Abstract
Although chimeric antigen receptor (CAR)-engineered T cell therapy has achieved encouraging clinical trial results for treating hematological cancers, further optimization can likely expand this therapeutic success to more patients and other cancer types. Most CAR constructs used in clinical trials incorporate single chain variable fragment (scFv) as the extracellular antigen recognition domain. The immunogenicity of nonhuman scFv could cause host rejection against CAR T cells and compromise their persistence and efficacy. The limited availability of scFvs and slow discovery of new monoclonal antibodies also limit the development of novel CAR constructs. Adnectin, a class of affinity molecules derived from the tenth type III domain of human fibronectin, can be an alternative to scFv as an antigen-binding moiety in the design of CAR molecules. We constructed adnectin-based CARs targeting epithelial growth factor receptor (EGFR) and found that compared to scFv-based CAR, T cells engineered with adnectin-based CARs exhibited equivalent cell-killing activity against target H292 lung cancer cells in vitro and had comparable antitumor efficacy in xenograft tumor-bearing mice in vivo. In addition, with optimal affinity tuning, adnectin-based CAR showed higher selectivity on target cells with high EGFR expression than on those with low expression. This new design of adnectin CARs can potentially facilitate the development of T cell immunotherapy for cancer and other diseases.
Keywords: chimeric antigen receptor (CAR), adnectin, single chain variable fragment (scFv), epithelial growth factor receptor (EGFR), adoptive T cell therapy (ACT)
Wang and colleagues show that a novel design of CAR based on adnectin, a class of scaffold molecules derived from the tenth type III domain of human fibronectin, can target EGFR-positive tumor cells and exhibit a similar functional profile as compared with the conventional scFv-derived CAR.
Introduction
Chimeric antigen receptor (CAR) is a synthetic chimeric receptor composed of an antigen recognition domain, typically an extracellular single chain variable fragment (scFv) derived from monoclonal antibody (mAb), a hinge, a transmembrane domain, intracellular signaling, and costimulatory domains. Adoptive transferred CAR-engineered T (CAR-T) cells can specifically bind to their targets, such as tumor-associated antigens (TAAs), via the antigen recognition domain and thus mediate cell-killing activity toward the target cells.1 CAR-T cell therapy has achieved notable success and has shown promise in cancer treatment, especially for treating hematological malignancies. In recent years, multiple clinical trials testing CD19-targeting CAR-T cells in adult and pediatric patients with relapsed and refractory B cell acute lymphoblastic leukemia (ALL) have exhibited a high rate of remissions (70%–90%).2, 3, 4, 5, 6, 7, 8 CD19 CAR-T cells have shown a very encouraging clinical outcome in chronic lymphocytic leukemia (CLL) and lymphoma patients as well;9, 10 the overall response rate in CLL patients is not as high, yet the responding patients have achieved durable remissions for years.11, 12, 13 Inspired by this impressive success of CAR-T cells in B cell malignancies, researchers in the field have been actively broadening indications for existing CARs, developing new CAR constructs and exploring new targets for this modality in order to treat a broader range of cancers. There are over 100 ongoing clinical trials worldwide evaluating CAR-T cell therapy in both liquid tumors and solid tumors,14 and the number of trials is still increasing.
However, because the most widely used CAR design strategy has some considerable limitations, it is necessary to develop an effective method for novel CAR construct design to meet fast-growing needs. CARs are typically designed to incorporate scFv as the ectodomain to confer specificity against target antigens. Due to the availability of existing well-characterized mouse monoclonal antibodies, many CAR constructs used in clinical trials contain murine-derived scFv.15 The immunogenicity of the xenogeneic regions in CAR molecules increases the risk of developing an undesired immune response against the CAR-T cells in host. Previous clinical experience has shown responses, such as human anti-mouse antibody (HAMA), and even one death caused by life-threatening anaphylaxis after multiple infusions of murine scFv-derived CAR-T cells targeting mesothelin.16 The immune responses also resulted in limited persistence and compromised efficacy of CAR-T cells in the clinic.17, 18 One strategy to reduce immunogenicity of CAR-T cells is to humanize the scFv. However, despite its time-consuming procedure, the humanization of scFv by CDR grafting alone might not completely preclude the potential immunogenicity.15, 18 Rather than nonhuman scFvs, human-derived proteins could be advantageous in preventing the unwanted immune responses to CARs. scFv has several other limitations as a key domain in CAR molecules, such as impaired stability19 and the potential to cause tonic signaling and exhaustion of CAR-T cells.20 Alternative affinity molecules with a simple and stable structure may be of advantage to substitute scFvs in CARs and overcome their limitations.
Adnectin is derived from a single domain scaffold, the tenth type III domain of human fibronectin (10Fn3). The 10Fn3 domain is a member of the immunoglobulin (Ig) superfamily and contains a beta sandwich protein fold, which resembles an Ig variable domain but has no disulfide bonds.21, 22 The protein fold contains three loops, BC, DE, and FG, which are structurally analogous to antibody complementarity-determining regions (CDRs).23 Introduction of mutations in these loops can confer different binding capacities in the 10Fn3-based variants.24 Hence, the 10Fn3 domain can be modified to mimic scFv to bind to proteins of interest other than its natural target integrin. By mRNA, phage, or yeast display, adnectins can be efficiently adapted to bind the target of interest with high affinity and specificity.24 Adnectins typically have a smaller size and more stable structure than scFv and are monomeric without disulfide bonds, which may mitigate the basal level CAR activation caused by random crosslinking or dimerization of scFv-derived extracellular domains.20 Adnectins originate from human fibronectin and therefore have minimal immunogenic potential. Because of these stated properties, adnectin has multiple advantages over scFv and may be suitable as an alternate antigen-binding domain of the CAR molecule.
