Abstract
Episodic memory involves coding of the spatial location and time of individual events. Coding of space and time is also relevant to working memory, spatial navigation, and the disambiguation of overlapping memory representations. Neurophysiological data demonstrate that neuronal activity codes the current, past and future location of an animal as well as temporal intervals within a task. Models have addressed how neural coding of space and time for memory function could arise, with both dimensions coded by the same neurons. Neural coding could depend upon network oscillatory and attractor dynamics as well as modulation of neuronal intrinsic properties. These models are relevant to the coding of space and time involving structures including the hippocampus, entorhinal cortex, retrosplenial cortex, striatum and parahippocampal gyrus, which have been implicated in both animal and human studies.
Introduction
The coding of space and time is essential for a number of different memory phenomena, including episodic memory, which by definition involves coding of where and when an event occurs. Studies in humans have analyzed memory in humans for spatial trajectories to goal locations [1–3], and the spatial location of objects and landmarks [4–6]. Performance of spatial memory tasks involves interactions of neural activity in structures including the hippocampus, paraphippocampal gyrus, retrosplenial cortex and prefrontal cortex [1–5]. Studies have also analyzed memory for the temporal order of events in episodic memory [6–8], showing involvement of the hippocampus and parahippocampal gyrus. Research has also examined the disambiguation of overlapping spatial and non-spatial representations in both humans and animals [1,7,9].
Consistent with the patterns of activity observed in humans using fMRI, neurophysiological studies have demonstrated neurons that respond on the basis of spatial location of an animal in foraging tasks or tasks that require a specific memory of spatial location such as spatial alternation. Neurons in the hippocampus fire as place cells coding location [10,11] or time cells coding the time interval during a behavioral task [12–14]. Neurons in the entorhinal cortex code spatial features including location in the environment [15–18], distance to an environmental boundary [10,19], allocentric direction [10,17], and running speed [20,21]. Neurons with more complex firing patterns incorporating location and head direction appear in structures such as the basal forebrain [22], retrosplenial cortex [23], and parietal cortex [24]. For many years, computational models have attempted to describe the mechanisms and functional role of coding of space and time in these structures and their potential role in behavior. This review will provide an overview of recent work addressing the coding of space and time in these structures.
Modeling of spatial memory (grid cells, place cells and splitter cells)
Models have addressed the neural coding of spatial dimensions relevant to spatial memory. This includes models of spatial coding in foraging tasks. Foraging tasks may not be considered traditional memory tasks, but the capacity of neurons to respond in a stable manner across different trials requires memory of the overall configuration of cues in an environment. Models have also addressed the memory of specific behaviorally relevant locations within an environment.
Modeling neural responses based on spatial location
Many studies have addressed the response of place cells and grid cells to spatial location [25,26]. Models have addressed the generation of place cells and grid cells by both path integration or the angle of visual cues. This includes sophisticated models of physiological mechanisms for grid cells based on path integration. These models use different mechanisms that include interactions of oscillatory dynamics as initially proposed by Burgess, Barry and O’Keefe [27–29] or based on attractor dynamics as proposed by researchers such as McNaughton or Fiete [30,31]. Many properties of these two model classes are compatible, so features of these models can be combined [27]. Other models have addressed the response of grid cells based on self-organization of input from place cells [32] or as a result of a probabilistic learning process [33] or the eigenvectors of the normalized graph Laplacian [34].
The loss of grid cell spatial selectivity with the inactivation of input that provides coding of heading direction [17] supports models that use path integration in generation of grid cell responses. Extensive data supports a role for attractor dynamics in generation of grid cell firing, including the modular properties of distinct populations of grid cells that share properties such as spacing and orientation and the ellipsoid shape of firing fields [15,18], and the change in correlation of grid cell firing with different anatomical distances between grid cells [35]. The role of oscillatory dynamics in coding of grid cells is supported by evidence that the intrinsic rhythmicity of entorhinal neurons differs with spatial scale [36] and shifts with running speed [20,36], and that inactivation of the medial septum causes loss of grid cell firing properties [37]. The effects of medial septum inactivation could be due to loss of GABAergic input that can drive network theta rhythm activity [38], or to loss of the cellular effects of cholinergic modulation that normally shift network dynamics from sharp-wave spiking to theta rhythm dynamics [38,39] and normally enhance the influence of external sensory input [40]. The loss of spatial coding could also be due to the loss of glutamatergic input from the medial septum that contributes to coding of running speed in the hippocampus [41,42].
