Abstract
Men are underrepresented in obesity services, suggesting current weight loss service provision is suboptimal. This systematic review evaluated evidence-based strategies for treating obesity in men. Eight bibliographic databases and four clinical trials’ registers were searched to identify randomized controlled trials (RCTs) of weight loss interventions in men only, with mean/median body mass index of ≥30 kg/m2 (or ≥28 kg/m2 with cardiac risk factors), with a minimum mean/median duration of ≥52 weeks. Interventions included diet, physical activity, behavior change techniques, orlistat, or combinations of these; compared against each other, placebo, or a no intervention control group; in any setting. Twenty-one reports from 14 RCTs were identified. Reducing diets produced more favorable weight loss than physical activity alone (mean weight change after 1 year from a reducing diet compared with an exercise program −3.2 kg, 95% confidence interval −4.8 to −1.6 kg, reported p < .01). The most effective interventions combined reducing diets, exercise, and behavior change techniques (mean difference in weight at 1 year compared with no intervention was −4.9 kg, 95% confidence interval −5.9 to −4.0, reported p < .0001). Group interventions produced favorable weight loss results. The average reported participant retention rate was 78.2%, ranging from 44% to 100% retention, indicating that, once engaged, men remained committed to a weight loss intervention. Weight loss for men is best achieved and maintained with the combination of a reducing diet, increased physical activity, and behavior change techniques. Strategies to increase engagement of men with weight loss services to improve the reach of interventions are needed.
Keywords: obesity, behavioral issues, men’s health interventions
Introduction
Obesity increases the risk of many serious illnesses, such as coronary heart disease, type 2 diabetes, and osteoarthritis. Men with a body mass index (BMI) of 30 kg/m2 or more and waist circumference of 102 cm or greater have an increased risk of at least one symptom of impaired physical, psychological, or sexual function (Han et al., 2011; Prospective Studies Collaboration, 2009; Renehan, Tyson, Egger, Heller, & Zwahlen, 2008). Men appear more likely than women to misperceive their weight, less likely to consider their body weight a risk for health, and less likely to consider trying to manage their weight (Duncan et al., 2011; Gregory, Blanck, Gillespie, Maynard, & Serdula, 2008).
Men are underrepresented in weight loss research. A recent systematic review of male inclusion in randomized controlled trials (RCT) of weight loss reported that men made up only 27% of participants in RCTs, although the percentage rose to 36% when interventions were targeted at participants with obesity related comorbidities (Pagoto et al., 2012). In the U.S. National Weight Control Registry (NWCR; 2014) only 20% of participants are men. The average woman in the registry is aged 45 years and weighs 145 lbs, while the average man is 49 years of age and weighs 190 lbs (NWCR, 2014). Given the dominance of weight loss interventions that appear to target women, it is unsurprising that fewer men than women are recruited to weight loss services. Where men are recruited to mixed-sex programs, active components are often the same for both sexes despite a lack of understanding about whether men and women will respond differently to interventions, or an understanding of why interventions that are effective for men work well (Lovejoy & Sainsbury, 2009). For example, a recent systematic review conducted by Young, Morgan, Plotnikoff, Callister, and Collins (2012) concluded that men-only interventions may appeal to men but that more high-quality research in this area was required.
Similarly, men have been underrepresented in physical activity research, where interventions designed to increase physical activity have also largely targeted women (George et al., 2012; Kassavou, Turner, & French, 2013) and, consequently, may not appeal to men (Kassavou et al., 2013; Wong, Gilson, van Uffelen, & Brown, 2012). Increased weight has been reported to be a significant determinant of future physical inactivity (Golubic et al., 2013), and physical activity is recognized as being important for the prevention of obesity and other negative health outcomes (Biswas et al., 2015; Byberg et al., 2009). Physical activity also has a recognized role in lifestyle management programs aimed at promoting weight loss or preventing weight regain (National Institute for Health and Care Excellence, 2013a, 2013b). That men have been neglected in the field of physical activity research is particularly pertinent as, when men do attempt to manage their weight, they are more likely to use exercise as a weight management strategy than women (George et al., 2012).
It, therefore, seems likely that current weight loss and maintenance service provision is suboptimal for men. This systematic review of the evidence base for the management of obesity in men aimed to understand which interventions are effective for achieving long-term weight loss in men to inform service provision.
Method
This study is an update of one of six systematic reviews undertaken for the ROMEO (Review Of MEn and Obesity) project, a mixed-methods synthesis of evidence for weight loss management for men (Robertson et al., 2014; PROSPERO Number CRD 42011001479), which searched for evidence up to May 2012. All the reviews were undertaken according to a prespecified protocol (Robertson et al., 2014). The review presented here is an update of our original search strategy to identify RCTs of weight loss and weight management interventions for men only and considers evidence up to March 2014. Four additional reports, three of which are from two newly identified RCTs, are included in this review. Ethical approval was not required for this study.
Search Strategy
Highly sensitive searches of MEDLINE, MEDLINE-in-Process, and Other Non-Indexed Citations and Embase for a previous review of RCTs were updated (Avenell et al., 2004). Additional searches were run in CINAHL, PsycINFO, the Cochrane Library, and the Database of Abstracts of Reviews of Effects (latest search March 2014). No language restrictions were imposed on the searches. The example literature search strategy is provided in Appendix A. The full search strategies are available from the first author.
Types of Study
RCTs or quasi-randomized trials (including trials with a cluster design) with a mean or median duration of 52 weeks or more were considered. This duration of data follow-up was chosen to ensure that long-term weight loss and maintenance interventions were evaluated for their associated effects on weight and obesity-related comorbidities (Avenell et al., 2004).
Types of Participants
The types of participants included were men aged 16 years or more, with no upper age limit, with a mean or median BMI of ≥30 kg/m2 (or ≥28 kg/m2 with cardiac risk factors based on criteria for receiving orlistat; Avenell et al., 2004). Studies particularly examining men with obesity related to psychotropic medication, learning disability, or diagnosed eating disorder were excluded. Studies that recruited both men and women were excluded as these studies typically recruit far fewer men than women and interventions have typically been developed with women in mind.
Types of Interventions and Comparators
Interventions in the form of diet, physical activity, behavior change techniques, orlistat, or combinations of any of these, in any setting, were considered. Interventions of complementary therapy, for example, acupuncture or non-diet products promoted for weight loss available solely over the counter, were excluded, as were studies evaluating bariatric surgery, as only studies concerned with lifestyle management interventions were eligible for inclusion. Studies examining interventions for a combination of health-related conditions, for example, smoking cessation and weight loss at the same time, were also excluded.
Types of Outcomes
Studies had to explicitly mention weight loss or maintenance as a main aim to be eligible for inclusion. The following types of outcome were considered:
Primary outcome: Weight change
Secondary outcomes: Waist circumference; cardiovascular risk factors (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides, fasting glucose, glycosylated hemoglobin [HbA1c], systolic and diastolic blood pressure); disease-specific outcomes (e.g., diabetes); adverse events; quality of life outcomes; process outcomes.
Data Extraction Strategy
One reviewer (CR) independently screened titles and abstracts of all potentially relevant reports and extracted details of study design, methods, participants, interventions, and outcomes of the included studies. The data extraction was then checked by a second reviewer (AA) and any errors were corrected.
Quality Assessment Strategy
AA and CR assessed the methodological quality of the included RCTs using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins & Green, 2011). An adapted version of the Campbell and Cochrane Equity Methods Group checklist was used to assess the effect of interventions on disadvantaged groups and/or their impact on reducing socioeconomic inequalities (Ueffing et al., 2011). Any disagreements or uncertainty were resolved by discussion between the two reviewers. A third reviewer was not required to act as an arbitrator.
Data Analysis
Where data were suitable for inclusion in a meta-analysis, the authors imported data into Review Manager Software (Version 5.1) for data synthesis. For continuous outcomes, mean difference and risk ratio for dichotomous data, with 95% confidence intervals (CIs), are reported. Due to the inherent heterogeneity in studies of obesity interventions, random effects meta-analysis was used throughout. Visual inspection and the I2 statistic were used to assess heterogeneity in forest plots (Centre for Reviews and Dissemination, 2009). Planned funnel plot analysis to investigate reporting biases for forest plots was not possible owing to the limited number of studies. Methods reported in our previous technology assessment (Avenell et al., 2004) were used to derive weight changes and standard deviations, where missing, and are detailed in Appendix B.
Subgroup analyses were planned to explore whether the effectiveness of interventions differed for participants with newly diagnosed or preexisting obesity-related comorbidities (e.g., diabetes, hypertension) compared with those without. This was not possible owing to the limited quantity of data and heterogeneity of the studies. Sufficient data were not available to explore the effect of deprivation, age, and ethnicity on effectiveness; or to explore the effect of assumed values for weight on meta-analyses.
Results
Quantity of Evidence
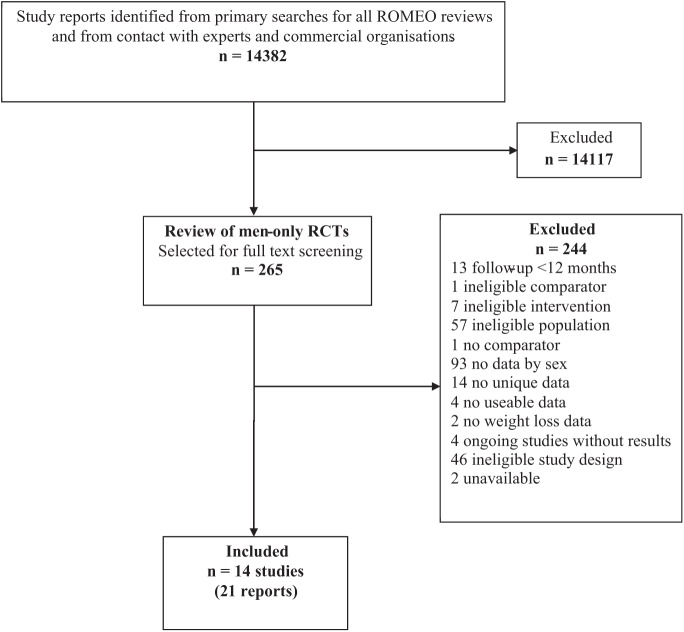
The primary literature search identified 14,382 potentially relevant titles and abstracts, from which 265 reports were selected for full text assessment. Of these, 21 reports (14 RCTs [Benassi-Evans, Clifton, Noakes, Keogh, & Fenech, 2009; Borg, Kukkonen-Harjula, Fogelholm, & Pasanen, 2002; Esposito et al., 2004; Hunt et al., 2014; Jeffery, Gerber, Rosenthal, & Lindquist, 1983; Khoo et al., 2011; King, Frey-Hewitt, Dreon, & Wood, 1989; Morgan, Lubans, Collins, Warren, & Callister, 2011; Patrick et al., 2011; Pavlou, Krey, & Steffee, 1989; Van Aggel-Leijssen, Saris, Hul, & van Baak, 2001; Wood et al., 1988; Wycherley, Brinkworth, Clifton, & Noakes, 2012] with seven linked reports [Collins et al., 2013; Fortmann, Haskell, & Wood, 1988; Jeffery, Bjornson-Benson, Rosenthal, Lindquist, & Johnson, 1984; Kukkonen-Harjula, Borg, Nenonen, & Fogelholm, 2005; Lejeune, Aggel-Leijssen, van Baak, & Westerterp-Plantenga, 2003; Lutze et al., 2013; Multiple Risk Factor Intervention Trial Research Group, 1982; Van Aggel-Leijssen, Saris, Hul, & van-Baak, 2002]) were eligible for inclusion. Details of the flow chart for the results of the literature search and a description of all the included trials are provided in Appendices C and D.
