Abstract
Objective:
The Medication Research Partnership (MRP), a collaboration between a national commercial health plan and nine addiction treatment centers, implemented organizational and system changes to promote use of federally approved medications for treatment of alcohol and opioid use disorders.
Method:
A difference-in-differences analysis examined change over time in the percentage of patients receiving a prescription medication for alcohol or opioid use disorders treated in MRP (n = 9) and comparison (n = 15) sites.
Results:
MRP clinics experienced a 2.4-fold increase in patients receiving an alcohol or opioid prescription (13.2% at baseline to 31.7% at 3 years after MRP initiation); comparison clinics experienced significantly less change (17.6% to 23.5%) with an adjusted difference-in-differences of 12.5% (95% CI [5.4, 19.6], p = .001). MRP sites increased the patients with prescriptions to treat opioid use disorder from 17.0% (baseline) to 36.8% (3 years after initiation), with smaller changes observed in comparison sites (23.2% to 24.0%) and a 3-year post-initiation adjusted difference-in-differences of 19% (95% CI [8.5, 29.5], p = .000). Medications for alcohol use disorders increased in both MRP (9.0% to 26.5%) and comparison sites (11.4% to 23.1%).
Conclusions:
Promoting the use of medications to support recovery required complex interventions. The Advancing Recovery System Change Model, initially developed in publicly funded systems of care, was successfully adapted for commercial sector use. The model provides a framework for providers and commercial health plans to collaborate and increase patient access to medications.
Six medications have u.s. Food and Drug Administration (FDA) approval for the treatment of alcohol and/or opioid use disorders: acamprosate, buprenorphine, disulfiram, methadone, oral naltrexone, and extended-release naltrexone (Lee et al., 2015). Pharmacotherapy for the treatment of alcohol and opioid use disorders, however, is underused in the United States. Although the number of prescriptions written for alcohol and opioid treatment medications has increased since 2002 (Mark et al., 2009), 56% of addiction treatment centers still do not prescribe addiction medications (Abraham et al., 2013). A minority of patients with alcohol use disorders (20%) or opioid use disorders (33%) received medications as part of treatment in privately funded treatment programs (Knudsen et al., 2011b). Slow uptake of medications to treat alcohol and opioid use disorders reflects limited access to physicians in many addiction treatment programs, the lack of mechanisms to reimburse physician time and purchase medications, the high costs of some medications, abstinence-only treatment philosophies, and persistent biases against their use (Abraham et al., 2013; Aletraris et al., 2015; Jones et al., 2015; Molfenter et al., 2015).
Private health insurance companies spent about $6.1 billion in 2011 treating substance use disorders (Mark et al., 2016). Approximately 20% of the 14,000 specialty addiction treatment programs in the United States provide short-term (less than 30 days) residential treatment and residential detoxification services (Substance Abuse and Mental Health Services Administration [SAMHSA], 2014). Analyses of utilization data from large health plans suggest that patients taking medication for alcohol and opioid use disorders (compared with patients in treatment without medication support) have lower health expenditures because of reduced use of emergency care and inpatient hospitalization (Hartung et al., 2014; Lynch et al., 2014; McCarty et al., 2010). Only a minority of private treatment programs, however, provide medications for alcohol or opioid use disorders; most patients do not receive prescriptions to support their long-term sobriety and recovery (Abraham et al., 2013; Knudsen & Roman, 2012; Knudsen et al., 2011a).
Advancing Recovery
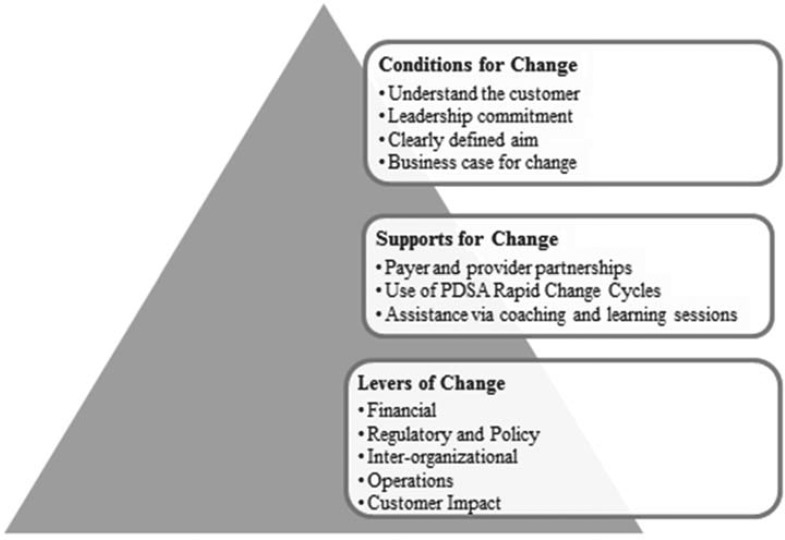
The Advancing Recovery System Change Model was developed in publicly funded systems of care to facilitate organizational change and promote the adoption of evidence-based practices for the treatment of alcohol and drug use disorders (Molfenter et al., 2013; Schmidt et al., 2012). As an extension of the Network for the Improvement of Addiction Treatment (NIATx) organizational change model (Gustafson et al., 2011), Advancing Recovery forged collaborative partnerships between addiction treatment providers and the state and county agencies that fund addiction treatment services. Twelve participating states and counties partnered with treatment providers using five levers to promote system change: (a) financial analysis (e.g., budget, cost, and reimbursement processes), (b) regulatory and policy analysis (e.g., licensing standards), (c) interorganizational analysis (e.g., stakeholder description, roles, and relationships), (d) operations analysis (e.g., organizational processes), and (e) customer impact analysis (e.g., customer feedback and preferences). States and counties modified contracts, revised regulations, and facilitated conversations with Medicaid and other payers. Treatment providers altered intake processes and workflows, networked with other providers, and sought patient input and participation (Figure 1). Five of the 12 partnerships addressed access to medications. Treatment providers in Missouri, for example, developed standard procedures to assess patients with alcohol use disorders and to prescribe naltrexone or acamprosate when appropriate; the state agency developed a centralized program for medication purchase and made medication and physician time allowable expenses in provider contracts. More patients received medications, and the state authority eventually required all addiction treatment programs to support patient use of medications (Schmidt et al., 2012).
Figure 1.
The key components of the Advancing Recovery Change Model are described, including the conditions, supports, and levers for change. Adapted from Schmidt et al., 2012. PDSA = Plan-Do-Study-Act.
The Medication Research Partnership (MRP) extended the Advancing Recovery System Change Model to a commercial health plan and tested the model’s generalizability in increasing the use of medications for members covered by the plan. Private treatment centers typically contract with multiple payers and confront complex reimbursement systems. Commercial health plans, conversely, have no regulatory authority and limited ability to mandate specific treatments. This article reports results from a 3-year assessment of quantitative outcomes of an active partnership between a private health insurance company that encouraged use of medication in short-term residential rehabilitation and outpatient facilities that contracted with the health plan.
