Abstract
Allergic disease prevalence has significantly increased in recent decades. Primary prevention efforts are being guided by the study of the exposome, or collective environmental exposures beginning during the prenatal period, to identify modifiable factors that impact allergic disease risk. In this review, we explore the evidence supporting a relationship between key components of the external exposome in the prenatal and early-life periods and their impact on atopy development, focused on microbial, allergen, and air pollution exposures. The abundance and diversity of microbial exposures during the first months and years of life have been linked with risk of allergic sensitization and disease. Indoor environmental allergen exposure during early life may also impact disease development, depending on the allergen type, dose, and timing of exposure. Recent evidence supports the role of ambient air pollution in allergic disease inception. The lack of clarity in the literature surrounding the relationship between environment and atopy reflects the complex interplay between cumulative environmental factors and genetic susceptibility, such that no one factor dictates disease development in all individuals. Understanding the impact of the summation of environmental exposures throughout a child's development is needed to identify cost-effective interventions that reduce atopy risk in children.
Keywords: environment, allergy, asthma, exposure, microbiome, infection, endotoxin, allergen, air pollution, tobacco smoke
Introduction
Like many chronic health conditions, allergic disease likely results from complex gene-environment interactions. Mapping of the human genome has advanced our understanding of genetic risk factors for allergic diseases. However, the increase in prevalence of allergic disease over the past few decades has occurred too rapidly to be accounted for by changes in the genome alone and is more likely to be the result of changes in environmental factors, in some cases accompanied by epigenetic changes. These observations have led to increasing interest in understanding the impact of the exposome on the development of atopic disease. In 2005, Christopher Wild framed our understanding of the exposome concept to include three types of exposures: 1) the general external environment including factors such as the urban–rural residence, climate factors, air pollution, social capital and education; 2) one's specific external environment including diet, physical activity, tobacco exposure, infection, and occupation; and 3) the internal environment that includes the biological and metabolic/toxicological manifestations of these exposures in the body 1. In this review, we explore the impact of a variety of environmental exposures in early life that have been found to influence the development of allergic disease, with particular focus on exposures to microbes, allergens, and ambient air pollutants.
Microbial Exposure
The rise in prevalence of allergic disease, particularly in the western world, has coincided with significant environmental changes that have reduced microbial exposure in early life, such as improved sanitation and increased rates of immunization. Many have proposed that among genetically-susceptible individuals, these changes in environmental conditions may alter normal development of the immune system and thus affect susceptibility to allergic disease, the basis of the “hygiene hypothesis” 2. In this section, we will discuss key findings from studies examining both endogenous and exogenous microbial exposures.
The host microbiome
The human microbiome consists of all microbial communities within the body, including the gut, airways, skin, and others. Alteration of the host microbiome is suspected to play a role in susceptibility to allergic disease, particularly during early life coinciding with maturation of the immune system. Establishment of the microbiome begins in utero and is likely the result of maternal transmission 3-5. During infancy, differences in the gut microbial environment between those who go on to develop atopy and those who do not are apparent in the first few months of life. Reduced diversity of stool flora at age one month was predictive of atopic eczema at age 2 and allergic sensitization and allergic rhinitis at age 6 6. Similarly, diversity of microbial species in the infant gut was shown to be inversely related to risk of atopic sensitization, allergic rhinitis, and eosinophilia 7. Atopic children showed reduced early life colonization with Lactobacilli 8, Bifidobacteria 8, 9 and Bacteroides and increased colonization with Clostridia 8 and yeasts 9. A greater abundance of Bacteroides and Lactobacillus have been associated with protection against allergy, while abundance of Clostridia has been associated with wheezing, allergic sensitization, and atopic eczema 10, 11.
Microbial colonization of the airways also begins early in life. Colonization with Streptococcus at age 2 months was associated with increased risk for earlier first lower respiratory tract illness (LRTI), which has been linked with later asthma development 12. Similarly, in a study from the COPSAC birth cohort, asymptomatic one-month-old neonates colonized with Streptococcus pneumoniae, Moraxella catarrhalis, or Haemophilus influenzae via hypopharyngeal aspirate were at greater risk of a first wheezing episode, persistent wheeze, severe exacerbation of wheeze, and hospitalization for wheeze 13. Lower airway colonization with these organisms was also associated with higher blood eosinophil counts and total IgE levels, but not specific IgE levels, at 4 years and with bronchodilator reversibility and development of asthma at 5 years. In a study of children under 3 years hospitalized for viral-induced wheezing, 60% demonstrated nasopharyngeal (NP) colonization with Streptococcus pneumoniae, Moraxella catarrhalis, or Haemophilus influenzae, and this was associated with increased risk of recurrent wheezing episodes during the following year 14. Importantly, antibiotic use may select for these organisms 12.
Many factors can impact microbial colonization in infants and young children, including prenatal and postnatal antibiotic exposure, mode of delivery, and early diet. Wu et al identified dose-dependent relationships between risk of childhood asthma and maternal urinary tract infections (UTIs) during pregnancy or infant antibiotic use during the first year of life 15. The increase in risk is presumably due to changes in the abundance and diversity of the host's commensal microbes, as demonstrated by Penders et al, who reported that antibiotic use in infancy was associated with decreased abundance of Bifidobacteria and Bacteroides 16. Mode of delivery is also an important determinant of the infant microbiome 17, though the impact of vaginal versus caesarean delivery on development of allergic disease is debated. Vaginally-delivered infants tended to be colonized with vaginal (Lactobacillus) and fecal (Prevotella) flora, whereas infants born by caesarean section tended to be colonized by skin flora (Staphylococcus, Corynebacterium) 17 with increased abundance of Clostridium difficile and reduced Bifidobacteria and Bacteroides 16. Meta-analyses of studies examining the association between delivery mode and allergic disease in Western countries found an increased risk of childhood asthma 18, 19, allergic rhinitis 18, and food allergy 18 in children born by caesarean section compared to vaginal births. However, studies from outside the U.S. and Europe have not consistently shown these effects 20-22. Diet during early life may also be important for establishing the infant's microbiome. Breastfeeding was associated with greater microbial diversity compared to formula feeding 23, and a recent study reported that breastfeeding was associated with a trend towards increased Bifidobacterium and reduced Clostridia at 3 to 6 months of age 24. Despite this evidence, it remains unclear whether these differences in the infant microbiome promote development of allergy or merely serve as a marker of immune dysregulation early in life that leads to allergic disease.
The external microbial environment
Exposure to abundant and diverse microbes in the environment appears to augment the risk of allergic disease. The “biodiversity hypothesis” suggests that reduced exposure during childhood to the rich environmental microbiome inherent within natural green spaces impedes the cultivation of a robust host microbial community, leading to immune dysregulation, though the exact mechanisms of this interplay are unknown 25. To this effect, Ruokolainen and colleagues demonstrated that children living in homes surrounded by forests and agricultural land had lower rates of aeroallergen sensitization compared to their counterparts in industrialized environments 26. In addition to environmental biodiversity, specific microbial products have been identified as key players in immune tolerance. Endotoxin, a component of gram negative bacterial cell walls and a marker of microbial exposure, was among the first microbial products implicated in protection against atopic asthma and other allergic diseases 27-30. The Allergy and Endotoxin (ALEX) cross-sectional survey found that higher levels of endotoxin in child mattresses were associated with reduced risk of allergic sensitization, hay fever symptoms, and atopic asthma 27. The precise mechanism for this effect is not known. One theory suggests that increased exposure to environmental endotoxin leads to down-regulation of inflammatory responses 27. Others have suggested the protective effect is the result of polymorphisms within the CD14 gene, which encodes a co-receptor for toll-like receptor 4 (TLR4) with high specificity for lipopolysaccharide, and in fact a recent systematic review found a significant gene-environment interaction between CD14 polymorphisms and microbial exposure 31. The timing of endotoxin exposure also appears to be important, with more significant protective effects seen with early life exposure 28, 32.
