Abstract
Introduction:
Diabetes is reaching epidemic levels in Afghanistan. This study identifies the risk factors associated with diabetes in Herat City, Afghanistan, and explores the prevalence of previously undiagnosed diabetes.
Methods:
A cross-sectional study was conducted using multistage cluster sampling by adopting the World Health Organization’s (WHO) STEPwise approach to Surveillance (STEPS). We enrolled 1129 participants aged 25–70 years between May and June of 2015 (47.4% males, 52.6% females). A structured questionnaire was used for data collection of demographic, socioeconomic, and behavioral factors. Investigators collected anthropometric measurements and blood samples from study participants. A multivariable logistic regression model was used to identify factors associated with diabetes prevalence.
Results:
We found that the prevalence of diabetes in Herat City was 9.9% (9.8% in males and 10.1% in females). Of the 1129 respondents, only 3.3% were previously diagnosed with diabetes or were under treatment, whereas 6.6% of respondents were previously undiagnosed. The multivariable analyses showed that age, frequency of rice consumption, type of cooking oil, and systolic blood pressure were associated with diabetes.
Conclusions:
This is one of the first studies to discuss the high prevalence of undiagnosed diabetes in Herat, Afghanistan. This study found several modifiable factors that were associated with diabetes in Herat, Afghanistan. Future reduction of disease burden should focus on these factors in the development of the most optimal diabetes prevention programs.
Keywords: Risk Factors, Diabetes, Chronic Disease, Afghanistan
Diabetes mellitus (DM) is a global public health problem and is listed as a priority noncommunicable disease (NCD) by the World Health Organization (WHO).1 The rising prevalence of diabetes is reaching epidemic proportions worldwide.2 Genetics, environmental factors, and lifestyle choices are contributing to the development of this chronic metabolic disorder.3 Diabetes was ranked as the sixth leading cause of death in 2015, with 1.6 million deaths attributed to this disease.4 The global prevalence of diabetes increased from 4.7% in 1980 to 8.5% in 2014, resulting in 422 million people in the world living with diabetes in 2014.5
According to a study of diabetes in Kuwait, the crude prevalence of total diabetes cases in this country was 21.4%, and almost one-fifth of these cases were previously undiagnosed.6 In Oman, the overall prevalence of diabetes appears to be on the rise with risk factors such as urban residence, obesity, age, and systolic blood pressure being significantly associated with diabetes.7 The study from Iran demonstrated that diabetes was more prevalent among older age groups, females, and urban dwellers.8 In China, the prevalence of diabetes in the 35–74 age group was 5.5%, with 5.8% of females and 5.2% of males being diagnosed with diabetes.9 A study in Pakistan found that central obesity, hypertension, and family history of the disease were risk factors for diabetes.10
In Afghanistan, due to years of war and conflict, few studies were conducted to estimate the burden of diabetes in the country. However, the Afghanistan Mortality Survey (AMS) in 2010 reported that one third of all deaths were attributed to NCDs.11 The prevalence of diabetes in the 20–79 year age group in Afghanistan was estimated to be 8.6% in 2010, whereas by 2030 it is projected to reach 9.9%.12 Moreover, studies reported that the prevalence of diabetes was 13.2% in Kabul (age group of ≥40 years), 11.8% in Jalalabad, and 22.4% in Kandahar in the early 2010s.13–16 We also previously reported an overall diabetes prevalence of 9.9% in a cohort of adults from Herat.15 However, the risk factors associated with diabetes in this population have not yet been explored. The aim of this study was to determine risk factors associated with diabetes among adults living in Herat, Afghanistan and to explore the prevalence of previously undiagnosed diabetes in this geographic region.
Methods
The Institutional Review Board of the Ministry of Public Health, Afghanistan, provided ethical approval of the study protocol. The study design was a cross-sectional survey of permanent residents of Herat, Afghanistan, using the STEPwise approach to Surveillance (STEPS) tool,17 which inludes demographic, physical, and biochemical measurements. Herat is the largest city in the Herat province of Afghanistan. The location of the study setting in Herat is shown in Figure 1. The urban population of Herat is estimated to be 491,967, (242,102 females and 249,865 males.18) Permanent residents of Herat between the ages of 25 and 70 were enrolled in this study. Participants signed informed consents prior to face-to-face interviews. For illiterate participants, the informed consent was read by the interviewer and the fingerprint of the respondent was taken to confirm agreement to participate.
