Abstract
Purpose
To compare the performance of 4 metrics of metabolic response on FDG-PET/CT against RECIST 1.0 for determining response and predicting overall survival (OS) following 90Y resin microspheres radioembolization of colorectal liver metastases (CLM).
Methods
We conducted an IRB-waived retrospective review of our radioembolization database to identify patients with unresectable CLM treated between December 2009 and December 2013. We included patients who had both PET/CT and contrast enhanced CT (CECT) available at baseline and on the first follow-up post-radioembolization. On baseline CECT up to five target tumors were chosen per patient according to RECIST 1.0. Four metrics of FDG-avidity (SUVmax, SUVpeak, metabolic tumor volume (MTV), and total lesion glycolysis (TLG)) on PET/CT were measured for the same target tumors. Using RECIST 1.0, patients were classified as no progression (partial response or stable disease) and progression. For each PET metric, a cut-off point of ≥30% decrease was chosen to define response. OS was calculated from the time of radioembolization using Kaplan-Meier methodology. The log-rank test was used for univariate analysis to identify predictors of OS.
Results
The study enrolled 49 patients with 119 target tumors; a median of 2 (range: 1–5) tumors were selected per patient. Median OS was 12.7 months (95%CI: 7.2–16.7). Response by MTV (P=0.035) and TLG (P=0.044) reached statistical significance in predicting OS. Response by SUVmax (P=0.21), SUVpeak (P=0.20) or no progression by RECIST1.0 (P=0.44) did not predict OS.
Conclusion
Metabolic response based on changes in MTV and TLG can predict OS post-radioembolization of CLM.
Keywords: Radioembolization, PET/CT, RECIST 1.0, colorectal liver metastases, overall survival
Introduction
Annually about 143,000 patients are diagnosed with colorectal cancer [1]. It is estimated that about half of colorectal cancer patients will develop liver metastases during the course of their disease [2, 3]. Surgical resection is considered the gold standard therapy providing 5-year survival rates of 28% – 58% [4–6]. However, only about 15% of patients have resectable disease [2]. Additionally, about 36% of those who undergo resection will experience liver recurrences [7]. Depending on the extent of liver involvement, several options are available for patients with unresectable disease. Patients may be candidates for local ablation therapy if their liver disease is limited to 1–3 tumors, ideally <3 cm, and with no or limited EHD [8–11]. Patients with colorectal liver metastases (CLM) that are not candidates for surgery or ablation may benefit from the addition of Yttrium-90 (90Y) radioembolization to systemic chemotherapy [12, 13]. A phase II [12] and a Phase III [13] randomized clinical trials have shown that the addition of radioembolization to fluorouracil based systemic chemotherapy significantly prolonged time to liver progression, time to disease progression and overall survival (OS) in unresectable patients. The toxicity profile and safety of radioembolization in unresectable CLM patients was found to be acceptable by several studies [14–17], even in the heavily pre-treated population [18, 19].Retrospective studies with the largest sample sizes to date (n=214–606) report a median survival of 7.2–10.6 months after radioembolization of CLM; mostly in the salvage setting [14–16].
Early recognition of radioembolization non-responders may help identify those patients that could benefit from extended systemic chemotherapy or a change in regimen. Several studies have concluded that response criteria based on tumor morphology – Response Evaluation Criteria In Solid Tumors (RECIST)—has a poor sensitivity in detecting response to radioembolization in CLM patients [20–24]. In addition, these studies showed that changes in tumor metabolic activity had a higher sensitivity [20–23] and could predict liver progression free survival or OS. [22, 23]. To quantify metabolic response on FDG-PET imaging, several SUV based semi-quantitative metrics are available. The pixel with the highest uptake or the SUVmax is the measure of metabolic activity most widely used in clinical practice. The EORTC proposed the use of the percentage change in SUVmax to categorize patients’ response to cancer therapy [25]. Later in 2009, Wahl et al described a new FDG-PET based response criteria to quantify the changes in metabolic activity. These criteria, named “PERCIST” (PET response criteria in solid tumors); advocate the use of a PET SUV metric termed the “SUVpeak”. The SUVpeak is defined as the meanSUV in an 1 cm3 sphere around the pixel with the highest uptake [26]. Two other metrics of metabolic activity that are available and can be used to evaluate and categorize tumor response, include the metabolic tumor volume (MTV) and the total lesion glycolysis (TLG); which is the product of MTV and meanSUV [26].
The aim of this study is to compare the performance of 4 metrics of metabolic response on FDG-PET/CT (SUVmax, SUVpeak, MTV, and TLG) and RECIST 1.0 for determining response and predicting overall survival (OS) following 90Y radioembolization of colorectal liver metastases (CLM).
Methods
Study population
We performed a retrospective review of our prospectively created HIPAA compliant clinical radioembolization database. We identified patients treated for unresectable CLM from December 2009 to December 2013 (n=59). We used Y90 loaded resin microspheres (SIR-Spheres; Sirtex Medical). For this study, we included only patients who had both contrast enhanced CT (CECT) and PET/CT studies available both at baseline and on the first follow-up post-radioembolization to allow for early assessment of response (n=52). We excluded three patients: one patient with tumors that were iso-metabolic to the liver background, one patient who had his first follow-up PET/CT scan done at an outside facility, and one patient who had his first follow-up scan more than 12 weeks post-radioembolization. The final study population consisted of 49 patients. The baseline characteristics of the enrolled patients are displayed in table1. These patients were treated with radioembolization for progressing unresectable CLM in the salvage setting. Patients were followed-up with serial imaging every 2–4 months. The study concluded at the end of October 2015.