The microbial environment within the home is dictated by its human and non-human occupants. For instance, homes with dogs contain richer more diverse bacterial communities compared to homes without pets 33. Differences in microbial exposure are seen with increasing family size 15 as well as with certain activities of the occupants, particularly farming. It has long been recognized that the prevalence of allergic disease amongst the children of farmers is lower than in non-farming families 28, 34-36. A number of studies have been conducted to identify farm-specific factors, such as consumption of raw milk 37-40 and exposure to high amounts of endotoxin in animal stables, that influence inception of allergic disease. A recently published study compared the incidence of allergic disease in children from an Amish traditional farming community to those from a Hutterite community that, while genetically similar to the Amish, practices modern industrial farming. The authors reported a significantly lower prevalence of allergic disease in Amish children. Amish homes were found to contain higher levels of endotoxin in airborne house dust, and comparisons of bacteria isolated from mattress dust showed distinct microbial profiles between Amish and Hutterite households. To further examine the effects of the Amish environment on allergy, the authors administered Amish house dust intranasally to OVA-sensitized mice and demonstrated reduced airway hyper-responsiveness (AHR) and bronchoalveolar lavage (BAL) eosinophilia in response to ovalbumin (OVA) challenge. Conversely, intranasal administration of Hutterite house dust led to exacerbation of OVA-induced AHR and eosinophilia. Though the precise components responsible for this protective effect are unknown, the inhibitory effects of Amish house dust for OVA-mediated inflammation were reduced in MyD88 and TRIF deficient mice, suggesting the protective effects were primarily mediated by the innate immune response 41.
Role of childhood respiratory infections
For asthma development in particular, there is abundant literature suggesting a critical role for viral respiratory infection in early life. Respiratory syncytial virus (RSV) and human rhinovirus (HRV) are most frequently associated with wheezing episodes in young children 42-44. In the prospective cohort RSV Bronchiolitis in Early Life (REBEL) study, approximately 50% of the 206 infants with LRTI due to RSV during the first 12 months of life subsequently developed persistent asthma up to age 7 45. Similarly, in a case control study of infants hospitalized with RSV versus healthy controls followed to age 18, a significantly higher prevalence of current asthma was seen in the RSV group compared to controls; RSV was the major risk factor in subsequent asthma development (OR 7.2, 95% CI 2.1, 23.9) 43.
Others have found that HRV infection may be equally or more important for development of asthma than RSV. Asthma development by school age was four times greater in wheezing infants with HRV infection compared to infants with other viruses in one study 44. In the COAST birth cohort, a group at high risk for development of asthma, HRV-induced wheezing in infancy was the strongest predictor of persistent wheezing at age 3 and diagnosis of asthma at age 6 46, 47. Prevention of viral infection in early life has been proposed as a strategy for primary prevention of asthma, but so far this has not been possible given the difficulties with synthesizing an effective vaccine against HRV. A few trials have been conducted with palivizumab, or RSV-specific IgG. Simoes et al reported a lower incidence of recurrent wheezing in premature infants treated with palivizumab compared to untreated controls 48. In a randomized controlled trial, Blanken et al found that premature infants receiving palivizumab had fewer wheezing days during the first year of life 49. Whether these effects would translate into reduced incidence of asthma remains unknown.
Indoor Allergen Exposure
The indoor environmental allergen milieu is of particular interest in the study of the determinants of allergic disease due to constant exposure during early childhood and the potential for intervention. In samples taken from 831 homes across the US, at least six detectable allergens were found in more than fifty percent of homes 50. Allergens from house dust mite (HDM), furred pets (cats and dogs), mice, cockroaches, and fungi comprise the most common indoor allergens implicated in atopic disease 51-53. The strong relationship between allergic sensitization and development of allergic rhinitis and asthma has been well documented 53-57. Recently, Rubner et al demonstrated that aeroallergen sensitization before the age of five significantly increased the risk of asthma with persistence into adolescence 54. While the role of allergen sensitization in the pathogenesis of allergic rhinitis and asthma is clear, the causal relationship between individual allergen exposure and the development of these conditions has been more difficult to delineate, likely due to the complexity of interactions between various environmental factors, the timing and dose of exposure, and genetic predisposition. Similarly, the direct effect of indoor allergens on the development of atopic dermatitis (AD) has not been clearly established, though the correlation between indoor allergen sensitization and disease activity is better understood 58-60. In turn, AD enhances development of allergic rhinitis and asthma by providing an epicutaneous route of sensitization to aeroallergens via transepidermal water loss and epidermal barrier dysfunction 61. In this section, we briefly discuss some of the existing literature surrounding each of the indoor allergens and their role in allergic disease.
House Dust Mite Exposure
House dust mite (HDM) allergen has long been implicated as an important determinant of atopic disorders. An early prospective study by Sporik et al followed 65 children from birth to 11 years of age and demonstrated increased risk of allergic sensitization and asthma in children exposed to high levels of HDM during the first year of life 62. Subsequently, similar studies have provided evidence for a causal relationship between HDM sensitization early in life and allergic rhinitis, persistent wheeze, and asthma 63-67. While sensitization has a positive correlation with asthma development, studies from European birth cohorts have established that exposure to HDM alone is not sufficient to incur an increased risk of asthma 68, 69, indicating that IgE sensitization is the bridge between allergen exposure and asthma development; however, innate immune responses triggered by HDM have also been implicated in allergic disease pathogenesis 70. Tovey et al suggest a nonlinear dose-response relationship between HDM exposure and development of allergic disease, with exposure to both very low and very high levels of allergen correlating with decreased risk of sensitization and asthma and exposure to intermediate levels of allergen correlating with increased risk 71. Whether host or concomitant environmental factors alter the otherwise positive association between HDM exposure and atopy at these critically low and high levels remains unknown. Exposure to intermediate concentrations of HDM allergen has also been linked to atopic disease severity 72, 73. More recent studies have focused on differing routes of early HDM exposure, such as placental and breastmilk transfer, as additional potential risk factors for development of allergic respiratory disease 74, 75.
Household Pest Exposure
In addition to HDM, exposure to mouse and cockroach allergens have been linked to allergic disease prevalence and severity, particularly in urban settings 76-86. In a prospective birth cohort of 505 infants of atopic parents from the metropolitan Boston area, Gold et al found that exposure to cockroach allergen levels in the family room greater than 0.05 units/gram of dust was an independent predictor for early wheeze 79. Evidence for cockroach exposure as a risk factor for persistent wheeze and asthma was provided by an evaluation of 222 siblings of the above infants, with those exposed to higher concentrations of allergen having the greatest risk 80. Data from the same Boston cohort also illustrated an association between early mouse allergen exposure and early wheeze 82 and a non-statistically significant trend towards predicting asthma, allergic rhinitis, and AD at 7 years of age 87. In a similar prospective study of infants followed for three years, Donohue and colleagues not only found a significant effect of mouse and cockroach sensitization on the prevalence of AD, allergic rhinitis, and asthma but also demonstrated a dose-response relationship between higher cockroach or mouse-specific IgE levels and increased prevalence of allergic disease 78. Thus, the evidence for mouse and cockroach allergen exposure predicting the development of atopic disorders, especially in inner city children, is compelling.
Furred Pet Exposure
In contrast, a plethora of contradictory associations exists between furred pet exposure and development of atopy. Studies examining the link between pet ownership and risk of atopic disease have generally focused on the most common household pets, cats and dogs, or examined pet keeping in general. The ubiquitous nature of cat and dog allergens 88-90 makes epidemiological studies assessing the risk of “exposure” quite difficult. To this effect, Liccardi et al questioned whether surveys regarding the presence of pets in the home or home allergen measurements are sufficient to accurately convey an individual's exposure 91. A number of systematic reviews examining the effect of pet exposure on allergic disease have been conducted, a few of which will be highlighted here. Takkouche et al reviewed cohort studies from 1996-2007 that assessed pet allergen exposure 92. While dog exposure had no significant effect on asthma, exposure to cat allergen yielded a relative risk (RR) for asthma of 0.72 (95% CI, 0.55, 0.93); exposure to either pet was found to be slightly protective for allergic rhinitis (RR 0.79, 95% CI, 0.68, 0.93). No associations between asthma and early exposures to cat and dog allergens were found in 17 and 13 birth cohorts included in a systematic review, respectively 93. Finally, pooled analysis of data from 11 European birth cohorts found no effect of early pet ownership on asthma development or allergic rhinitis when examining mutually-exclusive pet ownership categories (cat only, dog only, cat and dog only, bird only, or rodent only) 94. Overall, the cumulative evidence suggests no increase in risk of developing allergic disease from pet allergen exposure, with a possible decreased risk of asthma associated with cat allergen exposure in one study. More recent studies focus on subgroup analyses, which may explain some of the variability in results of existing data 95.