Six trials were conducted in the United States (Jeffery et al., 1983; King et al., 1989; Patrick et al., 2011; Pavlou et al., 1989; Wood et al., 1988). Four trials were carried out in Australia (Benassi-Evans et al., 2009; Khoo et al., 2011; Morgan et al., 2011; Wycherley et al., 2012); and studies conducted by Borg et al. (2002), Esposito et al. (2004), Hunt et al. (2014), and van Aggel-Leijssen et al. (2001) were conducted in Finland, Italy, the United Kingdom, and the Netherlands, respectively. The majority of trials investigated weight loss interventions. Two trials were conducted by the same authors and published in the same report (Pavlou et al., 1989). Only two investigated interventions for weight maintenance (Borg et al., 2002; King et al., 1989). Most men included in the trials were middle aged with a median (range) reported age of 46 years (36-62 years), weight of 112.7 kg (93.0-112.7 kg), and BMI of 32.4 kg/m2 (30.1-36.9 kg/m2). The mean reported participant retention rate for all trials was 78.2%, ranging from 44% to 100% retention.
Quality of the Evidence
Risk of bias
The assessments of risk of bias and equity and sustainability for the individual trials are provided in Appendix E.
Trials were of moderate quality with poor reporting of sequence generation and allocation concealment. Few reports gave details of the method of randomization. It is therefore not possible to judge the success of the randomization for these trials. Similarly, few authors used intention to treat analysis, choosing instead to present data for completers only both at baseline and for final outcome measurement. Equity and sustainability items, such as sociodemographic differences between withdrawals and exclusions, process measures or fidelity checks, were mostly not considered or reported.
Assessment of Effectiveness
Low Fat Reducing Diet With Behavior Change Techniques and Exercise Advice or Program Versus Control
Four studies examined low fat reducing diets, exercise, and behavior change training in comparison with a control group (Esposito et al., 2004; Hunt et al., 2014; Morgan et al., 2011; Patrick et al., 2011).
In the Self-Help, Exercise, and Diet using Information Technology (SHED-IT) trial by Morgan et al. (2011), men followed a free Internet-based weight loss program, with support and exercise advice for 3 months. The control group received an information booklet only. At 12 months, the internet group had lost more weight than the control group, mean difference of −2.2 kg (95% CI −5.7 to 1.3), but the difference in weight was not statistically significant. Change in erectile dysfunction of sexually active men was compared between groups; however, data were reported at 6 months only (Collins et al., 2013). Men in the intervention group reported significant improvements in erectile function, as measured by the International Index of Erectile Function-5 (IIEF-5) questionnaire, compared with the control group (mean difference 1.4 [95% CI 0.3 to 2.4], reported p = .018). Secondary analyses of only those sexually active men reporting dysfunction at baseline also identified a significant intervention effect (mean difference 4.2 [95% CI 1.7 to 6.6], reported p = .004). It should be noted that whether this beneficial effect remained at 12 months is unknown.
Patrick et al. (2011) also used the internet to deliver dietary and physical activity advice and behavior change training. Their intervention was developed by interviewing male weight loss experts and holding focus groups with men described as overweight. This resulted in an intervention that was individualized, fact-based, flexible, simple to understand, and used “business-like” language. Pedometers were provided to encourage physical activity and were enjoyed by the men for their novelty and assistance with self-monitoring their behavior. Men in the control group were given access to a website detailing general male-related health advice that was unlikely to lead to lifestyle changes that would promote weight loss (e.g., dealing with stress, hair loss, and worksite injury prevention). Men receiving the weight loss intervention lost more weight than the control group, but differences between groups at 12 months were also reported as not significant (mean −0.9 kg [SD 6.17] vs. −0.2 kg [SD 5.97] between group difference −0.69 kg [−1.52, 0.14], reported p = .101).
Hunt et al. (2014) developed the Football Fans In Training (FFIT) intervention to appeal to men in terms of the context of traditionally male-dominated football (soccer) clubs. The intervention included a simple presentation of the science of weight loss and a style of delivery that encouraged male banter (joshing). The authors reported that delivering the intervention in a humorous way facilitated the discussion of sensitive subjects among the men. Men were recruited through 13 professional soccer clubs and were randomized to receive either the FFIT weight loss program or to a waiting list control group, who received FFIT 12 months later. Men in the FFIT program attended 12 weekly sessions at their club training ground where they received personalized dietary, healthy eating, and behavior change advice, followed by structured exercise classes delivered by the club’s community coaches. Men were also encouraged to increase their walking activity gradually through the use of pedometers and were taught behavioral change techniques that are known to be effective at promoting improvements in physical activity and weight loss (e.g., goal setting and self-monitoring). The 12-week active phase was followed by a weight maintenance phase, which comprised six post-program email prompts over 9 months and a group reunion at 9 months after pre-program baseline measurements. The attrition rate was low, with 89% of the men who were randomly allocated to undertake the FFIT program and 95% of those allocated to the control waiting list arm remaining in the trial at 12 months.
After adjusting for baseline weight and club, the mean difference in weight loss was 4.9 kg (95% CI −5.9 to −3.9, reported p < .0001) and 4.4% (95% CI −3.6 to −5.1, p < .0001) for percentage weight loss at 12 months, both in favor of the FFIT intervention. Changes in cardiovascular risk factors and some measures of psychological health and physical, but not mental, quality of life were also significantly in favor of the intervention. Participants in the FFIT trial were provided with vouchers for their football club shop to the value of £40 for trial completers and £20 for dropouts if they provided 12-month measurements. Whether this incentive impacted on the effectiveness of the intervention is unclear.
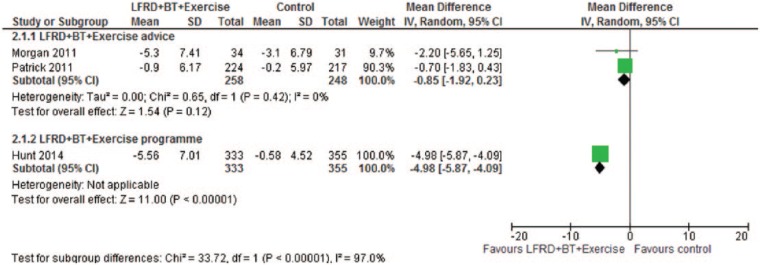
The 12-month results from these three RCTs, where the intervention comprised a low fat reducing diet, behavior change techniques, and either structured exercise advice or an exercise program compared with a control, were pooled in a meta-analysis to establish the overall effect (see Figure 1). Only the FFIT trial (Hunt et al., 2014) reported a significant effect in favor of the intervention. The I2 statistic of 97% indicates the presence of marked statistical heterogeneity, probably relating to differences between internet and football venue settings.
Figure 1.
Effect of low fat reducing diet with behavior change training and exercise versus control at 12 months.
A further trial by Esposito et al. (2004) examined a low fat reducing diet, behavior change techniques, and advice for increasing physical activity. The men were recruited because they were obese and had erectile dysfunction (determined by a score of 21 or less on the IIEF-5; Rosen et al., 1997). The men met in groups but received advice tailored to their individual requirements. The control group received general oral and written advice regarding healthy food choices and exercise at baseline. Results are not presented in the meta-analysis in Figure 1 due to differences in timing of outcome measurement. At 2 years, the intervention group had lost significantly more weight with a mean difference of −13 kg (95% CI [−18, −11], reported p = .007) and reported statistically significant improvements for cardiovascular risk factors compared with the control group. Esposito et al. (2004) also reported that 17/55 men in the intervention group compared with 3/55 in the control group reported an International Index of Erectile Dysfunction score of 22 or higher, indicating regained sexual function at 2 years (reported p = .001).
Exercise Versus Diet Versus Control
Wood et al. (1988) compared men in an exercise program with men in an energy-reducing diet and a control group. Men in the exercise program participated in supervised exercise three times per week in 1-hour sessions. Exercise activities included calisthenics, muscle stretching, brisk walking, and jogging. Men in the diet group had a daily deficit of 300 to 500 kcal/day and made no alteration to their level of physical activity. The control group made no change to either their diet or levels of physical activity.
At 1 year, the authors reported significant differences in weight in favor of the exercise and diet groups versus the control group (mean difference of −4.6 kg [95% CI −6.2 to −3.0] and −7.8 kg [95% CI −9.4 to −6.2], reported p < .01). Both exercise and diet groups significantly lowered their triglyceride levels and improved their HDL cholesterol compared with the control group (reported p < .05 and p < .01, respectively). Participants in the diet group lost significantly more weight than those in the exercise group, producing a mean difference in weight of −3.2 kg (95% CI −4.8 to −1.6, reported p < .01).
