Abstract
The reticular activating system (RAS) is not an amorphous region but distinct nuclei with specific membrane properties that dictate their firing during waking and sleep. The locus coeruleus and raphe nucleus fire during waking and slow wave sleep, with the pedunculopontine nucleus (PPN) firing during both waking and REM sleep, the states manifesting arousal-related EEG activity. Two important discoveries in the PPN in the last 10 years are, 1) that some PPN cells are electrically coupled, and 2) every PPN cell manifests high threshold calcium channels that allow them to oscillate at beta/gamma band frequencies. The role of arousal in drug abuse is considered here in terms of the effects of drugs of abuse on these two mechanisms. Drug abuse and the perception of withdrawal/relapse are mediated by neurobiological processes that occur only when we are awake, not when we are asleep. These relationships focus on the potential role of arousal, more specifically of RAS electrical coupling and gamma band activity, in the addictive process as well as the relapse to drug use.
Keywords: Cocaine, connexin 36, dopamine, modafinil, N- and P/Q-type calcium channels, preconscious awareness
1. The Control of Arousal
1.1 Waking and drug abuse
Drug abuse only occurs during waking. We experience withdrawal or relapse to drug abuse only when awake. Higher motor activation while awake has been associated with greater addiction liability. The ventral tegmental area (VTA), a key neural substrate for the modulation of drug abuse, is modulated by reticular activating system (RAS) output, particularly from the pedunculopontine nucleus (PPN). The RAS modulates oscillating rhythms between the thalamus, hypothalamus, basal forebrain, and cortex that are characterized in the EEG during wake-sleep states [1]. The RAS is not an amorphous, unspecific region but rather a group of distinct nuclei with specific intrinsic membrane properties that dictate their firing frequencies during waking and sleep. All three main nuclei in the RAS, the PPN that is partly cholinergic, the locus coeruleus (LC), which is mainly noradrenergic, and the raphe nucleus (RN), which is mainly serotonergic, have been described as affecting neural substrates related to addiction like the VTA. These VTA inputs are critical because the basal activity of VTA neurons can be functionally associated to vulnerability to drug abuse [2].
The PPN contains cholinergic, glutamatergic, and GABAergic neurons [3]. The cholinergic output has a net excitatory effect on LC and RN neurons [4, 5]. Importantly, one of the targets of PPN non-cholinergic neurons is the VTA [6]. Cholinergic efferents from the PPN to the VTA form a loop that includes the medial prefrontal cortex (mPFC). This loop is composed of mPFC glutamatergic efferents to dopaminergic (DA) and GABAergic neurons in the VTA and to the nucleus accumbens (NAcc) through a polysynaptic circuit that includes the PPN. In addition, VTA sends dopaminergic and GABAergic efferents to the NAcc. Activation of the PPN thus increases VTA dopaminergic output, and increases extracellular DA levels in the NAcc and mPFC [7], which suggests that the PPN in part regulates the reward and motivational functions of the VTA [8]. In turn, increased glutamatergic efferent activation from the mPFC would in turn reduce VTA dopaminergic output through its direct activation of local GABAergic interneurons in the VTA. Recent optogenetic experiments confirmed that PPN-VTA pathway stimulation can induce psychostimulant-like behavior in the absence of drug administration [9].
The noradrenergic input from the LC to the PPN is inhibitory, presumably via alpha 2 adrenergic receptors [10]. Furthermore, the RN sends inhibitory serotonergic projections to both PPN and the LC [11]. Other inhibitory inputs to the PPN come from GABAergic neurons in the substantia nigra (SN), an area also involved in the locomotor activation produced by psychostimulant administration. Since midbrain dopaminergic neurons originating in the VTA and SN pars compacta (SNc) have been previously described as the neural substrates underlying individual vulnerability to drug addiction [12, 13, 14], understanding the functional modulation of the VTA and SNc by the RAS is key to understanding how reinforcing, drug craving, and the effects of drugs of abuse are modulated by a wake-promoting nucleus such as the PPN.