Modeling the influence of boundaries
Physiological studies in animals demonstrate that place cell firing depends on the position of environmental boundaries [11]. This led to models that coded location based on selective firing of boundary cells [10,43]. This prediction was later supported by experimental evidence of boundary cells [19,44]. Modeling demonstrated how egocentric visual input about boundaries could be transformed to code allocentric spatial location [45]. These models demonstrate how place cells can be driven without grid cell input, since inactivation of grid cells does not abolish place cell firing [46].
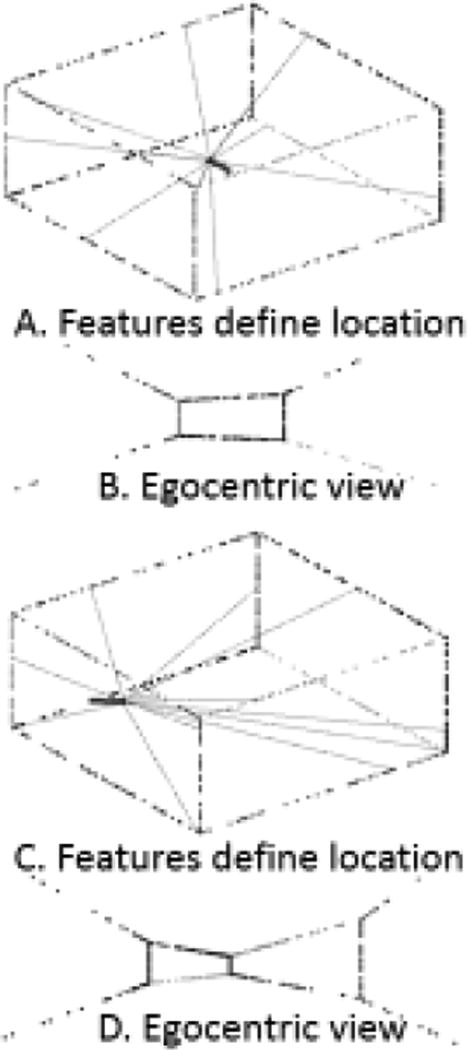
Alterations of visual boundaries also influence the location of grid cell firing fields. The spacing of grid cell firing fields is compressed or expanded by movements of the environment walls [18], and this change may be specific to grid cells with larger spacing while sparing smaller spacing [15] though the earlier study showed compression of cells with small spacing [18]. The response of grid cells to barrier movement has been modeled based on selective influences of the angle and optic flow of visual cues from different parts of the visual field [47]. The computation of location from visual angle is shown in Figure 1. The regular pattern of grid cell fields is lost when shifting from a rectangular environment to a trapezoid environment [16], and this has been modeled based on field boundary effects [48] or the normalized graph Laplacian [34].
Figure 1.
A. Computation of the location of an animal in a square environment with barriers can be determined from the angle and distance to visual features [47]. Movement of barriers has been shown to shift neurophysiological codes for location [15,18]. B. Figure showing the egocentric view of visual features at location in A. C–D. Same as A–B for a different location.
Grid cells, head direction and boundary cells have all been shown to fire in a stable manner when an animal is removed from the environment and then returned to the environment. This requires anchoring of the neural responses to features in the environment, as utilized in models showing how sensory cues observed when re-entering an environment can allow the accurate firing of grid cells [27,45,47] and head direction cells [49]. Consistent with this, grid cells exhibit greater consistency in the location of spatial firing near an environment boundary, and poorer consistency at greater distances from the boundary [50].
Modeling the encoding and retrieval of sequences
Models have also addressed the encoding and retrieval of sequences. In an influential model developed by Lisman and Jensen, the initial storage of a sequence of locations has been modeled using after-depolarization potentials triggered by high levels of cholinergic modulation [51,52]. This model includes separation of different elements of a sequence on different gamma cycles that are phase amplitude coupled with theta rhythm [52]. Considerable data supports this model, including evidence that different sequence positions exhibit greater gamma power at distinct phases of theta [8], and this segregation is related to successful temporal order memory. The maintenance of spiking activity associated with a sequence of locations allows strengthening of connections between place cells that can mediate later sequential replay of the sequence. This sequential replay of place cells occurs during sharp wave ripple activity observed in the local field potential in the hippocampus [53,54]. This sharp wave replay occurs during the low levels of cholinergic modulation that occur in quiet waking or slow wave sleep [51,55,56]. These replay events could mediate the consolidation of previously encoded information during periods of quiet waking or slow-wave sleep [6,38,53,54,57]. These replay events may correspond to information packets that form the basis of cortical cognitive processing [58]. Such information packets could use codes such as the residue number system suggested for grid cell coding [59].