We designed both scFv-based and adnectin-based CARs targeting human wild-type epidermal growth factor receptor and compared their functions in this study. Epithelial growth factor receptor (EGFR) is one of the most attractive targets for cancer therapy. It is widely overexpressed in a variety of cancers, and high level of EGFR expression is correlated with poor prognosis for many cancer types.25, 26, 27 The scFv-based CAR was derived from the scFv sequence of Cetuximab, a Food and Drug Administration (FDA)-approved chimeric monoclonal antibody against human EGFR, which has achieved considerable success in the clinic.28 We exploited the previous work of Emanuel et al.,29 in which they have evaluated different adnectin clones with high affinity to human EGFR to construct four adnectin-based EGFR CARs.
Results
Design and Generation of EGFR-Specific CAR Constructs
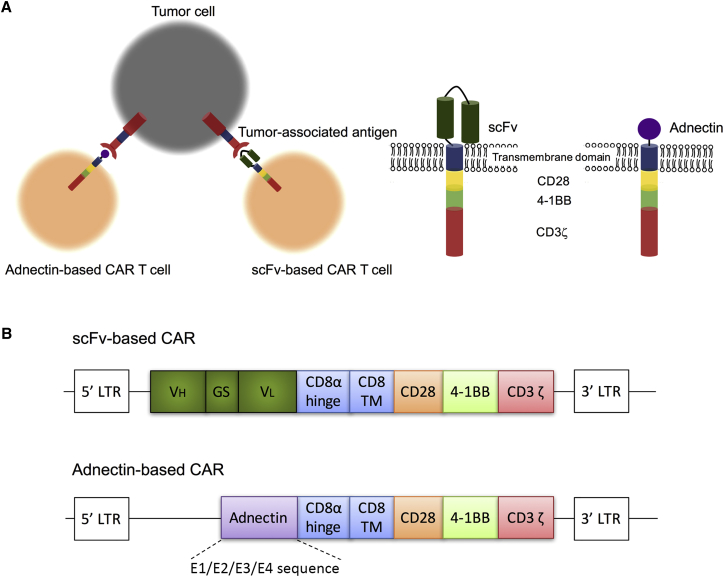
We have constructed several third-generation CARs targeting human wild-type EGFR. The conventional scFv-based CAR incorporates the scFv derived from cetuximab as the antigen-recognition domain and hence is designated as Cetux-CAR. The adnectin-CARs use EGFR-targeting adnectin sequences that were previously published by Emanuel et al.29 to substitute the scFv sequence. Using an mRNA display, they generated four adnectin clones (E1, E2, E3, and E4) targeting the human wild-type EGFR extracellular domain with a different affinity (KD = 0.7, 3.4, 9.92, and 0.13 nM).29 The corresponding CARs are designated as E1-CAR, E2-CAR, E3-CAR, and E4-CAR. The above scFv-based and adnectin-based ectodomains are linked through CD8α hinge and transmembrane domains, CD28 and 4-1BB costimulatory domains, and CD3ζ T cell receptor signaling domain (Figure 1).
Figure 1.
Engineering T Cells with scFv-Based CAR and Adnectin-Based CAR to Target Tumor
(A) Schematic representation of adnectin-based CAR structure. Adnectin can be used as the antigen-recognition domain in the CAR construct instead of scFv. (B) CAR construct targeting human wild-type EGFR. For scFv-based CAR, the scFv region of Cetuximab was fused in frame with the CD8α hinge and transmembrane domain, followed by the CD28/4-1BB/CD3ζ signaling domains. For adnectin-CAR, different clones of EGFR-targeting adnectins (E1/E2/E3/E4) were cloned upstream of the hinge domain to replace the Cetuximab scFv sequence.
Evaluation of Adnectin-Based CARs
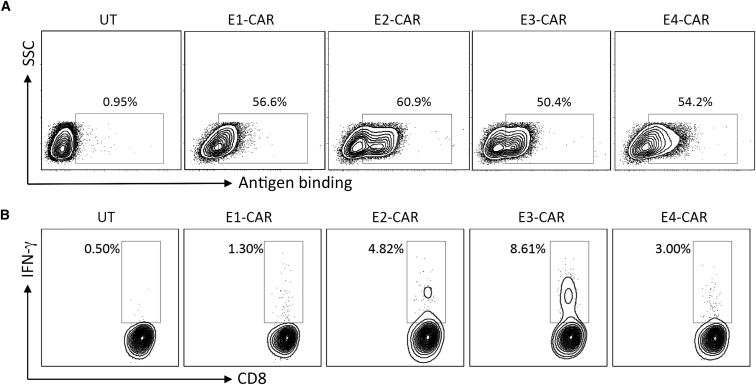
Human peripheral blood mononuclear cells (PBMCs) were activated for 2 days and transduced with viral vectors encoding different adnectin-CARs. All four groups of adnectin-CAR-T cells demonstrated expected surface expression of CARs and a similar CAR-positive percentage (approximately 50%–60%), measured by binding of recombinant human EGFR (Figure 2A). When cocultured with EGFR-overexpressing breast cancer cells MDA-MB-231,30 these four adnectin-CARs displayed different levels of reactivity against target cells. E3-CAR T cells had the highest percentage of interferon gamma (IFN-γ)-secreting CD8 T cells, measured by intracellular cytokine staining (Figure 2B). It indicates that although E3 adnectin has the lowest affinity toward EGFR among the four clones,29 E3-CAR exhibits the highest reactivity against the target MDA-MB-231 cells. The differences among these adnectin clones may be attributed to their different binding sites and hence distinct accessibility to the target antigen in the tumor cell surface. In the following experiments, E3-CAR was chosen as the adnectin-CAR to compare with Cetux-CAR.