Diet and Exercise Program Versus Diet Only
Calorie reducing diet and exercise program versus calorie reducing diet only
Two trials reported the effect of adding an exercise program to a calorie reducing diet compared with a calorie reducing diet only (Pavlou et al., 1989; Van Aggel-Leijssen et al., 2001). In the first trial, men followed a very low energy (500 kcal/day) formula diet for 6 weeks (Van Aggel-Leijssen et al., 2001). For Weeks 7 to 8, men consumed 330 kcal/day of the formula diet and 840 kcal/day from foods of their choice. During Weeks 9 to 10, men consumed 170 kcal/day of the formula diet and 1170 kcal/day from their chosen food. The men were then instructed to stabilize their body weights for Weeks 11 to 12. Men in the diet and exercise group followed the same dietary pattern but also participated in a low-intensity exercise program (40% VO2max) for 12 weeks, which was then continued to Week 52. The men trained four times per week in 1-hour sessions. Three of these sessions were supervised by a personal trainer in the research laboratory and the other session was unsupervised at home. The exercise sessions consisted of cycling, walking, and aqua-jogging. Attendance for supervised exercise sessions was 57% (SD 20%). Two of the men in the exercise group had to withdraw from the study due to knee injuries. At 12 months, men in the diet and exercise group did not lose as much weight as men in the diet only group (mean difference 4.2 kg, 95% CI −1.5 to 9.9; Lejeune et al., 2003).
In their main trial, Pavlou et al. (1989) investigated the effects of adding an exercise program to a variety of different diets ranging from 420 to 1000 kcal/day, including one very low carbohydrate diet, over 8 to 12 weeks, with long-term follow-up in a group of public sector workers in a police department. Combining results for all diet groups, the effect of adding exercise to diet was highly significant at 18 months (mean difference −7.6 kg, 95% CI −10.3 to −4.9) and at 36 months (mean difference −8.2 kg, 95% CI −15.3 to −1.2). There were no significant differences in weight lost between any of the types of calorie reducing diets at 18 or 36 months. At 18 months, systolic (mean difference −8.90 mmHg, 95% CI 13.7 to −4.2) and diastolic (mean difference −12.1 mmHg, 95% CI −15.2 to −9.0) blood pressure were significantly lower in the diet and exercise groups compared with the diet only groups.
Types of Calorie Reducing Diet Compared
The pilot trial conducted by Pavlou et al. (1989) and three other trials (Benassi-Evans et al., 2009; Khoo et al., 2011; Wycherley et al., 2012) examined varying the protein, carbohydrate, and fat proportions of reducing diets, or examined a more stringent initial calorie prescription of 900 kcal for 8 weeks followed by a 600 kcal/day deficit to 600 kcal/day deficit alone. None of the trials identified significant differences between the different dietary approaches after 12 months.
Group Versus Individual Monetary Contracts
The trial conducted by Jeffery et al. (1983) recruited men to a 15-week financial incentive intervention for weight loss with a goal of achieving a total weight loss of 30 lbs (13.6 kg). Using a factorial design, men in the trial were randomized to pay monetary deposits of US$30, $150, or $300 and to either a group or an individual contract. Men in the individual contract groups received refunds based on individual weight loss, while those with group contracts were refunded based on the mean weight loss of their group. Group contracts produced significantly more weight loss than individual contracts both at 1 and 2 years (reported p < .05). The size of contract did not have a significant effect at 1 year (Jeffery et al., 1983) or 2 years (Jeffery et al., 1984).
Weight Maintenance
Calorie reducing diet and exercise versus diet for weight maintenance
The trial conducted by Borg et al. (2002) examined whether adding walking or resistance training to a diet compared with diet alone improved weight maintenance following a weight reduction period. During the weight reduction period, participants followed a very low calorie diet of 500 kcal/day for 2 months. The mean weight loss at the end of the weight reduction period was 14.2 kg. Participants were then randomized to follow a low fat diet of 1200 kcal/day only; or to diet and walking; or diet and resistance training exercise groups. Exercise sessions were held three times a week and lasted 45 minutes, each aiming to expend 300 to 400 kcal per session. No statistically significant differences between any of the groups were reported for weight after 31 months, apart from HDL cholesterol and waist to hip ratio, which were better in the resistance training than the walking group.
Behavior change techniques for weight maintenance versus control
The trial conducted by King et al. (1989) randomized men from the diet only or exercise only arms at the end of the 1-year Wood trial (Wood et al., 1988). Men were randomized within their original intervention groups to receive behavioral change techniques or a control. The behavioral change techniques comprised monthly mailed information packs including a supportive letter, list of coping strategies for problems relevant to their original intervention, for example, holiday eating for dieters or finding time to engage in physical activity for exercisers. The men were telephoned regularly to discuss any concerns or questions related to their problem areas and were weighed at 6-monthly intervals. Men in the control group were given written information about their original weight loss method from the Wood trial (Wood et al., 1988) at the start of the weight maintenance period. The men received no other contact apart from the 6-monthly weight assessments.
The behavior change techniques produced greater weight maintenance success for the exercise only group compared with the control group than it did for the diet only group. After 1 year, exercisers who received the behavioral intervention had significantly lower weight than controls (−3.10 kg, 95% CI −5.0 to −1.2). Dieters in the behavioral intervention group were not significantly different from controls after 1 year (0.60 kg, 95% CI −1.3 to 2.5).
Discussion
Results from this systematic review should be treated with caution due to the limited number of trials, and thus limited statistical power. Nevertheless, the evidence indicates that weight reduction for men is best achieved through a combination of a reducing diet, physical activity advice, or an activity program and behavior change training. The high trial participant retention rates indicate that, although it might be harder to attract men to join weight loss programs than for women, once engaged, men will commit to the program. It is therefore important that programs are appealing to men to promote the effectiveness of interventions.
Tailoring the style of delivery could be as important as the content of the intervention, with men preferring simple, fact-based language with individual feedback (Hunt et al., 2014; Morgan et al., 2011; Patrick et al., 2011). A preference for individualized interventions and personal goals has also been reported in the physical activity literature (George et al., 2012; Newton, Griffith, Kearney, & Bennett, 2015). It could be that tailoring interventions for individual requirements or preferences offers men a greater sense of personal control than interventions that lack this personalized element, and this may appeal to men more than to women (Robertson et al., 2014). The inclusion of a physical activity element could also increase the appeal of interventions for men (Patrick et al., 2011). The trial by Borg et al. (2002) did not clearly demonstrate that the type of physical activity was important for weight maintenance. Interventions situated in sporting contexts, such as the FFIT trial (Hunt et al., 2014), may particularly encourage engagement through the association of long-standing loyalty, commitment, and camaraderie attained from collectively supporting a sports team. The motivation for supporting a team could consciously or subconsciously become associated with the motivation to lose weight with fellow team supporters. It is also possible that the sense of belonging and cohesiveness of the group was influential (Hoddinott, Allan, Avenell, & Britten, 2010). The success of this trial may be limited to men who enjoy physical activity or have a keen interest in sport. How best to engage men who are not sports fans, or prefer more sedentary activities, is still a challenging topic that should be addressed. Health benefits associated with weight loss could also help motivate men to lose weight, for example, the potential benefit on erectile function is not well known to men.
Trials of group interventions produced beneficial weight loss results. This is in keeping with findings of a systematic review comparing group and individual treatments for obesity in both men and women, which also reported that group-based interventions were more effective than interventions delivered to individuals only, although the reviewed population was predominantly female (Paul-Ebhohimhen & Avenell, 2009). Men tend to be reluctant to join groups (Jolly et al., 2011), and it may be important to ensure that groups are designed and tailored specifically to attract, engage, and retain men; however, very few trials reported that they had consulted men during the development of their interventions. Providing men with the opportunity to attend programs in men-only groups, in settings where they feel comfortable and are able to discuss individual concerns and receive individualized advice, could enhance men’s engagement with weight loss services (Robertson et al., 2014). The use of humor in groups can also encourage men to discuss sensitive or personal issues (Hunt et al., 2014), although humor should be used carefully to ensure issues or concerns are not trivialized as this can have an alienating effect (Paula Carroll, Men’s Health Forum Ireland, December 4, 2012, personal communication).
The strengths of this study are the systematic and rigorous methods taken to review the evidence. Despite these efforts, very few eligible studies were identified. Furthermore, data on men from deprived areas, ethnic minorities, or men who were unemployed, younger, disabled, gay, bisexual, transgender, and other minority groups were lacking. It therefore remains unclear what types of interventions or engagement strategies work best with hard to reach men or men from minority groups. Similarly, it is unclear whether the sex of the intervention provider contributes to intervention engagement and/or effectiveness. Future research should address these areas of uncertainty, while gathering information on patient reported quality of life and clinical and economic outcomes to assess the full value of an intervention other than amount of weight lost. Future research is also required to develop effective weight maintenance interventions to prevent men regaining weight in the long-term following successful weight loss.
Acknowledgments
We thank the Men’s Health Forums of Scotland, Ireland, England, and Wales, especially Tim Street, Paula Carroll, Colin Fowler, and David Wilkins. We also thank Kate Jolly for information about the Lighten Up trial.
Appendix A
Example Search Strategy
MEDLINE 1948 to March 14, 2014
MEDLINE In-Process & Other Non-Indexed Citations March 14, 2014
Embase 1974 to 2014 Week 13
Ovid multifile search: http://shibboleth.ovid.com/
obesity/
(obesity adj2 (morbid or diabet$)).tw.
obesity, morbid/ use prmz
morbid obesity/ use oemezd
obes$.tw.
weight loss/ use prmz
weight reduction/ use oemezd
(weight adj1 (los$ or reduc$ or maint$ or control or manag$)).tw.
(diet adj5 weight).tw.
overweight.tw.
(obesity adj1 management).tw.
(anti obesity or antiobesity).tw.
or/1-12
exp clinical trial/
Randomized Controlled Trials as Topic/
randomized controlled trial.pt.
controlled clinical trial.pt.
randomization/ use oemezd
randomi?ed.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/14-23
exp animals/ not humans/
25 not 26
13 and 27
(letter or editorial or comment or note).pt.
28 not 29
limit 30 to (“all infant (birth to 23 months)” or “all child (0 to 18 years)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)” or “adolescent (13 to 18 years)”)
30 not 31
limit 30 to (embryo or infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
30 not 33
32 or 34
limit 35 to yr=“2001 -Current”
36 not ((women not men) or (female not male)).tw.
36 and male/
37 or 38
Appendix B
Statistical Methods for Estimation of Standard Deviation of Change in Weight and Estimation of Standard Deviation of Change in Risk Factors
Statistical Methods for Estimation of Standard Deviation of Change in Weight
Introduction
The following provides an equation for deriving the standard deviation for the change in weight from baseline given the absolute value of the mean change in weight since baseline.
Method
Summary statistics were provided from a series of trials representing 62 trial-treatment combinations of which four had no data. A linear regression was made of the standard deviation of the mean change on the absolute mean change for weight.
