Abstract
Recent studies suggest that the use of preoperative β blockers in cardiac surgery may not provide improved mortality rates and may even contribute to negative clinical outcomes. We therefore assessed the role of β blockers on several outcomes after cardiac surgery (delirium, acute kidney injury [AKI], stroke, atrial fibrillation (AF), mortality, and hospital length of stay) in 4,076 patients who underwent elective coronary artery bypass grafting, coronary artery bypass grafting + valve, or valve cardiac surgery from November 1, 2009, to September 30, 2015, at Vanderbilt Medical Center. Clinical data from 2 prospectively collected datasets at our institution were reviewed: the Cardiac Surgery Perioperative Outcomes Database and the Society of Thoracic Surgeons Database. Preoperative β-blocker use was defined by Society of Thoracic Surgeons guidelines as patients receiving a β blocker within 24 hours preceding surgery. Of the included patients, 2,648 (65.0%) were administered a β blocker within 24 hours before surgery. Adjusting for possible confounders, preoperative β-blocker use was associated with increased odds of AKI stage 2 (odds ratio 1.96, 95% confidence interval 1.19 to 3.24, p <0.01). There was no evidence that β-blocker use had an independent association with postoperative delirium, AKI stages 1 and 3, stroke, AF, mortality, or prolonged length of stay. A secondary propensity score analysis did not show a marginal association between β-blocker use and any outcome. In conclusion, we did not find significant evidence that preoperative β-blocker use was associated with postoperative delirium, AF, AKI, stroke, or mortality.
The use of β blockers in patients who underwent vascular or cardiac surgery is common and driven in large part by current guidelines and clinician preference. The Society of Thoracic Surgeons (STS) uses preoperative β blockers as a quality measure1; however, recent studies suggest that the use of preoperative β blockers may not provide improved mortality rates and may even contribute to negative clinical outcomes, including postoperative delirium.1–3 The cardioprotective properties of β blockers may be offset by several undesirable side effects, including hypotension, lethargy, depression, sedation, and other adverse neurocognitive effects.4 Therefore, to address recent discrepancies in the literature, we assessed the impact of preoperative β blockers on several outcomes after cardiac surgery.
Methods
The Society of Thoracic Surgeons National Adult Cardiac Database (STS-NCD) was established in 1989 to compile perioperative data on patients who underwent cardiac surgery. Data are collected quarterly from our institution for the STS-NCD. The Cardiac Surgery Perioperative Outcomes Database (POD) at our institution is an institutional review board-approved data registry of cardiac surgical patients. Data from the STS database at our institution and the POD were linked using the electronic medical record number and the date of surgery to create a unique identifier. The data quality was verified by independent investigators (JBO and FTB) with frequent cross-checks for the completeness and consistency of the datasets. The study has been reviewed and approved by our institutional review board under protocol number 151362. The study was registered at ClinicalTrials.gov (NCT02548975) before data extraction.
This work was a retrospective cohort study of patients who underwent elective coronary artery bypass grafting (CABG), CABG + valve, or isolated cardiac valve surgery at our institution from November 1, 2009, to September 30, 2015. Emergency cases were not included in the study. Our institution is a 1,105-bed tertiary care facility located in Nashville, Tennessee. Over 50,000 cardiac and noncardiac surgeries are performed at our institution annually.
Preoperative β-blocker use was defined by STS-NCD guidelines as receipt of a β blocker within 24 hours preceding surgery. Delirium was defined as any positive confusion assessment method for the intensive care unit (CAM-ICU) examination during the postoperative intensive care unit (ICU) course. At our institution, ICU standard practice directs bedside nurses to perform a CAM-ICU twice per 12-hour shift. The duration of delirium was defined as the total number of days with CAM-ICU positive recorded in the patient’s chart (if a patient was positive for either 1 or both of the daily assessments, this was considered a day of delirium). Acute kidney injury (AKI) was defined using Kidney Disease: Improving Global Outcome Group (KDIGO) stages 1, 2, or 3 serum creatinine (SCr) criteria.5 Postoperative stroke was defined as any confirmed neurological deficit of abrupt onset caused by a disturbance in blood supply to the brain that did not resolve within 24 hours per STS-NCD guidelines. The development of postoperative atrial fibrillation (AF) was defined as a report of a newly diagnosed AF in the patient’s medical record after surgery. In-hospital 30-, 60-, and 90 day and 1-year mortality data were obtained from the POD. Hospital length of stay (LOS) was reported in days.