Figure 2.
Evaluation of Adnectin-CARs Based on Their Expression and Functional Activity in Human T Cells
(A) Different groups of adnectin-CAR T cells and untransduced T cells (UT) were incubated with recombinant human EGFR-Fc protein followed by staining with PE-conjugated goat anti-human Fc antibody to assess the CAR expression via antigen binding. (B) All four groups of adnectin-CAR T cells were cocultured with EGFR-overexpressing MDA-MB-231 breast cancer cells in the presence of GolgiPlug inhibitor for 6 hr to assess the CAR-T cell activity. Untransduced T cells were used as a negative control. Interferon-gamma (IFN-γ)-producing CD8+ T cells were detected by intracellular staining with anti-IFN-γ antibody and their percentage over the total CD8+ T cell population is shown in each panel.
E3-CAR Displayed Lower Binding Affinity toward EGFR Compared to Cetux-CAR
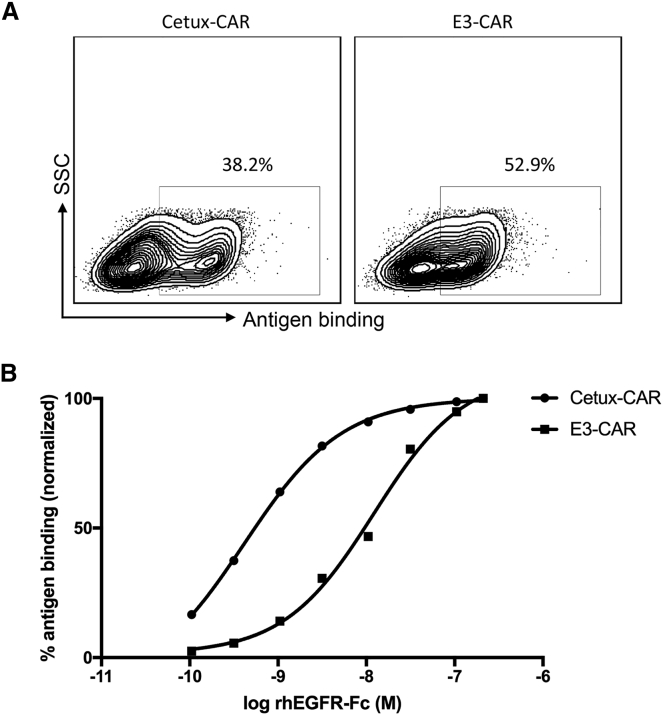
To compare scFv-CAR and adnectin-CAR, we activated and transduced human T cells with viral vectors encoding Cetux-CAR or E3-CAR in parallel and measured the CAR expression on the cell surface. E3-CAR had a higher rate of expression than did Cetux-CAR (52.9% versus 38.2%), whereas the CAR expression level was lower than that of Cetux-CAR. The E3-CAR+ population had a relatively lower median fluorescence intensity (MFI 600) than that of the Cetux-CAR+ population (MFI 973). Overall, their CAR expression in human primary T cells was comparable (Figure 3A).
Figure 3.
Comparison of Binding Affinity of Cetux-CAR and E3-CAR
(A) Comparison of Cetux-CAR and E3-CAR expression in human T cells. (B) The binding affinity of Cetux-CAR and E3-CAR to their target antigen EGFR was assessed. CAR-T cells were stained with recombinant human EGFR-Fc at different concentrations and subsequently with goat anti-human Fc antibody. The fluorescence was measured by flow cytometry and normalized into percentage of antigen binding.
We next determined whether the difference of Cetuximab scFv (KD = 0.39 nM)31 and adnectin E3 (KD = 9.92 nM)29 in their binding affinity toward EGFR remained in the CAR structure. The two groups of CAR-T cells were stained with recombinant human EGFR-Fc at serially diluted concentrations. As expected, Cetux-CAR exhibited about a 30-fold higher binding affinity to the target antigen compared to E3-CAR. Cetux-CAR achieved 50% of normalized antigen binding at 0.41 nM of target antigen EGFR, whereas E3-CAR reached a concentration of 12.21 nM (Figure 3B). The relatively lower binding affinity of E3 could be exploited for better distinction between high and low EGFR-expressing target cells.
