Abstract
OBJECTIVE
The purpose of the present study is to evaluate Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, tumor attenuation criteria, Choi criteria, and European Organization for Research and Treatment of Cancer (EORTC) PET criteria as measures of response and subsequent predictors of liver progression-free survival (PFS) after radioembolization (RE) of colorectal liver metastases (CLM). The study also assesses interobserver variability for measuring tumor attenuation using a single 2D ROI on a simple PACS workstation.
MATERIALS AND METHODS
We performed a retrospective review of the clinical RE database at our institution, to identify patients treated in the salvage setting for CLM between December 2009 and March 2013. Response was evaluated on FDG PET scans, with the use of EORTC PET criteria, and on portal venous phase CT scans, with the use of RECIST 1.1, tumor attenuation criteria, and Choi criteria. Two independent blinded observers measured tumor attenuation using a single 2D ROI. The intraclass correlation coefficient (ICC) for interobserver variability was assessed. Kaplan-Meier methodology was used to calculate liver PFS, and the log-rank test was used to assess the response criteria as predictors of liver PFS.
RESULTS
A total of 25 patients with 46 target tumors were enrolled in the study. The ICC was 0.95 at baseline and 0.98 at response evaluation. Among the 25 patients, more responders (i.e., partial response) were identified on the basis of EORTC PET criteria (n = 14), Choi criteria (n = 15), and tumor attenuation criteria (n = 13) than on the basis of RECIST 1.1 (n = 2). The median liver PFS was 3.0 months (95% CI, 2.1–4.0 months). Response identified on the basis of EORTC PET criteria (p < 0.001), Choi criteria (p < 0.001), or tumor attenuation criteria (p = 0.01) predicted liver PFS; however, response identified by RECIST 1.1 did not (p = 0.1).
CONCLUSION
RECIST 1.1 has poor sensitivity for detecting metabolic responses classified by EORTC PET criteria. EORTC PET criteria, Choi criteria, and tumor attenuation criteria appear to be equally reliable surrogate imaging biomarkers of liver PFS after RE in patients with CLM.
Keywords: Choi criteria, colorectal liver metastases, radioembolization, tumor attenuation
Approximately 45% of patients with colorectal cancer develop liver metastases [1]. These metastases are either synchronous (evident at the time of first diagnosis) in 25% of patients or metachronous (developing at some point after primary resection) in 20% of patients [1, 2]. The only potentially curative treatment is liver resection, which has been associated with 5-year survival rates of 20–58% [3–5]. However, only approximately 15% of patients are eligible to undergo resection [1].
Radioembolization (RE) is a locoregional therapy that can provide local control of liver disease in patients who cannot be treated by surgery or ablation [6, 7]. RE involves the delivery of radioactive 90Y-loaded microspheres directly into the hepatic arteries that feed the tumor(s) [8]. In clinical practice, RE is predominantly used in the salvage setting to extend liver progression-free survival (PFS) and overall survival in this patient population [9–14].
The Response Evaluation Criteria in Solid Tumors (RECIST) guideline was originally developed for assessment of cytotoxic therapies [15]. However, RECIST may be suboptimal in the evaluation of molecular targeting agents. Evidence of this has emerged from studies of several primary tumors in which improvements in overall survival and liver PFS were observed after molecular targeting agents therapy, despite poor response rates by RECIST [16–23]. Such improvements could be explained by the dominant cytostatic and necrotic effect of targeted therapies that may not result in tumor shrinkage [23, 24]. Changes in tumor metabolic activity on FDG PET scans have been proven to be a useful tool in the evaluation of response and the prediction of the oncologic endpoints for such therapies [18, 25]. The same observations also apply to liver locoregional therapy using RE, for which RECIST has underestimated the metabolic tumor response documented on FDG PET [26–28]. Of more importance, in some studies of patients with CLM, the metabolic response noted on FDG PET predicted liver PFS and overall survival, whereas RECIST did not [29–31].
However, PET still is not widely available and is expensive when compared with CT [32]. Researchers have therefore evaluated the use of CT morphologic criteria, which may offer higher sensitivity in assessing the response to targeted and locoregional therapies [18, 33, 34]. Notable examples of such criteria include the modified RECIST (mRECIST) guideline, which has been used to assess the response of hepatocellular carcinoma [34], and Choi tumor attenuation criteria, which originally were used to evaluate the response of gastrointestinal stromal tumors (GISTs) [18]. Both criteria proved to be significant predictors of overall survival and liver PFS [17, 18, 20, 35]. According to mRECIST, an arterial phase CT scan is used to measure the diameter of the enhancing (i.e., viable) part of the tumor instead of the maximum tumor diameter [34]. Thus, mRECIST would not be applicable to tumors that typically appear hypovascular on CT, such as CLM. On the other hand, the Choi criteria use a combination of a change in tumor attenuation (measured in Hounsfield units) and a change in maximum tumor diameter to assess response [18]. Thus, for evaluation of response in patients with hypovascular CLM, measurement of changes in tumor attenuation and the use of Choi criteria may be more applicable.
The goal of the present study is to evaluate RECIST version 1.1 (RECIST 1.1), changes in tumor attenuation, Choi criteria, and European Organization for Research and Treatment of Cancer (EORTC) PET criteria as measures of response and as subsequent predictors of liver PFS after RE for CLM. We also report the interobserver variability for measuring tumor attenuation with the use of a single 2D ROI on a simple PACS workstation.
Materials and Methods
Study Population
After the institutional review board at Memorial Sloan Kettering Cancer Center approved the present study, we conducted a retrospective review of our HIPAA-compliant RE clinical database.
We identified 53 patients with unresectable CLM who were treated in the salvage setting with the use of 90Y-loaded resin microspheres (SIR-Spheres, Sirtex Medical) from September 2009 to March 2013. Patients were included in the study if both FDG PET and CT scans were available both at baseline and at the first follow-up examination, and if FDG PET and CT scans were obtained within 3 weeks of each other at both baseline and the first follow-up examinations. A total of 29 patients were eligible for inclusion in the study.