Table 1.
Baseline patient characteristics.
| Character | No./Median | Percentage/Range |
|---|---|---|
|
| ||
| Age | 57 | (24–86) |
|
| ||
| Sex | ||
| Male | 28 | (57%) |
| Female | 21 | (43%) |
|
| ||
| Prior liver resection | ||
| Yes | 22 | (45%) |
| No | 27 | (55%) |
|
| ||
| Prior HAIC | ||
| Yes | 26 | (53%) |
| No | 23 | (47%) |
|
| ||
| Prior lines of systemic chemotherapy | ||
| 2 | 35 | (71%) |
| ≥3 | 14 | (29%) |
|
| ||
| Prior chemotherapeutic agents received | ||
| Capecitabine | 17 | (35%) |
| 5 FU/LV | 45 | (91%) |
| Oxaliplatin | 47 | (96%) |
| Irinotecan | 45 | (92%) |
|
| ||
| Prior biological agents received | ||
| Bevacizumab | 38 | (78%) |
| Cetuximab | 22 | (45%) |
| Panitumumab | 5 | 10%) |
|
| ||
| Prior treatments for liver metastasis | ||
| Resection + HAIC + systemic chemotherapy | 17 | (35%) |
| Resection + systemic chemotherapy | 5 | (10%) |
| HAIC + systemic chemotherapy | 9 | (18%) |
| Systemic chemotherapy | 18 | (37%) |
|
| ||
| Extrahepatic disease | ||
| No | 9 | (18%) |
| Lung only | 13 | (27%) |
| Lymph nodes only | 5 | (10%) |
| Lung + lymph nodes | 11 | (23%) |
| Other 2 sites | 4 | (8%) |
| Multiple sites | 7 | (14%) |
|
| ||
| Extent of liver replacement by tumor | ||
| <25% | 44 | (90%) |
| ≥25% | 5 | (10%) |
|
| ||
| Baseline CEA (ng/ml) | 82 | (2.7-23938) |
|
| ||
| Number of target lesions/patient | ||
| 1 | 17 | (33%) |
| 2 | 10 | (22%) |
| 3 | 9 | (18%) |
| 4 | 10 | (21%) |
| 5 | 3 | (6%) |
|
| ||
| Treatment | ||
| 1 session | 36 | (73%) |
| 2 sessions | 13 | (27%) |
HAIC: Hepatic Arterial Infusion Chemotherapy
CT imaging and morphologic response evaluation
All patients were scanned with a 16- and 64-slice multidetector CT scanner (VCT, Lightspeed, GE Healthcare). The following CT parameters were used: pitch/table speed = 0.984-1.375/39.37-27.50 mm; autoMA 220–380; noise index 12.5–14; rotation time 0.7-0.8 ms; and scan delay 80–85 s. Patients received intravenous (IV) administration of 150 mL of iodinated contrast material (Omnipaque 300, GE Healthcare, New Jersey). Axial slices reconstructed at 5 mm interval were used for the analysis
Target tumors in the liver were chosen on the portal venous phase of the baseline CECT study according to RECIST 1.0, and up to five target tumors were chosen per patient. The sum of the largest diameters was calculated at baseline and on the first follow-up imaging. The percentage change was calculated and patients were assigned a response category according to RECIST 1.0 (table2). Because there was only one patient with partial response (PR), we could not dichotomize these patients into responders and non-responders. Instead, we classified patients into either no progression (PR or stable disease (SD)) or progression of disease (PD) [23].
PET imaging and metrics of metabolic response
All patients were scanned on a dedicated PET/CT scanner (GE systems). Scanning was performed approximately 60 minutes following the intravenous injection of 10 mCi ± 10% of 18 fluorodeoxyglucose (FDG). A low dose CT scan (80 mA) was performed from mid skull to mid thigh followed by the emission scan (3 min/field of view). Images were reconstructed using iterative reconstruction and attenuation correction.
All images were reviewed on a GE PET VCAR station. A 3D volume of interest was generated based on the tumor morphology on the CECT (S.K. or S. G.). The MTV (cm3) was defined as the volume of FDG-avid disease with voxels that contain activity equal to or greater than 50% of the maximum tumor activity. Overlapping tumors were segmented manually to mirror the anatomic distribution on CECT. SUV measurements were corrected to the patient’s body weight. The four metrics (SUVmax (g/cm3), SUVpeak (g/cm3), MTV (cm3), and TLG (g)) were generated for each tumor. A mean value per patient was calculated for each of the 4 metrics on the baseline and follow-up scans. The percentage change of each PET metric was calculated, and the patient was assigned a response category based on the cut-off points proposed by PERCIST (table2). We classified patients into 2 groups: responders (PR) and non-responders (SD or PD).
Table 2.