E3-CAR T Cells Displayed Higher Selectivity against EGFR-Overexpressing Cancer Cells from Lower EGFR-Expressing Cells
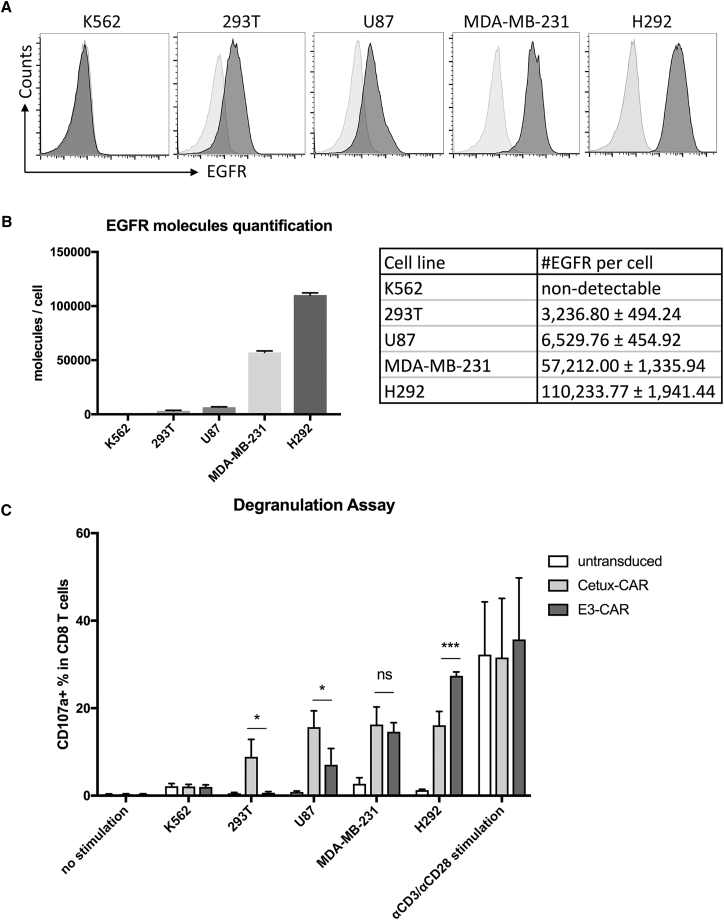
Affinity-tuned CARs bearing lower affinity scFvs have been demonstrated to be beneficial for enhanced selectivity against cells with a different target antigen density.32, 33 To evaluate the selectivity of E3-CAR toward different target cells, we first collected multiple EGFR-positive cell lines and one EGFR-negative cell line and quantified their EGFR expression levels. The EGFR-positive cell lines (293T, U87, MDA-MB-231, and H292) all express wild-type EGFR on the cell surface, but the expression levels are very different, ranging from 103 to 105 molecules per cell. Based on the quantification results, H292 and 293T cells had the highest and lowest EGFR expression levels, respectively, and MDA-MB-231 cells exhibited a medium-level EGFR expression. As expected, the EGFR-negative cell line K562 did not display detectable EGFR molecules on the surface (Figures 4A and 4B).
Figure 4.
Activation of E3-CAR T Cells Is Positively Correlated with EGFR Density on Target Cells
(A) Different cell lines (K562, 293T, U87, MDA-MB-231, and H292) were stained with PE anti-human EGFR antibody (dark gray histograms) and mouse IgG1-PE as isotype control (light gray histograms). The representative histograms from triplicates are shown above. (B) EGFR expression was measured and quantified by flow cytometry. Summarized statistics from triplicates are shown in the bar graph and table. (C) On day 10 post-activation, Cetux-CAR T, E3-CAR T, and untransduced T cells were cocultured with H292 cells at a 1:1 ratio for 4 hr with GolgiStop inhibitor and FITC-conjugated CD107a antibody against the degranulation marker CD107a. Unstimulated CAR-T cells were used as negative control, whereas anti-CD3 and anti-CD28 antibody stimulated CAR-T cells were used as positive control. The upregulated degranulation marker CD107a was identified, and the CD107a+ CD8+ T cell population was gated and its percentage over total CD8+ T cells is shown in each scatterplot. This degranulation assay was repeated with CAR T cells derived from three different donors, and the summarized statistics are shown in bar graphs (n = 3, mean ± SEM; ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA with Tukey’s multiple comparison).
Cetux-CAR displayed similar levels of reactivity against all the EGFR-positive cell lines, presumably due to the high affinity of Cetuximab scFv (Figure 4C). In contrast, E3-CAR T cells could distinguish target cells with a different EGFR density. E3-CAR T cells had the upregulated degranulation marker CD107a in the presence of EGFR-overexpressing cancer cells, such as U87, MDA-MB-231, and H292, and the percentage of degranulation with H292 was significantly higher than that of Cetux-CAR (p < 0.001). On the other hand, E3-CAR T cells did not show reactivity toward 293T cells, which only had low-level endogenous EGFR expression.34
E3-CAR Had Comparable Reactivity against H292 Lung Cancer Cells to That of Cetux-CAR
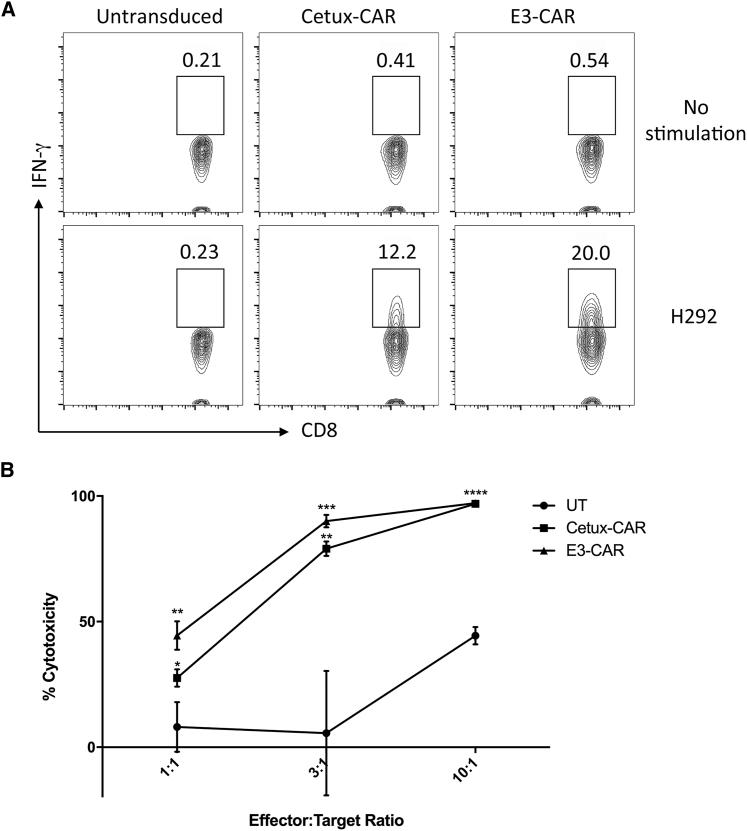
To further determine the function of E3-CAR T cells, we performed an intracellular staining assay and cytotoxicity assay with the lung cancer cell line H292, which has a high expression level of wild-type EGFR on the cell surface.12 In the presence of the GolgiPlug inhibitor, Cetux-CAR, E3-CAR, and untransduced T cells were cocultured with H292 cells for 6 hr and then the production of inflammatory cytokine IFN-γ in the CD8+ T cell population was measured and shown in the scatterplot. All the CD8+ T cell populations in different groups only displayed a background level of IFN-γ in the absence of stimulation. Upon stimulation of H292 target cells, Cetux-CAR T cells displayed 12.2% IFN-γ+ CD8 T cells, whereas E3-CAR T cells exhibited higher activity (20.0% IFN-γ+ CD8 T cells) (Figure 5A). This observed higher activity of E3-CAR T cells is consistent with the previous finding of the degranulation assay (Figure 4C).