Modeling of context-dependent disambiguation
Models have addresssed how the retrieval of spatial memory representations can be used to guide future decisions. This includes models in which retrieval of sequences guides spatial alternation and the selective retrieval of overlapping trajectories [56,60]. Models that allow selective retrieval of one out of many individual sequences can generate context-dependent neuronal activity [61,62]. These models can simulate the firing of splitter cells, that selectively respond on overlapping track segments based on the future or past turning response of a rat [9,63]. These circuit level mechanisms also allow simulation of the disambiguation of overlapping sequences in human subjects [1,64].
Modeling of goal-directed navigation
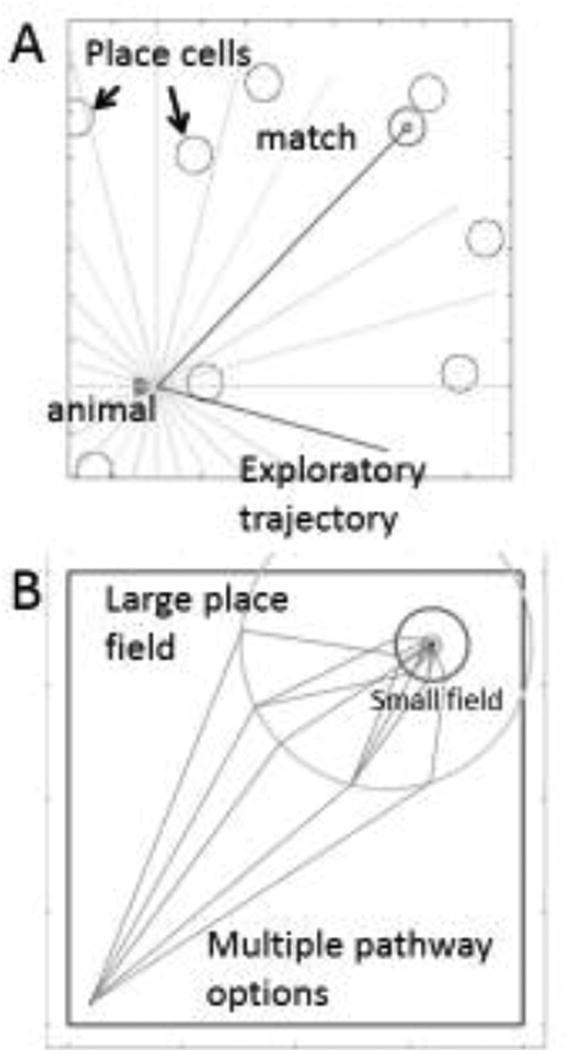
Models also address how the forward read-out of locations based on grid cell firing patterns allow selection of either the direct vector to a goal location [65–68] or a pathway dependent upon obstacles [67,69] as shown in Figure 2. These models are supported by experimental data indicating the replay of place cell sequences associated with goal-directed navigation [70–72]. The models are also supported by fMRI data in humans demonstrating hippocampal and parahippocampal activity at the start of a trajectory when a subject retrieves a previously learned overlapping trajectory [1,64]. Subsequent work showed that retrieval of a planned trajectory involves reactivation of intermediate locations on the trajectory [2]. Studies show coding of navigationally relevant information in fMRI activity, with hippocampus coding path length and entorhinal cortex coding the Euclidean distance to the goal [3,73]. Modeling shows how interaction of brain regions can allow performance based on semantic memory for the allocentric position of landmarks translated to the egocentric position of landmarks [45]. These models and other work on the influence of visual cues [47] are relevant to data showing the interaction of regions coding visual cues such as optic flow and feature angle with regions involved in navigation [73,74].
Figure 2.
A. Model for goal-directed behavior based on linear forward trajectory planning [68]. Circles represent the location where previous place cell firing took place. Triangle indicates animal location. Light gray lines indicate sequential sampling of forward trajectories through the environment. Darker line ending in circle indicates the trajectory that overlaps with the previously visited place cell firing field that was associated with a goal location, allowing selection of this trajectory. B. Different trajectories obtained by sampling at multiple spatial scales, allowing initial selection of trajectories to the large place cell firing field, followed by selection of trajectories to smaller place cell firing fields.