For binary outcomes, logistic regression was performed to assess the association between preoperative β-blocker use and the outcome, adjusting for possible confounders. Possible confounders were determined a priori and included exposure to cardiopulmonary bypass (CPB) and CPB duration (nested within CPB exposure), age, race, body mass index, current tobacco use, chronic lung disease, history of diabetes, dyslipidemia, end-stage renal disease, hypertension, congestive heart failure, myocardial infarction, peripheral arterial disease, cerebrovascular disease, and previous cardiac surgery. Preoperative hematocrit, SCr, and glomerular filtration rate were also included in the model. For the ordinal outcome, delirium duration, ordinal logistic regression was performed to assess the association between preoperative β-blocker use and delirium, adjusting for the same set of confounders. For the quantitative outcome, hospital LOS, linear regression analysis was implemented to assess the association between preoperative β-blocker use and LOS while again adjusting for the same set of confounders. The effects of quantitative covariates (i.e., CPB duration, body mass index, preoperative labs, and age) on each outcome (i.e., LOS or the odds of each categorical outcome) were modeled using a restricted cubic spline basis with 4 knots spaced over the covariate quintiles. This method allowed the effects of quantitative predictors to be nonlinear. Due to the rarity of postoperative stroke, death in hospital, and death within 30, 60, and 90 days and 1 year, no adjusted analysis was implemented. Fisher’s exact test was used to assess the unadjusted association between β blocker exposure and each of these rare outcomes.
In addition to the regression analysis, a secondary analysis was completed using a propensity score matching method. The propensity score for preoperative β-blocker use was estimated for each patient using logistic regression, adjusting for the potential confounders listed previously. Propensity scores were then used for 1:1 matching of treated (β blocker) and control patients. In the present study, there were fewer control patients than treated patients (1,203 non–β-blocker users and 2,294 β-blocker users) such that we matched treated patients to control patients. Matching was performed using a caliper method with a replacement of matched control subjects. Ties were broken uniformly at random. Then, weighted, unadjusted (ordinal) logistic regression was performed for the categorical outcomes, and weighted simple linear regression was performed for the quantitative outcome.
Results
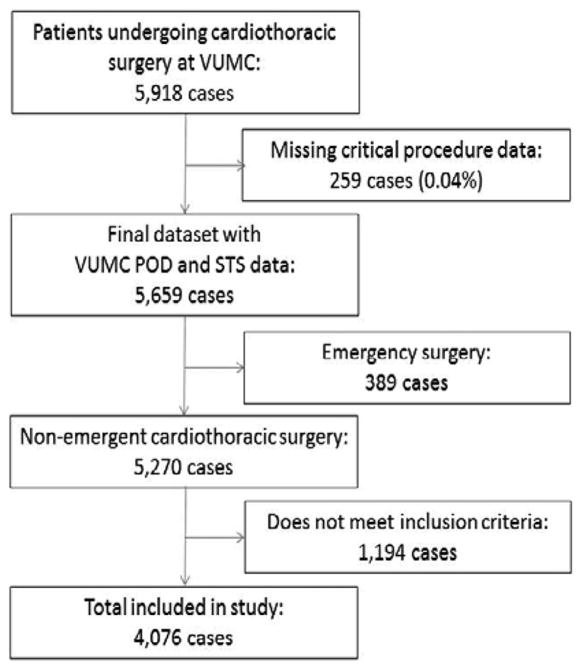
A total of 4,076 patients who underwent CABG, CABG + valve, or valve only cardiac surgeries were included in this analysis (Figure 1). All other cardiac surgery procedures were removed in an attempt to provide as homogenous a population as possible. A total of 2,648 patients (65.0%) received a β blocker within 24 hours before surgery. The patient characteristics are included in Table 1.
Figure 1.
Patient flow diagram. VUMC = Vanderbilt University Medical Center.
Table 1.