Method
Medication Research Partnership
The MRP was a collaboration between a national commercial health plan and treatment centers contracting with the health plan. The health plan invited 24 specialty addiction treatment centers to participate in the initiative; the programs were located on the northeastern seaboard of the United States. Nine programs accepted the invitation. Eight of the nine sites offered short-term residential care (i.e., detoxification plus 7–28 days for rehabilitation and stabilization), and one site offered only outpatient care. The 15 programs that declined to participate or failed to respond to the invitation served as a nonintervention comparison group and controlled for secular changes in the uptake of addiction medications. The health plan linked and de-identified claims data for analysis. A data use agreement permitted transfer of the data to Oregon Health & Science University (OHSU). Institutional Review Boards at OHSU, the University of Wisconsin, and the University of California San Francisco reviewed and approved the study protocol.
During a 21-month active intervention, MRP tested organizational and system change interventions to enhance implementation of FDA-approved medications for alcohol and opioid use disorders and monitored sustainability over an additional 15 months (see timeline in Supplemental Appendix A). Participants received training in the Advancing Recovery System Change Model and the NIATx model of process improvement (Gustafson et al., 2011; McCarty et al., 2007; Molfenter et al., 2013; Schmidt et al., 2012). Sites completed four face-to-face “learning sessions” before and after each of three, 6-month change cycles and shared strategies to facilitate the use of medication. Learning sessions provided specific content (e.g., implementing rapid change cycles, overcoming staff resistance) and included provider presentations about their change processes (Alanis-Hirsch et al., 2016). Nationally recognized experts in the substance abuse field provided technical assistance on the use of pharmacotherapy.
Study sites identified “change leaders” and formed “change teams” to implement strategies to promote use of medications in participating treatment centers and to communicate the value of medications to staff and leadership. Change teams ranged in size from two to five. External coaches assisted change leaders on organizational change strategies with periodic conference calls and site visits. Change teams used brief, rapid change cycles to test strategies to promote medication use and adopted, adapted, or abandoned these strategies based on their own monitoring of results. These organizational changes emerged organically as change teams examined barriers within their treatment center; investigators did not prescribe specific changes. Initial change cycles were used to educate staff, patients, and patients’ families about the medication choices and why they could be a useful addition to treatment and recovery plans.
Change teams tested brochures for patient education about medications in patient intake and orientation materials and developed family education materials. As medical officers who were reluctant to support the use of medications left the organization, they were replaced with physicians who championed medications to support recovery. Participating centers adjusted workflows and responsibilities. Specific physicians assumed responsibility for induction on buprenorphine or extended-release naltrexone. Nurses and aides needed time to address precertification and utilization review requirements. Some participating centers asked representatives from the pharmaceutical manufacturers to train staff in ordering and using the medication. Pharmaceutical representatives also facilitated linkages with prescribers in the community so that discharged patients could receive refills.
The commercial health plan contributed to the change processes by inviting treatment providers to participate in the study and hosting the initial launch meeting at its headquarters. When programs reported problems with the process for ordering extended-release naltrexone, the health plan facilitated conversations with the manufacturer, who intervened to resolve concerns. The health plan initiated a change project and developed a pilot initiative that permitted increased days of residential care for patients who received extended-release naltrexone. A change team within the health plan worked to improve access to medications and educated multiple levels of staff.
Study data
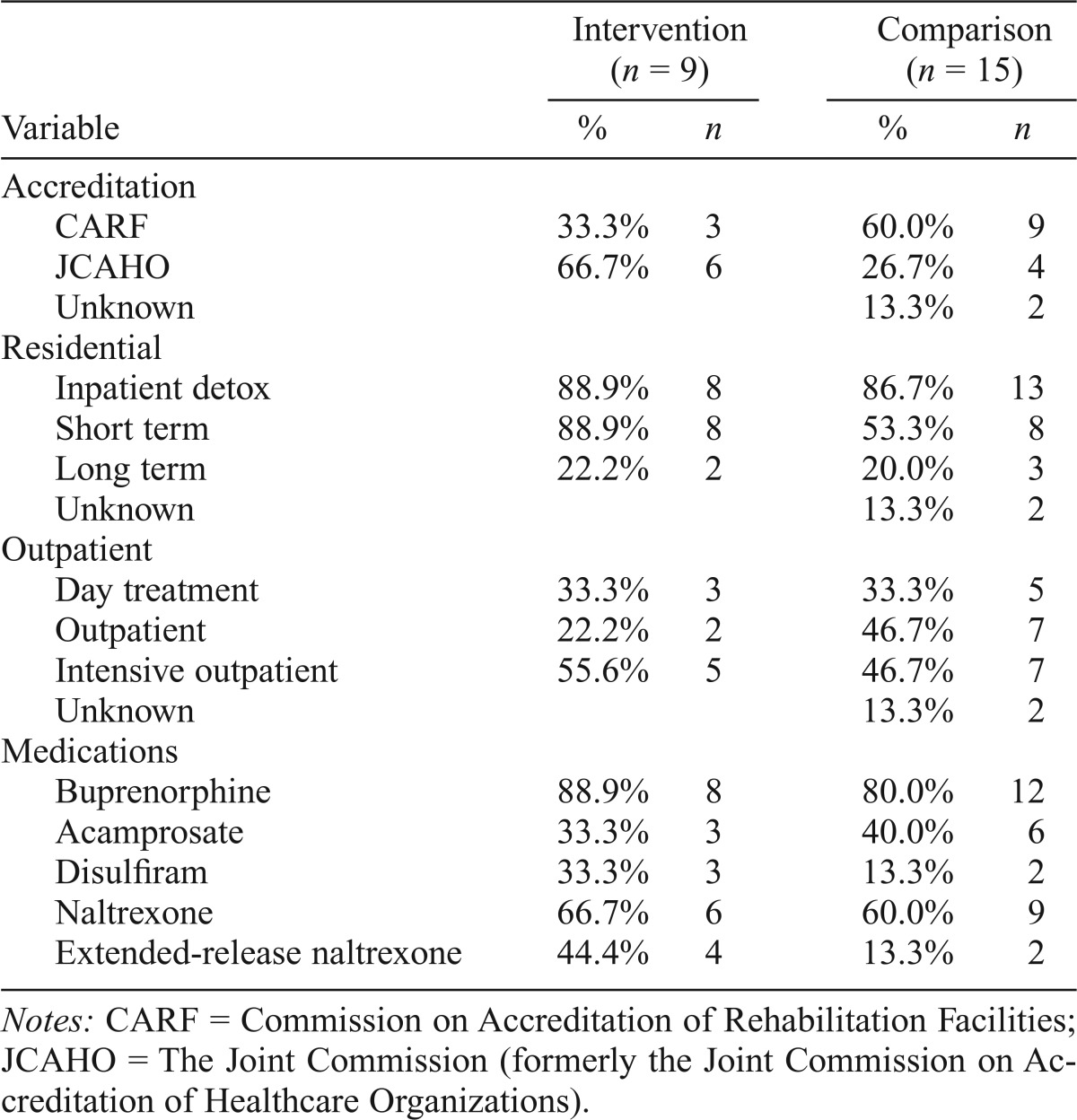
Available public information was used to prepare site profiles and to extract organizational characteristics (SAMHSA, 2015); baseline claims data assessed medication use at baseline (Table 1). MRP sites were more likely to have JCAHO (Joint Commission on Accreditation of Healthcare Organizations [now known as The Joint Commission]) accreditation (67% vs. 27%) and less likely to have CARF (Commission on Accreditation of Rehabilitation Facilities) accreditation (33% vs. 60%) (data were missing for two comparison sites). MRP sites were more likely to provide short-term residential care (89% vs. 53%) and less likely to provide standard outpatient care (22% vs. 47%). Rates for other residential and outpatient services were similar. Three MRP clinics provided only residential services, five offered a mix of residential and outpatient services, and one clinic offered only outpatient services. At the start of the study, 80% or more of the clinics offered buprenorphine to some patients. Acamprosate was offered by 33%–40% of the clinics and disulfiram by 13%–33% of participating clinics. Sixty percent of providers in the intervention clinics and two thirds of the control clinics offered oral naltrexone. The number of patients on any medication, however, was low. At baseline, four intervention clinics and two comparison clinics reported prior use of extended-release naltrexone (XR-NTX).
