Abstract
It is controversial whether African American men(AAM) with low-risk prostate cancer (PC) should be placed on active surveillance (AS). Recent literature indicates AAM diagnosed with low-risk disease have increased pathologic upgrading and disease progression. We evaluated the surgical pathology of AAM and Caucasians who underwent prostatectomy to assess the suitability of AAM for AS. We retrospectively reviewed 1,034 consecutive men who underwent open prostatectomy between 2004 and 2015; 345 Caucasians and 58 AAM met the American Urological Association criteria for low-risk PC. We excluded from analysis two men whose prostatectomies were aborted. Chi-square test, Fisher’s exact test, and Wilcoxon rank sum test were used for statistical analysis. AAM with low-risk PC have a lower rate of surgical upgrading and similar rates of adverse pathology compared with Caucasians. 29.8% of AAM (17/57) diagnosed with low-risk disease but 44.5% of Caucasians (153/344) had disease upgrading at prostatectomy (p < .04), although AAM overall were less likely to be clinically diagnosed with low-risk cancer (33.1 vs. 41.7%, p < .05). AAM with low-risk pathology were younger (median 55 vs. 59 years, p < .001) and had smaller prostates (32 vs. 35 g, p < .04). AAM with preoperative low-risk disease have lower rates of surgical upgrading and similar adverse pathology compared with Caucasians. There may be a Will-Rogers effect as AAM with aggressive disease appear more likely to be stratified into intermediate- and high-risk groups, leaving those AAM diagnosed with low-risk disease fully eligible for AS. Our results support that AS for AAM should remain a viable option.
Keywords: active surveillance, African American, Gleason score, prostate cancer, prostatectomy
Active surveillance (AS) has gained acceptance for clinically localized prostate cancer (PC) in recent years, with increasing utilization and support from systematic reviews and multi-institutional evaluations (Dall’Era et al., 2012; Eggener et al., 2013). Such assessments were designed to address the concerns of overtreatment of indolent PC in the elderly and among patients diagnosed with low-risk PC. Currently, the National Comprehensive Cancer Network (NCCN) guidelines support the use of AS in very low- and low-risk PC instead of immediate intervention (Mohler et al., 2016).
It remains controversial whether African American men (AAM) should proceed with AS under current protocols. A recent article by Sundi et al. showed African Americans with NCCN very-low-risk disease (prostate-specific antigen [PSA] ≤10 ng/mL, Gleason ≤6 on fewer than three biopsy cores, ≤50% cancer in any biopsy core, clinical stage T1c, and PSA density <0.15 ng/mL/g) had higher likelihood of pathologic Gleason upgrading and pT3 staging than other races (Sundi et al., 2013), while literature suggests that PC in African Americans have unique genetic and molecular expression patterns and undergo more rapid disease progression (Sundi & Schaeffer, 2014). Moreover, African Americans have at least two to three times the mortality rate from PC compared with Caucasians (Chornokur, Chornokur, Dalton, Borysova, & Kumar, 2011) and are severely underrepresented in the major active surveillance cohorts (Sormani, 2009). Current AS guidelines may not accurately represent African Americans and there is an urgent need to gather more data.
In this study, we examined the clinicopathologic features of African Americans and Caucasians clinically diagnosed with American Urological Association (AUA) low-risk disease (PSA ≤10 ng/mL, Gleason ≤6, and clinical stage ≤T2a).
Materials and Methods
We retrospectively reviewed clinicopathologic data from 1,034 consecutively operated men (828 Caucasian, 175 African American, 31 other) who underwent open radical retropubic prostatectomy by a single high-volume surgeon between October 2004 and August 2015. Pathology from 345 Caucasian (1 Hispanic, 344 non-Hispanic) and 58 AAM met AUA criteria for clinically diagnosed low-risk PC. We excluded from analysis two men whose prostatectomies were aborted. Research was approved by our institutional review board and compliant with the Health Insurance Portability and Accountability Act.