Indoor Fungal Exposure
Similar to pet allergens, fungi are ubiquitous in both indoor and outdoor environments, and both predictive and protective associations of indoor fungal exposure on atopic disorders have been discovered. Qualitative assessments of fungal exposure in the form of mildew odor or visible mold have been linked with increased risk of allergic rhinitis and asthma 96-98. This finding is corroborated by studies using quantitative fungal measures, such as DNA-based analyses 99 and β-1,3-glucan measurements 100, 101. In a longitudinal birth cohort of high-risk infants, Iossifova et al demonstrated an increased risk of asthma with exposure to low levels of β-1,3-glucan but a protective effect upon exposure to levels of β-1,3-glucan greater than 60 micrograms per gram of dust 97, 100, suggesting a possible nonlinear dose-response relationship similar to that observed for HDM allergen. However, while increased levels of β-1,3-glucan may certainly indicate greater fungal concentrations, it may also represent a more diverse fungal population. In fact, exposure to greater fungal diversity offered protection against sensitization to aeroallergens and early childhood wheeze in a German longitudinal birth cohort, mirroring the protective effects of microbial diversity in the human microbiome 102. Importantly, the predictive effect of fungal exposure on asthma development seems to occur independently of fungal sensitization. Zhang et al demonstrated that non-allergenic components of fungi promote T-helper type 17 (Th17) responses by direct activation of innate immune receptors 103. Fungal components also potentiate allergen-induced T-helper type 2 (Th2) responses through non-IgE mediated pathways. The mechanism by which greater fungal diversity confers protection against allergic disease remains unclear.
Overall, convincing data exists for the role of environmental indoor allergens in the pathogenesis of allergic disorders. The positive effect of multifaceted environmental interventions on disease prevalence and morbidity 104, 105 highlights this point. Further studies are needed that examine the complex interplay of environment and genetics to determine the most effective intervention strategies for reducing the risk of allergic disease.
Ambient Air Pollution Exposure
Great strides have been made in understanding the impact of environmental air pollutants on population health, which has impacted environmental health policy and consequently improved public health. However, despite overall improvements in air quality, indoor and outdoor air pollutants continue to cause adverse health effects and have been recently shown to promote the onset of atopic disease.
The World Health Organization (WHO) reported in 2016 that 92% of the world's population lives in places where air quality levels exceed the WHO's Ambient Air quality guidelines for annual mean of particulate matter (PM) with a diameter of less than 2.5 micrometers (PM2.5) 106. Those thought to be especially susceptible to the effects of air pollution include the very young and those of lower socioeconomic status, due to increased exposure to pollutants in poor housing conditions. The respiratory tract is particularly susceptible to air pollution, due to continuous exposure to the ambient environment. In this section, we will discuss the effects of two key sources of environmental air pollution, traffic related air pollution (TRAP) and environmental tobacco smoke (ETS), on the development of allergic airway disease. Markers of TRAP include (but are not limited to) carbon monoxide, nitrogen oxides (NOx), black carbon, PM, benzene, and ultrafine particles.
Effects of TRAP on Lung Development and Asthma Risk
Numerous studies throughout the world have shown that TRAP (particularly PM2.5) negatively impacts lung development 107-109 with potential consequences for the development of asthma and chronic obstructive pulmonary disease. The effects of early life exposure, both prenatal and postnatal, are of particular interest in efforts to prevent detrimental effects on lung development.
The Spanish Infancia y Medio Ambiente (INMA) cohort examined the association of TRAP exposure during specific trimesters of pregnancy on lung function in children aged 4.5 years 110. Exposure to higher levels of benzene and nitrogen dioxide (NO2) during the second trimester of pregnancy was associated with increased risk of clinically-significant low lung function (Forced expiratory volume in 1 second [FEV1] less than 80% of predicted). These studies collectively demonstrate that exposure to air pollution has systemic implications, with significant consequences for fetal lung development. Efforts to promote prevention focus on examination of prenatal exposures on asthma development. The Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, an urban pregnancy cohort, found that prenatal exposures to black carbon and PM were associated with a significant risk of wheezing by age 2 111. Moreover, exposure to increased PM2.5 levels during the second trimester of pregnancy was significantly associated with asthma at age 6, particularly in boys 112.
Postnatal exposure to TRAP, particularly during the first years of life, is also an important determinant of lung function and development of asthma. A birth cohort from the Boston metropolitan area recruited between 1999-2002 demonstrated that TRAP was associated with reduced lung development in elementary-school aged children as measured by spirometry 113. Despite improvements in PM2.5 levels (below current EPA standards) for most of the cohort during the study, lifetime and prior year exposure to TRAP was associated with a reduction in forced vital capacity (FVC), and exposure to PM2.5 was specifically associated with higher odds of clinically-significant airway obstruction. A Swedish birth cohort examined the impact of TRAP during the first year of life on lung function in later childhood 114, 115. At age 8, exposure to PM10 during the first year of life had a bigger impact on reduced FEV1 in children sensitized to food and aeroallergens, with less effect on lung function if exposure occurred later in childhood. Additionally, high exposure to NO2 during the first year of life was associated with increased odds of having significantly decreased FEV1 and FVC.
Although many studies have demonstrated an association between early life exposure to pollutants and asthma or persistent wheezing 116-123, conflicting evidence recently emerged from a cross-sectional examination of five European birth cohorts, where no associations were found between air pollutant exposure and asthma prevalence 124. To further examine these discrepancies, a recent longitudinal examination using birth cohort data from over 14,000 children from the Netherlands, Germany, and Sweden evaluated the relationship of annual air pollution concentrations (from birth through age 14-16 years) with asthma and rhinoconjunctivitis incidence and prevalence 125. This study, using both meta-analyses and pooled analyses, found that increasing exposure to NO2 and PM2.5 at the birth address were associated with increased asthma incidence through adolescence. There was no effect of air pollutants on rhinoconjunctivitis. Other European and North American cohorts have shown that increased childhood exposure to PM2.5 and black carbon were associated with increased risk of asthma at age 12 126.
Effects of ETS on Asthma and Allergy Risk
The most profound prenatal and early life influences of air pollution, though, emerge from ETS. Despite great improvements in reducing the rate of smoking and second hand smoke (SHS) exposure in the US population, approximately 25% of nonsmokers in the US (58 million people) were still exposed to SHS in 2011-2012 127. Of this number, 15 million of the exposed were children ages 3-11. ETS exposure has been identified as a major risk factor for asthma 128, 129 and allergic sensitization 130, especially with in utero or early-life exposures. The health effects of maternal exposure to SHS have more recently been elucidated. Pooled analyses from 15 European birth cohorts found that children whose mothers were exposed to SHS during pregnancy were more likely to wheeze at age 2; this risk was further increased by postnatal SHS exposure, and further increased in children of atopic families 131. SHS exposure during pregnancy alone (in nonsmoking mothers) was recently found to be associated with physician-diagnosed asthma at age 7 132. With the increasing popularity of e-cigarettes throughout the world, it will be imperative to examine the effects of vaporized nicotine, vehicles (such as propylene glycol) and an endless variety of flavoring agents on the development of asthma and airway disease.
