Abstract
The objective of this study was to assess the capacity of adipose-derived mesenchymal stem cells (ASCs) to mitigate disease progression in an experimental chronic pancreatitis mouse model. Chronic pancreatitis (CP) was induced in C57BL/6 mice by repeated ethanol and cerulein injection, and mice were then infused with 4 × 105 or 1 × 106 GFP+ ASCs. Pancreas morphology, fibrosis, inflammation, and presence of GFP+ ASCs in pancreases were assessed 2 weeks after treatment. We found that ASC infusion attenuated pancreatic damage, preserved pancreas morphology, and reduced pancreatic fibrosis and cell death. GFP+ ASCs migrated to pancreas and differentiated into amylase+ cells. In further confirmation of the plasticity of ASCs, ASCs co-cultured with acinar cells in a Transwell system differentiated into amylase+ cells with increased expression of acinar cell-specific genes including amylase and chymoB1. Furthermore, culture of acinar or pancreatic stellate cell lines in ASC-conditioned medium attenuated ethanol and cerulein-induced pro-inflammatory cytokine production in vitro. Our data show that a single intravenous injection of ASCs ameliorated CP progression, likely by directly differentiating into acinar-like cells and by suppressing inflammation, fibrosis, and pancreatic tissue damage. These results suggest that ASC cell therapy has the potential to be a valuable treatment for patients with pancreatitis.
Keywords: adipose stem cell therapy, differentiation, acinar cells, fibrosis, ethanol and cerulean-induced chronic pancreatitis mouse model, inflammation, mesenchymal stem cells
Could stem cell therapy have a role in managing chronic pancreatitis? In a murine chronic pancreatitis model, intravenous injection of stem cells improved chronic pancreatitis progression. Stem cells likely played a role through differentiation into acinar-like cells and suppressing inflammation and fibrosis. This is promising work in discovering new treatments for chronic pancreatitis.
Introduction
Chronic pancreatitis (CP) is characterized by continuous or recurrent inflammation, fibrosis, and irreversible morphological changes in the pancreas.1 Chronic abdominal pain is the principal symptom of CP, and has been reported in the majority of patients.2 Progressive loss of exocrine and endocrine function occurs during disease progression.3 Many patients require hospitalization at some stage in their illness.4 The etiology of CP is complex and not completely understood. For example, although development of CP is associated with alcohol use, only a small percentage of heavy drinkers develop CP.5, 6 An underlying genetic disorder has been identified in a minority of patients with CP.4 The poorly understood pathophysiology of CP makes identification of means to treat the underlying cellular disorder problematic. New insights into the pathogenesis of CP on a cellular level have focused attention on altering the mechanistic pathway from acute to CP. Current treatments for CP mainly target the symptoms rather than the cause of the disease and, apart from alcohol and smoking cessation, are not effective in controlling disease progression or inducing remission.7 Among all patients, 40%–75% will eventually require surgery, but only 34%–52% attain pain relief after pancreas resection.8 Thus, more effective therapies are needed to diminish the substantial CP disease burden.
Mesenchymal stromal/stem cells (MSCs) are a rare population of adult stem cells that can be isolated from multiple tissues of mesodermal origin including bone marrow, umbilical cord, dental pulp, and other organs.9, 10 MSCs can be expanded rapidly ex vivo and can be differentiated into osteocytes, chondrocytes, and adipocytes. They have immune-privileged and tissue-protective functions. MSC and hematopoietic stem cell therapy have been used in clinical trials for the treatment of pancreatic disorders including type 1 diabetes.11, 12 Protective roles of MSCs are thought to be mediated through their paracrine secretion of growth factors having anti-apoptotic, immunoregulatory, and angiogenic functions; cell-to-cell contact is also important.13
White adipose tissue is a promising source of MSCs because of its ease of sampling and ability to be expanded to clinically useful cell numbers in a short period of time. For example, 1 in 100,000 mononuclear bone marrow cells are MSCs, and their frequency decreases with age. In contrast, more than 108 adipose-derived mesenchymal stem cells (ASCs) can be obtained from 30 mL of initial liposuction in 2 weeks, even from patients with diabetes.14 Similar or even better therapeutic effects can be achieved with lower numbers of ASCs than bone marrow MSCs, which reduces the risks associated with ex vivo cell expansion.14 Compared with MSCs from other tissues, ASCs display greater regenerative capacity based on their differentiation and paracrine activities.15 The protective effects of ASCs have been shown in rodent models of severe acute pancreatitis,16 type 1 diabetes,17, 18 experimental autoimmune encephalomyelitis,19 rheumatoid arthritis, systemic lupus erythematosus, and other disorders.20, 21 ASCs are currently being tested in clinical trials for the treatment of Crohn’s-related rectovaginal fistula,22 refractory rheumatoid arthritis,23 severe osteoarthritis of the knee,24 systemic sclerosis,25 and other diseases.26
The pancreas is a logical target for cell therapy because it has an extremely low capacity to regenerate both exocrine and endocrine components. MSC therapy can intervene in multiple disease pathways because of their multifactorial protective functions.27 MSC therapy has been attempted in rodent models of severe acute pancreatitis and has been shown to promote regeneration of damaged pancreatic tissue.28, 29, 30 However, ASCs have yet to be attempted in patients with acute or CP in clinical trials. Here, we studied the ability of ASC therapy to rescue the pancreas from disease progression in a mouse model of CP. We found that ASCs effectively reversed CP progression. They migrated to the diseased pancreas, where they directly differentiated into amylase+ acinar-like cells. ASCs suppressed inflammation in acinar cells and in pancreatic stellate cells.
Results
CP Mouse Model
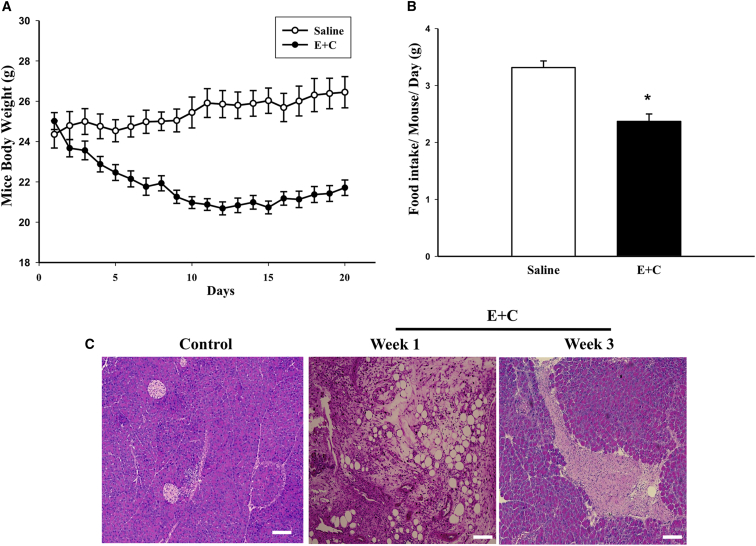
The CP mouse model was generated by repeated intraperitoneal injections of ethanol and cerulein. At 3 weeks after the initial injection, mice showed significantly reduced body weights (Figure 1A) and reduced food intake (Figure 1B), which is consistent with typical signs in CP patients. H&E staining showed vacuolization consistent with fatty degeneration of acinar cells within the pancreas at 1 week post-injection (Figure 1C), a typical pancreas injury induced by ethanol.31 At 3 weeks after CP induction, more severe damage including pancreas edema and fibrosis within the pancreas parenchyma was observed (Figure 1C). These data indicate that the CP mouse model exhibited typical characteristics of CP.
