Abstract
The current study sought to clarify the role of phosphodiesterase type 5 inhibitors (PDE-5i) and a vacuum erection device (VED) in penile rehabilitation after laparoscopic nerve-preserving radical proctectomy (LNRP) for rectal cancer. Participants were assigned to one of the following arms—no-intervention, nightly use of sildenafil 25 mg for 3 months after surgery, or concurrent use of nightly sildenafil 25 mg/day for 3 months and a vacuum erection device (VED) 10 to 15 minutes/day for 3 months—in a nonrandomized fashion. All participants had a follow-up of over 12 months prospectively, and patients had baseline, 3-, 6-, and 12-month assessment based on the International Index of Erectile Function–5 (IIEF-5). Seventy-one cases were included in final analyses. In the no-intervention group, the mean baseline IIEF-5 score of 21.9 decreased rapidly to 5.0 at 3 months (p < .001), 9.2 at 6 months (p < .001), and stayed at 10.9 at 12 months (p < .001). In the single therapy group, the mean baseline IIEF-5 score of 22.4 decreased dramatically to 9.0 at 3 months (p < .001), 14.9 at 6 months (p = .005), and stayed at 15.1 at 12 months (p = .005). In the combined therapy group, the mean baseline IIEF-5 score of 23.0 decreased slightly to 15.0 at 3 months (p = .005), 18.0 at 6 months (p = .038), and maintained at 18.7 at 12 months (p = .163). Findings suggested an over 50% decline in the quality of erection function of the patients after LNRP. The early use of PDE-5i alone or combined use of PDE-5i and VED after LNRP maintained erectile function at 12 months.
Keywords: penile rehabilitation, erectile dysfunction, rectal cancer, laparoscopic surgery
Introduction
Erectile dysfunction (ED) is an unfavorable comorbid outcome of radical resection for rectal cancer, and it has a significant adverse impact on the sexual health–related quality of life after these radical therapies (Andersson et al., 2014; Aoun, Peltier, & van Velthoven, 2015; Attaallah, Ertekin, Tinay, & Yegen, 2014; Ball et al., 2013; Dowswell et al., 2011). Although a high prevalence of ED was detected among male patients after treatment for colorectal cancer (CRC), published reports depicting the ED experience of patients with CRC to underpin service development are insufficient (Park et al., 2015). Unlike patients with prostate cancer, men with CRC are not routinely offered information and treatment for ED. Investigations on patients after surgery for prostate cancer could provide some potentially useful insights for postoperative care of CRC patients (Kimura et al., 2012; Pavlovich et al., 2013; Segal, Bivalacqua, & Burnett, 2013).
A phosphodiesterase type 5 inhibitor (PDE-5i) is commonly used as a first-line primary penile rehabilitation strategy for post–radical prostatectomy (RP) ED (Bergman, Gore, Penson, Kwan, & Litwin, 2009). A number of investigations report on the expanded role of using a vacuum erection device (VED) as combined therapy with a PDE5i for penile rehabilitation after RP (Kohler et al., 2007; Pahlajani et al., 2010). The ability of PDE5i and VED to aid in the return of erections after nerve-sparing RP has been established and may benefit rectal cancer patients with ED after surgery (Lindsey, George, Kettlewell, & Mortensen, 2002; Traa, De Vries, Roukema, & Den Oudsten, 2012). Penile rehabilitation, defined as the use of any drug or device after radical resection for rectal cancer to maximize the recovery of sexual function, is warranted.
Laparoscopic surgery, although technically demanding and associated with a long learning curve, has the advantage of clear visualization for the smallest structures, including the autonomic nerves. Laparoscopic resection for rectal cancer could facilitate preservation of the pelvic autonomic nerves, thus facilitating the retention of genitourinary function in a significant proportion of such patients (Liang, Lai, & Lee, 2007).
Given the lack of consensus regarding penile rehabilitation for post–radical rectal resection, the authors designed a prospective controlled trial evaluating the safety and efficacy of PDE5i and/or VED in rectal cancer patients after laparoscopic nerve-preserving radical proctectomy (LNRP).
Method
Study Design and Patients
This prospective, nonrandomized intervention study was carried out at the department of general surgery at a tertiary university hospital. The protocol was approved by The institutional review board of Nanfang Hospital, Southern Medical University before enrollment (NFEC-2013-035), and written informed consent was obtained from all participating patients. This study was registered on clinicaltrials.gov (NCT01912586).