1.2 State-dependent activity
The LC and RN fire during waking and also during slow wave sleep, with the pedunculopontine nucleus (PPN) being the only RAS cell group firing during both waking and rapid eye movement (REM) sleep, states of high frequency beta/gamma band EEG activity [15]. Single cell recordings in PPN in vivo identified several categories of thalamic projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital wave generation [16]. Some PPN neurons fired at low rates (<10 Hz), but most fired in the beta/gamma range (20–80 Hz). PPN neurons exhibit beta/gamma frequencies in vivo during active waking and REM sleep, but not during slow wave sleep [16–21]. Similarly, the presence of gamma band activity has been confirmed in the PPN in relation to the cortical EEG of the cat in vivo when the animal is active [16, 22]; and in the region of the PPN in humans during stepping, but not at rest [23]. A recent study showed that PPN neurons exhibited low firing frequencies ~10 Hz at rest, but the same neurons fired at gamma band frequencies when the animal woke up, or when the animal began walking on a treadmill [24]. That is, the same cells were involved in both arousal and motor control. Thus, there is ample evidence for gamma band activity during active waking and movement in the PPN in vitro, in vivo, and across species, including man.
PPN neurons showed increased firing rates during REM sleep, named “REM-on” cells, or both waking and REM sleep, named “Wake/REM-on” cells, and some cells fired only during waking, called “Wake-on” cells [16, 17, 19], suggestive of increased excitation only during activated states. Stimulation of the PPN potentiated the appearance of beta/gamma oscillations in the cortical EEG, outlasting stimulation by 10–20 seconds [25]. These findings emphasize the fact that PPN neurons are mainly active during states of arousal marked by high frequency EEG activity such as waking and REM sleep.
1.3 Major Breakthroughs
1.3.1 Electrical coupling in the RAS
We demonstrated the presence of cells in the PPN, as well as in its ascending target the Parafascicular nucleus (Pf), and in its descending target, the Subcoeruleus nucleus dorsalis (SunCD), a proportion (7–10%) of which were electrically coupled [26]. This was the first finding suggesting a role for electrical coupling in wake-sleep control. Electrical coupling through (Cx36) 36 gap junctions has been described in the reticular nucleus of the thalamus, the site of slow wave generation [27–29], so that its presence in wake-sleep regions is well known. In fact, gap junction blockers such as halothane and propofol are among the most rapidly acting anesthetics [30–31]. The role of electrical coupling is to allow neurons to fire together, regardless of frequency. Modafinil, a stimulant used for the treatment of narcolepsy and excessive daytime sleepiness, was found to increases gap junctions, driving coherence at high frequencies to promote arousal [32]. We also found that input resistance in PPN and SubCD, mainly GABAergic, neurons was reduced by modafinil [33]. Thus, electrical coupling in the RAS serves to synchronize activity across populations of cells, blockade of electrical coupling induces anesthesia, and such coupling is increased by the stimulant modafinil to induce arousal.
1.3.2 Gamma band intrinsic membrane oscillations
We also discovered that all PPN cells fired maximally at gamma band frequency when depolarized using current steps [34]. We identified the mechanism responsible for this ceiling in firing as high threshold, voltage-dependent calcium channels [35]. Basically, ramp-induced membrane depolarization resulted in beta/gamma frequency intrinsic membrane oscillations at −30 mV to −10 mV, suggesting that the location of these channels was away from the cell body [35]. This was confirmed using fast calcium imaging demonstrating that the channels are located all along the dendrites of these cells [36]. Pharmacological studies established that the calcium channels involved were N- and P/Q-type [35, 37, 38].
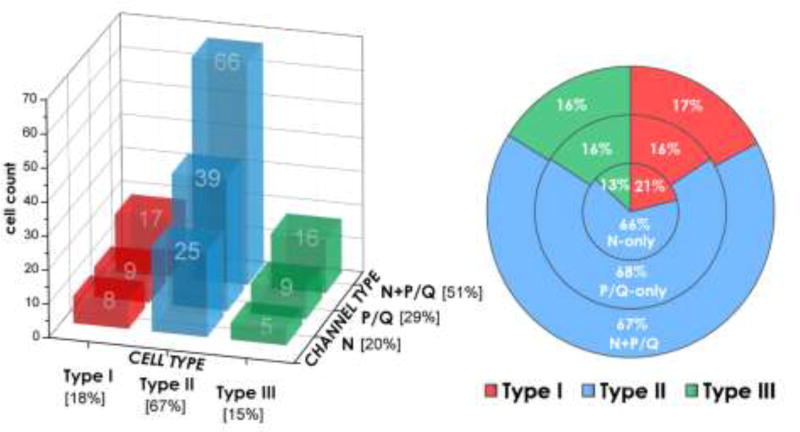
The most impressive element in these findings was that all PPN neurons manifested these channels, regardless of transmitter type, cholinergic, glutamatergic, or GABAergic [37, 38]. Moreover, there are three electrophysiological types of PPN cells, namely neurons with a low threshold spike (LTS) (type I, which are non-cholinergic), A current (type II, 2/3 of which are cholinergic), and both A+LTS currents (type III, 1/3 of which are cholinergic) [39–41], and they all bear these channels. However, not all PPN cells have both channels. Our studies showed that ~50% of PPN neurons have both channels, but 20–30% have either N-type channels or P/Q-type channels [42, 43]. Figure 1 shows the distribution of PPN cells by electrophysiological type I, type II, or type III, and by the presence of high threshold N+P/Q, P/Q only, or N only channels.