Results
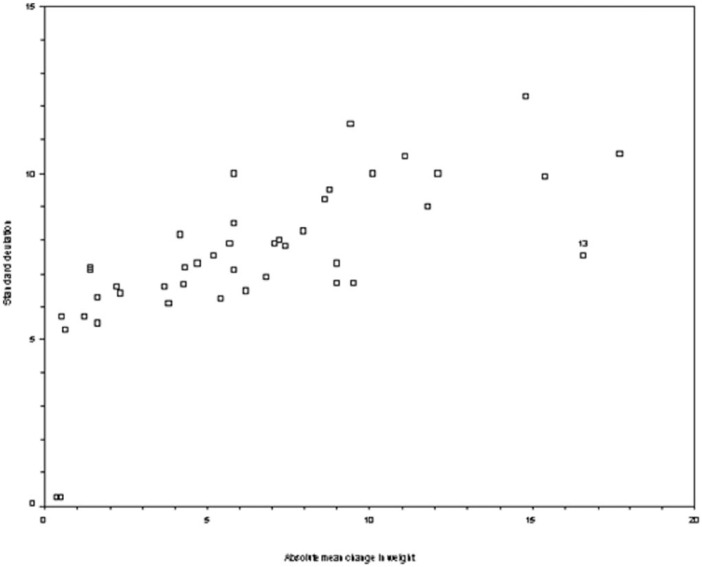
Of the 58 trial-treatment combinations, 43 reported both the mean change and the standard error of the mean change in body weight from baseline to the end of the first treatment phase while 8 only reported the mean and 7 reported neither. The plot of standard deviation by the absolute value of the mean change (see scatter plot below) shows two points where both the absolute mean and the standard deviation of the mean are close to zero, both were excluded from the linear regression giving n = 41. The linear regression was also repeated with Observation 13, which was influential, excluded to see of the regression coefficients changed.
Discussion
The results from the two linear regressions were similar. Diagnostic plots (not shown) suggested the regression could be improved by allowing for the increase in variation of the standard deviation with increasing mean; however, this is unlikely to change the results.
Conclusion
When the mean change in weight since baseline (mean) is known but its standard deviation (SD) is unknown then use the following equation to derive the standard deviation of the mean change:
SD = 5.915 + 0.283 * absolute (mean)
Table 1.
Summary Statistics and the Equations for the Predicted Values of the Standard Deviations of the Two Linear Regressions.
| N | R 2 | Constant | Slope | ||||
|---|---|---|---|---|---|---|---|
| 41 | 53.7% | SD | = | 5.915 | + | 0.283 | * abs(mean) |
| 40 | 63.4% | SD | = | 5.694 | + | 0.328 | * abs(mean) |
Figure 1.
Scatter plot of the standard deviation of the mean change in weight by the absolute mean change in weight. Observation 13 is labeled.
Statistical Methods for Estimation of Standard Deviation of Change in Risk Factors
Estimation of Standard Deviation of Change in Blood Pressure E
Introduction
The following short report describes the derivation of an equation for the standard deviation for the change in blood pressure (BP) from baseline given the mean change in blood pressure since baseline. Both systolic and diastolic blood pressures were available.
Method
Summary statistics were provided from a series of trials representing 96 trial-treatment-BP combinations. A linear regression was made of the standard deviation of the mean change on the absolute mean change for both systolic and diastolic data.
Results
Of the 96 trial-treatment-BP combinations (46 systolic BP and 50 diastolic BP), 51 (25, 26) reported both the mean change and the standard error of the mean change in BP from baseline to the end of the first treatment phase while 12 (6, 6) only reported the mean and 33 (15, 18) reported neither.
The plot of standard deviation by the absolute value of the mean change showed the systolic and diastolic data to be sufficiently different not to warrant a joint regression model. The systolic data showed greater variation among their standard deviations. One study reported three diastolic absolute means and the standard deviation of the mean that were close to zero and they were excluded, linear regression giving n = 25 for systolic and n = 23 for diastolic blood pressure.
Systolic
The absolute mean had no effect on the standard deviation. The overall mean for the standard deviation is reported below.
Diastolic
The absolute mean had no effect on the standard deviation. When two influential points were excluded there was no change in result. The overall mean for the standard deviation is reported below.
Discussion
Only over half of the trial-treatment-BP combinations were available for use in the regression models. Of the remaining 45, 33 had data on both the mean and standard deviation of the mean at the two time points available. Standard deviations for the change could be derived if some assumptions on correlation were made, possibly based on the 9 observations, where all three standard deviations were available.
Conclusion
Standard deviation of the mean change in systolic BP, use 12.7mmHg. Standard deviation of the mean change in diastolic BP, use 8.3mmHg.
Table 2.
Summary Statistics for the Mean Standard Deviation of the Mean Change in Blood Pressure.
| N | Minimum | Maximum | Mean | Standard Deviation | |
|---|---|---|---|---|---|
| Systolic | 25 | 6.80 | 23.97 | 12.7070 | 4.0164 |
| Diastolic I | 23 | 5.60 | 14.75 | 8.2958 | 2.1794 |
| Diastolic II | 21 | 5.60 | 9.40 | 7.7549 | 1.2773 |
Diastolic II was based on removing two influential data points.
Estimation of Standard Deviation of Change in Fasting Lipids and Plasma Glucose Level Control
Introduction
The following short report describes the derivation of an equation for the standard deviation for the change in total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, fasting plasma glucose, and HbA1c from baseline given the mean change since baseline.
Method
Summary statistics were provided from a series of trials representing 208 trial-treatment-blood measure combinations from 50 trial-treatment combinations. The relationship between the absolute mean change and the standard deviation of the mean change was examined for six types of blood measure: total cholesterol from 44 trial-treatment combinations, LDL from 30, HDL from 42, TGs from 42, fasting glucose from 30, and HbA1c from 20. The relationship could be affected by whether participants were diabetic or nondiabetic, in particular for fasting glucose and HbA1c.
The following analysis was done for each blood measure:
Plot of the number of observations versus the standard deviation.
Summary statistics for the standard deviation by treatment.
Where the SD varied with study size, summary statistics stratified by study size.
Results
The plots suggested that the standard deviations were quite stable, but below a threshold there were cases where some of the standard deviations were greater as the number of participants fell. The threshold varied for each measure. Causes for this were not reviewed.
Table 3.
Summary statistics for the mean standard deviation of the mean change in fasting lipids and plasma glucose level control.
| Blood Measure | Mean SD | Median SD | Details |
|---|---|---|---|
| HDL | 0.29 | 0.24 | Mostly below 0.4 except for five between 0.4 and 0.6 when n < 100 |
| LDL | 0.74 | 0.71 | No relationship with n |
| TGs | 0.96 | 0.81 | Mostly below 1.5 except for four between 1.5 and 3.5 when n < 50 |
| Cholesterol | 1.08 | 0.83 | A narrow band of SDs. One outlier. Four higher SDs, three from small trials (n = 30) and one (n ~ 100) |
| Fasting glucose | 2.43 | 1.42 | Clear threshold effect. One outlier (a possible typographic error). Two high values for two large studies (n ~ = 350). Most SDs below 2. |
| 3.11 | 3.49 | Where n <30 | |
| 1.98 | 0.95 | Where n ≥ 30 | |
| HbA1c | 1.96 | 1.60 | Clear threshold effect. SDs increase rapidly when n < 30. |
| 2.70 | 2.10 | Where n < 30 | |
| 0.76 | 0.66 | Where n ≥ 30 |
Discussion
The effect of study size needs to be reviewed when estimating standard deviations. For the blood lipids this appears to make little difference and either the mean or median standard deviation could be used. Erring on the side of caution would suggest using the mean value. There is, however, a study size effect for glucose and HbA1c. The possibility of using the stratified SDs should be considered.
The cause of the effect of the number of observations was not reviewed. The main candidate would be treatment. Plots were reviewed but there are numerous treatments and no clear way to group them.
Appendix C
Flow Chart of the Number of Potentially Relevant Reports and the Numbers Subsequently Included and Excluded From the Review.
Appendix D
Characteristics of the Included Trials.
