Abstract
Background
Bone marrow of blotchy mouse (blotchy marrow) reflects the function of transmembrane domain and relevant intramembrane sites of ATP7A in myeloid cells. By chronic infusion of angiotensin II, we previously found that blotchy marrow plays a minor role in regulating plasma copper. Moreover, the recipients of blotchy marrow presented a moderate reduction of plasma lipids and inflammatory mediator production. Little is known about whether these changes are a specific response to angiotensin II or reveal a more general role of ATP7A.
Objective and design
We investigated if blotchy marrow reduces plasma lipids and inflammatory mediators induced by high-fat diets. To test this hypothesis, blotchy and control marrows were reconstituted to the recipient mice (irradiated male LDLR−/− mice), followed by high-fat-diet feeding for 4 months. At the end points, plasma metals (copper, zinc and iron), lipid profiling (cholesterol, triglyceride, phospholipids and lipoprotein) and six inflammatory mediators (lymphotacin, MCP3, MCP5, TIMP1, VEGF-A and IP-10) were measured. Parallel experiments were performed using male LDLR−/− mice fed either high-fat diets or chow diets for 4 months.
Results
In addition to hyperlipidemia and low-grade inflammation, high-fat diets selectively increased plasma copper concentration compared to chow diets in LDLR−/− mice. After high-fat-diet feeding, the recipients with blotchy marrow showed a decrease in plasma copper (p < 0.01) and an increase in plasma iron (p < 0.05). The recipients with blotchy marrow also presented decreases in cholesterol (p < 0.01) and phospholipids (p < 0.05) in plasma. Surprisingly, plasma levels of MCP3 (p < 0.05), MCP5 (p < 0.05), TIMP1 (p < 0.01), VEGF-A (p < 0.01) and IP-10 (p < 0.01) were significantly increased in the recipients with blotchy marrow compared to controls; the increased levels of MCP3, MCP5 and TIMP1 were more than 50%.
Conclusion
Our studies showed that blotchy marrow counteracts the increased copper levels induced by high-fat diets, indicating that circulating myeloid cells can regulate blood copper levels via ATP7A. Moreover, transplantation of blotchy marrow followed by high-fat diets leads to a decrease in lipid profile and an increase in inflammatory mediator production. Overall, blotchy marrow mediates divergent responses to angiotensin II and high-fat diets in vivo.
Keywords: ATP7A, Copper, Lipid metabolism, Inflammation, Bone marrow
Introduction
The blotchy mouse is an animal model primarily recapitulating the connective tissue defects in human Menkes disease, which is an X-linked genetic disorder caused by loss-of-function mutations of ATP7A. ATP7A is located at Xq21.1, consists of 23 exons, and encodes a copper-transporting P-type ATPase. Its gene product, ATP7A, a single-chain 178-kDa polypeptide, is localized to the trans-Golgi apparatus and, to a lesser extent, plasma membrane, nucleus, lipid rafts and phagosome [1–3]. The similarity between mouse and human ATP7A is high: 88% in nucleotide and 90% in amino acid [4]. The studies from Menkes disease and its animal models reveal that the primary functions of ATP7A are to emigrate copper from cells [5–9] and to deliver dietary copper from intestine to blood [10–12]. The reduced activity of copper-dependent enzymes, such as dopamine-β-hydroxylase [13,14], cytochrome oxidase [13,15], lysyl oxidase [16,17] and extracellular superoxide dismutase [18–20], are well documented in the tissues of Menkes disease patients and animal models. The reduction appears partly due to ATP7A mutations failing to transfer copper to cellular secretory pathways, likely the first site for these enzymes to receive copper in a tightly regulated manner [21], and partly due to overall reduction of copper levels in extracellular space, likely the second site for these excreted enzymes to receive copper for the maintenance of their full activity. The later is important in the homeostasis of vascular walls, because a recent study indicated that elastic laminae in extracellular matrix serve as a major copper reservoir [22,23]. Furthermore, the copper deprivation caused by dysfunctional copper delivery in intestine of Menkes disease overshadows the functions of ATP7A in other tissues, bone marrow transplantation (BMT) offers a bypass around the intestine barrier and directly investigates the function of ATP7A in myeloid cells in vivo.
The functional domains of ATP7A include six metal-binding domains with MXCXXC binding motifs, a nucleotide-binding domain, an actuator domain, a phosphorylation domain, eight transmembrane domains and a C-terminal tail. These functional domains offer a glimpse how ATP7A functioning as a copper émigré. Copper emigration is efficiently (from few minutes to few hours) regulated via two mechanisms: translocation, ATP7A primarily locates at trans-Golgi apparatus at basal levels and has capacity to translocate to plasma membrane under the copper elevation [24]; and catalytic cycle, a cycle of phosphorylation to escalate copper affinity of ATP7A and dephosphorylation to reduce copper affinity [25]. ATP7A translocation can be regulated by metal-binding domains [24], which serves as a sensor of low copper levels [25]. Catalytic cycle contains two important components: the formation of a transient phosphorylated intermediate, affecting copper affinity of ATP7A [25]; and the role of a lumenal loop (Met672–Pro707) between the first two transmembrane domains, binding copper and facilitating copper releasing [21]. In addition, two transmembrane domains (six and seven) contain high affinity sites to copper [26]. Furthermore, the theory of catalytic cycle emphasizes the important role of transmembrane domains, which is further reiterated in blotchy mice with ATP7A mutations targeting to the fourth transmembrane domain [27]. Because the mutations in blotchy mice moderately reduced tissue amount of ATP7A (hypomorphic), but not change the protein size, the finding from bone marrow of blotchy mice (blotchy marrow) primarily represents the function of transmembrane domain and intramembrane sites of ATP7A in myeloid cells. In addition, we found that certain stimulants can gradually increase ATP7A protein levels within a few days, likely associated with increased copper emigration [8]. Thus, increasing protein levels may serve as a chronic mechanism to regulate copper emigration via an increase in functional domain availability to stimulate protein translocation and/or to enhance the copper affinity constantly.