Patient characteristics. Statistical significance for categorical variables tested using Pearson test and Deuchler-Wilcoxon procedure for continuous variables
| Characteristics | β-blocker
|
|
|---|---|---|
| Yes n = 3648 (65.0%) |
No n = 1428 (35.0%) |
|
| Age, Mean ±SD (years) | 64 ± 12† | 63 ± 13 |
| Body mass index, Mean ±SD (kg/m2) | 29.7 ± 7.0† | 28.8 ± 6.4 |
| Male | 1859 (70.2%)† | 936 (65.6%) |
| Black | 180 (7.4%) | 74 (5.8%) |
| Prior myocardial infarction | 1034 (39.1%)† | 291 (20.4%) |
| Congestive heart failure | 356 (13.5%)* | 156 (10.9%) |
| Hypertension | 2100 (79.3%)† | 893 (62.5%) |
| Cardiac surgery | 332 (12.5%) | 163 (11.4%) |
| Dyslipidemia | 1154 (43.6%)† | 461 (32.3%) |
| End stage renal disease | 83 (3.1%)* | 26 (1.8%) |
| Peripheral artery disease | 265 (10.0%)† | 101 (7.1%) |
| Diabetes mellitus | 1113 (42.0%)† | 442 (31.0%) |
| Chronic lung disease | 684 (25.8%)† | 285 (20.0%) |
| Current tobacco use | 74 (2.8%)† | 75 (5.3%) |
| Cerebrovascular disease | 491 (18.5%) | 232 (16.2%) |
| Number of narrowed coronary arteries | ||
| 0 | 474 (18.1%)† | 569 (40.6%) |
| 1 | 249 (9.5%)† | 182 (13.0%) |
| 2 | 569 (21.8%)† | 231 (16.5%) |
| 3 | 1323 (50.6%)† | 421 (30.0%) |
| Baseline Laboratory Values | ||
| Serum creatinine, Mean ±SD (mg/dL) | 1.31 ± 1.41† | 1.15 ± 0.97 |
| Estimated glomerular filtration rate, Mean ±SD (mL/min/1.73 m2) | 70 ± 25† | 74 ± 23 |
| Hematocrit, Mean ±SD (%) | 38.7 ± 5.6† | 39.0 ± 6.0 |
| Type of Surgery | ||
| Coronary surgery | 1909 (72.1%)† | 675 (47.3%) |
| Aortic valve surgery | 488 (18.4%)† | 512 (35.6%) |
| Mitral valve surgery | 473 (17.9%)† | 343 (24.0%) |
| Complex surgery | 293 (11.0%) | 177 (12.4%) |
| On-pump | 1215 (45.9%)† | 965 (67.6%) |
SD = standard deviation; Complex surgery includes coronary + valve procedures.
p < 0.05.
p < 0.01.
The unadjusted univariate analyses are outlined in Table 2. After adjusting for confounders, there was no evidence that preoperative β-blocker use had an independent association with postoperative delirium (odds ratio [OR] 0.95 [95%] confidence interval CI 0.79 to 1.15, p = 0.65) (Table 3). Patients who underwent β-blocker therapy did have a slightly decreased odds of a longer delirium duration (OR 0.72, 95% CI 0.52 to 0.96, p = 0.03). Preoperative β-blocker use was associated with greater odds of KDIGO stage 2 (OR 1.96, 95% CI 1.19 to 3.24, p = 0.009); however, after adjusting for confounders, the use of β blockers was not associated with either KDIGO stage 1 (OR 1.07, 95% CI 0.87 to 1.30, p = 0.53) or KDIGO stage 3 (OR1.22, 95% CI 0.56 to 2.60, p = 0.62). Comparable with the unadjusted analysis, the odds of postoperative AF, mortality, and stroke were not significantly increased in patients receiving β blockers. LOS was also not significantly prolonged with β-blocker use.
Table 2.
