by shared resource Translational Imaging Shared Resource at Roswell Park Cancer Institute, which was supported by the Cancer Center Support Grant (P30CA01656).
Footnotes
Authors’ contributions:
E.K. conceptualized, performed experiments and prepared the article.
O.R. prepared the article.
K.T. provided supervision of experiments and prepared the article.
Disclosure
There are no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rashid OM, Takabe K. Animal models for exploring the pharmacokinetics of breast cancer therapies. Expert opinion on drug metabolism & toxicology. 2015;11:221–230. doi: 10.1517/17425255.2015.983073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rashid OM, Maurente D, Takabe K. A Systematic Approach to Preclinical Trials in Metastatic Breast Cancer. Chemotherapy. 2016;5 doi: 10.4172/2167-7700.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer research. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashid OM, Nagahashi M, Ramachandran S, Dumur C, Schaum J, et al. An improved syngeneic orthotopic murine model of human breast cancer progression. Breast cancer research and treatment. 2014;147:501–512. doi: 10.1007/s10549-014-3118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rashid OM, Nagahashi M, Ramachandran S, Graham L, Yamada A, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153:771–778. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rashid OM, Takabe K. The evolution of the role of surgery in the management of breast cancer lung metastasis. Journal of thoracic disease. 2012;4:420–424. doi: 10.3978/j.issn.2072-1439.2012.07.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebos JM, Mastri M, Lee CR, Tracz A, Hudson JM, et al. Neoadjuvant antiangiogenic therapy reveals contrasts in primary and metastatic tumor efficacy. EMBO molecular medicine. 2014;6:1561–1576. doi: 10.15252/emmm.201403989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashid OM, Takabe K. Does removal of the primary tumor in metastatic breast cancer improve survival? Journal of women’s health (2002) 2014;23:184–188. doi: 10.1089/jwh.2013.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsuta E, DeMasi SC, Terracina KP, Spiegel S, Phan GQ, et al. Modified breast cancer model for preclinical immunotherapy studies. The Journal of surgical research. 2016;204:467–474. doi: 10.1016/j.jss.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terracina KP, Aoyagi T, Huang WC, Nagahashi M, Yamada A, et al. Development of a metastatic murine colon cancer model. The Journal of surgical research. 2015;199:106–114. doi: 10.1016/j.jss.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki H, Aoki M, Katsuta E, Ramanathan R, Idowu MO, et al. Host sphingosine kinase 1 worsens pancreatic cancer peritoneal carcinomatosis. The Journal of surgical research. 2016;205:510–517. doi: 10.1016/j.jss.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki H, Aoki M, Yang J, Katsuta E, Mukhopadhyay P, et al. Murine model of long-term obstructive jaundice. The Journal of surgical research. 2016;206:118–125. doi: 10.1016/j.jss.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology (Baltimore, Md) 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4:e156. doi: 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid OM, Nagahashi M, Ramachandran S, Dumur CI, Schaum JC, et al. Is tail vein injection a relevant breast cancer lung metastasis model? Journal of thoracic disease. 2013;5:385–392. doi: 10.3978/j.issn.2072-1439.2013.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, et al. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Molecular oncology. 2009;3:248–261. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones RJ, Young O, Renshaw L, Jacobs V, Fennell M, et al. Src inhibitors in early breast cancer: a methodology, feasibility and variability study. Breast cancer research and treatment. 2009;114:211–221. doi: 10.1007/s10549-008-9997-1. [DOI] [PubMed] [Google Scholar]
- 19.Gucalp A, Sparano JA, Caravelli J, Santamauro J, Patil S, et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clinical breast cancer. 2011;11:306–311. doi: 10.1016/j.clbc.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demasi SC, Katsuta E, Takabe K. Live animals for preclinical medical student surgical training. Edorium J Surg. 2016;5:24–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Dothager RS, Flentie K, Moss B, Pan MH, Kesarwala A, et al. Advances in bioluminescence imaging of live animal models. Current opinion in biotechnology. 2009;20:45–53. doi: 10.1016/j.copbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui K, Xu X, Zhao H, Wong ST. A quantitative study of factors affecting in vivo bioluminescence imaging. Luminescence: the journal of biological and chemical luminescence. 2008;23:292–295. doi: 10.1002/bio.1032. [DOI] [PubMed] [Google Scholar]
- 23.Sun A, Hou L, Prugpichailers T, Dunkel J, Kalani MA, et al. Firefly luciferase-based dynamic bioluminescence imaging: a noninvasive technique to assess tumor angiogenesis. Neurosurgery. 2010;66:751–757. doi: 10.1227/01.NEU.0000367452.37534.B1. discussion 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massague J, Batlle E, Gomis RR. Understanding the molecular mechanisms driving metastasis. Molecular oncology. 2017;11:3–4. doi: 10.1002/1878-0261.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malladi S, Macalinao DG, Jin X, He L, Basnet H, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Bioluminescence of with/without local recurrence and ex vivo. (A & B) Individual bioluminescence of the mice which received tumorectomy (A) or Halsted mastectomy (B). (C) 1POD bioluminescence of the mice which received tumorectomy and Halsted mastectomy. (D) Comparison of bioluminescence between tumorectomy and Halsted mastectomy. (E) The correlation of lung metastasis between in vivo and ex vivo in lung metastasis. Data are expressed as means ± SEM. *: p < 0.05.
Supplementary Figure 2. H&E staining of lung metastasis of AZD0530 treated orthotopic model for all mice. (A) Vehicle control and (B) AZD0530 treated mice.