Abstract
Early malnutritional status has been associated with reduced cognitive ability in childhood. However, there are almost no studies on the effect of malnutrition on positive social behavior, and no tests of possible mediating mechanisms. This study tests the hypothesis that poor nutritional status is associated with impaired social functioning in childhood, and that neurocognitive ability mediates this relationship. We assessed 1553 male and female 3‐year‐olds from a birth cohort on measures of malnutrition, social behavior and verbal and spatial neurocognitive functions. Children with indicators of malnutrition showed impaired social behavior (p < .0001) as compared with children in the control group with adequate nutritional status. These associations even persisted after controlling for social adversity and parental education. Findings were not moderated by gender or ethnicity, and there was no interaction effect with parental education. A dose–response relationship was observed between degree of malnutrition and degree of social behavior, with increased malnutrition associated with more impaired social behavior. Neurocognitive ability was found to mediate the nutrition–social behavior relationship. The mediation effect of neurocognitive functioning suggests that poor nutrition negatively impacts brain areas that play important roles in developing positive social behavior. Findings suggest that reducing poor nutrition, alternatively promoting good nutrition, may help promote positive social behavior in early childhood during a critical period for social and neurocognitive development, with implications for improving positive health in adulthood.
Keywords: nutritional status, malnutrition, social behavior, positive behavior, preschool, children
Introduction
An increasing body of evidence indicates that poor nutritional status during early childhood has a negative impact on cognitive development (Lozoff 2000; Grantham‐McGregor & Ani 2001; Liu et al. 2003; Laus et al. 2011; Galler et al. 2012; Bogale et al. 2013; Waber et al. 2014) externalizing behavior (Liu et al. 2004; Galler et al. 2011; Liu & Raine 2011) even after controlling for social adversity. Nutritional supplementation, on the other hand, has been shown to reduce behavioral problems in early childhood (Berglund et al. 2013) as well as in adolescence (Raine et al. 2015). A major gap that remains in the literature, however, concerns whether or not nutritional status is associated with positive social behaviors, and if so, what mechanisms may account for such a relationship.
Research on the effects of poor nutrition on playful and non‐playful social behavior in animals provides hypotheses on such effects in humans. In this context, playful social behavior is an indicator of better social functioning. Early experimental research on rats suggested that poor nutrition increases some non‐playful social behaviors (Whatson & Smart 1978). Later studies indicate that prenatal and postnatal malnutrition in rats decrease both playful and non‐playful social encounters (Whatson & Smart 1978; Almeida et al. 1996; Almeida & De Araujo 2001; Camargo & de Sousa Almeida 2005; Belluscio et al. 2014). However, later nutritional rehabilitation has been found to reverse the changes in non‐playful social behavior as well as increase playful social behavior (Almeida & De Araujo 2001; Soares et al. 2013; Soares et al. 2015). Studies on primates have shown that protein malnutrition increases aggression and reduces active social play behavior while rehabilitation of the nutritional deprivation increased duration of active social play behavior (Zimmermann et al. 1975). Overall, these studies suggest that malnutrition reduces social behavior in animals, although given the mixed findings in rats, definitive conclusions cannot be drawn.
Regarding humans, there are few studies on the effects of malnutrition on positive social behavior (Sigman et al. 1989; Espinosa et al. 1992; Lozoff et al. 1998; Black et al. 2004; Lozoff et al. 2007; Aburto et al. 2010; Engle & Fernández 2010). In a study of 110 Kenyan toddlers, reduced food intake was related to children's active social play and extent of verbalizations (Sigman et al. 1989), with better nutritional status associated with positive playground social behavior and activity levels (Espinosa et al. 1992). Furthermore, Lozoff et al. (Lozoff et al. 1998; Lozoff et al. 2007; Lozoff et al. 2014) have reported relationships between iron deficiency (one of the measures indicating malnutrition in our current study) and early childhood behavior, including displaying less pleasure and delight, and less playfulness in infants, as well as slowness to display positive affect and less social eye gazes toward their mothers in preschoolers. More recently, similar findings have been found in Chinese children where iron deficiency anemia was associated with less positive affect and more passive behavior (Chang et al. 2011). Furthermore, iron and zinc supplementation was reported to promote exploratory behavior in a group of 221 Bangladeshi infants (Black et al. 2004). In a separate study, multiple‐micronutrient supplementation benefitted physical activity and exploratory behavior in a sample of 109 Mexican infants (Aburto et al. 2010). More recently, Lozoff et al. (2014) found that iron supplementation in infancy promoted more adaptive behavior. These initial findings suggest that poor nutrition may impair playful and interactive behavior, which is closely intertwined with positive social functioning in childhood. Nevertheless, these studies are limited in small sample size and lack dose–response analyses. Despite these past studies, few have reported on the link between nutrition status and a broad range of social behavior in early childhood. Furthermore, little is known on the mediating effect of malnutrition and social behavior on neurocognitive function.
In the current study, we utilized laboratory and clinical indicators of malnutrition to go beyond food intake to assess whether the nutrition–social behavior relationship applies to early childhood in a large sample of 1553 3‐year‐old children. The authors hypothesized that (1) malnutrition would be associated with a reduction in positive social functioning; (2) a dose–response relationship would be observed; (3) effects would be independent of social adversity; and (4) IQ would mediate the malnutrition–social behavior relationship.
Key messages.
Previous research with animal models shows that poor nutritional status has adverse effects on playful and non‐playful social behavior in rats.
However, in humans, limited studies show that food intake is related to playground behavior in children, and iron deficiency anemia is associated with poor social emotional development.
This sample of preschool children found that those with poor nutritional status at age three years show significantly lower levels of positive social functioning compared with controls.
Our findings suggest a possible mechanism whereby poor nutrition leads to impaired social behavior.
The study suggests that having a good nutritional status may promote positive social behavior in children.
Methods
Participants
Participants were drawn from a birth cohort of 1795 children from the island of Mauritius lying off the coast of Africa (Raine et al. 2010). Based on vaccination records, all children born in 1969–1970 in two main towns, which were chosen in order to be representative of the ethnic distribution of the whole island, were recruited into the study at aged 3 years between September 1972 and August 1973. Detailed descriptions of the study setting and demographics, sampling procedures, characteristics of this cohort, parental consent, human subject consent and approvals are described elsewhere (Raine et al. 2010). The sample consisted of males (51.4%) and females (48.6%). Ethnic distribution was as follows: Indian 68.7%, Creoles (African origin) 25.7% and others (Chinese, English and French) 5.6%. Census data for the island as a whole indicated 66% Indian, 29% Creole and 5% other, indicating that the study largely achieved its goal of sampling a population representative in gender and ethnicity.
Following previous work on this population, data analyses were restricted to Indians and Creoles (Raine et al. 2002a). Verbal informed consent was obtained from the mothers of the participants in accordance with the principles outlined in the Declaration of Helsinki (WMA 2012) that prevailed in 1972 when the research was initiated. Institutional Review Board approval for later research phases and retrospective data analyses was obtained from the University of Pennsylvania. Complete data on both malnutrition and social behavior variables were available for 1535 subjects, the final sample used in this study.
Indicators of childhood malnutrition at age 3 years
Four early indicators of malnutrition were assessed in a clinical examination of 1559 of the children at the age of 3 years: (1) angular stomatitis, an indicator of vitamin B2 and niacin deficiency, with a base rate of 7.0%; (2) hair dyspigmentation, an indicator of protein malnutrition, with a base rate of 6.8%; (3) sparse/thin hair, an indicator of protein malnutrition, with a base rate of 5.8%; and (4) anemia, measured by blood hemoglobin levels, which had a base rate of 17%.