Figure 1.
Ethanol and Cerulean-Induced CP Mouse Model
(A) Body weight changes in mice after saline (CTR) or ethanol and cerulein (E+C) injections (n = 10 per group). (B) Food intake in mice treated with saline or ethanol and cerulein 2 weeks after CP induction. (C) Representative H&E staining of pancreatic tissue from mice receiving saline (control) or ethanol and cerulein 1 and 3 weeks after injection. Scale bars, 100 μm. Data presented are mean ± SEM. Differences were compared by one-way ANOVA; *p < 0.05.
ASC Infusion Restores Physical Activity and Ameliorates Pancreas Damage in CP Mice
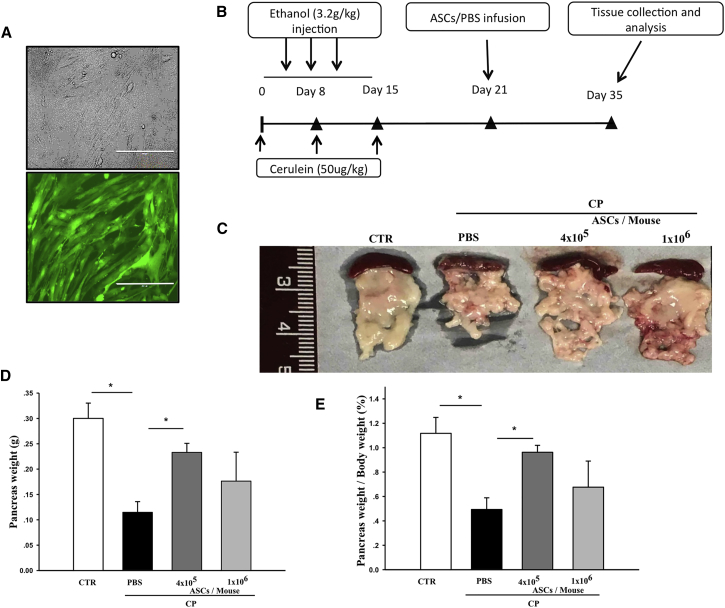
Mouse ASCs were collected from wild-type or C57BL/6-Tg (CAG-EGFP) mice. Phenotypic analysis showed that ASCs used in this study attached to cell culture plates (Figure 2A); expressed CD29, CD105, and CD166; lacked expression of CD45; and could be differentiated into adipocytes, chondrocytes, and osteocytes as we reported previously.32 ASCs (4 × 105 or 1 × 106 cells/mouse) were infused into CP mice via the tail vein. Control normal and CP mice received PBS on the same schedule (Figure 2B). CP mice demonstrated reduced physical activity compared with normal control mice. Physical activity of CP mice that received ASCs increased slightly at 2 weeks after cell injection, although the increase did not reach significance compared with CP mice receiving PBS (Figure S1).
Figure 2.
Characteristics of CP Mice
(A) Micrographs of GFP+ ASCs observed by light or fluorescent microscopy. Scale bars, 100 μm. (B) Schematic diagram of CP induction, cell infusion, and tissue collection and analysis. (C) Images of pancreases collected from normal control, CP control, or CP mice treated with ASCs (4 × 105 or 1 × 106 cells/mouse). (D and E) Pancreas weights (D) and percent ratio of pancreas weight to body weight (E) in control, CP control, or CP mice treated with ASCs (4 × 105 or 1 × 106 cells/mouse). Data presented are mean ± SEM. Differences were compared by one-way ANOVA; *p < 0.05.
CP induction led to irreversible pancreas damage in control mice. Pancreas size, pancreas weight, and pancreas weight per body weight in saline-treated CP mice were significantly reduced compared with normal controls (Figures 2C–2E). In contrast, mice that received ASCs, at either concentration, showed significant increases in both pancreas size (Figure 2C) and pancreas weight (Figure 2D). Although body weights of mice from both control or ASC-treated mice were increased after termination of ethanol and cerulein injections, mice that received ASCs had higher pancreas-to-body weight ratios (Figure 2E), suggesting that ASC treatment attenuated ethanol and cerulein-induced pancreas damage. We observed that treatment with 4 × 105 ASCs per mouse provided slightly better protection from pancreas damage; therefore, we used 4 × 105 cells per mouse in all mechanistic studies.
Treatment with ASCs Suppresses Pancreatic Fibrosis
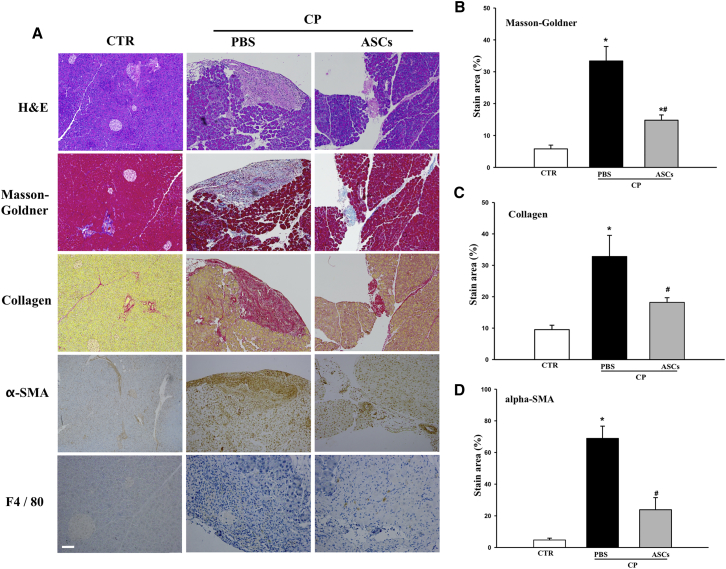
One of the hallmarks of CP is the destruction and replacement of acinar tissue by fibrotic tissue. We assessed the effects of ASC infusion on pancreatic fibrosis. The extent of fibrosis was clearly visible in the pancreas from CP mice treated with saline as analyzed by H&E and Masson-Goldner trichrome stains (Figure 3A). In contrast, pancreatic structure of mice treated with ASCs was better preserved and showed significantly reduced Masson-Goldner-positive staining (Figures 3A and 3B), indicating a potential role of ASCs in reversing fibrosis in the pancreas of CP mice.
