Abstract
Sexual health severely decreases with age. For males older than 40 years, erectile dysfunction (ED) is the most common sexual disorder. Although physical and psychological risk factors for ED have been identified, protective factors are yet to be determined. To date, no study has examined endocrine and psychosocial factors in parallel with regard to their modifying effect on the age-related increase in ED. Two hundred and seventy-one self-reporting healthy men aged between 40 and 75 years provided both psychometric data on sexual function and a set of potential psychosocial protective factors, and saliva samples for the analysis of steroid hormones and proinflammatory cytokines. Around 35% of the participants reported at least a mild form of ED. Direct associations with ED were identified for perceived general health, emotional support, relationship quality, intimacy motivation but not for steroid hormones or proinflammatory markers. Moderation analyses for the association between age and ED revealed positive effects for testosterone (T), dehydroepiandrosterone (DHEA), perceived general health, emotional support, intimacy motivation, and a negative effect for interleukin-6 (all p < .05; f2 > .17). Group differences between older men with and without ED emerged for T, DHEA, and psychometric measures such as perceived general health, emotional support, satisfaction with life, and intimacy motivation (all p < .05; d > .3). Both psychosocial and endocrine parameters moderated the association between age and sexual health. Perceived general health, emotional support, intimacy motivation, and relationship quality emerged as psychosocial protective factors against ED. Higher T and DHEA and lower interleukin-6 levels also buffered against an age-related increase in ED.
Keywords: erectile dysfunction, steroid hormones, inflammatory markers, protective factors, aging, cluster analysis
Introduction
Sexuality is an important quality-of-life consideration, also in late life (Hyde et al., 2012). Sexual health severely decreases with age. For males older than 40 years, erectile dysfunction (ED) is the most common sexual disorder, with a reported prevalence of 61% for 40- to 69-year-olds and 77% for ≥70-year-olds (Wagle, Carrejo, & Tan, 2012). ED is defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance (NIH Consensus Conference on Impotence, 1993), and its etiology is widely accepted to be multifactorial in nature, including organic, psychological, and social factors.
Besides vascular or neurogenic causes, hormonal factors seem to play a major role in the development and maintenance of ED (Mola, 2015). Higher levels of testosterone (T) and dehydroepiandrosterone (DHEA) as well as estradiol (E2) have been reported to be associated with better overall sexual function and less ED in different age groups (Finkelstein et al., 2013; Luo et al., 2015; Rabijewski, Papierska, Kuczerowski, & Piątkiewicz, 2015), although there is conflicting literature on the association between E2 and ED (El-Sakka, 2013; O’Connor et al., 2011; Vignozzi et al., 2014). A recent study reported that changes in T and E2 levels in response to T treatment were associated with improvements in sexual activity and sexual desire, but not erectile function in older men (Cunningham et al., 2016). Only a small number of studies have investigated the association between progesterone (P), a precursor of the primary sex hormones, and ED. Animal models suggest P to be a protective factor against ED (Andersen et al., 2007; Shirai et al., 2003). By contrast, cortisol (C) as well as proinflammatory cytokines such as interleukin-6 (IL6) have been reported to be positively associated with ED in healthy males and in males with urological problems (Kobori et al., 2009; Ückert et al., 2003; Zhao, Wang, Han, Li, & Yao, 2015).
Identified behavioral and psychosocial risk factors for ED are smoking, alcohol consumption, anxiety, stress, depression, mental illness, and dementia (Cho et al., 2006; Corona, Rastrelli, Maseroli, Forti, & Maggi, 2013). Literature on protective factors against ED is scarce. Although the importance of a multidisciplinary approach to the treatment of ED has been emphasized, few studies have investigated behavioral and psychosocial protective factors against ED (Shabsigh et al., 1998). The most frequently described such factors include exercise, healthy diet, good relationship quality, and engaging in sexual activity (Cho et al., 2006; Corona et al., 2013). As sexual health can be seen as a mirror of men’s overall health status (Capogrosso, Montorsi, & Salonia, 2015), protective factors against health deterioration in aging men might, at the same time, be protective factors against age-related ED. Positive affect, optimism, social and emotional support, relationship quality, and satisfaction with life are among the best described factors in this regard (Barrett, Putzke, & Richards, 2002; Berry & Worthington, 2001; Dainese et al., 2011; De Moor et al., 2006; Krokavcova et al., 2008).
To assess protective factors against ED, in the current study, steroid hormones, a proinflammatory cytokine and psychosocial protective factors were examined in parallel with regard to their association with ED and their modifying effect on the cross-sectional association between age and ED.
Material and Method
Participants
This study was part of the Men’s Health 40+ study (Walther, Philipp, Lozza, & Ehlert, 2016). The sample of the present study consisted of 271 self-reporting healthy men aged 40 to 75 years. Participants provided information about their current health status and medication intake via standardized questionnaires. Table 1 presents specific sample characteristics. Participants provided psychometric data on sexual health and psychosocial protective factors and endocrine and proinflammatory parameters. Informed consent was obtained from all participants before completing the measures. The local Ethics Committee of the Faculty of Arts at the University of Zurich approved the study protocol before data collection.
Table 1.
Descriptive Statistics of the Sample for Demographic, Sexual Health, and Psychosocial Protective Factors.