Response criteria
| Category | PET response criteria | RECIST 1.1 |
|---|---|---|
| PR | ≥30% decrease in PET metric | ≥30% decrease in sum of LD. |
| SD | Non PR or PD | Non PR or PD |
| PD | >30% increase in PET metric and/or New tumor(s). | >20% increase in sum of LD and/or New tumor(s). |
PR: Partial Response
SD: Stable Disease
PD: Progression of Disease
Statistical analysis
Overall survival (OS) was calculated from the time of radioembolization until the time of death or latest follow-up using the Kaplan-Meier methodology. Log-rank tests were used to evaluate for statistical significance of response in predicting OS. A Cox-regression model was used to report the hazard ratios. Kappa statistic was used to evaluate the diagnostic agreement between the four PET metrics in classifying the response of target tumors into PR, SD, and PD [27]. Statistical significance was defined as a P-value of <0.05. Analysis was performed using STATA version 12 (StataCorp LP, College Station, Texas).
Results
The study enrolled 49 patients with 119 target tumors; a median of 2 (range: 1–5) tumors per patient. Twenty-two patients (45%) were treated for recurrences post-hepatectomy. Baseline characteristics are summarized in table 1. Baseline imaging prior to radioembolization was obtained at a median of 4.1 weeks with an inter-quartile range of 3.0–5.6 weeks and a range of (0.4–14.6) weeks. Post-radioembolization imaging was obtained at a median of 6 weeks with an inter-quartile range of 3.4–8.9 and a range of (3–11.1) weeks.
The median follow-up period was 47.1 months and 7 patients (14%) were alive at the end of the study. Median OS was 12.7 months (95%CI: 7.2–16.7), with one-, two-, and three-year OS rates of 52%, 23% and 15% respectively. On the first follow-up scan, 16 patients (33%) showed evidence of new liver tumors either in the treated liver lobe (n=11) or in the untreated liver lobe (n=5).
The highest diagnostic agreement in classifying target tumor response was found between SUVmax and SUVpeak (K=0.77) (table 3). The second highest agreement was between MTV and TLG (K=0.62) (table 3). There was only fair agreement between TLG or MTV and SUVmax or SUVpeak (K=0.22-0.42) (table 3). The Kappa statistic for diagnostic agreement across the metrics increased when patients were classified as either response or no response: 0.84 (SUVmax versus SUVpeak), 0.57 (SUVmax versus MTV), 0.64 (SUVmax versus TLG), 0.40 (SUVpeak versus MTV), 0.63 (SUVpeak versus TLG), and 0.76 (MTV versus TLG).
Table 3.
The Diagnostic agreement across the 4 PET metrics in classifying the response of target lesions only (not taking into account new lesions).
| SUVmax | % agreement | Kappa | P-value | ||||
|---|---|---|---|---|---|---|---|
| SUVpeak | PR | SD | PD | Total SUVpeak |
86% | 0.77 | <0.001 |
| PR | 22 | 3 | 0 | 25 | |||
| SD | 0 | 12 | 2 | 14 | |||
| PD | 0 | 2 | 8 | 10 | |||
| Total SUVmax | 22 | 17 | 10 | ||||
| MTV | Total MTV | ||||||
| PR | 17 | 2 | 4 | 23 | 55% | 0.30 | 0.002 |
| SD | 1 | 9 | 5 | 15 | |||
| PD | 4 | 6 | 1 | 11 | |||