Subjects were defined as suffering from nutritional deficits if at least one of the four indicators was present (22.6%). The control group consisted of participants with no indicator present, and was viewed as relatively better nourished (77.4%). To assess for a dose–response relationship between malnutrition and social behavior, subjects with available behavior data were categorized into one of four groups: no malnutrition (N = 1189), one indicator (N = 261), two indicators (N = 68) and three indicators (N = 17).
These nutritional markers were consistent with those used in previously published studies of this cohort in which further details on assessment are also provided (Liu et al. 2003; Raine et al. 2003; Liu et al. 2004; Liu et al. 2009; Raine et al. 2010). Assessments were conducted using a structured protocol by local pediatricians, physicians and nutritionists who had received their medical training in the UK, USSR and India. All assessments were conducted at the research unit of the Joint Child Health Project in Quatre Bornes.
Positive social behavior measures
Positive social behavior consisted of four components measured at age 3 years: (1) exploratory behavior assessed as the children's interaction with toys in the presence of the mother in a controlled laboratory setting and rated on a four‐point scale (1 = passive, clings to mother, withdrawn; 2 = shows interest, examines toys but stays close to mother; 3 = leaves mother, mild independent exploration, comes and goes to mother; 4 = active independent exploration); (2) extent of verbalization to the research assistant during cognitive testing, rated on a 4‐point scale by the rater (1 = very reluctant to speak; 4 = many spontaneous comments); (3) friendliness with the rater during cognitive testing, rated on a 4‐point scale by the rater (1 = very reluctant or fearful; 4 = immediately friendly); and (4) active social play with other children during free play in a sandbox, rated by a rater on a 5‐point scale (1 = solitary; through 3 = associates with others; 5 = cooperative relationship with role reciprocity). Full details of measurement, procedure, factor structure, reliability and validity in this sample are given in Raine et al. (1998). Factor scores for the single factor solution were calculated for the four ratings. Cronbach's α reliability for the total scale was 0.88.
Neurocognitive functioning
Neurocognitive functioning was assessed from six sub‐tests of the Boehm Test of Basic Concepts‐Preschool Version (Boehm 1986), which measures basic verbal and visual–spatial concepts that are fundamental for early school achievement. Estimates of verbal and spatial IQ were derived from this test battery (Liu et al. 2003). Full details of size, measurement, factor structure, reliability and validity in this sample are given elsewhere (Boehm 1986; Liu et al. 2003; Raine et al. 2010).
Psychosocial adversity
Following our prior work, the measure of psychosocial adversity at age 3 years included nine variables collected by social workers who visited the homes of the children (Liu et al. 2003; Raine et al. 2010). Furthermore, a total index of psychosocial adversity (Raine et al. 1998) was created by adding one point for each of these nine variables (i.e. father uneducated, mother uneducated, father semiskilled or in unskilled occupation, single parent status, separation from parents, large family size, poor health of mother, teenaged mother and overcrowded home). The index was created along the lines similar to those described by Rutter (1997) and Moffitt (1990).
Statistical analyses
We first conducted multivariate analysis of variance (MANOVA) to test for the effects of malnutrition on social behavior using statistical software (SPSS; SPSS Inc., Chicago, Ill) and controlled for potential confounds which may mediate the effects of malnutrition on social behavior, including cognitive ability at age 3, father's education level, mother's education level, the nine psychosocial adversity variables, total social adversity score, ethnicity and gender. Analysis of variance (ANOVA) was used to ascertain which specific indicator of malnutrition was linked to social behavior and to test for a dose–response relationship between number of malnutrition indicators and social behavior. Both linear and quadratic relationships were tested. Also, MANOVA was used to test for the mediating effect of verbal and spatial neurocognitive ability. Two‐tailed tests of significance were used throughout. Furthermore, we used a generalized linear model (GLM) strategy to confirm the above MANOVA/ANOVA statistical approach. We used GLM to test the effect of malnutrition on social behavior, while controlling for the aforementioned confounders, as well as the significance of the interaction between malnutrition and parental education on social behavior. We further used GLM to ascertain which specific indicator of malnutrition was linked to overall social behavior (Z score), while controlling for the total adversity and parental education level.
Results
Sample characteristics
Complete data on both malnutrition and social behavior variables were available for 1535 subjects, somewhat less than the original sample of 1795. Those with and without complete data were compared on key demographic variables that were available on all subjects at age 3. Groups did not differ on gender (χ 2 = 0.98, df = 1, p > .33), ethnicity (χ 2 = 2.11, df = 1, p > 0.15) or total psychosocial adversity score (t = 0.27, p = .79). Thus, the sub‐sample was deemed to be representative of the larger sample on these measures at age 3.
Effect of malnutrition on social behavior
Means and standard deviations (SDs), together with results of specific t‐test comparisons for age 3 social behaviors for the malnourished and the control groups, are shown in Table 1 (see also Fig. 1). A MANOVA on the four dependent variables of social behavior indicated a main group effect (F = 35, 75, df = 4, 1553, p < .001), indicating reduced scores of social behavior at age three in the malnourished group. Results of ANOVAs for individual social behavior components show that all social behaviors were impacted by poor nutrition (see Table 1). Furthermore, adjusted GLM results indicated similar outcomes, except that the P‐value for exploratory behavior changed from 0.013 to 0.062 (see Table 2).
Table 1.
Components of social behavior and results of t test comparisons in the malnourished and control groups
| Measure | Malnourished group | Control group | t Test | df | P value |
|---|---|---|---|---|---|
| Social behavior | −.24 (0.65) | .0054 (.68) | 5.98 | 1534 | <.001 |
| (n = 347) | (n = 1189) | ||||
| Exploratory behavior | 2.11 (1.37) | 2.32 (1.43) | 2.483 | 1553 | .013 |
| (n = 352) | (n = 1203) | ||||
| Extent of verbalization | 2.30 (1.42) | 2.55 (1.33) | 3.178 | 1553 | .002 |
| (n = 325) | (n = 352) | ||||
| Friendliness | 2.41 (1.44) | 2.75 (1.36) | 4.025 | 1553 | <.001 |
| (n = 352) | (n = 1203) | ||||
| Active social play | 7.79 (3.58) | 9.01 (3.85) | 5.259 | 1510 | <.001 |
| (n = 346) | (n = 1166) |
Figure 1.
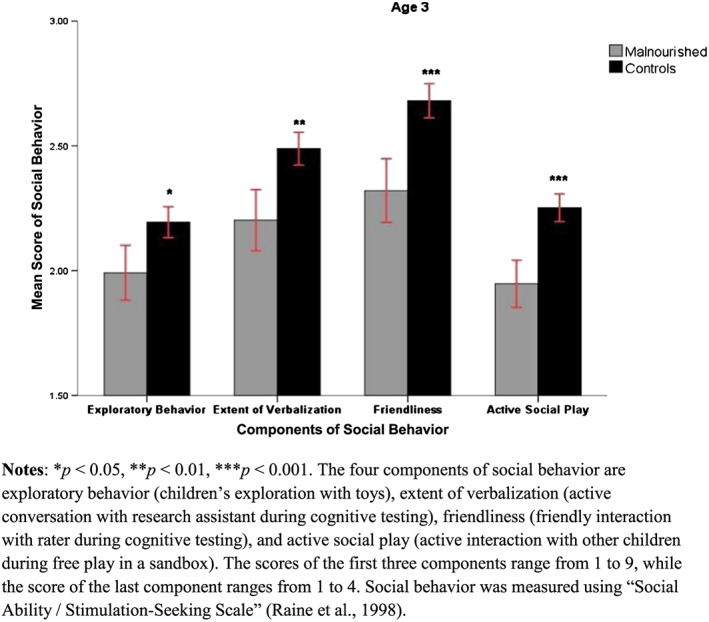
Four components of social behavior for malnourished and controlled group of children at three years of age.