Of the 29 patients eligible for inclusion, four were excluded from the study for the following reasons: the largest diameter of all measurable tumors was smaller than 1.5 cm (n = 2), all tumors were grossly calcified (n = 1), and all tumors were isometabolic to the liver (n = 1). These exclusions were made to avoid the partial volume effect that would accompany the measurement of tumor attenuation for small tumors (largest diameter, < 1.5 cm) and because gross tumor calcification would compromise objective detection of a change in tumor attenuation.
The final study population consisted of 25 patients with 46 target tumors. The response to RE was evaluated on the basis of RECIST 1.1, changes in tumor attenuation, Choi criteria, and EORTC PET criteria (Table 1).
TABLE 1.
Criteria Used in the Evaluation of Response to Radioembolization in 25 Patients With Colorectal Liver Metastases
| Response Category | EORTC PET Criteria | RECIST 1.1 Guidelines | Tumor Attenuation Criteria | Choi Criteria |
|---|---|---|---|---|
|
| ||||
| Partial response | ≥ 25% decrease in SUVmax | ≥ 30% decrease in sum of longest tumor diameters | ≥ 15% decrease in tumor attenuation (HU) | ≥ 15% decrease in tumor attenuation (HU) or ≥ 10% decrease in tumor size |
| Stable disease | No partial response or progressive disease | No partial response or progressive disease | Does not meet criteria for partial response | No partial response or progressive disease |
| Progressive disease | > 25% increase in SUVmax or development of new tumors | > 20% increase in sum of longest tumor diameters or development of new tumors | Does not meet criteria for partial response | Has ≥ 10% increase in tumor size but does not meet criteria for partial response as measured by change in tumor attenuation or development of new tumors |
Note—EORTC = European Organization for Research and Treatment of Cancer, RECIST 1.1 = Response Evaluation Criteria in Solid Tumors, HU = Hounsfield units, SUVmax = maximum standardized uptake value.
Contrast-Enhanced CT
Either a 16- or a 64-MDCT scanner (both Light-Speed VCT, GE Healthcare) was used. Portal venous phase CT was performed after IV administration of 150 mL of iodinated contrast material (iohexol, Omnipaque 300, GE Healthcare). The median rate of contrast material delivery was 3.5 mL/s (range, 1–4 mL/s) for the baseline scans and 3.5 mL/s (range, 2–4 mL/s) for the response evaluation scans. The CT parameters were as follows: pitch/table speed, 0.984–1.375/39.37–27.50 mm; automA, 220–380 mA; noise index, 12.5–14; rotation time, 0.7–0.8 ms; and scan delay, 80–85 seconds. Axial slices reconstructed at 5-mm intervals were used for analysis.
Measuring the Anatomic Criteria
The first reviewer chose the target tumors on the portal venous phase of the CT scans obtained at baseline, in accordance with RECIST 1.1. For the purposes of the present study, only tumors for which the largest diameter was 1.5 cm or more (n = 46) were included. The first reviewer then proceeded with measurements of the maximum tumor diameter for response assessment by RECIST 1.1; the series and image numbers used were recorded. The reviewer was blinded to the response identified using EORTC PET criteria.
Two independent reviewers then measured the attenuation of the target tumors with the use of the same exact series and image numbers recorded by the first observer for the portal venous phase CT scan. This was done using a single 2D ROI that was drawn to encompass as much of the tumor as possible without encroaching on the margin of the normal liver, to minimize partial volume effects. The two independent reviewers were blinded to the responses identified using both RECIST 1.1 and EORTC PET criteria. Interobserver variability was assessed for the measurements of tumor attenuation on the baseline and response evaluation scans.
FDG PET/CT
For all patients, imaging was performed using a dedicated PET/CT scanner (D600/D690/ D710, GE Healthcare). Scanning was performed approximately 60 minutes after IV injection of 10–12 mCi (370–444 MBq) of FDG. A low-dose (80-mA) CT scan of the area from the head to the thighs was performed and was followed by a PET scan of the area from the head to the thighs. The images were reconstructed using iterative reconstruction and attenuation correction. The maximum standardized uptake value (SUVmax) normalized to body weight was measured with the use of a 3D ROI for the same target tumors chosen on CT by at least one experienced board-certified nuclear medicine physician.
Date of Liver Tumor Progression
Follow-up imaging was performed 4–8 weeks after RE, to evaluate response. This first follow-up scan was regarded as the new baseline to be used for future comparisons. All patients subsequently underwent both CT and FDG PET imaging every 2 months to detect the first sign of liver tumor progression on either CT or PET. Both RECIST 1.1 and EORTC PET criteria were used to document the date of disease progression in the treated liver. On the first follow-up examinations, observation of discrepant findings between the two modalities were dealt with as follows: first, if stable disease was noted on the basis of EORTC PET criteria but progressive disease was noted on the basis of RECIST 1.1, the finding was documented as progressive disease; second, if progressive disease was noted on the basis of EORTC PET criteria but stable disease was noted on the basis of RECIST 1.1, the finding was documented as progressive disease; and third, if a partial response was noted on the basis of EORTC PET criteria but progressive disease was noted on the basis of RECIST 1.1, the finding was documented as a response. The rationale for defining the first two examples of discrepant findings as progressive disease was that liver PFS could be recorded on the basis of the earliest imaging evidence of progression (anatomic or metabolic) without being restricted to a specific modality. The rationale for defining the third example of discrepant findings as a response was based on the findings of previously published studies, which revealed that FDG PET had a higher sensitivity for detecting response [28, 29, 36, 37] and that a paradoxic increase in tumor size resulting from changes noted after RE (e.g., edema, cystic degeneration, or both) can occur despite a reduction in tumor metabolic activity [27]. Liver PFS was defined as the interval from the date of RE to the date of evidence of radiologic progression (as previously defined).
Statistical Analysis
The intraclass correlation coefficient (ICC) for interobserver variability was assessed using ANOVA. Information on each response metric was displayed as the mean (± SD) value. The nonparametric Wilcoxon signed rank test and Mann-Whitney U test were used to detect significant changes in metrics after treatment. The Spearman rank correlation test was used to assess the statistical significance of and the degree of correlation between the percentage changes of maximum tumor diameter, tumor attenuation, and metabolic tumor activity (SUVmax). The kappa statistic was used to assess diagnostic agreement among the response categories across the criteria used. The McNemar test was used to compare the sensitivity of Choi criteria or tumor attenuation criteria versus RECIST 1.1 in detecting metabolic response. Liver PFS was calculated using Kaplan-Meier methodology from the date of RE until the date of liver progression. The log-rank test was used to detect whether a statistically significant difference in liver PFS existed between responders (i.e., patients with a partial response) and nonresponders (i.e., patients who had stable disease or progressive disease), for each of the response criteria used. A Cox regression model was used to report hazard ratios (HRs). Analysis was performed using statistical software (Stata, version 12, StataCorp).