| Total (n = 271) | |
| Age, M (SD) | 57.1 (10.7) |
| Current health condition, n (%) | |
| Very good | 97 (35.8) |
| Good | 148 (54.6) |
| Fair | 26 (9.6) |
| Bad | 0 (0.0) |
| Very bad | 0 (0.0) |
| Body mass index, kg/m2, M (SD) | 25.4 (3.4) |
| Education, n (%) | |
| Tertiary education | 106 (39.1) |
| Postsecondary nontertiary education | 57 (21.0) |
| Higher secondary school | 76 (28.0) |
| Lower secondary education | 32 (11.1) |
| Did not finish regular school | 2 (0.8) |
| Current smoking status, n (%) | |
| Nonsmoker | 224 (82.7) |
| Occasional smoker | 25 (9.1) |
| Smoker | 22 (8.2) |
| Alcohol consumption, n (%) | |
| Never | 56 (20.4) |
| 1-6 Drinks per week | 161 (59.4) |
| ≥1 Drinks per day | 54 (20.2) |
| Medication intake, n (%) | |
| No | 180 (66.4) |
| Yes | 91 (33.6) |
| Sexual health with the IIEF, M (SD) | |
| Erectile function | 43.3 (16.8) |
| Sexual desire | 9 (3.0) |
| Additional sexual health-related questionsa, n (%) | |
| Sexual thoughtsa | |
| Never | 10 (3.7) |
| 1-3 Times a month | 34 (12.6) |
| 1-6 Times a week | 142 (52.4) |
| ≥1 Times a day | 85 (31.4) |
| Sexual intercoursea | |
| Never | 68 (25.2) |
| 1-3 Times a month | 103 (38.1) |
| 1-3 Times a week | 91 (33.6) |
| >3 Times a week | 9 (3.3) |
| Masturbationa | |
| Never | 30 (11.1) |
| 1-3 Times a month | 111 (41.0) |
| 1-6 Times a week | 120 (44.3) |
| ≥1 Times a day | 10 (3.7) |
| Metrics of psychosocial protective factors, M (SD) | |
| Perceived general health (SF-36-PGH) | 22.9 (2.9) |
| Satisfaction with life (SWLS) | 27.7 (4.7) |
| Resilience (RS-11) | 61.1 (8.0) |
| Optimism (LOT-R) | 17.6 (3.6) |
| Emotional support (BSSS-ES) | 3.5 (0.5) |
| Motivation (GOALS) | |
| Affiliation (GOALS-A) | 12.1 (3.6) |
| Intimacy (GOALS-I) | 17.5 (2.4) |
| Engaged in a romantic relationship, n (%) | 240 (88.5) |
| Relationship quality (RAS) | 4.2 (0.7) |
Additional sexual health-related questions were obtained from the European Male Aging Study–Sexual Function Questionnaire (Wiltink, Hauck, Phädayanon, Weidner, & Beutel, 2003).
Erectile Dysfunction
The status of ED was assessed with the International Index of Erectile Function (IIEF), a self-report measure that is well validated and widely used in clinical and epidemiological studies (Rosen, Wagner, & Riley, 1997). The German version of the IIEF was applied, for which the authors suggest calculating a main scale including all 15 items, a scale for sexual desire consisting of 3 items (IIEF-SD), and a scale for sexual function (IIEF-SF)—mainly reflecting erectile function—consisting of 12 items (Wiltink et al., 2003). A main score lower than 53 is considered as a clinically relevant cutoff for ED, indicating at least a mild ED. For the scale used to assess sexual function, the internal consistency was satisfactory, with Cronbach’s alpha of .82. Participants also responded to additional questions related to sexual health derived from the questionnaire of the European Male Aging Study, addressing the frequency of sexual thoughts, the frequency of sexual intercourse, the frequency of masturbating, the frequency of having woken up with an erection, the decrease in having woken up with an erection, whether the amount of sexual intercourse was satisfying, and whether sexual desire has changed, all referring to the past year (O’Connor et al., 2008).
Psychosocial Protective Factors
Psychosocial protective factors were assessed via self-report measures. Well-validated psychometric instruments were completed for perceived general health (SF-36-PGH; Ruta, Abdalla, Garratt, Coutts, & Russell, 1994), emotional support (BSSS-ES; Schulz & Schwarzer, 2003), relationship quality (RAS; Barrett et al., 2002), affiliation and intimacy motivation (GOALS-A/-I; Pöhlmann & Brunstein, 1997), optimism (LOT-R; Glaesmer, Hoyer, Klotsche, & Herzberg, 2008), resilience (RS-11; Schumacher, Leppert, Gunzelmann, Strauß, & Brähler, 2005), and satisfaction with life (SWLS; Glaesmer, Grande, Braehler, & Roth, 2011). For descriptive information on the psychometric instruments, see the appendix. For all psychometric instruments, satisfactory internal consistency has been reported, with Cronbach’s alpha ranging from .72 to .91.
Salivary Analytes
Saliva samples were obtained in a standardized procedure between 8:00 a.m. and 8:15 a.m. Participants were asked to successively fill 3 SaliCaps of 2 mL capacity (SaliCaps, IBL International GmbH, Hamburg, Germany) with saliva. The following parameters were used for the present study: T, DHEA, E2, P, C, and IL6.
All saliva samples were stored at −20°C until required for biochemical analysis. Salivary analytes were analyzed either in the biochemical laboratory of the Department of Psychology of the University of Zurich or in the biochemical laboratory of the Technical University of Dresden.
Potential Covariates and Confounders
The included covariates were age, BMI, marital status, smoking status and alcohol consumption, medication intake, drug consumption, potency medication intake, education, and having had a cold or an injury or bleeding in the mouth in the past 7 days. Due to occasionally occurring delays in participants’ saliva sampling and the circadian rhythmicity of different hormones, the exact starting time of saliva collection was used as a covariate. The current health condition and the amount of hours of physical activity per week were further included as covariates.
Statistical Analysis
First, outliers were identified and removed for the salivary parameters. Values that were greater or less than 3 standard deviations around the mean were removed (Rousseeuw & Croux, 1993). For T, 1.1% (n = 3); for DHEA, 1.8% (n = 5); for E2, 0.4% (n = 1); for P, 2.6% (n = 7); for C, 3.3% (n = 9); and for IL6, 0.8% (n = 2) of values were removed.
Quantile regressions for sexual function by age were computed for the 10% and the 25% quantile of the sample. To illustrate the risk groups of low levels of sexual function, the areas between the regression lines were colored red (0% to 10%), orange (10% to 25%), and green (25% to 100%).
Associations between ED, age, salivary analytes, and psychosocial protective factors were analyzed using Pearson bivariate and partial correlations. To examine whether the association between age and ED was influenced by either endocrine and inflammatory markers or psychosocial protective factors, moderation analyses were conducted according to Hayes (Hayes, 2013). Additionally, the potential covariates and confounders introduced were included in the analyses. In a next step, homogeneous groups of participants were searched for using a hierarchical cluster analysis based on the variables of age and sexual function. Both variables were standardized for this analysis to account for their different ranges. The Euclidean distance was employed as a measure of distance in this analysis. In a first step, single-linkage clustering was used to determine possible outliers. Based on a visual inspection of the dendrogram of this analysis, one participant was identified as outlier and excluded from the further data analysis, reducing the sample to 270 participants. In a second step, clusters of participants were formed using the Ward method. The number of clusters for all subsequent analyses was determined with Mojena’s test and the elbow method (Backhaus, Erichson, Plinke, & Weiber, 2015); both methods indicated the presence of four clusters. To compare the different clusters, t tests for independent samples were calculated (Backhaus et al., 2015).