Prostate biopsy specimens were taken both from our institution and from outside institutions. All biopsy cores taken from outside institutions were re-reviewed by genitourinary pathologists at our institution, with grading from our institution taking precedence in the event of a discrepancy. Biopsies taken after 2005 were read in accordance with 2005 International Society of Urological Pathology (ISUP)–modified Gleason guidelines. Prostatectomy specimens were weighed, inked, and sectioned at 3-mm intervals according to our institutional protocol. Gleason scoring and pathological variables were assessed after evaluating the entire prostate.
Upgrading was defined as an increase in the Gleason score between the highest Gleason sum on biopsy and the final overall Gleason score on prostatectomy specimen.
Preoperative clinical and pathological variables chosen for this study included AUA low versus not low risk, patient age, PSA at biopsy, and clinical staging on rectal examination.
Surgical pathology included Gleason scoring, Gleason upgrading, pathological stage, extracapsular extension, surgical margins, seminal vesicle invasion, prostate size, and percent of prostate that was tumor. Adverse surgical pathology was also specifically defined as pT2 and Gleason ≥4 + 3, or pT3a and Gleason 3 + 3 and positive surgical margins, or pT3a and Gleason ≥3 + 4, or ≥pT3b (Sundi et al., 2013).
Categorical variables were compared between Caucasian and African American patients and analyzed with Chi-squared test and Fisher’s exact test. Because the continuous variables were all not normally distributed, they were compared with the Mann-Whitney U test and Spearman correlation coefficient. Statistical significance was set at p < .05. Statistics were analyzed using JMP Pro 12. Post hoc power analysis was performed using GPower 3.1.
Results
Clinical Risk Stratification
33.1% (58/175) of AAM were clinically diagnosed with AUA low-risk PC, compared with 41.7% (345/828) of Caucasian men (p < .05; Figure 1). AAM were more likely than Caucasian men were to be clinically diagnosed with intermediate-risk disease (41.1% [72/175] vs. 38.3% [317/828]) or high-risk disease (25.7% [45/174] vs. 20.0% [166/828]).
Figure 1.
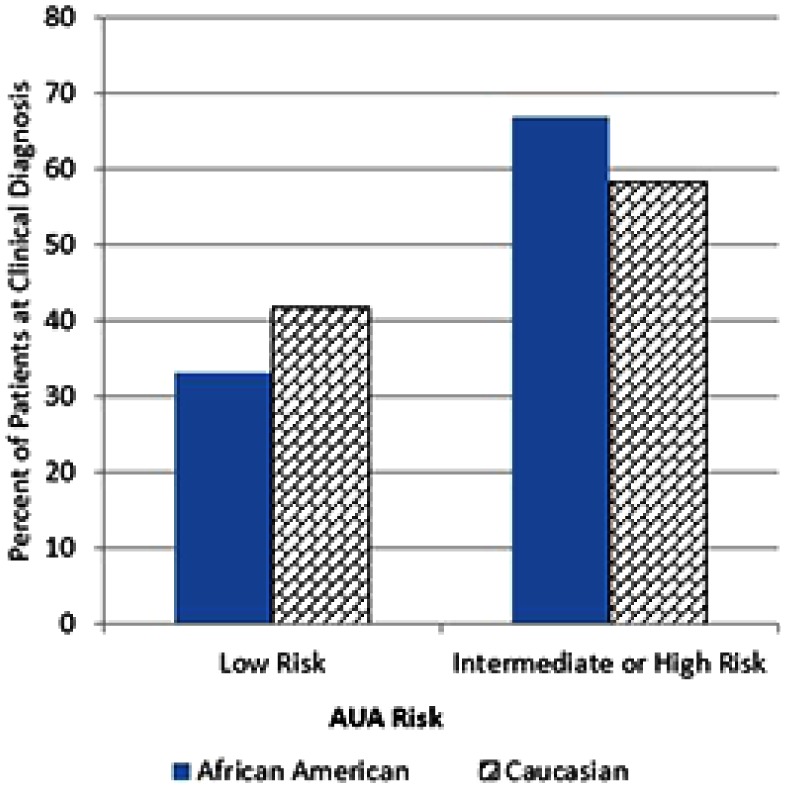
Clinical AUA risk stratification by race.