Figure 5.
Activity of Cetux-CAR and E3-CAR against H292 Lung Cancer Cells
(A) On day 10 after activation and expansion ex vivo, Cetux-CAR T, E3-CAR T, and untransduced T cells were cocultured with H292 cells with GolgiPlug inhibitor for 6 hr. Intracellular cytokine staining of CAR T cells was performed, and the gated IFN-γ+CD8+ T cells are shown in each scatterplot. (B) Cetux-CAR T, E3-CAR T, or untransduced T cells were cocultured with H292 cells for 18 hr at different effector-to-target ratios of 1:1, 3:1, or 10:1. Cytotoxicity of CAR T cells against target cells was measured. The summarized statistics were shown as mean ± SEM (n = 3; ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; one-way ANOVA with Tukey’s multiple comparison).
To test the cell killing activity of both CAR T cells, they were cocultured with H292 cells at different effector to target ratios (E:T) (1:1, 3:1, and 10:1) for 18 hr and the specific target cell lysis was calculated. Both Cetux-CAR and E3-CAR T cells had significantly higher cytotoxicity toward H292 than did untransduced T cells at all three E:T ratios (Figure 5B). There was no significant difference in the cytotoxicity between Cetux-CAR and E3-CAR T cells.
E3-CAR T Cells Showed Similar Antitumor Efficacy to Cetux CAR-T Cells In Vivo
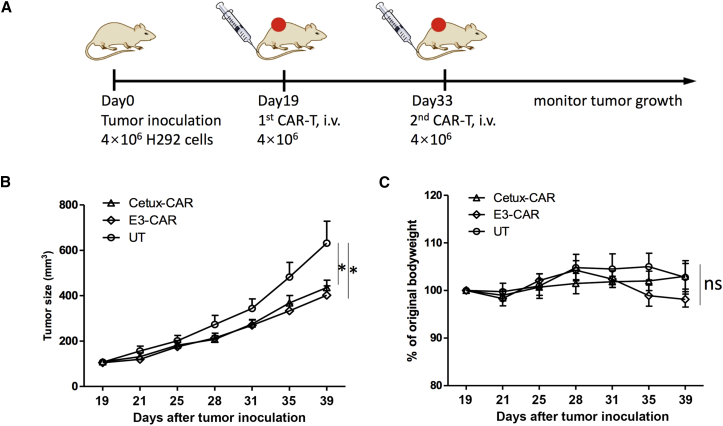
Given the comparable cytotoxicity of E3-CAR T cells toward H292 cells in vitro, we next sought to investigate the antitumor efficacy in vivo. We created a subcutaneous human lung cancer xenograft model in immunodeficient NOD.Cg-PrkdcscidIL2Rgtm1Wjl/Sz (NSG) mice. On day 0, NCI-H292 cells were injected into the right flank of NSG mice. When the average tumor size reached around 120 mm3 on day 19 after tumor inoculation, all the tumor-bearing mice were randomized into tumor size rank matched cohorts (n = 8 per treatment group), and then CAR-T treatment was started. Mice were treated with four million CAR-T cells through intravenous injection on day 19 and 33, and tumor growth was monitored (Figure 6A). Although all groups of animals showed tumor progression, mice receiving Cetux-CAR or E3-CAR T cells had significant tumor growth inhibition compared to those receiving untransduced T cells (one-way ANOVA: p < 0.05, Cetux-CAR versus UT; p < 0.05, E3-CAR versus UT) (Figure 6B). The average body weight change throughout the study was not significantly different in all three groups (Figure 6C). On day 42, the tumor tissues were harvested and tumor-infiltrating T cells were enumerated. The Cetux-CAR group had a significantly higher percentage of T cells trafficking to the tumor site than did the control group, whereas the E3-CAR group was not significantly different from either the control or Cetux-CAR group (Figure S1).
Figure 6.