Because blotchy mice develop aortic aneurysm and rupture [28], we recently studied the contribution of blotchy marrow to aortic pathologies in a well-accepted model of aortic aneurysm and dissection (chronic infusion of angiotensin II) [29]. This study revealed that blotchy marrow has less influence on plasma copper and aortic aneurysm and dissection, although there was an increased tendency of aortic rupture. Moreover, this study revealed novel functions of blotchy marrow – the recipients of blotchy marrow present a moderate reduction of plasma triglycerides and a subset of inflammatory mediators. However, little is known about whether these changes are a specific response to angiotensin II or reveal a more general role of blotchy marrow. We therefore investigated how blotchy marrow regulates plasma lipids and inflammatory mediator production under the induction of high-fat diets. Surprisingly, this study also revealed an opposite regulation of plasma copper levels by high-fat diets and blotchy marrow.
Materials and methods
Mice
ATP7A-deficient heterozygous female mice (B6Ei.Cg-Atp7aMo–blo), green fluorescent protein (GFP) transgenic mice [C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ] and low-density lipoprotein receptor (LDLR)−/− mice (B6.129S7-Ldlrtm1Her) were obtained from the Jackson Laboratory (Bar Harbor, ME). ATP7A-deficient female heterozygotes were crossed with GFP transgenic males to produce two new mouse strains: blotchy; GFP and wild type (WT); GFP mice [29]. A normal chow diet and an atherogenic high-fat diet (TD.88137) were used (Table 1). The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Table 1.
Comparison of dietary composition between the chow diets and high-fat diets used in this study.
| Dietary composition | Unit | Chow dieta (Harlan 7012) | High-fat dieta (TD.88137) |
|---|---|---|---|
| Protein | % by weight | 19 | 17 |
| Fat (ether extract) | % by weight | 6 | 49 |
| Carbohydrate | % by weight | 44 | 21 |
| Energy density | kcal/g | 3 | 5 |
| Calories from protein | % | 25 | 15 |
| Calories from fat | % | 17 | 42 |
| Calories from carbohydrate | % | 58 | 43 |
| Total saturated fatty acid | % of diet | 1 | 13 |
| Total monounsaturated fatty acid | % of diet | 1 | 6 |
| Total polyunstaturated fatty acid | % of diet | 3 | 1 |
| Copper | mg/kg | 23 | 6 |
| Zinc | mg/kg | 63 | 35 |
| Iron | mg/kg | 240 | 36 |
The data were collected from datasheet of 7012 and TD.88137 as well as personal communication (Dr. Ramesh Khanal) from Harlan Laboratories, Inc., Madison, WT 53744.
Genotyping
Genomic DNAs were obtained from either mouse-tail snips or blood samples. Polymerase chain reaction (PCR) was employed for the genotyping of LDLR −/− mice and GFP transgenic mice, whereas PCR followed by restriction enzyme (AvaII, New England Biolabs, Ipswich, MA) digestion was conducted for the genotyping of blotchy mice and mice reconstituted with blotchy marrow as described [29].
Bone marrow transplantation
Blotchy; GFP or WT; GFP mice at 3–4 months of age (donors) and LDLR−/− mice at 2–3 months of age (recipients) were used to create chimeras. One wk prior to radiation, the recipient mice were given acidified (pH 2.6) water containing neomycin (0.2 mg/mL; Sigma–Aldrich, St. Louis, MO) and polymyxin B (0.02 mg/mL; Sigma–Aldrich). During radiation, a total of 900 centiGrays (cGy) was administered at a rate of 98.1 cGy/min to eradicate bone marrow from the recipient mice using a 137Cesium source. Bone marrow was isolated from the donor mice by flushing their femurs with Dulbecco’s modified Eagle’s medium (GIBCO™, Invitrogen, Carlsbad, CA), followed by disrupting the marrow through a 25-gauge needle and filtering through a 40-μm-cell strainer (BD Biosciences, Franklin Lakes, NJ). Following centrifugation at 250 × g for 5 min, the pellet was resuspended in Hank’s balanced salt solution (Invitrogen). Four hrs after radiation, these marrow cells (5 × 106) were injected into the tail veins of recipient mice. The mice were kept on antibiotic water for 4 wks post-radiation. There was no significant difference in the engraftment efficiency of donor cells (>90%) between the two groups of recipient mice, which was determined by measuring the percentage of GFP+ cells in the peritoneal exudates of recipient mice using flow cytometry as described [29].