Figure 1.
Location of study setting in western province of Herat, Afghanistan
Sampling Strategy
The statistical software program Epi Info v. 7 was used to calculate the sample size for this study. Although the national burden of diabetes is not well known in Afghanistan, a WHO report estimated the prevalence of diabetes at 8.6%.12 Although information on diabetes risk factors is scarce for Afghanistan, epidemiological research from other developing countries has reported that physical activity, blood pressure, dietary factors, obesity, age, level of education, smoking status, and other factors were associated with diabetes. The sample size was calculated to be 1200 based on the proportion of these risk factors and the cluster sampling technique. Inclusion criteria were as follows: adult population aged 25–70 (as outlined in WHO survey tool), must be Herat residents during the study period, and must consent to participate. Exclusion criteria included: temporary residents (less than six months in the city) and those living in the institutionalized settings or unsafe areas.
Study Variables and Data Collection
The primary outcome variable was the presence of diabetes (yes/no). Participants with a fasting blood sugar (FBS) of ≥126mg/dl or undergoing diabetes treatment during the data collection appointment were considered as diabetic.19 Main factors such as age, sex, ethnicity, educational status, income, job type, proxy for physical activity (vigorous or moderate physical activity), dietary factors, tobacco use, obesity, hypertension, and blood lipid levels were assessed and analyzed.
A structured (standardized) STEPS questionnaire was adopted and translated into the Dari language, the official language of Afghanistan. The answer options in the questionnaire were previously coded to facilitate the data entry and data analysis. Training and field testing was conducted ahead of time, and the questionnaire was adjusted accordingly before the actual data collection period of May–June 2015.
A household was defined as a group of people who are cooking together in same kitchen, sharing the same food pot but no necessarily the same roof. In each household, the interviewer counted all persons eligible for the study based on the inclusion and exclusion criteria. In the households with more than one eligible person, a lottery system was used to select the respondent for the survey. If that individual refused to participate, the interviewers approached the next household on the list. Anthropometric measurements (height and weight) were used to calculate body mass index (BMI). A BMI of >30 kg/m2 was considered as obese, 25–30 kg/m2 as overweight, and 18.5–25 kg/m2 as normal weight.20 A waist circumference of 94 cm for men and 80 cm for women was considered as central obesity.21 Systolic blood pressure of 140 mmHg and diastolic blood pressure of 90 mmHg were considered as hypertensive.22 Blood samples were collected and processed by lab technicians under supervision of the lab coordinator. After samples were shipped to the Central Public Health Laboratory (CPHL) in Kabul, they were stored at −80°C until glucose measurements were completed. To enhance the quality of the data, close monitoring of all procedures was carried out throughout the study.
Statistical Analysis
Data entry was done using Epi Info v. 7 and data analysis was done using SPSS version 20. Participants with missing data and specimens were excluded from the final analysis. Our final sample size for statistical analysis was 1,129 participants. Central tendencies, proportions, and frequencies were calculated and tabulated. The prevalence of diabetes was calculated in all subgroups, and different tables were developed including tables of demographic, socio-economic, and behavioral data using descriptive analyses. Statistical analyses were conducted using student t-test, chi-square, univariate, and multivariable logistic regression. In the univariate model, the relationship between the variables and the outcome was analyzed individually. Then, based on the level of significance and biological plausibility, a multivariable analysis was conducted to address confounding and to find independent associations of factors with the outcome. Statistical significance was based on an α level of <0.05 and 95% confidence interval.