Analyses were conducted using R version 3.2.2 (R Core Team, 2016) and SPSS 23 (IBM Corp; Statistics). The level of significance was set at α = .05.
Results
Demographic, sexual, and psychosocial characteristics of the sample consisting of 271 participants are presented in Table 1. Table 2 presents descriptive statistics of the salivary analytes. Figure 1 shows quantile regressions for sexual function by age.
Table 2.
Descriptive Statistics of Salivary Analytes (Sex Steroids and IL6 Are Depicted in pg/mL and C in nmol/L).
| T | DHEA | E2 | P | C | IL6 | |
|---|---|---|---|---|---|---|
| N a | 268 | 266 | 270 | 264 | 262 | 248 |
| M | 67.4 | 256.3 | 1.32 | 28.43 | 18.3 | 3.9 |
| SD | 26.7 | 224.3 | 1.0 | 18.8 | 8.2 | 3.4 |
| Minimum | 7.8 | 8.1 | 0.16 | 0.05 | 1.8 | 0.08 |
| Maximum | 165.1 | 1129.5 | 6.0 | 104.2 | 47.3 | 17.4 |
Note. T = testosterone; DHEA = dehydroepiandrosterone; E2 = estradiol; P = progesterone; C = cortisol; IL6 = interleukin-6.
Absolute number of analyzed hormonal parameters included for statistical analyses of 271 included participants. The numbers vary slightly among different salivary analytes due to exclusion of extreme values. A total of 21 participants had IL6 levels below the detection range.
Figure 1.
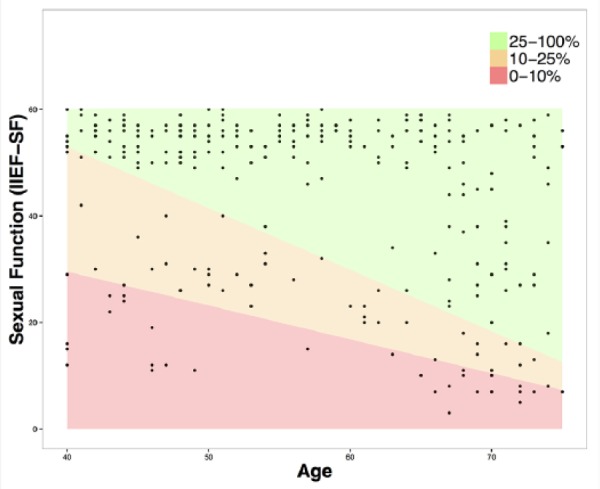
Quantile regressions for sexual function by age for the 10% and the 25% quantile of the sample.
Note. IIEF-SF = Sexual Function.
A score below 53 on the main scale of the IIEF is considered to indicate a clinically relevant ED (Wiltink et al., 2003). In the current sample, 34.7% of participants scored below this cutoff. Partial correlation analysis identified that age was significantly associated with ED (IIEF-SF; r = −.272, p = .000). As presented in Table 3, partial correlation analysis identified that ED was neither directly associated with steroid hormones nor with IL6 (all p > .05). For psychosocial protective factors, positive associations emerged for perceived general health (SF-36-PGH; r = .119, p = .028), emotional support (BSSS-ES; r = −.188, p = .001), relationship quality (RAS; r = .305, p = .000), intimacy motivation (GOALS-I; r = .170, p = .003), resilience (RS-11; r = .131, p = .018), and satisfaction with life (SWLS; r = .282, p = .000; see Table 4). No associations between hormonal or inflammatory parameters and psychosocial variables were identified after inclusion of confounders.
Table 3.
Partial Correlations for Sexual Health and Hormonal and Inflammatory Protective Factors.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. IIEF-SF | ||||||
| 2. T | −.029 | |||||
| 3. DHEA | .053 | .353** | ||||
| 4. E2 | −.084 | .288** | .152* | |||
| 5. P | −.011 | .319** | .216* | .395** | ||
| 6. C | −.088 | .246** | .285** | .112 | .228** | |
| 7. IL6 | −.046 | .136* | .212* | .215* | .171* | .023 |
Note. IIEF-SF = sexual function; T = testosterone; DHEA = dehydroepiandrosterone; E2 = estradiol; P = progesterone; C = cortisol; IL6 = interleukin-6. Pearson correlations.
p < .05. **p < .01.
Table 4.
Partial Correlations for Sexual Health and Psychological Protective Factors.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. IIEF-SF | ||||||||
| 2. SF-36-PGH | .119* | |||||||
| 3. BSSS-ES | .188** | .107 | ||||||
| 4. RAS | .305** | .099 | .397** | |||||
| 5. GOALS-A | .054 | .063 | .215* | .002 | ||||
| 6. GOALS-I | .170** | .155* | .391** | .106 | .335** | |||
| 7. LOT-R | .097 | .264** | .282** | .270** | .049 | .175* | ||
| 8. RS-11 | .131** | .266** | .347** | .241** | .217** | .326** | .526** | |
| 9. SWLS | .282** | .214* | .263** | .483** | −.057 | .097 | .540** | .386** |
Note. IIEF-SF = sexual function; SF-36-PGH = perceived general health; BSSS-ES = emotional support; RAS = relationship quality; GOALS-A = affiliation motivation; GOALS-I = intimacy motivation; LOT-R = optimism; RS-11 = resilience; SWLS = satisfaction with life. Number of participants vary slightly among different variables. Pearson correlations controlled for confounders.
p < .05. **p < .01.