| TLG | Total TLG | ||||||
| PR | 18 | 3 | 1 | 22 | 59% | 0.36 | <0.001 |
| SD | 3 | 8 | 6 | 17 | |||
| PD | 1 | 6 | 3 | 10 | |||
| SUVpeak | % agreement | Kappa | P-value | ||||
| MTV | PR | SD | PD | 51% | 0.22 | 0.02 | |
| PR | 17 | 2 | 4 | ||||
| SD | 3 | 7 | 5 | ||||
| PD | 5 | 5 | 1 | ||||
| TLG | |||||||
| PR | 19 | 2 | 1 | 63% | 0.42 | <0.001 | |
| SD | 4 | 8 | 5 | ||||
| PD | 2 | 4 | 4 | ||||
| MTV | % agreement | Kappa | P-value | ||||
| TLG | PR | SD | PD | 75% | 0.62 | <0.001 | |
| PR | 20 | 2 | 0 | ||||
| SD | 3 | 10 | 4 | ||||
| PD | 0 | 3 | 7 | ||||
PR: Partial Response
SD: Stable Disease
PD: Progression of Disease
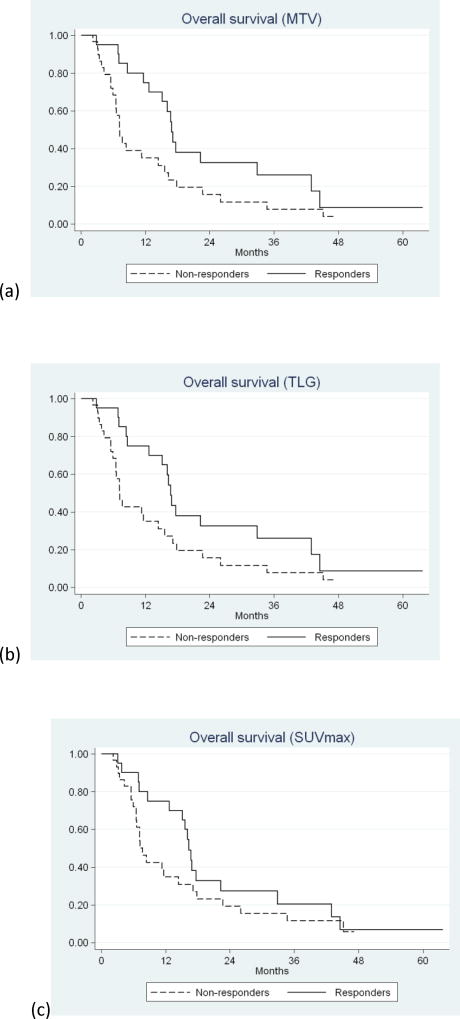
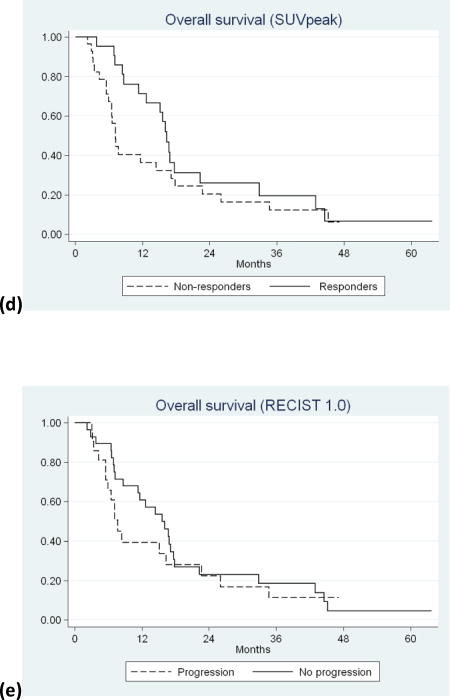
On univariate analysis, only response by MTV (P=0.035) (HR=0.72; 95%CI: 0.52-0.98) and TLG (P=0.044) (HR=0.73; 95%CI: 0.53-1.0) reached statistical significance in predicting OS (table 4) (Figure 1a and b). Response by SUVmax (P=0.21) (HR=0.82; 95%CI: 0.60-1.1) and SUVpeak (P=0.20)) (HR=0.82; 95%CI: 0.60-1.1) did not reach statistical significance (Table 4) (Figure 1c and d). No progression (PR or SD) according to RECIST1.0 did not reach statistical significance in predicting OS (P=0.44) (HR=0.78; 95%CI: 0.42-1.5) (table3) (Figure 1e).
Table 4.
Survival of responders and non-responders as predicted by RECIST1.1 and the PET metrics
| Metric of response | No. | Median survival (months) |
1 year survival % |
2 year survival % |
3 year survival % |
P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| MTV (cm3) | ||||||
| Response | 20 | 16.9 | 75 | 33 | 26 | 0.035 |
| No response | 29 | 7.2 | 35 | 16 | 8 | |
|
| ||||||
| TLG (g) | ||||||
| Response | 20 | 16.7 | 75 | 33 | 26 | 0.044 |
| No response | 29 | 7.2 | 35 | 16 | 8 | |
|
| ||||||
| SUVmax (g/cm3) | ||||||
| Response | 20 | 16.3 | 75 | 27 | 20 | 0.21 |
| No response | 29 | 7.7 | 35 | 19 | 11 | |
|
| ||||||
| SUVpeak (g/cm3) | ||||||
| Response | 21 | 16.3 | 71 | 26 | 19 | 0.20 |
| No response | 28 | 7.2 | 36 | 20 | 12 | |
|
| ||||||
| REICST 1.0 | ||||||
| No progression | 28 | 15.6 | 61 | 23 | 18 | 0.44 |
| Progression | 21 | 7.7 | 39 | 22 | 11 | |
Figure 1.
Kaplan-Meier survival curves of responders versus non-responders classified according to: (a) MTV (P=0.035), (b) TLG (P=0.044), (c) SUVmax (P=0.21), (d) SUVpeak (P=0.20), and (e) RECIST 1.0 (P=0.44).
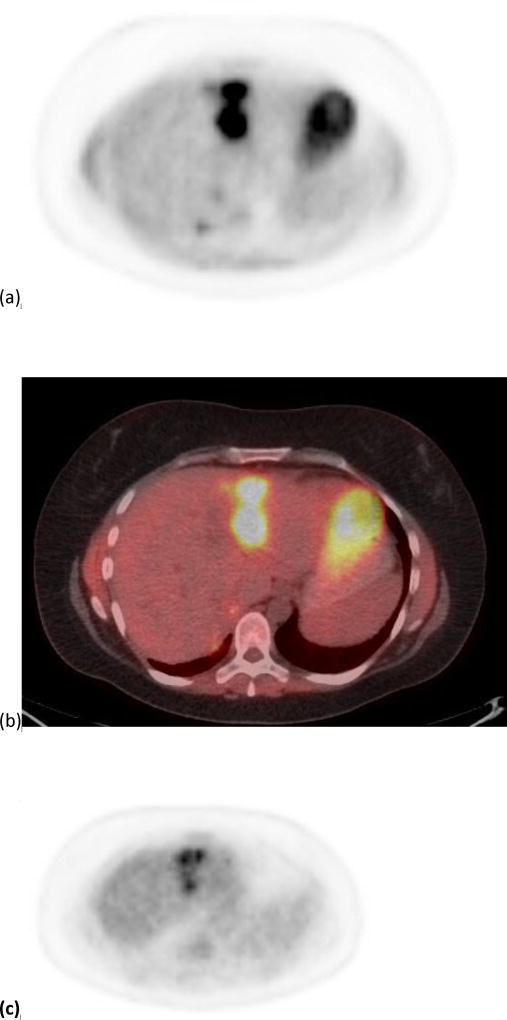
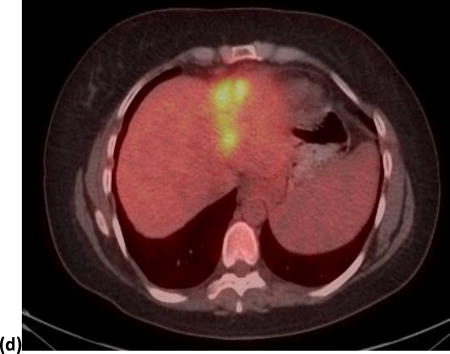
Using both MTV and/or TLG, 22 patients showed PR; out of which four patients were classified as non-responders by SUVmax and/or SUVpeak. The median survival of these four patients was 11.6 months; range 2.9–40.3 months. On the other hand, using both SUVmax and/or SUVpeak, 22 patients were classified as PR; out of which four patients were classified as non-responders by MTV and/or TLG. The median survival of these four patients was only 3.8 months; range 3.1–15.6 months. Figure 2 shows an example of a tumor responding by SUVmax but not by MTV, and figure 3 shows an example of a tumor responding by MTV but not by SUVmax.