Results
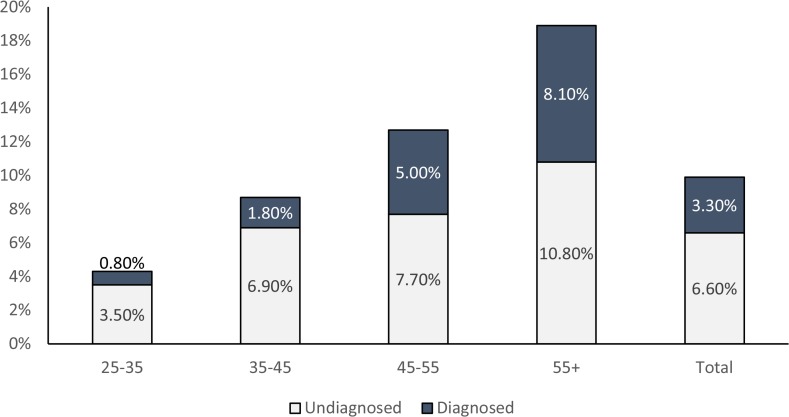
Table 1 summarizes all of the participant characteristics. Out of 1129 participants included in this analysis, 594 (52.6%) were females and 535 (47.4%) were males. The participants had a mean age of 41.7±13.1 years, and 60% were aged less than 45 years. About 48% of study respondents were overweight or obese and 52.3% had central obesity. High blood pressure was recorded in 35.6% of the participants. Approximately 28.4% had high cholesterol and 45% had high triglycerides. Furthermore, high levels of low density lipoprotein (LDL) and high levels of high density lipoprotein (HDL) were observed in 47% of the study participants. More than half of the respondents (54%) were illiterate, and 82.7% of the participants had a monthly income lower than 10,000 Afghanis (USD 146). As previously reported, the overall prevalence of diabetes was 9.9%.15 When stratified by sex, we found that 9.8% of males and 10.1% of females had diabetes. We found that 6.6% of the participants were previously undiagnosed with diabetes, while 3.3% had been previously diagnosed and were undergoing treatment (Figure 2). The mean level of fasting blood sugar was 96.20 mg/dl, with a range of 22–388 mg/dl. The main diabetes management modalities reported by the participant were insulin (28%), oral drugs (74%), dietary restriction (68%), and weight loss recommendation (38%). Identification of diabetes type (Type I vs. Type II) was not the objective of the study and is not reported.
Table 1.
Univariate analysis of demographic, socio-economic and behavioral factors associated with diabetes among study participants in Herat, Afghanistan
| Variables | Categories | No diabetes | Diabetes | Odds Ratio | CI 95% Lower Limit | CI 95% Upper Limit | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | % | N | % | |||||
| Age in years | ||||||||
|
| ||||||||
| 25 – 34 | 382 | 37.6 | 17 | 15.2 | 1.00 | Reference | ||
| 35 – 44 | 263 | 25.9 | 25 | 22.3 | 2.14 | 1.13 | 4.03 | |
| 45 – 54 | 192 | 18.9 | 28 | 25.0 | 3.28 | 1.75 | 6.14 | |
| 55 + | 180 | 17.7 | 42 | 37.5 | 5.24 | 2.91 | 9.46 | |
|
| ||||||||
| Gender | ||||||||
|
| ||||||||
| Female | 536 | 52.7 | 58 | 51.8 | 1.00 | Reference | ||
| Male | 481 | 47.3 | 54 | 48.2 | 1.84 | 1.15 | 2.95 | |
|
| ||||||||
| Fruit serving days per week | ||||||||
|
| ||||||||
| ≤3 days | 793 | 84.0 | 77 | 74.0 | 1.00 | Reference | ||
| > 3 days | 151 | 16.0 | 27 | 26.0 | 0.96 | 0.72 | 1.27 | |
|
| ||||||||
| Vegetable serving days per week | ||||||||
|
| ||||||||
| ≤3 days | 692 | 71.9 | 70 | 66.7 | 1.00 | Reference | ||
| >3 days | 270 | 28.1 | 35 | 33.3 | 1.28 | 0.83 | 1.97 | |
|
| ||||||||
| Cooking Oil in Kitchen | ||||||||
|
| ||||||||
| Liquid | 491 | 60.1 | 37 | 40.7 | 1.00 | Reference | ||
| Solid | 326 | 39.9 | 54 | 59.3 | 2.20 | 1.41 | 3.42 | |
|
| ||||||||
| Obesity based on BMI | ||||||||
|
| ||||||||
| No | 858 | 84.6 | 90 | 81.1 | 1.00 | Reference | ||
| Yes | 156 | 15.4 | 21 | 18.9 | 1.28 | 0.78 | 2.13 | |
|
| ||||||||
| High Blood Pressure | ||||||||
|
| ||||||||
| No | 687 | 67.6 | 40 | 35.7 | 1.00 | Reference | ||
| Yes | 330 | 32.4 | 72 | 64.3 | 3.75 | 2.49 | 5.64 | |
|
| ||||||||
| Triglycerides | ||||||||
|
| ||||||||
| <150 mg/dL | 574 | 56.4 | 47 | 42.0 | 1.00 | Reference | ||
| ≥150 mg/dL | 443 | 43.6 | 65 | 58.0 | 1.79 | 1.21 | 2.66 | |
|
| ||||||||
| Rice consumption in days per week | ||||||||
|
| ||||||||
| ≤3 days | 290 | 29.1 | 53 | 48.2 | 1.00 | Reference | ||
| > 3 days | 708 | 70.9 | 57 | 51.8 | 0.44 | 0.30 | 0.66 | |
|
| ||||||||
| Vigorous Physical Activity | ||||||||
|
| ||||||||
| No | 903 | 89.5 | 101 | 91.0 | 1.00 | Reference | ||
| Yes | 106 | 10.5 | 10 | 9.0 | 0.84 | 0.43 | 1.67 | |
|
| ||||||||
| Moderate Physical Activity | ||||||||
|
| ||||||||
| No | 781 | 77.6 | 96 | 85.7 | 1.00 | Reference | ||
| Yes | 225 | 22.4 | 16 | 14.3 | 0.58 | 0.33 | 1.00 | |
Figure 2.