Male patients aged 18 to 70 years and scheduled for total mesorectal excision (TME) for rectal cancer within 11 cm from the anal verge were recruited for this study. The International Index of Erectile Function–5 (IIEF-5) questionnaire (Rosen, Cappelleri, Smith, Lipsky, & Pena, 1999) was assessed both preoperatively and 3, 6, and 12 months after surgery. Eligible patients had to be sexually active men without the consistent use of erectile aids preoperatively, with an IIEF-5 domain score greater than 21 preoperatively. Exclusion criteria included a history of cardiac failure, angina, or life-threatening arrhythmia within the past 6 months, taking or having been prescribed nitrate medication in any form in the last 6 months, contraindication to sildenafil (e.g., nitrates, hypersensitivity), contraindication to VED (e.g., coagulation abnormality, stick cell disease), men with a history of known penile deformity or Peyronie’s disease, and pre- or postoperative androgen therapy.
Grouping and Interventions
The participants were assigned to one of the following arms—no-intervention, nightly use of sildenafil 25 mg for 3 months after surgery, or the concurrent use of nightly sildenafil 25 mg/day for 3 months and a VED 10 to 15 minutes/day for 3 months—in a nonrandomized fashion. Treatment was started within 1 or 2 weeks (at catheter removal) after surgery to minimize penile tissue degeneration. All participants had a follow-up of over 12 months prospectively, and patients had baseline of 3-, 6-, and 12-month assessment based on IIEF-5.
Study Outcomes
The primary outcome was the change in IIEF-5 score from the baseline (before starting medication) to the end of the treatment. The IIEF-5, which is a five-item version of the IIEF questionnaire (composed of four questions about erectile function and one question about intercourse satisfaction) was used to evaluate erectile function. Nocturnal penile tumescence was used to distinguish psychogenic from organic impotence. Patients were assessed with IIEF-5 at the end of treatment and at 3, 6, and 12 months based on IIEF-5 scores after surgery. Secondary outcomes were compliance and overall patient satisfaction.
Safety Variables
Patients were monitored closely for any signs of adverse events. Safety assessment included the presence of all reported adverse events regardless of relationship to the study drug, monitoring of vital signs, abnormality in laboratory results, and complete physical examination.
Statistical Methods
Baseline clinical data were analyzed using the t test for continuous data and Fisher exact test or the chi-square test for categorical data. Data for the total IIEF scores and the domain scores were analyzed using a two-way analysis of variance. All analyses were performed using SPSS Version 18.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and p values <.05 were considered statistically significant.
Results
Patient Characteristics
A total of 90 patients were enrolled and followed up in the study between April 2013 and August 2014. After excluding 19 cases lost to follow-up, 71 cases were included in the final analyses (14 in the combined therapy group, 23 in the single therapy group, 34 in the no-intervention group (Figure 1). The mean age was 37.0 years in the combined therapy group, 42.5 years in the single therapy group, and 50.9 years in the no-intervention group (p = .013). The patient characteristics between the three groups were not significantly different in terms of body mass index, tumor stage, type of resection, preoperative chemoradiotherapy, stoma status, or nerve preservation (Table 1).
Figure 1.
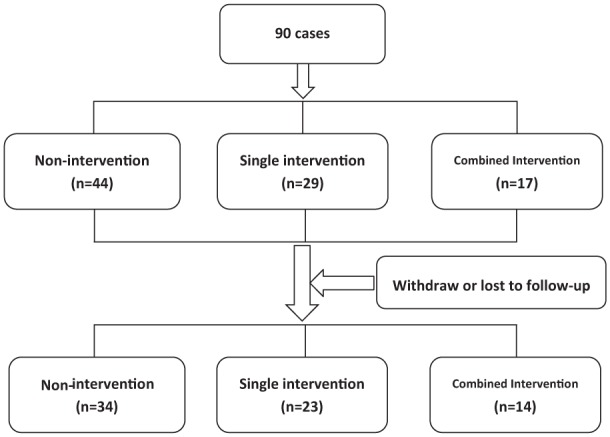
Flowchart for inclusion in the study.
Table 1.