Results
Study Population
We evaluated 25 patients (18 men and seven women) who received RE for chemorefractory CLM in the salvage setting. These patients were heavily pretreated. Of the 25 patients, 10 (40%) were treated for recurrences after hepatectomy, 12 (48%) received prior hepatic arterial infusion chemotherapy, and seven (28%) more than three previous chemotherapy regimens (i.e., lines). In addition, 19 patients (76%) had evidence of extrahepatic disease at the time of RE. Eighteen of the 25 patients (72%) were treated in one session, whereas seven patients (28%) received bilobar treatments in two sessions separated by a median interval of 5.7 weeks (range, 4.9–8 weeks), with response assessment performed after the second session (i.e., at the time of treatment completion). The median time between the baseline scans and RE was 4.1 weeks (range, 1.1–6.9 weeks), whereas the median time between RE and the response evaluation scans was 7.0 weeks (range, 3.4– 9.9 weeks). The demographic and clinical characteristics of the patients at baseline are summarized in Table 2.
TABLE 2.
Demographic and Clinical Characteristics of the Study Population(n = 25) at Baseline
| Characteristic | Value |
|---|---|
|
| |
| Sex | |
| Male | 18 (72) |
| Female | 7 (28) |
| Age (y), median (range) | 56 (24–86) |
| Prior liver resection | |
| Yes | 10 (40) |
| No | 15 (60) |
| Prior HAIC | |
| Yes | 12 (48) |
| No | 13 (52) |
| Prior lines of systemic chemotherapy | |
| 2–3 | 18 (72) |
| > 3 | 7 (28) |
| Prior RE chemotherapeutic or biologic agents | |
| Capecitabine | 7 (28) |
| 5-Fluorouracil/leucovorin | 22 (88) |
| Oxaliplatin | 25 (100) |
| Irinotecan | 22 (88) |
| Bevacizumab | 22 (88) |
| Cetuximab or panitumumab | 13 (52) |
| Received post-RE chemotherapeutic or biologic agents | |
| Capecitabine | 2 (8) |
| 5-Fluorouracil/leucovorin | 10 (40) |
| Oxaliplatin | 6 (24) |
| Irinotecan | 12 (48) |
| Bevacizumab | 9 (36) |
| Cetuximab or panitumumab | 9 (36) |
| Extrahepatic disease | |
| Yes | 19 (76) |
| No | 6 (24) |
| Extent of liver replacement by tumor | |
| < 25% | 23 (92) |
| ≥ 25% | 2 (8) |
| No. of treatment sessions | |
| 1 | 18 (72) |
| 2 | 7 (28) |
| No. of target tumors per patient | |
| 1 | 12 (48) |
| 2 | 6 (24) |
| 3 | 6 (24) |
| 4 | 1 (4) |
| Previously received treatments for liver metastasis | |
| Resection, HAIC, and systemic chemotherapy | 8 (32) |
| Resection and systemic chemotherapy | 2 (8) |
| HAIC and systemic chemotherapy | 4 (16) |
| Systemic chemotherapy only | 11 (44) |
Note—Except for patient age, data are number (%) of patients. HAIC = hepatic arterial infusion chemotherapy, RE = radioembolization.
Changes in Response Parameters After Treatment
A high ICC was noted between the two observers for measurements of tumor attenuation, both at baseline (0.95) and after RE (0.98). Thus, a mean value of the measurements of both observers was calculated and used in further analysis.
A statistically significant decrease in tumor attenuation occurred after RE, with a mean attenuation of 68.7 ± 17.4 HU noted at baseline, compared with a mean attenuation of 58.3 ± 18.9 HU noted after RE (p < 0.001). The mean change in attenuation per patient was therefore −10.7 ± 12.5 HU after RE. For responders, the mean percentage change in tumor attenuation was −27.5% ± 8.8%, compared with 0% ± 9.0% for nonresponders (p < 0.001). At baseline, no statistically significant difference in mean tumor attenuation was noted between responders and nonresponders (p = 0.8). There was no statistically significant difference in the sum of the longest tumor diameters noted after RE; the sum of the longest diameters was 8.0 ± 5.2 cm at baseline versus 8.5 ± 6.0 cm after RE (p = 0.3). At baseline, there was no difference in the sum of the longest tumor diameters for responders versus nonresponders (p = 0.2).
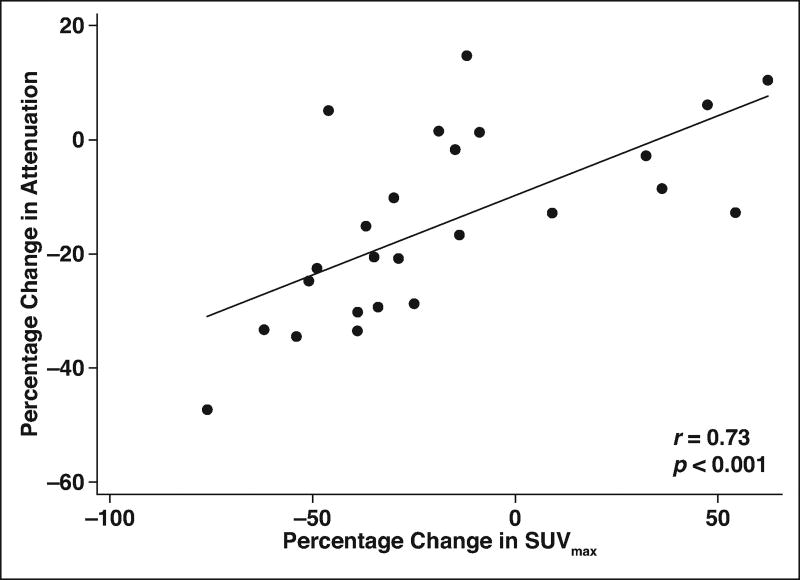
A statistically significant decrease in the SUVmax was noted after RE (mean SUVmax, 11.0 ± 6.4 at baseline vs 9.0 ± 8.3 after RE; p = 0.02). The mean percentage change in the SUVmax of responders was −43.3% ± 14.1%, compared with 15.5% ± 31.2% for nonresponders (p < 0.001). No statistically significant difference in the SUVmax at baseline was observed when responders were compared with nonresponders (p = 0.4). A statistically significant moderate-to-high correlation existed between the percentage change in tumor attenuation and the SUVmax (r = 0.73; p < 0.001) (Fig. 1), but no such correlation existed between the percentage change in the sum of the longest tumor diameters and the SUVmax (r = 0.1; p = 0.6).