Outcome events. Statistical significance for categorical variables tested using Pearson test and Deuchler-Wilcoxon procedure for continuous variables
| Outcome | β-blocker
|
|
|---|---|---|
| Yes | No | |
| Delirium incidence | 615 (23.2%) | 326 (22.8%) |
| Delirium duration, Mean ±SD (days) | 0.47 ± 1.14 | 0.53 ± 1.32 |
| Kidney Disease: Improving Global Outcome | ||
| Stage 1 | 626 (24.5%)* | 269 (20.0%) |
| Stage 2 | 158 (6.2%)* | 37 (2.7%) |
| Stage 3 | 85 (3.3%)* | 24 (1.8%) |
| Atrial fibrillation | 820 (31.0%) | 413 (28.9%) |
| Length of stay, Mean ±SD (days) | 9.7 ± 5.8* | 9.6 ± 6.4 |
| Mortality | ||
| In hospital | 18 (0.7%) | 11 (0.8%) |
| 30 days | 49 (1.9%) | 25 (1.8%) |
| 60 days | 61 (2.3%) | 31 (2.2%) |
| 90 days | 76 (2.9%) | 34 (2.4%) |
| 1-year | 119 (4.5%) | 57 (4.0%) |
| Stroke | 44 (1.7%) | 27 (1.9%) |
SD = standard deviation.
p < 0.01.
Table 3.
Adjusted odds for outcomes (primary analysis). Statistical significance for categorical variables tested using Pearson test and Deuchler-Wilcoxon procedure for continuous variables
| Outcome | β-blocker
|
|---|---|
| Adjusted OR (95% CI) | |
| Delirium incidence | 0.95 (0.79 to 1.15) |
| Delirium duration | 0.72 (0.52 to 0.96)* |
| Kidney Disease: Improving Global Outcome | |
| Stage 1 | 1.07 (0.87 to 1.30) |
| Stage 2 | 1.96 (1.19 to 3.24)† |
| Stage 3 | 1.22 (0.56 to 2.60) |
| Atrial fibrillation | 1.13 (0.95 to 1.34) |
| Mortality | |
| 90 days | 1.20 (0.71 to 2.03) |
| 1-year | 1.10 (0.73 to 1.65) |
| Length of stay (Δdays) | −0.11 (−0.51 to 0.28) |
| Stroke‡ | 0.88 |
OR = odds ratio; CI = confidence interval.
p < 0.05.
p < 0.01.
Fisher test used to determine statistical significance.
Of the original 4,076-patient study cohort, 3,497 (1,203 non–β-blocker users and 2,294 β-blocker users) had values for all of the designated confounders. Propensity scores with matching and replacements were performed on the complete cases. There was no evidence of an association with preoperative β-blocker use on any outcome including postoperative delirium, duration of delirium, AKI, postoperative AF, LOS, or mortality, after propensity score matching (Table 4).
Table 4.
Adjusted odds for outcomes (secondary analysis). Statistical significance for categorical variables tested using Pearson test and Deuchler-Wilcoxon procedure for continuous variables
| Outcome | β-blocker
|
|---|---|
| Adjusted OR (95% CI)* | |
| Delirium incidence | 0.96 (0.79 to 1.17) |
| Delirium duration | 0.94 (0.77 to 1.14) |
| Kidney Disease: Improving Global Outcome | |
| Stage 1 | 1.11 (0.89 to 1.38) |
| Stage 2 | 1.27 (0.71 to 2.27) |
| Stage 3 | 0.70 (0.30 to 1.64) |
| Atrial fibrillation | 1.16 (0.98 to 1.38) |
| Mortality | |
| 90 days | 1.04 (0.59 to 1.86) |
| 1-year | 1.01 (0.66 to 1.55) |
| Length of stay (Δdays) | 0.08 (−0.15 to 0.31) |
| Stroke | 1.02 (0.58 to 1.80) |
CI = confidence interval; OR = odds ratio.
p < 0.05.
Discussion
In our study, β-blocker use was not significantly associated with any outcomes after cardiac surgery except possibly for increased odds of AKI stage 2, after adjusting for potential confounders. However, a secondary propensity score analysis did not reveal a marginal association between β-blocker use and AKI stage 2, nor with any other outcome.