Note. AUA = American Urological Association. African American men were significantly less likely to be diagnosed with AUA low-risk prostate cancer than Caucasian men (33.1 [58/175] vs. 41.7% [345/828], p < .05).
Of the African Americans diagnosed with AUA low-risk PC, 13.8% (8/58) met the criteria for NCCN very-low-risk disease, while 72.4% (42/58) did not. Of the Caucasians diagnosed with AUA low-risk PC, 21.2% (73/345) met the criteria for NCCN very-low-risk disease, while 64.9% (224/345) did not. It is unconfirmed whether 13.8% (8/58) of African Americans and 13.6% (47/345) Caucasians met the NCCN very-low-risk disease criteria because the total number of biopsy cores were not submitted from an outside institution for review or because multiple biopsy cores were aggregated to provide overall Gleason scores for the right and left prostate.
Gleason Score Upgrading on Surgical Pathology for Clinical Low-Risk Disease
29.8% (17/57) of AAM diagnosed with clinical AUA low-risk disease had Gleason upgrading on surgical pathology, compared with 44.5% (153/344) of Caucasian men (p < .04; Figure 2). The majority of Gleason upgrading were from clinical Gleason 3 + 3 = 6 to pathological Gleason 7 disease for both African Americans (16/57) and Caucasians (143/344; Table 1). The majority of pathological Gleason 7 disease was Gleason 3 + 4 = 7 (15/57 African Americans, 128/344 Caucasians).
Figure 2.
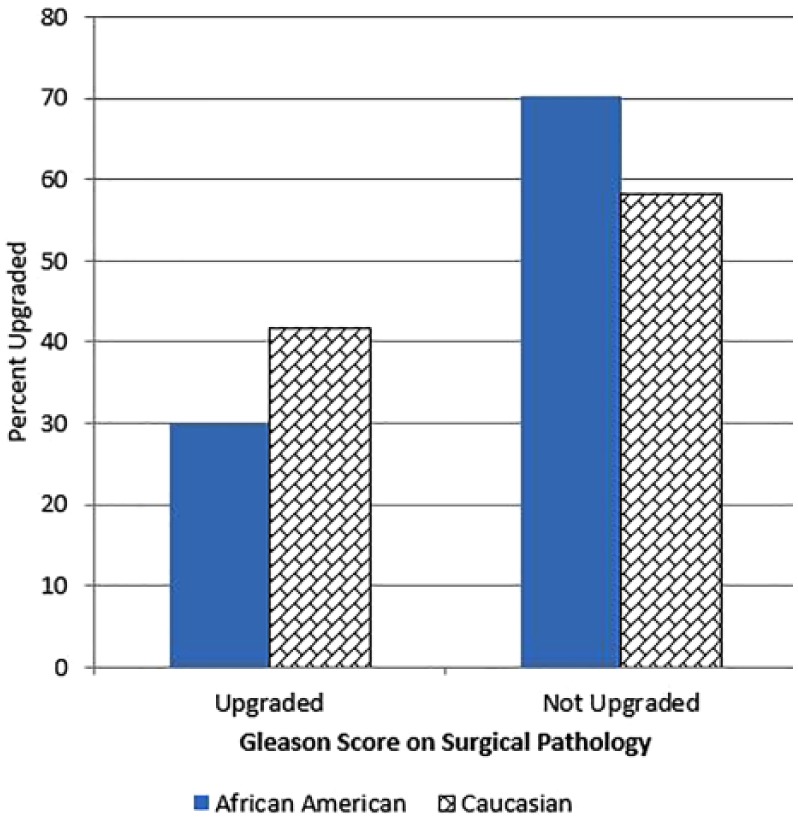
Gleason score upgrading on surgical specimen for clinical AUA low-risk disease by race.