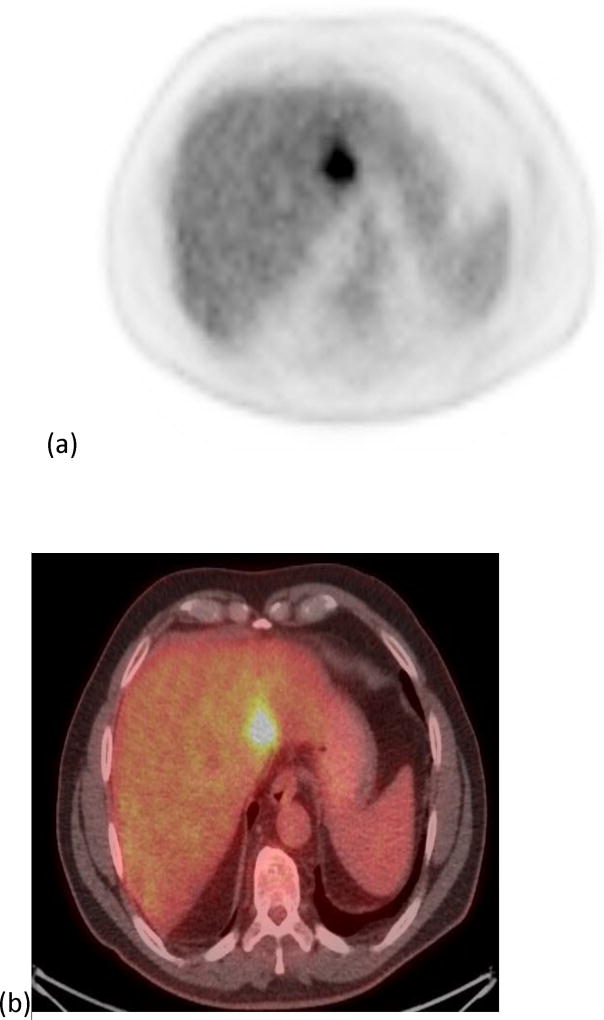
Figure 2.
A 40 year old female patient with one of the target tumors in segment 2. Images. (a) and (b) show the PET and PET/CT fused imaged prior to radioembolization with a SUVmax of 11.02 g/cm3 and a MTV of 33.25 cm3. Images (c) and (d) show the PET and PET/CT fused images post-radioembolization with a decrease in SUVmax to 6.8 g/cm3 (38%) and an increase in MTV to 55.64 cm3 (67%).
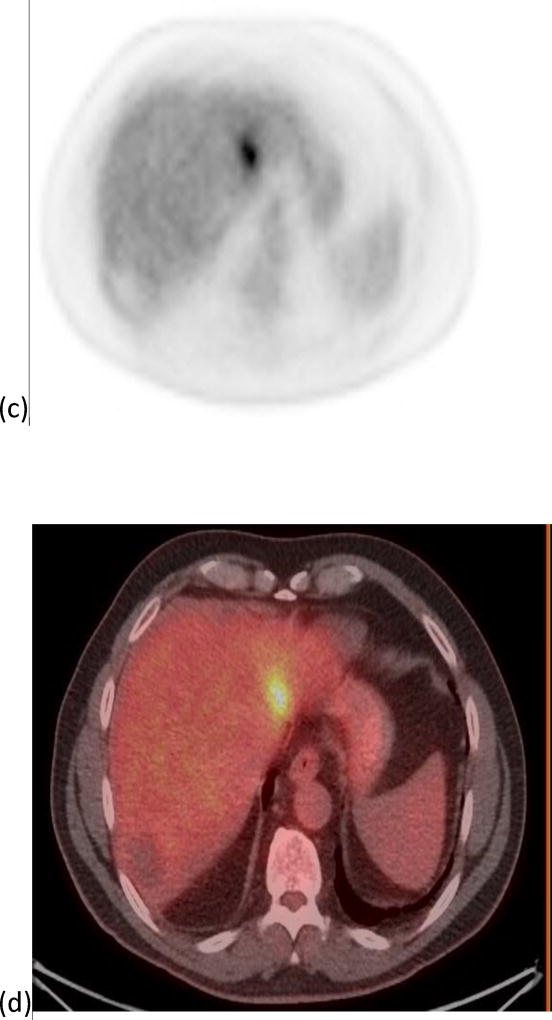
Figure 3.