The use of preoperative β blockers in patients who underwent CABG surgery is considered a quality standard by the STS. This recommendation is supported by a previous study by Ferguson et al on patients who underwent CABG surgery from 1996 to 1999 using the STS-NCD to evaluate the effect of preoperative β-blocker use on outcomes, which demonstrated a survival benefit in patients taking β blockers.6 Other retrospective analyses from the 1990s also showed benefits from preoperative β-blocker use.7,8 However, more recent studies do not support the necessity of preoperative β blockers in patients who underwent CABG surgery.9,10
Delirium was diagnosed in 23.1% of the patients included in our study population, which corresponds with previous studies.11,12 In a study on patients who underwent vascular surgery, Katznelson et al found an increased incidence of delirium in patients taking β blockers (26.5% vs 17.4%, p = 0.03).3 Another study that included on-pump and off-pump CABG surgeries, valve surgery, and also patients who underwent transcatheter aortic valve implantation cardiac surgery found an association between β-blocker use and delirium (OR 1.7, 95% CI 1.1 to 2.6, p = 0.01).11 Within our cohort, no association between patients taking β blockers and the incidence of delirium was evident. Our primary analysis showed an association with decreased duration of delirium; however, the secondary analysis was not consistent with this finding. Of note, our study population did not include patients who underwent transcatheter aortic valve implantation procedures, which is a population of patients often deemed too frail for open-heart surgery. Our analyses did include and account for an extensive list of known confounders in the model such as age, previous cerebrovascular accident, diabetes, peripheral arterial disease, and hematocrit.13
In the primary analysis, patients taking β blockers were shown to have increased odds of AKI stage 2. A previous retrospective analysis on 506,110 patients who underwent nonemergent CABG surgery did not find a difference in patients developing AKI when receiving β blockers (2.33% vs 2.24%; OR 1.04, 95% CI 0.97 to 1.11, p = 0.30).2 The criteria to define AKI in the present study were taken from the STS-NCD guidelines, which included only patients with an increase in the SCr level to at least 3 times the preoperative level or an SCr level of at least 4.0 mg/dL with a minimum increase of 0.5 mg/dL relative to the last preoperative value and/or a new requirement for dialysis. These criteria exclude KDIGO stages 1 and 2; however, the criteria are similar to KDIGO stage 3 for which our study also did not find an association with β-blocker use. Thus, our findings are consistent with previous studies in regard to severe AKI. Incorporating stages 1 and 2 capture cases of mild and moderate AKI and, in addition to stage 3, are considered predictors of worse outcomes after cardiac surgery.14 A weak adjusted association was shown between β-blocker use and stage 2 AKI, but no association with stage 1 AKI was found. Our secondary analysis found no marginal association between β-blocker use and any stage of AKI. Therefore, our results are probably due to residual confounding and should not be taken as evidence of a harm signal for β blockers that manifest as AKI.
In the present study, preoperative β-blocker use was not shown to be a predictor of several tested negative outcomes after cardiac surgery. A meta-analysis by Devereaux et al on the use of perioperative β blockers in noncardiac surgery found increased risks of bradycardia and hypotension in patients receiving β blockers.15 The mechanism behind β blockers contributing to AKI may be hypotension with a subsequent impairment of renal perfusion during the surgery due to β-blocker administration. The POISE trial found an increased incidence in stroke in patients randomized to preoperative β-blocker administration and also suggested hypoperfusion to the brain as a mechanism for this finding.16 The cohort for our study does not have intraoperative hemodynamic data available for incorporation into the analysis, which may provide further insight to results from these trials and offer a potential direction for future investigations of β-blocker use in cardiac surgery.
The study was completed at a single medical center, and one must consider the generalizability of our findings. Using the STS database for our institution only included whether or not a patient had received a β blocker within 24 hours of surgery. The β-blocker type, the dose, and the duration of therapy were not available for the study. The analyses were completed by adjusting for several confounders; however, residual confounding could be present as the study was nonrandomized. Known delirium risk factors such as dementia, decreased functional status, and psychiatric illness were not incorporated into the model.17 Delirium was recorded by nurses in the ICU using the CAM-ICU method. Although using the CAM-ICU by nurses was previously validated,18 the consistency in which delirium was measured may not have been similar across all patients. The outcome of postoperative AF was reliant on the accuracy of bedside charting of the available electrocardiogram.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under award numbers T32GM108554, K23GM102676, and R01GM112871, and also by Vanderbilt Institute for Clinical and Translational Research grant number UL1 TR000445, from NCATS/NIH.