Antitumor Efficacy of CAR-T Cells in a Human Lung Cancer Xenograft Model
(A) The CAR-T treatment timeline. H292 cells were injected into the right flank of NSG mice on day 0. On day 19, when the xenografted tumors were established, mice were randomized into three groups (n = 8 each group) and treated with four million Cetux-CAR T, E3-CAR T, or untransduced T cells on day 19 and day 33. Tumor size was measured by caliper twice every week. (B) Tumor growth curve in each group was shown as mean ± SEM (ns, not significant; *p < 0.05). (C) The average body weight change of each group was shown as mean ± SEM (ns, not significant; *p < 0.05).
Discussion
Adoptive transfer of CAR-T cells has achieved considerable success in multiple clinical trials; however, the inherent limitations of conventional CAR design and the lack of a more efficient method for novel construct design have curbed the development of this promising therapy. Our study has shown that compared to scFv-based CARs, CARs using adnectin as the antigen recognition domain are equally effective to kill tumor cells. It indicates that the novel method to derive CAR constructs from adnectin sequences is feasible and has potential value for future CAR development.
This method certainly offers some advantages and opportunities for CAR design. The flexibility of adnectin selection could allow optimal affinity tuning of the antigen-binding moiety to enhance CAR specificity to tumors. Affinity tuning is critical for CAR to discriminate tumor cells that overexpress target antigens from normal tissues that express target antigens at physiological levels. Traditionally, CARs are mostly designed to incorporate high-affinity scFvs derived from monoclonal antibodies. However, previous studies have demonstrated that the activation threshold of CARs is inversely correlated with the scFv affinity and that high-affinity CARs show a poor discrimination power among target cells with different levels of antigen expression.35, 36 Different from high-affinity therapeutic antibodies, high-affinity CARs may result in much more serious on-target off-tumor toxicity in clinical trials.37 This is presumably caused by the higher sensitivity of CAR-T cells to cells with low target expression than antibody-based therapy.38 Previous studies have provided evidence that by tuning down the affinity of CARs toward the target antigen via low-affinity scFvs, both anti-EGFR or anti-human epidermal growth factor receptor 2 (HER2) CARs could distinguish tumor cells from normal tissues and only recognize and eradicate tumor cells with high expression levels of target antigens.32, 33, 35 One example is the nimotuzumab scFv-derived CAR-targeting EGFR designed by Caruso et al.33 Nimotuzumab has a 10-fold lower Kd than cetuximab, resulting from a 59-fold reduced on-rate of binding, which imparts a requirement for (at least) bivalent binding to EGFR and restricts the binding to cells expressing high-density EGFR.29 It should be pointed out that nimotuzumab-derived CAR bears a similar level of affinity against EGFR as that of E3 adnectin (2-fold difference). Both nimotuzumab-CAR and E3-CAR display a similar level of biological function in vitro as that of high-affinity scFv CARs, and their relatively low affinity leads to the enhanced selectivity toward EGFR-overexpressing tumor cells over normal cells with the endogenous level of EGFR expression. Therefore, developing low-affinity adnectin-CARs with enhanced tumor selectivity might be a promising strategy to improve the safety profile of CAR-T therapy. Paradoxically, low-affinity adnectins may have disadvantages in terms of T cell persistence and proliferation. E3-CAR had a lower percentage of tumor-infiltrating T cells compared to Cetux-CAR, although the difference was not statistically significant, and it may compromise the antitumor efficacy of E3-CAR T cells in vivo. It highlights the importance to optimize the affinity range for the antigen-recognition domain of CAR to achieve a balance of both efficacy and safety.
In addition, the human-derived sequences of adnectin render relatively low immunogenicity and could potentially allow longer persistence and higher efficacy of adnectin-CAR-T cells. It has been more well-known that the routine lymphodepletion procedure before adoptive transfer of engineered T cells is one of the key factors leading to clinical success of CAR-T therapy.39 In the short time window provided by lymphodepletion for infused cells to evade host rejection, a less immunogenic CAR might not be more advantageous; however, in the long run, the construct may be more durable; this requires further clinical test to be demonstrated though. The newly formed epitopes in adnection-binding loops and junction sites in CARs might still cause immune responses, but we expect to see a much lower chance for life-threatening responses to occur. Other antigen-binding moieties, such as naturally occurring receptor ligands, have been used to design less immunogenic CARs40, 41 before and there are some successful examples in the clinic, such as interleukin-13 (IL-13)(E13Y)-zetakine CAR-targeting IL-13Rα2 in glioblastoma treatment.42 Yet the availability of candidate ligands is very limited and cannot meet a broad variety of needs for recognizing tumor cells, and the ligand-based CARs may bind to other receptors with lower affinity and cause undesired off-target toxicity.41
Multiple clinical trials of CAR-T cells have shown antigen escape of cancer cells and relapses in patients4, 6 due to the heterogeneous target antigen expression in cancer cells. Previous study has demonstrated that combinational antigen recognition by bispecific OR-Gate CAR may provide a safeguard against antigen escape.43 With similar rationale, multi-domain adnectin, such as EGFR and insulin-like growth factor 1 receptor (IGF-IR) bispecific adnectin in a previous report,29 can also be used to enhance the efficacy of CAR. The small size of adnectin and its native structure derived from fibronectin also make it very adaptable to develop a multi-domain adnectin that is multi-specific to different targets.29 It provides a new means to explore adnectin CAR design.