Figure 3.
Effects of ASC Injection on Pancreas Damage, Fibrosis, and Macrophage Infiltration
(A) Histological analysis of pancreas tissue sections from normal control or CP mice treated either with PBS or ASCs at 4 × 105 cells/mouse. H&E staining, Masson-Goldner-trichrome staining, and staining with antibodies against collagen, α-SMA, and F4/80 are shown. Scale bar, 100 μm. (B–D) Percentages of positive area in total pancreatic area of (B) Masson-Goldner, (C) collagen, and (D) α-SMA. Data are means ± SEMs. *p < 0.05 compared with normal control; #p < 0.05 compared with CP-PBS group, one-way ANOVA test.
Fibrosis in the CP pancreas is mainly caused by the activation of pancreatic stellate cells (PSCs) within the pancreas. Activated PSCs secrete extracellular matrix proteins, including collagen type 1 and α-smooth muscle actin (α-SMA), that contribute to fibrosis. This was also observed in our model; CP induction resulted in significantly increased collagen and α-SMA expression in the pancreas. Again, ASC infusion significantly attenuated ethanol and cerulein-induced collagen deposition and α-SMA expression in the CP pancreas (Figures 3A–3C), suggesting a protective role of ASCs in reducing pancreas fibrosis.
During CP progression, necrosis of acinar cells leads to infiltration of monocytes/macrophages into the injured pancreas.33 We observed increased macrophage (F4/80+) infiltration in CP mice (Figure 3A). In contrast, when CP mice were treated with ASCs, the numbers of infiltrating macrophages in the pancreas were significantly reduced (Figure 3A). We also measured serum tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 levels in normal controls, CP controls, and ASC-treated CP mice. No detectable amounts of TNF-α or IL-6 were observed in mice from any group (data not shown).
ASC Infusion Protects against Pancreatic Cell Death
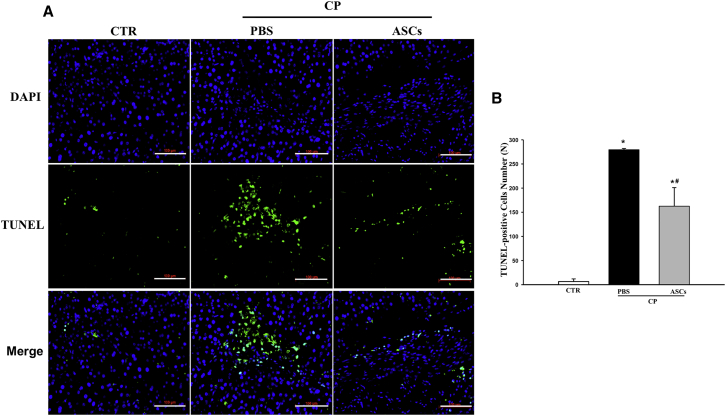
Next, we measured the impact of ASC infusion on pancreatic cell death. Treatment with ethanol and cerulein caused dramatic cell death among pancreatic cells shown by increased terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ staining within the pancreatic parenchyma (Figures 4A and 4B). In contrast, TUNEL+ staining was significantly reduced in CP mice receiving ASCs (Figures 4A and 4B), indicating that ASCs protected pancreatic cells from ethanol and cerulein-induced cell death.
Figure 4.
ASC Infusion Protects against Pancreatic Cell Death
(A) Immunostaining of TUNEL+ cells in the pancreas from normal control, CP mice treated with PBS, or ASCs (4 × 105 cells/mouse) 2 weeks after infusion. Scale bars, 100 μm. (B) Average numbers of TUNEL+ cells in pancreas sections. Data presented are mean ± SEM. Differences were compared by one-way ANOVA; *p < 0.05 compared with normal control; #p < 0.05 compared with CP-PBS group.
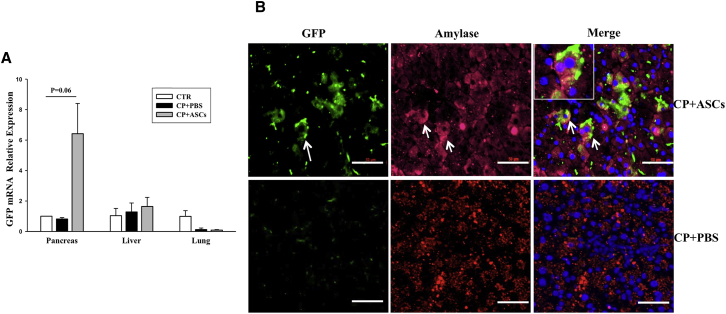
ASCs Migrate to the Injured Pancreas and Differentiate into Amylase+ Cells
MSCs have been shown to migrate to injured tissues after systemic injection. To determine whether ASCs migrated to the injured pancreas in CP mice, we measured expression of GFP in lung, liver, and pancreas tissues harvested from normal controls, CP controls, and GFP+ ASC-treated CP mice 2 weeks after cell injection. GFP expression was found in the pancreas, but not in liver or lung tissues, of mice receiving GFP+ ASCs and not in any tissue from control mice (Figure 5A). GFP+ cells in the pancreas of GFP+ ASC-treated CP mice were further investigated by immunohistological staining using the anti-GFP and the anti-amylase antibodies. GFP+ cells were observed in the pancreas, and most GFP+ cells also stained positive for amylase, suggesting that GFP+ ASCs not only migrated to the injured pancreas, but also differentiated into amylase+ acinar-like cells (Figure 5B).
Figure 5.
Differentiation of Infused ASCs into Amylase+ and Insulin+ Cells in the Treated Pancreas
(A) Real-time RT-PCR analysis of mRNA expression of GFP in the pancreas, liver, and lungs from normal controls, CP controls, and ASC-treated CP mice 2 weeks after injection. (B) Immunohistochemical staining of pancreatic tissues from mice receiving ASCs (upper panels) or PBS (lower panels) with the anti-GFP and the anti-amylase antibodies. Green represents GFP+ cells, and red identifies amylase+ cells. Nuclei are stained blue (DAPI). Scale bar, 50 μm. Data presented are mean ± SEM. Differences were compared by one-way ANOVA.
The Impact of CP Induction and ASC Infusion on Endocrine Cells within the Pancreas
To assess the impact of CP induction and ASC therapy on the endocrine compartment, we measured blood glucose levels in normal controls, CP controls, and ASC-treated CP mice 2 weeks after cell infusion. All mice were normoglycemic during the course of the experiment, exhibiting well-preserved pancreatic β cell mass and similar numbers of islets per pancreatic section (Figure S2). These data further indicated that ethanol and cerulein treatment selectively destroyed the exocrine compartment but had little effect on β cell mass, the endocrine compartment of the pancreas in this model.