Patient Characteristics.
| Characteristic | Nonintervention (n = 44) | Single intervention (n = 29) | Combined interventions (n = 29) | p |
|---|---|---|---|---|
| Age (years), mean ± SD | 50.1 ± 9.4 | 42.5 ± 6.5 | 37.0 ± 10.5 | .013 |
| Body mass index (kg/m2), mean ± SD | 22.3 ± 2.9 | 23.1 ± 4.0 | 20.5 ± 4.9 | .415 |
| Tumor stage; n (%) | ||||
| I | 9 (20.5) | 4 (13.8) | 3 (17.6) | .915 |
| II | 25 (56.8) | 16 (55.2) | 10 (58.9) | |
| III | 10 (22.7) | 9 (31.0) | 4 (23.5) | |
| Type of resection; n (%) | ||||
| Low anterior resection | 32 (72.7) | 19 (65.6) | 13 (76.5) | |
| Abdominoperineal resection | 12 (27.3) | 10 (34.4) | 4 (23.5) | .692 |
| Preoperative chemoradiotherapy; n (%) | 17 (43.2) | 11 (37.9) | 6 (35.3) | .971 |
| Stoma status; n (%) | 28 (63.6) | 16 (55.2) | 9 (52.9) | .662 |
| Perioperative nerve preservation; n (%) | 42 (95.4) | 28 (96.5) | 17 (100) | .674 |
| Operative time (minutes), mean ± SD | 153.5 ± 35.0 | 144.3 ± 27.4 | 175.3 ± 20.2 | .349 |
Sexual Function
In the no-intervention group, the mean baseline IIEF-5 score of 21.9 decreased rapidly to 5.0 at 3 months (p < .001), 9.2 at 6 months (p < .001), and stayed at 10.9 at 12 months (p < .001). In the single therapy group, the mean baseline IIEF-5 score of 22.4 decreased dramatically to 9.0 at 3 months (p < .001), 14.9 at 6 months (p = .005), and stayed at 15.1 at 12 months (p = .005). In the combined therapy group, the mean baseline IIEF-5 score of 23.0 decreased slightly to 15.0 at 3 months (p = .005), 18.0 at 6 months (p = .038), and maintained 18.7 at 12 months (p = .163) (Figure 2).
Figure 2.
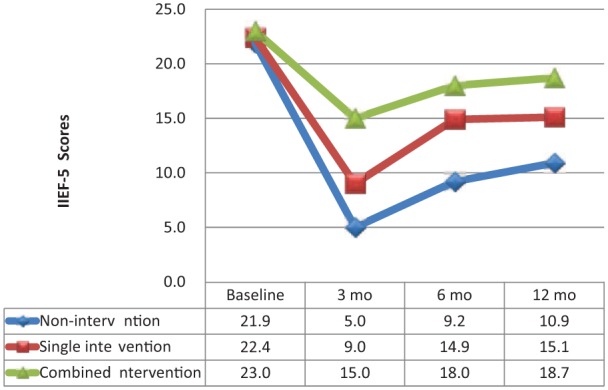
Erectile function changes after laparoscopic nerve-preserving radical proctectomy (LNRP).
Significant differences were identified in IIEF-5 scores between the three groups at 6 months (p = .010) and 12 months (p = .043) after LNRP, after adjusting for age (data not shown).
Discussion
After pelvic cancer treatment, nerve and blood vessel injury, or manipulation can impair penile erections, penile oxygenation, and long-term penile and sexual health. Penile rehabilitation helps minimize the negative impacts on male sexual function and expedite recovery of sexual function (Aoun et al., 2015). The interventions aimed at preserving sexual function must allow regular erections. Clinical studies support early and aggressive therapies for ED after surgery, which can help more rapid and complete recovery of sexual function. Based on available evidence, penile rehabilitation programs usually included oral medications, injection therapy, urethral suppositories, VED, or testosterone replacement (Segal et al., 2013). PED-5is have recently been used not only as a treatment of ED in the RP population but also as a preventive strategy in penile rehabilitation programs (Hatzimouratidis et al., 2009). The current study, which included 71 patents with rectal cancer, identified an over 50% decline in quality of erection function of the patients after LNRP, providing an opportunity to initiate early intervention with PED-5i or VED in selected patients.