Fig. 1.
Scatterplot showing correlation between percentage change in tumor attenuation (measured in Hounsfield units) and percentage change in metabolic activity (measured as maximum standardized uptake value [SUVmax]). Line denotes mean.
Response Categories, as Defined by Different Criteria
Table 3 shows the interagreement of response categories as defined by EORTC PET criteria, Choi criteria, and RECIST 1.1. A statistically significant moderate diagnostic agreement existed between Choi criteria and EORTC PET criteria (κ = 0.49; p < 0.001). Poor agreement was noted between EORTC PET criteria and RECIST 1.1 (κ = 0.11), but it did not reach statistical significance (p = 0.1). Choi criteria correctly classified the response in 13 of 14 patients who, on the basis of EORTC PET criteria, had a partial response, whereas RECIST 1.1 correctly identified only two of these 14 patients.
TABLE 3.
Agreement of Categories of Tumor Response for 25 Patients, as Assessed Across Three Sets of Response Criteria
| Criteria, Response Category |
EORTC PET Criteria | ||
|---|---|---|---|
|
| |||
| Partial Response | Stable Disease | Progressive Disease | |
|
| |||
| RECIST 1.1 | |||
| Partial response | 2 | 0 | 0 |
| Stable disease | 9 | 1 | 3 |
| Progressive disease | 3 | 2 | 5 |
| Choi criteria | |||
| Partial response | 13 | 1 | 1 |
| Stable disease | 0 | 0 | 2 |
| Progressive disease | 1 | 2 | 5 |
Note—Data are number of patients. EORTC = European Organization for Research and Treatment of Cancer, RECIST 1.1 = Response Evaluation Criteria in Solid Tumors version 1.1.
Table 4 presents data on agreement across all criteria for classifying patients as responders. Statistically significant substantial agreement existed between EORTC PET criteria and both Choi criteria (κ = 0.75; p < 0.001) and tumor attenuation criteria (κ = 0.76; p < 0.001) in detecting responders. The sensitivity and specificity of tumor attenuation criteria in detecting response on the basis of EORTC PET criteria were 86% (95% CI, 57–98%) and 91% (95% CI, 59– 100%), respectively. With the use of Choi criteria, the sensitivity increased by one patient to 93% (95% CI, 66–100%), whereas the specificity decreased by one patient to 82% (95% CI, 48–98%). Although RECIST 1.1 identified only two of 14 responders identified by EORTC PET criteria, for a sensitivity of 14% (95% CI, 2–43%), its specificity was 100% (95% CI, 72–100%), with 11 of 11 patients identified as nonresponders. In the detection of metabolic tumor response, tumor attenuation criteria (p = 0.006) and Choi criteria (p = 0 .001) had a statistically significantly higher sensitivity than did RECIST 1.1.
TABLE 4.
Agreement in Classifying 25 Patients as Responders or Nonresponders Across Four Sets of Response Criteria
| Criteria, Patient Classification | Patient Classification According to EORTC PET Criteria | |
|---|---|---|
|
| ||
| Responders | Nonresponders | |
|
| ||
| RECIST 1.1 | ||
| Responders | 2 | 0 |
| Nonresponders | 12 | 11 |
| Tumor attenuation criteria | ||
| Responders | 12 | 1 |
| Nonresponders | 2 | 10 |
| Choi criteria | ||
| Responders | 13 | 2 |
| Nonresponders | 1 | 9 |
Note—Data are number of patients. Responders were defined as patients with a partial response to treatment, whereas nonresponders were defined as patients who had stable disease or progressive disease. EORTC = European Organization for Research and Treatment of Cancer, RECIST 1.1 = Response Evaluation Criteria in Solid Tumors version 1.1.
Table 5 shows individual patient response, as defined in accordance with each of the four sets of criteria used in the present study. Figure 2 presents PET and contrast-enhanced CT images of the tumor response in a patient, as evidenced by decreases in both SUVmax and tumor attenuation but not by RECIST 1.1.
TABLE 5.
Individual Patient Response According to Each of the Four Criteria Evaluated
| Patient | EORTC PET Criteria | Tumor Attenuation Criteria |
Choi Criteria | RECIST 1.1 |
|---|---|---|---|---|
|
| ||||
| 1 | Partial response | Responder | Partial response | Stable disease |
| 2 | Partial response | Responder | Partial response | Stable disease |
| 3 | Partial response | Responder | Partial response | Stable disease |
| 4 | Partial response | Responder | Partial response | Stable disease |
| 5 | Partial response | Responder | Partial response | Stable disease |
| 6 | Progressive disease | Nonresponder | Progressive disease | Progressive disease |
| 7 | Progressive disease | Nonresponder | Progressive disease | Progressive disease |
| 8 | Progressive disease | Nonresponder | Progressive disease | Progressive disease |
| 9 | Stable disease | Nonresponder | Progressive disease | Progressive disease |
| 10 | Partial response | Responder | Partial response | Stable disease |
| 11 | Partial response | Nonresponder | Partial response | Partial response |
| 12 | Partial response | Responder | Partial response | Partial response |
| 13 | Progressive disease | Responder | Partial response | Stable disease |
| 14 | Partial response | Responder | Partial response | Stable disease |
| 15 | Progressive disease | Nonresponder | Stable disease | Stable disease |
| 16 | Partial response | Responder | Partial response | Progressive disease |
| 17 | Partial response | Responder | Partial response | Progressive disease |
| 18 | Progressive disease | Nonresponder | Stable disease | Stable disease |
| 19 | Progressive disease | Nonresponder | Progressive disease | Progressive disease |
| 20 | Progressive disease | Nonresponder | Progressive disease | Progressive disease |
| 21 | Partial response | Responder | Partial response | Stable disease |
| 22 | Progressive disease | Nonresponder | Progressive disease | Progressive disease |
| 23 | Partial response | Nonresponder | Progressive disease | Progressive disease |
| 24 | Partial response | Responder | Partial response | Stable disease |
| 25 | Progressive disease | Nonresponder | Partial response | Stable disease |
Note—Responders were defined as patients with a partial response to treatment, whereas nonresponders were defined as patients who had stable disease or progressive disease. EORTC = European Organization for Research and Treatment of Cancer, RECIST 1.1 = Response Evaluation Criteria in Solid Tumors version 1.1.