Table 1.
Organizational characteristic comparison: Implementation vs. control clinics
| Variable | Intervention (n = 9) |
Comparison (n = 15) |
||
| % | n | % | n | |
| Accreditation | ||||
| CARF | 33.3% | 3 | 60.0% | 9 |
| JCAHO | 66.7% | 6 | 26.7% | 4 |
| Unknown | 13.3% | 2 | ||
| Residential | ||||
| Inpatient detox | 88.9% | 8 | 86.7% | 13 |
| Short term | 88.9% | 8 | 53.3% | 8 |
| Long term | 22.2% | 2 | 20.0% | 3 |
| Unknown | 13.3% | 2 | ||
| Outpatient | ||||
| Day treatment | 33.3% | 3 | 33.3% | 5 |
| Outpatient | 22.2% | 2 | 46.7% | 7 |
| Intensive outpatient | 55.6% | 5 | 46.7% | 7 |
| Unknown | 13.3% | 2 | ||
| Medications | ||||
| Buprenorphine | 88.9% | 8 | 80.0% | 12 |
| Acamprosate | 33.3% | 3 | 40.0% | 6 |
| Disulfiram | 33.3% | 3 | 13.3% | 2 |
| Naltrexone | 66.7% | 6 | 60.0% | 9 |
| Extended-release naltrexone | 44.4% | 4 | 13.3% | 2 |
Notes: CARF = Commission on Accreditation of Rehabilitation Facilities; JCAHO = The Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations).
Episodes of care were created for members with a primary diagnosis of an alcohol use disorder, opioid use disorder, or other drug use disorder (Supplemental Appendix B). Episode initiation claims had revenue codes for detoxification, rehabilitation, or behavioral health services. The place of service was either an MRP or comparison clinic. Member episodes (N = 3,887) were equally distributed across the intervention (51%; n = 1,986) and the comparison (49%; n = 1,901) groups. MRP and comparison clinics had similar patient characteristics; mean patient age in years (MRP = 34.6; comparison = 34.5), and percentage of women (MRP = 31%; comparison = 32%) did not differ significantly. Counts of members with an alcohol and/or opioid disorder are shown in Supplemental Table A. The study analysis included all episodes of care.
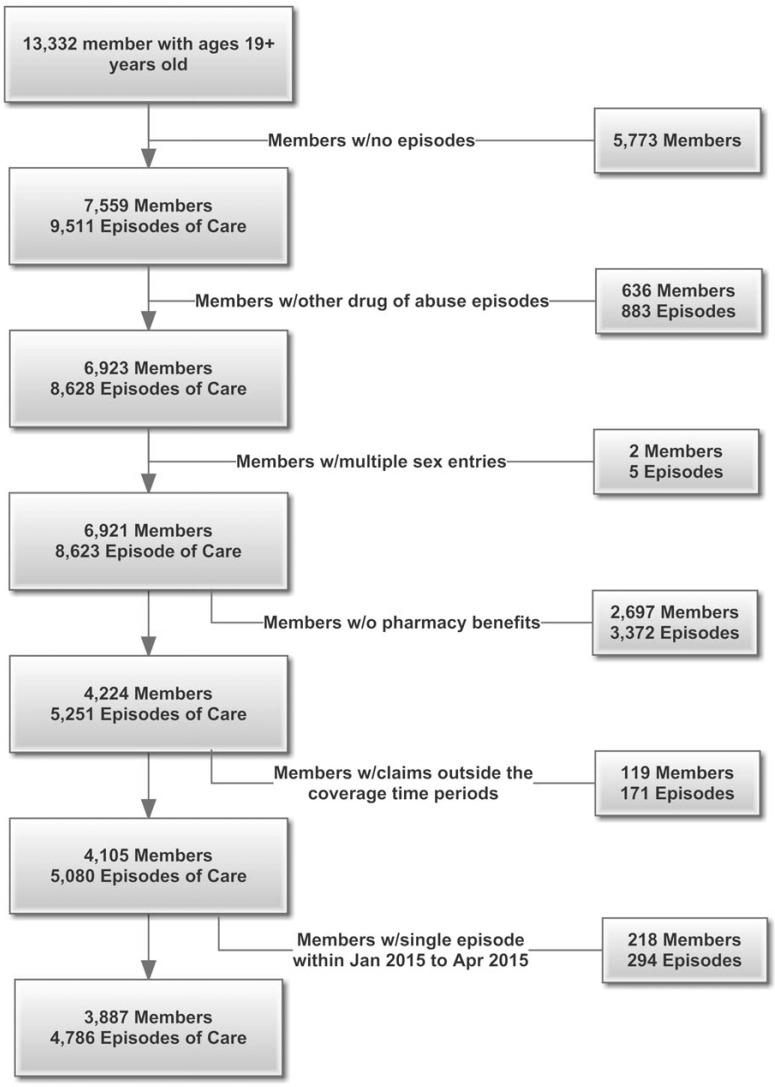
The study population was restricted to health plan members (and/or their dependents) with pharmacy benefits managed by either the health plan or by a pharmacy management program that reported utilization to the health plan. Member-level data were extracted from the commercial health plan’s utilization and claims records (see Figure 2 for a consort diagram detailing the sample selection). The initial sample started with 13,332 members age 19 or older and extracted all episodes of care for the members. A total of 5,773 members with no episodes were eliminated, leaving 7,559 members with 9,511 episodes of care. Members with diagnostic codes for other drugs of abuse (n = 636 members; 883 episodes) or with inconsistent demographic information (n = 2 members; 5 episodes) were not included in the analysis. Member episodes in which the pharmacy benefit was not managed by the health plan or a pharmacy management plan that reported to the health plan were excluded (n = 2,697 members; 3,372 episodes). Claims outside the study time-frame were also excluded (n = 337 members; 465 episodes). Among members included and excluded from the analysis, age and gender did not differ significantly. After exclusions, the final sample was 3,887 members with 4,786 episodes of care. No individual episode of care exceeded 90 days. (Note: The word member is used within a health plan when describing the claims selection process. Going forward, the word patient refers to the member and/or his or her dependents.)