Table 2.
Adjusted* generalized linear regression coefficients of malnutrition on social behavior
| Responses | Predictors: malnutrition vs. controls | ||
|---|---|---|---|
| B | P value | 95% CI | |
| Social behavior † | −.22 | <0.001 | (−.30, −.14) |
| Exploratory behavior | −.16 | 0.062 | (−.33, .01) |
| Extent of verbalization | −.21 | 0.010 | (−.37, −.052) |
| Friendliness | −.30 | <0.001 | (−.47, −.14) |
| Active social play | −1.01 | <0.001 | (−1.47, −.56) |
The generalized linear model adjusted for age, sex, ethnicity and the nine psychosocial adversity variables, including parental education level.
The total behavior used z scores, whereas the four components of social behavior used the total score.
Neurocognitive ability as a mediator of the malnutrition–social behavior relationship
IQ was identified as a potential mediator, i.e. variable accounting for a significant portion of the relationship between the predictor (malnutrition, correlation coefficient = −.107) and the outcome variable (social behavior, correlation coefficient = .357). After entering verbal and spatial neurocognitive ability into the MANOVA, the main effect of malnutrition on social behavior was abolished, F (12, 3441), df = 1.01, p = .429), indicating that neurocognitive ability mediates the link between malnutrition and social behavior.
Potential confounds and effects of moderators
Malnourished children were more likely to suffer overall psychosocial adversity (t = 4.13, p < .001), to have a less‐educated mother (t = 3.29; p < .001), a less‐educated father (t = 3.21; p < .001) and to have lower scores on IQ than controls at age 3 (t = 3.69; p < .001). In addition, Creoles were more likely to be slightly more malnourished (27%) than Indians (20.6%) (χ 2 = 6.97; p < .011). Consequently, it is possible that psychosocial adversity, parents' education level, the child's cognitive ability and ethnicity could account for the main effects of malnutrition on social behavior.
This possibility was tested by entering these variables as covariates. There was no interaction between malnutrition grouping and any of the potential moderators. MANOVAs conducted on gender (F = 0.108, df = 4, 1505, p = .980) and ethnicity (F = .385, df = 4, 1446, p = .82) were non‐significant, indicating that these measures did not enhance or decrease the effects of malnutrition on social behavior. Furthermore, after controlling for psychosocial adversity, the main effect of malnutrition remained significant (F = 6.75, df = 4, 1506, p < 0.0001), indicating that even after accounting for these cofounding variables, the main effect of malnutrition remains. Further GLM results showed that there was no significant interaction between malnutrition and parental education on social behavior.
Dose–response relationship
To assess for a dose–response relationship between number of indicators of malnutrition and the degree of reduced social behavior, a MANOVA (with four groups: 0, 1, 2 and 3 and more malnutrition indicators) was conducted on social behavior. This relationship is depicted in Fig. 2. Results show a significant main effect of number of indicators on social behavior, F (12,4518), df = 3.33, p < .0001, with increasing degree of malnutrition associated with decreasing social behavior. There were both linear (R 2 = 0.024) and quadratic (R 2 = 0.025) relationships between malnutrition indicators and social behavior (Ps < 0.001).
Figure 2.
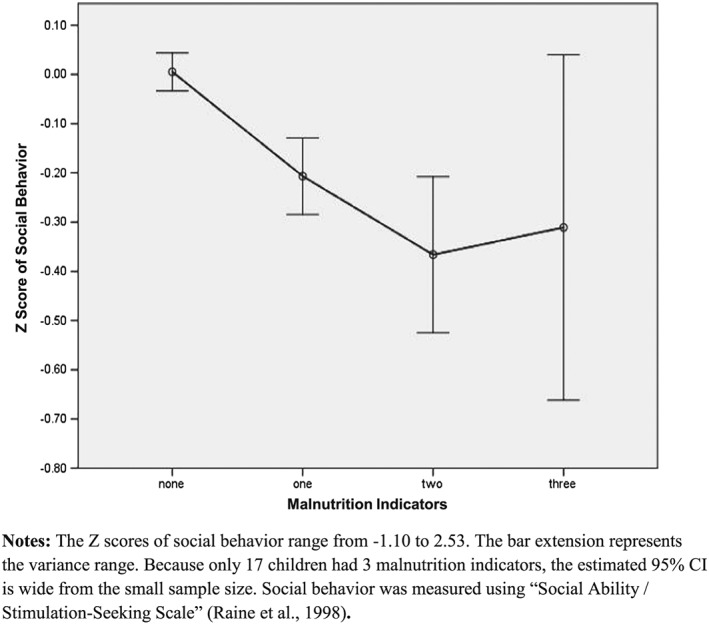
Dose–response relationships between number of malnutrition indicators present and social behavior at three years of age.
Effect of individual indicators of malnutrition on social behavior
Results of MANOVAs and univariate ANOVAs conducted on each of the four malnutrition indicators for age 3 social behavior measures are shown in Table 3. All four malnutrition indicators were generally associated with reduced functioning on all four social behavior components, although findings were less robust for the sparse, thin hair indicator (see Table 3). Moreover, the GLM results showed that all indicators of malnutrition were associated with total social behavior scores after controlling for social adversity and parental education, with anemia (β = −0.11, p < 0.00), angular stomatitis (β = −0.07, p < 0.003), kwashiorkor (β = −0.07, p < 0.005) and sparse/thin hair (β = −0.05, p < 0.030). However, anemia was more consistently associated with sub‐scores of social behavior, suggesting that anemia was the most important indicator of malnutrition affecting social behavior. Summary of the generalized linear regression models for malnutrition indicators and social behavior components are presented in Table 4 .
Table 3.
Malnutrition indicators in relation to social behavior components
| Measure | Anemia | Angular stomatitis | Kwashiorkor | Sparse, thin hair | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F test | df | P value | F test | df | P value | F test | df | P value | F test | df | P value | |
| Social behavior | ||||||||||||
| Overall ANOVA | 26.03 | (1,1551) | .001 | 12.1 | (1,1722) | .001 | 10.75 | (1,1724) | .001 | 6.22 | (1,1723) | .013 |
| Univariate | ||||||||||||
| Exploratory behavior | 3.80 | (1,1527) | .073 | 6.83 | (1,1695) | .009 | 4.32 | (1,1697) | .038 | .732 | (1,1696) | .392 |
| Extent of verbalization | 15.37 | (1,1527) | .001 | 5.55 | (1,1695) | .019 | 5.074 | (1,1697) | .024 | 4.99 | (1,1696) | .026 |
| Friendliness | 22.64 | (1,1527) | .001 | 4.29 | (1,1695) | .039 | 10.43 | (1,1697) | .001 | 4.28 | (1,1696) | .039 |
| Active social play | 25.13 | (1,1527) | .001 | 9.47 | (1,1695) | .002 | 3.83 | (1,1697) | .051 | 3.027 | (1,1696) | .082 |
Univariate analysis of variance was conducted on overall social behavior to assess the effect of individual malnutrition indicators. Multivariate analysis of variance was conducted to assess the effect of malnutrition indicators on individual social behavior components.
Table 4.