| Study ID | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|
| Benassi-Evans 2009 |
Location: One nutrition clinic in Adelaide, Australia Period of study: NR Inclusion criteria: Male; age 20-65 years; BMI 27-40; at least one cardiovascular disease risk factor other than obesity Exclusion criteria: History of metabolic or coronary disease, diabetes 1 or 2 Age, years: mean (SEM): a: 54.94 (1.17), b: 52.94 (1.5) BMI: mean (SEM): a: 32.42 (0.79), b: 31.47 (0.96) Weight, kg: mean (SEM) a: 99.84 (2.45); b: 99.58 (3.61) Baseline comparability: Yes |
Description of interventions: a: High protein (red meat) diet comprising 35% protein, 40% carbohydrate, 25% fat, 7MJ with some adjustment in energy to achieve approximate weight loss of 1 kg per week. b: High carbohydrate diet comprising 17% protein, 58% carbohydrate, 25% fat, 7MJ with some adjustment in energy to achieve approximate weight loss of 1 kg per week. Timing of active intervention: a + b: 0 to 12 weeks intensive weight loss with fortnightly clinic visits followed by monthly weight maintenance visits up to 1 year Number of times contacted: a + b: 15 Number allocated: a: 16; b: 17 Completed: a: 16; b: 17 % dropout: 0% Number assessed: a: 16; b: 17 |
Length of follow-up: 1 year Outcomes: Weight |
|
|
Borg 2002
Kukkonen-Harjula 2005 |
Location: Single research clinic, Finland Period of study: NR Inclusion criteria: Aged 35-50 years, BMI 30-40 kg/m2, waist circumference >100 cm, clinically healthy other than obesity Exclusion criteria: Regular medication, participation in leisure time exercise more than twice weekly, smoker, resting BP > 160/105 mmHg Age, years: mean (SD) a + b + c: 42.6 (4.6) BMI: (after 2 months VLCD and prior to randomization) mean (SD): a: 33.1 (2.7); b: 33.3 (2.8); c: 32.4 (2.4) Weight, kg: mean (SD) a + b + c: 106.0 (9.9) Baseline comparability: Yes |
Description of interventions: All men participated in a 2 month weight reduction program consisting of a very low energy diet of 2 MJ per day for 8 weeks followed by a low energy diet of 5 MJ per day. Men attended small group meetings led by a nutritionist weekly. Men were then randomized to groups a, b, and c: a: Control: Men advised not to increase their physical activity. b: Walking: An exercise instructor supervised 1 group training session per week; 10 minute warm-up + 45 minutes training + 5 minute cool down. Heart rate monitors used to ensure target training intensity of 60% to 70% maximum oxygen consumption. Energy expenditure per exercise session equal to 1.7 MJ. c: Resistance exercise: An exercise instructor supervised 1 group training session per week; 10 minute warm-up + 45 minutes training + 5 minute cool down. Resistance load set at 60% to 80% of 1 repetitive maximum with 8 repetitions and 3 sets in each exercise. |
Length of follow-up: 21 months Outcomes: BMI, WHR, LDL and HDL cholesterol; systolic and diastolic BP; fasting plasma glucose |
|
| Each session included 6 exercises aimed at large muscle groups. Energy expenditure per exercise session equal to 1.2 MJ. Men continued to meet weekly in small groups in their intervention group throughout the 6-month weight maintenance phase. Men in all groups were given the same instruction to follow ad libitum a high-carbohydrate, low-fat diet and received written material. No special instructions for diet or physical activity were given during follow up after the weight maintenance period. Timing of active intervention: 6 months (preceded by 2 month pre-treatment phase) Number of times contacted: a: 24 times, weekly for 6 months; b and c: 96 times, with exercise sessions up to 3 times per week for 6 months Number allocated: a: 30; b: 30; c: 30 Completed: a: 22; b: 20; c: 26 % dropout: a: 27%; b: 33%; c: 13% Number assessed: a: 22; b: 20; c: 26 |
||||
| Esposito 2004 |
Location: One University Hospital, Naples, Italy Period of study: 2000 to 2003 Inclusion criteria: Aged 35 to 55 years with erectile dysfunction; International Index of Erectile Function (IIEF-5) score <22; no participation in diet reduction programs within previous 6 months; sedentary (<1 hour per week physical activity); BMI ≥30 |
Description of interventions: a: General oral and written advice regarding healthy food choices and exercise given at baseline. b: Group sessions led by a nutritionist and exercise trainer providing individually tailored advice giving detailed advice about reducing calorie intake, goals setting and self-monitoring to achieve a 10% reduction in body weight. Dietary advice and guidance for increasing physical activity tailored to each individual man. Behavioral and psychological counselling offered. Diet composition per 1,000 kcal comprised carbohydrate 50% to 60%, protein 15% to 20%, total fat <30%, fiber 18 g. |
Length of follow-up: Two years Outcomes: Weight, BMI, total cholesterol, HDL-C, triglycerides, systolic and diastolic BP, erectile function, glucose |
The trial objective was to determine the effect of weight loss and increased physical activity on erectile function in obese men. |
|
Exclusion criteria: Diabetes mellitus/impaired glucose tolerance; impaired renal function/macroalbuminuria; pelvic trauma; prostatic disease; peripheral neuropathy; hypertension; cardiovascular disease; psychiatric problems; drug/alcohol abuse; taking medication for erectile dysfunction. Age, years: mean (SD) a: 43 (5.1); b: 43.5 (4.8) BMI: mean (SD) a: 36.4 (2.3); b: 36.9 (2.5) Baseline comparability: Yes |
Timing of active intervention: a + b: Two years—monthly visits for year 1, bimonthly visits for year 2. Number of times contacted: a + b: 18 Number allocated: a: 55; b: 55 Completed: a: 52; b: 52 % dropout: a: 5.5%; b: 5.5% Number assessed: a: 55; b: 55 |
|||
| Hunt 2014 |
Location: Thirteen SPFL clubs, UK Period of study: June 2011 to June 2012 Inclusion criteria: Aged 35-65 years; BMI ≥28; completed physical activity readiness questionnaire; consented to weight, height and waist measurements; not previously participated in FFIT. Exclusion criteria: Participants with systolic BP ≥160 mmHg and diastolic BP ≥100 mmHg were excluded from intense physical activity during the program. Age, years: mean (SD) a: 47.2 (7.89); b: 47.0 (8.07) BMI: mean (SD) a: 35.1 (4.8); b: 35.5 (5.1) Baseline comparability: Yes |
Description of interventions: a: Waiting list: A comparison group were randomized to receive the intervention 12 months later b: Gender-sensitized weight management, physical activity and health living program (Football Fans In Training, FFIT) based on control theory and components of behavioral change techniques. Men attended 12 weekly classroom-based group discussions held at the men’s SPFL club training grounds where men received personalized advice on diet (portion control and healthy eating) to suit individual circumstances and preferences. Following these discussions men engaged in pitch side/in stadia structured aerobic, muscle strengthening, and flexibility exercise training sessions which were tailored to individual fitness and abilities. Weekly sessions lasted 90 minutes. Outside the weekly sessions men were given an incremental walking program. Pedometers were issued to the men to monitor individual daily goals and progress was reported at the weekly meetings. Men were encouraged to supplement walking with more strenuous activity if they were able and to meet up outside the program to train together. Aimed to achieve 45 minutes of moderate physical activity most days. Avoidance of behaviors that would undermine weight loss was also encouraged. |
Length of follow-up: 1 year Outcomes: Mean weight change; BMI; waist circumference; adverse events; change in self-reported physical activity and dietary habits; psychological and HRQOL. |
|
|
Timing of active intervention: 12 weeks Number allocated: a: 374; b: 374 Completed: a: 355; b: 333 % dropout: a: 5.1%; b: 11% Number assessed: a: 374; b: 374 |
||||
|
Jeffery 1983
Jeffery 1984 |
Location: One university research center, Minnesota, USA Period of study: 1974 to 1975 Inclusion criteria: Aged 35 to 75; self-reported body weight >30lbs (13.6kg) above ideal weight Exclusion criteria: Uncontrolled diabetes, heart disease, concurrent dietary or psychological treatment, self-report <6 alcoholic drinks per day. Age, years: Mean; a (i) 52.0, (ii) 53.8, (iii) 52.4; b (i) 54.1, (ii) 50.5, (iii) 53.8 BMI: Mean; a (i) 30.5, (ii) 31.8, (iii) 32.8; b (i) 31.0, (ii) 32.3, (iii) 32.7 Weight, kg: Mean a (i) 93.07, (ii) 99.38, (iii) 104.83; b (i) 96.17, (ii) 102.87, (iii) 107.86 Baseline comparability: Yes |
Description of interventions: All groups participated in a 15-week educational program emphasizing reduced eating and increased exercise equally. Three levels of monetary deposit were made at the first meeting. a: Individual monetary contracts (i) $30 (ii) $150 (iii) $300 b: Group monetary contracts (i) $30 (ii) $150 (iii) $300 Refunds at a rate of $1, $5, or $10 per pound, up to a maximum cumulative weight loss of 2 lbs per week. Individual contracts based on individual weight loss. Group contracts based on average group weight loss Timing of active intervention: 0 to 15 weeks Number of times contacted: a + b 16 Number allocated: a: (i) 16 (ii) 15 (iii) 14 b: (i) 17 (ii) 14 (iii) 13 Completed: a: (i) 16 (ii) 14 (iii) 14 b: (i) 17 (ii) 13 (iii) 12 |
Length of follow-up: 2 years Outcomes: Weight |
One year data reported in Jeffery 1983. Two year data reported in Jeffery 1984. |
|
% dropout: a: 2.2%; b: 4.5% Number assessed: a: (i) 16 (ii) 15 (iii) 14 b: (i) 17 (ii) 14 (iii) 13 |
||||
| Khoo 2011 |
Location: Community, Adelaide, Australia Period of study: June 2007 to May 2008 Inclusion criteria: BMI >30; waist circumference ≥102 cm, type 2 diabetes mellitus, HbA1c on diet or oral medication stable for 3 months ≤7% Exclusion criteria: Smoker, previous or current treatment for sexual problems or LUTS, glomerular filtration rate <60 mL/min, alcohol >500 g/w in previous 12 months Age, years: mean (SD) a: 58.1 (11.4); b: 62.3 (5.9) BMI: NR Weight, kg: mean (SD) a: 112.7 (19.2); b: 109.6 (14.9) Baseline comparability: Poorer IIEF-5 score in a. |