Plasma analyses
Whole blood was collected through the right ventricle of the heart and transferred to a microtainer™ tube with ethylenediaminetetraacetic acid (BD Biosciences, Franklin Lakes, NJ). Plasma was collected by centrifugation at 2000 × g at 4°C for 10 min. Plasma samples were analyzed to determine the biometal concentration via inductively coupled plasma mass spectrometry. Colorimetric assays were used to determine the concentrations of triglycerides, total cholesterol and phospholipids in plasma as described [30]. Lipid distribution among various lipoproteins in pooled plasma samples (n = 4–5/group) was analyzed by applying plasma samples to fast performance liquid chromatography (FPLC) gel filtration on two Superose 6 HR columns as described [31]. Each fraction (0.5 mL) was collected for triglyceride and cholesterol measurements. Inflammatory mediators were analyzed in plasma samples through a bead-based multiplexing immunoassay as described [32,33]. These mediators included interferon-γ-inducible protein (IP)-10, lymphotactin, monocyte chemotactic protein (MCP)3, MCP5, tissue inhibitor of metalloproteinases (TIMP)1 and vascular endothelial growth factor (VEGF)-A.
Statistics
Quantitative variables, including body weight, plasma biometal concentrations, lipid profiles and inflammatory mediator concentrations, were expressed as the mean ± standard error (SE) and were compared in a paired Student’s t-test (one-tailed). Significant differences were defined as those with p values <0.05. All statistical analysis was performed with Graphpad Prism version 6 (GraphPad Software, Inc., La Jolla, CA).
Results
We first compared the body weight and plasma analytes in LDLR−/− mice fed either chow diets or high-fat diets for 4 mon (n = 3 for each group). Compared to chow diets, high-fat diets resulted in a 1.6-fold increase in body weight: 28.8 ± 1.3 g vs. 44.3 ± 1.3 g, p < 0.001. Hyperlipidemia was developed following high-fat-diet feeding: a 5.1-fold increase in plasma triglyceride (172 ± 22 mg/dL vs. 884 ± 222 mg/dL, p < 0.05), a 19.8-fold increase in plasma cholesterol (148 ± 19 mg/dL vs. 2913 ± 278 mg/dL, p < 0.01) and a 3.8-fold increase in plasma phospholipids (337 ± 37 mg/dL vs. 1288 ± 99 mg/dL, p <0.01). Among six inflammatory mediators studied, three of them were significantly increased following high-fat feeding: lymphotactin (155 ± 20 pg/mL vs. 243 ± 28 pg/mL, p <0.05, 1.6-fold), TIMP1 (3.2 ± 0.4 ng/mL vs. 1.2 ± 0.3 ng/mL, p <0.05, 2.7-fold) and IP-10 (72 ± 8 pg/mL vs. 131 ± 25 pg/mL, p <0.05, 1.8-fold). More important, copper concentration was increased 2.4-fold over high-fat feeding (439 ± 31 μg/L vs. 1028 ± 110 μg/L, p <0.05), whereas zinc and iron concentrations were not significantly altered. Note although the copper content in high-fat diets was lower than that in chow diets (Table 1), copper contents in both diets were adequate because only the diet with copper content less than 0.4 mg/kg is considered as copper deficient [34–36].
Next, we compared the body weight and plasma analytes in LDLR−/− mice reconstituted with either blotchy (n = 5) or control (n = 4) marrow, followed by high-fat diets for 4 mon. Note that all parameters detected from mice with BMT were markedly lower than those from mice without BMT, and this is likely due to that BMT reduced the dietary uptake and relevant metabolism. Our results showed that there was no significant difference in body weight between these two groups (32.2 ± 1.2 g vs. 31.4 ± 1.1 g, p >0.05). Compared to reconstitution with control marrow, reconstitution with blotchy marrow resulted in a significant decrease in plasma cholesterol (2316 ± 72 mg/dL vs. 1917 ± 75 mg/dL, p < 0.01, 17% reduction) and phospholipids (1001 ± 51 mg/dL vs. 881 ± 28 mg/dL, p <0.05, 12% reduction) (Fig. 1).
Fig. 1.
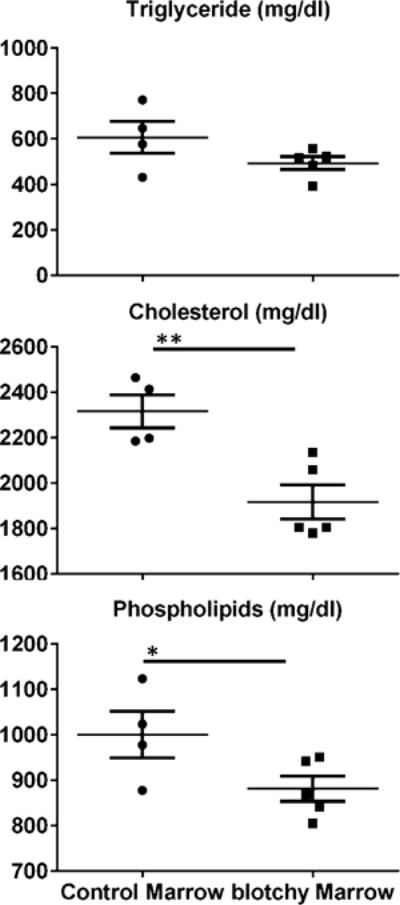
Concentrations of triglycerides, total cholesterol and phospholipids in the plasma of LDLR−/− mice reconstituted with control or blotchy marrow following by 4 mon of high-fat feeding. Fifteen microliters of plasma (nonfasting) was used to determine the concentrations of triglycerides, total cholesterol and phospholipids through specific colorimetric assays. Dots and squares represent values from individual mice, thin long lines are mean values of each group, and bars are SE. n = 4–5 for each group. *p < 0.05 and **p <0.01.