Summary of the generalized linear regression models: malnutrition indicators and social behavior components*
| Measure | Anemia | Angular stomatitis | Kwashiorkor | Sparse, thin hair | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95%CI | P value | Beta | 95%CI | P value | Beta | 95%CI | P value | Beta | 95%CI | P value | |
| Social behavior † | −.11 | (−.28,−.10) | .000 | −.07 | (−.32,−.06) | .003 | −.07 | (−.31,−.05) | .005 | −.05 | (−.29,−.02) | .030 |
| Exploratory behavior | −.03 | (−.31,.08) | .228 | −.04 | (−.50,.03) | .085 | −.03 | (−.44,.11) | .241 | .02 | (−.17,.41) | .422 |
| Extent of verbalization | −.08 | (−.47,−.11) | .002 | −.03 | (−.42,.08) | .172 | −.03 | (−.41,.09) | .216 | −.001 | (−.27,.27) | .981 |
| Friendliness | −.10 | (−.55,−.18) | .000 | −.03 | (−.40,.11) | .256 | −.05 | (−.55,−.03) | .027 | −.002 | (−.29,.26) | .931 |
| Active social play | −.10 | (−1.49,−.48) | .000 | −.06 | (−1.59,−.17) | .016 | −.03 | (−1.16,.28) | .235 | −.03 | (−1.28,.28) | .207 |
These models were all controlled for age, sex, ethnicity and the nine psychosocial adversity variables, including parental education level.
The total behavior used z scores, whereas the four components of social behavior used the total score.
Discussion
This study documents that children with physical indicators of malnutrition at the age of three years showed significantly lower levels of positive social functioning compared with controls. Importantly, the malnutrition–social behavior relationship was mediated by neurocognitive ability, suggesting that malnutrition predisposes children to poorer neurocognitive functioning, which in turn predisposes them to reduced positive social behavior. Neurocognitive measures were found to account for 55.6% of the malnutrition–social behavior relationship. A dose–response relationship between degree of malnutrition and degree of reduced social behavior problems was also documented, although the quadratic relationship was noticed when three malnutrition indicators were present, which might be because of the group's small sample size (N = 17). All above relationships were not an artifact of psychosocial adversity and were not influenced by parental education. Analyses are consistent with the notion that malnutrition first impairs neurocognition and brain functioning, which in turn hampers the development of positive social functioning skills in children. To the authors' knowledge, these findings are the first to indicate a possible mechanism in humans whereby poor nutrition translates itself into impaired social behavior.
Past research has reported on the relationship between nutrition and behavior, particularly externalizing behaviors (Liu & Raine 2011). In this same sample, we previously found that malnutrition at age 3 years was associated with externalizing behavior across childhood (Liu et al. 2004). More recently, Galler et al. (2011) reported that early childhood malnutrition was linked to problems in executive functioning in later childhood and adolescence, as well as increased levels of aggression (Galler et al. 2013). Furthermore, iron deficiency in children has been linked to adverse social–emotional behavioral outcomes (Lozoff et al. 2007; Lozoff et al. 2008; Corapci et al. 2010; Chang et al. 2011; Lozoff et al. 2014; Bakoyiannis et al. 2015). Conversely, nutritional supplements of omega‐3 fatty acids reduced behavioral problems among children aged 8 to 16 years old (Raine et al. 2015). Nevertheless, to our knowledge, the findings in our current study are among the first studies in humans to document malnutrition–social behavior relationships and are the first to indicate a possible mechanism whereby poor nutrition translates itself to impaired social behavior. These findings in turn have potential implications for public health efforts to promote better nutrition in the early years in order to promote positive social behavior and potentially enhance later health outcomes (Taylor et al. 1985). Importantly, increasing evidence demonstrates that physical activity and exercise might provide benefits to overall mental health (Stanton et al. 2014), including improving self‐image and reducing the symptoms of anxiety (Taylor et al. 1985), which can ultimately reduce the developmental child psychopathology.
Nutrition, brain and social behavior
How nutritional deficits contribute to reduced social behavior is not well understood, and this issue represents a significant gap in the literature. We hypothesize that poor nutrition negatively affects brain functioning which in turn impairs the development of positive social behavior. Malnutrition has been increasingly recognized as a factor that may play a role in altering brain function via three independent mechanisms: retarding brain cell growth/development (reducing the number of neurons), altering brain neurochemistry (neurotransmitters) and increasing the effects of neurotoxicity (e.g. lead toxicity) (Liu & Raine 2006; Laus et al. 2011). The finding that neurocognitive functioning accounted for 55.6% of the nutrition–social behavior relationship supports the partial mediation hypothesis that poor nutrition results in poorer brain functioning (as indicated by reduced neurocognitive functioning), which in turn impairs positive social behavior.
A key question concerns the functional neuroanatomical underpinnings of the nutrition – social behavior relationship – which specific brain areas are implicated? The burgeoning new field of social neuroscience is documenting the role of the ‘social brain’ in orchestrating social behavior in humans (Adolphs 2003; Kennedy & Adolphs 2012). Brain areas linked with positive social behavioral characteristics include the inferior parietal lobule (Rapp et al. 2008), the anterior cingulate gyrus (Haas et al. 2006), the prefrontal cortex (Wright et al. 2006) and the temporal–parietal junction (Decety & Lamm 2007). Recent experimental animal research has revealed cellular activation in limbic brain systems during active social play behavior (van Kerkhof et al. 2014). These brain regions have in turn been found to be particularly affected by a reduction in food intake. For example, patients with anorexia nervosa have been shown to have reduced gray matter volumes specifically in frontal and parietal brain regions, deficits which are remediated by weight normalization (Swayze et al. 2003). Consequently, through its negative effects on prefrontal and parietal brain networks critical to social behavior, poor nutrition in children could reduce positive social behaviors (Alamy & Bengelloun 2012; Plaven‐Sigray et al. 2014). We caution however that this is a provisional hypothesis that requires further substantiation and testing in future research that integrates functional brain imaging, nutrition and social behavior.
The current findings also raise questions concerning the issue of causality, and whether the negative effect of poor nutrition on social behavior can be reversed. One experimental intervention study has shown that vitamin E supplementation results in increased verbalizations and social play behavior in toddlers in Indonesia (Pollitt et al. 2000). Supplemental iron in infancy has been shown to increase adaptive behavior at 10 years of age (Lozoff et al. 2014). Recent experimental animal studies have also shown that mouse sociability and brain serotonin turnover are reduced by acute tryptophan depletion and can be enhanced by supplementation (Zhang et al. 2015). Taken together with the current findings of reduced verbalizations, friendliness and active social play behavior in malnourished children, these findings suggest that poor nutrition may predispose to reduced positive social interaction in children. In terms of indicators of malnutrition, anemia was found to have the most important influence on social behavior, because it was more significantly associated across the four components of social behavior. A future longitudinal study which includes nutritional supplementation such as iron could help further tease out the causal ordering of the associations observed in this study.