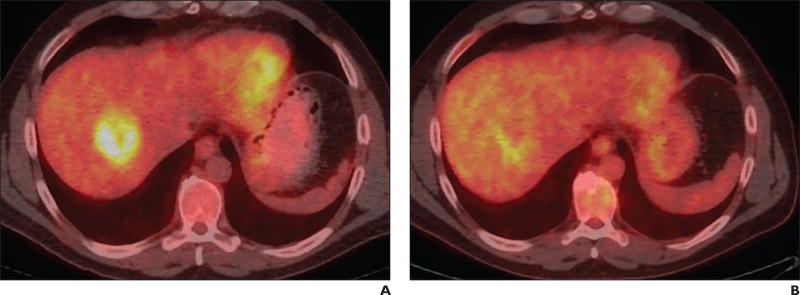
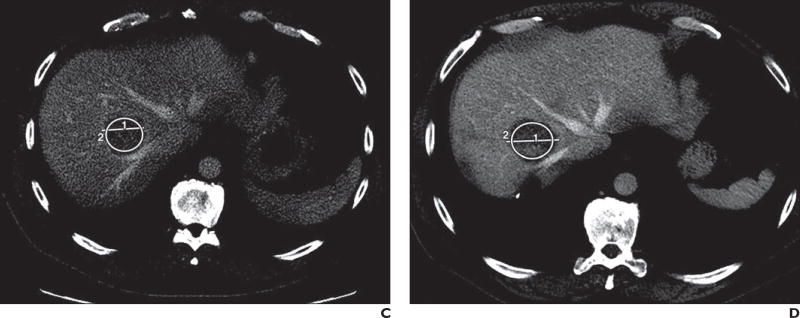
Fig. 2.
52-year-old man with colorectal liver metastases with tumor in right lobe.
A, PET/CT scan shows tumor before radioembolization (RE). Maximum standardized uptake value (SUVmax) is 6.
B, PET/CT scan shows tumor exhibiting partial response to RE, with SUVmax decreasing to 3.9 (for decrease of 35%).
C, Contrast-enhanced CT image shows tumor before RE. Longest tumor diameter is 4.3 cm, tumor area is 840.6 mm2, and mean tumor attenuation is 68.1 ± 13.4 HU. Circle labeled “2” denotes ROI; line labeled “1” denotes maximum diameter.
D, Contrast-enhanced CT image shows tumor after RE. Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 falsely classified this tumor as stable disease on basis of misleading increase in longest tumor diameter to 4.7 cm (for increase of 6%). However, tumor attenuation criteria correctly classified tumor as showing response, which is evident by decrease in attenuation to 55.3 HU (for decrease of 19%). Tumor area is 864.1 mm2 and mean tumor attenuation is 55.3 ± 15.7 HU. Circle labeled “2” denotes ROI; line labeled “1” denotes maximum diameter.
Liver Progression-Free Survival
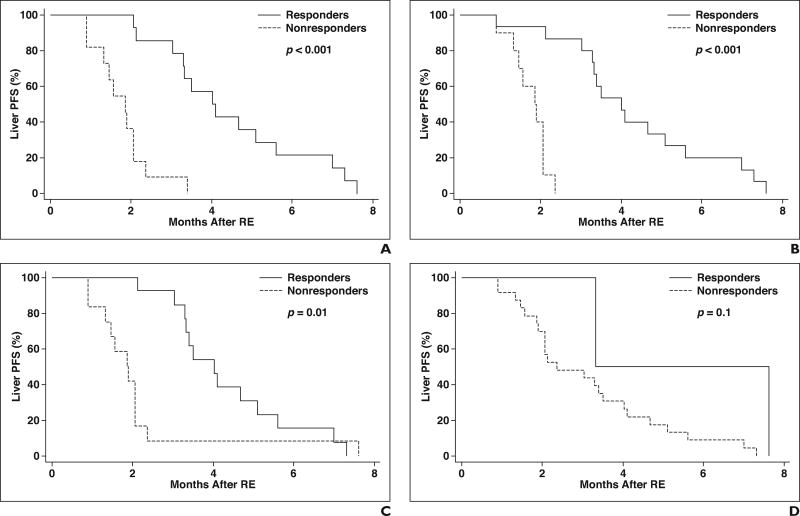
The median liver PFS was 3.0 months (95% CI, 2.1–4.0 months). Among the 25 patients evaluated, the first sign of liver tumor progression was the detection of new tumors in six patients (24%) and the progression of the treated target tumors in 19 patients (76%). Response determined by EORTC PET criteria was a statistically significant predictor of liver PFS (HR, 0.1; 95% CI, 0.03–0.33; p < 0.001), with a median liver PFS of 4.0 months noted for responders and 1.9 months noted for nonresponders (Fig. 3A). Response identified using Choi criteria predicted liver PFS (HR, 0.05; 95% CI, 0.01–0.26; p < 0.001), with a median liver PFS of 4.0 months noted for responders and 1.9 months noted for nonresponders (Fig. 3B). Response identified by tumor attenuation criteria was also statistically significant (HR, 0.35; 95% CI, 0.15– 0.83; p = 0.01), with a median liver PFS of 4.0 months noted for responders and 1.9 months noted for nonresponders (Fig. 3C). Response determined by RECIST 1.1 did not predict liver PFS (HR, 0.22; 95% CI, 0.03–1.7; p = 0.1), with a median liver PFS of 3.3 months noted for responders and 2.4 months noted for nonresponders (Fig. 3D).