ED is a prevalent negative issue reported in 10% to 60% of patients after rectal cancer surgery (Traa et al., 2012). In a historic prospective case series study, ED was reported in 48% of patients after abdominoperineal resection (Danzi, Ferulano, Abate, & Califano, 1983), which carries a higher risk of postoperative ED than low anterior resection procedures with reported rates varying from 15% to 92% (Keating, 2004; Pocard et al., 2002), in agreement with the current study. The stoma made after abdominoperineal resection has also been reported to affect body image and increase the rate of postoperative sexual dysfunction, but there is also controversy in a small series reporting no difference between patients with or without a stoma (Nishizawa et al., 2011). In the current study, more than 50% of the patients had stoma, which may contribute to the increased rates of ED. Patients with an age of less than 50 years had a decreased risk of ED (Fazio, Fletcher, & Montague, 1980). The mean age of the current study was less than 50 years; however, the incidence of ED at 3 months after surgery in nonintervention group was more than 50%. Surgical expertise is another influencing factor of ED with case series from high surgeon volume and high cancer center volume reporting lower rates of ED (Havenga et al., 1996). Furthermore, a laparoscopic approach can be used in pelvic autonomic nerve-preserving surgery for patients with low rectal cancer (Kim et al., 2012; Liang et al., 2007). The center where the current study was conducted can be considered a high-volume center for colorectal surgery as it performs more than 800 CRC resections by 3 units per year, and this trial was performed by one experienced laparoscopic surgeon from the first unit, which has high-volume activity.
Rehabilitation programs for these patients are complex. Psychological evaluation and support of the patient and his or her partner are mandatory, resulting in enhancing the response to pharmacologic therapy (Eveno, Lamblin, Mariette, & Pocard, 2010). Among the medications available, the efficacy of sildenafil was demonstrated in a study where 32 patients were randomized to medical treatment or placebo after rectal resection (Lindsey et al., 2002). Erectile potency improved in 80% of patients treated with sildenafil compared with 17% of patients treated with placebo (Lindsey et al., 2002). To date, most experienced surgeons perform TME with preservation of the neurovascular bundles with improved reported rates of ED (Asoglu et al., 2009; Breukink et al., 2009; Kim et al., 2012; Pocard et al., 2002). However, only one prospective study conducted in Japan examined the outcome of postoperative treatment with sildenafil for potent male patients after TME for low rectal cancers (Nishizawa et al., 2011). Forty out of 49 sexually active patients preoperatively presented ED at 3 months postoperatively, and only 4 patients regained their erection at 12 months. Sildenafil was administered to 16 patients who requested the drug during follow-up, and sexual dysfunction was improved in 11 of these patients. The current study identified an over 50% decline in the quality of erection function of the patients after LNRP at 3 months postoperatively and the early use of PDE-5i alone or combined use of PDE-5i and VED after LNRP maintained erectile function at 12 months. Sildenafil has also been reported to improve anal function (Fritz, Hammer, Schmidt, Eherer, & Hammer, 2003; Milone & DiBaise, 2005), but further experimental research is needed to understand the mechanism of action and its impact on postoperative anal function.
The current study has some strengths. Medication administration began immediately after surgery, and usage was evaluated throughout. The quality of nerve-preserving surgery can be qualified by experienced surgeons. In addition, two complete validated instruments (IIEF and nocturnal penile tumescence) were adopted to account for the disparity that existed among patients classified as potent by the IIEF. There are also limitations including nonrandomized design and small sample size. Notably, the disparity of age among the three groups may have arisen from the nonrandomized design, which may lead to bias. Patients with strong preferences for “usual care” often do not get into a trial because randomisation does not guarantee that they will get what they want.
Still, erectile function changes showed that the curves of changes of IIEF-5 scores of 3, 6, 12 months in the intervention groups were smoother than that in the nonintervention group, suggesting that the intervention works irrespective of age.
Conclusion
The current findings reported an over 50% decline in the quality of erection function of patients after LNRP, providing an opportunity to initiate early intervention with PDE-5i or VED in selected patients. In present study, the early use of PDE-5i alone or the combined use of PDE-5i and VED after LNRP maintained erectile function at 12 months. Randomized controlled trials are warranted to further confirm the findings.
Footnotes
Author’s Note: Authors Haijun Deng, Dong Liu, and Xiangming Mao contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Key Clinical Specialty Discipline Construction Program from National Health and Family Planning Commission of China and the program of Guangdong Provincial Science and Technology Department (no. 2013B022000019).
