Abstract
Phthalates are endocrine-disrupting compounds detectable in more than 75% of the U.S. population with differential distributions across racial and ethnic groups, and they have been linked with reduced levels of serum testosterone. This study aims to investigate the associations of phthalate metabolites with erectile dysfunction (ED) and to determine whether these associations vary by race/ethnicity among men in the United States. Analyzed data for 12 phthalate metabolites from 3,746 men (≥20 years old), who participated in the National Health and Nutrition Examination Survey 2001-2004 cross-sectional study, were included. Metabolites included MBP, MCHP, MEP, MEHP, MiNP, MBzP, MMP, MCPP, MEHHP, MEOHP, MiBP, and MECPP. Racial/ethnic groups included non-Hispanic Blacks (n = 770), non-Hispanic Whites (n = 2,147), and Mexican Americans (n = 829). ED was assessed by a single question during a self-paced, computer-assisted self-interview. In racial/ethnic stratified analyses, there were higher MBP and MBzP concentrations that had a strong-dose response association with lower prevalence odds of ED among Mexican Americans, ptrend < .01, and ptrend = .03, respectively. Similarly, a significant inverse association between MEHHP and likelihood of ED among non-Hispanic Black men (ptrend < .04) was observed. Furthermore, significant inverse associations between higher concentrations of phthalates and ED were identified only in minority populations. Further investigations, particularly prospective studies, are warranted to determine the role of phthalates on the biological mechanism(s) associated with ED. A focus may be placed on testosterone levels which are suggested to be affected by phthalates, and also low levels of testosterone are suggested to increase the risk of ED.
Keywords: erectile dysfunction, phthalates, race and ethnicity, NHANES
Introduction
Phthalates are a family of diester compounds of 1,2-benzenedicarboxylic acid made by man and found mainly in plastics (i.e., food and beverage containers) and medical and personal care products (i.e., cosmetics, shampoos, soaps; Centers for Disease Control and Prevention [CDC], 2009). Phthalates are quickly metabolized and excreted in humans (Anderson, Castle, Scotter, Massey, & Springall, 2001); and they have been detected in urine in >75% of the U.S. population (Silva et al., 2004) and there is an unclear disproportionate burden of these phthalates among racial and ethnic groups (CDC, 2009; Silva et al., 2004). A population-based cross-sectional study reported differences in the distribution of phthalates among non-Hispanic Whites, non-Hispanic Blacks, and Mexican Americans identifying minority populations as having the highest concentrations (Huang, Saxena, Isganaitis, & James-Todd, 2014; Silva et al., 2004).
Phthalates are known to be the most widely studied endocrine-disrupting compounds and to disrupt the male reproductive development in an antiandrogenic fashion compounds (Zoeller et al., 2012). Phthalates metabolites have been associated with a reduction in levels of testosterone (Meeker, 2010; Meeker, Calafat, & Hauser, 2009; Pan et al., 2006). In addition, testosterone concentrations have been reported to vary by race and ethnicity among young and middle-aged men; previous research reported that Mexican American men had higher levels of testosterone compared with non-Hispanic Whites (Lopez et al., 2013; Rohrmann et al., 2007).
Low levels of testosterone have been associated with reduced sexual desire, decreased spontaneous erections, and erectile dysfunction (ED; Sansone, Romanelli, Gianfrilli, & Lenzi, 2014; Seftel, Kathrins, & Niederberger, 2015; Traish, Miner, Morgentaler, & Zitzmann, 2011). Prevalence of ED in men aged ≥20 years in the United States is 18.4% suggesting that more than 18 million are affected (Lue, 2000; Selvin, Burnett, & Platz, 2007). In addition, prevalence of ED varies by race/ethnicity identifying non-Hispanic Whites (22.6%) and Hispanics (20.1%) with higher rates than non-Hispanic Blacks (Weinberg, Eisenberg, Patel, Chertow, & Leppert, 2013). In older men, the prevalence of ED increases significantly affecting their overall quality of life (Francis, Kusek, Nyberg, & Eggers, 2007); at ages 40 and 70 years, approximately 44% and 70%, respectively, are affected by this condition (Guay et al., 2003; Selvin et al., 2007). Although modifiable and nonmodifiable factors have been associated with ED, the role of phthalates metabolites as potential risk factors for ED remains undetermined. The current study investigated the associations of 12 urinary phthalate metabolites with ED and aimed to determine whether these associations vary by race and ethnicity in the National Health and Nutrition Examination Survey (NHANES) 2001-2004.