Figure 2.
Inclusion and exclusion criteria for selecting analytical study sample. The figure provides an overview of the consort diagram for the identification and selection of the episodes of care to be included in the analysis.
The analysis included two patient groups: (a) patients with an alcohol use disorder diagnosis during their episode of care, and (b) patients with an opioid use disorder diagnosis during their episode of care. To avoid double counting patients with both an alcohol and an opioid use disorder diagnosis during the same episode of care, patients with both alcohol and opioid use disorders were included in the opioid cohort. The two groups were combined to assess the overall effect. Alcohol patients could receive prescriptions for acamprosate, disulfiram, oral naltrexone, and/or extended-release naltrexone; opioid patients could receive prescriptions for buprenorphine, oral naltrexone, and/or extended-release naltrexone. Patients treated at least once at an intervention site were assigned to the intervention group. Patients treated in a comparison clinic and without care from an intervention treatment center were assigned to the comparison group.
Claims were categorized as pre-intervention (claims from October 1, 2010, to September 30, 2011— the Partnership’s initial learning session was held in October 2011), Year 1 (January 1, 2012 to December 31, 2012), Year 2 (January 1, 2013, to December 31, 2013), and Year 3 (January 1, 2014, to December 31, 2014). Age, gender, patient’s treatment status (i.e., intervention vs. control clinic), and type of coverage (i.e., employee, spouse, significant other, or child) were extracted from the claims data and used as covariates in the analysis.
Statistical analysis
The analysis included the intervention sites that provided short-term residential rehabilitation services, outpatient services, or a combination of residential and outpatient services. Differences in demographic characteristics were assessed using chi-square tests and z tests, for categorical and continuous outcomes, respectively. The descriptive analysis examined unadjusted medication prescription rates in the MRP and comparison patient groups (Alcohol, Opioid, and Alcohol and Opioid combined). Change over time was assessed at 1 year, 2 years, and 3 years after initiation of the intervention against the pre-intervention baseline.
Three regression analyses examined the alcohol and opioid patients together and separately (see Supplemental Appendix C for analytic model). A difference-in-differences framework assessed the change in percentage of patients receiving medication over time for the MRP sites relative to the comparison group (Ai & Norton, 2003; Dimick & Ryan, 2014; Ryan et al., 2015). The difference-in-differences is equivalent to the average difference (after the baseline assessment) in medication prescribing within the intervention group minus the average difference within the comparison group (i.e., the interaction of time and the intervention assignment). The first difference reflects changes in prescribing that occur in the intervention group after the implementation of the medication-assisted treatment intervention. By subtracting the second difference—the changes that occur in the comparison group—we account for secular change that may have occurred for reasons not related to the implementation of the change intervention. Significant differences in prescribing—the difference-in-differences—are attributed to the study intervention. Difference-in-differences analyses are an established method for observational studies of policy interventions (Goldman et al., 2006; McConnell et al., 2012).
The difference-in-differences analysis constructed linear probability models using observations for each patient in each episode of care and adjusted for the demographic covariates and time variables. The linear probability model has the advantage of generating coefficient estimates that can be directly interpreted as the impact of a covariate on the probability of receiving a prescription (Ai & Norton, 2003; Angrist & Pischke, 2008; Karaca-Mandic et al., 2012). For example, a coefficient of 0.03 on the Treatment x Post Period 1 interaction would indicate that the treated group had a 3% higher probability of receiving a prescription after the intervention, compared with the control group. Following a sensitivity analysis, we also display results from models that used a logistic regression in conjunction with Stata’s “Margin” command. These analyses suggest that the estimates from the linear probability models are almost identical to those generated from the logistic modeling approach.
The independent variables included in the models were (a) program type (MRP vs. comparison), (b) period (2011 vs. 2012, 2013, and 2014), (c) type of coverage (subscriber, spouse, child, or other), (d) a statistical interaction term between period and program type (the difference-in-differences), (e) age (in years), and (f) gender (male/female). The dependent variable assessed presence or absence of a filled prescription for a study medication during the episode of care. The interaction term determined if changes in prescribing for the intervention group differed significantly from changes in prescribing for the comparison group (Karaca-Mandic et al., 2012).
Sub-analyses examined the alcohol and opioid patients separately. Linear and logistic regression models were constructed using observations of each patient in each episode of care within each patient group.
Results
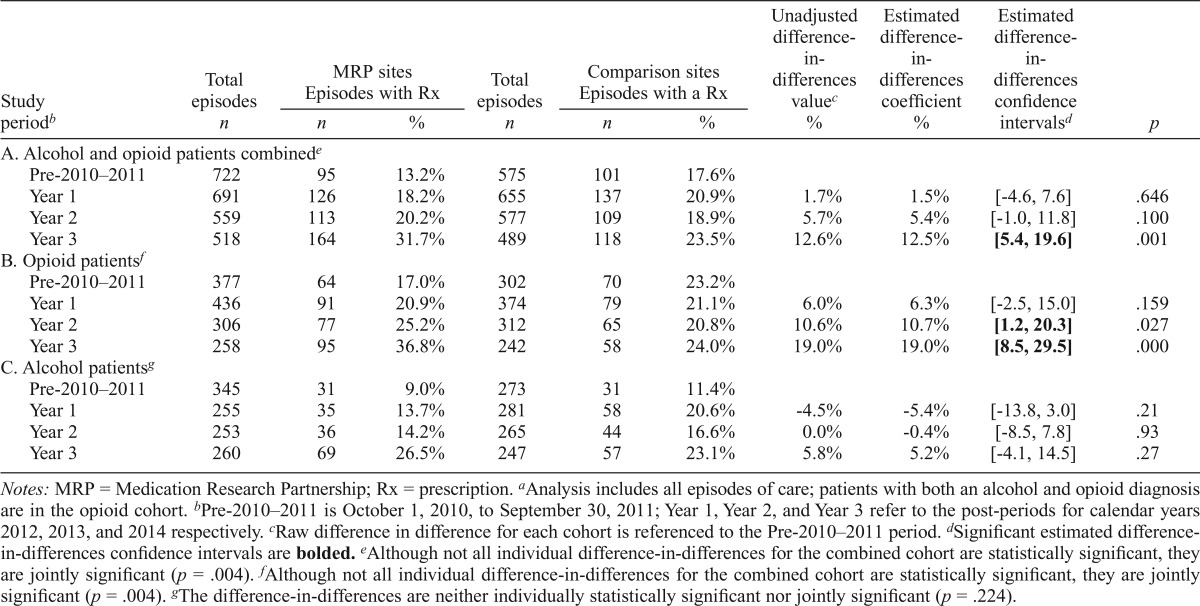
Use of medications for alcohol and opioid use disorders increased over the study period in both MRP and comparison sites. The difference-in-differences analysis, using all episodes of care and the interaction of time and intervention assignment, suggested that by Year 3, gains were greater within MRP clinics than in the comparison clinics. Table 2, section A, provides descriptive data on intervention and comparison sites. The percentage of treatment episodes for patients receiving a prescription for medications incrementally increased from baseline (MRP = 13%; comparison = 18%) through Year 1 (MRP = 18%; comparison = 21%), Year 2 (MRP = 20%; comparison = 19%), and Year 3 (MRP = 32%; comparison = 24%). Although the comparison treatment centers initially had a higher percentage of treatment episodes for patients receiving a prescription for an approved medication at baseline, the intervention clinics surpassed them by Year 3. The adjusted difference-in-differences reached a significant difference from baseline in Year 3 (12.5%, p = .001).