A 64 year old male with one segment 2 tumor. Images (a) and (b) show the PET and PET/CT fused imaged prior to radioembolization with a SUVmax of 6.36 g/cm3 and a MTV of 18.29 cm3. Images (c) and (d) show the PET and PET/CT fused images post-radioembolization with an increase in SUVmax to 7.0 g/cm3 (10%) and a decrease in MTV to 12.62 cm3 (31%).
Discussion
Our results show that early metabolic response by MTV or TLG can significantly predict OS post-radioembolization of CLM. In addition, RECIST 1.0 showed poor performance in detecting response and predicting OS at an early time point. Several studies for CLM patients treated with radioembolization have shown that PET/CT had a higher sensitivity than CT in detecting response [22, 28–31]. Lewandowski et al and Kennedy et al described this in earlier reports [31, 32]. Lewandowski et al found that FDG-PET had a higher sensitivity than RECIST in detecting response post-radioembolization for both the initial and subsequently treated lobes (73–88% versus 35–36%) [31]. In a multi-institutional study of 208 patients, Kennedy et al reported a higher metabolic response rate on FDG-PET than by anatomic criteria on CT (85% versus 36%) [32]. More recently, Tochetto et al published two sequential studies first evaluating response on a tumor basis then on a patient basis [29, 30]. In the first study, FDG-PET response by SUVmax was detected in 38/74 tumors, of those RECIST could only detect 8/38 (21%) responders [29]. In the second study, a patient-based analysis revealed response by SUVmax in 15/20 patients at 3 months post-radioembolization; of those, RECIST detected response only in 2/15 (13%) patients on the CT performed at 1 month and in 6/15 (40%) patients on the CT performed at 3 months post-radioembolization [30]. Similarly, in a study by Zerizer et al, 15/25 (60%) patients had PR by SUVmax on FDG-PET/CT while only 2/25 (8%) had a PR by RECIST [22]. Finally, Sofocleous et al reported only a 2% response rate by RECIST versus 45% by PET on the 1st 4–8 weeks scans, and a 7% response rate by RECIST versus a 47% response rate by PET on the 2nd 12–16 weeks scans post-radioembolization [19]. These studies pointed out the relative tendency of RECIST to underestimate response to radioembolization in patients with CLM at an early time point, and highlighted the importance of metabolic assessment of tumor response in this setting
There are several semi-quantitative measurements of metabolic response on FDG-PET/CT. In 1999, the EORTC (European Organization for Research and Treatment of Cancer) published a study reviewing the data available at the time on the use of FDG-PET in Oncology [25]. The group recommended the use of the SUVmax as the measure of tumor metabolic activity and a cut-off point of 25% decrease to define response to oncologic treatments. The SUVmax has the advantages of ease of measurement and least affection by partial volume effects. Since then, the SUVmax has become the most commonly reported PET metric in metabolic response evaluation studies [26]. More recently, in 2009, Wahl et al published a study describing the PET Response Criteria in Solid Tumors “PERCIST”. In this extensive review, the authors advocated for the use of the SUVpeak, which is the meanSUV in a sphere of a 1.2 cm diameter drawn around the maximal pixel, to measure the tumor metabolic activity. The SUVpeak, being a small volume based measurement, has the advantage of less statistical variance when compared to the single pixel SUVmax. They also recommended the use of a higher cut-off point of 30% for response. Other measurements of tumor metabolic activity available include the metabolic tumor volume (MTV) and the total lesion glycolysis (TLG); calculated by multiplying the MTV by the meanSUV in that volume. These volume-based metrics have the advantage of assessing data from the entire tumor, while the SUVmax or SUVpeak assess only the most active part of the tumor. The question posed by this study, is which of these four metrics would have a predictive value for OS post-radioembolization of CLM, and thus would be the most useful surrogate image biomarker in this setting.
A prior study by Fendler et al also sought to answer this question. The study enrolled 80 patients and compared the four PET metrics (MTV, TLG, SUVmax, and SUVpeak) and RECIST1.1 as predictors of OS post-radioembolization of CLM. In their study, the MTV (P=0.006) (HR: 0.51; 95% CI: 0.31-0.84) and TLG (P=0.025) (HR: 0.59; 95% CI: 0.36-0.94) were the only significant predictors of OS [23]. These were followed by SUVmax (P=0.16) which could separate the median OS of responders from non-responders (91 weeks versus 57 weeks) better than the SUVpeak (P=0.314) (67 versus 51 weeks). RECIST 1.1 apparently had a low sensitivity in detecting PR, similar to our own study, as the authors resorted to classifying patients into any response (partial response and stable disease) and progression. Any response by RECIST1.1 failed to reach statistical significance (P=0.086) in predicting OS in their study [23]. Fendler et al also found a higher sensitivity in predicting PR by MTV (n=27) and TLG (n=30) over SUVmax (n=17) and SUVpeak (n=18). However, in our data we did not notice this difference in sensitivity between the metrics. This may be attributed to our smaller sample size. The authors identified a subset of patients (n=15/80; 19%) showing response by MTV and/or TLG with no response by SUVmax and/or SUVpeak, and reported a high median survival of 104 weeks for this subset of patients [23]. Similarly in our data, patients with response only by MTV and/or TLG but not by SUVmax and/or SUVpeak (n=4/46; 8%) had a median survival of 11.2 months. This exceeded the median survival of the opposite situation in our data, the four patients with response by SUVmax and/or SUVpeak but not by TLG and/or MTV, with a lower median survival (4.3 months). This may indicate that in the setting of discrepant findings between SUVmax/SUVpeak and volume based metrics of metabolic activity (MTV/TLG), MTV/TLG maybe more valuable. However, the number of patients in each group in our study was too small to test for statistical significance.