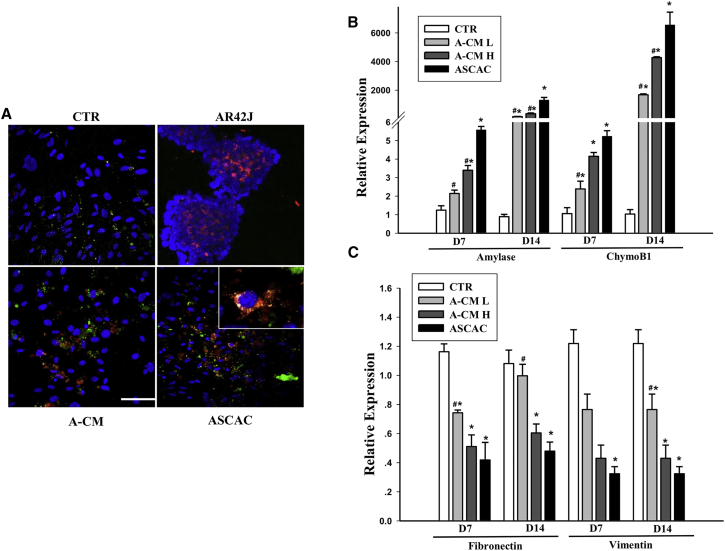
GFP+ ASCs Differentiate into Amylase+ Cells In Vitro When Co-cultured with Acinar Cells or in Acinar Cell-Conditioned Medium
MSCs possess considerable plasticity, and they can differentiate into multiple cell types.34 To confirm that ASCs can differentiate into amylase+ cells, we co-cultured GFP+ ASCs with AR42J cells in the Transwell system. We also cultured ASCs in acinar cell-conditioned medium. After 7 days of co-culture, ASCs were stained with anti-GFP and anti-amylase antibodies. GFP+ amylase+ cells were observed among ASCs co-cultured with acinar cells or cultured in acinar cell-conditioned medium, but were not seen among ASCs cultured in medium alone (Figure 6A). These results suggest that ASCs differentiated into acinar-like cells when exposed to acinar cells directly or to acinar cell-conditioned medium. In a separate set of experiments, we measured expression of acinar cell markers, amylase 1 and chymoB1, and stem cell markers, fibronectin and vimentin, in ASCs after 7 or 14 days of co-culture. ASCs co-cultured with acinar cells or in acinar conditioned medium exhibited increased expression of amylase-1 and chymoB1 (Figure 6B) and decreased expression of fibronectin and vimentin (Figure 6C). Thus, when cultured in F-12K medium alone, ASCs retained their expression of stem cell markers, but when cultured in the presence of acinar cells or acinar cell-conditioned medium, ASCs gradually lost their stem cell attributes and progressed toward other cell fates. Changes in gene expression profiles in ASCs in the presence of acinar cells were consistent with differentiation into acinar-like cells.
Figure 6.
ASCs Differentiate into Amylase+ Cells In Vitro When Co-cultured with Acinar Cells or in Acinar Cell-Conditioned Medium
(A) ASCs cultured alone (CTR), cultured in AR42J-conditioned medium (A-CM), or co-cultured with AR42J cells (ASCAC) were stained for GFP (green) and amylase (red). AR42J cells were used as a positive control for amylase staining. Nuclei are stained blue (DAPI). Scale bar, 50 μm. (B and C) Relative expression of amylase 1 and chymoB1 (B) and fibronectin and vimentin (C) after 7 or 14 days of culture, in ASCs cultured alone (CTR), in ASCs cultured in low-dose AR42J-conditioned medium (A-CM-L), ASCs cultured in high-dose AR42J-conditioned medium (A-CM-H), or ASCs co-cultured with acinar cells. Data presented are mean ± SEM. Differences were compared by one-way ANOVA; *p < 0.05 versus CTR, #p < 0.05 versus ASCAC.
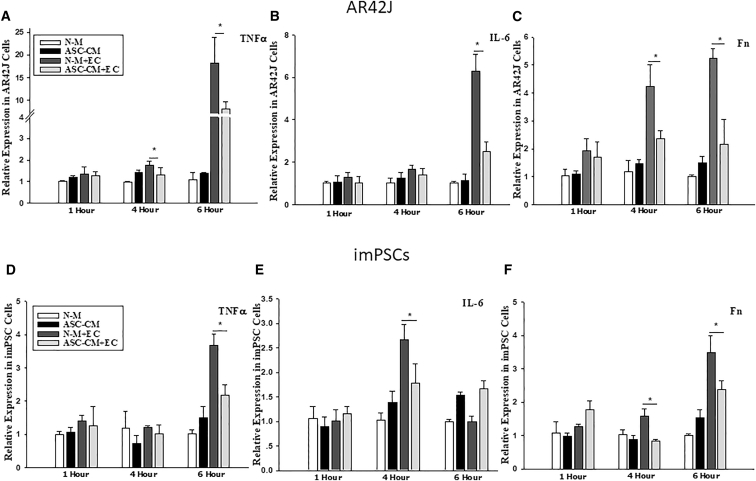
Co-culture in ASC-Conditioned Medium Suppresses Ethanol and Cerulein-Induced Production of Proinflammatory Cytokines by AR42J Cells and imPSCs
To determine the capacity of ASCs to suppress ethanol and cerulein-induced inflammation in AR42J cells and immortalized mouse PSCs (imPSCs), we measured expression of TNF-α, IL-6, and fibronectin mRNA in cells cultured in normal or ASC-conditioned medium in the presence or absence of ethanol and cerulein. The results indicated that culture of AR42J cells with ASC-conditioned medium significantly suppressed expression of TNF-α, IL-6, and fibronectin (Figures 7A–7C). Similarly, culture of imPSCs with ASC-conditioned medium significantly suppressed expression of TNF-α, IL-6, and fibronectin (Figures 7D–7F). These data suggest that co-culture with ASCs suppresses ethanol and cerulein-induced pro-inflammatory cytokine production in both acinar cells and imPSCs.
Figure 7.
Co-culture with ASC-Conditioned Medium Inhibits Ethanol and Cerulein-Induced Expression of Proinflammatory Cytokines in AR42J Cells and imPSCs
(A–F) Expression of TNF-α (A and D), IL-6 (B and E), and fibronectin (C and F) in AR42J cells or imPSCs. Data are representative of at least three individual experiments. Data presented are mean ± SEM. Differences were compared by one-way ANOVA; *p < 0.05. ASC-CM, cells cultured in ASC-conditioned medium; ASC-CM+EC, cells cultured in ASC-conditioned medium challenged with ethanol and cerulein; N-M, cells cultured in DMEM/F12; N-M+EC, cells cultured in DMEM/F12 challenged with ethanol and cerulein.