Prevalence of diagnosed and undiagnosed diabetes by age group
Table 1 shows the results of univariate analysis of risk factors and diabetes among study participants. Odds of having diabetes were 2.14 (95% CI: 1.13 – 4.03) times higher in the 35–45 year age group, 3.28 (95% CI: 1.75 – 6.14) times higher in the 45–55 year age group, and 5.24 (95% CI: 2.91 – 9.46) times higher in the ≥55 year age group as compared to 25–35 age category. Gender, level of education, marital status, income, and other socioeconomic factors were not significantly associated with diabetes in this study. There was a statistically significant association between type of cooking oil used (liquid that was assumed to be unsaturated oil and solid that was assumed to be saturated oil) and diabetes with an OR=2.20 (95% CI: 1.41 – 3.42) (Table 1). Greater frequency of rice consumption was significantly associated with reduced odds of diabetes with an OR = 0.44 (95% CI: 0.30–0.66). Other dietary habits such as frequency of consuming vegetables, fruits, chicken, red meat, and table salt were not significantly associated with diabetes. High blood pressure and high levels of triglycerides were associated with diabetes with OR=3.75 (95% CI: 2.49 – 5.64) and OR=1.79 (95% CI: 1.21 – 2.66) respectively. Furthermore, blood lipids (except triglycerides) and proxies for physical activity as defined by WHO STEPwise approach to Surveillance were not associated with diabetes (Table 1).
In the multivariable analysis, only the variables of biological and statistical significance (Table 1) were included in the model. Table 2 summarizes the variables that were significantly associated with diabetes, including age group, blood pressure, triglyceride, type of cooking oil, and rice consumption. The multivariate model was run with all variables in one model to identify the independent associations in logistic regression. Increasing age group was associated with greater odds of having diabetes, with the 55+ age group having the greatest odds (OR = 4.17, 95% CI: 2.02–8.61). Greater rice consumption was significantly associated with increased odds of having diabetes with an OR = 1.53 (95% CI: 1.03–2.26). High blood pressure, high triglycerides, and solid cooking oil were all associated with lower odds of having diabetes (all p < 0.05).
Table 2.