References
- Andersson J., Abis G., Gellerstedt M., Angenete E., Angeras U., Cuesta M. A., . . . Haglind E. (2014). Patient-reported genitourinary dysfunction after laparoscopic and open rectal cancer surgery in a randomized trial (COLOR II). British Journal of Surgery, 101, 1272-1279. doi: 10.1002/bjs.9550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoun F., Peltier A., van Velthoven R. (2015). Penile rehabilitation after pelvic cancer surgery. Scientific World Journal, 2015, 876046. doi: 10.1155/2015/876046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asoglu O., Matlim T., Karanlik H., Atar M., Muslumanoglu M., Kapran Y., . . . Parlak M. (2009). Impact of laparoscopic surgery on bladder and sexual function after total mesorectal excision for rectal cancer. Surgical Endoscopy, 23, 296-303. doi: 10.1007/s00464-008-9870-7 [DOI] [PubMed] [Google Scholar]
- Attaallah W., Ertekin C., Tinay I., Yegen C. (2014). High rate of sexual dysfunction following surgery for rectal cancer. Annals of Coloproctology, 30, 210-215. doi: 10.3393/ac.2014.30.5.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball M., Nelson C. J., Shuk E., Starr T. D., Temple L., Jandorf L., . . . DuHamel K. (2013). Men’s experience with sexual dysfunction post-rectal cancer treatment: A qualitative study. Journal of Cancer Education, 28, 494-502. doi: 10.1007/s13187-013-0492-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J., Gore J. L., Penson D. F., Kwan L., Litwin M. S. (2009). Erectile aid use by men treated for localized prostate cancer. Journal of Urology, 182, 649-654. doi: 10.1016/j.juro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Breukink S. O., Wouda J. C., van de Werf-Eldering M. J., van de Wiel H. B., Bouma E. M., Pierie J. P., . . . Weijmar Schultz W. C. (2009). Psychophysiological assessment of sexual function in women after radiotherapy and total mesorectal excision for rectal cancer: A pilot study on four patients. Journal of Sexual Medicine, 6, 1045-1053. doi: 10.1111/j.1743-6109.2008.00990.x [DOI] [PubMed] [Google Scholar]
- Danzi M., Ferulano G. P., Abate S., Califano G. (1983). Male sexual function after abdominoperineal resection for rectal cancer. Diseases of the Colon & Rectum, 26, 665-668. [DOI] [PubMed] [Google Scholar]
- Dowswell G., Ismail T., Greenfield S., Clifford S., Hancock B., Wilson S. (2011). Men’s experience of erectile dysfunction after treatment for colorectal cancer: Qualitative interview study. BMJ, 343, d5824. doi: 10.1136/bmj.d5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveno C., Lamblin A., Mariette C., Pocard M. (2010). Sexual and urinary dysfunction after proctectomy for rectal cancer. Journal of Visceral Surgery, 147(1), e21-e30. doi: 10.1016/j.jviscsurg.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Fazio V. W., Fletcher J., Montague D. (1980). Prospective study of the effect of resection of the rectum on male sexual function. World Journal of Surgery, 4, 149-152. [DOI] [PubMed] [Google Scholar]
- Fritz E., Hammer J., Schmidt B., Eherer A. J., Hammer H. F. (2003). Stimulation of the nitric oxide-guanosine 3′,5′-cyclic monophosphate pathway by sildenafil: Effect on rectal muscle tone, distensibility, and perception in health and in irritable bowel syndrome. American Journal of Gastroenterology, 98, 2253-2260. doi: 10.1111/j.1572-0241.2003.07661.x [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K., Burnett A. L., Hatzichristou D., McCullough A. R., Montorsi F., Mulhall J. P. (2009). Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: A critical analysis of the basic science rationale and clinical application. European Urology, 55, 334-347. doi: 10.1016/j.eururo.2008.10.028 [DOI] [PubMed] [Google Scholar]
- Havenga K., Enker W. E., McDermott K., Cohen A. M., Minsky B. D., Guillem J. (1996). Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. Journal of the American College of Surgeons, 182, 495-502. [PubMed] [Google Scholar]