Pollutants and Allergen Sensitization
A Swedish birth cohort 133 found that high exposure to NO2 during the first year of life was associated with increased risk of sensitization to pollens (assessed by blood IgE) at age 4, and food sensitization at age 8. In North American cohorts, diesel exhaust particle exposure in the first year of life was associated with greater aeroallergen sensitization in early childhood than children with low exposure 134, 135. Children who were aeroallergen sensitized and had high exposure to TRAP during the first year of life had an almost 3-fold higher risk of asthma development compared to children who were not sensitized to allergens 135, nor did sensitization alone increase the risk of asthma in children exposed to low levels of TRAP, suggesting that the combination of early life pollutant exposure and allergic sensitization contributes to asthma development. Exposure to elevated levels of TRAP during infancy has also been associated with atopy to foods and perennial aeroallergens at age 1 136. These effects may extend to the prenatal period. The EDEN birth cohort study found that increased maternal exposure to PM10 was associated with reduced number of infant regulatory T cells (Tregs) and increased CD8+ T cells at birth 137, with potential to increase risk of atopy development and/or affecting responses to viral infection in these infants.
The strongest links between pollutant exposure and allergic sensitization relate to ETS exposure. In the absence of maternal smoking, exposure to SHS in infancy was associated with an increased risk of food sensitization at ages 4, 8 and 16 years 138 and an increased risk of eczema with allergic sensitization. The German Lifestyle and Environmental Factors and their Influence on Newborns Allergy risk (LINA) birth cohort found that increased exposure to products of ETS during pregnancy was associated with increased Eosinophil/Basophil (Eo/B) progenitor cells in cord blood, and that cord blood IL-4 and IL-13 levels were associated with the development of these progenitor cells 139. This group later described that maternal smoking or ETS exposure during pregnancy was associated with reduced numbers of infant regulatory T (Treg) cell numbers at birth, and that these infants with reduced numbers of Tregs had increased risk of allergic sensitization in the first year of life 140.
Potential Mechanisms for effects of Air Pollution on Development of Atopic Disease
Recent mechanistic studies investigating the effect of pollutant exposure on the development of allergic disease have been guided by key findings from birth cohorts, including the role of oxidative stress on promoting epigenetic modifications that regulate gene expression of Tregs through microRNAs (miRNAs) and DNA methylation 129, 141. miRNAs are small, noncoding RNAs that repress target protein expression through numerous mechanisms, including destabilizing mRNA and translational silencing 142 . ETS exposure during pregnancy was associated with increased maternal and cord blood miRNA-223 expression 143, a miRNA previously linked with Treg development and function 144-146. Increased miRNA-223 was associated with reduced Tregs in maternal and cord blood; in turn, these reduced Treg numbers at birth were associated with increased risk of atopic dermatitis during the first 3 years of life.
Among the most common mechanisms for epigenetic modifications is DNA methylation, where increased methylation at the promoter and at the 3′ end silences a gene, negatively correlating with gene expression. Increased FOXP3 methylation from salivary DNA was associated with increased pollutant exposure during childhood, impacting both increased risk of asthma diagnosis at age 7 147 and asthma severity 148. Although pollutant-induced epigenetic modifications are not restricted to Treg development and function, their dysregulation has potential to impact the development of allergic disease over the lifespan 149.
Conclusions and Future Directions
The impact of microbial, allergen, and air pollutant exposures have been artificially subdivided in this review; in reality, these exposures and many others interact simultaneously with each other to promote or prevent allergic disease (Figure 1). For example, air pollution and climate change can promote oxidative stress in pollen-producing plants, increasing both the amounts and allergenicity of pollen grains 150. Individual factors identified in this review as protective for or promoting the development of atopy are summarized in Table 1. The types of exposures explored here are in no way exhaustive, as many other exposures in early life have been linked with allergy and asthma development, including diet, obesity, pharmaceuticals, lifestyle factors, and maternal psychological stress in the prenatal and postnatal period. For example, Vitamin D deficiency in the first decade of life was recently associated with increased susceptibility to sensitization and AD by age 3 and increased risk of persistent asthma by age 10 151. Additionally, the impact of maternal psychological stress on atopic disease development is being pursued by the South Korean Cohort for Childhood Origin of Asthma and Allergic Diseases (COCOA), who recently demonstrated that increased prenatal maternal depression or anxiety were associated with increased risk of infant atopic dermatitis by age 6 months 152.
Figure 1. Interplay of Early Life Exposures that Impact Allergic Disease Development.
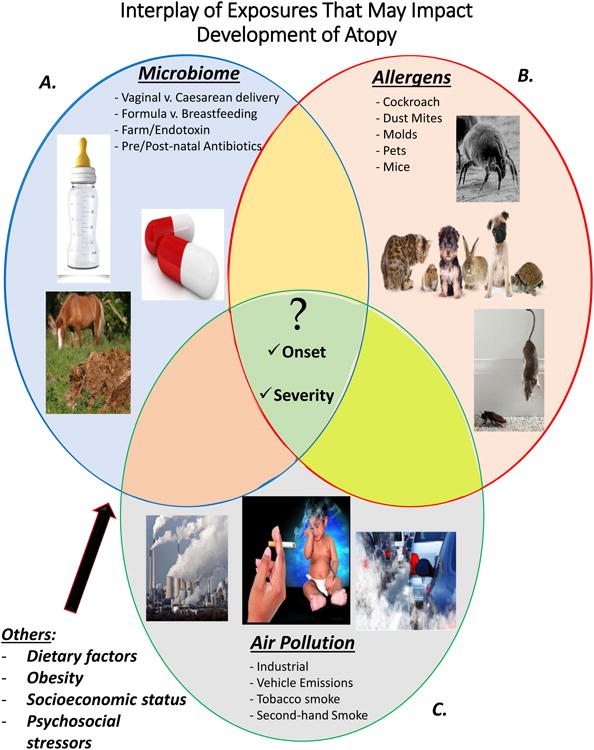
Allergic disease development is influenced by many different factors. This figure displays several of the identified environmental triggers that increase susceptibility to allergic disease. A) Common behaviors and determinants that influence an individual's microbiome and external microbial environment, thereby influencing susceptibility to allergic disease. B) Allergens that have been linked to development of atopy. C) Air pollution exposures that not only influence lung function but also contribute to the immune response. Collectively, this Venn diagram demonstrates the overlapping contributions of each exposure. The center of the figure shows the questions that have yet to be elucidated, including how any of the numerous combinations of these common exposures influence the onset of disease or impact severity of disease.
Table 1. Early Life Exposures Impacting Allergic Disease Development.
| Protective against Allergic Disease | Ref. | Promoting Allergic Disease | Ref. |
|---|---|---|---|
| Vaginal Birth | 16-19 | Prenatal/Postnatal Antibiotics | 15-16 |
| Increased Microbial/Fungal Diversity | 6-11; 23-26; 102 | Viral lower respiratory tract infection (RSV, HRV) | 42-47 |
| Increased number of siblings; later birth order | 15 | House Dust Mite, Cockroach, Mouse, and Fungal Exposure | 62-67; 71-75; 76-86; 96-101 |
| Farm Exposure | 28; 34-40 | Prenatal/Postnatal Exposure to Air Pollution | 110-123; 125-126; 133-136 |
| Endotoxin Exposure | 27-33 | Prenatal /Postnatal Tobacco Smoke Exposure | 131-132; 138-140 |
Because humans do not develop in exposure silos, future studies should focus on identifying the impact of the summation of these exposures with different degrees of interaction amongst them in addition to the epigenetic effects of these exposures. This approach can more effectively identify the interventions that will have the greatest impact, both at the legislative and individual levels, on the development and sequelae of atopic disease. Examples of proposed interventions to date are provided in Figure 2. Several research initiatives focused on the exposome are being pursued in Europe (EXPOsOMICS, HELIX, and Heals) and the United States (Hercules) to simultaneously assess a large set of exposures while linking these exposures with the body's response via ‘-omics approaches. These investments may identify atopy risk factors that are underestimated, or perhaps are yet undiscovered. Use of environment-wide association study (EWAS) approaches to identify associations with adverse health effects will bring their own challenges, highlighted by recent publications noting that some exposures may be highly correlated amongst each other, and some may be synergistic to promote adverse health effects 153. Additionally, it will be challenging to identify the effect of an early life exposure on the sensitivity to future exposures over the lifespan. Exposome-focused projects are inherently expensive at this time given the large sample sizes required to assess numerous exposures, in addition to the use of numerous tools required to assess both external and internal exposome components, including the use of environmental monitoring technology (using both geographic information system-based pollutant models and personal sensors) and ‘-omics technologies. The cost and efficiency of applying an exposome-focused approach to human disease will have to be improved in order to make discoveries with the highest impact.