Method
Study Population
NHANES is a program of studies undertaken by the National Center for Health Statistics (NCHS) of the CDC to assess the health and nutritional status of adults and children in the United States. NHANES uses a multistage, stratified, and clustered probability sampling strategy in which Mexican Americans, non-Hispanic Blacks, and the elderly are oversampled to ensure adequate sample size and to represent the total U.S. civilian, noninstitutionalized population (NCHS, 1994). For the purpose of this study, data from continuous NHANES waves 2001-2002 and 2003-2004 were combined because information on ED was reported only on those years.
Assessment of Erectile Dysfunction
NHANES participants were in a private room using a self-paced audio computer–assisted self-interview system that enabled them to both hear questions through earphones and read questions on the computer screen related to ED. To assess ED, men (≥20 years) were asked the following question (that has been previously validated by O’Donnell Araujo, Goldstein, and McKinlay [2005]) who suggested that it can be added in large ongoing national epidemiologic surveys to provide information about the prevalence of ED: “Many men experience problems with sexual intercourse. How would you describe your ability to get and keep an erection adequate for satisfactory intercourse?” The following answers were provided: “Would you say that you are . . . always or almost always able, usually able, sometimes able, or never able?” For the purpose of this study, positive ED was dichotomized from the answers “sometime able” or “never able” to keep an erection, and subsequently negative ED was derived from the answers “almost always able” or “usually able” to maintain an erection (Selvin et al., 2007). In this study, men were excluded if they have the following conditions as they could influence the condition of ED: Men who were diagnosed with prostate cancer (n = 94) or underwent surgery/radiation treatment (n = 85) for the same disease. In addition, men who did not identify themselves as non-Hispanic White, non-Hispanic Black, or Mexican American (n = 261) were excluded leaving a total sample size of 3,746 men with valid data on ED.
Assessment of Phthalate Exposure Data
Phthalate metabolites were measured in urine samples in a random one-third subsample of the NHANES participants (Latini, 2005). After collection, urine samples were stored at −20 °C and later shipped to CDC’s National Center for Environmental Health for analysis. Laboratory methods and quality control statistics regarding specific phthalate measurement are reported elsewhere (Silva et al., 2004). For laboratory testing results below the limit of detection, they were replaced with limit of detection divided by the square root of 2 (CDC, 2009); this method of handling nondetectable values produces reasonably nonbiased means and standard deviations (Finkelstein & Verma, 2001).
The following 12 phthalate metabolites that were selected and measured in the two NHANES waves 2001-2004: mono-n-butyl phthalate (MBP), mono-cyclohexyl phthalate (MCHP), mono-ethyl phthalate (MEP), mono-(2-ethyl)-hexyl phthalate (MEHP), mono-isononyl phthalate (MiNP), mono-benzyl phthalate (MBzP), mono-n-methyl phthalate (MMP), mono-(3-carboxypropyl) phthalate (MCPP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-isobutyl phthalate (MiBP), and mono-2-ethyl-5-carboxypentyl phthlate (MECPP). Because only the NHANES wave 2003-2004 included the four metabolites of di-2-ethylhexyl phthalate (DEHP), MEHP, MEHHP, MEOHP and MECPP, they were summed using molar concentrations in one single phthalate variable for analysis labeled by ΣDEHP2003-2004. Phthalates were categorized into quartiles and the lowest category was chosen as the reference group. Adjustment of these values for urinary dilution took place by using measured creatinine concentrations from enzymatic assays. Thus, urinary creatinine–corrected levels by dividing urinary phthalate concentrations by creatinine/100 for final units of micrograms per gram creatinine were provided (Meeker & Ferguson, 2014).
Assessment of Covariates
Age, race/ethnicity, smoking status, education, and physical activity during the past 30 days (moderate and vigorous) were self-reported during the NHANES interview. NHANES categorizes race/ethnicity as non-Hispanic White, non-Hispanic Black, Mexican American, and “other” (other Hispanics and all others). Due to the small number of the latter group, it was not included in the final analyses. Participants were classified as never, former, and current smokers. Participants were asked if they had smoked more than 100 cigarettes in their lifetime and if they were current smokers; serum cotinine was measured using high-performance liquid chromatography/atmospheric-pressure ionization tandem mass spectrometry (Bernert et al., 1997). Cotinine is a more accurate marker of exposure to environmental tobacco smoke; thus, by controlling for this marker, there is a reduction in residual confounding that could be present when there is only adjustment for active smoking status. Current smokers consisted of those who self-reported smoking habits, smoked more than 100 cigarettes in their lifetime and cotinine (ng/ml) that was dichotomized as ≥10 ng/ml (actively exposed). Information about vigorous physical activity was obtained from the questions on whether participants did any activity that caused heavy sweating or large increases in breathing or heart rate (e.g., swimming, aerobics, or fast cycling), while moderate physical activity was determined from the questions on whether they did any activities that caused light sweating or a moderate increases in the heart rate, such as playing golf, dancing, bicycling for pleasure, or walking. Body mass index (BMI) was calculated from measured weight and height (weight in kilograms divided by height in meters squared). Individuals were classified as having type 2 diabetes if their fasting plasma glucose levels were ≥126 mg/dl, or if they responded positively to questions about medication treatment or being “told by a doctor you have diabetes or sugar diabetes.” Fasting plasma glucose concentration was measured in the morning session after an overnight fast of at least 8 hours (Ford & Giles, 2003), details related to the laboratory procedures are reported elsewhere (NCHS, 1994).