Modeling working memory for spatial location
Many spatial tasks require working memory for spatial location of an object either in allocentric or egocentric coordinates [75]. This has been modeled using attractor dynamics in which recurrent synaptic connections maintain a bump of activity representing the location of an object in a scene. Most models of attractor dynamics have focused on maintaining sustained activity in a full population of neurons for the full delay period. However, models show how activity might shift between different minicolumns [76]. The addition of inhibitory interneurons to the earlier model generates bursts of gamma frequency oscillations [77]. Experimental data on gamma oscillations [78] indicates that the pattern of neural activity during delay periods shows brief bursts of high frequency gamma oscillations similar to the model, in contrast to a background state of lower frequency beta oscillations.
Modeling of temporal memory (time cells)
As noted above, neurophysiological data indicates that neurons in a number of different structures including hippocampus and entorhinal cortex respond selectively at specific temporal intervals within behavioral tasks [12–14]. In particular, the neural activity occurring at specific intervals during delay periods of behavioral tasks have been described as time cells. These temporal interval phenomena have been modeled in a number of different ways which could be tested experimentally by evaluating the relative contribution of synaptic properties, intrinsic neuronal properties or oscillatory dynamics.
Temporal intervals coded by synaptic interactions
The sequence of neural activity observed in some tasks can be modeled by complex synaptic interactions. Sequence retrieval based on the fast time constants of glutamatergic synaptic potentials would cause fast sequence retrieval such as that observed during brief sharp-wave ripple events observed in the hippocampal EEG [53,54,57], in contrast to the slow transitions of time cells [12]. Therefore, models of the slow transitions between different cells over a time scale of seconds require complex network dynamics, such as the slow activation of different attractors referred to as context cells [79,80]. Similarly, slow transitions between active time cells can be obtained by modeling different dynamics of synaptic influences within recurrent neural network models [81]. A number of mechanisms have been proposed for coding position within a sequence, which includes addressing data on the verbal memory for serial order lists [80]
Temporal intervals coded by intrinsic properties
Another mechanism for obtaining slow transitions between neuronal populations is to utilize intrinsic conductances with slower time constants. For example, models have replicated time cells using exponential decay with slow time constants [82,83], and have shown how this could arise from slow decay of a calcium-activated non-specific cation current [55,84] or a change in spiking threshold due to the calcium activated potassium current [83]. These models could be tested by altering the level of neuromodulators such as acetylcholine that regulate these intrinsic properties.
Temporal coding by oscillatory interactions
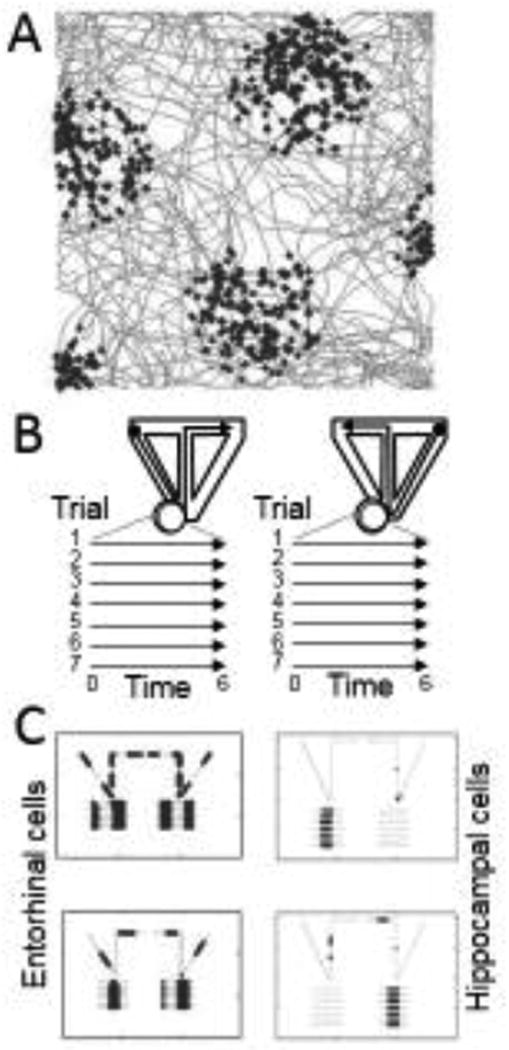
Models have also shown how temporal intervals can be coded by oscillatory interactions. This includes models in which the discrete temporal order of stimuli is coded by the position of gamma frequency oscillations within a theta frequency oscillation [52], which has been supported by recent data showing phase specificity of gamma oscillations related to stimulus order [8]. Another set of models show that a continuous representation of temporal interval can be coded by interference between different oscillations [60,85,86]. Simulations using these models demonstrated the same oscillatory dynamics that generate grid cell firing patterns could also generate time cell firing patterns [60] as shown in Figure 3. These models generated predictions of multiple time cell firing fields that are supported by experimental data [87]. The models that depend upon oscillatory interactions for coding of both space and time are supported by data showing that the inactivation of the medial septum causes loss of both grid cell firing in the entorhinal cortex [37] and the loss of time cell firing in the hippocampus [88]. However, further studies must determine whether these effects are due to loss of oscillatory input or loss of cholinergic modulation arising from the medial septum.