Figure 1. Distribution of PPN neurons according to electrophysiological type (I, II, or III) and high threshold calcium channel type (N+P/Q, N only, P/Q only).
Left side. Graph of the distribution of cells by cell type (Type I red, Type II blue, Type III green columns) and by channel type (N only, P/Q only, N+P/Q). Note that the numbers in each column are cell counts of recorded cells, and the percentage of cell type or channel type are in brackets in the axis legends. Right side. Pie chart of the percentage of cells by cell type and channel type. Note that the numbers inside the chart represent percent, not cell counts. Basically, the sample of almost 200 PPN cells shows that all cell types manifest all three types of channel expression. Since Type I cells are non-cholinergic, Type II cells are 2/3 cholinergic, and Type III cells are 1/3 cholinergic, it is highly likely that all three transmitter types (cholinergic, glutamatergic, and GABAergic) manifest all three channel types.
But there is a yet more surprising detail to the manifestation of these calcium channels in PPN cells. Injections of glutamate into the PPN increased waking and REM sleep [44], while injections of the glutamatergic receptor agonist N-methyl-D-aspartic acid (NMDA) increased only waking [45], and injections of the glutamatergic receptor agonist kainic acid (KA) increased only REM sleep [46]. These findings suggest that waking is modulated by NMDA, while REM sleep is modulated by kainic acid, input to the PPN. Intracellularly, protein kinase C (PKC), which modulates KA receptors, enhances N-type channel activity and has no effect on P/Q-type channel function [47], but CaMKII, which modulates NMDA receptors, was shown to modulate P/Q-type channel function [48]. That is, the two calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. The implications from all of these results are that, a) there is a “waking” pathway mediated by CaMKII and P/Q-type channels and a “REM sleep” pathway mediated by cAMP/PK and N-type channels, and b) different PPN cells fire during waking (those with N+P/Q and only P/Q-type) vs REM sleep (those with N+P/Q and only N-type) [37, 38, 49, 50].
We should note that the main ascending target of the PPN, the Pf, also manifests gamma band activity mediated by high threshold calcium channels [51], which are localized in the dendrites [52], while the main descending target of the PPN, the SubCD, manifests sodium-dependent subthreshold oscillations [53]. That is, all of these cell groups exhibit beta/gamma frequency intrinsic membrane oscillations and export beta/gamma band activity to their targets.
1.4 Gamma band activity
Gamma band activity appears to participate in sensory perception, problem solving, and memory [54–57]. Gamma band activation among thalamocortical networks [58], and in other neuronal groups is thought to contribute to the merger, or “binding”, of information originating from separate regions [59]. On the other hand, gamma oscillation deficits have been suggested as a pathophysiologic feature of a number of diseases [60–62]. The question then becomes, is gamma band activity in the PPN expressed at the level of the cortex? As far as the cortex is concerned, the difference between gamma band activity during waking vs REM sleep appears to be a lack of coherence [63]. That is, brainstem driving of gamma band activity during waking carries with it coherence across distant cortical regions, while driving of gamma band activity during REM sleep does not include coherence across distant regions [63, 64]. Also, carbachol-induced REM sleep with cataplexy is characterized by decreased gamma band coherence in the cortex [65]. These results suggest that, a) brainstem centers drive gamma band activity that is manifested in the cortical EEG, and b) during waking, brainstem-thalamic projections include coherence across regions. Moreover, during REM sleep, which is controlled by the SubCD region since lesion of this region eliminates REM sleep, and injections of carbachol induce REM sleep signs, the PPN-SubCD pathway drives cortical EEG rhythms without coherence [64].