Note. AUA = American Urological Association. African American men clinically diagnosed with AUA low-risk prostate cancer were significantly less likely to have Gleason upgrading on surgical pathology than their Caucasian counterparts (29.8 [17/57] vs. 44.5% [153/344], p < .04).
Table 1.
Pathological Gleason Scores of Clinically Diagnosed AUA Low-Risk Disease.
| Surgical Gleason score | African American (n = 57) | Caucasian (n = 344) |
|---|---|---|
| 5 | 5.26% (n = 3) | 4.07% (n =14) |
| 6 | 64.91% (n = 37) | 52.03% (n = 179) |
| 7 | 28.07% (n = 16) | 41.57% (n = 143) |
| 3 + 4 | 26.32% (n = 15) | 37.21% (n = 128) |
| 4 + 3 | 1.75% (n = 1) | 4.36% (n = 15) |
| 8 | 1.75% (n = 1) | 1.45% (n = 5) |
| 9 | 0% | 0.87% (n = 3) |
Note. AUA = American Urological Association.
Preoperative Clinicopathologic Characteristics
African Americans were significantly more likely to be younger than Caucasians (55 vs. 59 years, p < .04; Table 2). There were no statistically significant differences in median PSA at biopsy or clinical staging.
Table 2.
Clinicopathologic Characteristics of Clinically Diagnosed AUA Low-Risk Disease.
| Characteristic | African American % (n = 57) | Caucasian % (n = 344) | p value |
|---|---|---|---|
| Age (median years) | 55 | 59 | p < .001 |
| PSA at biopsy (median ng/mL) | 4.7 | 4.8 | p < .17 |
| Clinical stage | p < .48 | ||
| T1c | 13.6% (n = 43) | 86.4% (n = 274) | |
| T2a | 16.7% (n = 14) | 83.3% (n = 70) | |
| Pathologic upgrading | 29.8% (n = 17) | 44.5% (n = 153) | p < .04 |
| Pathological stage* | p < .92 | ||
| T2x | 14.3% (n = 48) | 85.7% (n = 287) | |
| T3x | 14.1% (n = 9) | 85.9% (n = 55) | |
| Extracapsular extension | 7.0% (n = 4) | 14.8% (n = 51) | p < .16 |
| Margins | 38.6% (n = 22) | 44.7% (n = 116) | p < .76 |
| Seminal vesicles | 5.3% (n = 3) | 1.7% (n = 6) | p < .10 |
| Adverse pathology | 15.8% (n = 9) | 17.7% (n = 61) | p < .72 |
| Prostate weight (median, g) | 32 | 35 | p < .04 |
| % Tumor (median) | 5 | 5 | p < .86 |
Note. AUA = American Urological Association; PSA = prostate-specific antigen.
One Caucasian listed as pT4.
Prostatectomy Pathology Characteristics
AAM had significantly smaller prostates than Caucasian men did (32 vs. 35 g, p < .04; Table 2). There were no statistically significant pathological differences in staging, extracapsular extension, positive margins, seminal vesicle invasion, percent tumor, or adverse surgical pathology between African American and Caucasian men.
Multivariate Correlation and Power Analysis
On multivariate analysis, Spearman coefficient between age and prostate weight was 0.31 (p < .0001). Spearman coefficient between prostate weight and percent tumor was -0.29 (p < .0001). Coefficient between age and mean pathologic Gleason score was 0.11 (p < .03), and coefficient between prostate weight and mean pathologic Gleason score was -0.12 (p < .02). Coefficient between prostate weight and pathologic staging was -0.17 (p < .001). Age was not significantly correlated with percent tumor or PSA at biopsy. Post hoc analysis showed that power for the mean pathologic Gleason score was 0.50. Post hoc analysis showed that power for the mean pathological staging was 0.07.