Fig. 3.
Liver progression-free survival (PFS) after radioembolization (RE) in patients with colorectal liver metastases, as stratified by response defined on basis of each set of criteria used for evaluation.
A, Kaplan-Meier survival curve shows liver PFS based on European Organization for Research and Treatment of Cancer PET criteria.
B, Kaplan-Meier survival curve shows liver PFS based on Choi criteria.
C, Kaplan-Meier survival curve shows liver PFS based on tumor attenuation criteria.
D, Kaplan-Meier survival curve shows liver PFS based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
Discussion
RECIST has been shown to underestimate response to molecular targeting agents [16–23]. A clear example of this can be observed in a phase 3 randomized study of the addition of bevacizumab to either capecitabine plus oxaliplatin (Xelox, Roche) or a regimen of oxaliplatin plus folinic acid and 5-fluorouracil (FOLFOX-4) in the management of metastatic colorectal cancer [22]. Significant improvement in outcomes was observed in the bevacizumab treatment arm, even though RECIST identified almost identical response rates in both treatment arms [22]. For such therapies, evaluating the changes in tumor attenuation and morphologic findings on contrast-enhanced CT can be a viable alternative to RECIST.
An interesting study evaluated the use of RECIST and devised morphologic response criteria in detecting pathologic response and predicting overall survival for patients with CLM [33]. The patients in this study received a regimen containing bevacizumab before undergoing liver resection [33]. The morphologic criteria were more specific than RECIST in detecting minor pathologic response, and, more importantly, they predicted overall survival, whereas RECIST did not [33]. The pathologic response and the percentage of viable tumor cells were significantly correlated with the morphologic appearance (p < 0.001).
Choi and colleagues devised CT criteria that included both changes in tumor attenuation and maximum tumor diameter in predicting response after treatment of GIST with imatinib [18]. In their study, a decrease of 15% or more in tumor attenuation correctly identified 82% of 33 patients with a metabolic response identified on the basis of a change in the SUVmax. In contrast, RECIST could identify only 17 these 33 patients, resulting in a much lower sensitivity of 52% and clearly underestimating response [18]. An even higher sensitivity (97%) was achieved using either a decrease in tumor attenuation of 15% or more or a decrease in tumor size of 10% or more as a requirement for defining response (i.e., Choi criteria). Choi criteria and the percentage decrease in the SUVmax significantly predicted PFS, whereas RECIST did not [18]. This finding was validated in another study of GIST tumors treated with imatinib. In that study, Choi criteria predicted PFS and disease- specific survival, but RECIST failed to predict any of these endpoints [17].
Measurement of changes in tumor attenuation after the use of molecular targeting therapies has been used for primary tumors other than GIST. For patients with hepatocellular carcinoma treated with sorafenib, Choi criteria were more sensitive than RECIST in detecting response and were a significant predictor of overall survival [20]. Similarly, for patients with metastatic renal cell carcinoma treated with sunitinib, Choi criteria identified more responders than did RECIST at the time of the first evaluation, and they also predicted PFS and overall survival [38]. For patients with CLM, Choi criteria, when compared with RECIST, could significantly classify more patients as responders in both treatment arms (i.e., chemotherapy only [80% vs 31%; p < 0.001] and chemotherapy plus bevacizumab [79% vs 41%; p = 0.003]) [24]. Thus, if RECIST only was used, a significant proportion of patients would have been mistakenly classified as nonresponders. Choi criteria also significantly predicted time to disease progression in both treatment arms [24].
Similar to reports of findings for molecular targeting therapies, studies have shown that, after RE, evaluation of tumor shrinkage in patients with CLM with the use of RECIST seems to underestimate response [28, 29, 36, 37, 39]. These studies have also shown that changes in tumor metabolic activity detect a higher response rate [28, 29, 36, 37, 39]. In one study of CLM treated with RE, the metabolically active tumor volume decreased by 80%, compared with a decrease of only 39% in anatomic tumor volume at 4 weeks [37]. Another study even reported a paradoxically false increase in the size of several tumors (probably attributable to treatment-induced cystic degeneration, edema, or hemorrhage), despite metabolic tumor response [27]. The lower response rates noted when RECIST was used are probably caused by post-RE changes, such as peritumoral edema and necrosis [27, 40]. In a recent study, these changes occurred in as many as 57.3% of patients [40]. In two studies, changes in tumor metabolic activity predicted overall survival and liver PFS after RE, whereas RECIST failed to do so [29, 30]. In addition, on the basis of limited available data, it appears that, for patients with CLM, early metabolic response at 4–6 weeks may be a reliable predictor of overall survival after RE [37, 41, 42]. However, validation of these findings is needed from more studies comparing the predictive ability of metabolic response at several time points. An interesting study has reviewed the value of FDG PET/CT examination for patients with CLM treated with RE [43]. In general, FDG PET/CT has been a valuable tool for evaluating patients with CLM in several settings [32, 44–47].
The present study evaluates the use of EORTC PET criteria [48], RECIST 1.1 [49], tumor attenuation criteria [18], and Choi criteria [18] as measures of response and predictors of subsequent liver PFS in patients with CLM treated with RE. The EORTC PET criteria showed the highest sensitivity in detecting response and significantly predicted liver PFS. We compared the ability of three anatomic criteria to detect the metabolic response. RECIST 1.1 showed a poor sensitivity and a high specificity. In contrast, changes in tumor attenuation showed high sensitivity and high specificity.
When Choi criteria were used as compared with tumor attenuation criteria, the sensitivity increased (by one patient with a 31% decrease in tumor size), whereas the specificity decreased (by one patient with a 14% increase in size). This finding might raise the following question: what is the best threshold of change in tumor size to include in Choi criteria applied to CLM to complement the changes in tumor attenuation? Although the present study is underpowered to answer this question, its results showed that the 10% change in tumor size suggested for GISTs by Choi criteria was accompanied by both a slight increase in sensitivity (n = 1) and a slight decrease in specificity (n = 1). Another viable alternative is to use the thresholds provided by RECIST: a minimum decrease in tumor size of 30% to denote response and a minimum 20% increase in tumor size to denote disease progression. Applying these RECIST parameters to our study population could have provided the gain in sensitivity while avoiding the loss in specificity. Naturally, the answer to this question requires a larger sample size to compare various thresholds of change in tumor size, to arrive at more robust conclusions.