Analysis of plasma cholesterol lipoproteins by FPLC showed that under high-fat diets, there was a marked accumulation of larger lipoproteins in both groups, whose size corresponded to very low density lipoproteins (VLDLs) and low-density lipoproteins (LDLs) (Fig. 2, top panel). Fortuitously, due to the difference in the recovery rate of FPLC between samples, we can compare lipoprotein contents under approximate equal amounts of cholesterol between both groups and found that more LDL and more high density lipoprotein (HDL) in the subsets of fractions in blotchy marrow recipients (Fig. 2, top panel). For quantification, factions from FPLC were pooled and classified them as VLDL, LDL and HDL. The largest amount in cholesterol content was seen in LDL fraction of both groups, whereas the largest amount in triglyceride content was seen in VLDL fraction of both groups (Table 2). A lesser amount of lipoproteins was seen in blotchy-marrow recipients except triglyceride-HDL (Table 2).
Fig. 2.
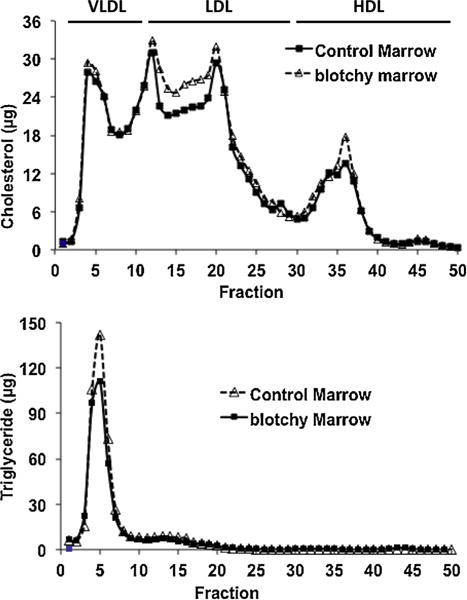
Plasma lipoproteins in LDLR−/− mice reconstituted with control or blotchy marrow following by 4 mon of high-fat feeding. Pooled plasma from each group (nonfasting) was centrifuged at d = 1.215 g/mL, the lipoprotein fractions (d < 1.215 g/mL) were subjected to FPLC, and the total cholesterol and triglyceride content of each fraction was measured using an enzymatic assay kit.
Table 2.
Cholesterol and triglyceride contents of plasma lipoprotein fractions from recipient mice fed high-fat diets.a
| Concentration (mg/dL)
|
|||
|---|---|---|---|
| Control marrow | Blotchy marrow | ||
| Cholesterol | |||
| VLDL | 737 | 517 | |
| LDL | 949 | 733 | |
| HDL | 313 | 239 | |
| Triglyceride | |||
| VLDL | 681 | 490 | |
| LDL | 83 | 59 | |
| HDL | 2 | 11 | |
Lipoprotein fractions isolated by FPLC in Fig. 2 were used for measurement of triglyceride and total cholesterol contents. The following FPLC fractions were pooled for analysis: VLDL, fractions 1–12; LDL, fractions 13–29; HDL, fractions 30–50. Data were corrected by the recovery rate of FPLC and were expressed as mg per dL of original plasma sample.
Surprisingly, compared to reconstitution with control marrow, reconstitution with blotchy marrow led to increases in the plasma concentrations of MCP3 (175 ± 7 pg/mL vs. 319 ± 58 pg/mL, p < 0.05, 82% increase), MCP5 (19 ± 2 pg/mL vs. 37 ± 7 pg/mL, p < 0.05, 93% increase), TIMP1 (1.83 ± ng/mL vs. 3.22 ± ng/mL, p <0.01, 76% increase), VEGF-A (723 ± 69 pg/mL vs. 1021 ± 66 pg/mL, p<0.01, 41% increase) and IP-10 (155 ± 19 pg/mL vs. 201 ± 20 pg/mL, p < 0.01, 29% increase). Plasma lymphotactin was not significantly altered (Fig. 3).
Fig. 3.