Discussion
In this study, we identify that AAM are less likely to be clinically diagnosed with AUA low-risk disease than Caucasian men. However, in contrast with recent literature, we report that African Americans with clinical low-risk disease are less likely to have Gleason upgrading on prostatectomy than Caucasians. Such upgrading is predominately from clinical Gleason 3 + 3 = 6 to pathological Gleason 3 + 4 = 7. AAM with clinical low-risk disease were also significantly younger and had smaller prostates than their Caucasian counterparts. Our results indicate that African Americans are viable candidates for AS as initial management and support that this topic should be further investigated.
Our results may be explained by the Will-Rogers phenomenon. This phenomenon states that moving an element from one set to another set raises (or lowers) the average value of both sets. Classic examples include changes in classification criteria or diagnostic techniques that can produce apparent improvements in medical outcomes despite no true progress (Sormani, 2009). Both Powell et al. and Iremashvili et al. have suggested that PC is more aggressive in African Americans, growing faster with quicker disease progression (Iremashvili, Iremashvili, Soloway, Rosenberg, & Manoharan, 2012; Powell,Bock, Ruterbusch, & Sakr, 2010). In our study, AAM with more aggressive PC may be more likely to be initially stratified into intermediate- or high-risk classification and rendered ineligible for AS. This potentially leaves only African Americans with truly less aggressive cancer to preferentially be diagnosed with low-risk disease at biopsy, leaving them less likely to be upgraded on surgical pathology and thus eligible for initial AS under current protocols.
Current AS protocols are based on the 2005 ISUP-modified Gleason scoring system (Epstein, Allsbrook, Amin, Egevad, & ISUP Grading Committee, 2005). The 2005 ISUP updates widened the range of pathologies classified as Gleason 4 pattern and decreased the range of pathologies classified as Gleason pattern 3, which has resulted in increased Gleason grade migration (Helpap & Egevad, 2006; Ozok et al., 2010; Zareba et al., 2009). For example, Danneman et al. reported that of PCs with cT1c and PSA 4 to 10 ng/mL, 19% were Gleason ≥7 before 2005, while 33% were Gleason ≥7 after 2005 (Danneman, Drevin, Robinson, Stattin, & Egevad, 2015). Such changes in Gleason pattern criteria in 2005 may have reclassified relatively more aggressive but previously classified Gleason pattern 3 disease as Gleason pattern 4. The more aggressive PCs of African Americans may have been disproportionately affected by the 2005 ISUP guidelines, with those who would have been diagnosed with clinical Gleason pattern 3 cancer before the 2005 ISUP guidelines classified clinical Gleason pattern 4 cancer under the expanded criteria. These men would thus be removed from AS eligibility. Consequently, indolent PC in African Americans may be disproportionately classified as clinical Gleason pattern 3 under the expanded 2005 ISUP criteria, thereby contributing to the Will Rogers phenomenon.
Kryvenko et al. recently concluded that the Gleason 3 + 3 = 6 PC of AAM produce less PSA than the Gleason 3 + 3 = 6 cancer of Caucasian men (Kryvenko, Balise, Soodana Prakash, & Epstein, 2016). This conclusion was based on results showing that African Americans and Caucasians had similar serum PSA, PSA mass, and tumor volume despite African Americans having significantly larger prostates and lower PSA densities. The authors suggested lowering contemporary PSA density thresholds for African Americans to compensate for their findings when determining AS eligibility. The results of Kryvenko et al. may support ours: The AAM in their cohort with pathological Gleason 3 + 3 = 6 disease may have had less aggressive cancers (as suggested by PSA output) compared with Caucasian men and were perhaps better candidates for AS had they chosen that management.