Figure 3.
Coding of space and time by the oscillatory interference model [29,60]. A. The model simulates grid cell firing as a simulated rat runs on a foraging trajectory (gray lines). Black dots show the location of the simulated rat each time the grid cell model generates a spike. B. The same model addresses activity during spatial alternation on a T-maze (top) with a period of running on a running wheel during the delay period between choices (lines on bottom). C. The same model shows location dependent firing on the T-maze (dark segments in top plot) as well as consistent firing at specific intervals of time or distance during running on the running wheel (dark segments on bottom lines).
Temporal coding between episodes
Note that most of the models described here focus on the timing of events within specific episodes, rather than the temporal order between different episodes, which requires larger scales of temporal coding. Modeling studies have proposed that neurogenesis within the dentate gyrus could provide different populations of granule cells allowing contextual coding between memories on a much larger temporal scale [89]. Work in humans suggests that adult neurogenesis additionally impacts the entorhinal cortex [90].
Conclusions
In summary, models have demonstrated potential mechanisms for a range of different spatial and temporal coding properties within cortical structures, and experimental data supports aspects of these different mechanisms. In particular, some classes of models can account for both the coding of spatial location in an environment as well as the coding of time during running in one location on a treadmill, including the oscillatory interference models [27,29,60], attractor dynamic models [83,91] and models using Laplace transforms [82]. These models have inspired and guided many experiments testing physiological mechanisms of spatial and temporal coding [13,15,17,18,20,21,87,88]. Modeling also demonstrates how coding of spatial location and time can form a framework to form the associations necessary to link events to the spatiotemporal trajectory of an episodic memory [56,82]. Modeling of episodic memories as trajectories has inspired experimental tests of the coding of memory in humans [1–3,69,73]. However, a full understanding of the network dynamics for coding space and time in episodic memory will require both more sophisticated and detailed models as well as more explicit experimental tests of the different mechanistic components of these computational models.
Highlights.
Neurons show coding of time and spatial location in memory-guided tasks
Models demonstrate how spatial coding could arise from integration of self-motion
Models show how spatial coding could depend on angle and distance of visual stimuli.
Models show how the same neuron can code both location and time interval.
Acknowledgments
This work supported by the National Institutes of Health, grant numbers R01 MH60013, R01 MH61492 and by the Office of Naval Research MURI N00014-16-1-2832.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests.
References
- 1*.Brown TI, Stern CE. Contributions of medial temporal lobe and striatal memory systems to learning and retrieving overlapping spatial memories. Cereb Cortex. 2014;24:1906–1922. doi: 10.1093/cercor/bht041. Study showing fMRI activation in the hippocampus when viewing the initial location of an overlapping trajectory and retrieving the full trajectory to generate a correct response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Brown TI, Carr VA, LaRocque KF, Favila SE, Gordon AM, Bowles B, Bailenson JN, Wagner AD. Prospective representation of navigational goals in the human hippocampus. Science. 2016;352:1323–1326. doi: 10.1126/science.aaf0784. Multi-voxel pattern analysis of fMRI data showing that retrieval of a trajectory to a different location on a circular pathway involves reactivation of the representation of intermediate locations along that trajectory. [DOI] [PubMed] [Google Scholar]
- 3.Howard LR, Javadi AH, Yu Y, Mill RD, Morrison LC, Knight R, Loftus MM, Staskute L, Spiers HJ. The hippocampus and entorhinal cortex encode the path and Euclidean distances to goals during navigation. Curr Biol. 2014;24:1331–1340. doi: 10.1016/j.cub.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchette SA, Vass LK, Ryan J, Epstein RA. Outside Looking In: Landmark Generalization in the Human Navigational System. J Neurosci. 2015;35:14896–14908. doi: 10.1523/JNEUROSCI.2270-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horner AJ, Bisby JA, Zotow E, Bush D, Burgess N. Grid-like Processing of Imagined Navigation. Curr Biol. 2016;26:842–847. doi: 10.1016/j.cub.2016.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig M, Dewar M, Della Sala S, Wolbers T. Rest boosts the long-term retention of spatial associative and temporal order information. Hippocampus. 2015;25:1017–1027. doi: 10.1002/hipo.22424. [DOI] [PubMed] [Google Scholar]