Discussion
Inflammation in CP activates PSCs, leading to pancreatic fibrosis and exocrine tissue damage, and at late stages, β cell death and endocrine dysfunction. Current pharmacological and surgical treatments for CP mainly target the symptoms rather than rescuing damaged pancreatic tissue from destruction.8 There is a need for more effective therapeutic options that have the capability of repairing damage of multiple cell types within the pancreas. In this study, we found that ASC therapy has great potential for use as a treatment of CP. A single systemic infusion of ASCs significantly reduced inflammation, fibrosis, and pancreatic tissue damage. This effect is likely mediated via their paracrine secretion of growth factors, which was indicated by the fact that acinar cell and PSC lines cultured in ASC-conditioned medium exhibited reduced proinflammatory cytokine production when stimulated with ethanol and cerulein. The most dramatic finding was that ASCs differentiated into amylase+ cells, which occurred both in vivo after cell infusion and in vitro when ASCs were co-cultured with acinar cells or cultured in acinar cell-conditioned medium. This suggests that ASC therapy attenuated pancreas damage through suppression of inflammation and fibrosis and through direct differentiation into pancreatic cells.
There have been a number of approaches to generating mouse models that mimic the symptoms of CP patients, including pancreatic duct ligation, duct infusion with trinitrobenzene sulfonic acid, injection of dibutyltin dichloride, and injection of cerulein and/or ethanol.35 Ethanol and cerulein are the most frequently used agents for CP induction in rodents.36 When repeatedly injected, cerulein can cause excessive edema, increased levels of pancreatic enzymes, inflammation, necrosis, and exocrine insufficiency.37 Although ethanol is one of the major causes of CP, fewer than 10% of chronic alcoholics develop CP.38 Ethanol is more likely a cofactor for the development of fibrosis, which is a constant histopathological feature of CP of all etiologies in “pre-injured” animals.37 Consistent with another study,36 we found that consecutive injections of ethanol together with cerulein destroyed the exocrine compartment of the mouse pancreas and induced fibrosis, enabling us to study the effects of ASC therapy on both pancreas damage and fibrosis.
As shown in acute pancreatitis models,16, 29, 39, 40, 41 we also found that the protective effects of MSC therapy were multifactorial and targeted several cell types in the pancreas. First, ASCs protected pancreatic cells from ethanol- and cerulean-induced cell death. ASCs also protected acinar cells and PSCs from inflammation in vitro when cells were challenged with ethanol and cerulein. ASCs suppressed pancreatic fibrosis, which was confirmed by immunohistochemical analysis of collagen and α-SMA and by inhibiting PSC activation. Our data are consistent with another study, which suggested that fibrosis is not merely an end product of chronic injury, but is a dynamic process that could be reversible in its early stages.30
Our study showed for the first time that systemically injected ASCs migrated and homed to the diseased pancreas and directly differentiated into amylase+ cells. The fate of MSCs after intravenous infusion is uncertain. In a study that followed the fate of MSCs for 72 hr after intravenous infusion, Eggenhofer et al.42 showed that MSCs were short-lived; they were found in the lung 1 hr after infusion and in the liver 24 hr after infusion, but then disappeared. In contrast, others have shown that MSCs passed through the liver and migrated to sites of injury.43, 44 We found significant numbers of GFP+ ASCs in the diseased pancreas at 14 days after cell infusion. The discrepancy between these studies and Eggenhofer et al.’s42 study might have been caused by the timing of analysis and different methods used for detection of MSCs. First, in Eggenhofer et al’s42 study, tissues were collected only until 72 hr after infusion; it is thus unknown whether DS-red+ MSCs can be found if tissues were analyzed 14 days post-infusion. Second, Eggenhofer et al.’s42 study cultured cells for 2–7 days before observation. The cell culture system might have favored growth and proliferation of some cell types other than MSCs, and therefore if there were only small numbers of MSCs, it might not be able to be detected.
In vivo models showed that MSCs after infusion can migrate to an inflammatory or injured site in mouse models of cardiac infarct45 and radiation-induced bone marrow injuries.46 Migration and homing require that cells can attach to and migrate between endothelial cells to enter the target tissue.47 The binding and rolling of MSCs were shown mediated by P-selectin, whereas migration involved the binding of very late antigen (VLA)-4 (or integrin-β1 and integrin-α4 dimer) on MSCs with vascular cell adhesion molecule (VCAM)-1 and interceullar adhesion molecule (ICAM)-1 found on endothelial cells.48 The precise mechanisms of MSC homing are largely unclear. It is believed that receptors and cell adhesion molecules expressed on MSCs facilitate their migration and homing. For example, the chemokine receptor C-X-C motif chemokine receptors (CXCR)4 was expressed in a subpopulation of MSCs that aid the CXCR-12-dependent migration and homing of MSCs.49, 50 In addition, MSCs also express C-C chemokine receptor (CCR)1, CCR7, and CCR9, as well as CXC chemokines receptors CXCR4, CXCR5, and CXCR7, integrins that are also involved in MSC migration.48, 51, 52, 53 Nevertheless, as shown in the acute pancreatitis model, an interaction of locally produced stromal cell-derived factor 1 and CXCR4 receptor-induced migration of bone marrow MSCs to the injured pancreas54 is a potential molecular mechanism responsible for the migration of injected MSCs into the diseased pancreas.
The ability of MSCs to mediate tissue repair is believed to be dependent on their paracrine functioning and on interactions with specific factors present in the microenvironment of the injured tissue.55 We argue that the impact is mutual, and the microenvironment in the diseased pancreas also determines the fate of MSCs. In our study, MSCs, which are of mesodermal origin with high plasticity, differentiated into cells of endodermal origin (i.e., acinar cells) in the diseased pancreas. ASCs preferably differentiated into acinar cells. The self-functional repair stimuli of stem cells might have been responsible for the presence of ASC-derived acinar in our study. However, further studies beyond the scope of this one have to be conducted to answer this question. This is consistent with the fact that CP specifically destroys the exocrine compartment of the pancreas during the early stages of disease progression.56, 57 In addition, we do not exclude the possibility that ASCs might have differentiated into other cell types of the pancreas such as endocrine cells and duct cells.
Our in vitro cell culture studies provide further evidence that growth factors and signaling molecules secreted by acinar cells can lead to the differentiation of ASCs. Rat bone marrow MSCs, human amniotic epithelial cells, and human ASCs have been shown to undergo differentiation into salivary acinar cells in vitro when co-cultured with acinar cells.58, 59, 60 However, not all MSCs appear to have equal capacity for differentiation. Other studies have been consistent with our findings. For example, it has been reported that 13%–18% of ASCs differentiated into amylase+ cells in a salivary gland model.27, 61 It will be important to determine whether there is a subset of the MSC population that has the potential to differentiate into specific cell types in specific disease situations.