African Americans had larger prostates than Caucasians in the study by Kryvenko et al., while African Americans had smaller prostates in our cohort (32 vs. 35 g, p < .04). Multiple factors could contribute to this discrepancy, including different patient populations and that African Americans in our study population were younger than Caucasians (correlation coefficient between age and prostate weight was 0.31). Given their smaller prostates, there may be less sampling error on biopsy in our African American patients; consequently, our AAM could potentially have more accurate risk assessment prior to prostatectomy compared with their Caucasian counterparts.
Other studies on the eligibility of AAM for AS have reported conflicting evidence. Sundi et al. studied 359 men from 2004 to 2012 clinically diagnosed with NCCN very-low-risk PC who underwent prostatectomy (Sundi et al., 2013). In their cohort, AAM were more likely to have pathologic Gleason upgrading, pathologic stage T3a, positive margins, and adverse surgical pathology. The same group reported that at median 4 years follow-up after prostatectomy, African Americans were more likely to have biochemical recurrence, while African Americans diagnosed with very-low- or low-risk disease had biochemical recurrence-free survival similar to Caucasians of low- and intermediate-risk groups, respectively (Faisal et al., 2014). Sanchez-Ortez et al. compared 37 African American and 35 Caucasian men with PC before 2006 (Sanchez-Ortiz et al., 2006); 56.8% of African Americans and 62.8% of Caucasians had Gleason ≤6 disease at biopsy, but only 21.6% of African Americans and 57.1% of Caucasians had pathologic Gleason ≤6. In contrast, Resnick et al. studied 1,146 patients with low-risk disease from 1991 to 2007 who underwent radical prostatectomy (Resnick et al., 2009). They did not identify any racial differences in Gleason upgrading, extracapsular extension, margins, seminal vesicle invasion, or tumor volume.
In comparison, AAM were less likely to have Gleason upgrading compared with Caucasians in our cohort. Moreover, there were no significant differences in pathologic staging, margins, or adverse surgical pathology. There are several potential sources of difference between our study and the ones from Sundi et al., Sanchez-Ortez et al., and Resnick et al. There may be differences in patient population undergoing treatment by our institutions. Our patients were younger than the population of other studies, and our African American patients were younger than our Caucasian ones in contrast to previous studies. With smaller prostates, our African American patients could have had more accurate biopsy diagnoses than our Caucasian patients. Finally, there may be interobserver variability among the various institutional pathologists.
African American low-risk men were significantly younger than Caucasian men were in our cohort (55 vs. 59 years, p < .04). Powell et al. has long suggested that AAM should be screened for PC at younger ages than Caucasian men (Powell et al., 1999). It is possible that disease progression was diagnosed earlier in the younger AAM at an earlier stage when such cancers did not have as much time to progress and spread into the prostate, and these men may have been recommended more aggressive treatment either due to racial or life expectancy factors. Nonetheless, there was no systemic effort to screen AAM for PC from a younger age compared with Caucasian men.
Abern et al. from our institution reported that African Americans on AS were more likely to proceed to treatment, although a majority (63%) remained on surveillance (Abern et al., 2013). Iremashvili et al. studied 249 patients (24 African Americans, 225 Caucasians) with clinical low-risk disease from 1994 to 2011 who underwent at least 1 repeat biopsy (Iremashvili et al., 2012). Of 24 African Americans, 14 had disease progression, and the authors reported that African American race was significantly associated with disease progression. Sundi et al. reported that AAM on AS were more likely than Caucasians to have Gleason upgrading on serial biopsies (36 vs. 16%, p < .001), and the African American race was an independent predictor of upgrading (Sundi et al., 2015). Nonetheless, all three studies reported that large percentages of AAM with contemporary low-risk disease did not have disease progression. They suggest that many African Americans are still eligible for AS, and the potential overemphasis on dangers of delaying intervention could unnecessarily sway such patients toward overtreatment and unnecessary morbidity risks.
Strengths of this study include that a decade of recent data has been presented, with the majority of patients undergoing procedures after 2005 and thus the 2005 ISUP guidelines being applied. Biopsy and surgical pathologies were all reviewed by our institution’s genitourinary pathologists. Our patient population includes a higher percentage of African Americans (57/344; 14.2%) than the large randomized-controlled trials in which this group is underrepresented.