Description of interventions: a: Low calorie diet: Total intake of 900 kcal/day from 2 liquid meals (Kicstart, Pharmacy Health Solutions) replacements consumed daily, providing a maximum of 450 kcal, 0.8 g protein per kg of ideal body weight and the recommended daily allowance of vitamins, minerals, and omega 3 and 6 fatty acids, plus one other small meal to give a total of approximately 900 kcal/day. After 8 weeks men changed to follow b: b: High protein, low fat diet: Daily energy reduction of approximately 600 kcal/day. Daily consumption of 300 g lean meat, poultry or fish, 3 servings of cereals/breads and low-fat dairy and 2 servings of fruit and vegetables. All men received a written plan with diet information, menu plan, recipes and advice for cooking and eating out and all maintained their usual daily activity levels. Timing of active intervention: a + b 1 year Number of times contacted: a + b 16 to 29 Number allocated: a: 19; b:12 Completed: a: 9; b:7 % dropout: a: 52.63%; b:41.67% Number assessed: a: 9; b: 7 |
Length of follow-up: 1 year Outcomes: Weight, waist circumference, erectile function, adverse events |
|
|
Morgan 2011
Collins 2013 |
Location: One university center, University of Newcastle, Australia Period of study: 2007 to 2008 Inclusion criteria: BMI 25-37 kg/m2 Exclusion criteria: History of major medical problems preventing physical activity, recent weight loss ≥4.5 kg, taking medications that might affect body weight. |
Description of interventions: a: Researcher delivered one 60 minute face-to-face weight loss information session. Participants given a self-help weight loss program booklet. |
Length of follow-up: 12 months Outcomes: Weight change; waist circumference; BMI, systolic and diastolic BP; 6-month erectile function |
|
|
Age, years: mean (SD): a: 34 (11.6); b: 37.5 (10.4) BMI: mean (SD): a: 30.5 (3.0); b: 30.6 (2.7) Weight, kg: mean (SD) a: 99.2 (13.7); b: 99.1 (12.2) Baseline comparability: Yes |
b: Researcher delivered one 75-minute information session (60 minutes weight loss + 15 minute Internet instruction). Participants given self-help weight loss program booklet and 3 months online support from the study website, Calorie King. Participants received personalized online feedback and responses to any questions posted on the website noticeboard, including anecdotes and weight loss strategies for men. Weight loss information sessions in both groups covered instruction relating to the modification of diet and physical activity habits and behavior change, based on Bandura’s Social Cognitive Theory. Timing of active intervention: 3 months Number of times contacted: a: 4 times for 3 monthly assessments; b: 11 times for 3 monthly assessments and 7 feedback sessions Number allocated: a: 31; b: 34 Completed: a: 20; b: 26 % dropout: a: 35.5%; b: 23.5% Number assessed: a: 31; b: 34 |
|||
| Patrick 2011 |
Location: Universities of California, and San Diego, San Diego State, USA Period of study: February 2004 to March 2005 Inclusion criteria: BMI ≥25, age 25-55 Exclusion criteria: NR Age, years: mean (SD) a: 42.8 (8.0); b: 44.9 (7.8) BMI: mean (SD) a: 34.3 (4.0); b: 34.2 (4.2) Weight, kg: mean (SD) a: 104.6 (15.3); b: 104.7 (15.3) Baseline comparability: yes |
Description of interventions: a: Wait list/general Internet advice: Participants given access to a website giving general male health advice that was unlikely to produce a change in diet or physical activity (e.g., stress, hair loss, worksite injury prevention). Men were given the option to swap to the weight loss intervention after 12 months. b: Internet-based diet and physical activity advice and behavioral support: based on social cognitive theory and informed by the behavioral determinants model and designed to improve diet and physical activity in five key areas to promote weight loss, rather than directly targeting calorie restriction: 1. Increase fruit and vegetable intake to 5-9 servings per day 2. Decrease saturated fat intake to ≤20g per day 3. Increase wholegrain intake to ≥3 servings per day 4. Increase physical activity to >10,000 steps per day using a pedometer for at least 5 days per week 5. Participate in upper and lower body strength training at least twice per week. |
Length of follow-up: 12 months Outcomes: weight, BMI |
Trial conducted focus groups with men and 2 weight loss experts to tailor the intervention for men (not published). |
| Men met with a case manager to set goals at baseline and completed weekly web-based activities, including behavior change skills, and reading diet and physical activity information. Men also had the opportunity to email study experts (dietician, physical activity expert, and a clinical psychologist). Both groups paid $20 for completing 6 months and $100 for completing 12 months. Timing of active intervention: 12 months Number of times contacted: a: 3; b: 55 Number allocated: a: 217; b: 224 Completed: a + b: 309 % dropout: a + b: 29.9% Number assessed: a: 217; b: 224 |
||||
| Pavlou 1989 (main trial) |
Location: One University center, Boston University Medical Centre, USA Period of study: NR Inclusion criteria: Male, aged 26-52 years, euthyroid, free from any physical, psychological, or metabolic impairment Exclusion criteria: NR Age, years: mean (SD): a: 41.5 (7.59), b: 42.9 (6.63), c: 45.1 (10.0), d: 49.6 (8.4), e: 41.8 (10.44), f: 41.8 (7.57), g: 46.1 (9.33), h: 44.5 (9.6) (completers) BMI: mean: a: 32.54, b: 32.4, c: 32.07, d: 31.5, e: 30.13, f: 34.82, g: 31.89, h: 33.78 (completers) Weight, kg: mean, SEM (SD): a: 103.1, 3.1 (9.80), b: 105.0, 4.4 (14.59), c: 100.8, 2.3 (9.2), d: 98.8, 2.6 (10.4), e: 96.1, 3.3 (10.44), f: 103.0, 3.7 (13.34), g: 100.8, 2.3 (9.76), h: 105.7, 3.4 (13.6) (completers) Baseline comparability: Yes |
Description of interventions: Subjects were randomly assigned to 4 diets and exercise and non-exercise groups for 8 weeks. Exercise consisted of 90 minutes supervised exercise program 3 times/week from baseline to week 8 which consisted of 35-60 minutes of aerobic activity, e.g., walk-jog-run (70% to 85% max HR), calisthenics and relaxation techniques. Non-exercise groups were instructed to continue normal daily activity and not to participate in any form of additional supervised and/or unsupervised physical activity during initial 8 weeks a: Balanced caloric-deficit, low calorie diet (BCDD). 1,000 kcal/day selected from usual 4 food groups in quantities thought to meet basic requirements. b: BCDD + exercise c: Protein-sparing modified fast, low carbohydrate (PSMF). Ketogenic diet of meat, fish, and fowl used as only dietary source to provide equivalent of 1.2 g high biologic value protein per kg of ideal body weight or 1,000 kcal/day, no carbohydrate and all fat ingested from meat, fish and fowl. Participants prescribed 2.8 g potassium chloride daily. d: PSMF + exercise e: DPC-70; A very low calorie diet of 420 kcal powdered protein carbohydrate mix derived from calcium caseinate, egg albumin and fructose, formulated with vitamins and minerals to meet the U.S. Recommended Dietary Allowances (RDA) dissolved in water or other noncaloric liquid. Fat content zero. Participants instructed to consume 5 packets/day and consume no other nutrients. Participants prescribed 2.8g potassium chloride daily. f: DPC-70 + exercise g: DPC 800; A very low calorie diet of 800 kcal/day diet provided in powdered form consumed similarly to DPC-70, provided a complete mixture of nutrients and similar nutritionally to BCDD except for fewer calories. h: DPC-800 + exercise All participants attended weekly education sessions up to week 8 that included behavior modification, diet and general nutrition, and exercise education. All participants were given multivitamins, daily food and activity records up to week 8. Noncalorific liquids, including coffee, were allowed in unrestricted amounts. Timing of active intervention: 8 weeks + 18 months follow-up Number of times contacted: a-h: 11 times, weekly 0-8 weeks then at 8 and 18 months Number allocated: 160 men (20 in each intervention group) Completed: a: 10, b: 11, c: 16, d: 16, e: 10, f: 13, g: 18, h: 16 at 18 months post-treatment % dropout: 31% at 18 months Number assessed: a: 10, b: 11, c: 16, d: 16, e: 10, f: 13, g: 18, h: 16 at 18 months (completers) |
Length of follow-up: 18 months Outcomes: Weight, total cholesterol, HDL cholesterol, triglycerides, systolic and diastolic BP |
|
| Pavlou 1989 (pilot) |
Location: As above Period of study: As above Inclusion criteria: As above Exclusion criteria: As above Age, years: Mean (SD): a: 49.2 (6.48), b: 44.8 (7.84), c: 46.1 (5.14), d: 48.1 (4.65) (data for completers) BMI: mean: a: 31.75, b: 31.92, c: 31.11, d: 30.4 (completers) Weight, kg: mean (SEM) a: 102.3 (2.1), b: 99.2 (4.2), c: 101.7 (3.1), d: 97.3 (4.1) Baseline comparability: Yes |
Description of interventions: As above but a, b, c, d only Timing of active intervention: 12 weeks Number of times contacted: 16 times, weekly 0-12 weeks and then at 6, 18, and 36 months Number allocated: a + b + c + d: 24 Completed: a: 5, b: 6, c: 5, d: 5 at 36 months post-treatment % dropout: 13% at 36 months post-treatment Number assessed: a: 5, b: 6, c: 5, d: 5 at 36 months post treatment |
Length of follow-up: 162 weeks Outcomes: Weight |
|
|
Van Aggel-Leijssen 2001
Van Aggel-Leijssen 2002 Lejeune 2003 |
Location: one University research department, Maastricht, The Netherlands Period of study: Not reported Inclusion criteria: Good health; ≤2 hours per week sports activities; stable body weight over previous 3 months (<3 kg change) Exclusion criteria: Physically demanding job; taking medication known to influence measured variables Age, years: mean (SD), a: 38.6 (6.5), b: 39.3 (7.7) BMI: mean (SD) a: 32.0 (2.1), b: 32.6 (2.5) Weight, kg: mean (SD), a: 103.6 (11.7), b: 102.6 (9.8) Baseline comparability: Yes |
Description of interventions: a: 12-week energy restriction period followed by 40-week weight maintenance period: Weeks 1-6 Very low energy diet of 2.1 MJ/day protein enriched formula (Modifast, Novartis) diet consisting of 50 g carbohydrate, 52 g protein, 7 g fat. Weeks 7-8 1.4 MJ/day formula + 3.5 MJ/day free choice. Weeks 9-10 0.7 MJ/day formula + 4.9 MJ/day free choice. Weeks 11-12 Participants received dietary instruction to stabilize their body weight and not to change their habitual activity pattern. b: 12-week energy restriction period (as per a) combined with an exercise training program (40% VO2max) for 1 hour, 4 times per week either cycling, walking or aqua-jogging (3 sessions supervised by a personal trainer in the lab and 1 session at home). Exercise training program continued during the weight maintenance period to week 52. |
Length of follow-up: 52 weeks Outcomes: Weight, BMI |
|
| Timing of active intervention: a: Weeks 0-12 b: Weeks 0-12 Number of times contacted: a: 12 times at weekly intervals; b: 168 times up to 4 times per week for 52 weeks Number allocated: a: 20; b: 20 Completed: a: 15; b: 14 % dropout: a: 25%; b: 30% Number assessed: a:15; b: 14 |