- 7.Ross RS, Brown TI, Stern CE. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus. 2009;19:790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heusser AC, Poeppel D, Ezzyat Y, Davachi L. Episodic sequence memory is supported by a theta-gamma phase code. Nat Neurosci. 2016;19:1374–1380. doi: 10.1038/nn.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 10.Hartley T, Lever C, Burgess N, O'Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 12.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal "Time Cells": Time versus Path Integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal "time cells" bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stensola H, Stensola T, Solstad T, Froland K, Moser MB, Moser EI. The entorhinal grid map is discretized. Nature. 2012;492:72–78. doi: 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- 16.Krupic J, Bauza M, Burton S, Barry C, O'Keefe J. Grid cell symmetry is shaped by environmental geometry. Nature. 2015;518:232–235. doi: 10.1038/nature14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Winter SS, Clark BJ, Taube JS. Spatial navigation. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science. 2015;347:870–874. doi: 10.1126/science.1259591. Data showing that inactivation of a region providing input coding the head direction of the animal causes a loss of the spatial specificity of grid cell firing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–684. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- 19.Lever C, Burton S, Jeewajee A, O'Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinman JR, Brandon MP, Climer JR, Chapman GW, Hasselmo ME. Multiple Running Speed Signals in Medial Entorhinal Cortex. Neuron. 2016;91:666–679. doi: 10.1016/j.neuron.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Kropff E, Carmichael JE, Moser MB, Moser EI. Speed cells in the medial entorhinal cortex. Nature. 2015;523:419–424. doi: 10.1038/nature14622. Data showing neurons that selectively code running speed in entorhinal cortex, consistent with previous studies showing coding of running speed by grid cells and head direction cells. [DOI] [PubMed] [Google Scholar]
- 22.Tingley D, Alexander AS, Kolbu S, de Sa VR, Chiba AA, Nitz DA. Task-phase-specific dynamics of basal forebrain neuronal ensembles. Front Syst Neurosci. 2014;8:174. doi: 10.3389/fnsys.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander AS, Nitz DA. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci. 2015;18:1143–1151. doi: 10.1038/nn.4058. [DOI] [PubMed] [Google Scholar]
- 24.Nitz DA. Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nat Neurosci. 2012;15:1365–1367. doi: 10.1038/nn.3213. [DOI] [PubMed] [Google Scholar]
- 25.Giocomo LM, Moser MB, Moser EI. Computational models of grid cells. Neuron. 2011;71:589–603. doi: 10.1016/j.neuron.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Bush D, Barry C, Burgess N. What do grid cells contribute to place cell firing? Trends Neurosci. 2014;37:136–145. doi: 10.1016/j.tins.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Bush D, Burgess N. A hybrid oscillatory interference/continuous attractor network model of grid cell firing. J Neurosci. 2014;34:5065–5079. doi: 10.1523/JNEUROSCI.4017-13.2014. Biologically detailed spiking model of network dynamics showing how oscillatory interference can account for multiple aspects of physiological data on grid cells, including theta phase precession and accurate firing location when reintroduced to the environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasselmo ME. Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120523. doi: 10.1098/rstb.2012.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess N, Barry C, O'Keefe J. An oscillatory interference model of grid cell firing. Hippocampus. 2007;17:801–812. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widloski J, Fiete IR. A model of grid cell development through spatial exploration and spike time-dependent plasticity. Neuron. 2014;83:481–495. doi: 10.1016/j.neuron.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 31.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the 'cognitive map'. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 32.Si B, Kropff E, Treves A. Grid alignment in entorhinal cortex. Biol Cybern. 2012;106:483–506. doi: 10.1007/s00422-012-0513-7. [DOI] [PubMed] [Google Scholar]
- 33.Cheung A. Probabilistic Learning by Rodent Grid Cells. PLoS Comput Biol. 2016;12:e1005165. doi: 10.1371/journal.pcbi.1005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stachenfeld KL, Botvinick MM, Gershman SJ. Design principles of the hippocampal cognitive map. Advances in Neural Information Processing Systems. 2014;27:2528–2536. [Google Scholar]