The authors thank Leslie Busbee, BS (Department of Cardiac Surgery, Vanderbilt University Medical Center) for assistance with the data retrieval.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Number: NCT02548975.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Toppen W, Sareh S, Satou N, Shemin R, Hunter C, Buch E, Benharash P. Do preoperative beta-blockers improve postoperative outcomes in patients undergoing cardiac surgery? Challenging societal guidelines. Am Surg. 2014;80:1018–1021. [PubMed] [Google Scholar]
- 2.Brinkman W, Herbert MA, O’Brien S, Filardo G, Prince S, Dewey T, Magee M, Ryan W, Mack M. Preoperative beta-blocker use in coronary artery bypass grafting surgery: national database analysis. JAMA Intern Med. 2014;174:1320–1327. doi: 10.1001/jamainternmed.2014.2356. [DOI] [PubMed] [Google Scholar]
- 3.Katznelson R, Djaiani G, Mitsakakis N, Lindsay TF, Tait G, Friedman Z, Wasowicz M, Beattie WS. Delirium following vascular surgery: increased incidence with preoperative beta-blocker administration. Can J Anaesth. 2009;56:793–801. doi: 10.1007/s12630-009-9148-0. [DOI] [PubMed] [Google Scholar]
- 4.Keller S, Frishman WH. Neuropsychiatric effects of cardiovascular drug therapy. Cardiol Rev. 2003;11:73–93. doi: 10.1097/01.CRD.0000053453.89776.2D. [DOI] [PubMed] [Google Scholar]
- 5.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson TB, Jr, Coombs LP, Peterson ED. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA. 2002;287:2221–2227. doi: 10.1001/jama.287.17.2221. [DOI] [PubMed] [Google Scholar]
- 7.ten Broecke PW, De Hert SG, Mertens E, Adriaensen HF. Effect of pre-operative beta-blockade on perioperative mortality in coronary surgery. Br J Anaesth. 2003;90:27–31. [PubMed] [Google Scholar]
- 8.Zaugg M, Tagliente T, Lucchinetti E, Jacobs E, Krol M, Bodian C, Reich DL, Silverstein JH. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology. 1999;91:1674–1686. doi: 10.1097/00000542-199912000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Brinkman WT, Herbert MA, Prince SL, Magee MJ, Dewey TM, Smith RL, Edgerton JR, Head SJ, Ryan WH, Mack MJ. Preoperative beta-blocker usage: is it really worthy of being a quality indicator? Ann Thorac Surg. 2011;92:788–795. doi: 10.1016/j.athoracsur.2011.03.088. discussion 795–786. [DOI] [PubMed] [Google Scholar]
- 10.Vaishnava P, Eagle KA. Surgery. beta—blockers—still a trusted ally or time for retirement? Nat Rev Cardiol. 2014;11:502–503. doi: 10.1038/nrcardio.2014.112. [DOI] [PubMed] [Google Scholar]
- 11.Tse L, Schwarz SK, Bowering JB, Moore RL, Barr AM. Incidence of and risk factors for delirium after cardiac surgery at a quaternary care center: a retrospective cohort study. J Cardiothorac Vasc Anesth. 2015;29:1472–1479. doi: 10.1053/j.jvca.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 12.O’Neal JB, Shaw AD. Predicting, preventing, and identifying delirium after cardiac surgery. Perioper Med (Lond) 2016;5:7. doi: 10.1186/s13741-016-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollinger A, Siegemund M, Goettel N, Steiner LA. Postoperative delirium in cardiac surgery: an unavoidable menace? J Cardiothorac Vasc Anesth. 2015;29:1677–1687. doi: 10.1053/j.jvca.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Machado MN, Nakazone MA, Maia LN. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29:299–307. doi: 10.5935/1678-9741.20140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devereaux PJ, Beattie WS, Choi PT, Badner NH, Guyatt GH, Villar JC, Cina CS, Leslie K, Jacka MJ, Montori VM, Bhandari M, Avezum A, Cavalcanti AB, Giles JW, Schricker T, Yang H, Jakobsen CJ, Yusuf S. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;331:313–321. doi: 10.1136/bmj.38503.623646.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Malaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 17.Bin Abd Razak HR, Yung WY. Postoperative delirium in patients undergoing total joint arthroplasty: a systematic review. J Arthroplasty. 2015;30:1414–1417. doi: 10.1016/j.arth.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]