Table 2.
Intervention impact: All episodes of care for members filling prescriptions for approved medicationsa
| Total episodes | MRP sites Episodes with Rx |
Total episodes | Comparison sites Episodes with a Rx |
Unadjusted difference-in-differences valuec | Estimated difference-in-differences coefficient | Estimated difference-in-differences confidence intervalsd | p | |||
| Study periodb | n | n | % | n | n | % | % | % | ||
| A. Alcohol and opioid patients combinede | ||||||||||
| Pre-2010–2011 | 722 | 95 | 13.2% | 575 | 101 | 17.6% | ||||
| Year 1 | 691 | 126 | 18.2% | 655 | 137 | 20.9% | 1.7% | 1.5% | [-4.6, 7.6] | .646 |
| Year 2 | 559 | 113 | 20.2% | 577 | 109 | 18.9% | 5.7% | 5.4% | [-1.0, 11.8] | .100 |
| Year 3 | 518 | 164 | 31.7% | 489 | 118 | 23.5% | 12.6% | 12.5% | [5.4,19.6] | .001 |
| B. Opioid patientf | ||||||||||
| Pre-2010–2011 | 377 | 64 | 17.0% | 302 | 70 | 23.2% | ||||
| Year 1 | 436 | 91 | 20.9% | 374 | 79 | 21.1% | 6.0% | 6.3% | [-2.5, 15.0] | .159 |
| Year 2 | 306 | 77 | 25.2% | 312 | 65 | 20.8% | 10.6% | 10.7% | [1.2, 20.3] | .027 |
| Year 3 | 258 | 95 | 36.8% | 242 | 58 | 24.0% | 19.0% | 19.0% | [8.5, 29.5] | .000 |
| C. Alcohol patientsg | ||||||||||
| Pre-2010–2011 | 345 | 31 | 9.0% | 273 | 31 | 11.4% | ||||
| Year 1 | 255 | 35 | 13.7% | 281 | 58 | 20.6% | -4.5% | -5.4% | [-13.8, 3.0] | .21 |
| Year 2 | 253 | 36 | 14.2% | 265 | 44 | 16.6% | 0.0% | -0.4% | [-8.5, 7.8] | .93 |
| Year 3 | 260 | 69 | 26.5% | 247 | 57 | 23.1% | 5.8% | 5.2% | [-4.1, 14.5] | .27 |
Notes: MRP = Medication Research Partnership; Rx = prescription.
Analysis includes all episodes of care; patients with both an alcohol and opioid diagnosis are in the opioid cohort.
Pre-2010–2011 is October 1, 2010, to September 30, 2011; Year 1, Year 2, and Year 3 refer to the post-periods for calendar years 2012, 2013, and 2014 respectively.
Raw difference in difference for each cohort is referenced to the Pre-2010–2011 period.
Significant estimated difference- in-differences confidence intervals are bolded.
Although not all individual difference-in-differences for the combined cohort are statistically significant, they are jointly significant (p = .004).
Although not all individual difference-in-differences for the combined cohort are statistically significant, they are jointly significant (p = .004).
The difference-in-differences are neither individually statistically significant nor jointly significant (p = .224).
The impact of the intervention was more substantial for opioid use disorders. Medications to treat opioid use disorders (Table 2, section B) increased from 17% at baseline to 37% at the 3-year follow-up within MRP sites; comparison sites saw a slight gain (from 23% to 24%). MRP sites experienced the largest increase in medication use in study Years 2 and 3. The difference-in-differences increased from 6.3% to 10.7% (Year 2, p = .027, CI [1.2%, 20.3%]) and to 19% (Year 3, p = .000, CI [8.5%, 29.5%]). Patients prescribed medications to treat alcohol use disorders (Table 2, section C) increased from 9% (baseline) to 27% (Year 3) within MRP sites; comparison sites increased from 11% to 23%. The adjusted difference-in-differences did not differ significantly. Similar results were found when individuals with both an alcohol and an opioid diagnosis were included in the Alcohol cohort (Supplemental Table B).
A sensitivity analysis (Supplemental Table C) examined the last member episodes of care (n = 3,887). Within each group of patients, the total episodes and episodes with a prescription increased for the intervention and comparison clinics. Although the percentage of episodes with a prescription changed slightly, the results were similar to the assessment of all episodes of care. Supplemental Figures A (all episodes of care) and B (last episodes of care) provide pictorial representations of the change in percentage of members with a prescription. Using all episodes of care, we also examined changes in specific medication prescribing (Supplemental Table D). By 2014, the intervention clinics had a higher overall use of oral naltrexone and extended-release naltrexone for opioid patients versus the comparison clinics and of extended-release naltrexone for alcohol patients.