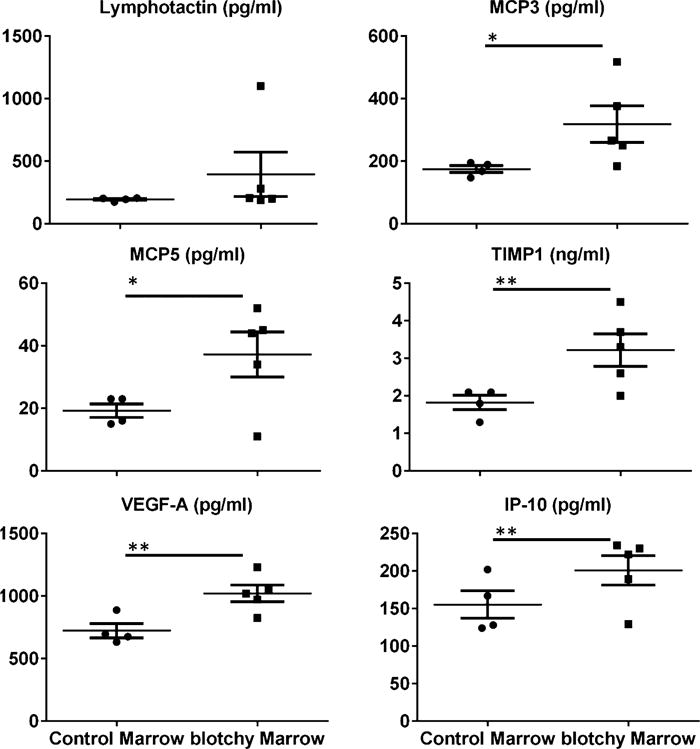
Concentrations of inflammatory mediators in the plasma of LDLR−/− mice reconstituted with control or blotchy marrow following 4 mon of high-fat feeding. Six inflammatory mediators were detected in plasma using a bead-based multiplexing immunoassay as previously described [33]. Dots and squares represent values from individual mice, thin long lines are mean values of each group, and bars are SE. n = 4–5 for each group. *p < 0.05 and **p <0.01.
Finally, compared to reconstitution with control marrow, reconstitution with blotchy marrow resulted in a 10% decrease in plasma copper concentration: 687 ± 12 μg/L vs. 618 ± 22 μg/L, p < 0.01. In contrast, reconstituted with blotchy marrow resulted in a 60% increase in the iron concentration: 4.5 ± 0.6 mg/L vs. 7.3 ± 0.9 mg/L, p < 0.05. However, zinc concentration was not significantly altered (Fig. 4).
Fig. 4.

Concentration of copper, iron and zinc in plasma of LDLR−/− mice reconstituted with control or blotchy marrow following 4 mon of high-fat feeding. Fifty microliters of plasma were analyzed to determine biometal concentrations via inductively coupled plasma mass spectrometry. Three replicate measurements were performed for each sample, and the final reported concentration was the mean value of these three replicates. Dots and squares represent values from individual mice, thin long lines are mean values of each group, and bars are SE. n = 4–5 for each group. *p <0.05 and **p <0.01.
Discussion
Our studies showed that blotchy marrow counteracts the increased copper levels induced by high-fat diet, indicating that circulating myeloid cells can regulate blood copper levels. Moreover, transplantation of blotchy marrow followed by high-fat diets leads to a decrease in lipid profile and an increase in inflammatory mediator production. By comparing our previous study in chronic angiotensin II infused model [29], the role of blotchy marrow in copper and iron metabolism, lipid profile and inflammation was discussed here.
Role of blotchy marrow in the regulation of blood copper levels
The plasma copper concentration in blotchy mice was decreased approximately 40% compared to control mice (data not shown). In our high-fat feeding models (4 mon), the plasma copper concentration in chimeras with blotchy marrow was decreased approximately 10% compared to those with control marrow. Although intestine is believed to play a major role to regulate tissue copper levels, our finding indicates that ATP7A in blood cells has moderate abilities (approximately 25%) to regulate the blood copper levels and hints that the dysfunction of ATP7A in blood cells also contribute to the reduced copper levels in blood of Menkes disease.
In our previously study using angiotensin II infused model (1 mon), there was not significant changed in blood copper levels between chimeras with blotchy marrow or control marrow. The different results between high-fat feeding and angiotensin II infusion can be generated from two factors: time and stimulation. First, after BMT, it may require a longer period of recovery (between 1 and 4 mon) to allow blotchy myeloid cells to interfere blood copper levels significantly. In the early stage of post-BMT, the components of vascular walls such as endothelial cells, smooth muscle cells and elastin may well compensate the dysfunction of blotchy myeloid cells on blood copper regulation. Second, certain stimulations may be needed to unmask the consequence of loss-of-function mutations of ATP7A. This possibility is supported by a recent in vitro experiment showing that the copper levels were not changed in rat intestinal epithelial cells after inhibition of ATP7A using siRNA technique; whereas the copper levels were markedly changed only after these cells treated with copper chloride [37]. Because high-fat-diet feeding (4 mon), but not angiotensin II infusion (data not shown), can significantly increase blood copper levels (Fig. 4), the increased copper may serve as a stimulant to efficiently reveal the dysfunction of blotchy myeloid cells. Overall, our findings provide an in vivo evidence to support the concept that the function of ATP7A on copper emigration is on idle mode at basal condition and is activated under specific stimulations such as increased copper levels.
Cross-talking between copper and iron metabolism
The cross-talking between copper and iron metabolism was suggested previously, e.g., hepatic copper levels may increase in rats with iron-deprived diets, and conversely, hepatic iron levels may increase in rats with copper-deprived diets [38]. Furthermore, ATP7A offers a mechanistic insight to understand the event, e.g., the increased intestinal ATP7A protein in rats with iron-deprived diets [39] and in Belgrade rat model with genetic iron deprivation [40]. Thus, liver and intestine have been considered as two major organs to host the cross-talking [41]. Our current study showed that the decreased copper levels in plasma of chimeras with blotchy marrow are associated with increased iron levels. Because BMT bypasses intestinal copper absorption and most likely hepatic copper storage, myeloid cells are likely another organ to host the cross-talking between copper and iron.