- 35.Heys JG, Rangarajan KV, Dombeck DA. The functional micro-organization of grid cells revealed by cellular-resolution imaging. Neuron. 2014;84:1079–1090. doi: 10.1016/j.neuron.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeewajee A, Barry C, O'Keefe J, Burgess N. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus. 2008;18:1175–1185. doi: 10.1002/hipo.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H. Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015;35:8394–8410. doi: 10.1523/JNEUROSCI.4460-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandecasteele M, Varga V, Berenyi A, Papp E, Bartho P, Venance L, Freund TF, Buzsaki G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci U S A. 2014;111:13535–13540. doi: 10.1073/pnas.1411233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1516134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron. 2015;86:1253–1264. doi: 10.1016/j.neuron.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, Williams S. Optogenetic Activation of Septal Glutamatergic Neurons Drive Hippocampal Theta Rhythms. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016;36:3016–3023. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgess N, Jackson A, Hartley T, O'Keefe J. Predictions derived from modelling the hippocampal role in navigation. Biol Cybern. 2000;83:301–312. doi: 10.1007/s004220000172. [DOI] [PubMed] [Google Scholar]
- 44.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 45.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82:789–796. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Raudies F, Hasselmo ME. Differences in Visual-Spatial Input May Underlie Different Compression Properties of Firing Fields for Grid Cell Modules in Medial Entorhinal Cortex. PLoS Comput Biol. 2015;11:e1004596. doi: 10.1371/journal.pcbi.1004596. Model showing how the differential response of grid cells to movement of environmental barriers could be due to differential coding of visual features in the dorsal versus ventral visual field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krupic J, Bauza M, Burton S, Lever C, O'Keefe J. How environment geometry affects grid cell symmetry and what we can learn from it. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130188. doi: 10.1098/rstb.2013.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bicanski A, Burgess N. Environmental Anchoring of Head Direction in a Computational Model of Retrosplenial Cortex. J Neurosci. 2016;36:11601–11618. doi: 10.1523/JNEUROSCI.0516-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Hardcastle K, Ganguli S, Giocomo LM. Environmental boundaries as an error correction mechanism for grid cells. Neuron. 2015;86:827–839. doi: 10.1016/j.neuron.2015.03.039. Data showing that the accuracy of grid cell firing decreases with distance from boundaries, supporting a role of boundaries in resetting the location code. [DOI] [PubMed] [Google Scholar]
- 51.Koene RA, Hasselmo ME. First-in-first-out item replacement in a model of short-term memory based on persistent spiking. Cereb Cortex. 2007;17:1766–1781. doi: 10.1093/cercor/bhl088. [DOI] [PubMed] [Google Scholar]
- 52.Lisman JE, Jensen O. The theta-gamma neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 54.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 55.Saravanan V, Arabali D, Jochems A, Cui AX, Gootjes-Dreesbach L, Cutsuridis V, Yoshida M. Transition between encoding and consolidation/replay dynamics via cholinergic modulation of CAN current: A modeling study. Hippocampus. 2015;25:1052–1070. doi: 10.1002/hipo.22429. [DOI] [PubMed] [Google Scholar]
- 56.Hasselmo ME. A model of episodic memory: mental time travel along encoded trajectories using grid cells. Neurobiol Learn Mem. 2009;92:559–573. doi: 10.1016/j.nlm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luczak A, McNaughton BL, Harris KD. Packet-based communication in the cortex. Nat Rev Neurosci. 2015;16:745–755. doi: 10.1038/nrn4026. [DOI] [PubMed] [Google Scholar]
- 59.Sreenivasan S, Fiete I. Grid cells generate an analog error-correcting code for singularly precise neural computation. Nat Neurosci. 2011;14:1330–1337. doi: 10.1038/nn.2901. [DOI] [PubMed] [Google Scholar]
- 60.Hasselmo ME. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18:1213–1229. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz Y, Kath WL, Spruston N, Hasselmo ME. Coincidence detection of place and temporal context in a network model of spiking hippocampal neurons. PLoS Comput Biol. 2007;3:e234. doi: 10.1371/journal.pcbi.0030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 64.Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubie JL, Fenton AA. Linear look-ahead in conjunctive cells: an entorhinal mechanism for vector-based navigation. Front Neural Circuits. 2012;6:20. doi: 10.3389/fncir.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bush D, Barry C, Manson D, Burgess N. Using Grid Cells for Navigation. Neuron. 2015;87:507–520. doi: 10.1016/j.neuron.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erdem UM, Hasselmo M. A goal-directed spatial navigation model using forward trajectory planning based on grid cells. Eur J Neurosci. 