Based on the presence of electrical coupling, of intrinsic membrane properties, and of circuitry capable of generating and maintaining beta/gamma band activity throughout our waking hours, we proposed a novel role for beta/gamma band activity in the RAS. While the usual role for gamma band activity in the cortex is that of sensory or motor binding, we suggested that tonic activation of the RAS during waking manifests gamma activity essential to a state capable of reliably assessing the world around us [1, 37, 38, 49]. We suggested that a mechanism similar to that found in the cortex for achieving temporal coherence at high frequencies is present in the PPN and its subcortical targets (e.g., Pf and SubCD), and that gamma band activity and electrical coupling generated in the PPN stabilizes the coherence that is essential for a stable activation state. That is, sensory input and ongoing beta/gamma band activity in the RAS participates in preconscious awareness [1, 37, 38, 49].
2. Drug Abuse, Gap Junctions, and Calcium Channels
Virtually no attention has been paid to the relationship between gap junctions and high threshold calcium channels in the PPN and drug abuse aside from its potential role in activating reward pathways [14]. There will likely be changes in these mechanisms during acute and/or chronic exposure to certain drugs of abuse, but the more critical question is: what changes in the PPN are related to the addictive process or to its relapse?
Some studies have addressed gap junctions and cell adhesion molecules in the VTA. VTA neurons express Cx36 gap junctions [66, 67], and more recent results showed that Cx36 gap junctions play a critical role in regulating inhibition of VTA dopaminergic neurons [66]. Acute exposure to ethanol decreased electrical coupling between VTA neurons, an effect duplicated by the gap junction blocker mefloquine [66, 68]. These results suggest that the dopaminergic pathway most involved in attention to rewarding stimuli, learned behavior, and drug addiction [69], is modulated by gap junctions. It is possible that, just as electrical coupling in the RAS provides coherence related to a stable arousal state, gap junctions in the reward circuit provide synchronization of activity related to appropriate reward perception. Disturbances in this modulation could be responsible for skewed responses to rewarding stimuli. Moreover, cell adhesion molecules that are involved in intercellular connections are over-represented in addiction-associated genes [70]. Thus cell adhesion molecule expression specifically in dopamine neurons could impact electrical coupling in circuits related to addiction and drug abuse.
These findings, coupled with the discovery of electrical coupling in the RAS, point to the coherence in the maintenance of arousal and in VTA reward responses as potential contributors to dysregulation in reward perception. The PPN-VTA pathway thus gains added importance in determining the background of arousal on which rewarding stimuli are evaluated. Disturbances in the maintenance of gamma band activity in the PPN or the VTA will permit peripatetic firing patterns unable to maintain high frequency activity necessary for appropriately evaluating the world around us. Therefore, the manner in which drugs of abuse alter, acutely or in the long run, the coherence of PPN-VTA circuitry needs considerably more attention.
Some interesting results on the role of high threshold calcium channels in VTA have been forthcoming. Voltage-dependent calcium channels, specifically N-type channels, were found to increase dopamine release following their pharmacological blockade [71]. In N-type channel knockout animals there is reduced alcohol consumption and resistance to the acute intoxicating effects of alcohol [72]. Although the two studies would seem to imply opposite actions, these findings point to N-type calcium channels as a possible target for abuse intervention. Of course, the cellular localization of these channels is still lacking, i.e. dopaminergic or non-dopaminergic, as is any role for P/Q-type calcium channels. It remains to be determined if N- and P/Q-type channels modulate high frequency membrane oscillations in VTA neurons. For example, we know that PPN cells project to intralaminar thalamus (Pf) and activate Pf neurons with N- and P/Q-type calcium channels, which are expected to pass on the high frequency activity. It is not clear if the PPN input to VTA also acts to promote high frequency activity in the VTA.
These and additional studies are needed to explore the role of electrical coupling and high threshold calcium channels in the actions of drugs of abuse. For example, do P/Q-type calcium channels also alter dopamine release (as do N-type channels [71]? While P/Q-type channel knockouts reduce gamma band activity, is that one mechanism behind the soporific effect of agents such as alcohol? It has been suggested that P/Q-type calcium channel over expression may be one mechanism behind insomnia [1]. Do drugs of abuse such as stimulants affect the expression of these channels or of N-type channels for that matter? Since the most plausible manner for modulating these channels is through their respective intracellular pathways, do drugs of abuse alter CaMKII (that regulates P/Q-type channels) and/or cAMP/PK (that regulates N-type channels) specifically in the PPN? These are only a few of the potential avenues for developing a more complete idea of the role of arousal in drug abuse.