Statistical Analysis
Sampling weights were applied to take into account selection probabilities, oversampling, nonresponse, and differences between the sample and the total U.S. population. Population distributions of categorical and continuous covariates were adjusted for the complex NHANES sampling design. Computed median and interquartile ranges for phthalate metabolite levels, adjusted for urinary creatinine concentrations were reported. These values were also stratified by race and ethnicity. Distribution of phthalate metabolite concentrations was log-transformed to normalize the data. Adjusted odds ratios and 95% confidence intervals (CI) for ED using weighted logistic regression models were estimated in relation of continuous phthalate metabolites and quartiles of these continuous phthalates, respectively. In the multiple logistic regression models, the variables vigorous and moderate physical activity, age (categorical), smoking status, education, race/ethnicity (only in total population), obesity (BMI ≥ 30 kg/m2), diabetes (yes/no), and urinary creatinine (continuous) were included. The Wald test for trend across quartiles was conducted by entering quartiles values (e.g., 1, 2, 3, and 4) as an ordinal variable into the weighted logistic regression models. Adjustment for multiple comparisons was not conducted due to the exploratory nature of this study (Rothman, 1990).
Stratified analyses were conducted by race and ethnicity (Mexican American, non-Hispanic White, and non-Hispanic Black) because there are differences in ED across racial/ethnic groups (CDC, 2009; Silva et al., 2004; Weinberg et al., 2013). Quartiles of continuous phthalate metabolite levels were categorized according to their distribution in each specific racial and ethnic group. With the exception of the race and ethnicity variable, the multiple regression logistic models were identical as described above. All p values were two-sided; alpha = .05 was considered the cutoff for statistical significance. All statistical analyses were performed using STATA version 12.0 (College Station, TX).
Results
The distribution of baseline characteristics in the study population after applying sample weights is reported in Table 1. The mean age was approximately 45 years with a higher frequency for the 20 to 30 years old age category (n = 3,746; 39.4%) and the majority of the participants were non-Hispanic White (n = 2,147; 80.5%). The prevalence of overweight and obesity based on BMI ≥25 to 29.99 and ≥30 kg/m2 was 40.0% (n = 1,492) and 31.4% (n = 1,145), respectively. Approximately 9% (n = 456) of the participants were diabetic, and 36.9% (n = 1,314) were current smokers with a weighted mean value of serum cotinine of 82.6 ng/ml. Twenty-nine percent (n = 966) of the participants had some college education and 27.5% (n = 934) had a high school diploma or GED equivalent. Approximately 43% (n = 1,833) performed moderate physical activity, while 59.5% (n = 2,343) reported vigorous physical activity.
Table 1.
Selected Characteristics of the U.S. Population of Adult Men Aged 20 Years and Older.
| Characteristics | Unweighted sample size (N) | Weighted mean or percentage (SE)a |
|---|---|---|
| Age, years | 3,746 | 45.1 (±0.38) |
| Age, categorical | ||
| 20-30 | 1,249 | 39.4% |
| 40-49 | 712 | 24.1% |
| 50-59 | 521 | 17.6% |
| ≥60 | 1,264 | 18.9% |
| Race/ethnicity | ||
| Mexican American | 829 | 8.6% |
| Non-Hispanic White | 2,147 | 80.5% |
| Non-Hispanic Black | 770 | 10.9% |
| Education | ||
| Less than 9th grade | 505 | 5.8% |
| 9th Grade-11th grade | 562 | 10.7% |
| High school graduate/GED or equivalent | 934 | 27.5% |
| Some college or associate degree | 966 | 29.2% |
| College graduate or above | 778 | 26.8% |
| Cigarette smoking | ||
| Never | 1,363 | 38.8% |
| Former | 1,066 | 24.4% |
| Current | 1,314 | 36.9% |
| Body mass index (kg/m2) | ||
| <25 | 1,046 | 28.6% |
| ≥25-29.99 | 1,492 | 40.0% |
| ≥30 | 1,145 | 31.4% |
| Type 2 diabetes | ||
| No | 3,290 | 91.4% |
| Yes | 456 | 8.6% |
| Physical activity status | ||
| Moderate | ||
| No | 1,820 | 57.1% |
| Yes | 1,833 | 42.9% |
| Vigorous | ||
| No | 1,251 | 40.5% |
| Yes | 2,343 | 59.5% |
| Serum cotinine (ng/ml) | 3,591 | 82.8 (±5.42) |
| Creatinine (mg/dl) | 3,691 | 4.8 (±0.02) |
Note. SE = standard error.