Figure 2. Potential Interventions Reducing Risk of Allergic Disease Development.
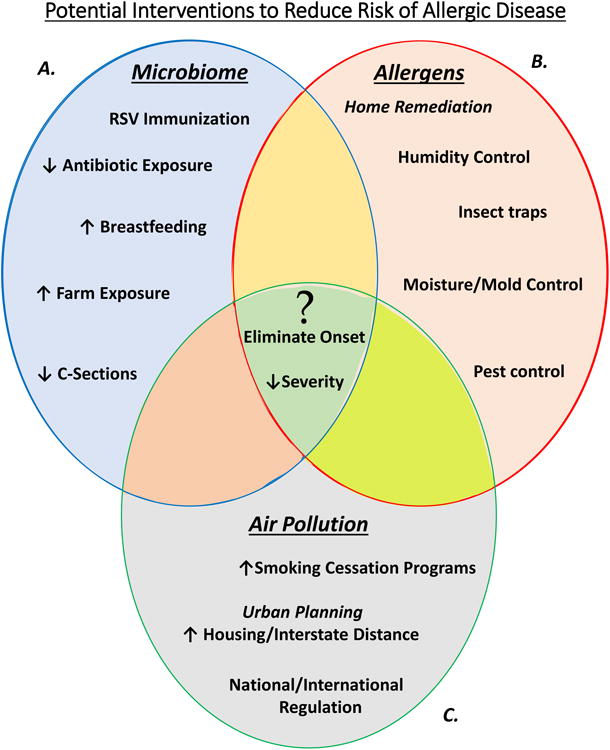
A) Certain modifications can positively influence an individual's microbiome or select for a protective external microbial environment. B) Reducing the quantity of allergen exposure may reduce development of allergic disease. C) Although the highest pollutant exposures occur from exposure to environmental tobacco smoke, mitigating industrial and traffic related air pollution will have to come from national and local regulatory agencies throughout the world. The center of the figure highlights the questions that have yet to be elucidated. Could these interventions impact the incidence of allergic disease, and if so, which are the most pragmatic, cost-effective, and have the highest potential impact in different societies?
In the interim, integrating data from numerous birth cohort studies throughout the world will aide in more clearly defining the environmental determinants driving atopy and perhaps elucidate the optimal time to implement interventions. The Mechanisms of the Development of ALLergy (MeDALL) is incorporating data from over 44,000 European children participating in birth cohort studies, and the Environmental Influences on Child Health Outcomes (ECHO) program in the United States includes over 50,000 children. Large-scale birth cohort studies with harmonized exposure and outcome assessments throughout the world will need to be pursued as we define the peak susceptibility periods to a variety of exposures during the prenatal and postnatal periods and which exposures relevant to specific geographical areas should be the primary targets for interventions. An important example of harmonized outcome assessments is clearly delineating between allergic vs non-allergic asthma when assessing the effect of various exposures on this outcome. Once key exposures and potential interventions are identified, an integrative approach amongst clinicians, children and their caregivers, health care organizations, insurance providers, government agencies, and urban planners must be undertaken to establish cost-effective primary and secondary prevention strategies to reduce these risks and promote wellness.
Search Strategy.
Relevant publications were retrieved from the PubMed Databases using the search terms below as well from review of references of the publications identified with this search.
(microbiome) OR biodiversity) OR endotoxin) OR farm) OR infection) OR viral)) AND (wheeze) OR asthma) OR atop*) OR eczema) OR allergy) OR sensitization)) AND (((early life) OR child*) OR infant)
(Indoor allergen) OR dust mite) OR cockroach) OR mouse) OR pet) OR cat) OR dog) OR fungi)) AND (wheeze) OR asthma) OR atop*) OR eczema) OR allerg*) OR sensitization)) AND (((early life) OR child*) OR infant)
(TRAP) OR Pollut*) OR Tobacco smoke) OR second hand smoke) OR PM) OR NO)) AND (Lung function) OR Spirometry) OR FEV) OR Pulmonary function)) AND (((early life) OR child*) OR infant)
(TRAP) OR Pollut*) OR Tobacco smoke) OR second hand smoke) OR PM) OR NO)) AND (wheeze) OR asthma) OR atop*) OR eczema) OR allerg*) OR sensitization)) AND (early life) OR child*) OR infant)
| What do we know? |
| The microbiome differs between atopic and non-atopic people, and these differences are seen in the first few months of life. |
| Children exposed to farms and biodiversity in green spaces during early life are at reduced risk of developing allergic disease, while early life exposure to HDM, cockroach, mice, and fungi promotes development of allergic disease. |
| Wheezing episodes associated with RSV and HRV infection increase the risk of asthma in later childhood. |
| There is no clear evidence that cat or dog exposure promotes or prevents development of atopy. |
| Prenatal and early life exposure to both indoor and outdoor air pollutants negatively impacts lung development and increases risk of wheezing and asthma. |
| Maternal smoking or exposure to SHS increases the risk of asthma, allergic sensitization, and atopic dermatitis. |
| What is still unknown? |
| Does alteration of the diversity and abundance of commensal microbes in early life directly impact development of allergic disease or are these differences merely markers of immune dysregulation? |
| What are the mechanisms by which early life farm exposure imparts protection against allergic disease? |
| Can asthma development be prevented by RSV and/or HRV immunization? |
| What air quality standards are sufficient to prevent detrimental effects on lung development and atopic disease in children? |
| When are children most susceptible to the effects of air pollution (prenatal, first year of life, or cumulative lifetime exposure), and what personalized interventions can be developed to reduce the risks associated with pollution exposure? |
| What are the most cost-effective environmental intervention strategies to prevent allergic disease, and how do these strategies vary geographically? |
Acknowledgments
Sources of support: AJB is supported by 2T32GM086330. DBP is supported by R01ES023349 and P30ES010126. MLH is supported by the AAAAI ARTrust™ Gail G. Shapiro Clinical Faculty Development Award.
Abbreviations
- LRTI
lower respiratory tract illness
- UTI
urinary tract infection
- NP
nasopharyngeal
- TLR-4
toll-like receptor 4
- OVA
ovalbumin
- BAL
bronchoalveolar lavage
- AHR
airway hyper-responsiveness
- RSV
respiratory syncytial virus
- HRV
human rhinovirus
- HDM
house dust mite
- AD
atopic dermatitis
- Th17
T-helper type 17
- Th2
T-helper type 2
- TRAP
traffic-related air pollution
- PM
particulate matter
- ETS
environmental tobacco smoke
- NOx
nitrogen oxides
- NO2
nitrogen dioxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- SHS
second-hand smoke
- Eo/B
eosinophil/basophil progenitor cell
- Treg
regulatory T cell
- miRNA
microRNA
Footnotes
Conflicts of Interest: The authors have nothing to disclose.