Despite the great potential of adnectin-CAR, there are also some limitations and concerns of this design method. It remains unknown whether it is a widely applicable strategy to design CAR constructs based on adnectin sequences. Although we have demonstrated that EGFR-targeting adnectin-CAR has equivalent efficacy against target cancer cells both in vitro and in vivo, it still requires further evaluation of its efficacy and extensive study of its reactivity to normal tissues in a clinical setting. It is especially crucial for low-affinity adnectins because they may bear less specificity at the same time. In addition, many existing therapeutic monoclonal antibodies and their targeted epitopes in the antigens are extensively studied and the understanding of their structure and potential side effects can facilitate rational design of CARs.44, 45 In contrast, although a number of adnectins binding to different targets, such as EGFR, IGF-IR, tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor receptor 2 (VEGFR-2), have already been developed,29, 46, 47, 48 little is known about the structural information of adnectins and their interactions with target antigens.49 The unpredictability is a double-edged sword. If the soluble extracellular domain of a membrane-bound target protein is used as the antigen during selection, it is possible that the resultant adnectin may not recognize the target in the physiological condition. It may also cause unexpected toxicity. On the other hand, it offers opportunities to select out targeting sites that are only exposed in transformed cells but not in normal cells. Therefore, a preliminary screening of multiple candidate adnectin-CARs, based on their potential to react to tumor cells and normal tissues, may be necessary.
In summary, CAR-T therapy is rapidly evolving and our study has provided a novel strategy to develop CAR molecules from adnectins rather than the conventional CAR design from scFvs. The results demonstrate that bearing equivalent potency to traditional CARs, adnectin-based CARs may benefit from reduced immunogenicity, increased tumor selectivity, and improved safety profile due to optimal affinity tuning. The method to design CAR molecules based on the adnectin sequence may help expand the applicability of CAR therapy to a wide range of tumor types.
Materials and Methods
Construction of Plasmids
The third generation of Cetuximab scFv.28BBz CAR was cloned into the MP71 retroviral vector as previously described.50 The amino acid sequences of adnectins targeting EGFR were published previously29 and shown as follows. The bolded sequences in each clone represent different BC, DE, and FG binding loops. The adnectin CAR constructs were constructed as follows. The corresponding adnectin DNA sequences were codon optimized (see Supplemental Information) and synthesized by Integrated DNA Technologies. Then, PCR-amplified DNA sequences were assembled together with all the other fragments encoding CD28, 4-1BB, CD3ζ, and MP71 backbone vector using the Gibson Assembly Cloning Kit (New England Biolabs).
E1(105aa):MGVSDVPRDLEVVAATPTSLLISWDSGRGSYQYYRITYGETGGNSPVQEFTVPGPVHTATISGLKPGVDYTITVYAVTDHKPHADGPHTYHESPISINYRTEIDK; E2(100aa):MGVSDVPRDLEVVAATPTSLLISWLPGKLRYQYYRITYGETGGNSPVQEFTVPHDLRTATISGLKPGVDYTITVYAVTNMMHVEYSEYPISINYRTEIDK;
E3(100aa):MGVSDVPRDLEVVAATPTSLLISWVAGAEDYQYYRITYGETGGNSPVQEFTVPHDLVTATISGLKPGVDYTITVYAVTDMMHVEYTEHPISINYRTEIDK;
E4(105aa):MGVSDVPRDLEVVAATPTSLLISWWAPVDRYQYYRITYGETGGNSPVQEFTVPRDVYTATISGLKPGVDYTITVYAVTDYKPHADGPHTYHESPISINYRTEIDK
Cell Lines and Culture Media
Cell lines 293T, MDA-MB-231, and U87 were cultured in D10 medium consisting of DMEM medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. H292 cells were cultured in R10 medium consisting of RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 mM HEPES. The above culture media and supplements were purchased from Hyclone. Human PBMCs from healthy donors were obtained from AllCells. PBMCs were cultured in T cell medium (TCM) consisting of X-Vivo 15 (Lonza) supplemented with 5% human AB serum (GemCell), 1% HEPES (Gibco), 1% Pen-Strep (Gibco), 1% GlutaMax (Gibco), and 0.2% N-acetyl cysteine (Sigma-Aldrich).
Retroviral Vector Production
As previously described, retroviral vectors were prepared by transient transfection of 293T cells using a standard calcium phosphate precipitation protocol.51 Fresh supernatant-containing retroviral vectors were collected 48 hr after transfection and used to transduce activated T cells.
T Cell Transduction and Expansion
Frozen human PBMCs were thawed in TCM and rested overnight. PBMCs were activated by culturing with Dynabeads Human T-Activator CD3/CD28 (Thermo Fisher Scientific) at a bead-to-cell ratio of 3:1 and 10 ng/mL recombinant human IL-7 and IL-15 (PeproTech). After 2 days, activated T cells were added onto retroviral vector loaded non-tissue-culture-treated 12-well plates as previously described50 and spun at 1,000 × g at 32°C for 10 min and incubated overnight. On the following day, transduced T cells were harvested in fresh TCM and the same transduction procedure was repeated to enhance the transduction rate. During transduction and ex vivo expansion, culture medium was supplemented with 10 ng/mL IL-7 and IL-15 and replenished every 2 days. Cell density was adjusted to 0.5 million cells/mL for optimal T cell growth.
Surface Immunostaining and Flow Cytometry
To detect CAR expression on the cell surface, 1 × 106 cells were harvested and washed three times with fluorescence-activated cell sorting (FACS) buffer (PBS containing 4% bovine serum albumin fraction V) and then stained with recombinant human EGFR-Fc (R&D Systems) in FACS buffer at 4°C for 30 min. After two washes, cells were stained with phycoerythrin (PE)-AffiniPure F(ab’)2 fragment of goat anti-human IgG (Jackson ImmunoResearch) in FACS buffer at 4°C for 30 min. Cells were washed twice and resuspended in PBS. Fluorescence was assessed using a Miltenyi Biotec flow cytometer, and the FACS data were analyzed with FlowJo software.