Sampling weights were applied.
The distribution of creatinine-adjusted concentrations of phthalate metabolites in the total U.S. population and stratified by race and ethnicity is reported in Table 2. Concentrations of phthalates varied widely by race and ethnicity with Mexican Americans having the highest levels of MEP, MiNP, MMP, MiBP; non-Hispanic Whites having the highest levels of MBP, MCPP, and MECPP; and non-Hispanic Blacks having the highest concentrations of MCHP, MEHP, MBzP, MEHHP, and MEOHP. The two minority populations, Mexican Americans and non-Hispanic Blacks, had higher phthalate concentrations of MEP, MEHP, MBzP, MMP, and MiBP than non-Hispanic Whites.
Table 2.
Creatinine-Corrected Urinary Phthalate Metabolite (ng/ml) Medians (25th-75th Percentiles) by Race and Ethnicity in National Health and Nutrition Examination Survey 2001-2004.
| Mono-n-butyl phthalate (MBP) | Mono-cyclo hexyl phthalate (MCHP) | Mono-ethyl phthalate (MEP) | Mono-2-ethyl-hexyl-phthalate (MEHP) | Mono-isonyl-phthalate (MiNP) | Mono-benzyl phthalate (MBzP) | |
|---|---|---|---|---|---|---|
| Total population | 13.38 (8.63-22.43) | 0.26 (0.18-0.44) | 107.14 (39.6-310.2) | 2.35 (1.06-5.38) | 0.71 (0.50-1.10) | 7.25 (4.10-13.16) |
| Mexican American | 14.18 (9.24-22.38) | 0.28 (0.19-0.44) | 138.12 (51.91-370.39) | 2.63 (0.98-5.48) | 0.76 (0.54-1.08) | 7.20 (3.83-12.75) |
| Non-Hispanic White | 15.43 (10.66-22.82) | 0.30 (0.20-0.46) | 89.57 (34.76-269.10) | 2.07 (1.04-5.00) | 0.75 (0.53-1.21) | 7.06 (3.90-13.58) |
| Non-Hispanic Black | 14.29 (7.62-23.85) | 0.33 (0.19-0.50) | 127.52 (58.16-359.79) | 2.80 (1.28-6.57 | 0.54 (0.38-0.83) | 8.07 (4.69-14.77) |
| Mono-n-methyl phthalate (MMP) | Mono-3-carboxypropyl phthalate (MCPP) | Mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) | Mono-2-ethyl-5-oxohexyl phthalate (MEOHP) | Mono-isobutyl Phthalate (MiBP) | Mono-2-Ethyl 5-carboxypentyl phthalate (MECPP)a | |
| Total population | 1.27 (0.57-2.50) | 2.02 (1.31-3.13) | 13.86 (8.38-26.67) | 9.10 (5.48-17.01) | 2.47 (1.41-4.33) | 21.95 (13.39-43) |
| Mexican American | 1.28 (0.56-2.77) | 1.93 (1.26-2.97) | 13.13 (8.26-22.14) | 8.67 (5.59-14.36) | 2.98 (1.52-5.30) | 22.48 (14.47-35.35) |
| Non-Hispanic White | 1.25 (0.57-2.45) | 2.19 (1.44-3.38) | 13.56 (8.59-27.20) | 9.18 (5.58-17.91) | 2.11 (1.23-3.53) | 22.55 (14.81-47.05) |
| Non-Hispanic Black | 1.27 (0.58-2.50) | 1.72 (1.05-2.81) | 14.87 (7.69-13.24) | 9.35 (4.98-17.91) | 2.98 (1.78-5.02) | 19.30 (10.10-37.03) |
Only data available for 2003 to 2004.