Blotchy marrow and plasma lipid metabolism
The change of lipid metabolism in Menkes disease appears controversial, e.g., cerebral free cholesterol and phospholipids were markedly decreased in a patient with Menkes disease [42]. In contrast, plasma triglycerides were markedly increased in another set of two patients with Menkes disease, whereas plasma total cholesterols were normal or increased [43]. The exact reason for these opposite results is unknown. The variability of plasma copper levels was dramatic between patients (approximately between 10% and 60% of normal levels), which likely partly contributes to these opposite results. In addition, to make the event more complicated, lipid profile was measured in the patients commonly after extensive copper treatment; thus, it is unclear if abnormal lipid metabolism is due to the disease itself or a combined effect of copper treatment. To overcome the limitation, animal models should be employed. Our current study indicates that blotchy marrow counteracts the higher levels of total cholesterol and phospholipids induced by high-fat feeding (Fig. 1). Overall, these findings support a role of blotchy marrow in regulation of plasma lipid levels and the insight mechanisms deserve a further investigation.
Blotchy marrow and inflammatory mediator production
We recently employed a high-throughput multiplexing immunoassay to investigate the inflammatory mediator production in various murine models. In the thioglycollate-induced acute peritonitis, we detected higher-than-5-fold increases of most of inflammatory mediators in blood and peritoneal exudates [32], indicating a high-grade inflammation. Interestingly, the peak levels of all inflammatory mediator productions were not associated with the peak accumulation of macrophages [32]. Although it is not clear in which extent the finding from acute inflammation reflects the pathogenesis of chronic inflammation, such as obesity and atherosclerosis, inflammatory mediator production provides a better indicator of the degree of inflammation over the usage of macrophage number. By using the same immunoassay, our current study showed that high-fat diets selectively induced the production of a subset of inflammatory mediators with lesser-than-3-fold increases, indicating a low-grade inflammation. A similar pattern was also revealed in angiotensin II-infused mice (data not shown). Thus, the advantage of multiplex assay is two folds: global, examination of a large number of inflammatory mediators simultaneously; and selective, revealing the differential response of inflammatory mediator production.
During angiotensin II infusion in vivo, we found that blotchy marrow mediates moderate hypo-response on inflammation: productions of a subset of inflammatory mediators were decreased (less than 50% in all mediators) [29]. In contrast, during high-fat-diet feeding in our current study, blotchy marrow mediates potent hyper-response on inflammation: productions of a subset of inflammatory mediators were increased (higher than 50% in three mediators). Although both angiotensin II and high-fat diets evoke low-grade pro-inflammatory responses in vascular walls, they are mediated via different mechanisms, such as thrombi [44,45] and saturated fatty acid [46,47], respectively. Thus, blotchy marrow may regulate divergent effects of thrombi vs. saturated fatty acid mediated inflammation.
Overall, by using hypercholesterolemia sensitive mice, our study revealed that plasma copper levels can be robustly upregulated by high-fat diet. Moreover, the upregulation of plasma copper can be reversed by ATP7A mutations in myloid cells, in conjunction with decreased lipid metabolism and increased inflammation. These findings offer a glimpse into the delicate regulation of copper metabolism and its complex interaction with iron and lipid metabolisms and inflammation.
Acknowledgments
This work was partly supported by an AHA National Scientist Development Grant (0835268N). The lipid profile service from the Mouse Metabolic Phenotyping Center of the University of Cincinnati was supported by U24 DK059630.
Abbreviations
- BMT
bone marrow transplantation
- cGy
centiGray
- FPLC
fast performance liquid chromatography
- GFP
green fluorescent protein
- HDL
high density lipoprotein
- IP
interferon-γ-inducible protein
- LDL
low density lipoprotein
- LDLR
low-density lipoprotein receptor
- MCP
monocyte chemotactic protein
- PCR
polymerase chain reaction
- TIMP
tissue inhibitor of metalloproteinases
- VEGF
vascular endothelial growth factor
- VLDL
very low density lipoprotein
- WT
wild type
Footnotes
Conflict of interest
There was no conflict of interest in the manuscript.