||||
|
Wood 1988
Fortmann 1988 |
Location: One research center, Stanford, USA Period of study: NR Inclusion criteria: 120% to 160% ideal body weight; nonsmoker; plasma total cholesterol <8.28 mmol/L; triglycerides <5.65; normal electrocardiogram, alcohol intake <4 drinks per day; sedentary activity; weight stable (±5 lbs) over the past year Exclusion criteria: Blood pressure >160/100; taking medications affecting lipids; plasma cholesterol >300 mg/dL; triglycerides >500 mg/dL; exercise ≥3 times per week Age, years: Mean (SD) a: 45.2 (7.2), b: 44.2 (8.2), c: 44.1 (7.8) BMI: NR Weight, kg: mean (SD) a: 95.4 (10.6), b: 93.0 (8.8), c: 94.1 (8.6) Baseline comparability: Yes |
Description of interventions: a: Control: Weight stable, no added energy restriction or exercise. b: Diet: Energy restriction (reduced food quantity but not proportions of fat, carbohydrate, protein or alcohol). Energy intake reduced by 300-500 kcal/day depending on weight loss goals, energy needs and baseline food intake to achieve approximately 0.3-0.6 kg fat loss per week. No added exercise weight loss goals. c: Exercise: Increased activity with instruction to maintain exercising heart rate of 65% to 85% of peak heart rate (approximately caloric output of 8-10 calories per minute). Supervised sessions 3 times per week starting with fast walking for 25 minutes, gradually adding jogging for 0-3 months with continuous jogging increasing to 40-50 minutes. Two additional days of unsupervised walking or jogging added by month 6. Program aimed to decrease body fat by 2-3 kg in the first 3 months, 4-5 kg in months 3-6, and the remainder in months 6-9. Timing of active intervention: b + c: 0 to 9 months Number allocated: a: 52, b 51, c: 52 Completed: a: 51, b: 49, c: 49 % dropout: a 1.9%, b: 5.8%, c: 3.9% Number assessed: a: 44, b: 41, c: 36 |
Length of follow-up: 1 year Outcomes: Mean change in weight, mean change in systolic and diastolic blood pressure |
|
| King 1989 (continuation of Wood 1988) | At the end of the one year Wood 1988 trial phase participants from groups a and b were randomized within intervention groups to weight maintenance follow-up interventions. Age, years: Mean (SD) a (i): 45.5 (9.6); a (ii): 44.4(4.8); b (i) 46.1 (7.6); b (ii) 42.9 (7.3) BMI: NR Weight, kg: mean (SD) a (i): 85.7 (9.1); a (ii): 83.4 (8.8); b (i): 91.0 (10.6); b (ii): 86.2 (7.6) Baseline comparability: Yes |
Description of interventions: a (i): Diet mail and telephone contact. Participants received monthly mailings consisting of a supportive letter; self-scored assessment of an energy restriction problem area; list of energy restriction coping suggestions (e.g., holiday eating, emotional eating, eating away from home, stress eating). Mailings supplemented by telephone calls monthly for the first 3 months, then at 6, 9, and 12 months. a (ii): Diet control. Participants received no mailings or telephone calls. b (i): Exercise mail and telephone contact. As a (i) but self-scored assessment of an exercise problem area and list of exercise coping strategies (e.g., time pressure, boredom, illness or injury). b (ii): Exercise control (as a (ii)) At the beginning of the weight maintenance phase, participants in all groups given written information for the weight control method they received during the weight loss trial period and were encouraged to seek support from members of their original intervention group. Timing of active intervention: 1 year weight maintenance following end of 1 year weight loss phase Number of times contacted: a: 3 times at 6 monthly intervals; b: 21 times at monthly intervals Number allocated: a (i): 24; a (ii) 20; b (i) 24; b (ii) 22 Completed: a (i): 20; a (ii): 16; b (i): 21; b (ii): 15 % dropout: a (i): 16.7%; a (ii): 20%; b (i): 12.5%; b (ii): 31.8% Number assessed: a (i): 20; a (ii): 16; b (i): 21; b (ii): 15 |
Length of follow-up: 1 year Outcomes: Mean change in weight |
|
|
Wycherley 2012
Lutze 2013 |
Location: One center (community setting), Australia Period of study: NR Inclusion criteria: Overweight or obese males Exclusion criteria: BMI <27 or >40; Aged <20 years or >65 years; diabetes, uncontrolled hypertension, history of gastrointestinal, renal, coronary, metabolic, or hepatic disease; malignancy; taking hypoglycemic medication or drugs affecting insulin sensitivity or may interfere with bowel function; smokers; history of heavy alcohol consumption (>5 standard drinks/day). Age, years: Mean (SD) a: 51.3 (9.4); b: 50.2 (9.3) BMI (calculated): Mean a: 33.8; b: 32.5 Weight, kg: mean (SD) a: 106.0 (12.9); b: 101.6 (14.9) Baseline comparability: Triglycerides lower in group a (reported p = .05). |
Description of interventions: a: High protein, low fat energy restricted diet (1672 kcal/day). Diet consisted of: 35% protein (142 g/day), 40% carbohydrate (135 g/day), 25% fat (total 53 g/day, saturated 14 g/day). b: High carbohydrate, low fat isocaloric diet. Diet consisted of: 17% protein (88 g/day), 58% carbohydrate (198 g/day), 25% fat (51 g total, 14 g saturated). a + b: Participants were supplied with diet-specific foods, representing 60% of energy intake, for the first 12 weeks. Participants met individually with a qualified dietician at baseline and fortnightly for 12 weeks and monthly thereafter. Participants received detailed dietary and meal planning advice and recipes. Participants were required to keep daily semiquantitative food records. Timing of active intervention: a + b, 52 weeks Number allocated: a: 59; b: 64 Completed: a: 33; b: 35 % dropout: a: 56%; b: 55% Number assessed: a: 59; b: 64 |
Length of follow-up: 1 year Outcomes: Mean weight change; total, LDL and HDL cholesterol; triglycerides; systolic and diastolic BP |
Appendix E
Risk of Bias and Equity Assessment for Individual Studies Included in the Review
Table 1.
Risk of Bias Assessment.
| Benassi-Evans 2009 | Borg 2002 | Esposito 2004 | Hunt 2014 | Jeffrey 1983 | Khoo 2011 | King 1989 | Morgan 2011 | Patrick 2011 | Pavlou 1989 | Van Aggel-Leijssen 2001 | Wood 1988 | Wycherley 2012 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation (selection bias) | ? | ? | ? | ✓ | ? | ? | ? | ✓ | ✓ | ? | ? | ✓ | ✓ |
| Allocation concealment (selection bias) | ? | ✓ | ✓ | ✓ | ? | ? | ? | ✓ | ✓ | ? | ? | ✓ | ✓ |
| Blinding of participants (performance bias) | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Blinding of health care providers (performance bias) | ? | × | × | × | × | × | × | × | × | × | × | × | × |
| Blinding of outcome assessment (detection bias) | ? | × | ✓ | ? | ? | ? | ✓ | ✓ | ✓ | ? | ? | ? | ? |
| Groups treated identically (performance bias) | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Incomplete outcome data (attrition bias) | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ? | ? | ✓ | ? | ✓ |
| Intention to treat (attrition bias) | ? | × | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | × | × | ✓ |
| Selective reporting (reporting bias) | ✓ | ✓ | ✓ | ✓ | ✓ | × | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ? |
| Other bias | ? | ? | ✓ | ✓ | × | ✓ | ✓ | × | ✓ | ? | ✓ | × | ✓ |
Note. ✓ = low risk of bias; × = high risk of bias; ? = unclear risk of bias.
Table 2.
Equity Assessment.
| Benassi-Evans 2009 | Borg 2002 | Esposito 2004 | Hunt 2014 | Jeffrey 1983 | Khoo 2011 | King 1989 | Morgan 2011 | Patrick 2011 | Pavlou 1989 | Van Aggel-Leijssen 2001 | Wood 1988 | Wycherley 2012 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equity pointer: Social context of the study, e.g., was study conducted in a particular setting that might target/exclude specific populations? | ? | × | × | ✓ | ✓ | × | × | ✓ | ✓ | × | × | × | ? |
| Representativeness of sample: Are participants in the study likely to be representative of the target population? | ✓ | ✓ | × | ✓ | ? | ✓ | × | × | ? | × | ✓ | × | × |
| Sociodemographic differences between withdrawals and exclusions? | ? | ? | ? | ? | ? | × | ? | ? | ✓ | ? | ? | ? | ? |
| PROGRESS categories reported at baseline (indicate letters of those reported: Place of residence, race, occupation, gender, religion, education and literacy, SES, social capital) | × | × | ? | ✓ | ✓ | × | × | ✓ | ✓ | ? | ? | × | ? |
| Did the intervention include strategies to address diversity/disadvantage? | × | × | × | × | × | × | × | × | ? | × | × | × | × |
| Was there a fidelity check? | × | ✓ | × | ✓ | × | × | × | × | × | ✓ | ✓ | ✓ | ✓ |
| Were process measures taken? | × | ✓ | × | ✓ | × | × | ✓ | ✓ | × | × | × | × | × |
| Providers (who, number, education/training in intervention delivery, ethnicity, etc. if potentially relevant to acceptance and uptake by participants | ✓ | ? | ✓ | ✓ | ? | × | ? | ✓ | ✓ | ? | ✓ | ✓ | ✓ |
| Was sustainability discussed by the authors? Was it a consideration in study development? | × | × | × | ✓ | ? | × | × | × | × | × | × | × | × |
| Do the authors describe any political or organizational context? | × | × | × | × | ? | × | × | × | × | × | × | × | × |
| Were any partnerships referred? | × | × | × | ✓ | ? | × | × | × | × | × | × | × | × |
| Potential for author conflict, i.e., evidence that author or data collectors would benefit if results favored the intervention under study or the control? | ? | ? | ? | × | × | × | ? | × | ✓ | ? | ? | ? | × |
| Were outcomes relating to harms/unintended effects of the intervention described? | ? | ✓ | ? | ✓ | × | ✓ | ? | × | × | ? | ✓ | ? | × |
Note. ✓ = yes; × = no; ? = unclear/not reported.
Footnotes
Authors’ Note: The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the Department of Health. HERU, HSRU, and NMAHP are funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. The authors accept full responsibility for this publication.
© Queen’s Printer and Controller of HMSO 2012. This work was produced by the ROMEO (Review Of MEn and Obesity) Group under the terms of a commissioning contract issued by the Secretary of State for Health. This journal issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC, HTA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review is an update of one of a series of systematic reviews for the ROMEO project (Review Of MEn and Obesity), funded by the National Institute for Health Research, Health Technology Assessment Programme (NIHR HTA Project 09/127/01; Systematic reviews and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men http://www.nets.nihr.ac.uk/projects/hta/0912701).
Systematic Review Registration: This study was registered as PROSPERO CRD42011001479.