One limitation of our study is that it is unknown whether pain has been induced in our mouse model, because metrics to measure mouse pain are problematic. The changes in physical activities we observed in CP mice and the effect of cell therapy on activity were suggestive but were not a direct measurement of pain.
In conclusion, systemic infusion of ASCs reduced ethanol and cerulein-induced pancreatic fibrosis and cellular injury in a mouse model of CP. ASCs exerted their protection both via direct differentiation into amylase+ cells and via their paracrine effects. ASC therapy has potential as a treatment option for reversing pancreatic destruction in patients with CP and other diseases.
Materials and Methods
CP Induction in Mice
CP was induced in male C57BL/6 mice at 6–8 weeks of age (The Jackson Laboratory) by repeated injections of ethanol and cerulein as previously described.36 In brief, mice received intraperitoneal (i.p.) injection of ethanol (Life Technology) (3.2 g/kg) once per day, six times per week for 2 weeks. On the first day of each week, mice received six i.p. injections of cerulein (Sigma-Aldrich) (50 μg/kg) at hourly intervals. Mice were housed under a standard light-dark cycle with ad libitum access to chow and water. Body weight and food intake were measured daily, and physical activities were measured weekly using the Accuscan Versamax Analyzer. Blood glucose levels in mice were measured using a glucometer (Abbott). The Animal Care and Use Committee at the Medical University of South Carolina approved all mouse experiments.
ASC Preparation and Infusion
Inguinal fat tissues were collected from C57BL/6 mice or from C57BL/6-Tg (CAG-EGFP) mice in which GFP expression can be detected in most cell types including the MSCs. Tissues were washed in PBS and digested in 0.1% collagenase type II at 37°C for 20 min with occasional shaking. Digestion was stopped by addition of complete medium containing DMEM/F12 (1:1; Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Cells were filtered through 100 μm nylon mesh and cultured at 1,000–3,000 cells per cm2 in 5% CO2 at 37°C. Floating cells were removed 8 hr later. Cells were split when the population reached 80%–90% confluence. ASCs at passage 3 (4 × 105 or 1 × 106 cells per mouse) were infused into CP mice via tail vein injection. CP and normal mice injected with PBS were used as controls. Two weeks after cell injection, serum, pancreas, liver, and lungs of treated mice were collected for further analysis.
Immunohistochemical Analysis
Paraffin-embedded pancreatic tissues were deparaffinized by treating with xylene for 5 min and then dehydrated. Serial 10 μm sections were collected for every 100 μm pancreas tissue spanning the entire pancreas. Consecutive sections were stained with each individual antibody. Antigen retrieval was performed by treating the slides in 95°C water for 5 min, followed by treatment in 0.5% Triton X-100 in PBS for 30 min. Endogenous peroxidases were blocked by incubation in 3% hydrogen peroxide for 30 min. Slides were then rinsed in PBS and blocked with BSA. H&E staining was performed using a standard protocol. Masson-Goldner trichrome staining was performed using a Masson-Goldner staining kit (Millipore) according to the manufacturer’s recommendation. Area positive for Masson trichrome was measured, and the ratio of the Masson trichrome-positive area to total pancreas area was calculated using ImageJ software. For detection of α-SMA, samples were incubated with a mouse anti-α-SMA antibody overnight at 4°C, followed by incubation with a goat anti-mouse secondary antibody conjugated with horseradish peroxidase (HRP) and 3,3' diaminobenzidine (DAB) for signal detection. For collagen detection, slides were incubated with Wiegert’s hematoxylin, followed by staining with picrosirius red in saturated aqueous solution of picric acid. Slides were observed using an Olympus BX40 microscope. For each pancreas, 30–100 individual slides were stained and analyzed to calculate SMA+, Masson-Goldner+, and insulin+ areas.
For detection of GFP+ cells within the pancreas, tissue sections were incubated with a chicken anti-GFP antibody (Abcam) and a rabbit anti-amylase antibody (Sigma-Aldrich). Secondary antibodies were Alex Fluor 488 goat anti-chicken and Alexa Fluor 568 goat anti-rabbit (Life Technologies). Slides were observed using a Leica SP5 confocal microscope. Percentage of GFP+ amylase+ cells in all amylase+ cells was calculated. Slides from three to four pancreases were analyzed in each group.
TUNEL Assay
Cell death was measured in situ in deparaffinized tissue using a fluorescein TUNEL Cell Death Detection kit (Roche Life Science) according to the manufacturer’s recommendation. On each slide, six areas were randomly selected for analysis. At least 10 slides were counted per pancreas, and average percentages of TUNEL+ cells among total cells were observed and calculated using the Zeiss AxioImager M2 Imaging System.
Beta Cell Mass Measurement
Serial pancreas sections were collected, and islets were identified by staining with the anti-insulin antibody by immunohistochemistry. The number of islets per pancreas section was counted. For each pancreas, at least 10 slides spanning the pancreas were analyzed.
Conditioned Medium
For ASC-conditioned medium (ASC-CM), ASCs were grown in DMEM/F12 with 10% FBS until reaching 90% confluence. Cell culture medium was then replaced by serum-free DMEM/F12 containing only antibiotics. Conditioned medium was collected 48 hr after medium replacement, centrifuged at 500 × g for 3 min, filtered through a 0.22 μm filter, aliquoted, frozen, and stored at −80°C for future use. For acinar cell-conditioned medium (A-CM), an acinar cell line, AR42J (ATCC), was cultured in serum-free F-12K, and conditioned medium was collected using the same protocol.
In Vitro Co-culture of ASCs and AR42J
Mouse ASCs at passage 2 were seeded on a cover slide and co-cultured with AR42J in a Transwell system in a 12-well plate. ASCs cultured in DMEM/F12 or AR42J-conditioned F-12K mediums were used as controls. Media were replaced every 3 days during co-culture. At 7 days of co-culture, ASCs were removed from the co-culture system, fixed, and analyzed by immunohistochemistry for GFP and amylase expression. In a separate set of experiments, ASCs were harvested at 7 and 14 days after co-culture with AR42J or in AR42J-conditioned media, and total RNA was extracted. Expression of amylase 1, chymoB1, and MSC markers, including fibronectin and vimentin, were analyzed by real-time RT-PCR.
Stimulation of AR42J and imPSCs by Ethanol and Cerulein
AR42J or imPSCs (kindly gifted by Dr. Raul Urrutia, Mayo Clinic) were seeded into 12-well cell culture plates and were cultured in F-12K medium. In some wells, cells were pre-incubated with ASC condition medium. Cells were treated with ethanol (2.4 mg/mL) and cerulein (0.13 mg/mL), and were collected at 1, 4, 6, and 24 hr after treatment. Expression of TNF-α, IL-6, and fibronectin were measured in samples by real-time RT-PCR analysis.