3. The Role of the Specific and Non-Specific Thalamus
Psychostimulants like cocaine have been found to affect thalamocortical networks through their well-known inhibition of cathecholamine reuptake from RAS afferents to the thalamocortical system [73,74]. In rodents, an enhancement of spontaneous GABA release from thalamic reticular nucleus neurons was described after repetitive cocaine administration (i.e., binge-like administration: 3 cocaine injections at 15 mg/kg; i.p., one hour apart) [75–78]. Higher levels of GABA release at thalamic levels were correlated with abnormally high levels of low frequency oscillations in the cortical EEG [75]. Such abnormal rhythmicity of thalamocortical columns has been previously described as one of the underlying mechanisms associated with several neurological and psychiatric conditions termed thalamocortical dysrhythmia syndrome [79, 80].
Cocaine has been found to mediate alterations of the level of expression of CaV3.1 T-type calcium channels in the membrane of thalamocortical neurons [75, 76]. These channels are widely expressed in thalamocortical and thalamic reticular neurons [81, 82], and play a crucial role in maintaining sleep [83]. The combination of higher T-type channel activation plus higher synaptic GABAergic activity induced by cocaine administration in mice was suggested to “lock” the whole thalamocortical system at low frequencies, an effect demonstrated in EEG recordings [75], and which is associated with thalamocortical dysrhythmia syndrome [79, 80].
4. The Role of the Hypothalamus
A role for the hypocretin system of the hypothalamus in the response to acute stress has been convincingly advanced [84]. The idea is that this system, which was proposed to participate in the stability of arousal [85], is part of the circuitry involved in the stress response and may facilitate the resumption of drug seeking behavior. Much attention has focused on hypothalamic neurons in the control of waking, a leading lab optogenetically engineered animals that have rhodopsin cation channels in orexin and in noradrenergic LC cells [86]. They showed that light activation of orexin neurons requires ~20 seconds of stimulation before waking ensues, implying that orexin output must travel elsewhere before the animal awakens. When they light activated LC cells, the animals awoke immediately, within 1–2 seconds, but if LC was light-inactivated by expressed anion channels, orexin neuron stimulation failed to awaken the animals. This result suggests that orexin neurons must first affect a descending RAS target, the LC, to induce a waking effect in vivo. That is, the lateral hypothalamic system acts through the RAS to elicit arousal. Moreover, the long latency required to induce arousal after orexin neuron activation suggests that this region “recruits” rather than “induces” waking. The result showing that optogenetic inactivation/inhibition of the LC in advance of activation of optogenetically altered orexin neurons fails to induce waking [86] suggest that, in the absence of the RAS, orexin cells cannot induce arousal. That is, descending orexin projections to the RAS may be essential for these cells to ultimately have an effect on waking.
In addition, the role of these neurons is modulated by sleep deprivation. Optogenetic stimulation experiments found that sleep deprivation blocked the ability of orexin to activate its downstream targets and induce waking [87], suggesting that these neurons can be prevented from eliciting waking by sleep deprivation. Therefore, rather than a specific role in arousal, hypocretin neurons have been implicated in the integration of motor, metabolic, circadian, and limbic inputs that can influence sleep to wake transitions [88]. It is clear, however, that orexin projections are not the final common pathway for arousal.
5. A Note About Optogenetics
Optogenetic tools derived from microbial (type I) or animal (type II) opsins are widely used in a fast growing area of neuroscience that combines several animal models in order to target subpopulations of neurons within particular tissues/brain areas [89]. While this is a powerful technology, there are a number of caveats that need to be remembered. First, the combination of behavioral with optogenetics methods require in many cases the implantation of an optical fiber to deliver blue light capable of reaching subcortical structures. Recent studies show that blue light activates cells that do not expressed opsins [90]. Then there is the issue of latency. Although rapid resolution of opsins like channelrhodopsin or archaerhodopsin allow the study of fast interactions between cell groups, their association with behavioral events is not always rapid or direct. This makes it difficult to interpret multifactorial behavioral assays (i.e., sleep, anxiety, fear, or depression tests) due to the long latency between stimulation and behavioral event. Thirdly, the methodology employs the genetic introduction of the opsin using a vector, usually viral or transgenic, attached to a promoter that overexpresses the protein. Therefore, expression of opsins might permanently alter synaptic activity among brain nuclei by simply changing the lipid environment required for the normal membrane expression of channels and/or receptors [91]. These manipulations may not affect membrane potential, but when voltage-dependent channels, for example, their activation could easily be altered.