- Keating J. P. (2004). Sexual function after rectal excision. ANZ Journal of Surgery, 74, 248-259. doi: 10.1111/j.1445-2197.2004.02954.x [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Kim N. K., Lee K. Y., Hur H., Min B. S., Kim J. H. (2012). A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: Laparoscopic versus robotic surgery. Annals of Surgical Oncology, 19, 2485-2493. doi: 10.1245/s10434-012-2262-1 [DOI] [PubMed] [Google Scholar]
- Kimura M., Caso J. R., Banez L. L., Koontz B. F., Gerber L., Senocak C., . . . Polascik T. J. (2012). Predicting participation in and successful outcome of a penile rehabilitation programme using a phosphodiesterase type 5 inhibitor with a vacuum erection device after radical prostatectomy. BJU International, 110(11 Pt C), E931-E938. doi: 10.1111/j.1464-410X.2012.11168.x [DOI] [PubMed] [Google Scholar]
- Kohler T. S., Pedro R., Hendlin K., Utz W., Ugarte R., Reddy P., . . . Monga M. (2007). A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU International, 100, 858-862. doi: 10.1111/j.1464-410X.2007.07161.x [DOI] [PubMed] [Google Scholar]
- Liang J. T., Lai H. S., Lee P. H. (2007). Laparoscopic pelvic autonomic nerve-preserving surgery for patients with lower rectal cancer after chemoradiation therapy. Annals of Surgical Oncology, 14, 1285-1287. doi: 10.1245/s10434-006-9052-6 [DOI] [PubMed] [Google Scholar]
- Lindsey I., George B., Kettlewell M., Mortensen N. (2002). Randomized, double-blind, placebo-controlled trial of sildenafil (Viagra) for erectile dysfunction after rectal excision for cancer and inflammatory bowel disease. Diseases of the Colon & Rectum, 45, 727-732. [DOI] [PubMed] [Google Scholar]
- Milone M., DiBaise J. K. (2005). A pilot study of the effects of sildenafil on stool characteristics, colon transit, anal sphincter function, and rectal sensation in healthy men. Digestive Diseases and Sciences, 50, 1005-1011. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y., Ito M., Saito N., Suzuki T., Sugito M., Tanaka T. (2011). Male sexual dysfunction after rectal cancer surgery. International Journal of Colorectal Disease, 26, 1541-1548. doi: 10.1007/s00384-011-1247-z [DOI] [PubMed] [Google Scholar]
- Pahlajani G., Raina R., Jones J. S., Burdick M., Ali M., Li J., . . . Zippe C. (2010). Early intervention with phosphodiesterase-5 inhibitors after prostate brachytherapy improves subsequent erectile function. BJU International, 106, 1524-1527. doi: 10.1111/j.1464-410X.2010.09343.x [DOI] [PubMed] [Google Scholar]
- Park S. Y., Choi G. S., Park J. S., Kim H. J., Park J. A., Choi J. I. (2015). Efficacy and safety of udenafil for the treatment of erectile dysfunction after total mesorectal excision of rectal cancer: A randomized, double-blind, placebo-controlled trial. Surgery, 157, 64-71. doi: 10.1016/j.surg.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Pavlovich C. P., Levinson A. W., Su L. M., Mettee L. Z., Feng Z., Bivalacqua T. J., Trock B. J. (2013). Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: Results of a randomized double-blind trial with placebo. BJU International, 112, 844-851. doi: 10.1111/bju.12253 [DOI] [PubMed] [Google Scholar]
- Pocard M., Zinzindohoue F., Haab F., Caplin S., Parc R., Tiret E. (2002). A prospective study of sexual and urinary function before and after total mesorectal excision with autonomic nerve preservation for rectal cancer. Surgery, 131, 368-372. [DOI] [PubMed] [Google Scholar]
- Rosen R. C., Cappelleri J. C., Smith M. D., Lipsky J., Pena B. M. (1999). Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. International Journal of Impotence Research, 11, 319-326. [DOI] [PubMed] [Google Scholar]
- Segal R. L., Bivalacqua T. J., Burnett A. L. (2013). Current penile-rehabilitation strategies: Clinical evidence. Arab Journal of Urology, 11, 230-236. doi: 10.1016/j.aju.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traa M. J., De Vries J., Roukema J. A., Den Oudsten B. L. (2012). Sexual (dys)function and the quality of sexual life in patients with colorectal cancer: A systematic review. Annals of Oncology, 23, 19-27. doi: 10.1093/annonc/mdr133 [DOI] [PubMed] [Google Scholar]