Implications
The potential importance and implications of the current findings can be viewed on a number of levels. While an increasing body of evidence indicates that malnutrition negatively impacts cognitive development (Alamy & Bengelloun 2012), positive social behavior has been relatively ignored. Positive social interactions have been linked with later physical and mental health (Uvnas‐Moberg 1998; Brülde 2007; Segrin & Taylor 2007; Umberson 2010; Thoits 2011). Empirical evidence supports the notion that positive social functioning is related to better psychological well‐being (Segrin & Taylor 2007), quality of life (Bowling et al. 2003; Brülde 2007; Levasseur et al. 2009) and longevity (Anme et al. 2007). Cross‐sectional studies have shown an association between positive social behavior and better health‐related quality of life (Guyatt et al. 1993), while longitudinal studies have shown that early positive social behavior is positively related to reduced adolescent deviance (Ary et al. 1999), better quality of life and less chronic disorders in adulthood (Dawson et al. 1994; Ary et al. 1999), as well as slowed decline of mental status in the elderly (Lowenthal 1964). Positive social interactions have also been linked with health‐promoting effects and reduced stress (Uvnas‐Moberg 1998). Because reduced stress can combat hypertension and heart disease (Turner et al. 1995; Spence et al. 1999), early positive social behavior may protect against the development of chronic adult physical illness. Stress load has also been linked to increased psychopathology, and studies have implicated stress as a risk factor in increased depressive symptoms and substance abuse (Weber et al. 2008; Brietzke et al. 2012; Lewandowski et al. 2014); this association suggests that reduced stress may have the added benefit of leading to reduced psychopathology.
With respect to neurocognitive functioning, positive social behavior in early childhood has been associated with increased cognitive competency, scholastic ability and neurocognitive test performance in later childhood (Raine et al. 2002a; Bornstein et al. 2010). These effects are not trivial in that effect sizes (Cohen's d) of previous studies range from .52 to .87 (Raine et al. 2002a). It has been hypothesized that young children who physically explore their environment, engage socially with other children and verbally interact with adults create for themselves an enriched, stimulating, varied and challenging environment. This environmental enrichment in turn is hypothesized to result in enhanced brain functioning, resulting in better neurocognitive ability and better school performance (Raine et al. 2002a; Pang & Hannan 2013). In particular, children who scored high on the exploratory behavior component in this study would be expected to engage in more physical activity. Physical exercise in turn is known to promote neurogenesis in the dentate gyrus of the hippocampus (van Praag et al. 1999; Kim et al. 2010; Erickson et al. 2011), a brain area of importance in both attention and memory. In humans, there is increasing evidence that exercise can be beneficial to cognitive functioning (Best 2010; Smith et al. 2010; Biddle & Asare 2011; Donnelly & Lambourne 2011). Recent studies suggest that the beneficial effects of physical activity/fitness during childhood and adolescence carry over into adulthood, resulting in an improved health status as indicated by cardiovascular health, musculoskeletal function and body composition (Boreham & Riddoch 2001; Malina 2001; Janssen & Leblanc 2010; Gunter et al. 2012). Physical activity has also been linked to improvements in mental health (Stathopoulou et al. 2006; Stanton et al. 2014), such as the alleviation of depression (Deslandes et al. 2009) and anxiety symptoms (De Moor et al. 2006) and increased quality of life (Anokye et al. 2012). Moreover, research has also shown that psychosocial stimulation (play sessions) during early childhood is linked to reduced involvements in fights and serious violent behavior later in life (Walker et al. 2011; Tremblay 2012).
Low IQ represents the best‐replicated cognitive risk factor for externalizing behavior problems. Furthermore, longitudinal studies have shown that early neurocognitive deficits precede the onset of antisocial behavior (Cottle et al. 2001; Raine et al. 2002b; DeLisi & Vaughn 2011; Liu 2011). For example, low spatial IQ at age 3 (as well as and low verbal and spatial IQ at age 11) predicted life‐course persistent antisocial behavior in community children, with similar findings observed by others (Raine et al. 2002b). Neuropsychological deficits have been found to characterize child and adult violent and antisocial behavior (Cottle et al. 2001; Raine et al. 2002b). Similarly, poor school achievements and failure to complete school have also been shown to be positively related to juvenile delinquency (Farrington 1989; Henry et al. 2012; Jakobsen et al. 2012).
Taken together, there is reason to believe that positive social behavior early in life can positively impact later health, school performance and IQ. As such, the current findings suggest that the effect of poor nutrition on the child's positive social behavior has much broader implications for the child's later well‐being than previously suspected. This in turn suggests that early adequate nutrition, by impacting positive social behavior, has implications for well‐being that extend beyond physical health.
Limitations and conclusions
Several limitations need to be acknowledged. First, findings are restricted to childhood, and we did not assess the long‐term effects of malnutrition on social–behavioral outcomes. Second, while the assessments used included a biochemical measure of hemoglobin that can be deemed a strength of the study, we may not have detected more subtle nutritional deficiencies in children lacking objective malnutrition indicators. Furthermore, anemia is considered relatively severe as defined by our hemoglobin levels, and blood loss can be potentially caused by malaria and infestation as opposed to poor nutrition. Third, although children's nutritional status and social behavior were blindly evaluated, the social behavior raters might not have been completely blind to children's nutritional status based on children's physical appearance, although they were blind to the hypothesis under study. Finally, findings from Mauritius need to be replicated and extended in Western cultures.
Set against these limitations are several strengths that support the robustness of our findings. First, nutrition status included both a biochemical assessment (hemoglobin) and clinical evaluation by pediatricians. Second, social behavior was measured by four different components to cover a broad construct of social functioning in a controlled laboratory setting and had been previously psychometrically established using confirmatory factor analysis (Raine et al. 2010). Third, the fact that gender and ethnicity did not moderate the multivariate findings indicates that the nutrition–social behavior relationship is not specific to one gender or ethnic grouping. Fourth, while a significant linear dose–response relationship confirms and extends the finding of the relationship between malnutrition and social behavior, a quadratic effect was also observed indicating that three indicators of malnutrition did not impair social behavior any further. This latter effect should be treated with caution because of small sample size of the group with three indicators (N = 17). Fifth, findings are based on a large community sample of over 1500 participants. For these reasons, we believe that our findings reflect a reliable relationship between adequate nutrition status and positive social behavior.
In conclusion, this study extends and amplifies findings from the only other study conducted on children, and to our knowledge is the first to attempt to understand the mechanism of action by which poor nutrition translates into reduced positive social behaviors. Findings in turn provide an initial model upon which future studies may build. Because positive social behaviors subsequently influence mental health, which has a lifelong impact on individuals, research in this area is particularly important. We suggest that our findings provide an initial ‘proof of principle’ that the effects of malnutrition on behavior are mediated through cognitive impairment. Nevertheless, future studies need to ascertain whether this model is supported at more marginal levels of nutritional impairments.
Source of funding
This study was supported by National Institutes of Health/National Institute of Environmental Health Sciences USA (NIH/NIEHS) 1K02ES019878 and National Institute of Nursing Research USA (NINR) 5F32NR008661 to Dr. Liu; and National Institute of Mental Health USA (NIMH) Independent Scientist Award (K02 MH‐01114), a grant from the Borchard Foundation and a grant from NIMH (R01 MH‐46435) to Dr. Raine; and a grant from the Ministry of Health of the Mauritian Government. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
Jianghong Liu: Dr. Liu conceptualized and designed the study, performed statistical analyses, developed the manuscript, drafted the initial manuscript and approved the final manuscript as submitted. Adrian Raine: Dr. Raine conceptualized and designed the study, and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Marie‐Clare Calambay, Meena Calinghen, Athene Chiriaca, Cyril Dalais, Fazila Dinally, Devi Jaganathen, Goorah Rajah and Charles Yip Tong for help in data collection and scoring and Cyril Dalais for help in conducting research.
Liu, J. , and Raine, A. (2017) Nutritional status and social behavior in preschool children: the mediating effects of neurocognitive functioning. Maternal & Child Nutrition, 13: 12321. doi: 10.1111/mcn.12321.