Many of these issues are particularly critical when studying the PPN. For example, in some groups of neurons like the PPN [1, 37, 38, 49] high frequency subthreshold oscillations require the use of a ramp-like sustained depolarization that cannot be achieved using trains of light stimulation. Therefore, the kind of light-stimulated activity induced may be far from that induced by the normal synaptic activation of calcium channels mediating intrinsic oscillations. Moreover, these channels are expressed all along the dendrites of PPN neurons [36, 52]. The initial overexpression of the channelrhodopsin could interfere with the lipid rafts that organize, for example, high threshold, voltage-dependent channels [92]. Finally, since expressing these proteins in a cell could, a) alter protein expression, b) opsins could induce heat that changes the cell, and c) channel and pump activity can be altered [93], the long-term insertion of these calcium leak channels could gradually change calcium intracellular concentrations, leading to abnormal responses to afferent inputs.
6. Conclusions
Hopefully, the case has been made for considering the roles of alterations by drugs of abuse and by the process of acquiring dependence as well as of relapse, on two of the most important new findings on the control of arousal. The discoveries of electrical coupling that promotes coherence, and of intrinsic membrane oscillations at beta/gamma frequencies remain virtually unexplored in the field of drug abuse. These two issues, coherence and gamma frequency, are critical to waking as well as attention, and higher functions. As such, their dysregulation may facilitate the initial susceptibility as well as the relapse to drug abuse, and could account for a number of as yet investigated consequences of the normal process of arousal.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P30 GM110702 to Dr. Garcia-Rill. In addition, this work was supported by grants from FONCYT Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT 2007-1009; PICT-2012-1769, PICT-2012-0924, PICT-2015-2594 and UBACYT 2014-2017 #20120130101305BA (to Dr. Urbano); and by grants from CONICET- PIP 2011-2013-11420100100072 and from FONCYT Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT-2012-0924 (to Dr. Bisagno).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia-Rill E. Waking and the Reticular Activating System. Academic Press; New York: 2015. [Google Scholar]
- 2.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J. Neurosci. 2000;200:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J. Comp. Neurol. 1996;7:2353–2356. doi: 10.1002/(SICI)1096-9861(19960729)371:3<345::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Egan TM, North RA. Actions of acetylcholine and nicotine on rat locus coeruleus neurons in vitro. Neurosci. 1986;19:565–571. doi: 10.1016/0306-4522(86)90281-2. [DOI] [PubMed] [Google Scholar]
- 6.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to the drugs of abuse and pathology. Brit. J. Pharmacol. 2008;1:438–445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 8.Good CH, Lupica CR. Properties of distinct ventral tegmental area synapses activated via pedunculopontine or ventral tegmental area stimulation in vitro. J. Physiol. 2009;587:1233–1247. doi: 10.1113/jphysiol.2008.164194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JA, Reiner PB. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J. Neurosci. 1993;13:3878–3883. doi: 10.1523/JNEUROSCI.13-09-03878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neurosci. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 13.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 14.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Rill E. Sleep and arousal states: reticular activating system. In: Squire LR, Bloom F, Spitzer N, Gage F, Albright T, editors. New Encyclopedia of Neuroscience. Vol. 8. Elsevier; Oxford, England: 2009. pp. 137–143. [Google Scholar]
- 16.Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 18.Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 20.Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraix V, Bastin J, David O, Goetz L, Ferraye M, Benabid A, Chabardes S, Pollak P, Debû B. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE. 2013;8:e83919. doi: 10.1371/journal.pone.0083919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, Chabardes S. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J. Neural Transm. 2016;123:667–678. doi: 10.1007/s00702-016-1577-7. [DOI] [PubMed] [Google Scholar]
- 25.Steriade M, Curro Dossi R, Paré D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc. Nat. Acad. Sci. USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Rill E, Heister D, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J. Neurosci. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentealba P, Steriade M. The reticular nucleus revisited: Intrinsic network properties of a thalamic pacemaker. Prog. Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Liu XB, Jones EG. Fine structural localization of Connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J. Comp. Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- 30.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap junctional intercellular communication. Bioch. Soc. Trans. 2001;29:606–612. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 31.He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ. Res. 2000;86:1–10. doi: 10.1161/01.res.86.11.e104. [DOI] [PubMed] [Google Scholar]