References
- 1.White C, Lee J, Kambe T, Fritsche K, Petris M. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–56. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins JF, Hua P, Lu Y, Ranganathan PN. Alternative splicing of the Menkes copper ATPase (ATP7A) transcript in the rat intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;297(4):G695–707. doi: 10.1152/ajpgi.00203.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, et al. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res. 2010;107(6):787–99. doi: 10.1161/CIRCRESAHA.110.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercer JF, Grimes A, Ambrosini L, Lockhart P, Paynter JA, Dierick H, et al. Mutations in the murine homologue of the Menkes gene in dappled and blotchy mice. Nat Genet. 1994;6(4):374–8. doi: 10.1038/ng0494-374. [DOI] [PubMed] [Google Scholar]
- 5.Goka TJ, Stevenson RE, Hefferan PM, Howell RR. Menkes disease: a biochemical abnormality in cultured human fibroblasts. Proc Natl Acad Sci U S A. 1976;73(2):604–6. doi: 10.1073/pnas.73.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riordan JR, Jolicoeur-Paquet L. Metallothionein accumulation may account for intracellular copper retention in Menkes’ disease. J Biol Chem. 1982;257(8):4639–45. [PubMed] [Google Scholar]
- 7.Berg GJ, Kroon JJ, Wijburg FA, Sinjorgo KMC, Herzberg NH, Bolhuis PA. Muscle cell cultures in Menkes’ disease: copper accumulation in myotubes. J Inherit Metab Dis. 1990;13(2):207–11. doi: 10.1007/BF01799687. [DOI] [PubMed] [Google Scholar]
- 8.Afton SE, Caruso JA, Britigan BE, Qin Z. Copper egress is induced by PMA in human THP-1 monocytic cell line. Biometals. 2009;22(3):531–9. doi: 10.1007/s10534-009-9210-y. [DOI] [PubMed] [Google Scholar]
- 9.Kim HW, Chan Q, Afton SE, Caruso JA, Lai B, Weintraub NL, et al. Human macrophage ATP7A is localized in the trans-Golgi apparatus: controls intracellular copper levels, and mediates macrophage responses to dermal wounds. Inflammation. 2012;35(1):167–75. doi: 10.1007/s10753-011-9302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly EJ, Palmiter RD. A murine model of Menkes disease reveals a physiological function of metallothionein. Nat Genet. 1996;13(2):219–22. doi: 10.1038/ng0696-219. [DOI] [PubMed] [Google Scholar]
- 11.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87(3):1011–46. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 12.Phillips M, Camakaris J, Danks DM. A comparison of phenotype and copper distribution in blotchy and brindled mutant mice and in nutritionally copper deficient controls. Biol Trace Elem Res. 1991;29(1):11–29. doi: 10.1007/BF03032670. [DOI] [PubMed] [Google Scholar]
- 13.Hunt DM. Catecholamine biosynthesis and the activity of a number of copper-dependent enzymes in the copper deficient mottled mouse mutants. Comp Biochem Physiol C. 1977;57(1):79–83. doi: 10.1016/0306-4492(77)90082-x. [DOI] [PubMed] [Google Scholar]
- 14.Hunt DM, Johnson DR. An inherited deficiency in noradrenaline biosynthesis in the brindled mouse. J Neurochem. 1972;19(12):2811–9. doi: 10.1111/j.1471-4159.1972.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 15.Rezek DL, Moore CL. Depletion of brain mitochondria cytochrome oxidase in the mottled mouse mutant. Exp Neurol. 1986;91(3):640–5. doi: 10.1016/0014-4886(86)90060-9. [DOI] [PubMed] [Google Scholar]
- 16.Rowe DW, McGoodwin EB, Martin GR, Grahn D. Decreased lysyl oxidase activity in the aneurysm-prone: mottled mouse. J Biol Chem. 1977;252(3):939–42. [PubMed] [Google Scholar]
- 17.Starcher BC, Madaras JA, Tepper AS. Lysyl oxidase deficiency in lung and fibroblasts from mice with hereditary emphysema. Biochem Biophy Res Commun. 1977;78(2):706–12. doi: 10.1016/0006-291x(77)90236-4. [DOI] [PubMed] [Google Scholar]
- 18.Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEBJ. 2006;20(2):334–6. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 19.Qin Z, Gongora MC, Ozumi K, Itoh S, Akram K, Ushio-Fukai M, et al. Role of Menkes ATPase in angiotensin II-induced hypertension: a key modulator for extracellular superoxide dismutase function. Hypertension. 2008;52(5):945–51. doi: 10.1161/HYPERTENSIONAHA.108.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudhahar V, Urao N, Oshikawa J, McKinney RD, Llanos RM, Mercer JFB, et al. Copper transporter ATP7A protects against endothelial dysfunction in type 1 diabetic mice by regulating extracellular superoxide dismutase. Diabetes. 2013;62(11):3839–50. doi: 10.2337/db12-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry AN, Otoikhian A, Bhatt S, Shinde U, Tsivkovskii R, Blackburn NJ, et al. The lumenal loop Met672–Pro707 of copper-transporting ATPase ATP7A binds metals and facilitates copper release from the intramembrane sites. J Biol Chem. 2011;286(30):26585–94. doi: 10.1074/jbc.M111.229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Z, Lai B, Landero J, Caruso JA. Coupling transmission electron microscopy with synchrotron radiation X-ray fluorescence microscopy to image vascular copper. J Synchrotron Radiat. 2012;19(Pt 6):1043–9. doi: 10.1107/S090904951203405X. [DOI] [PubMed] [Google Scholar]
- 23.Qin Z, Toursarkissian B, Lai B. Synchrotron radiation X-ray fluorescence microscopy reveals a spatial association of copper on elastic laminae in rat aortic media. Metallomics. 2011;3(8):823–8. doi: 10.1039/c1mt00033k. [DOI] [PubMed] [Google Scholar]