Gulec et al, in a study enrolling 20 patients, also pointed out the predictive value of measuring the MTV and TLG for CLM patients treated with radioembolization. The absolute values of MTV and TLG at baseline and at 4 weeks post-radioembolization were significant predictors of OS (P<0.05) [33]. Patients with baseline MTV <200 cm3 or TLG <600 g had an extended survival (26.9 versus 11.2 months), and those with post treatment MTV <30 cm3 or post treatment TLG <100 g had an extended survival (26.9 versus 10.9 months). However, they did not report on the value of the percentage change of MTV or TLG post-radioembolization, nor did they evaluate the performance of SUVmax or SUVpeak. Another study by Soydal et al showed that a 26.5% decline in TLG post-radioembolization of CLM can significantly predict survival (P=0.016) [34]. Together, the preceding data and our own data support the use of MTV and TLG as prognostic surrogate imaging biomarkers in the setting of CLM patients treated with radioembolization.
There is no consensus on the optimal time point to obtain PET/CT imaging post-radioembolization to evaluate response and predict outcomes. The study by Fendler et al used the PET/CT acquired at 3 months for the evaluation of response and showed an ability to predict OS [23]. Alternatively, other authors have shown that metabolic response detected at earlier time points can similarly predict OS [34, 35]. Soydal et al in a study with 35 patients with CLM showed that metabolic response evaluated by TLG at 6 weeks post-radioembolization did predict survival [34]. The OS of responders was 20.8 months versus 11.3 months for non-responders (P=0.016) [34]. In addition, Sabet et al in a larger study (n=51) showed that metabolic response as early as 4 weeks post-radioembolization can predict survival [35]. The OS for responders was 10 months versus 4 months for non-responders (P<0.001) [35]. The authors defined metabolic response as a >50% decline in the tumor SUVmax to liver SUVmean ratio [35]. Zerizer et al showed that metabolic response by SUVmax at 6–8 weeks is a significant predictor of liver progression free survival post-radioembolization of CLM [22]. Similarly, our results show that an early metabolic response by TLG or MTV at a median of 6 weeks can predict survival. Thus, based on limited evidence from the literature and our results, it appears that metabolic response on FDG PET/CT as early as 4–6 weeks post-radioembolization can predict outcomes.
This study had several limitations. First, it is a retrospective study performed in a single center, and the second limitation is the relatively small sample size (n=49). A Multi-institutional study can help overcome the limitation of small sample sizes of single institution studies, and provide a more diverse population for more universal application of the conclusions. Another limitation was the wide range of the time interval at which the post-radioembolization PET/CT was acquired (3–11.1 weeks). This limitation is a direct consequence of the retrospective nature of the study. Finally, other confounding factors such as prior systemic chemotherapy and/or HAIC may have contributed to the OS achieved in this population.
In conclusion, volume based metrics of metabolic activity i.e. metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are significant predictors of overall survival post-radioembolization of CLM, and seem to be more valuable than SUVmax and SUVpeak in this setting. RECIST 1.0 has a low sensitivity in detecting early response in CLM patients treated with radioembolization in the salvage setting, and fails to predict overall survival at an early time point. The need to define and assess surrogate-imaging biomarkers in the setting of CLM treated by image-guided therapies is a priority of the NIH and the society of interventional radiology (SIR) [36]. Additional studies in larger populations, ideally prospectively designed, with pathologic validation of imaging findings are very much needed. Such studies would provide a higher level of evidence for the prognostic value of surrogate-imaging biomarkers in the setting of CLM patients treated with radioembolization.
Acknowledgments
[BLINDED] Center is supported by the [BLINDED] from the [BLINDED].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Possible conflict of interest for Constantinos T. Sofocleous: A consultant for SIRTEX ((Woburn MA 01801 United States), owns SIRTEX stock, and has received research support from SIRTEX in the past.