References
- 1.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–50. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 2.Fishbein AB, Fuleihan RL. The hygiene hypothesis revisited: does exposure to infectious agents protect us from allergy? Curr Opin Pediatr. 2012;24:98–102. doi: 10.1097/MOP.0b013e32834ee57c. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez E, Fernandez L, Marin ML, Martin R, Odriozola JM, Nueno-Palop C, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51:270–4. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez E, Marin ML, Martin R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159:187–93. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Rautava S, Collado MC, Salminen S, Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102:178–84. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–40. 40 e1–2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Boyce JA, Bochner B, Finkelman FD, Rothenberg ME. Advances in mechanisms of asthma, allergy, and immunology in 2011. J Allergy Clin Immunol. 2012;129:335–41. doi: 10.1016/j.jaci.2011.12.968. [DOI] [PubMed] [Google Scholar]
- 8.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–91. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55 e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132:601–7 e8. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 14.Jartti T, Kuneinen S, Lehtinen P, Peltola V, Vuorinen T, Leinonen M, et al. Nasopharyngeal bacterial colonization during the first wheezing episode is associated with longer duration of hospitalization and higher risk of relapse in young children. Eur J Clin Microbiol Infect Dis. 2011;30:233–41. doi: 10.1007/s10096-010-1075-z. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, Feldman AS, Rosas-Salazar C, James K, Escobar G, Gebretsadik T, et al. Correction: Relative Importance and Additive Effects of Maternal and Infant Risk Factors on Childhood Asthma. PLoS One. 2016;11:e0156473. doi: 10.1371/journal.pone.0156473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38:634–42. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 19.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629–33. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 20.Park YH, Kim KW, Choi BS, Jee HM, Sohn MH, Kim KE. Relationship between mode of delivery in childbirth and prevalence of allergic diseases in Korean children. Allergy Asthma Immunol Res. 2010;2:28–33. doi: 10.4168/aair.2010.2.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menezes AM, Hallal PC, Matijasevich AM, Barros AJ, Horta BL, Araujo CL, et al. Caesarean sections and risk of wheezing in childhood and adolescence: data from two birth cohort studies in Brazil. Clin Exp Allergy. 2011;41:218–23. doi: 10.1111/j.1365-2222.2010.03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan AM, de Bruyne J, Khalid F, Arumugam K. Caesarean section and asthma in Malaysian children: a case-control study. Asian Pac J Allergy Immunol. 2012;30:204–8. [PubMed] [Google Scholar]
- 23.Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB, et al. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13:r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3-6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART) J Allergy Clin Immunol. 2017;139:482–91 e14. doi: 10.1016/j.jaci.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A. 2012;109:8334–9. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruokolainen L, von Hertzen L, Fyhrquist N, Laatikainen T, Lehtomaki J, Auvinen P, et al. Green areas around homes reduce atopic sensitization in children. Allergy. 2015;70:195–202. doi: 10.1111/all.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 28.Riedler J, Eder W, Oberfeld G, Schreuer M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy. 2000;30:194–200. doi: 10.1046/j.1365-2222.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- 29.Gehring U, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med. 2002;166:939–44. doi: 10.1164/rccm.200203-256OC. [DOI] [PubMed] [Google Scholar]
- 30.Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–3. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 31.Lau MY, Dharmage SC, Burgess JA, Lowe AJ, Lodge CJ, Campbell B, et al. CD14 polymorphisms, microbial exposure and allergic diseases: a systematic review of gene-environment interactions. Allergy. 2014;69:1440–53. doi: 10.1111/all.12454. [DOI] [PubMed] [Google Scholar]
- 32.Mendy A, Cohn RD, Thorne PS. Endotoxin exposure, serum vitamin D, asthma and wheeze outcomes. Respir Med. 2016;114:61–6. doi: 10.1016/j.rmed.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126:410–2. 2 e1–3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012;129:1470–7.e6. doi: 10.1016/j.jaci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Ernst P, Cormier Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am J Respir Crit Care Med. 2000;161:1563–6. doi: 10.1164/ajrccm.161.5.9908119. [DOI] [PubMed] [Google Scholar]
- 36.Karadag B, Ege MJ, Scheynius A, Waser M, Schram-Bijkerk D, van Hage M, et al. Environmental determinants of atopic eczema phenotypes in relation to asthma and atopic sensitization. Allergy. 2007;62:1387–93. doi: 10.1111/j.1398-9995.2007.01505.x. [DOI] [PubMed] [Google Scholar]
- 37.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014;133:551–9. doi: 10.1016/j.jaci.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Schroder PC, Illi S, Casaca VI, Lluis A, Bock A, Roduit C, et al. A switch in regulatory T cells through farm exposure during immune maturation in childhood. Allergy. 2016 doi: 10.1111/all.13069. [DOI] [PubMed] [Google Scholar]
- 39.Brick T, Schober Y, Bocking C, Pekkanen J, Genuneit J, Loss G, et al. omega-3 fatty acids contribute to the asthma-protective effect of unprocessed cow's milk. J Allergy Clin Immunol. 2016;137:1699–706 e13. doi: 10.1016/j.jaci.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 40.Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol. 2011;128:766–73 e4. doi: 10.1016/j.jaci.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 41.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375:411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–81. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 44.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100.e3. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Blanken MO, Rovers MM, Bont L Dutch RSVNN. Respiratory syncytial virus and recurrent wheeze. N Engl J Med. 2013;369:782–3. doi: 10.1056/NEJMc1307429. [DOI] [PubMed] [Google Scholar]
- 50.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–84 e2. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck AF, Huang B, Kercsmar CM, Guilbert TW, McLinden DJ, Lierl MB, et al. Allergen sensitization profiles in a population-based cohort of children hospitalized for asthma. Ann Am Thorac Soc. 2015;12:376–84. doi: 10.1513/AnnalsATS.201408-376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–22. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 54.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139:501–7. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–6. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 56.Stoltz DJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy. 2013;43:233–41. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 58.Schafer T, Heinrich J, Wjst M, Adam H, Ring J, Wichmann HE. Association between severity of atopic eczema and degree of sensitization to aeroallergens in schoolchildren. J Allergy Clin Immunol. 1999;104:1280–4. doi: 10.1016/s0091-6749(99)70025-4. [DOI] [PubMed] [Google Scholar]
- 59.Peroni DG, Piacentini GL, Bodini A, Rigotti E, Pigozzi R, Boner AL. Prevalence and risk factors for atopic dermatitis in preschool children. Br J Dermatol. 2008;158:539–43. doi: 10.1111/j.1365-2133.2007.08344.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim J, Lee S, Woo SY, Han Y, Lee JH, Lee IY, et al. The indoor level of house dust mite allergen is associated with severity of atopic dermatitis in children. J Korean Med Sci. 2013;28:74–9. doi: 10.3346/jkms.2013.28.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boralevi F, Hubiche T, Leaute-Labreze C, Saubusse E, Fayon M, Roul S, et al. Epicutaneous aeroallergen sensitization in atopic dermatitis infants - determining the role of epidermal barrier impairment. Allergy. 2008;63:205–10. doi: 10.1111/j.1398-9995.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 62.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 63.Carter PM, Peterson EL, Ownby DR, Zoratti EM, Johnson CC. Relationship of house-dust mite allergen exposure in children's bedrooms in infancy to bronchial hyperresponsiveness and asthma diagnosis by age 6 to 7. Ann Allergy Asthma Immunol. 2003;90:41–4. doi: 10.1016/S1081-1206(10)63612-5. [DOI] [PubMed] [Google Scholar]