Real-Time RT-PCR Analysis
Total RNA was extracted from cells using the QIAGEN RNA extraction kit (QIAGEN). Samples were reverse transcribed into cDNA. cDNA was amplified using a SYBR Green RT-PCR system from Bio-Rad. Primer pairs used were described previously.60 Expression of specific genes was normalized to the expression of β-actin in each sample.
Statistical Analyses
Data presented are mean ± SEM. Differences between groups were analyzed by one-way ANOVA.
Author Contributions
Z.S., W.G., and D.K. performed conception design, data collection, and analysis. X.D. and Y.T. conducted data analysis. D.B.A. conducted provision of study material or patients and data analysis. C.S. performed data analysis and manuscript writing. H.W. performed study design, data interpretation, and manuscript writing.
Acknowledgments
The authors thank the Reeves family for their generous donation. This study was supported in part by NIH grants DK097544, DK105183, and DK099696.
Footnotes
Supplemental Information includes two figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.06.016.
Supplemental Information
References
- 1.Lankisch P.G. Natural course of chronic pancreatitis. Pancreatology. 2001;1:3–14. doi: 10.1159/000055786. [DOI] [PubMed] [Google Scholar]
- 2.Adams D.B., Davis B.R., Anderson M.C. Colonic complications of pancreatitis. Am. Surg. 1994;60:44–49. [PubMed] [Google Scholar]
- 3.Etemad B., Whitcomb D.C. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 4.Tieftrunk E., Demir I.E., Simon P., Friess H., Ceyhan G.O. Evidence of pancreatic neuropathy and neuropathic pain in hereditary chronic pancreatitis. Pancreatology. 2013;13:629–630. doi: 10.1016/j.pan.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Marks I.N., Bank S. The aetiology, clinical features and diagnosis of pancreatitis in the South Western Cape; a review of 243 cases. S. Afr. Med. J. 1963;37:1039–1053. [PubMed] [Google Scholar]
- 6.Wilson J.S., Korsten M.A., Pirola R.C. Alcohol-induced pancreatic injury (part I). Unexplained features and ductular theories of pathogenesis. Int. J. Pancreatol. 1989;4:109–125. [PubMed] [Google Scholar]
- 7.Andrén-Sandberg A., Hoem D., Gislason H. Pain management in chronic pancreatitis. Eur. J. Gastroenterol. Hepatol. 2002;14:957–970. doi: 10.1097/00042737-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Dumonceau J.M., Devière J., Le Moine O., Delhaye M., Vandermeeren A., Baize M., Van Gansbeke D., Cremer M. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest. Endosc. 1996;43:547–555. doi: 10.1016/s0016-5107(96)70189-x. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 11.D’Addio F., Valderrama Vasquez A., Ben Nasr M., Franek E., Zhu D., Li L., Ning G., Snarski E., Fiorina P. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes. 2014;63:3041–3046. doi: 10.2337/db14-0295. [DOI] [PubMed] [Google Scholar]
- 12.Frumento D., Ben Nasr M., El Essawy B., D’Addio F., Zuccotti G.V., Fiorina P. Immunotherapy for type 1 diabetes. J. Endocrinol. Invest. 2017 doi: 10.1007/s40618-017-0641-y. Published online March 4, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Amuro N., Ooki K., Ito A., Goto Y., Okazaki T. Nucleotide sequence of rat liver glutamate dehydrogenase cDNA. Nucleic Acids Res. 1989;17:2356. doi: 10.1093/nar/17.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bura A., Planat-Benard V., Bourin P., Silvestre J.S., Gross F., Grolleau J.L., Saint-Lebese B., Peyrafitte J.A., Fleury S., Gadelorge M. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Mizuno H., Tobita M., Uysal A.C. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.W., Song W.J., Li Q., Han S.M., Jeon K.O., Park S.C., Ryu M.O., Chae H.K., Kyeong K., Youn H.Y. Canine adipose tissue-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating T cells in rats. J. Vet. Sci. 2016;17:539–548. doi: 10.4142/jvs.2016.17.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang H.M., Kim J., Park S., Kim J., Kim H., Kim K.S., Lee E.J., Seo S.I., Kang S.G., Lee J.E., Lim H. Insulin-secreting cells from human eyelid-derived stem cells alleviate type I diabetes in immunocompetent mice. Stem Cells. 2009;27:1999–2008. doi: 10.1002/stem.127. [DOI] [PubMed] [Google Scholar]
- 18.Bassi E.J., Moraes-Vieira P.M., Moreira-Sá C.S., Almeida D.C., Vieira L.M., Cunha C.S., Hiyane M.I., Basso A.S., Pacheco-Silva A., Câmara N.O. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–2545. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constantin G., Marconi S., Rossi B., Angiari S., Calderan L., Anghileri E., Gini B., Bach S.D., Martinello M., Bifari F. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 20.Park M.J., Kwok S.K., Lee S.H., Kim E.K., Park S.H., Cho M.L. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015;24:2367–2377. doi: 10.3727/096368914X685645. [DOI] [PubMed] [Google Scholar]
- 21.González M.A., Gonzalez-Rey E., Rico L., Büscher D., Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 22.Sanz-Baro R., García-Arranz M., Guadalajara H., de la Quintana P., Herreros M.D., García-Olmo D. First-in-human case study: pregnancy in women with Crohn’s perianal fistula treated with adipose-derived stem cells: a safety study. Stem Cells Transl. Med. 2015;4:598–602. doi: 10.5966/sctm.2014-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Álvaro-Gracia J.M., Jover J.A., García-Vicuña R., Carreño L., Alonso A., Marsal S., Blanco F., Martínez-Taboada V.M., Taylor P., Martín-Martín C. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2016;76:196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 24.Pers Y.M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F., Sensebe L., Casteilla L., Fleury S., Bourin P., ADIPOA Consortium Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl. Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillaume-Jugnot P., Daumas A., Magalon J., Jouve E., Nguyen P.S., Truillet R., Mallet S., Casanova D., Giraudo L., Veran J. Autologous adipose-derived stromal vascular fraction in patients with systemic sclerosis: 12-month follow-up. Rheumatology (Oxford) 2016;55:301–306. doi: 10.1093/rheumatology/kev323. [DOI] [PubMed] [Google Scholar]
- 26.Díez-Tejedor E., Gutiérrez-Fernández M., Martínez-Sánchez P., Rodríguez-Frutos B., Ruiz-Ares G., Lara M.L., Gimeno B.F. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 2014;23:2694–2700. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Susman S., Rus-Ciuca D., Soritau O., Tomuleasa C., Buiga R., Mihu D., Pop V.I., Mihu C.M. Pancreatic exocrine adult cells and placental stem cells co-culture. Working together is always the best way to go. Rev. Roum. Morphol. Embryol. 2011;52(Suppl 3):999–1004. [PubMed] [Google Scholar]