References
- Aburto N.J., Ramirez‐Zea M., Neufeld L.M. & Flores‐Ayala R. (2010) The effect of nutritional supplementation on physical activity and exploratory behavior of Mexican infants aged 8–12 months. European Journal of Clinical Nutrition 64 (6), 644–651. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2003) Cognitive neuroscience of human social behavior. Nature Reviews Neuroscience 4 (3), 165–178. [DOI] [PubMed] [Google Scholar]
- Alamy M. & Bengelloun W.A. (2012) Malnutrition and brain development: an analysis of the effects of inadequate diet during different stages of life in rat. Neuroscience and Biobehavioral Reviews 36 (6), 1463–1480. [DOI] [PubMed] [Google Scholar]
- Almeida S.S. & De Araujo M. (2001) Postnatal protein manutrition affects play behavior and other social interactions in juvenile rats. Physiology & Behavior 74 (1–2), 45–51. [DOI] [PubMed] [Google Scholar]
- Almeida S.S., Tonkiss J. & Galler J.R. (1996) Prenatal protein malnutrition affects the social interactions of juvenile rats. Physiology & Behavior 60 (1), 179–201. [DOI] [PubMed] [Google Scholar]
- Anme T., Shinohara R., Sugisawa Y. & McCall M.E. (2007) Social interaction and longevity: an eleven‐year longitudinal study of older persons in a Japanese village. Hallym International Journal of Aging 9 (2), 89–105. [Google Scholar]
- Anokye N.K., Trueman P., Green C., Pavey T.G. & Taylor R.S. (2012) Physical activity and health related quality of life. BMC Public Health 12 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary D.V., Duncan T.E., Biglan A., Metzler C.W., Noell J.W. & Smolkowski K. (1999) Development of adolescent problem behavior. Journal of Abnormal Child Psychology 27 (2), 141–150. [DOI] [PubMed] [Google Scholar]
- Bakoyiannis I., Gkioka E., Daskalopoulou A., Korou L.M., Perrea D. & Pergialiotis V. (2015) An explanation of the pathophysiology of adverse neurodevelopmental outcomes in iron deficiency. Reviews in the Neurosciences. DOI: 10.1515/revneuro-2015-0012. [DOI] [PubMed] [Google Scholar]
- Belluscio L.M., Berardino B.G., Ferroni N.M., Ceruti J.M. & Canepa E.T. (2014) Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive‐like behaviors. Physiology & Behavior 129, 237–254. DOI: 10.1016/j.physbeh.2014.02.051. [DOI] [PubMed] [Google Scholar]
- Berglund S.K., Westrup B., Hagglof B., Hernell O. & Domellof M. (2013) Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics 131 (1), 47–55. DOI: 10.1542/peds.2012-0989. [DOI] [PubMed] [Google Scholar]
- Best J.R. (2010) Effects of physical activity on children's executive function: contributions of experimental research on aerobic exercise. Developmental Review 30 (4), 331–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle S.J.H. & Asare M. (2011) Physical activity and mental health in children and adolescents: a review of reviews. British Journal of Sports Medicine 45, 886–895. [DOI] [PubMed] [Google Scholar]
- Black M.M., Baqui A.H., Zaman K., Ake Persson L., El Arifeen S., Le K. et al. (2004) Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. American Journal of Clinical Nutrition 80 (4), 903–910. [DOI] [PubMed] [Google Scholar]
- Boehm A. (1986) Boehm Test of Basic Concepts—Preschool Version. Psychological Corporation: San Antonio, TX. [Google Scholar]
- Bogale A., Stoecker B.J., Kennedy T., Hubbs‐Tait L., Thomas D., Abebe Y. et al. (2013) Nutritional status and cognitive performance of mother–child pairs in Sidama, Southern Ethiopia. Maternal & Child Nutrition 9 (2), 274–284. DOI: 10.1111/j.1740-8709.2011.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham C. & Riddoch C. (2001) The physical activity, fitness and health of children. Journal of Sports Science 19 (12), 915–929. [DOI] [PubMed] [Google Scholar]
- Bornstein M.H., Hahn C.S. & Haynes O.M. (2010) Social competence, externalizing, and internalizing behavioral adjustment from early childhood through early adolescence: developmental cascades. Development and Psychopathology 22 (4), 717–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A., Gabriel Z., Dykes J., Dowding L.M., Evans O., Fleissig A. et al. (2003) Let's ask them: a national survey of definitions of quality of life and its enhancement among people aged 65 and over. International Journal of Aging & Human Development 56 (4), 269–306. [DOI] [PubMed] [Google Scholar]
- Brietzke E., Kauer‐Sant'anna M., Jackowski A., Grassi‐Oliveira R., Bucker J., Zugman A. et al. (2012) Impact of childhood stress on psychopathology. Revista Brasileira de Psiquiatria 34 (4), 480–488. [DOI] [PubMed] [Google Scholar]
- Brülde B. (2007) Happiness and the good life: introduction and conceptual framework. Journal of Happiness Studies 8 (1), 1–14. [Google Scholar]
- Camargo, L.M.M. , & de Sousa Almeida, S. (2005). Early postnatal protein malnutrition changes the development of social play in rats. Physiology & behavior, 85(3), 246–251. [DOI] [PubMed] [Google Scholar]
- Chang S., Wang L., Wang Y., Brouwer I.D., Kok F.J., Lozoff B. et al. (2011) Iron‐deficiency anemia in infancy and social emotional development in preschool‐aged Chinese children. Pediatrics 127 (4), e927–e933. DOI: 10.1542/peds.2010-1659. [DOI] [PubMed] [Google Scholar]
- Corapci F., Calatroni A., Kaciroti N., Jimenez E. & Lozoff B. (2010) Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. Journal of Pediatric Psychology 35 (3), 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle C.C., Lee R.J. & Heilbrun K. (2001) The prediction of criminal recidivism in juveniles: a meta‐analysis. Criminal Justice and Behavior 28 (3), 367–394. [Google Scholar]
- Dawson G., Hessl D. & Frey K. (1994) Social influences on early developing biological and behavioral systems related to risk for affective disorder. Development and Psychopathology 6 (4), 759–779. [Google Scholar]
- De Moor M.H.M., Beem A.L., Stubbe J.H., Boomsma D.I. & De Geus D. (2006) Regular exercise, anxiety, depression and personality: a population‐based study. Preventive Medicine 42, 273–279. [DOI] [PubMed] [Google Scholar]
- Decety J. & Lamm C. (2007) The role of the right temporoparietal junction in social interaction: how low‐level computational processes contribute to meta‐cognition. The Neuroscientist 13 (6), 580–593. [DOI] [PubMed] [Google Scholar]
- DeLisi M. & Vaughn M.G. (2011) The importance of neuropsychological deficits relating to self‐control and temperament to the prevention of serious antisocial behavior. International Journal of Child, Youth and Family Studies 2 (1/2), 12–35. [Google Scholar]
- Deslandes A., Moraes H., Ferreira C., Veiga H., Silveira H., Mouta R. et al. (2009) Exercise and mental health: many reasons to move. Neuropsychobiology 59 (4), 191–198. [DOI] [PubMed] [Google Scholar]
- Donnelly J.E. & Lambourne K. (2011) Classroom‐based physical activity, cognition, and academic achievement. Preventive Medicine 52 (Suppl 1), S36–S42. [DOI] [PubMed] [Google Scholar]
- Engle P.L. & Fernández P.D. (2010) INCAP studies of malnutrition and cognitivee behavior. Food and Nutrition Bulletin 31 (1), 83–94. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L. et al. (2011) Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 108 (7), 3017–3022. DOI: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa M.P., Sigman M.D., Neumann C.G. & Bwibo N.O. (1992) Playground behaviors of school‐age children in relation to nutrition, schooling and family characteristics. Developmental Psychology 28 (6), 1188–1195. [Google Scholar]