Multivariable analysis of risk factors and diabetes among study participants in Herat, Afghanistan
| Variables | Categories | B | Odds Ratio | CI 95% Lower Limit | CI 95% Upper Limit | P Value |
|---|---|---|---|---|---|---|
| Age Groups | ||||||
| 25–35 | 1 | Reference | ||||
| 35–45 | 0.90 | 2.45 | 1.17 | 5.15 | 0.02 | |
| 45–55 | 0.94 | 2.56 | 1.20 | 5.48 | 0.02 | |
| 55+ | 1.43 | 4.17 | 2.02 | 8.61 | <0.01 | |
|
| ||||||
| High Blood Pressure | ||||||
|
| ||||||
| No | 1 | Reference | ||||
| Yes | −0.88 | 0.42 | 0.26 | 0.67 | <0.01 | |
|
| ||||||
| Triglyceride | ||||||
|
| ||||||
| <150 mg/dL | 1 | Reference | ||||
| ≥150 mg/dL | −0.57 | 0.56 | 0.36 | 0.90 | 0.02 | |
|
| ||||||
| Cooking oil | ||||||
|
| ||||||
| Liquid | 1 | Reference | ||||
| Solid | −0.75 | 0.47 | 0.30 | 0.75 | <0.01 | |
|
| ||||||
| Consuming rice three times per week | ||||||
|
| ||||||
| ≤3 per week | 1 | Reference | ||||
| >3 per week | 0.63 | 1.53 | 1.03 | 2.26 | 0.01 | |
Discussion
The prevalence of diabetes in Herat City was found to be 9.9%, which was lower than reported in similar studies from Kabul13 and Jalalabad14; and it is less than half of the rate recorded in Kandahar, in southern Afghanistan.16 This may be due to age differences and/or cultural variations. Although blood samples were collected after fasting for 10–12 hours, outliers could be present due to non-fasting status of some of the participants (noncompliance to fasting requirement) or higher level of diabetes. However, our findings are consistent with similar reports from India, Pakistan, and China.10,23–25 Global studies reported that low-and middle-income countries have a greater burden of diabetes.5 Countries from the Eastern Mediterranean region including the UAE, Saudi Arabia, Bahrain, Kuwait, and Oman have reported a higher prevalence of diabetes ranging from 13.4–18.7% among wider age ranges.11
In a multivariatable analysis, age was a significant non-modifiable risk factor for diabetes, similar to results reported by other studies.12 Our findings show a higher prevalence of diabetes in women (10.1%) as compared to men (9.8%); however, gender was not statistically significant in both the univariate and multivariable analyses. Other studies have supported the statistical significance of gender at the national level in Kabul13 and in other countries.25 Significant increases in the global age-standardized prevalence of diabetes was observed in both men and women, with the greatest increase and highest prevalence reported in men. This increase could be due to the factors such as global population aging and sex differences in prevalence of risk factors, such as smoking and BMI.5
Analysis of dietary habits showed that consuming rice more frequently is associated with higher levels of diabetes. Systolic blood pressure was significantly associated with diabetes. Hypertension, frequency of vegetable consumption, and obesity was associated with diabetes in other studies conducted in Jalalabad, Kabul, and Kandahar cities.13,14,16,26 Triglycerides were found to be significant risk factors for diabetes, which may be due to their association with obesity as supported by published research.14,27
There were several limitations to our study. The main limitation was the inability to do follow-up visits. In addition, offering blood tests and blood pressure checks could have encouraged those with pre-existing diabetes or hypertension to be over enrolled. Physical activity levels were not significantly associated with diabetes; however, this association has been supported by other studies.8,14,28,29 Our future studies may need to assess physical activity using standardized data collection tools.
Afghanistan is traditionally viewed as a conflict zone, with health issues receiving inadequate attention. The design, implementation, and reporting of scientific studies, particularly epidemiological research in health, is an essential step in improving healthcare and the public health system of Afghanistan. To our knowledge, the current study was the first epidemiological cross-sectional investigation with the objective to identify the risk factors associated with diabetes among Herat residents in Afghanistan.
The high prevalence of diabetes among the adult population and the presence of modifiable risk factors are of importance for planning and implementing effective public health interventions. The findings of this study could be used as a baseline or starting point to design and implement nationwide studies to reflect the national burden of diseases and risk factors in Afghanistan.
References
- 1.Global Report on Diabetes. WHO Press: World Health Organization; 2016. [Google Scholar]
- 2.IDF Atlas Brussels, Belgium: International Diabetes Federation. 2015 [Google Scholar]
- 3.American Diabetes Association Clinical Practice Recommendations 2003. Diabetes Care. 2003;26(suppl 1) [PubMed] [Google Scholar]
- 4.WHO Mortality Database. http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed 29 July 2017.