We are aware of two previously published studies that compared EORTC PET criteria, RECIST, and tumor attenuation criteria as measures of patient response after RE of CLM [29, 39]. Tochetto et al. [39] used a 3D ROI to measure changes in tumor attenuation. In their study, a 15% decrease in attenuation resulted in a sensitivity of 93% (14/15 patients) for the prediction of metabolic response on the basis of the SUVmax, whereas RECIST 1.1 had a much lower sensitivity of 40% (6/15 patients). However, these findings were not correlated with liver PFS. Alternatively, Zerizer et al. [29] found that the use of a single 2D ROI to measure changes in tumor attenuation offered no additional value when compared with RECIST; both criteria identified only two of the 15 patients that were responders on the basis of EORTC PET criteria. In their study, Zerizer and colleagues noted that only EORTC PET criteria predicted liver PFS. The results of the present study seem to agree with the findings of Tochetto and colleagues. In addition, in the present study, we found both tumor attenuation criteria and Choi criteria, together with EORTC PET criteria, to be predictors of liver PFS.
We measured tumor attenuation with the use of a single 2D ROI and noted high interobserver agreement (ICC range, 0.95–0.98). In an interesting study, Chalian et al. [50] compared the intraobserver and interobserver variabilities for three methods of measuring tumor attenuation associated with CLM: use of a single 3D ROI, use of the mean of three 2D ROIs, and use of a single 2D ROI. The method using the 3D ROI was significantly more precise than the other methods evaluated, resulting in higher interobserver and intraobserver agreements. This finding is not surprising because all available data points are assessed with 3D measurements, whereas some data are ignored with the 2D measurements. Regardless, the method using the single 2D ROI still had high interobserver and intraobserver agreement, with ICCs of 0.96 and 0.95, respectively. This finding is consistent with the findings of the present study and suggests that the use of a 2D ROI is acceptable and has high reproducibility without the need for dedicated software required for 3D assessments.
The current study has several limitations. These include the small heterogeneous sample and the retrospective nature of the study. Another limitation is the range in the time interval at which the baseline and the first follow-up imaging scans were obtained; this range is a consequence of the retrospective study design. The fact that most patients who had evidence of extrahepatic disease before undergoing RE continued to receive systemic chemotherapy after RE may have contributed to the liver tumor response, PFS, or both, in this cohort. This contributory effect is probably minimal, because the tumors were initially chemo-resistant (as evident by failure of multiple lines of chemotherapy) and most of the patients restarted chemotherapy only after the acquisition of the response evaluation scans.
An important limitation of the present study, along with most similar studies, is the lack of histopathologic analysis to correlate and validate post-RE imaging findings. This correlation can provide a higher level of evidence when evaluating tumor attenuation as a possible surrogate image biomarker of response after RE of CLM patients. The use of CT scanners (16- and 64-slice) may also be a limitation. Another limitation is the exclusion of tumors with a diameter of 1.5 cm or smaller. This was done to avoid the partial volume effect that would be encountered when attempting to measure the attenuation for such smaller tumors. This was also a common limitation of other studies that assessed tumor attenuation changes, where only target tumors with a diameter of at least 1.5 or 2 cm were included [29, 36].
In conclusion, both tumor attenuation changes and Choi criteria show a higher sensitivity than RECIST 1.1 in predicting metabolic response after RE of CLM. Both criteria, in addition to EORTC PET criteria, were significant predictors of liver PFS, whereas RECIST 1.1 was not. The present study highlights the known limitations of RECIST in the assessment of tumor response to locoregional therapies and sets the ground for prospective studies that will assess other anatomic and metabolic imaging findings that may serve as surrogate biomarkers of oncologic outcomes, ideally with histopathologic validation of tumor response after therapy [31].
Acknowledgments
C. T. Sofocleous is a consultant for and receives research support from SIRTEX Medical, Inc.
Memorial Sloan Kettering Cancer Center is supported by grant P30 CA008748 from the National Cancer Institute.
Footnotes
Based on a presentation at the 2014 World Conference on Interventional Oncology, New York, NY.
References
- 1.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023–1033. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 2.Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1:398–407. doi: 10.1016/s1091-255x(97)80126-6. [DOI] [PubMed] [Google Scholar]
- 3.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion, 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 7.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278:601–611. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prompers L, Bucerius J, Brans B, Temur Y, Berger L, Mottaghy FM. Selective internal radiation therapy (RE) in primary or secondary liver cancer. Methods. 2011;55:253–257. doi: 10.1016/j.ymeth.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 10.Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951–959. doi: 10.1007/s00330-008-1211-7. [DOI] [PubMed] [Google Scholar]
- 11.Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13:27–36. doi: 10.1016/j.clcc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for 90Y resin microspheres. J Gastrointest Oncol. 2015;6:134–142. doi: 10.3978/j.issn.2078-6891.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1861–1869. doi: 10.1007/s00259-014-2799-2. [DOI] [PubMed] [Google Scholar]
- 14.Sofocleous CT, Violari EG, Sotirchos VS, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin Colorectal Cancer. 2015;14:296–305. doi: 10.1016/j.clcc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors (in French) Bull Cancer. 2000;87:881–886. [PubMed] [Google Scholar]
- 16.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR. 2012;198:737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 18.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 19.Faivre S, Zappa M, Vilgrain V, et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504–4512. doi: 10.1158/1078-0432.CCR-10-1708. [DOI] [PubMed] [Google Scholar]