Potential limitations include that our study is retrospective in nature and only comprises patients from a high-volume single-surgeon series at one academic institution, reducing our study’s power. Our research and contemporary studies also only used patients who ultimately underwent prostatectomy; patients who elected AS or other treatment methods were not included. There may be interobserver variability among our institution’s genitourinary pathologists when interpreting specimens, which potentially introduces detection bias, although review by an institution’s multiple pathologists better reflects practice in the general community. Finally, age was weakly correlated with and prostate size was negatively weakly correlated with mean pathologic Gleason scoring.
Given the controversies about the racial dimensions of AS and the diverse outcomes of previous studies, additional research into this topic is clearly warranted. One potential area is the impact anterior tumors have on pathology concordance. African Americans are more likely to have anterior tumors, which are more likely to have Gleason upgrading (Sundi et al., 2014). However, Kim et al. recently reported that anterior tumors have similar extracapsular extension, positive margins, and lymph node invasion compared with posterior tumors and that anterior tumors have significantly higher rates of 5-year biochemical-free recurrence survival (Kim et al., 2016). They hypothesized that a barrier formed by the anterior horn of the peripheral zone or anterior fibromuscular stroma and fewer lymphatics and blood vessels could be the reason for such results. Unfortunately, our data did not specify the location of tumors or the dominant nodule, nor did the data specify which patients specifically underwent anterior biopsies. Investigation into this topic could further contribute to refining the use of AS in African Americans.
Overall, the eligibility of AAM for AS remains an area of research and debate. We contribute a novel suggestion that the Will-Rogers phenomenon means that fewer African Americans are diagnosed with low-risk disease, but those with clinical low-risk disease are more likely to have truly low-risk, less aggressive cancer. Our results further support that many African Americans still are eligible for AS and that undue weight should not be given to emphasizing the risks of PC lest these men are overtreated and face undue morbidity. Further research into the role of AS in African Americans is certainly warranted, and our data begs for the need for a prospective randomized controlled trial of AS in African American and Caucasian men.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Abern M. R., Bassett M. R., Tsivian M., Bañez L. L., Polascik T. J., Ferrandino M. N., . . . Moul J. W. (2013). Race is associated with discontinuation of active surveillance of low-risk prostate cancer: Results from the Duke Prostate Center. Prostate Cancer and Prostatic Diseases, 16, 85–90. [DOI] [PubMed] [Google Scholar]
- Chornokur G., Dalton K., Borysova M. E., Kumar N. B. (2011). Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 71, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Era M. A., Albertsen P. C., Bangma C., Carroll P. R., Carter H. B., Cooperberg M. R., . . . Soloway M. S. (2012). Active surveillance for prostate cancer: A systematic review of the literature. European Urology, 62, 976–983. [DOI] [PubMed] [Google Scholar]
- Danneman D., Drevin L., Robinson D., Stattin P., Egevad L. (2015). Gleason inflation 1998-2011: A registry study of 97,168 men. BJU International, 115, 248–255. [DOI] [PubMed] [Google Scholar]
- Eggener S. E., Mueller A., Berglund R. K., Ayyathurai R., Soloway C., Soloway M. S., et al. (2013). A multi-institutional evaluation of active surveillance for low risk prostate cancer. Journal of Urology, 189(Suppl 1), S19–S25. [DOI] [PubMed] [Google Scholar]
- Epstein J. I., Allsbrook W. C., Amin M. D., Egevad L. L., & ISUP Grading Committee. (2005). The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. American Journal of Surgical Pathology, 29, 1228–1242. [DOI] [PubMed] [Google Scholar]