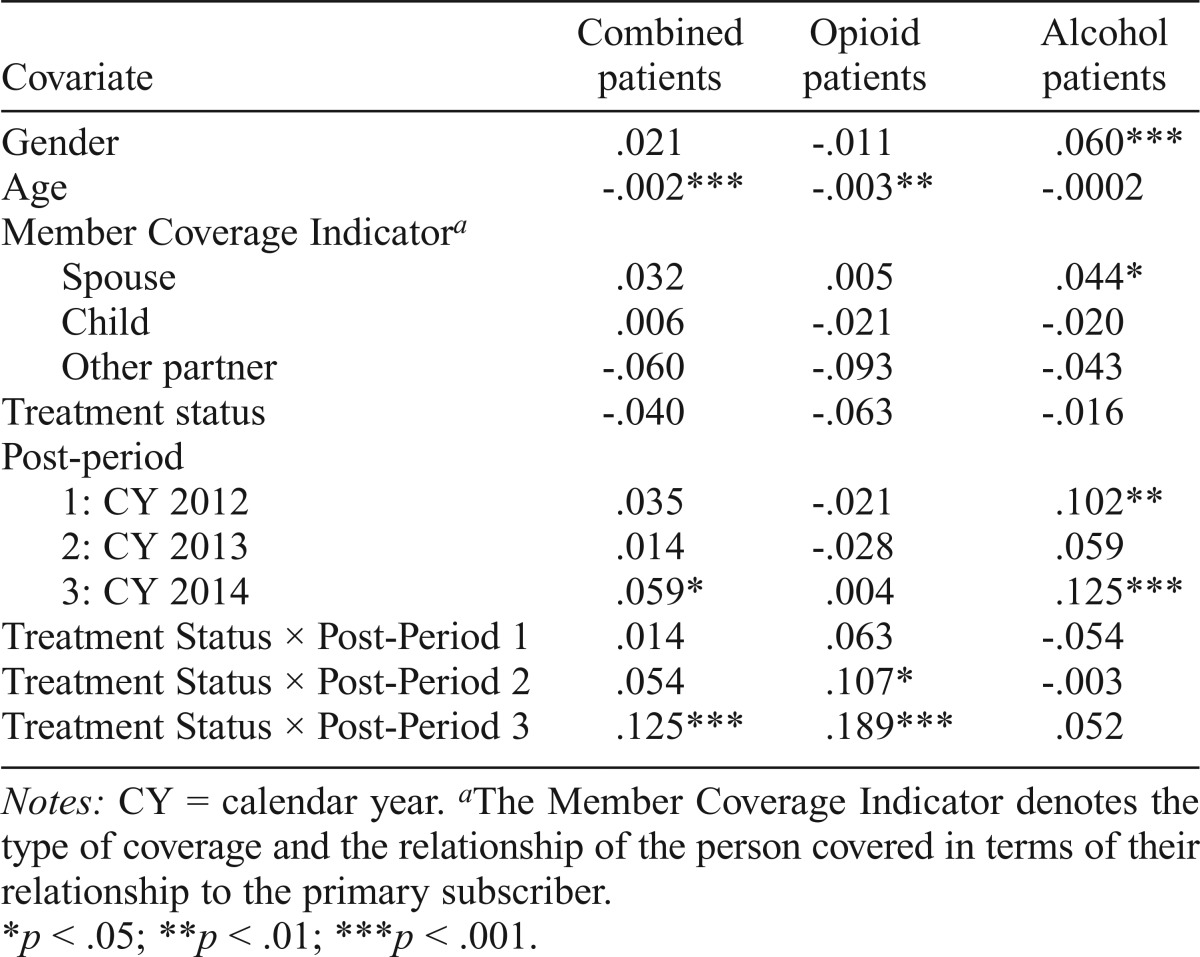
Patient demographics were associated with the differential use of medications (Table 3). In the alcohol group analysis, individuals with insurance coverage under their spouses were 4% (p < .05) more likely to receive a medication than the covered member. In the combined analyses, patients were -0.2% (p < .001) less likely to receive a medication for each year difference between their age and the average age of the cohort. A similar effect was seen in the opioid cohort. Women in the alcohol group were 6% (p < .001) more likely than men to receive medications for an alcohol use disorder. The interaction between the treatment status (intervention) and time periods (the “difference-in-difference” estimate) was significant in Year 3 for the combined group (12.5%, p < .001) and for Year 2 and Year 3 in the opioid group (18.9%, p< .001). We did not observe any statistically significant increase in the probability of receiving a prescription among patients in the alcohol group. See Supplemental Table E for logistic regression results.
Table 3.
Linear probability model coefficients by patient group (all episodes of care)
| Covariate | Combined patients | Opioid patients | Alcohol patients |
| Gender | .021 | -.011 | .060*** |
| Age | -.002*** | -.003** | -.0002 |
| Member Coverage Indicatora | |||
| Spouse | .032 | .005 | .044* |
| Child | .006 | -.021 | -.020 |
| Other partner | -.060 | -.093 | -.043 |
| Treatment status | -.040 | -.063 | -.016 |
| Post-period | |||
| 1: CY 2012 | .035 | -.021 | .102** |
| 2: CY 2013 | .014 | -.028 | .059 |
| 3: CY 2014 | .059* | .004 | .125*** |
| Treatment Status × Post-Period 1 | .014 | .063 | -.054 |
| Treatment Status × Post-Period 2 | .054 | .107* | -.003 |
| Treatment Status × Post-Period 3 | .125*** | .189*** | .052 |
Notes: CY = calendar year.
The Member Coverage Indicator denotes the type of coverage and the relationship of the person covered in terms of their relationship to the primary subscriber.
p < .05; **p < .01; ***p < .001.
Discussion
U.S. and international organizations advocate for promoting access to medication-assisted treatment for alcohol and opioid use disorders (European Monitoring Centre for Drugs and Drug Addiction, 2015; Frenk et al., 2015; Jones et al., 2015; Office of National Drug Control Policy, 2015; United Nations Office on Drugs and Crime, 2015). The MRP found that implementation of medications to support recovery required complex multilevel interventions to improve low rates of adoption within specialty addiction treatment programs. Several participating sites requested staff training and assistance with ordering and linking to community-based pre- scribers from pharmaceutical companies manufacturing the medications. Other providers addressed organizational philosophies against the use of any medications before changes could be implemented. When such supports were provided and barriers were removed, programs achieved increased use of medications. Using the Advancing Recovery multicomponent implementation strategy, providers and payers identified and implemented changes to increase access to medications. Participants used operational, regulatory, financial, and policy changes to increase access to medications for individuals with alcohol and opioid use disorders in a commercially funded context (Molfenter et al., 2013, 2015; Rieckmann et al., 2011; Schmidt et al., 2012).
The current analysis supports adaptation of the Advancing Recovery System Change Model for providers in the commercial sector. At baseline, providers in the MRP had smaller proportions of patients receiving medications than providers in the comparison group. Over time, medication utilization increased in the intervention sites to the point of overtaking those in the comparison sites, suggesting that changes were attributable to participation in the MRP rather than to secular trends. Intervention clinics experienced a 240% increase in episodes of care receiving an alcohol or opioid prescription, whereas comparison clinics experienced significantly less change, with an adjusted difference-indifference of 12.5%. The effects of the intervention were most visible among patients with an opioid use disorder. The overall difference was driven by the use of medications to treat opioid use disorders. In intervention sites, these prescriptions increased from 13% at baseline to 31.7% at 3 years.