- 5.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (London, England) 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Khalaf MM, Eid MM, Najjar HA, Alhajry KM, Doi SA, Thalib L. Screening for diabetes in Kuwait and evaluation of risk scores. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2010;16(7):725–731. [PubMed] [Google Scholar]
- 7.Al-Moosa S, Allin S, Jemiai N, Al-Lawati J, Mossialos E. Diabetes and urbanization in the Omani population: an analysis of national survey data. Population health metrics. 2006;4:5. doi: 10.1186/1478-7954-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteghamati A, Gouya MM, Abbasi M, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31(1):96–98. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 9.Gu D, Reynolds K, Duan X, et al. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA) Diabetologia. 2003;46(9):1190–1198. doi: 10.1007/s00125-003-1167-8. [DOI] [PubMed] [Google Scholar]
- 10.Shera AS, Basit A, Fawwad A, et al. Pakistan National Diabetes Survey: prevalence of glucose intolerance and associated factors in the Punjab Province of Pakistan. Primary care diabetes. 2010;4(2):79–83. doi: 10.1016/j.pcd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Afghan Public Health Institute at the Ministry of Public Health - APHI/MoPH CSO-CA, ICF Macro, Indian Institute of Health Management Research - IIHMR, and World Health Organization Regional Office for the Eastern Mediterranean - WHO/EMRO . Afghanistan Mortality Survey 2010. Calverton, Maryland, USA: APHI/MoPH, CSO, ICF Macro, IIHMR and WHO/EMRO; 2011. [Google Scholar]
- 12.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Saeed KMI, Asghar RJ, Sahak MN, Ansari J. Prevalence and risk factors associated with diabetes mellitus among Kabul citizens— Afghanistan, 2012. International Journal of Diabetes in Developing Countries. 2015;35(3):297–303. [Google Scholar]
- 14.Mir Islam SK. Prevalence and Predictors of Diabetes Mellitus in Jalalabad City, Afghanistan-2013. Iranian Journal of Diabetes and Obesity. 2014;6(1):1–8. [Google Scholar]
- 15.Saeed KMI, Rasooly M. Prevalence of Risk Factors for Non-Communicable Diseases (NCD) Using WHO STEP-Wise Approach in Herat City Afghanistan. IOSR Journal of Pharmacy. 2016;6(10):34–40. [Google Scholar]
- 16.Saeed KMI. Prevalence of Diabetes and its Risk Factors in Urban Setting of Kandahar City, Afghanistan-2015. IOSR Journal of Pharmacy. 2016;6(11):53–60. [Google Scholar]
- 17.Bonita R, Winkelmann R, Douglas KA, de Courten M. The WHO Stepwise Approach to Surveillance (Steps) of Non-Communicable Disease Risk Factors. In: McQueen DV, Puska P, editors. Global Behavioral Risk Factor Surveillance. Boston, MA: Springer US; 2003. pp. 9–22. [Google Scholar]
- 18.Central Statistics of Afghanistan (CSO) Population: Estimated Settled Population by Civil Division, Urban, Rural and Sex-2015–16. http://www.cso.gov.af/en/page/demography-and-socile-statistics/demograph-statistics/3897111. Accessed 29 July 2017.
- 19.Diabetes Fact Sheet no. 312. 2015 http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed 29 July 2017. [Google Scholar]
- 20.Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 21.The IDF consensus worldwide definitions of the metabolic syndrome. 2006. https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome. Accessed 29 July 2017.
- 22.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. Journal of hypertension. 2003;21(11):1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Aekplakorn W, Abbott-Klafter J, Premgamone A, et al. Prevalence and management of diabetes and associated risk factors by regions of Thailand: Third National Health Examination Survey 2004. Diabetes Care. 2007;30(8):2007–2012. doi: 10.2337/dc06-2319. [DOI] [PubMed] [Google Scholar]
- 24.Zafar J, Bhatti F, Akhtar N, et al. Prevalence and risk factors for diabetes mellitus in a selected urban population of a city in Punjab. JPMA The Journal of the Pakistan Medical Association. 2011;61(1):40–47. [PubMed] [Google Scholar]
- 25.Ning F, Pang ZC, Dong YH, et al. Risk factors associated with the dramatic increase in the prevalence of diabetes in the adult Chinese population in Qingdao, China. Diabetic medicine : a journal of the British Diabetic Association. 2009;26(9):855–863. doi: 10.1111/j.1464-5491.2009.02791.x. [DOI] [PubMed] [Google Scholar]
- 26.Khawaldeh A. Hyperlipidemia in Non-Insulin-Dependent Diabetes Mellitus. Bahrain Medical Bulletin. 1999;21(4) [Google Scholar]
- 27.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. The Journal of urology. 2004;171(6 Pt 1):2341–2345. doi: 10.1097/01.ju.0000125198.32936.38. [DOI] [PubMed] [Google Scholar]
- 28.Asgari F, Agajani H, Haghazali M, Heidarian H. Non-communicable diseases risk factors surveillance in Iran. Iranian Journal of Public Health. 2009;38(Suppl 1):119–122. [Google Scholar]
- 29.Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes research and clinical practice. 2009;84(1):99–106. doi: 10.1016/j.diabres.2009.02.003. [DOI] [PubMed] [Google Scholar]