- 32.Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc. Natl. Acad. Sci. USA. 2007;104:12554–1255. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: The role of transmitters and electrical coupling. Sleep. 2008;31:673–690. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, Garcia-Rill E. Gamma band unit activity and population responses in the pedunculopontine nucleus. J. Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur. J. Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J. Appl. Physiol. 2013;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med. Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbano FJ, D’Onofrio S, Luster B, Hyde J, Bisagno V, Garcia-Rill E. Pedunculopontine nucleus gamma band activity- preconscious awareness, waking, and REM sleep. Frontiers Sleep Chronobiol. 2014;5:210. doi: 10.3389/fneur.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonard CS, Llinas R R. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Steriade M, Biesold D, editors. Brain Cholinergic Systems. Oxford: Oxford Science; 1990. pp. 205–223. [Google Scholar]
- 40.Kamondi A, Williams J, Hutcheon B, Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J. Neurophysiol. 1992;68:1359–1372. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- 41.Takakusaki K, Kitai ST. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neurosci. 1997;78:771–794. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- 42.Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol. Rep. 2015;3:e12431. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN) Physiol. Rep. 2016;4:e12787. doi: 10.14814/phy2.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Datta S, Spoley E, Patterson E. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer. J. Physiol. Reg. Integ. Comp. Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 45.Datta S, Patterson E, Spoley E. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J. Neurosci. Res. 2002;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 46.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainite receptor. J. Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 47.Stea A, Soomg T, Snutch T. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 48.Jiang X, Lautermilch N, Watari H, Westenbroek R, Scheuer T, Catterall WA. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc. Nat. Acad. Sci. USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, Bisagno V, Urbano FJ. Gamma band activity in the RAS-intracellular mechanisms. Exp. Brain Res. 2014;232:1509–1522. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, Urbano FJ. Implications of gamma band activity in the pedunculopontine nucleus. J. Neural Transm. 2015;123:655–665. doi: 10.1007/s00702-015-1485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kezunovic N, Hyde J, Simon C, Urbano FJ, Garcia-Rill E. Gamma band activity in the developing parafascicular nucleus (Pf) J. Neurophysiol. 2012;107:772–784. doi: 10.1152/jn.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Visualization of fast calcium oscillations in the parafascicular nucleus. J. Eur. Physiol. (Pflug. Arch.) 2013;465:1327–1340. doi: 10.1007/s00424-013-1264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon C, Kezunovic N, Williams DK, Urbano FJ, Garcia-Rill E. Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Amer. J. Physiol. Cell Physiol. 2011;301:C327–C335. doi: 10.1152/ajpcell.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Retboeck H. Coherent oscillations: a mechanism of feature linking in the visual system? Biol. Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 55.Gray C, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Nat. Acad. Sci. USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int. J. Psychophysiol. 2009;73:350–354. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Palva S, Monto S, Palva J. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2009;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 58.Llinas R, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc. Natl. Acad. Sci. USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Llinás R, Paré D. Of dreaming and wakefulness. Neurosci. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 60.Ribary U, Ioannides A, Singh K, Hasson R, Bolton J, Lado F, Mogliner A, Llinas R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc. Nat. Acad. Sci. USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stam C, van Cappellen van Walsum A, Pijnenburg Y, Berendse H, de Munck J, Scheltens P, van Dijk B. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J. Clin. Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Uhlhaas P, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dial. Clin. Neurosci. 2013;15:301–313. doi: 10.31887/DCNS.2013.15.3/puhlhaas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castro S, Falconi A, Chase M, Torterolo P. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur. J. Neurosci. 2013;37:1330–1339. doi: 10.1111/ejn.12143. [DOI] [PubMed] [Google Scholar]
- 64.Cavelli M, Castro S, Schwartzkopf N, Chase M, Falconi A, Torterolo P. Coherent cortical oscillations decrease during REM sleep in the rat. Behav. Brain Res. 2015;281:318–325. doi: 10.1016/j.bbr.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 65.Torterolo P, Castro-Zaballa S, Cavelli M, Chase M, Falconi A. Neocortical 40 Hz oscillations during carbachol-induced rapid eye movement sleep and cataplexy. Eur. J. Neurosci. 2015;281:318–325. doi: 10.1111/ejn.13151. [DOI] [PubMed] [Google Scholar]