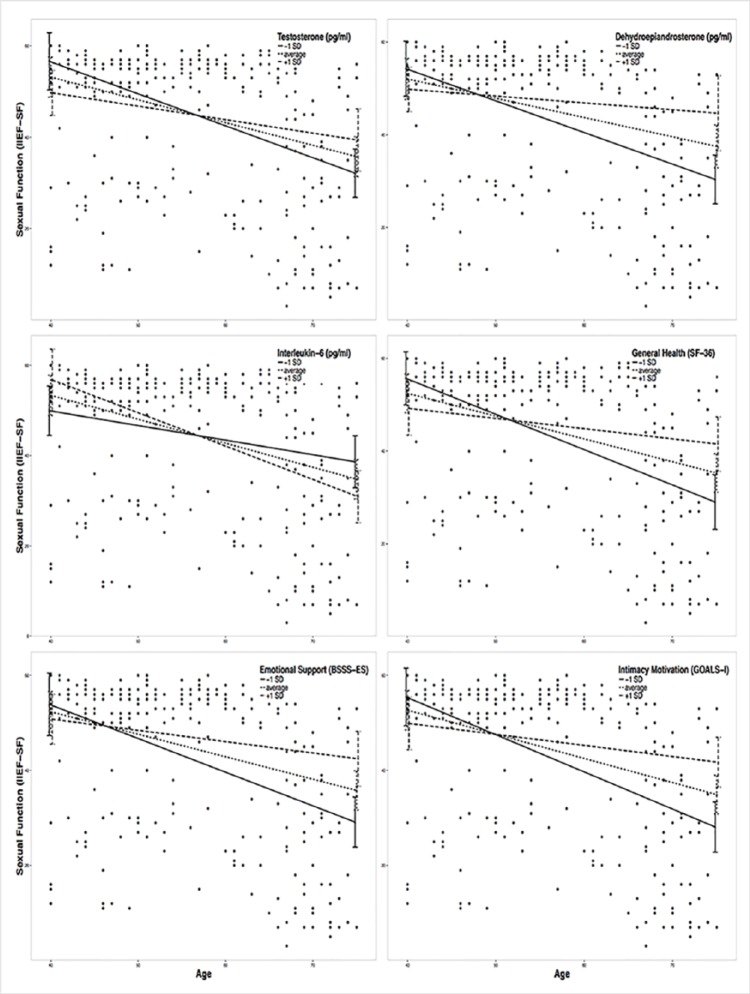
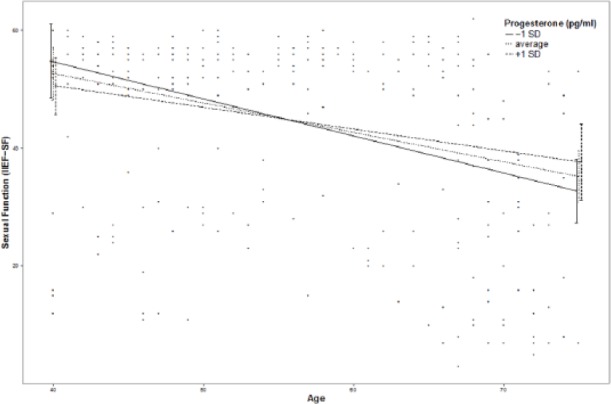
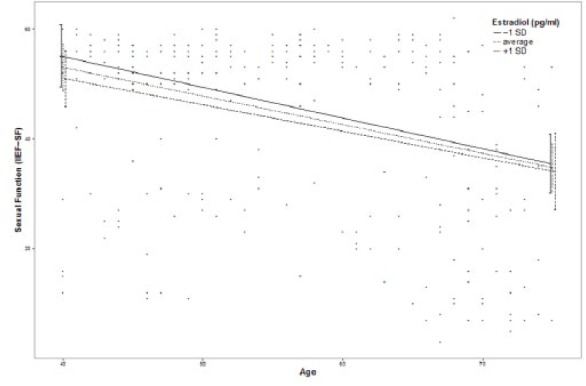
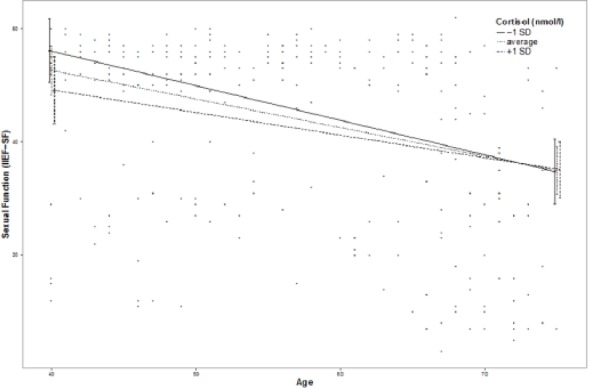
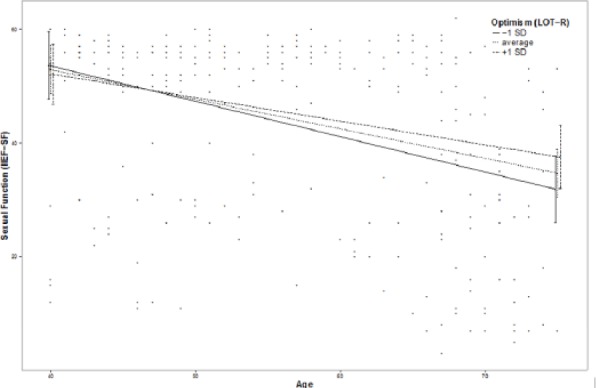
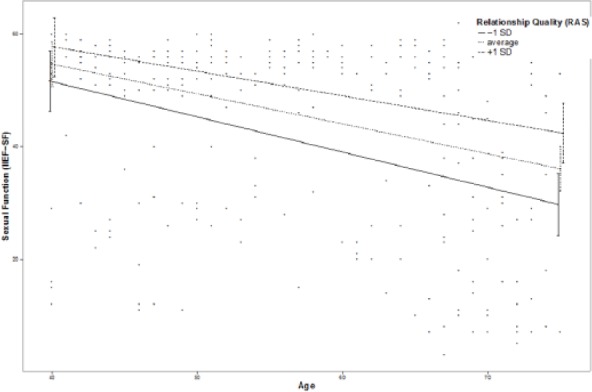
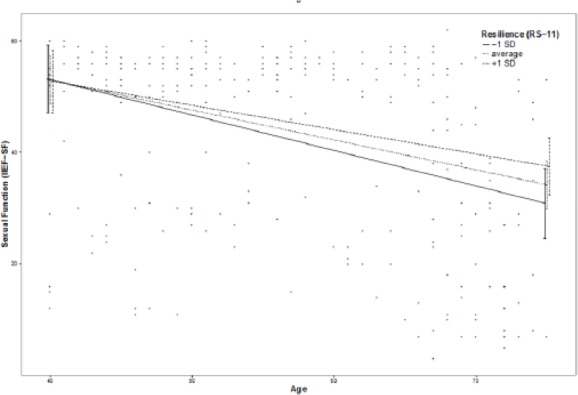
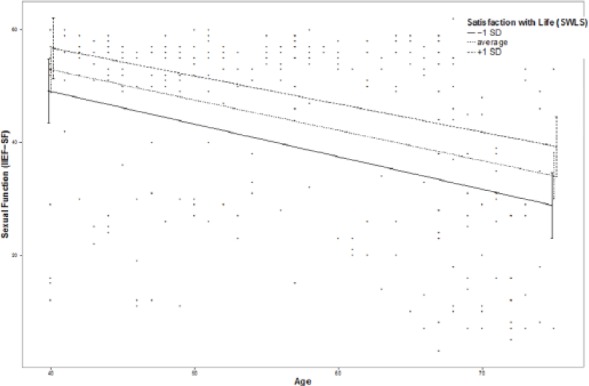
Moderation effects of protective factors on the relationship between age and ED were analyzed with moderation analyses according to Hayes by including the introduced confounders. Moderation analyses for the endocrine and inflammatory parameters identified significant associations between age and ED for T (β = .0076, p = .035, f2 = .0178), DHEA (β = .001, p = .007, f2 = .029), and IL6 (β = −.0614, p = .039, f2 = .0184; see Figure 2). Further moderation analyses for E2, P, and C failed to reach statistical significance (see the appendix). Moderation analyses for the psychosocial parameters identified significant associations between age and ED for perceived general health (β = .0928, p = .004, f2 = .0328), emotional support (β = .4321, p = .017, f2 = .0228), and intimacy motivation (β = .1129, p = .003, f2 = .0351; see Figure 2), while for relationship quality, affiliation motivation, optimism, resilience, and satisfaction with life, moderation analyses did not reach statistical significance (see the appendix).