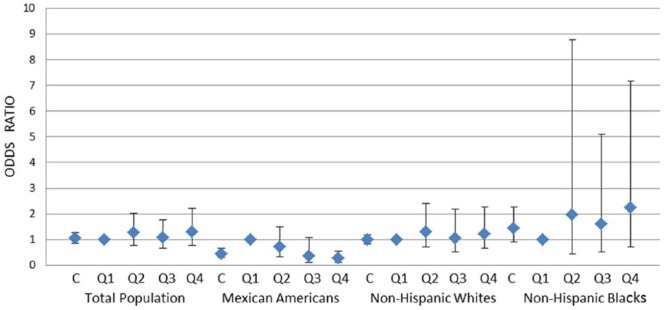
Multivariable logistic regression analyses were conducted to investigate the association of the 12 phthalate metabolites with ED after adjusting for age, race/ethnicity (only in total population), physical activity (vigorous and moderate), smoking status, education, obesity, diabetes, and urinary creatinine (Figures 1-3 and Online Supplemental Table 1). In the total population, none of the associations between urinary phthalates and ED reached statistical significance. However, in racial and ethnic-specific stratified analyses, three phthalate metabolites, MBP, MBzP, and MEHHP, were inversely associated with ED (Figures 1-3). In detail, MBP was analyzed in two forms, continuous and categorical (fourth-highest vs. first-lowest quartile), with ED among Mexican American, non-Hispanic Black, and non-Hispanic White men (Figure 1). Only in Mexican American men, there were significant inverse associations between MBP and ED in both statistical models, continuous (odds ratio [OR] = 0.45; 95% CI [0.31, 0.66], p < .01) and categorical (fourth-highest vs. first-lowest quartile: OR = 0.26; 95% CI [0.12, 0.54], ptrend < .01). This association did not reach statistical significance among non-Hispanic White and non-Hispanic Black men.
Figure 1.
Association of mono-n-butyl phthalate (MBP) with erectile dysfunction (ED) in total population and stratified by race and ethnicity.
Note. MBP was analyzed in continuous (C) and in quartiles (Q1-Q4) format. Adjusted for age, race/ethnicity (only in total population), physical activity (vigorous and moderate), smoking status, education, obesity, diabetes, and urinary creatinine. Diamonds and errors bars indicate point estimates and 95% confidence intervals, respectively. Only among Mexican American men, there was a significant inverse association between MBP and ED (ptrend < .01).
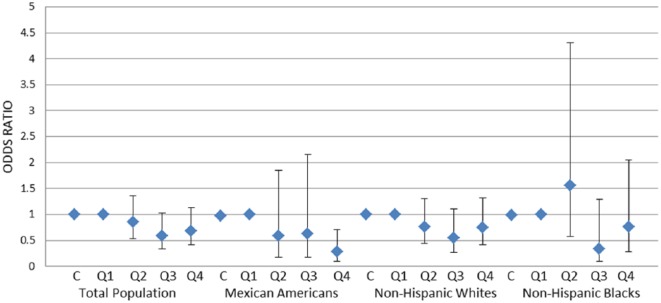
Figure 2.
Association of mono-benzyl phthalate (MBzP) with erectile dysfunction (ED) in total population and stratified by race and ethnicity.
Note. MBP was analyzed in continuous (C) and in quartiles (Q1-Q4) format. Adjusted for age, race/ethnicity (only in total population), physical activity (vigorous and moderate), smoking status, education, obesity, diabetes, and urinary creatinine. Diamonds and errors bars indicate point estimates and 95% confidence intervals, respectively. Only among Mexican American men, there was a significant inverse association between MBzP and ED (ptrend = .03).
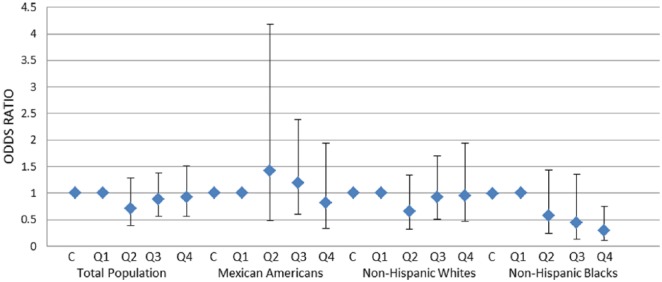
Figure 3.
Association of Mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) with erectile dysfunction (ED) in total population and stratified by race and ethnicity.
Note. MEHHP was analyzed in continuous (C) and in quartiles (Q1-Q4) format. Adjusted for age, race/ethnicity (only in total population), physical activity (vigorous and moderate), smoking status, education, obesity, diabetes, and urinary creatinine. y-Axis indicates odds ratio. Diamonds and errors bars indicate point estimates and 95% confidence intervals, respectively. Only among non-Hispanic Black men, there was a significant inverse association between MEHHP and ED (ptrend = .04).
The association between MBzP and ED in total population and by race and ethnicity is reported in Figure 2. Similar to the aforementioned analyses with MBP, no significant association was identified in the total population and among non-Hispanic White and non-Hispanic Black men, but among Mexican American men, there was significant strong-dose response inverse association between MBzP and ED (fourth-highest vs. first-lowest quartile: OR = 0.28; 95% CI [0.09, 0.70], and ptrend < .03).