- Farrington D.P. (1989) Early predictors of adolescent aggression and adult violence. Violence and Victimes 4 (2), 79–100. [PubMed] [Google Scholar]
- Galler J.R., Bryce C.P., Waber D.P., Medford G., Eaglesfield G.D. & Fitzmaurice G. (2011) Early malnutrition predicts parent reports of externalizing behaviors at ages 9–17. Nutritional Neuroscience 14 (4), 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler J.R., Bryce C.P., Zichlin M.L., Fitzmaurice G., Eaglesfield G.D. & Waber D.P. (2012) Infant malnutrition is associated with persisting attention deficits in middle adulthood. Journal of Nutrition 142 (4), 788–794. DOI: 10.3945/jn.111.145441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler J.R., Bryce C.P., Zichlin M.L., Waber D.P., Exner N., Fitzmaurice G.M. et al. (2013) Malnutrition in the first year of life and personality at age 40. Journal of Child Psychology and Psychiatry 54 (8), 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham‐McGregor S. & Ani C. (2001) A review of studies on the effect of iron deficiency on cognitive development in children. Journal of Nutrition 131 (2S‐2), 649S–666S. [DOI] [PubMed] [Google Scholar]
- Gunter K.B., Almstedt H.C. & Janz K.F. (2012) Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exercise and Sport Sciences Reviews 40 (1), 13–21. DOI: 10.1097/JES.0b013e318236e5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G.H., Feeny D.H. & Patrick D.L. (1993) Measuring health‐related quality of life. Annals of Internal Medicine 118 (8), 622–629. [DOI] [PubMed] [Google Scholar]
- Haas B.W., Omura K., Amin Z., Constable R.T. & Canli T. (2006) Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Social Neuroscience 1 (1), 16–24. [DOI] [PubMed] [Google Scholar]
- Henry K.L., Knight K.E. & Thornberry T.P. (2012) School disengagement as a predictor of dropout, delinquency, and problem substance use during adolescence and early adulthood. Journal of Youth and Adolescence 41 (2), 156–166. DOI: 10.1007/s10964-011-9665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen I.S., Fergusson D. & Horwood J.L. (2012) Early conduct problems, school achievement and later crime: findings from a 30‐year longitudinal study. New Zealand Journal of Educational Studies 47 (1), 123. [Google Scholar]
- Janssen I. & Leblanc A.G. (2010) Systematic review of the health benefits of physical activity and fitness in school‐aged children and youth. International Journal of Behavioral Nutrition and Physical Activity 7, 40 DOI: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P. & Adolphs R. (2012) The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences 16 (11), 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.E., Ko I.G., Kim B.K., Shin M.S., Cho S., Kim C.J. et al. (2010) Treadmill exercise prevents aging‐induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Experimental Gerontology 45 (5), 357–365. DOI: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Laus M.F., Vales L.D.M.F., Costa T.M.B. & Almeida S.S. (2011) Early postnatal protein‐calorie malnutrition and cognition: a review of human and animal studies. International Journal of Environmental Research and Public Health 8 (2), 590–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur M., St‐Cry Tribble D. & Desrosiers J. (2009) Meaning of quality of life for older adults: importance of human functioning components. Archives of Gerontology and Geriatrics 49 (2), e91–e100. [DOI] [PubMed] [Google Scholar]
- Lewandowski G.W. Jr., Mattingly B.A. & Pedreiro A. (2014) Under pressure: the effects of stress on positive and negative relationship behaviors. Journal of Social Psychology 154 (5), 463–473. DOI: 10.1080/00224545.2014.933162. [DOI] [PubMed] [Google Scholar]
- Liu J. (2011) Early health risk factors for violence: conceptualization, evidence, and implications. Aggression and Violent Behavior 16 (1), 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. & Raine A. (2006) The effect of childhood malnutrition on externalizing behavior. Current Opinion in Pediatrics 18 (5), 565–570. DOI: 10.1097/01.mop.0000245360.13949.91. [DOI] [PubMed] [Google Scholar]
- Liu J. & Raine A. (2011) Malnutrition and externalizing behavior In: Lifetime Nutritional Influences on Cognition, Behaviour and Psychiatric Illness (ed. Benton D.), pp 301–322. Woodhead Publishing: Cambridge. [Google Scholar]
- Liu J., Raine A., Venables P.H., Dalais C. & Mednick S.A. (2003) Malnutrition at age 3 years and lower cognitive ability at age 11 years: independence from psychosocial adversity. Archives of Pediatrics & Adolescent Medicine 157 (6), 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Raine A., Venables P.H. & Mednick S.A. (2004) Malnutrition at age 3 years and externalizing behavior problems at ages 8, 11, and 17 years. American Journal of Psychiatry 161 (11), 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Raine A., Wuerker A., Venables P.H. & Mednick S. (2009) The association of birth complications and externalizing behavior in early adolescents: direct and mediating effects. Journal of Research on Adolescence 19 (1), 93–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal M.F. (1964) Social isolation and mental illness in old age. American Sociological Review 29 (1), 54–70. [Google Scholar]
- Lozoff B. (2000) Perinatal iron deficiency and the developing brain. Pediatric Research 48 (2), 137–139. [DOI] [PubMed] [Google Scholar]
- Lozoff B., Castillo M., Clark K.M., Smith J.B. & Sturza J. (2014) Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. Journal of Nutrition 144 (6), 838–845. DOI: 10.3945/jn.113.182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B., Clark K.M., Jing Y., Armony‐Siven R., Angelilli M.L. & Jacobson S.W. (2008) Dose–response relationships between iron deficiency with or without anemia and infant social–emotional behavior. Journal of Pediatrics 152 (5), 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B., Corapci F., Burden M.J., Kaciroti N., Angulo‐Barroso R., Sazawal S. et al. (2007) Preschool‐aged children with iron deficiency anemia show altered affect and behavior. Journal of Nutrition 137 (3), 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B., Klein N.K., Nelson E.C., McClish D.K., Manuel M. & Chacon M.E. (1998) Behavior of infants with iron‐deficiency anemia. Child Development 69 (1), 24–36. [PubMed] [Google Scholar]
- Malina R.M. (2001) Physical activity and fitness: pathways from childhood to adulthood. American Journal of Human Biology 13 (2), 162–172. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E. (1990) Juvenile delinquency and attention deficit disorder: boys' developmental trajectories from age 3 to age 15. Child Development 61 (3), 893–910. [DOI] [PubMed] [Google Scholar]
- Pang T.Y. & Hannan A.J. (2013) Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology 64, 515–528. DOI: 10.1016/j.neuropharm.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Plaven‐Sigray P., Gustavsson P., Farde L., Borg J., Stenkrona P., Nyberg L. et al. (2014) Dopamine D1 receptor availability is related to social behavior: a positron emission tomography study. NeuroImage 102 (Pt 2), 590–595. DOI: 10.1016/j.neuroimage.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Pollitt E., Saco‐Pollitt C., Jahari A., Husaini M.A. & Huang J. (2000) Effects of an energy and micronutrient supplement on mental development and behavior under natural conditions in undernourished children in Indonesia. European Journal of Clinical Nutrition 54, S80–S90. [DOI] [PubMed] [Google Scholar]