In contrast to the public payers who developed Advancing Recovery, the commercial payer appeared to have a less direct influence on provider prescribing. In the public sector, the state and county payers operate in what are effectively “single payer” systems of addiction treatment. Their influence is centralized and powerful. In contrast, any one commercial payer in the private sector typically accounted for a small proportion of a treatment center’s total revenue. Without an influence similar to payers in the public sector, a commercial payer may not exert a similar influence on prescribing rates. However, the health plan in our study is using value-based contracting and provider participation in an Institute of Quality to continue to promote use of medication in the treatment of alcohol and opioid use disorders. Study findings provide evidence that treatment providers, in collaboration with a commercial health plan, can achieve measurable impacts on the adoption of medications that support recovery from opioid and alcohol use disorders. Importantly, commercial health plans note reductions in the cost of care for members on medications versus counseling only (Hartung et al., 2014).
Limitations
The study used a quality improvement strategy and assessed change over time; neither the participating programs nor the patients were randomly assigned to study conditions. Staff turnover and changes in the insurance market were uncontrolled during the 36-month observation period. Admission of patients with alcohol and opioid use disorders declined over time in both the intervention and comparison clinics, but the decline was more apparent in the intervention group.
An astute reviewer asked if there was a “denominator management” problem—analyses using data from the Veterans Administration (VA) suggested that performance on incentive metrics improved because clinics became more selective of who qualified for the denominator of patients eligible to be included in the metric (Harris et al., 2016). We began by asking our contacts at the participating commercial health plan if there were industry trends that contributed to the reduction in admissions in the intervention clinics. They speculated, “We have seen the growth of additional substance abuse facilities so there may be a potential shift of admissions to other organizations. In addition, more patients may be going to lower levels of care and are not being captured in the data” (A. J. Rocchino, personal communication, November 7, 2016). Our data were restricted to claims submitted to the health plan. It appears that over time a smaller portion of the patients treated in the intervention clinics had coverage from the participating health plan. More generally, there was no incentive for denominator management. Unlike the situation in the VA clinics, there was no reward for being more or less selective in who qualified to be included in the analysis. The decline in admissions appears to be attributable to changes in who was being served rather than denominator management.
The pharmacy data extract does not include information about the provider location where the prescription was written. As such, we cannot determine if there were changes in the number of prescribers at a given provider location. Also, a criterion for patient inclusion in the study sample was having a pharmacy benefit provided by the payer—3,372 episodes from 2,697 members without a pharmacy benefit were excluded from the analysis. The study included only clinics located in the Northeast region that had a contract with a specific payer; tests of the intervention in different geographic regions or with different commercial payers may yield different results. Strategies used by the comparison clinics to implement changes were not explored.
Implications
The payer–provider partnership is the foundation of the Advancing Recovery System Change Model. This study successfully adapted the model for use in the commercial sector. Payers can identify and change policies and/or regulations or introduce new payment models designed to increase access to medications for individuals with an alcohol and/or opioid disorder. Providers can leverage these payer-initiated changes in support of efforts to offer medications to more patients. At a time when the opioid epidemic is taxing the capacity of the addiction treatment system, the results from this study may offer encouragement to providers and payers that the Advancing Recovery System Change Model can promote access to medications, an important evidence-based treatment (Ducharme et al., 2016).
Acknowledgments
The authors thank our participating providers and change leaders: Bowling Green Brandywine (Lisa Olander), Eagleville Hospital (Charlie Folks), Horsham Clinic (Billie-Jo Sellman), Livengrin Foundation (Charlie Wolfe), Mirmont Treatment Centers (Pam Fries-Coffey), Maryland Treatment Centers (Meghan Westwood), Pace (Bruce Johnson), Penn Foundation (Christopher Squillaro, DO), and White Deer Run Allenwood (Amber Dissinger and Kieran Pelletier). In addition, we would like to thank Aetna Behavioral Health (Dr. Hyong Un, Danielle Yem, Bruce D. Condit, and Dawn R. Keiser) for their support in the development and implementation of the project. We also express our appreciation to Dr. John McConnell for his statistical consultation on the manuscript. Last, we thank Dr. Kimberly A. Johnson, who was an active member of the research team.
Conflict of Interest Statement
Dr. McCarty was the Principal Investigator on Research Service Agreements from Purdue Pharma and Alkermes, Inc., and he receives support on awards from the National Institutes of Health (UG1 DA015815, R33 DA035640, P50 DA018165, R01 MH1000001, and R01 DA036522). The other authors report no potential conflicts of interest.
Footnotes
An award from the National Institute on Drug Abuse (R01-DA029716) supported the study design, implementation, and analysis.
References
- Abraham A. J., Knudsen H. K., Rieckmann T., Roman P. M. Disparities in access to physicians and medications for the treatment of substance use disorders between publicly and privately funded treatment programs in the United States. Journal of Studies on Alcohol and Drugs. 2013;74:258–265. doi: 10.15288/jsad.2013.74.258. doi:10.15288/jsad.2013.74.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai C., Norton E. C. Interaction terms in logit and probit models. Economics Letters. 2003;80:123–129. doi:10.1016/S0165-1765(03)00032-6. [Google Scholar]
- Alanis-Hirsch K., Croff R., Ford J. H., II, Johnson K., Chalk M., Schmidt L., McCarty D. Extended-release naltrexone: A qualitative analysis of barriers to routine use. Journal of Substance Abuse Treatment. 2016;62:68–73. doi: 10.1016/j.jsat.2015.10.003. doi:10.1016/j.jsat.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aletraris L., Edmond M. B., Roman P. M. Adoption of injectable naltrexone in U.S. substance use disorder treatment programs. Journal of Studies on Alcohol and Drugs. 2015;76:143–151. doi:10.15288/ jsad.2015.76.143. [PMC free article] [PubMed] [Google Scholar]
- Angrist J. D., Pischke J.-S. Princeton, NJ: Princeton University Press; 2008. Mostly harmless econometrics: An empiricist's companion. [Google Scholar]
- Dimick J. B., Ryan A. M. Methods for evaluating changes in health care policy: The difference-in-differences approach. JAMA. 2014;312:2401–2402. doi: 10.1001/jama.2014.16153. doi:10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- Ducharme L. J., Chandler R. K., Harris A. H. S. Implementing effective substance abuse treatments in general medical settings: Mapping the research terrain. Journal of Substance Abuse Treatment. 2016;60:110–118. doi: 10.1016/j.jsat.2015.06.020. doi:10.1016/j.jsat.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. European drug report 2015: Trends and developments. Lisbon, Portugal: Author. 2015 Retrieved from http://www.emcdda.europa.eu/publications/edr/trends-developments/2015. [Google Scholar]