The association of MEHHP with ED is reported in Figure 3. Similar to MBP and MBzP, no significant association was identified in total population. However, in non-Hispanic Blacks, there was a significant inverse association (fourth-highest vs. first-lowest quartile: OR = 0.29; 95% CI [0.11, 0.75], and ptrend < .02), but this relationship was not significant in non-Hispanic White and Mexican American men.
In an online supplement (Supplemental Data Table 1), detailed information on the independent associations of the 12 urinary phthalates with ED was provided. In general and with the exception of MBP, MBzP, and MEHHP metabolites, no significant associations and patterns were reported for the remaining nine phthalates. The association of ΣDEHP2003-2004 (MEHP, MEHHP, MEOHP, and MECPP) was not significant or trend was observed in total population or among different racial/ethnic groups (data not reported).
Discussion
In this population-based NHANES 2001-2004 cross-sectional study, there were no significant associations between 12 urinary phthalate metabolites and ED in the total population. However, when these associations were stratified by race and ethnicity, two phthalate metabolites, MBP and MBzP, had a strong inverse dose–response relationship with lower odds prevalence of ED among Mexican Americans. In addition, higher concentrations of MEHHP were associated with lower odds of ED among non-Hispanic Black men. Interestingly, no significant associations were observed among non-Hispanic White men.
To the best of our knowledge, this is the first study to investigate the association of phthalate metabolites with ED across racial and ethnic groups. Although a recent study identified that women exposed to higher concentrations of phthalates had a reduced libido (Barrett et al., 2014), little is known about the role of phthalates on men’s libido. It is important to note that phthalates were previously reported to have a detrimental effect on the male’s reproductive system by acting on an antiandrogenic fashion (Meeker, 2010; Pan et al., 2006; Zoeller et al., 2012). Therefore, the suggested biological mechanism for the phthalate metabolites-ED association was that phthalates reduces levels of testosterone (Duty, Calafat, Silva, Ryan, & Hauser, 2005; Meeker, 2010) and subsequently, these low levels of testosterone could lead to the development of ED (Sansone et al., 2014); this suggested mechanism is under the assumption that low levels of testosterone are biologically responsible for increasing the risk of ED (Sansone et al., 2014).
Several aspects of this study merit discussion. Although the sample size in racial/ethnic stratification was small, the men in this study were sampled from the U.S. population to be nationally representative. In addition, racial and ethnic differences in phthalate concentrations have been previously reported. Huang et al. (2014) concluded that minority populations may be more susceptible to high concentrations of phthalates with respect to metabolic disturbances. Thus, in the present study, phthalate metabolite levels were categorized into quartiles according to their distribution in each specific racial and ethnic group. Only significant associations among minority populations were identified. However, unexpectedly these associations were inversed.
There are several possible explanations for the observed inverse associations of some phthalate metabolites with ED in minority populations. First, one possibility is that phthalate metabolites had little or no effect at all on reducing levels of testosterone, therefore, there was no increased risk in the development of ED, but this is only true under the hypothesis that low levels of testosterone is the main underlying mechanism in the etiology of ED. Note that the null effect of phthalates on testosterone levels is feasible and reported previously (Han et al., 2014; Jonsson, Richthoff, Rylander, Giwercman, & Hagmar, 2005; Li et al., 2011). Recently, Meeker et al. demonstrated in NHANES 20112012 (Meeker & Ferguson, 2014) that within the 20- to 40-year and 60- to- 80-year age groups, the associations between phthalate metabolites and testosterone levels were not statistically significant in men; and among the 40- to 60-year age group only one phthalate (i.e., MBP) was inversely associated with testosterone. Because the mean age of the participants in the present study was approximately 45 years old, it is possible that this population is still considered relatively young to experience any significant urological health-related problem attributed to low levels of testosterone.
Another possibility in the present study is that high levels of phthalates may have reduced levels of estradiol concentrations among men and this reduction led to a decreased likelihood of ED. This is a feasible hypothesis as a body of literature suggests that high levels of estradiol are associated to a more severe ED and greater sexual distress (El-Sakka, 2013; Sansone et al., 2014; Srilatha & Adaikan, 2011); however, in an animal study model, Lovekamp and Davis (2001) reported that phthalates may interfere with the production of estradiol.
Second, a study investigated the association of prenatal DEHP metabolite exposure with testosterone, and reported a positive and significant association (Jensen et al., 2015), 18% higher testosterone level in the highest MECPP tertile compared with the lowest tertile (p < .01). This observation seems to contradict the notion that phthalates negatively influence testicular Leydig cells function in their production of testosterone. Therefore, given the inevitable and constant exposure of phthalates during the life course, it is possible that adult phthalate exposure may not operate as a risk factor in reducing levels of testosterone and subsequently affect sexual and reproductive life.