- Faisal F. A., Sundi D., Cooper J. L., Humphreys E. B., Partin A. W., Han M., . . . Schaeffer E. M. (2014). Racial disparities in oncologic outcomes after radical prostatectomy: Long-term follow-up. Urology, 84, 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpap B., Egevad L. (2006). The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Archive: The European Journal of Pathology, 449, 622–627. [DOI] [PubMed] [Google Scholar]
- Iremashvili V., Soloway M. S., Rosenberg D. L., Manoharan M. (2012). Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. Journal of Urology, 187, 1594–1599. [DOI] [PubMed] [Google Scholar]
- Kim M., Choi S. K., Park M., Shim M., Song C., Jeong I. G., . . . Ahn H. (2016). Characteristics of anteriorly located prostate cancer and the usefulness of multiparametic magnetic resonance imaging for diagnosis. Journal of Urology, 196, 367–373. [DOI] [PubMed] [Google Scholar]
- Kryvenko O. N., Balise R., Soodana Prakash N., Epstein J. I. (2016). African-American men with Gleason score 3+3=6 prostate cancer produce less prostate specific antigen than Caucasian men: A potential impact on active surveillance. Journal of Urology, 195, 301–306. [DOI] [PubMed] [Google Scholar]
- Mohler J. L., Armstrong A., Bahnson R. R., D’Amico A. V., Davis B. J., Eastham J. A., Freedman-Cass D. A. (2016). Prostate cancer, version 1. Journal of the National Comprehensive Cancer Network, 4, 19–30. [DOI] [PubMed] [Google Scholar]
- Ozok H. U., Sagnak L., Tuygun C., Oktay M., Karakoyunlu N., Ersoy H., Alper M.(2010). Will the modification of the Gleason grading system affect the urology practice? International Journal of Surgical Pathology, 18, 248–254. [DOI] [PubMed] [Google Scholar]
- Powell I. J., Banerjee M., Sakr W., Grignon D., Wood D. P., Novallo M., Pontes E. (1999). Should African-American men be tested for prostate carcinoma at an earlier age than white men? Cancer, 85, 472–477. [PubMed] [Google Scholar]
- Powell I. J., Bock C. H., Ruterbusch J. J., Sakr W. (2010). Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in Black than in White American men, and influences racial progression and mortality disparity. Journal of Urology, 183, 1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. J., Canter D. J., Guzzo T. J., Brucker B. M., Bergey M., Sonnad S. S., Malkowicz S. B. (2009). Does race affect postoperative outcomes in patients with low-risk prostate cancer who undergo radical prostatectomy? Urology, 73, 620–623. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ortiz R. F., Troncoso P., Babaian R. J., Lloreta J., Johnston D. A., Pettaway C. A. (2006). African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer, 107, 75–82. [DOI] [PubMed] [Google Scholar]
- Sormani M. P. (2009). The Will Rogers phenomenon: The effect of different diagnostic criteria. Journal of the Neurological Sciences, 287 (Suppl 1), S46–S49. [DOI] [PubMed] [Google Scholar]
- Sundi D., Schaeffer E. M. (2014). Active surveillance for African-American men with prostate cancer: Proceed with caution. Con. Oncology, 28, 83–85. [PubMed] [Google Scholar]
- Sundi D., Faisal F. A., Trock B. J., Landis P. K., Feng Z., Ross A. E., Schaeffer E. M. (2015). Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology, 85, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundi D., Kryvenko O. N., Carter H. B., Ross A. E., Epstein J. I., Schaeffer E. M. (2014). Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in Black American men. Journal of Urology, 191, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundi D., Ross A. E., Humphreys E. B., Han M., Partin A. W., Carter H. B., Schaeffer E. M. (2013). African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: Should active surveillance still be an option for them? Journal of Clinical Oncology, 31, 2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareba P., Zhang J., Yilmaz A., Trpkov K. (2009). The impact of the 2005 International Society of Urological Pathology (ISUP) consensus on Gleason grading in contemporary practice. Histopathology, 55, 384–391. [DOI] [PubMed] [Google Scholar]