- 64.Celedon JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TA, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;120:144–9. doi: 10.1016/j.jaci.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Llanora GV, Ming LJ, Wei LM, van Bever HP. House dust mite sensitization in toddlers predict persistent wheeze in children between eight to fourteen years old. Asia Pac Allergy. 2012;2:181–6. doi: 10.5415/apallergy.2012.2.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lodge CJ, Lowe AJ, Gurrin LC, Hill DJ, Hosking CS, Khalafzai RU, et al. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. J Allergy Clin Immunol. 2011;128:782–8.e9. doi: 10.1016/j.jaci.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 67.Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139:541–9.e8. doi: 10.1016/j.jaci.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Casas L, Sunyer J, Tischer C, Gehring U, Wickman M, Garcia-Esteban R, et al. Early-life house dust mite allergens, childhood mite sensitization, and respiratory outcomes. Allergy. 2015;70:820–7. doi: 10.1111/all.12626. [DOI] [PubMed] [Google Scholar]
- 69.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–7. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 70.Jacquet A. Innate immune responses in house dust mite allergy. ISRN Allergy. 2013;2013:735031. doi: 10.1155/2013/735031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tovey ER, Almqvist C, Li Q, Crisafulli D, Marks GB. Nonlinear relationship of mite allergen exposure to mite sensitization and asthma in a birth cohort. J Allergy Clin Immunol. 2008;122:114–8. 8 e1–5. doi: 10.1016/j.jaci.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Harris JM, Williams HC, White C, Moffat S, Mills P, Newman Taylor AJ, et al. Early allergen exposure and atopic eczema. Br J Dermatol. 2007;156:698–704. doi: 10.1111/j.1365-2133.2006.07710.x. [DOI] [PubMed] [Google Scholar]
- 73.Huang JL, Chen CC, Kuo ML, Hsieh KH. Exposure to a high concentration of mite allergen in early infancy is a risk factor for developing atopic dermatitis: a 3-year follow-up study. Pediatr Allergy Immunol. 2001;12:11–6. doi: 10.1034/j.1399-3038.2001.012001011.x. [DOI] [PubMed] [Google Scholar]
- 74.Baiz N, Macchiaverni P, Tulic MK, Rekima A, Annesi-Maesano I, Verhasselt V. Early oral exposure to house dust mite allergen through breast milk: A potential risk factor for allergic sensitization and respiratory allergies in children. J Allergy Clin Immunol. 2017;139:369–72.e10. doi: 10.1016/j.jaci.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 75.Richgels PK, Yamani A, Chougnet CA, Lewkowich IP. Maternal house dust mite exposure during pregnancy enhances severity of HDM-induced asthma in murine offspring. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132:830–5.e1-2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Vera MJ, Drapkin S, Moy JN. Association of recurrent wheezing with sensitivity to cockroach allergen in inner-city children. Ann Allergy Asthma Immunol. 2003;91:455–9. doi: 10.1016/S1081-1206(10)61513-X. [DOI] [PubMed] [Google Scholar]
- 78.Donohue KM, Al-alem U, Perzanowski MS, Chew GL, Johnson A, Divjan A, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008;122:914–20. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–36. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 80.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001;107:41–7. doi: 10.1067/mai.2001.111143. [DOI] [PubMed] [Google Scholar]
- 81.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97:514–20. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 82.Phipatanakul W, Celedon JC, Sredl DL, Weiss ST, Gold DR. Mouse exposure and wheeze in the first year of life. Ann Allergy Asthma Immunol. 2005;94:593–9. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr. 2017;171:31–8. doi: 10.1001/jamapediatrics.2016.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fishbein AB, Lee TA, Cai M, Oh SS, Eng C, Hu D, et al. Sensitization to mouse and cockroach allergens and asthma morbidity in urban minority youth: Genes-environments and Admixture in Latino American (GALA-II) and Study of African-Americans, Asthma, Genes, and Environments (SAGE-II) Ann Allergy Asthma Immunol. 2016;117:43–9.e1. doi: 10.1016/j.anai.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Silva JM, Camara AA, Tobias KR, Macedo IS, Cardoso MR, Arruda E, et al. A prospective study of wheezing in young children: the independent effects of cockroach exposure, breast-feeding and allergic sensitization. Pediatr Allergy Immunol. 2005;16:393–401. doi: 10.1111/j.1399-3038.2005.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torjusen EN, Diette GB, Breysse PN, Curtin-Brosnan J, Aloe C, Matsui EC. Dose-response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents. Indoor Air. 2013;23:268–74. doi: 10.1111/ina.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phipatanakul W, Celedon JC, Hoffman EB, Abdulkerim H, Ryan LM, Gold DR. Mouse allergen exposure, wheeze and atopy in the first seven years of life. Allergy. 2008;63:1512–8. doi: 10.1111/j.1398-9995.2008.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Friedman W, Zeldin DC. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2004;114:111–7. doi: 10.1016/j.jaci.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 89.Zahradnik E, Raulf M. Animal allergens and their presence in the environment. Front Immunol. 2014;5:76. doi: 10.3389/fimmu.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perzanowski MS, Ronmark E, James HR, Hedman L, Schuyler AJ, Bjerg A, et al. Relevance of specific IgE antibody titer to the prevalence, severity, and persistence of asthma among 19-year-olds in northern Sweden. J Allergy Clin Immunol. 2016;138:1582–90. doi: 10.1016/j.jaci.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liccardi G, Salzillo A, Calzetta L, Piccolo A, Menna G, Rogliani P. Can the presence of cat/dog at home be considered the only criterion of exposure to cat/dog allergens? A likely underestimated bias in clinical practice and in large epidemiological studies. Eur Ann Allergy Clin Immunol. 2016;48:61–4. [PubMed] [Google Scholar]
- 92.Takkouche B, Gonzalez-Barcala FJ, Etminan M, Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy. 2008;63:857–64. doi: 10.1111/j.1398-9995.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 93.Chen CM, Tischer C, Schnappinger M, Heinrich J. The role of cats and dogs in asthma and allergy--a systematic review. Int J Hyg Environ Health. 2010;213:1–31. doi: 10.1016/j.ijheh.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Lodrup Carlsen KC, Roll S, Carlsen KH, Mowinckel P, Wijga AH, Brunekreef B, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One. 2012;7:e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wegienka G, Havstad S, Kim H, Zoratti E, Ownby D, Woodcroft KJ, et al. Subgroup differences in the associations between dog exposure during the first year of life and early life allergic outcomes. Clin Exp Allergy. 2017;47:97–105. doi: 10.1111/cea.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho SH, Reponen T, LeMasters G, Levin L, Huang J, Meklin T, et al. Mold damage in homes and wheezing in infants. Ann Allergy Asthma Immunol. 2006;97:539–45. doi: 10.1016/S1081-1206(10)60947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iossifova YY, Reponen T, Bernstein DI, Levin L, Kalra H, Campo P, et al. House dust (1-3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–13. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharpe RA, Thornton CR, Tyrrell J, Nikolaou V, Osborne NJ. Variable risk of atopic disease due to indoor fungal exposure in NHANES 2005-2006. Clin Exp Allergy. 2015;45:1566–78. doi: 10.1111/cea.12549. [DOI] [PubMed] [Google Scholar]
- 99.Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, et al. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann Allergy Asthma Immunol. 2011;107:120–6. doi: 10.1016/j.anai.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 100.Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, et al. Mold exposure during infancy as a predictor of potential asthma development. Ann Allergy Asthma Immunol. 2009;102:131–7. doi: 10.1016/S1081-1206(10)60243-8. [DOI] [PubMed] [Google Scholar]
- 101.Maheswaran D, Zeng Y, Chan-Yeung M, Scott J, Osornio-Vargas A, Becker AB, et al. Exposure to Beta-(1,3)-D-glucan in house dust at age 7-10 is associated with airway hyperresponsiveness and atopic asthma by age 11-14. PLoS One. 2014;9:e98878. doi: 10.1371/journal.pone.0098878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tischer C, Weikl F, Probst AJ, Standl M, Heinrich J, Pritsch K. Urban Dust Microbiome: Impact on Later Atopy and Wheezing. Environ Health Perspect. 2016;124:1919–23. doi: 10.1289/EHP158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Z, Biagini Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA, et al. beta-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. J Allergy Clin Immunol. 2017;139:54–65.e8. doi: 10.1016/j.jaci.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maas T, Kaper J, Sheikh A, Knottnerus JA, Wesseling G, Dompeling E, et al. Mono and multifaceted inhalant and/or food allergen reduction interventions for preventing asthma in children at high risk of developing asthma. Cochrane Database Syst Rev. 2009:Cd006480. doi: 10.1002/14651858.CD006480.pub2. [DOI] [PubMed] [Google Scholar]
- 105.Capristo C, Romei I, Boner AL. Environmental prevention in atopic eczema dermatitis syndrome (AEDS) and asthma: avoidance of indoor allergens. Allergy. 2004;59(78):53–60. doi: 10.1111/j.1398-9995.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 106.2016 Available from http://www.who.int/mediacentre/news/releases/2016/air-pollution-estimates/en/