Third, this is a cross-sectional study and there may be residual and unknown confounders that could be driving these findings in an inverse direction; therefore, this can be a potential alternative explanation that cannot be ruled out. However, the adjustment for major confounders for ED such as age, race/ethnicity (only in total population), physical activity, smoking status, education, obesity, and diabetes was conducted.
The current study has a number of strengths. NHANES includes a representative sample of the civilian noninstitutionalized U.S. men population, which aids in the generalizability of these results. The oversampling of minorities and the elderly allowed for reasonably stable estimates of the U.S. male population. In addition, phthalate concentrations were detected using a solid-phase extraction, isotope dilution, and liquid chromatography separation followed by tandem mass spectrometry. This results in an increased accuracy and precision of the data (Silva et al., 2004). Despite these strengths, the current study has some possible limitations. First, NHANES is a cross-sectional study; therefore, causality cannot be inferred in any of the observed associations or suggest any clinical practice change. Plus, there is an inherent bias in the use of surveys for data collection, ED for this matter. Second, single time point measurement of the phthalate metabolites were obtained, which may provide an imperfect estimate of a man’s usual or total phthalate exposure status. This is important to note as it has been previously suggested to perform frequent biological monitoring of phthalates to help physicians perform better health risk assessments (Latini, 2005). Finally, the adjustment for multiple comparisons was not conducted due to the exploratory nature of this study, therefore, the possibility that some of the statistical findings are due to chance cannot be ruled out.
In conclusion, there were inverse associations between higher concentrations of phthalates and ED only in Mexican American and non-Hispanic Black men. These findings seem to raise more questions related to the role of androgens in the development of ED. Therefore, further investigations, particularly prospective studies, are warranted to identify if and how phthalate metabolites influence ED.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The supplementary material for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/1557988316641370.
References
- Anderson W. A., Castle L., Scotter M. J., Massey R. C., Springall C. (2001). A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Additives & Contaminants, 18, 1068-1074. [DOI] [PubMed] [Google Scholar]
- Barrett E. S., Parlett L. E., Wang C., Drobnis E. Z., Redmon J. B., Swan S. H. (2014). Environmental exposure to di-2-ethylhexyl phthalate is associated with low interest in sexual activity in premenopausal women. Hormones and Behavior, 66, 787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert J. T., Jr., Turner W. E., Pirkle J. L., Sosnoff C. S., Akins J. R., Waldrep M. K., . . . Sampson E. J. (1997). Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clinical Chemistry, 43, 2281-2291. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2009). Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Author. [Google Scholar]
- Duty S. M., Calafat A. M., Silva M. J., Ryan L., Hauser R. (2005). Phthalate exposure and reproductive hormones in adult men. Human Reproduction (Oxford, England), 20, 604-610. [DOI] [PubMed] [Google Scholar]
- El-Sakka A. I. (2013). Impact of the association between elevated oestradiol and low testosterone levels on erectile dysfunction severity. Asian Journal of Andrology, 15, 492-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M. M., Verma D. K. (2001). Exposure estimation in the presence of nondetectable values: Another look. AIHAJ: A Journal for the Science of Occupational and Environmental Health and Safety, 62, 195-198. [DOI] [PubMed] [Google Scholar]
- Ford E. S., Giles W. H. (2003). A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care, 26, 575-581. [DOI] [PubMed] [Google Scholar]
- Francis M. E., Kusek J. W., Nyberg L. M., Eggers P. W. (2007). The contribution of common medical conditions and drug exposures to erectile dysfunction in adult males. Journal of Urology, 178, 591-596. [DOI] [PubMed] [Google Scholar]
- Guay A. T., Spark R. F., Bansal S., Cunningham G. R., Goodman N. F., Nankin H. R., . . . American Association of Clinical Endocrinologists Male Sexual Dysfunction Task Force. (2003). American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: A couple’s problem—2003 Update. Endocrine Practice, 9, 77-95. [DOI] [PubMed] [Google Scholar]
- Han X., Cui Z., Zhou N., Ma M., Li L., Li Y., . . . Cao J. (2014). Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China. International Journal of Hygiene and Environmental Health, 217, 271-278. [DOI] [PubMed] [Google Scholar]