References
- 1.Benson AB, 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Freedman-Cass DA, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2013;11:519–28. doi: 10.6004/jnccn.2013.0069. [DOI] [PubMed] [Google Scholar]
- 2.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur. J. Cancer. 2002;38:1023–33. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 3.Steele G, Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann. Surg. 1989;210:127–38. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Jaeck D, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. discussion 18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Hess K, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. discussion 25-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Pawlik TM, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann. Surg. 2009;250:440–8. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 8.Sofocleous CT, Petre EN, Gonen M, Brown KT, Solomon SB, Kemeny NE, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J. Vasc. Interv. Radiol. 2011;22:755–61. doi: 10.1016/j.jvir.2011.01.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Sofocleous CT, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes-A 10-year Experience at a Single Center. Radiology. 2015:142489. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–68. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 11.Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Solbiati L, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur. Radiol. 2015;25:3438–54. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Gary B, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J. Surg. Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 13.Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Flamen P, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J. Clin. Oncol. 2010;28:3687–94. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 14.Lewandowski RJ, Memon K, Mulcahy MF, Hickey R, Marshall K, Salem R, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1861–9. doi: 10.1007/s00259-014-2799-2. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy AS, Ball D, Cohen SJ, Cohn M, Coldwell DM, Wang EA, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J. Gastrointest. Oncol. 2015;6:134–42. doi: 10.3978/j.issn.2078-6891.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann. Surg. Oncol. 2015;22:794–802. doi: 10.1245/s10434-014-4164-x. [DOI] [PubMed] [Google Scholar]
- 17.Hickey R, Lewandowski RJ, Prudhomme T, Ehrenwald E, Baigorri B, Salem R, et al. Y90 Radioembolization of Colorectal Hepatic Metastases using Glass Microspheres: Safety and Survival Outcomes from a 531-Patient Multicenter Study. J. Nucl. Med. 2015 doi: 10.2967/jnumed.115.166082. [DOI] [PubMed] [Google Scholar]
- 18.Sofocleous CT, Garcia AR, Pandit-Taskar N, Do KG, Brody LA, Kemeny NE, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin. Colorectal Cancer. 2014;13:27–36. doi: 10.1016/j.clcc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Sofocleous CT, Violari EG, Sotirchos VS, Shady W, Gonen M, Kemeny NE, et al. Radioembolization as a Salvage Therapy for Heavily Pretreated Patients With Colorectal Cancer Liver Metastases: Factors That Affect Outcomes. Clin. Colorectal Cancer. 2015;14:296–305. doi: 10.1016/j.clcc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y–glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:815–20. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]
- 21.Szyszko T, Al-Nahhas A, Canelo R, Habib N, Jiao L, Tait P, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl. Med. Commun. 2007;28:15–20. doi: 10.1097/MNM.0b013e328011453b. [DOI] [PubMed] [Google Scholar]
- 22.Zerizer I, Al-Nahhas A, Towey D, Tait P, Ariff B, Barwick T, et al. The role of early (1)(8)F-FDG PET/CT in prediction of progression-free survival after (9)(0)Y radioembolization: comparison with RECIST and tumour density criteria. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:1391–9. doi: 10.1007/s00259-012-2149-1. [DOI] [PubMed] [Google Scholar]
- 23.Fendler WP, Philippe Tiega DB, Ilhan H, Paprottka PM, Heinemann V, Haug AR, et al. Validation of several SUV-based parameters derived from 18F–FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J. Nucl. Med. 2013;54:1202–8. doi: 10.2967/jnumed.112.116426. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy AS, Ball DS, Cohen SJ, Cohn M, Coldwell DM, Wang EA, et al. Hepatic imaging response to radioembolization with yttrium-90-labeled resin microspheres for tumor progression during systemic chemotherapy in patients with colorectal liver metastases. J. Gastrointest. Oncol. 2015;6:594–604. doi: 10.3978/j.issn.2078-6891.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Price P, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 26.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam. Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 28.Annunziata S, Treglia G, Caldarella C, Galiandro F. The role of 18F–FDG-PET and PET/CT in patients with colorectal liver metastases undergoing selective internal radiation therapy with yttrium-90: a first evidence-based review. ScientificWorldJournal. 2014;2014:879469. doi: 10.1155/2014/879469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tochetto SM, Rezai P, Rezvani M, Nikolaidis P, Berggruen S, Yaghmai V, et al. Does multidetector CT attenuation change in colon cancer liver metastases treated with 90Y help predict metabolic activity at FDG PET? Radiology. 2010;255:164–72. doi: 10.1148/radiol.09091028. [DOI] [PubMed] [Google Scholar]
- 30.Tochetto SM, Tore HG, Chalian H, Yaghmai V. Colorectal liver metastasis after 90Y radioembolization therapy: pilot study of change in MDCT attenuation as a surrogate marker for future FDG PET response. AJR Am. J. Roentgenol. 2012;198:1093–9. doi: 10.2214/AJR.11.6622. [DOI] [PubMed] [Google Scholar]
- 31.Lewandowski RJ, Thurston KG, Goin JE, Wong CY, Gates VL, Salem R, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135–150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J. Vasc. Interv. Radiol. 2005;16:1641–51. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE, Jr, Sailer S, et al. Resin 90Y–microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:412–25. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 33.Gulec SA, Suthar RR, Barot TC, Pennington K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:1289–95. doi: 10.1007/s00259-011-1758-4. [DOI] [PubMed] [Google Scholar]
- 34.Soydal C, Kucuk ON, Gecim EI, Bilgic S, Elhan AH. The prognostic value of quantitative parameters of 18F–FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl. Med. Commun. 2013;34:501–6. doi: 10.1097/MNM.0b013e32835f9427. [DOI] [PubMed] [Google Scholar]
- 35.Sabet A, Meyer C, Aouf A, Sabet A, Ghamari S, Ezziddin S, et al. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:370–6. doi: 10.1007/s00259-014-2935-z. [DOI] [PubMed] [Google Scholar]
- 36.Janned’Othee B, Sofocleous CT, Hanna N, Lewandowski RJ, Soulen MC, Kee ST, et al. Development of a research agenda for the management of metastatic colorectal cancer: proceedings from a multidisciplinary research consensus panel. J. Vasc. Interv. Radiol. 2012;23:153–63. doi: 10.1016/j.jvir.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]