- 24.Strausak D, Fontaine SL, Hill J, Firth SD, Lockhart PJ, Mercer JFB. The role of GMX-CXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J Biol Chem. 1999;274(16):11170–7. doi: 10.1074/jbc.274.16.11170. [DOI] [PubMed] [Google Scholar]
- 25.Voskoboinik I, Mar J, Strausak D, Camakaris J. The regulation of catalytic activity of the Menkes copper-translocating P-type ATPase: role of high affinity copper-binding sites. J Biol Chem. 2001;276(30):28620–7. doi: 10.1074/jbc.M103532200. [DOI] [PubMed] [Google Scholar]
- 26.González-Guerrero M, Eren E, Rawat S, Stemmler TL, Argüello JM. Structure of the two transmembrane Cu+ transport sites of the Cu+-ATPases. J Biol Chem. 2008;283(44):29753–9. doi: 10.1074/jbc.M803248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet. 1995;56(3):570–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews EJ, White WJ, Bullock LP. Spontaneous aortic aneurysms in blotchy mice. Am J Pathol. 1975;78(2):199–210. [PMC free article] [PubMed] [Google Scholar]
- 29.Harris D, Liang Y, Chen C, Li S, Patel O, Qin Z. Bone marrow from blotchy mice is dispensable to regulate blood copper and aortic pathologies but required for inflammatory mediator production in LDLR-deficient mice during chronic angiotensin II infusion. Ann Vasc Surg. 2015;29(2):328–40. doi: 10.1016/j.avsg.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollie NI, Konaniah ES, Goodin C, Hui DY. Group 1B phospholipase A2 inactivation suppresses atherosclerosis and metabolicdiseases in LDL receptor-deficient mice. Atherosclerosis. 2014;234(2):377–80. doi: 10.1016/j.atherosclerosis.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdes LU, Gerdes C, Klausen IC, Faergeman O. Generation of analytic plasma lipoprotein profiles using two prepacked superose 6B columns. Clin Chim Acta. 1992;205(1–2):1–9. doi: 10.1016/0009-8981(92)90348-t. [DOI] [PubMed] [Google Scholar]
- 32.Lam D, Harris D, Qin Z. Inflammatory mediator profiling reveals immune properties of chemotactic gradients and macrophage mediator production inhibition during thioglycollate elicited peritoneal inflammation. Mediators Inflamm. 2013;2013:931562. doi: 10.1155/2013/931562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel OV, Wilson WB, Qin Z. Production of LPS-induced inflammatory mediators in murine peritoneal macrophages: neocuproine as a broad inhibitorand ATP7A as a selective regulator. Biometals. 2013;26(3):415–25. doi: 10.1007/s10534-013-9624-4. [DOI] [PubMed] [Google Scholar]
- 34.Che H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, et al. Decreased hep-haestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. 2006;136(5):1236–41. doi: 10.1093/jn/136.5.1236. [DOI] [PubMed] [Google Scholar]
- 35.Klevay LM. Is the Western diet adequate in copper? J Trace Elem Med Biol. 2011;25(4):204–12. doi: 10.1016/j.jtemb.2011.08.146. [DOI] [PubMed] [Google Scholar]
- 36.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127(5):838S–41S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 37.Gulec S, Collins JF. Silencing of the Menkes copper-transporting ATPase (Atp7a) gene increases cyclin D1 protein expression and impairs proliferation of rat intestinal epithelial (IEC-6) cells. J Trace Elem Med Biol. 2014;28:459–64. doi: 10.1016/j.jtemb.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sourkes TL, Lloyd K, Birnbaum H. Inverse relationship of hepatic copper and iron concentrations in rats fed deficient diets. Can J Biochem. 1968;46(3):267–71. doi: 10.1139/o68-038. [DOI] [PubMed] [Google Scholar]
- 39.Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum: and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem. 2005;280(43):36221–7. doi: 10.1074/jbc.M506727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang L, Ranganathan P, Lu Y, Kim C, Collins JF. Exploration of the copper-related compensatory response in the Belgrade rat model of genetic iron deficiency. Am J Physiol Gastrointest Liver Physiol. 2011;301:G877–86. doi: 10.1152/ajpgi.00261.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulec S, Collins JF. Molecular mediators governing iron–copper interactions. Ann Rev Nutr. 2014;34(1):95–116. doi: 10.1146/annurev-nutr-071812-161215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara A, Taketomi T. Cerebral lipid and protein abnormalities in Menkes’ steely-hairdisease. Jpn J Exp Med. 1986;56(6):277–84. [PubMed] [Google Scholar]
- 43.Blackett PR, Lee DM, Donaldson DL, Fesmire JD, Chan WY, Holcombe JH, et al. Studies of lipids: lipoproteins, and apolipoproteins in Menkes’ disease. Pediatr Res. 1984;18(9):864–70. doi: 10.1203/00006450-198409000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aorticdissection precedes formation of aneurysms and atherosclerosis inangiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(9):1621–6. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 45.Sagan A, Mrowiecki W, Mikolajczyk TP, Urbanski K, Siedlinski M, Nosalski R, et al. Local inflammation is associated with aortic thrombus formation in abdominal aortic aneurysms. Relationship to clinical risk factors Thromb Haemost. 2012;108(11):812–23. doi: 10.1160/TH12-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pejnovic NN, Pantic JM, Jovanovic IP, Radosavljevic GD, Milovanovic MZ, Nikolic IG, et al. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62(6):1932–44. doi: 10.2337/db12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15(4):518–33. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]