Figure 2.
Moderation plots for psychobiological protective factors on the association between age and erectile dysfunction.
As presented in Figure 3, a hierarchical cluster analysis using the Ward method for sexual function by age identified four clusters (middle-aged men with [n = 31] and without ED [n = 77] and older men with [n = 70] and without ED [n = 92]). The subsequently computed group comparisons using t tests revealed group differences between older men with and without ED for T (t = 2.078, p = .039, d = .34), and DHEA (t = 3.864, p = .000, d = .64), but not for E2, P, C, or IL6. With regard to psychometric measures, group differences were reported for constructs such as perceived general health (t = 3.980, p = .000, d = .63), emotional support (t = 3.340, p = .001, d = .53), intimacy motivation (t = 3.264, p = .001, d = .53), satisfaction with life (t = 3.308, p = .001, d = .52), resilience (t = 2.068, p = .040, d = .33), and relationship quality (t = 3.064, p = .003, d = .51). No differences emerged for optimism and affiliation motivation. Taken together, older men without ED, in comparison with older men with ED, exhibited higher T and DHEA levels and reported higher perceived general health, emotional support, intimacy motivation, satisfaction with life, and resilience, and a better relationship quality.
Figure 3.
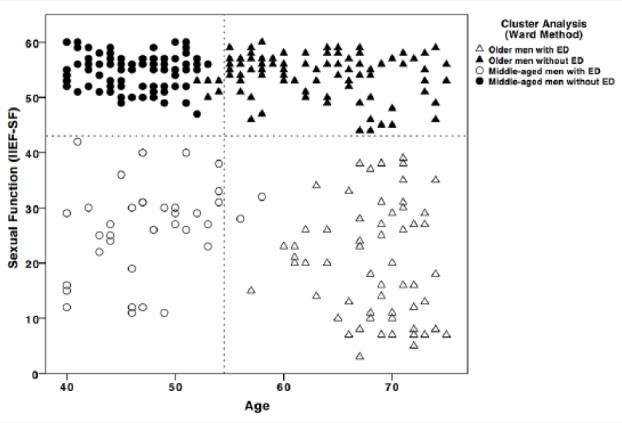
Hierarchical cluster analysis for sexual function by age.
Note. ED = erectile dysfunction. Black filled circles represent Cluster 1 = middle-aged men without ED. Black empty circles represent Cluster 2 = middle-aged men with ED. Black filled triangles represent Cluster 3 = older men without ED. Black empty triangles represent Cluster 4 = older men with ED.
No group differences between middle-aged men with and without ED emerged for sex steroids, or for C or IL6. The only psychometric construct that differed significantly between the two groups was satisfaction with life (t = 2.755, p = .009, d = .66), indicating more satisfaction with life in middle-aged men without ED. However, relationship satisfaction reached a statistical trend (t = 1.751, p = .096, d = .54), indicating higher relationship satisfaction in middle-aged men without ED. Further analyses of group differences are reported in the appendix.
Discussion
In this sample of self-reporting healthy men aged between 40 and 75 years, 34.7% reported at least a mild form of ED. Neither steroid hormones nor the proinflammatory marker IL6 were directly associated with ED. However, ED was negatively associated with perceived general health, emotional support, intimacy motivation, relationship quality, resilience, and satisfaction with life, but not with affiliation motivation and optimism. Both endocrine and psychosocial parameters modified the association between age and ED. Higher T and DHEA and lower IL6 levels buffered the association between age and ED. A buffering effect on the cross-sectional age-related increase in ED was also revealed for higher perceived general health, emotional support, and intimacy motivation. Furthermore, a hierarchical cluster analysis on age and sexual function indicated four clusters, representing middle-aged men with and without ED and older men with and without ED. This finding raises the question of potentially different forms of ED in these age groups. Group differences in sex steroid levels with regard to ED status were present in older men but not in middle-aged men. Furthermore, several psychosocial protective factors differed between older men with and without ED, while in middle-aged men only satisfaction with life was significantly higher in those men without ED. Besides higher sex steroid levels, for middle-aged men with ED, higher intimacy motivation and a trend toward more emotional support than in older men with ED was also reported. This indicates an age-related loss of intimacy motivation and potentially emotional support in men suffering from ED. In addition, older men with ED differed from middle-aged men without ED not only with regard to sex steroids but also in C and IL6 levels suggesting an ED-related increase in C and IL6 levels from middle-aged to older men (see the appendix).
Age is the most influential risk factor for ED. In the current sample of self-reporting healthy men, ED was present in 34.7% and demonstrated a strong cross-sectional increase with age. Therefore, the identification of protective factors decelerating the age-related increase in ED is of major interest for men’s sexual health. The findings are in accordance with previous research reporting that higher endogenous levels of T and DHEA are beneficially related to sexual health in men and seem to decelerate the age-related increase in ED (Walther & Ehlert, 2015). As ED is often related to vasculogenic disturbances, T and DHEA might exert their beneficial effects on ED via their vasodilative properties. Furthermore, lower levels of IL6 were associated with a buffered age-related increase in symptoms of ED, which further confirms the hypothesis of an association between inflammation and ED. The levels of a number of proinflammatory cytokines are also elevated in heart failure or heart dysfunction, and the administration of potency medication such as sildenafil was reported to cause a sustained reduction of proinflammatory cytokines, linking the influence of proinflammatory cytokines on vasculogenic function and ED (Zhao et al., 2015).