- Raine A., Liu J., Venables P.H., Mednick S.A. & Dalais C. (2010) Cohort profile: the Mauritius child health project. International Journal of Epidemiology 39 (6), 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Mellingen K., Liu J., Venables P. & Mednick S.A. (2003) Effects of environmental enrichment at 3–5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. American Journal of Psychiatry 160 (9), 1627–1635. [DOI] [PubMed] [Google Scholar]
- Raine A., Portnoy J., Liu J., Mahoomed T. & Hibbeln J.R. (2015) Reduction in behavior problems with omega‐3 supplementation in children aged 8–16 years: a randomized, double‐blind, placebo‐controlled, stratified, parallel‐group trial. Journal of Child Psychology and Psychiatry 56 (5), 509–520. DOI: 10.1111/jcpp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Reynolds C., Venables P.H. & Mednick S.A. (2002a) Stimulation seeking and intelligence: a prospective longitudinal study. Journal of Personality and Social Psychology 82 (4), 663–674. [PubMed] [Google Scholar]
- Raine A., Reynolds C., Venables P.H., Mednick S.A. & Farrington D.P. (1998) Fearlessness, stimulation‐seeking, and large body size at age 3 years as early predispositions to childhood aggression at age 11 years. Archives of General Psychiatry 55 (8), 745–751. [DOI] [PubMed] [Google Scholar]
- Raine A., Yaralian P.S., Reynolds C., Venables P.H. & Mednick S. (2002b) Spatial but not verbal cognitive deficits at age 3 years in persistently antisocial individuals. Development and Psychopathology 14 (1), 25–44. [DOI] [PubMed] [Google Scholar]
- Rapp A.M., Wild B., Erb M., Rodden F.A., Ruch W. & Grodd W. (2008) Trait cheerfulness modulates bold response in lateral cortical but not limbic brain areas: a pilot fMRI study. Neuroscience Letters 445 (3), 242–245. [DOI] [PubMed] [Google Scholar]
- Rutter M. (1997) Family area and school influences in the genesis of conduct disorders In: Aggression and Anti‐Social Behavior in Childhood and Adolescence (ed. Conner D.F.), pp 95–114. Pergamon Press: Oxford, England. [PubMed] [Google Scholar]
- Segrin C. & Taylor M. (2007) Positive interpersonal relationships mediate the association between social skills and psychological well‐being. Personality and Individual Differences 43 (4), 637–646. DOI: 10.1016/j.paid.2007.01.017. [DOI] [Google Scholar]
- Sigman M., Neumann C., Baksh M., Bwibo N. & McDonald M.A. (1989) Relationship between nutrition and development in Kenyan toddlers. Journal of Pediatrics 115 (3), 357–364. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Blumenthal J.A., Hoffman B.M., Cooper H., Strauman T.A., Welsh‐Bohmer K. et al. (2010) Aerobic exercise and neurocognitive performance: a meta‐analytic review of randomized controlled trials. Psychosomatic Medicine 72 (3), 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares R.O., Oliveira L.M., Marchini J.S., Antunes‐Rodrigues J., Elias L.L. & Almeida S.S. (2013) Effects of early protein malnutrition and environmental stimulation on behavioral and biochemical parameters in rats submitted to the elevated plus‐maze test. Nutritional Neuroscience 16 (3), 104–112. [DOI] [PubMed] [Google Scholar]
- Soares R.O., Rorato R.C., Padovan D., Lachat J.J., Antunes‐Rodrigues J., Elias L.L. et al. (2015) Environmental enrichment reverses reduction in glucocorticoid receptor expression in the hippocampus of and improves behavioral responses of anxiety in early malnourished rats. Brain Research 1600, 32–41. DOI: 10.1016/j.brainres.2014.12.047. [DOI] [PubMed] [Google Scholar]
- Spence J.D., Barnett P.A., Linden W., Ramsden V. & Taenzer P. (1999) Lifestyle modifications to prevent and control hypertension: recommendations on stress management. CMAJ 160 (9 Suppl), S46–S50. [PMC free article] [PubMed] [Google Scholar]
- Stanton R., Happell B. & Reaburn P. (2014) The mental health benefits of regular physical activity, and its role in preventing future depressive illness. Nursing: Research & Reviews 445, 53. [Google Scholar]
- Stathopoulou G., Powers M.B., Berry A.C., Smits J.A.J. & Otto M.W. (2006) Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology 13 (2), 179–193. [Google Scholar]
- Swayze V.W., Andersen A.E., Andreasen N.C., Arndt S., Sato Y. & Ziebell S. (2003) Brain tissue volume segmentation in patients with anorexia nervosa before and after weight normalization. International Journal of Eating Disorders 33 (1), 33–44. [DOI] [PubMed] [Google Scholar]
- Taylor C.B., Sallis J.F. & Needle R. (1985) The relation of physical activity and exercise to mental health. Public Health Reports 100 (2), 195–202. [PMC free article] [PubMed] [Google Scholar]
- Thoits P.A. (2011) Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior 52 (2), 145–161. [DOI] [PubMed] [Google Scholar]
- Tremblay R.E. (2012) A 2‐year early childhood psychosocial stimulation programme improves cognitive outcomes and decreases violent behaviour at 22 years for children with growth retardation. Evidence Based Medicine 17 (2), 49–50. [DOI] [PubMed] [Google Scholar]
- Turner L., Linden W., van der Wal R. & Schamberger W. (1995) Stress management for patients with heart disease: a pilot study. Heart & Lung 24 (2), 145–153. [DOI] [PubMed] [Google Scholar]
- Umberson D. (2010) Social relationships and health behavior across life course. Annual Review of Sociology 36, 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas‐Moberg K. (1998) Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology 23 (8), 819–835. [DOI] [PubMed] [Google Scholar]
- van Kerkhof L.W., Trezza V., Mulder T., Gao P., Voorn P. & Vanderschuren L.J. (2014) Cellular activation in limbic brain systems during social play behaviour in rats. Brain Structure and Function 219 (4), 1181–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G. & Gage F.H. (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience 2 (3), 266–270. [DOI] [PubMed] [Google Scholar]
- Waber D.P., Bryce C.P., Fitzmaurice G.M., Zichlin M.L., McGaughy J., Girard J.M. et al. (2014) Neuropsychological outcomes at midlife following moderate to severe malnutrition in infancy. Neuropsychology 28 (4), 530–540. DOI: 10.1037/neu0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.P., Chang S.M., Vera‐Hernández M. & Grantham‐McGregor S. (2011) Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics 127 (5), 849–857. [DOI] [PubMed] [Google Scholar]
- Weber K., Rockstroh B., Borgelt J., Awiszus B., Popov T., Hoffmann K. et al. (2008) Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry 8 (63), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatson T.S. & Smart J.L. (1978) Social behavior of rats following pre‐ and early postnatal undernutrition. Physiology & Behavior 20 (6), 749–753. [DOI] [PubMed] [Google Scholar]
- WMA . WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2012, from http://www.wma.net/en/30publications/10policies/b3/ [PubMed]
- Wright C.I., Williams D., Feczko E., Barret L.F., Dickerson B.C., Schwartz C.E. et al. (2006) Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex 16 (12), 1809–1819. [DOI] [PubMed] [Google Scholar]
- Zhang W.Q., Smolik C.M., Barba‐Escobedo P.A., Gamez M., Sanchez J.J., Javors M.A. et al. (2015) Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology 90, 1–8. DOI: 10.1016/j.neuropharm.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R.R., Strobel D.A., Steere P. & Geist C.R. (1975) Behavior and malnutrition in the rhesus monkey In: Primate Behavior: Developments in Field and Laboratory Research (ed. Rosenblum L.), Vol. 4, pp 241–306. Academic Press: New York. [Google Scholar]