- 107.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 108.Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J, et al. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect. 2013;121:1357–64. doi: 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pino-Yanes M, Thakur N, Gignoux CR, Galanter JM, Roth LA, Eng C, et al. Genetic ancestry influences asthma susceptibility and lung function among Latinos. J Allergy Clin Immunol. 2015;135:228–35. doi: 10.1016/j.jaci.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morales E, Garcia-Esteban R, de la Cruz OA, Basterrechea M, Lertxundi A, de Dicastillo MD, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax. 2015;70:64–73. doi: 10.1136/thoraxjnl-2014-205413. [DOI] [PubMed] [Google Scholar]
- 111.Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133:713–22 e4. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am J Respir Crit Care Med. 2015;192:1052–9. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rice MB, Rifas-Shiman SL, Litonjua AA, Oken E, Gillman MW, Kloog I, et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am J Respir Crit Care Med. 2016;193:881–8. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schultz ES, Gruzieva O, Bellander T, Bottai M, Hallberg J, Kull I, et al. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186:1286–91. doi: 10.1164/rccm.201206-1045OC. [DOI] [PubMed] [Google Scholar]
- 115.Schultz ES, Hallberg J, Bellander T, Bergstrom A, Bottai M, Chiesa F, et al. Early-Life Exposure to Traffic-related Air Pollution and Lung Function in Adolescence. Am J Respir Crit Care Med. 2016;193:171–7. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 116.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;188:309–18. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brunekreef B, Stewart AW, Anderson HR, Lai CK, Strachan DP, Pearce N, et al. Self-reported truck traffic on the street of residence and symptoms of asthma and allergic disease: a global relationship in ISAAC phase 3. Environ Health Perspect. 2009;117:1791–8. doi: 10.1289/ehp.0800467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 119.Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Kunzli N, Avol E, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–8. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118:1021–6. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishimura KK, Iwanaga K, Oh SS, Pino-Yanes M, Eng C, Keswani A, et al. Early-life ozone exposure associated with asthma without sensitization in Latino children. J Allergy Clin Immunol. 2016;138:1703–6 e1. doi: 10.1016/j.jaci.2016.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brunst KJ, Ryan PH, Brokamp C, Bernstein D, Reponen T, Lockey J, et al. Timing and Duration of Traffic-related Air Pollution Exposure and the Risk for Childhood Wheeze and Asthma. Am J Respir Crit Care Med. 2015;192:421–7. doi: 10.1164/rccm.201407-1314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ranciere F, Bougas N, Viola M, Momas I. Early Exposure to Traffic-Related Air Pollution, Respiratory Symptoms at 4 Years of Age, and Potential Effect Modification by Parental Allergy, Stressful Family Events, and Gender: A Prospective Follow-up Study of the PARIS Birth Cohort. Environ Health Perspect. 2016 doi: 10.1289/EHP239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Molter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2015;45:610–24. doi: 10.1183/09031936.00083614. [DOI] [PubMed] [Google Scholar]
- 125.Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Bruske I, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3:933–42. doi: 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- 126.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70:245–56. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- 127.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Vital signs: disparities in nonsmokers' exposure to secondhand smoke--United States, 1999-2012. MMWR Morb Mortal Wkly Rep. 2015;64:103–8. [PMC free article] [PubMed] [Google Scholar]
- 128.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 129.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010;126:453–65. doi: 10.1016/j.jaci.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lannero E, Wickman M, van Hage M, Bergstrom A, Pershagen G, Nordvall L. Exposure to environmental tobacco smoke and sensitisation in children. Thorax. 2008;63:172–6. doi: 10.1136/thx.2007.079053. [DOI] [PubMed] [Google Scholar]
- 131.Vardavas CI, Hohmann C, Patelarou E, Martinez D, Henderson AJ, Granell R, et al. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur Respir J. 2016;48:115–24. doi: 10.1183/13993003.01016-2015. [DOI] [PubMed] [Google Scholar]
- 132.Simons E, To T, Moineddin R, Stieb D, Dell SD. Maternal second-hand smoke exposure in pregnancy is associated with childhood asthma development. J Allergy Clin Immunol Pract. 2014;2:201–7. doi: 10.1016/j.jaip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 133.Gruzieva O, Bellander T, Eneroth K, Kull I, Melen E, Nordling E, et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol. 2012;129:240–6. doi: 10.1016/j.jaci.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 134.Codispoti CD, LeMasters GK, Levin L, Reponen T, Ryan PH, Biagini Myers JM, et al. Traffic pollution is associated with early childhood aeroallergen sensitization. Ann Allergy Asthma Immunol. 2015;114:126–33. doi: 10.1016/j.anai.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol. 2015;136:295–303 e7. doi: 10.1016/j.jaci.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sbihi H, Allen RW, Becker A, Brook JR, Mandhane P, Scott JA, et al. Perinatal Exposure to Traffic-Related Air Pollution and Atopy at 1 Year of Age in a Multi-Center Canadian Birth Cohort Study. Environ Health Perspect. 2015;123:902–8. doi: 10.1289/ehp.1408700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baiz N, Slama R, Bene MC, Charles MA, Kolopp-Sarda MN, Magnan A, et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn's cord blood lymphocyte subpopulations. The EDEN study cohort BMC. Pregnancy Childbirth. 2011;11:87. doi: 10.1186/1471-2393-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thacher JD, Gruzieva O, Pershagen G, Neuman A, van Hage M, Wickman M, et al. Parental smoking and development of allergic sensitization from birth to adolescence. Allergy. 2016;71:239–48. doi: 10.1111/all.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Junge KM, Hornig F, Herberth G, Roder S, Kohajda T, Rolle-Kampczyk U, et al. The LINA cohort: Cord blood eosinophil/basophil progenitors predict respiratory outcomes in early infancy. Clin Immunol. 2014;152:68–76. doi: 10.1016/j.clim.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 140.Hinz D, Bauer M, Roder S, Olek S, Huehn J, Sack U, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67:380–9. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- 141.Gruzieva O, Xu CJ, Breton CV, Annesi-Maesano I, Anto JM, Auffray C, et al. Epigenome-Wide Meta-Analysis of Methylation in Children Related to Prenatal NO2 Air Pollution Exposure. Environ Health Perspect. 2017;125:104–10. doi: 10.1289/EHP36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 143.Herberth G, Bauer M, Gasch M, Hinz D, Roder S, Olek S, et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2014;133:543–50. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 144.Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T, et al. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–27. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liston A, Lu LF, O'Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 147.Brunst KJ, Leung YK, Ryan PH, Khurana Hershey GK, Levin L, Ji H, et al. Forkhead box protein 3 (FOXP3) hypermethylation is associated with diesel exhaust exposure and risk for childhood asthma. J Allergy Clin Immunol. 2013;131:592–4 e1-3. doi: 10.1016/j.jaci.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–52 e10. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 149.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–45. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schiavoni G, D'Amato G, Afferni C. The dangerous liaison between pollens and pollution in respiratory allergy. Ann Allergy Asthma Immunol. 2017;118:269–75. doi: 10.1016/j.anai.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 151.Hollams EM, Teo SM, Kusel M, Holt BJ, Holt KE, Inouye M, et al. Vitamin D over the first decade and susceptibility to childhood allergy and asthma. The Journal of allergy and clinical immunology. 2017;139:472–81.e9. doi: 10.1016/j.jaci.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 152.Chang HY, Suh DI, Yang SI, Kang MJ, Lee SY, Lee E, et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. 2016;138:468–75 e5. doi: 10.1016/j.jaci.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 153.Siroux V, Agier L, Slama R. The exposome concept: a challenge and a potential driver for environmental health research. Eur Respir Rev. 2016;25:124–9. doi: 10.1183/16000617.0034-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