As middle-aged men’s ED is assumed to differ from older men’s ED (Mola, 2015), there is a need for tailored treatment approaches. In the present sample, the computed cluster analysis indicates a cutoff at the age of 54.5 years for a separation between middle-aged and older men with ED. While younger than 54.5 years, ED might be more associated with psychosocial and lifestyle factors, age-related biological changes occurring after the age of 54.5 years might be responsible for ED in the majority of older men. Therefore, for ED prevention in older men, and to help target the development of disorders early on, it might be crucial to begin monitoring endocrine and inflammatory levels, and neurogenic or anatomic function, at the age of 54.5 years.
Calculated effect sizes for the moderating effects of endocrine, inflammatory, or psychosocial protective factors on the age-related increase in ED were in general medium to large, indicating a similar level of influence of biological and psychosocial factors on the age-related increase in ED. These results further add and contribute to previous research reporting that directly after age, modifiable lifestyle factors and comorbidities are the most important contributors to the development of ED (Kupelian, Araujo, Chiu, Rosen, & McKinlay, 2010). The relatedness of biological, psychosocial, and lifestyle factors in terms of the age-related increase in ED seems to be best explained by their concomitant influence on general health, which in turn is reflected by erectile function (Capogrosso et al., 2015). This is supported by the present results, which indicate that perceived general health, as a proxy of general health, is associated with a buffered age-related increase in ED. However, lifestyle and psychosocial interventions are discussed to have beneficial effects on endothelial function and nitric oxide bioavailability, indicating that glycometabolic disorder is involved in the underlying pathophysiology of organic ED (Capogrosso et al., 2015; Chitaley, Kupelian, Subak, & Wessells, 2009). Furthermore, weight loss, causing a decrease in inflammation and an increase in T levels, has also been mentioned as possible mechanism through which erectile function could be reestablished (Lamina, Okoye, & Dagogo, 2009).
There are several limitations that need to be taken into consideration when interpreting the results. The cross-sectional design of the study does not allow causal conclusions, and further longitudinal examinations need to be carried out to confirm the obtained between-subject results using within-subject designs. A further limitation is the small sample size, which limits the statistical power and interpretation of the data. Apart from the self-report measurement of erectile function, there was no examination of ED by a clinician. Future studies should combine self-report measurements and clinical examination to ensure high sensitivity and specificity of ED diagnosis. A final limitation is that the results only apply to self-reporting healthy men aged between 40 and 75 years. As ED has been strongly related to general health and vascular disorders (Mola, 2015), the described sample might represent a less vulnerable sample to ED than the general population.
Conclusion
Studies attempting to determine the factors that lead to ED have been able to formulate numerous risk factors for ED, but efficient prevention and treatment strategies integrating endocrine, inflammatory and psychosocial protective factors for prevention, and early targeting of the very common disorder remain elusive. Combating the age-related increase in ED requires a combined intervention strategy consisting of androgen supplementation, anti-inflammatory interventions, diabetes and hypertension control in older males, alongside psychosocial interventions to improve emotional support, intimacy motivation, relationship quality, and satisfaction with life, as well as healthy lifestyle interventions addressing physical activity, smoking, and alcohol consumption both in middle-aged and older males.
Acknowledgments
We thank all participants who took part in the study. We would also like to thank Firouzeh Farahmand for performing the hormone analyses. Special thanks go to Isabelle Brunner and Sarah Mannion-Hernandez for helping with the preparation and the editing of the manuscript.
Appendix
Psychosocial Protective Factors
To measure participants’ satisfaction in an intimate relationship or marriage the RAS was used, consisting of seven Likert-type items, for which satisfactory reliability and validity were reported (Sander & Böcker, 1993). In this study, Cronbach’s alpha of .91 was reported.
The motivational questionnaire (GOALS) evaluates the six domains: power, achievement, diversification, altruism, intimacy (GOALS-I), and affiliation (GOALS-A). Cronbach’s alpha for the two domains used in our study (intimacy and affiliation) were .70 and .90 (Pöhlmann & Brunstein, 1997).
For evaluating optimism in participants, the German version of the Life-Orientation-Test (LOT-R) was used, that consists of 10 items on a 5-point Likert-type scale; 3 are optimistic formulated, 3 are pessimistic formulated, and 4 are neutral formulated. The LOT-R reaches Cronbach’s alpha of .92 (Glaesmer et al., 2008). Cronbach’s alpha in this study was .71.
To measure the resilience of participants, the German version of the resilience scale was used. It contains 11 one-dimensional items and shows Cronbach’s alpha of .91 (Schumacher et al., 2005). In this study, Cronbach’s alpha of .84 was reported.
To evaluate life satisfaction, the Satisfaction with Life Scale (SWLS) with five items on a 7-point Likert-type scale was used. The SWLS reaches Cronbach’s alpha of .92 (Glaesmer et al., 2011). In this study, Cronbach’s alpha of .89 was reported.
The Short Form (SF-36) was used to evaluate the participants’ perceived general health by 36 self-administrated items. The multi-item Likert-type scale assess eight health concepts including general health perception (SF-36-PGH) with a reported Cronbach’s alpha higher than .80 (Jenkinson, Coulter, & Wright, 1993).
The Berlin Social Support Scales (BSSS) were used to assess participants’ emotional (BSSS-ES), instrumental (BSSS-IS), and total social support (BSSS). The questionnaire consists of 17 items on a 4-point Likert-type scale. Cronbach’s alpha for the three BSSS scales ranged in this study between .83 and .86 (Schulz & Schwarzer, 2003).