2012;35:916–931. doi: 10.1111/j.1460-9568.2012.08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erdem UM, Hasselmo ME. A biologically inspired hierarchical goal directed navigation model. J Physiol Paris. 2014;108:28–37. doi: 10.1016/j.jphysparis.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spiers HJ, Gilbert SJ. Solving the detour problem in navigation: a model of prefrontal and hippocampal interactions. Front Hum Neurosci. 2015;9:125. doi: 10.3389/fnhum.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olafsdottir HF, Barry C, Saleem AB, Hassabis D, Spiers HJ. Hippocampal place cells construct reward related sequences through unexplored space. Elife. 2015;4:e06063. doi: 10.7554/eLife.06063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chrastil ER, Sherrill KR, Hasselmo ME, Stern CE. There and Back Again: Hippocampus and Retrosplenial Cortex Track Homing Distance during Human Path Integration. J Neurosci. 2015;35:15442–15452. doi: 10.1523/JNEUROSCI.1209-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sherrill KR, Chrastil ER, Ross RS, Erdem UM, Hasselmo ME, Stern CE. Functional connections between optic flow areas and navigationally responsive brain regions during goal-directed navigation. Neuroimage. 2015;118:386–396. doi: 10.1016/j.neuroimage.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schon K, Tinaz S, Somers DC, Stern CE. Delayed match to object or place: an event-related fMRI study of short-term stimulus maintenance and the role of stimulus pre-exposure. Neuroimage. 2008;39:857–872. doi: 10.1016/j.neuroimage.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fransén E, Lansner A. A model of cortical associative memory based on a horizontal network of connected columns. Network. 1998;9:235–264. [PubMed] [Google Scholar]
- 77.Lundqvist M, Compte A, Lansner A. Bistable, irregular firing and population oscillations in a modular attractor memory network. PLoS Comput Biol. 2010;6:e1000803. doi: 10.1371/journal.pcbi.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK. Gamma and Beta Bursts Underlie Working Memory. Neuron. 2016;90:152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 80.Burgess N, Hitch G. Computational models of working memory: putting long-term memory into context. Trends Cogn Sci. 2005;9:535–541. doi: 10.1016/j.tics.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 81.Rajan K, Harvey CD, Tank DW. Recurrent Network Models of Sequence Generation and Memory. Neuron. 2016;90:128–142. doi: 10.1016/j.neuron.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Howard MW, MacDonald CJ, Tiganj Z, Shankar KH, Du Q, Hasselmo ME, Eichenbaum H. A unified mathematical framework for coding time, space, and sequences in the hippocampal region. J Neurosci. 2014;34:4692–4707. doi: 10.1523/JNEUROSCI.5808-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itskov V, Curto C, Pastalkova E, Buzsaki G. Cell assembly sequences arising from spike threshold adaptation keep track of time in the hippocampus. J Neurosci. 2011;31:2828–2834. doi: 10.1523/JNEUROSCI.3773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tiganj Z, Hasselmo ME, Howard MW. A simple biophysically plausible model for long time constants in single neurons. Hippocampus. 2015;25:27–37. doi: 10.1002/hipo.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miall R. The storage of time intervals using oscillating neurons. Neural Comput. 1989;1:359–371. [Google Scholar]
- 86.Hasselmo ME, Stern CE. Theta rhythm and the encoding and retrieval of space and time. Neuroimage. 2014;85(Pt 2):656–666. doi: 10.1016/j.neuroimage.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87**.Kraus BJ, Brandon MP, Robinson RJ, 2nd, Connerney MA, Hasselmo ME, Eichenbaum H. During Running in Place, Grid Cells Integrate Elapsed Time and Distance Run. Neuron. 2015;88:578–589. doi: 10.1016/j.neuron.2015.09.031. Data showing that neurons that fire as grid cells during foraging will fire as time cells during running on a treadmill, confirming predictions of the oscillatory interference model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88**.Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci. 2015;18:282–288. doi: 10.1038/nn.3904. Study showing that the same inactivation of medial septum that impairs the spatial firing of grid cells will also prevent the temporal specificity of hippocampal firing during running in a running wheel. [DOI] [PubMed] [Google Scholar]
- 89.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whiteman AS, Young DE, Budson AE, Stern CE, Schon K. Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morphometry study. Neuroimage. 2016;126:229–238. doi: 10.1016/j.neuroimage.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burak Y, Fiete IR. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput Biol. 2009;5:e1000291. doi: 10.1371/journal.pcbi.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]