- 28.Lazebnik L.B., Trubitsyna I.E., Agafonov M.A., Kniazev O.V., Liundup A.V. [Mesenchymal stromal cells transplantation in acute and chronic pancreatitis in rats] Eksp. Klin. Gastroenterol. 2011:28–31. [PubMed] [Google Scholar]
- 29.Jung K.H., Song S.U., Yi T., Jeon M.S., Hong S.W., Zheng H.M., Lee H.S., Choi M.J., Lee D.H., Hong S.S. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C.H., Li M.L., Qin A.L., Lv S.X., Wen-Tang, Zhu X.Y., Li L.Y., Dong Y., Hu C.Y., Hu D.M., Wang S.F. Reduction of fibrosis in dibutyltin dichloride-induced chronic pancreatitis using rat umbilical mesenchymal stem cells from Wharton’s jelly. Pancreas. 2013;42:1291–1302. doi: 10.1097/MPA.0b013e318296924e. [DOI] [PubMed] [Google Scholar]
- 31.Ammann R.W., Heitz P.U., Klöppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224–231. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- 32.Cao M., Pan Q., Dong H., Yuan X., Li Y., Sun Z., Dong X., Wang H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res. Ther. 2015;6:208. doi: 10.1186/s13287-015-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apte M., Pirola R., Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid. Redox Signal. 2011;15:2711–2722. doi: 10.1089/ars.2011.4079. [DOI] [PubMed] [Google Scholar]
- 34.Maria O.M., Tran S.D. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev. 2011;20:959–967. doi: 10.1089/scd.2010.0214. [DOI] [PubMed] [Google Scholar]
- 35.Lerch M.M., Gorelick F.S. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 36.Charrier A.L., Brigstock D.R. Connective tissue growth factor production by activated pancreatic stellate cells in mouse alcoholic chronic pancreatitis. Lab. Invest. 2010;90:1179–1188. doi: 10.1038/labinvest.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aghdassi A.A., Mayerle J., Christochowitz S., Weiss F.U., Sendler M., Lerch M.M. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4:26. doi: 10.1186/1755-1536-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnelldorfer T., Adams D.B. Surgical treatment of alcohol-associated chronic pancreatitis: the challenges and pitfalls. Am. Surg. 2008;74:503–507. discussion 508–509. [PubMed] [Google Scholar]
- 39.Schneider G., Saur D. Mesenchymal stem cells: therapeutic potential for acute pancreatitis. Gastroenterology. 2011;140:779–782. doi: 10.1053/j.gastro.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Yin F., Battiwalla M., Ito S., Feng X., Chinian F., Melenhorst J.J., Koklanaris E., Sabatino M., Stroncek D., Samsel L. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem Cells. 2014;32:1278–1288. doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z., Lu F., Fang H., Huang H. Effect of mesenchymal stem cells on renal injury in rats with severe acute pancreatitis. Exp. Biol. Med. (Maywood) 2013;238:687–695. doi: 10.1177/1535370213490629. [DOI] [PubMed] [Google Scholar]
- 42.Eggenhofer E., Benseler V., Kroemer A., Popp F.C., Geissler E.K., Schlitt H.J., Baan C.C., Dahlke M.H., Hoogduijn M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson J.S., Golding J.P., Chapon C., Jones W.A., Bhakoo K.K. Homing of stem cells to sites of inflammatory brain injury after intracerebral and intravenous administration: a longitudinal imaging study. Stem Cell Res. Ther. 2010;1:17. doi: 10.1186/scrt17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapel A., Bertho J.M., Bensidhoum M., Fouillard L., Young R.G., Frick J., Demarquay C., Cuvelier F., Mathieu E., Trompier F. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 2003;5:1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 45.Shake J.G., Gruber P.J., Baumgartner W.A., Senechal G., Meyers J., Redmond J.M., Pittenger M.F., Martin B.J. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 46.François S., Bensidhoum M., Mouiseddine M., Mazurier C., Allenet B., Semont A., Frick J., Saché A., Bouchet S., Thierry D. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 47.Sohni A., Verfaillie C.M. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rüster B., Göttig S., Ludwig R.J., Bistrian R., Müller S., Seifried E., Gille J., Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 49.Wynn R.F., Hart C.A., Corradi-Perini C., O’Neill L., Evans C.A., Wraith J.E., Fairbairn L.J., Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 50.Fiorina P., Jurewicz M., Vergani A., Petrelli A., Carvello M., D’Addio F., Godwin J.G., Law K., Wu E., Tian Z. Targeting the CXCR4-CXCL12 axis mobilizes autologous hematopoietic stem cells and prolongs islet allograft survival via programmed death ligand 1. J. Immunol. 2011;186:121–131. doi: 10.4049/jimmunol.1000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A.M., Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 52.Von Lüttichau I., Notohamiprodjo M., Wechselberger A., Peters C., Henger A., Seliger C., Djafarzadeh R., Huss R., Nelson P.J. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- 53.Krampera M., Pizzolo G., Aprili G., Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Gong J., Meng H.B., Hua J., Song Z.S., He Z.G., Zhou B., Qian M.P. The SDF-1/CXCR4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol. Med. Rep. 2014;9:1575–1582. doi: 10.3892/mmr.2014.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prockop D.J. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol. Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goto M., Nakano I., Kimura T., Miyahara T., Kinjo M., Nawata H. New chronic pancreatitis model with diabetes induced by cerulein plus stress in rats. Dig. Dis. Sci. 1995;40:2356–2363. doi: 10.1007/BF02063237. [DOI] [PubMed] [Google Scholar]
- 57.Dong H., Morgan K., Adams D., Wang H. Prevention of beta cell death in chronic pancreatitis. Adv. Biosci. Biotechnol. 2012;3:782–787. [Google Scholar]
- 58.Huang G.L., Zhang N.N., Wang J.S., Yao L., Zhao Y.J., Wang Y.Y. Transdifferentiation of human amniotic epithelial cells into acinar cells using a double-chamber system. Cell. Reprogram. 2012;14:377–383. doi: 10.1089/cell.2011.0096. [DOI] [PubMed] [Google Scholar]
- 59.Lin C.Y., Lee B.S., Liao C.C., Cheng W.J., Chang F.M., Chen M.H. Transdifferentiation of bone marrow stem cells into acinar cells using a double chamber system. J. Formos. Med. Assoc. 2007;106:1–7. doi: 10.1016/S0929-6646(09)60209-6. [DOI] [PubMed] [Google Scholar]
- 60.Lee J., Park S., Roh S. Transdifferentiation of mouse adipose-derived stromal cells into acinar cells of the submandibular gland using a co-culture system. Exp. Cell Res. 2015;334:160–172. doi: 10.1016/j.yexcr.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Lim J.Y., Ra J.C., Shin I.S., Jang Y.H., An H.Y., Choi J.S., Kim W.C., Kim Y.M. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS ONE. 2013;8:e71167. doi: 10.1371/journal.pone.0071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.