Results not in Included in the Main Text: Further Analyses of Group Differences
Group differences between middle-aged men with ED and older men with ED revealed higher levels of T (t = −3.114, p = .003, d = .74), DHEA (t = −3.688, p = .001, d = .91), and P (t = −2.124, p = .046, d = .46), and a higher intimacy motivation (t = −2.523, p = .014, d = .50), as well as a trend toward more emotional support (t = −1.869, p = .065, d = .41) in middle-aged men with ED. Analysis of group differences between middle-aged men without ED and older men without ED identified higher levels of T (t = −3.114, p = .003, d = .49), DHEA (t = −3.688, p = .001, d = .38), and P (t = −2.124, p = .046, d = .50) in middle-aged men without ED, and no differences in psychosocial constructs. Analysis of group differences between middle-aged men without ED and older men with ED revealed significant differences for all salivary analytes (T: t = 5.020, p = .000, d = .83; DHEA: t = 5.579, p = .000, d = .92; E2: t = 2.129, p = .035, d = .35; P: t = 3.364, p = .001, d = .56; C: t = −2.151, p = .034, d = .36; IL6: t = −2.464, p = .015, d = .42). Taken together, middle-aged men without ED in comparison with older men with ED exhibited higher levels of T, DHEA, E2, and P and lower levels of C and IL6. With regard to psychosocial constructs, middle-aged men without ED reported higher perceived general health (t = 2.408, p = .017, d = .40) emotional support (t = 4.342, p = .000, d = .71), intimacy motivation (t = 2.410, p = .017, d = .40), and satisfaction with life (t = 2.049, p = .043, d = .34) compared with older men with ED.
Additional moderation plots for psychobiological protective factors on the association between age and ED:
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was fully funded by the University Research Priority Program “Dynamics of Healthy Aging” of the University of Zurich. The funding source had no role in the study design; the collection, analysis, and interpretation of the data; the writing of the report or the decision to submit the article for publication.
References
- Andersen M. L., Martins R. C., Alvarenga T. A., Antunes I. B., Papale L. A., Tufik S. (2007). Progesterone reduces erectile dysfunction in sleep-deprived spontaneously hypertensive rats. Reproductive Biology and Endocrinology, 5(1), 7. doi: 10.1186/1477-7827-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus K., Erichson B., Plinke W., Weiber R. (2015). Multivariate analysis methods: An application-oriented introduction. Heidelberg, Germany: Springer-Verlag. [Google Scholar]
- Barrett J. J., Putzke J. D., Richards J. S. (2002). Early versus late onset of spinal cord injury among the elderly. Journal of Clinical Psychology in Medical Settings, 9, 219-226. [Google Scholar]
- Berry J. W., Worthington E. L., Jr. (2001). Forgivingness, relationship quality, stress while imagining relationship events, and physical and mental health. Journal of Counseling Psychology, 48, 447-455. [Google Scholar]
- Capogrosso P., Montorsi F., Salonia A. (2015). Erectile dysfunction in young patients is a proxy of overall men’s health status. Current Opinion in Urology, 26, 140-145. [DOI] [PubMed] [Google Scholar]
- Chitaley K., Kupelian V., Subak L., Wessells H. (2009). Diabetes, obesity and erectile dysfunction: Field overview and research priorities. Journal of Urology, 182, 45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N. H., Ahn C. W., Park J. Y., Ahn T. Y., Lee H. W., Park T. S., . . . Choi D. S. (2006). Prevalence of erectile dysfunction in Korean men with Type 2 diabetes mellitus. Diabetic Medicine, 23, 198-203. [DOI] [PubMed] [Google Scholar]
- Corona G., Rastrelli G., Maseroli E., Forti G., Maggi M. (2013). Sexual function of the ageing male. Best Practice & Research: Clinical Endocrinology & Metabolism, 27, 581-601. [DOI] [PubMed] [Google Scholar]
- Cunningham G. R., Stephens-Shields A. J., Rosen R. C., Wang C., Bhasin S., Matsumoto A. M., . . . Cella D. (2016). Testosterone treatment and sexual function in older men with low testosterone levels. Journal of Clinical Endocrinology & Metabolism, 101, 3096-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainese S. M., Allemand M., Ribeiro N., Bayram S., Martin M., Ehlert U. (2011). Protective factors in midlife: How do people stay healthy? GeroPsych, 24, 19-29. [Google Scholar]
- De Moor J. S., De Moor C. A., Basen-Engquist K., Kudelka A., Bevers M. W., Cohen L. (2006). Optimism, distress, health-related quality of life, and change in cancer antigen 125 among patients with ovarian cancer undergoing chemotherapy. Psychosomatic Medicine, 68, 555-562. [DOI] [PubMed] [Google Scholar]
- El-Sakka A. I. (2013). Impact of the association between elevated oestradiol and low testosterone levels on erectile dysfunction severity. Asian Journal of Andrology, 15, 492-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. S., Lee H., Burnett-Bowie S. A. M., Pallais J. C., Yu E. W., Borges L. F., . . . Leder B. Z. (2013). Gonadal steroids and body composition, strength, and sexual function in men. New England Journal of Medicine, 369, 1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaesmer H., Grande G., Braehler E., Roth M. (2011). The German version of the Satisfaction with Life Scale (SWLS): Psychometric properties, validity, and population-based norms. European Journal of Psychological Assessment, 27, 127-132. doi: 10.1027/1015-5759/a000058 [DOI] [Google Scholar]
- Glaesmer H., Hoyer J., Klotsche J., Herzberg P. Y. (2008). Die deutsche Version des Life-Orientation-Tests (LOT-R) zum dispositionellen Optimismus und Pessimismus [The German version of the Life-Orientation-Test (LOT-R) for dispositional optimism and pessimism]. Zeitschrift für Gesundheitspsychologie, 16, 26-31. doi: 10.1026/0943-8149.16.1.26 [DOI] [Google Scholar]
- Hayes A. (2013). Introduction to mediation, moderation, and conditional process analysis. New York, NY: Guilford. [Google Scholar]
- Hyde Z., Flicker L., Hankey G. J., Almeida O. P., McCaul K. A., Chubb S. A., Yeap B. B. (2012). Prevalence and predictors of sexual problems in men aged 75-95 years: A population-based study. Journal of Sexual Medicine, 9, 442-453. [DOI] [PubMed] [Google Scholar]