- 66.Allison D, Wilcox R, Ellefsen K, Askwe C, Hansen D, Wilcox J, Sandoval S, Eggett D, Yanagawa Y, Steffensen S. Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: a physiologic role for connexin-36 gap junctions. Synapse. 2011;65:804–813. doi: 10.1002/syn.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steffensen S, Bradley K, Hansen D, Wilcox J, Wilcox R, Allison D, Merrill C, Edwards J. The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse. 2011;65:695–707. doi: 10.1002/syn.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stobbs S, Ohran A, Lassen M, Allison D, Brown J, Steffensen S. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves NMDA receptors. J. Pharmacol. Exp. Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- 69.Fields H, Hjelmstad G, Margolis E, Nicola S. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Ann. Rev. Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 70.Zhong X, Drgonova J, Li C, Uhl G. Human cell adhesion molecules: annotated functional subtypes and overrepresentation of addiction-associated genes. Ann. N. Y. Acad. Sci. 2015;1349:83–95. doi: 10.1111/nyas.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belardetti F, Ahn S, So K, Snutch T, Phillips A. Block of voltage-gated calcium channels stimulates dopamine efflux in rat corticolimbic system. Neuropharmacol. 2009;56:984–993. doi: 10.1016/j.neuropharm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Newton P, Messing R. The N-type calcium channel is a novel target for treating alcohol use disorders. Channels. 2009;3:77–81. doi: 10.4161/chan.3.2.8037. [DOI] [PubMed] [Google Scholar]
- 73.Pan D, Gatley S, Dewey S, Chen R, Alexoff D, Ding Y, Fowler J. Binding of bromine-substituted analogs of methylphenidate to monoamine transporters. Eur. J. Pharmacol. 1994;264:177–182. doi: 10.1016/0014-2999(94)00460-9. [DOI] [PubMed] [Google Scholar]
- 74.Howes S, Dalley J, Morrison C, Robbins T, Everitt B. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacol. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- 75.Urbano FJ, Bisagno V, Wikinski S, Uchitel O, Llinás R. Cocaine acute “binge” administration results in altered thalamocortical interactions in mice. Biol. Psychiat. 2009;66:769–776. doi: 10.1016/j.biopsych.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 76.Bisagno V, Raineri M, Peskin V, Wikinski S, Uchitel O, Llinás R, Urbano FJ. Effects of T-type calcium channel blockers on cocaine-induced hyperlocomotion and thalamocortical GABAergic abnormalities in mice. Psychopharmacol. 2010;212:205–214. doi: 10.1007/s00213-010-1947-z. [DOI] [PubMed] [Google Scholar]
- 77.Goitia B, Raineri M, González L, Rozas I, Garcia-Rill E, Bisagno V, Urbano FJ. Differential effects of methylphenidate and cocaine on GABA transmission in sensory thalamic nuclei. J. Neurochem. 2013;124:602–612. doi: 10.1111/jnc.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goitia B, Rivero-Echeto M, Weisstaub N, Gingrich J, Garcia-Rill E, Bisagno V, Urbano FJ. Modulation of GABA release from the thalamic reticular nucleus by cocaine and caffeine: role of serotonin receptors. J. Neurochem. 2016;136:526–535. doi: 10.1111/jnc.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Llinás R, Ribary U, Jeanmonod D, Kronberg E, Mitra P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Nat. Acad. Sci. USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Llinás R, Urbano FJ, Leznik E, Ramírez R, van Marle H. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Catterall W. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 82.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 83.Anderson M, Mochizuki T, Xie J, Fischler W, Manger J, Talley E, Scammell T, Tonegawa S. Thalamic Cav3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc. Nat. Acad. Sci. USA. 2005;102:1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boutrel B, de Lecea L. Addiction and arousal: the hypocretin connection. Physiol. Behav. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Lecea L, Jones BE, Boutrel B, Borgland S, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J. Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carter M, Brill J, Bonnavion P, Huguenard J, Huerta R, de Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc. Nat. Acad. Sci. USA. 2012;109:E2635–2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carter M, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front. Pharmacol. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Ann. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng K, Kiernan E, Eliceiri K, Williams J, Watters J. Blue Light Modulates Murine Microglial Gene Expression in the Absence of Optogenetic Protein Expression. Sci. Rep. 2016;6:21172. doi: 10.1038/srep21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brady J, Rich T, Le X, Stafford K, Fowler C, Lynch L, Karpen J, Brown R, Martens J. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol. 2004;65:503–511. doi: 10.1124/mol.65.3.503. [DOI] [PubMed] [Google Scholar]
- 92.Guéguinou M, Gambade A, Félix R, Chantôme A, Fourbon Y, Bougnoux P, Weber G, Potier-Cartereau M, Vandier C. Lipid rafts, KCa/ClCa/Ca2+ channel complexes and EGFR signaling: Novel targets to reduce tumor development by lipids? Biochim. Biophys. Acta. 2015;1848:2603–2620. doi: 10.1016/j.bbamem.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 93.Allen B, Singer A, Boyden E. Principles of designing interpretable optogenetic behavior experiments. Learn. Mem. 2015;22:232–238. doi: 10.1101/lm.038026.114. [DOI] [PMC free article] [PubMed] [Google Scholar]