- 20.Ronot M, Bouattour M, Wassermann J, et al. Alternative Response Criteria (Choi, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394–402. doi: 10.1634/theoncologist.2013-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhani N, Tu D, Sargent DJ, Seymour L, Moore MJ. Alternate endpoints for screening phase II studies. Clin Cancer Res. 2009;15:1873–1882. doi: 10.1158/1078-0432.CCR-08-2034. [DOI] [PubMed] [Google Scholar]
- 22.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 23.Desar IM, van Herpen CM, van Laarhoven HW, Barentsz JO, Oyen WJ, van der Graaf WT. Beyond RECIST: molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev. 2009;35:309–321. doi: 10.1016/j.ctrv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Chung WS, Park MS, Shin SJ, et al. Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR. 2012;199:809–815. doi: 10.2214/AJR.11.7910. [DOI] [PubMed] [Google Scholar]
- 25.Lastoria S, Piccirillo MC, Caraco C, et al. Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med. 2013;54:2062–2069. doi: 10.2967/jnumed.113.119909. [DOI] [PubMed] [Google Scholar]
- 26.Miller FH, Keppke AL, Reddy D, et al. Response of liver metastases after treatment with yttrium-90 microspheres: role of size, necrosis, and PET. AJR. 2007;188:776–783. doi: 10.2214/AJR.06.0707. [DOI] [PubMed] [Google Scholar]
- 27.Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815–820. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]
- 28.Szyszko T, Al-Nahhas A, Canelo R, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl Med Commun. 2007;28:15–20. doi: 10.1097/MNM.0b013e328011453b. [DOI] [PubMed] [Google Scholar]
- 29.Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression- free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391–1399. doi: 10.1007/s00259-012-2149-1. [DOI] [PubMed] [Google Scholar]
- 30.Fendler WP, Philippe Tiega DB, Ilhan H, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after RE of hepatic metastases from colorectal cancer. J Nucl Med. 2013;54:1202–1208. doi: 10.2967/jnumed.112.116426. [DOI] [PubMed] [Google Scholar]
- 31.Janne d’Othée B, Sofocleous CT, Hanna N, et al. Development of a research agenda for the management of metastatic colorectal cancer: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2012;23:153–163. doi: 10.1016/j.jvir.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahani DV, Bajwa MA, Andrabi Y, Bajpai S, Cusack JC. Current status of imaging and emerging techniques to evaluate liver metastases from colorectal carcinoma. Ann Surg. 2014;259:861–872. doi: 10.1097/SLA.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 33.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 35.Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 36.Tochetto SM, Rezai P, Rezvani M, et al. Does multidetector CT attenuation change in colon cancer liver metastases treated with 90Y help predict metabolic activity at FDG PET? Radiology. 2010;255:164–172. doi: 10.1148/radiol.09091028. [DOI] [PubMed] [Google Scholar]
- 37.Gulec SA, Pennington K, Wheeler J, et al. Yttrium-90 microsphere-selective internal radiation therapy with chemotherapy (chemo-RE) for colorectal cancer liver metastases: an in vivo double-arm-controlled phase II trial. Am J Clin Oncol. 2013;36:455–460. doi: 10.1097/COC.0b013e3182546c50. [DOI] [PubMed] [Google Scholar]
- 38.Thian Y, Gutzeit A, Koh DM, et al. Revised Choi imaging criteria correlate with clinical outcomes in patients with metastatic renal cell carcinoma treated with sunitinib. Radiology. 2014;273:452–461. doi: 10.1148/radiol.14132702. [DOI] [PubMed] [Google Scholar]
- 39.Tochetto SM, Tore HG, Chalian H, Yaghmai V. Colorectal liver metastasis after 90Y radioembolization therapy: pilot study of change in MDCT attenuation as a surrogate marker for future FDG PET response. AJR. 2012;198:1093–1099. doi: 10.2214/AJR.11.6622. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy AS, Ball DS, Cohen SJ, et al. Hepatic imaging response to radioembolization with yttrium-90-labeled resin microspheres for tumor progression during systemic chemotherapy in patients with colorectal liver metastases. J Gastrointest Oncol. 2015;6:594–604. doi: 10.3978/j.issn.2078-6891.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabet A, Meyer C, Aouf A, et al. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur J Nucl Med Mol Imaging. 2015;42:370–376. doi: 10.1007/s00259-014-2935-z. [DOI] [PubMed] [Google Scholar]
- 42.Turkmen C, Ucar A, Poyanli A, et al. Initial outcome after selective intraarterial radionuclide therapy with yttrium-90 microspheres as salvage therapy for unresectable metastatic liver disease. Cancer Biother Radiopharm. 2013;28:534–540. doi: 10.1089/cbr.2012.1455. [DOI] [PubMed] [Google Scholar]
- 43.Annunziata S, Treglia G, Caldarella C, Galiandro F. The role of 18F-FDG-PET and PET/CT in patients with colorectal liver metastases undergoing selective internal radiation therapy with yttrium-90: a first evidence-based review. Scientific World Journal. 2014;2014:879469. doi: 10.1155/2014/879469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metser U, You J, McSweeney S, Freeman M, Hendler A. Assessment of tumor recurrence in patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast-enhanced 64-MDCT of the chest and abdomen. AJR. 2010;194:766–771. doi: 10.2214/AJR.09.3205. [DOI] [PubMed] [Google Scholar]
- 45.Lau LF, Williams DS, Lee ST, Scott AM, Christophi C, Muralidharan V. Metabolic response to preoperative chemotherapy predicts prognosis for patients undergoing surgical resection of colorectal cancer metastatic to the liver. Ann Surg Oncol. 2014;21:2420–2428. doi: 10.1245/s10434-014-3590-0. [DOI] [PubMed] [Google Scholar]
- 46.Yip VS, Poston GJ, Fenwick SW, Wieshmann H, Athwal T, Malik HZ. FDG-PET-CT is effective in selecting patients with poor long term survivals for colorectal liver metastases. Eur J Surg Oncol. 2014;40:995–999. doi: 10.1016/j.ejso.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Marcus C, Marashdeh W, Ahn SJ, Taghipour M, Subramaniam RM. 18F-FDG PET/CT and colorectal cancer: value of fourth and subsequent posttherapy follow-up scans for patient management. J Nucl Med. 2015;56:989–994. doi: 10.2967/jnumed.115.156240. [DOI] [PubMed] [Google Scholar]
- 48.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 49.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Chalian H, Tochetto SM, Tore HG, Rezai P, Yaghmai V. Hepatic tumors: region-of-interest versus volumetric analysis for quantification of attenuation at CT. Radiology. 2012;262:853–861. doi: 10.1148/radiol.11110106. [DOI] [PubMed] [Google Scholar]