- Huang T., Saxena A. R., Isganaitis E., James-Todd T. (2014). Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001-2008. Environmental Health: A Global Access Science Source, 13(1), 6. doi: 10.1186/1476-069X-13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. S., Anand-Ivell R., Norgaard-Pedersen B., Jonsson B. A., Bonde J. P., Hougaard D. M., . . . Toft G. (2015). Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology (Cambridge, Mass.), 26, 91-99. [DOI] [PubMed] [Google Scholar]
- Jonsson B. A., Richthoff J., Rylander L., Giwercman A., Hagmar L. (2005). Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology (Cambridge, Mass.), 16, 487-493. [DOI] [PubMed] [Google Scholar]
- Latini G. (2005). Monitoring phthalate exposure in humans. Clinica Chimica Acta: International Journal of Clinical Chemistry, 361(1-2), 20-29. [DOI] [PubMed] [Google Scholar]
- Li S., Dai J., Zhang L., Zhang J., Zhang Z., Chen B. (2011). An association of elevated serum prolactin with phthalate exposure in adult men. Biomedical and Environmental Sciences, 24(1), 31-39. [DOI] [PubMed] [Google Scholar]
- Lopez D. S., Peskoe S. B., Joshu C. E., Dobs A., Feinleib M., Kanarek N., . . . Platz E. A. (2013). Racial/ethnic differences in serum sex steroid hormone concentrations in US adolescent males. Cancer Causes & Control: CCC, 24, 817-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp T. N., Davis B. J. (2001). Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicology and Applied Pharmacology, 172, 217-224. [DOI] [PubMed] [Google Scholar]
- Lue T. F. (2000). Erectile dysfunction. New England Journal of Medicine, 342, 1802-1813. [DOI] [PubMed] [Google Scholar]
- Meeker J. D. (2010). Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas, 66, 236-241. [DOI] [PubMed] [Google Scholar]
- Meeker J. D., Calafat A. M., Hauser R. (2009). Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. Journal of Andrology, 30, 287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Ferguson K. K. (2014). Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011-2012. Journal of Clinical Endocrinology & Metabolism, 99, 4346-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. (1994). Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: Programs and collection procedures. Vital and Health Statistics, 1, 1-407. [PubMed] [Google Scholar]
- O’Donnell A. B., Araujo A. B., Goldstein I., McKinlay J. B. (2005). The validity of a single-question self-report of erectile dysfunction: Results from the Massachusetts male aging study. Journal of General Internal Medicine, 20, 515-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G., Hanaoka T., Yoshimura M., Zhang S., Wang P., Tsukino H., . . . Takahashi K. (2006). Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): A cross-sectional study in China. Environmental Health Perspectives, 114, 1643-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann S., Nelson W. G., Rifai N., Brown T. R., Dobs A., Kanarek N., . . . Platz E. A. (2007). Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. Journal of Clinical Endocrinology & Metabolism, 92, 2519-2525. [DOI] [PubMed] [Google Scholar]
- Rothman K. J. (1990). No adjustments are needed for multiple comparisons. Epidemiology (Cambridge, Mass.), 1, 43-46. [PubMed] [Google Scholar]
- Sansone A., Romanelli F., Gianfrilli D., Lenzi A. (2014). Endocrine evaluation of erectile dysfunction. Endocrine, 46, 423-430. [DOI] [PubMed] [Google Scholar]
- Seftel A. D., Kathrins M., Niederberger C. (2015). Critical update of the 2010 endocrine society clinical practice guidelines for male hypogonadism: A systematic analysis. Mayo Clinic Proceedings, 90, 1104-1115. [DOI] [PubMed] [Google Scholar]
- Selvin E., Burnett A. L., Platz E. A. (2007). Prevalence and risk factors for erectile dysfunction in the US. American Journal of Medicine, 120, 151-157. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Barr D. B., Reidy J. A., Malek N. A., Hodge C. C., Caudill S. P., . . . Calafat A. M. (2004). Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environmental Health Perspectives, 112, 331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srilatha B., Adaikan P. G. (2011). Endocrine milieu and erectile dysfunction: Is oestradiol-testosterone imbalance, a risk factor in the elderly? Asian Journal of Andrology, 13, 569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish A. M., Miner M. M., Morgentaler A., Zitzmann M. (2011). Testosterone deficiency. American Journal of Medicine, 124, 578-587. [DOI] [PubMed] [Google Scholar]
- Weinberg A. E., Eisenberg M., Patel C. J., Chertow G. M., Leppert J. T. (2013). Diabetes severity, metabolic syndrome, and the risk of erectile dysfunction. Journal of Sexual Medicine, 10, 3102-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. T., Brown T. R., Doan L. L., Gore A. C., Skakkebaek N. E., Soto A. M., . . . Vom Saal F. S. (2012). Endocrine-disrupting chemicals and public health protection: A statement of principles from the endocrine society. Endocrinology, 153, 4097-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.