- Frenk S. M., Porter K. S., Paulozzi L. J. Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS Data Brief No. 2015;189:1–8. Retrieved from https://www.cdc.gov/nchs/products/databriefs/db189.htm. [PubMed] [Google Scholar]
- Goldman H. H., Frank R. G., Burnam M. A., Huskamp H. A., Ridgely M. S., Normand S.-L. T.…Blasinsky M.2006Behavioral health insurance parity for federal employees The New England Journal of Medicine 3541378–1386.doi:10.1056/NEJMsa053737 [DOI] [PubMed] [Google Scholar]
- Gustafson D., Johnson K., Capoccia V, Cotter F., Ford J. H., II, Holloway D.…Owens B.2011The NIATx model: Process improvement in behavioral health Madison, WI: University of Wisconsin-Madison [Google Scholar]
- Harris A. H. S., Chen C., Rubinsky A. D., Hoggatt K. J., Neuman M., Vanneman M. E.2016)Are improvements in measured performance driven by better treatment or denominator management? Journal of General Internal Medicine 31Supplement 121–27.doi:10.1007/ s11606-015-3558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung D. M., McCarty D., Fu R., Wiest K., Chalk M., Gastfriend D. R. Extended-release naltrexone for alcohol and opioid dependence: A meta-analysis of healthcare utilization studies. Journal of Substance Abuse Treatment. 2014;47:113–121. doi: 10.1016/j.jsat.2014.03.007. doi:10.1016/j.jsat.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Campopiano M., Baldwin G., McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health. 2015;105:e55–e63. doi: 10.2105/AJPH.2015.302664. doi:10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca-Mandic P., Norton E. C., Dowd B. Interaction terms in nonlinear models. Health Services Research. 2012;47:255–274. doi: 10.1111/j.1475-6773.2011.01314.x. doi:10.1111/j.1475-6773.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H. K., Abraham A. J., Oser C. B. Barriers to the implementation of medication-assisted treatment for substance use disorders: The importance of funding policies and medical infrastructure. Evaluation and Program Planning. 2011a;34:375–381. doi: 10.1016/j.evalprogplan.2011.02.004. doi:10.1016/j. evalprogplan.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H. K., Abraham A. J., Roman P. M. Adoption and implementation of medications in addiction treatment programs. Journal of Addiction Medicine. 2011b;5:21–27. doi: 10.1097/ADM.0b013e3181d41ddb. doi:10.1097/ADM.0b013e3181d41ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H. K., Roman P. M. Financial factors and the implementation of medications for treating opioid use disorders. Journal of Addiction Medicine. 2012;6:280–286. doi: 10.1097/ADM.0b013e318262a97a. doi:10.1097/ADM.0b013e318262a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kresina T. F., Campopiano M., Lubran R., Clark H. W. Use of pharmacotherapies in the treatment of alcohol use disorders and opioid dependence in primary care. BioMed Research International. 2015;2015:137020. doi: 10.1155/2015/137020. doi:10.1155/2015/137020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch F. L., McCarty D., Mertens J., Perrin N. A., Green C. A., Parthasarathy S.…Pating D.2014Costs of care for persons with opioid dependence in commercial integrated health systems Addiction Science & Clinical Practice 916.doi:10.1186/1940-0640-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark T. L., Kassed C. A., Vandivort-Warren R., Levit K. R., Kranzler H. R. Alcohol and opioid dependence medications: Prescription trends, overall and by physician specialty. Drug and Alcohol Dependence. 2009;99:345–349. doi: 10.1016/j.drugalcdep.2008.07.018. doi:10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark T. L., Yee T., Levit K. R., Camacho-Cook J., Cutler E., Carroll C. D. Insurance financing increased for mental health conditions but not for substance use disorders, 1986–2014. Health Affairs. 2016;35:958–965. doi: 10.1377/hlthaff.2016.0002. doi:10.1377/hlthaff.2016.0002. [DOI] [PubMed] [Google Scholar]
- McCarty D., Gustafson D. H., Wisdom J. P., Ford J., Choi D., Molfenter T.…Cotter F.2007The Network for the Improvement of Addiction Treatment (NIATx): Enhancing access and retention Drug and Alcohol Dependence 88138–145.doi:10.1016/j.drugalcdep.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D., Perrin N. A., Green C. A., Polen M. R., Leo M. C., Lynch F. Methadone maintenance and the cost and utilization of health care among individuals dependent on opioids in a commercial health plan. Drug and Alcohol Dependence. 2010;111:235–240. doi: 10.1016/j.drugalcdep.2010.04.018. doi:10.1016/j.drugalcdep.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell K. J., Gast S. H. N., Ridgely M. S., Wallace N., Jacuzzi N., Rieckmann T.…McCarty D.2012Behavioral health insurance parity: Does Oregon’s experience presage the national experience with the Mental Health Parity and Addiction Equity Act? American Journal of Psychiatry 16931–38.doi:10.1176/appi.ajp.2011.11020320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter T., McCarty D., Capoccia V, Gustafson D. Development of a multilevel framework to increase use of targeted evidence-based practices in addiction treatment clinics. Public Health Front. 2013;2:11–20. doi: 10.5963/PHF0201002. doi:10.5963/PHF0201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter T., Sherbeck C., Zehner M., Quanbeck A., McCarty D., Kim J.-S., Starr S. Implementing buprenorphine in addiction treatment: Payer and provider perspectives in Ohio. Substance Abuse Treatment, Prevention, and Policy. 2015;10:13. doi: 10.1186/s13011-015-0009-2. doi:10.1186/s13011-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. Washington D.C: Author; 2015. 2015 National Drug Control Strategy. [Google Scholar]
- Rieckmann T. R., Kovas A. E., Cassidy E. F., McCarty D. Employing policy and purchasing levers to increase the use of evidence-based practices in community-based substance abuse treatment settings: Reports from single state authorities. Evaluation and Program Planning. 2011;34:366–374. doi: 10.1016/j.evalprogplan.2011.02.003. doi:10.1016/j.evalprogplan.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. M., Burgess J. F., Jr., Dimick J. B. Why we should not be indifferent to specification choices for difference-in-differences. Health Services Research. 2015;50:1211–1235. doi: 10.1111/1475-6773.12270. doi:10.1111/1475-6773.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. A., Rieckmann T., Abraham A., Molfenter T., Capoccia V, Roman P.…McCarty D.2012Advancing recovery: implementing evidence-based treatment for substance use disorders at the systems level Journal of Studies on Alcohol and Drugs 73413–422.doi:10.15288/jsad.2012.73.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Author; 2014. National Survey of Substance Abuse Treatment Services (N-SSATS): 2013: Data on substance abuse treatment facilities. BHSIS Series S-73, HHS Publication No. (SMA) 14–489. Retrieved from https://www.samhsa.gov/data/sites/default/files/2013_N-SSATS/2013_N-SSATS_National_Survey_of_Substance_Abuse_Treatment_Services.pdf. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Author; 2015. National directory of drug and alcohol abuse treatment facilities 2015. HHS Publication No. (SMA) 16–4940. Retrieved from https://www.samhsa.gov/data/sites/default/files/2015_National_Directory_of_Drug_and_Alcohol_Abuse_Treatment_Centers_v1.pdf. [Google Scholar]
- United Nations Office on Drugs and Crime. Vienna, Austria: Author; 2015. World drug report 2015. Retrieved from https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. [Google Scholar]