- Jenkinson C., Coulter A., Wright L. (1993). Short form 36 (SF36) health survey questionnaire: Normative data for adults of working age; British Medical Journal, 306, 1437-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori Y., Koh E., Sugimoto K., Izumi K., Narimoto K., Maeda Y., . . . Namiki M. (2009). The relationship of serum and salivary cortisol levels to male sexual dysfunction as measured by the International Index of Erectile Function. International Journal of Impotence Research, 21, 207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokavcova M., van Dijk J. P., Nagyova I., Rosenberger J., Gavelova M., Middel B., . . . Groothoff J. W. (2008). Social support as a predictor of perceived health status in patients with multiple sclerosis. Patient Education & Counseling, 73, 159-165. [DOI] [PubMed] [Google Scholar]
- Kupelian V., Araujo A. B., Chiu G. R., Rosen R. C., McKinlay J. B. (2010). Relative contributions of modifiable risk factors to erectile dysfunction: Results from the Boston Area Community Health (BACH) Survey. Preventive Medicine, 50, 19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamina S., Okoye C. G., Dagogo T. T. (2009). Therapeutic effect of an interval exercise training program in the management of erectile dysfunction in hypertensive patients. Journal of Clinical Hypertension, 11, 125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Zhang H., Liao M., Tang Q., Huang Y., Xie J., . . . Yao Z. (2015). Sex hormones predict the incidence of erectile dysfunction: From a population-based prospective cohort study (FAMHES). Journal of Sexual Medicine, 12, 1165-1174. [DOI] [PubMed] [Google Scholar]
- Mola J. R. (2015). Erectile dysfunction in the older adult male. Urologic Nursing, 35, 87-94. [PubMed] [Google Scholar]
- NIH Consensus Conference on Impotence. (1993). NIH consensus development panel on impotence. Journal of the American Medical Association, 270, 83-90. [PubMed] [Google Scholar]
- O’Connor D. B., Corona G., Forti G., Tajar A., Lee D. M., Finn J. D., . . . Huhtaniemi I. T. (2008). Assessment of sexual health in aging men in Europe: Development and validation of the European male ageing study sexual function questionnaire. Journal of Sexual Medicine, 5, 1374-1385. [DOI] [PubMed] [Google Scholar]
- O’Connor D. B., Lee D. M., Corona G., Forti G., Tajar A., O’Neill T. W., . . . Finn J. D. (2011). The relationships between sex hormones and sexual function in middle-aged and older European men. Journal of Clinical Endocrinology & Metabolism, 96, 1577-1587. [DOI] [PubMed] [Google Scholar]
- Pöhlmann K., Brunstein J. C. (1997). GOALS: Ein Fragebogen zur Messung von Lebenszielen [GOALS: A questionnaire for assessing life goals]. Diagnostica, 43, 63-79. [Google Scholar]
- Rabijewski M., Papierska L., Kuczerowski R., Piątkiewicz P. (2015). Hormonal determinants of erectile dysfunction and lower urinary tract symptoms in middle-aged and elderly men with prediabetes. Aging Male, 18, 256-264. [DOI] [PubMed] [Google Scholar]
- Rosen R. C., Wagner G., Riley A. (1997). A multidimensional scale for assessment of erectile dysfunction. Urology, 49, 822-830. [DOI] [PubMed] [Google Scholar]
- Rousseeuw P. J., Croux C. (1993). Alternatives to the median absolute deviation. Journal of the American Statistical Association, 88, 1273-1283. [Google Scholar]
- Ruta D. A., Abdalla M. I., Garratt A. M., Coutts A., Russell I. T. (1994). SF 36 health survey questionnaire: I. Reliability in two patient based studies. Quality in Health Care, 3, 180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J., Böcker S. (1993). Die Deutsche Form der Relationship Assessment Scale (RAS): Eine kurze Skala zur Messung der Zufriedenheit in einer Partnerschaft [The German version of the Relationship Assessment Scale (RAS): A short scale for measuring satisfaction in a dyadic relationship]. Diagnostica, 39, 55-62. [Google Scholar]
- Schulz U., Schwarzer R. (2003). Social support in coping with illness: The Berlin Social Support Scales (BSSS) [Social support in coping with illness: The Berlin Social Support Scales (BSSS)]. Diagnostica, 49, 73-82. doi: 10.1026//0012-1924.49.2.73 [DOI] [Google Scholar]
- Schumacher J., Leppert K., Gunzelmann T., Strauß B., Brähler E. (2005). Die Resilienzskala—Ein Fragebogen zur Erfassung der psychischen Widerstandsfähigkeit als Personmerkmal [The Resilience Scale—A questionnaire to assess resilience as a personality characteristic]. Zeitschrift für Klinische Psychologie, Psychiatrie und Psychotherapie, 53(1), 16-39. Retrieved from http://www.mentalhealthpromotion.net/resources/resilienzskala2.pdf [Google Scholar]
- Shabsigh R., Klein L. T., Seidman S., Kaplan S. A., Lehrhoff B. J., Ritter J. S. (1998). Increased incidence of depressive symptoms in men with erectile dysfunction. Urology, 52, 848-852. [DOI] [PubMed] [Google Scholar]
- Shirai M., Yamanaka M., Shiina H., Igawa M., Fujime M., Lue T. F., Dahiya R. (2003). Downregulation of androgen, estrogen and progesterone receptor genes and protein is involved in aging-related erectile dysfunction. International Journal of Impotence Research, 15, 391-396. [DOI] [PubMed] [Google Scholar]
- Ückert S., Fuhlenriede M. H., Becker A. J., Stief C. G., Scheller F., Knapp W. H., Jonas U. (2003). Is there an inhibitory role of cortisol in the mechanism of male sexual arousal and penile erection? Urological Research, 31, 402-406. [DOI] [PubMed] [Google Scholar]
- Vignozzi L., Filippi S., Comeglio P., Cellai I., Morelli A., Marchetta M., Maggi M. (2014). Estrogen mediates metabolic syndrome-induced erectile dysfunction: A study in the rabbit. Journal of Sexual Medicine, 11, 2890-2902. [DOI] [PubMed] [Google Scholar]
- Wagle K. C., Carrejo M. H., Tan R. S. (2012). The implications of increasing age on erectile dysfunction. American Journal of Men’s Health, 6, 273-279. [DOI] [PubMed] [Google Scholar]
- Walther A., Ehlert U. (2015). Steroid secretion and psychological well-being in men 40+. In Rice T., Sher L. (Eds.), Neurobiology of men’s mental health (pp. 287-322). New York, NY: Nova. [Google Scholar]
- Walther A., Philipp M., Lozza N., Ehlert U. (2016). The rate of change in declining steroid hormones: A new parameter of healthy aging in men? Oncotarget, 7, 1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltink J., Hauck E. W., Phädayanon M., Weidner W., Beutel M. E. (2003). Validation of the German version of the International Index of Erectile Function (IIEF) in patients with erectile dysfunction, Peyronie’s disease and controls. International Journal of Impotence Research, 15, 192-197. [DOI] [PubMed] [Google Scholar]
- Zhao S. G., Wang J. M., Han Q. F., Li T., Yao H. C. (2015). Association between sildenafil and inflammation markers/mediators. International Journal of Cardiology, 198, 178-179. [DOI] [PubMed] [Google Scholar]