References
- Avenell A., Broom J., Brown T. J., Poobalan A., Aucott L., Stearns S. C., . . . Grant A. M. (2004). Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technology Assessment, 8(21), 1-182. [DOI] [PubMed] [Google Scholar]
- Benassi-Evans B., Clifton P. M., Noakes M., Keogh J. B., Fenech M. (2009). High protein-high red meat versus high carbohydrate weight loss diets do not differ in effect on genome stability and cell death in lymphocytes of overweight men. Mutagenesis, 24, 271-277. [DOI] [PubMed] [Google Scholar]
- Biswas A., Oh P. I., Faulkner G. E., Bajaj R. R., Silver M. A., Mitchell M. S., Alter D. A. (2015). Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults a systematic review and meta-analysis. Annals of Internal Medicine, 162, 123-132. [DOI] [PubMed] [Google Scholar]
- Borg P., Kukkonen-Harjula K., Fogelholm M., Pasanen M. (2002). Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: A randomized trial. International Journal of Obesity, 26, 676-683. [DOI] [PubMed] [Google Scholar]
- Byberg L., Melhus H., Gedeborg R., Sundstrom J., Ahlbom A., Zethelius B., . . . Michaëlsson K. (2009). Total mortality after changes in leisure time physical activity in 50 year old men: 35 Year follow-up of population based cohort. British Medical Journal, 338, 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination. (2009). Systematic reviews: CRD’s guidance for undertaking reviews in health care. York, England: University of York; Retrieved from http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf [Google Scholar]
- Collins C. E., Jensen M. E., Young M. D., Callister R., Plotnikoff R. C., Morgan P. J. (2013). Improvement in erectile function following weight loss in obese men: The SHED-IT randomized controlled trial. Obesity Research & Clinical Practice, 7, e450-e454. [DOI] [PubMed] [Google Scholar]
- Duncan D. T., Wolin K. Y., Scharoun-Lee M., Ding E. L., Warner E. T., Bennett G. G. (2011). Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. International Journal of Behavioral Nutrition & Physical Activity, 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K., Giugliano F., Di Palo C., Giugliano G., Marfella R., D’Andrea F., . . . Giugliano D. (2004). Effect of lifestyle changes on erectile dysfunction in obese men. Journal of the American Medical Association, 291, 2978-2984. [DOI] [PubMed] [Google Scholar]
- Fortmann S. P., Haskell W. L., Wood P. D. (1988). Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. American Journal of Cardiology, 62, 89-93. [DOI] [PubMed] [Google Scholar]
- George E. S., Kolt G. S., Duncan M. J., Caperchione C. M., Mummery W. K., Vandelanotte C., . . . Noakes M. (2012). A review of the effectiveness of physical activity interventions for adult males. Sports Medicine, 42, 281-300. [DOI] [PubMed] [Google Scholar]
- Golubic R., Ekelund U., Wijndaele K., Luben R., Khaw K. T., Wareham N. J., Brage S. (2013). Rate of weight gain predicts change in physical activity levels: A longitudinal analysis of the EPIC-Norfolk cohort. International Journal of Obesity, 37, 404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. O., Blanck H. M., Gillespie C., Maynard L. M., Serdula M. K. (2008). Perceived health risk of excess body weight among overweight and obese men and women: Differences by sex. Preventive Medicine, 47, 46-52. [DOI] [PubMed] [Google Scholar]
- Han T. S., Tajar S., O’Neill T. W., Jiang M., Bartfai G., Boonen S. . . . EMAS Group. (2011). Impaired quality of life and sexual function in overweight and obese men: The European Male Ageing Study. European Journal of Endocrinology, 164, 1003-1011. [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (2011). Cochrane handbook for systematic reviews of interventions (version 5.1.0 [updated March 2011]). The Cochrane Collaboration. Retrieved from http://www.cochrane-handbook.org/
- Hoddinott P., Allan K., Avenell A., Britten J. (2010). Group interventions to improve health outcomes: A framework for their design and delivery. BMC Public Health, 10, 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt K., Wyke S., Gray C. M., Anderson A. S., Brady A., Bunn C., . . . Treweek S. (2014). A gender-sensitised weight loss and healthy living programme for overweight and obese men delivered by Scottish Premier League football clubs (FFIT): A pragmatic randomised controlled trial. Lancet, 383, 1211-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery R. W., Bjornson-Benson W. M., Rosenthal B. S., Lindquist R. A., Johnson S. L. (1984). Behavioral treatment of obesity with monetary contracting: Two-year follow-up. Addictive Behaviors, 9, 311-313. [DOI] [PubMed] [Google Scholar]
- Jeffery R. W., Gerber W. M., Rosenthal B. S., Lindquist R. A. (1983). Monetary contracts in weight control: Effectiveness of group and individual contracts of varying size. Journal of Consulting and Clinical Psychology, 51, 242-248. [DOI] [PubMed] [Google Scholar]
- Jolly K., Lewis A., Beach J., Denley J., Adab P., Deeks J. J., . . . Aveyard P. (2011). Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. British Medical Journal, 343, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavou A., Turner A., French D. P. (2013). Do interventions to promote walking in groups increase physical activity? A meta-analysis. International Journal of Behavioral Nutrition and Physical Activity, 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo J., Piantadosi C., Duncan R., Worthley S. G., Jenkins A., Noakes M., . . . Wittert G. A. (2011). Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. Journal of Sexual Medicine, 8, 2868-2875. [DOI] [PubMed] [Google Scholar]
- King A. C., Frey-Hewitt B., Dreon D. M., Wood P. D. (1989). Diet vs. exercise in weight maintenance. The effects of minimal intervention strategies on long-term outcomes in men. Archives of Internal Medicine, 149, 2741-2746. [DOI] [PubMed] [Google Scholar]
- Kukkonen-Harjula K. T., Borg P. T., Nenonen A. M., Fogelholm M. G. (2005). Effects of a weight maintenance program with or without exercise on the metabolic syndrome: A randomized trial in obese men. Preventive Medicine, 41, 784-790. [DOI] [PubMed] [Google Scholar]
- Lejeune M. P. G. M., Aggel-Leijssen D. P. C., van Baak M. A., Westerterp-Plantenga M. S. (2003). Effects of dietary restraint vs exercise during weight maintenance in obese men. European Journal of Clinical Nutrition, 57, 1338-1344. [DOI] [PubMed] [Google Scholar]
- Lovejoy J. C., Sainsbury A. (2009). Sex differences in obesity and the regulation of energy homeostasis: Etiology and pathophysiology. Obesity Reviews, 10, 154-167. [DOI] [PubMed] [Google Scholar]
- Lutze J., Taylor P., Brinkworth G. D., Wyld B., Syrette J., Wilson C. J., . . . Noakes M. (2013). Psychological well-being response to high protein and high carbohydrate weight loss diets in overweight and obese men: A randomised trial. e-SPEN Journal, 8(6), e235-e240. [Google Scholar]
- Morgan P. J., Lubans D. R., Collins C. E., Warren J. M., Callister R. (2011). 12-Month outcomes and process |evaluation of the SHED-IT RCT: An internet-based weight loss program targeting men. Obesity, 19, 142-151. [DOI] [PubMed] [Google Scholar]
- Multiple Risk Factor Intervention Trial Research Group. (1982). Multiple Risk Factor Intervention Trial. Risk factor changes and mortality results. Journal of the American Medical Association, 248, 1465-1477. [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2013. a). PH46: Assessing body mass index and waist circumference thresholds for intervening to prevent ill health and premature death among adults from black, Asian and other minority ethnic groups in the UK. London, England: Author. Retrived; from http://www.nice.org.uk/guidance/ph46 [Google Scholar]
- National Institute for Health and Care Excellence. (2013. b). PH53: Managing overweight and obesity in adults—Lifestyle weight management services. London, England: Author; Retrieved from http://www.nice.org.uk/guidance/ph53 [Google Scholar]
- National Weight Control Registry. (2014). NWCR Facts. Providence, RI: Author; Retrieved from http://www.nwcr.ws/Research/default.htm [Google Scholar]
- Newton R. L., Jr., Griffith D. M., Kearney W., Bennett G. G. (2015). A systematic review of physical activity, dietary, and weight loss interventions involving African American men. Obesity Reviews, 15(Suppl. 4), 93-106. [DOI] [PubMed] [Google Scholar]
- Pagoto S. L., Schneider K. L., Oleski J. L., Luciani J. M., Bodenlos J. S., Whited M. C. (2012). Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity, 20, 1234-1239. [DOI] [PubMed] [Google Scholar]
- Patrick K., Calfas K. J., Norman G. J., Rosenberg D., Zabinski M. F., Sallis J. F., . . . Dillon L. W. (2011). Outcomes of a 12-month web-based intervention for overweight and obese men. Annals of Behavioral Medicine, 42, 391-401. [DOI] [PubMed] [Google Scholar]
- Paul-Ebhohimhen V., Avenell A. (2009). A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obesity Facts, 2(1), 17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou K. N., Krey S., Steffee W. P. (1989). Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. American Journal of Clinical Nutrition, 49, 1115-1123. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration. (2009). Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet, 373, 1083-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan A. G., Tyson M., Egger M., Heller R. F., Zwahlen M. (2008). Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet, 371, 569-578. [DOI] [PubMed] [Google Scholar]
- Robertson C., Archibald D., Avenell A., Douglas F., Hoddinott P., Van Teijlingen E., . . . Fowler C. (2014). Systematic reviews and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technology Assessment, 18(35), 1-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen R. C., Riley A., Wagner G., Osterloh I. H., Kirkpatrick J., Mishra A. (1997). The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology, 49, 822-830. [DOI] [PubMed] [Google Scholar]
- Ueffing E., Tugwell P., Welch V., Petticrew M., Kristjansson E. & Campbell Equity Methods Group. (2011). Equity checklist for systematic review authors (Version 2011-11-08). Cochrane and Campbell Equity Methods Group. Retrieved from http://equity.cochrane.org/sites/equity.cochrane.org/files/uploads/Equity%20Checklist%20for%20Systematic%20Review%20Authors%20-%202011-11-08.doc
- Van Aggel-Leijssen D. P., Saris W. H., Hul G. B., van-Baak M. A. (2002). Long-term effects of low-intensity exercise training on fat metabolism in weight-reduced obese men. Metabolism: Clinical & Experimental, 51, 1003-1010. [DOI] [PubMed] [Google Scholar]
- Van Aggel-Leijssen D. P. C., Saris W. H. M., Hul G. B., van Baak M. A. (2001). Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. American Journal of Clinical Nutrition, 73, 523-531. [DOI] [PubMed] [Google Scholar]
- Wong J. Y., Gilson N. D., van Uffelen J. G., Brown W. J. (2012). The effects of workplace physical activity interventions in men: A systematic review. American Journal of Men’s Health, 6, 303-313. [DOI] [PubMed] [Google Scholar]
- Wood P. D., Stefanick M. L., Dreon D. M., Frey-Hewitt B., Garay S. C., Williams P. T., . . . Haskell W. L. (1988). Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. New England Journal of Medicine, 319, 1173-1179. [DOI] [PubMed] [Google Scholar]
- Wycherley T. P., Brinkworth G. D., Clifton P. M., Noakes M. (2012). Comparison of the effects changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutrition and Diabetes, 2, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. D., Morgan P. J., Plotnikoff R. C., Callister R., Collins C. E. (2012). Effectiveness of male-only weight loss and weight loss maintenance interventions: A systematic review with meta-analysis. Obesity Reviews, 13, 393-408. [DOI] [PubMed] [Google Scholar]