EGFR Surface Staining and Quantification
To detect EGFR expression on the cell surface, 1 × 106 cells from different cell lines (K562, 293T, U87, MDA-MB-231, and H292) were harvested, washed, and then stained with PE anti-human EGFR antibody (BioLegend) or mouse IgG1-PE (BioLegend) as the isotype control. The EGFR molecules were quantified based on the mean fluorescence intensity of stained cells. The calibration was performed with Sphero Rainbow Calibration Particles (Spherotech) following the manufacturer’s instructions.
Intracellular Cytokine Staining
1 × 106 T cells were cocultured with target cells at a ratio of 1:1 for 6 hr at 37°C and 5% CO2 with GolgiPlug (BD Biosciences) in 96-well round-bottom plates. PE-Cy5.5 anti-CD3 antibody, APC-Cy7 anti-CD4 antibody, Pacific Blue anti-CD8 antibody, and PE anti-IFN-γ antibody were used for immunostaining. All the antibodies were purchased from BioLegend. Cytofix/Cytoperm Fixation and Permeabilization Kit (BD Biosciences) was used to permeabilize the cell membrane and perform intracellular staining according to the manufacturer’s instructions.
Degranulation Assay
0.5 × 106 T cells were cocultured with target cells at a ratio of 1:1 for 4 hr at 37°C and 5% CO2 with GolgiStop (BD Biosciences) and FITC anti-CD107a antibody in 96-well round-bottom plates. PerCP/Cy5.5 anti-CD4 antibody and Pacific Blue anti-CD8 antibody were used for immunostaining of the T cell surface marker. All the antibodies were purchased from BioLegend.
Cytotoxicity Assay
Target cells H292 were resuspended at the concentration of 1 × 106 cells/mL and labeled with 5 μM fluorescent dye CFSE in PBS+0.1% BSA. After a 30-min incubation at 37°C, the same volume of FBS was added into the cell suspension and incubated for 2 min at room temperature to stop the labeling reaction. The labeled target cells were then washed twice and suspended in fresh R10 medium. Cocultures were set up in round-bottom 96-well plates in triplicates at the following effector-to-target ratios: 1:1, 3:1, and 10:1, and each well had 5 × 104 target cells. After an 18-hr incubation at 37°C, the suspended cells were directly harvested, whereas the attached cells were obtained by trypsinization. All the cells were stained with 7-AAD and then flow cytometric analysis was performed to quantify remaining live (7-AAD negative) target cells. The cytotoxicity was calculated as 100%, the percentage of alive target cells/alive target cells in control wells without effectors. The statistics were presented in mean ± SEM.
Anti-tumor Efficacy of CAR-T Cells in a Non-Small Cell Lung Cancer Xenograft Mouse Model
The animal experiments were conducted according to the animal protocol approved by USC Institutional Animal Care and Use Committee (IACUC). 6- to 8-week-old female NSG mice (Jackson Laboratory) were used in this study. On day 0, 4 × 106 H292 cells were injected into the right flank of NSG mice in PBS. When the average tumor size reached 120 mm3 on day 19, all the mice were randomized based on the tumor size and assigned into four groups (n = 8). Mice were treated with four million CAR-T cells via tail vein injection on day 19 and day 33. CAR expression was normalized to 30% in all the CAR groups by the addition of donor-matched untransduced T cells. Tumor size was monitored twice every week by calipers and calculated by the following formula: L × W × H/2. Mice were euthanized when they displayed obvious weight loss, ulceration of tumors, or tumor size larger than 1,000 mm3.
Statistical Analysis
Statistical analysis was performed in GraphPad Prism, version 5.01. One-way ANOVA with Tukey’s multiple comparison was performed to assess the differences among different groups in the cytotoxicity assays. Tumor growth curve was analyzed using one-way ANOVA with repeated measures (Sidak’s multiple comparison method). A p value less than 0.05 was considered statistically significant. Significance of findings was defined as ns, not significant, p > 0.05; *p < 0.05.
Author Contributions
Conceptualization, X.H. and P.W.; Methodology, X.H. and P.W.; Investigation, X.H., G.E.C., Y.Z., and X.Z.; Formal Analysis, X.H. and Y.G.; Writing – Original Draft, X.H.; Writing – Review and Editing, X.H., P.W., and Y.G.; Funding Acquisition, P.W.; Resources, P.W.; Supervision, P.W.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Dr. Ite Laird-Offringa at the University of Southern California for providing the NCI-H292 cell line and Dr. Wolfgang Uckert at Humboldt University Berlin in Germany for providing retroviral plasmid MP71. This work was supported by NIH grants (R01AI068978, R01CA170820, R01EB017206, and P01CA132681) and a translational acceleration grant from the Joint Center for Translational Medicine.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and one figure and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.07.009.
Supplemental Information
References
- 1.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupp S.A., Maude S.L., Shaw P.A., Aplenc R., Barrett D.M., Callahan C., Lacey S.F., Levin B.L., Melenhorst J.J., Motley L. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019) Blood. 2015;126:681. [Google Scholar]
- 4.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer J.N., Somerville R.P.T., Lu T., Shi V., Bot A., Rossi J., Xue A., Goff S.L., Yang J.C., Sherry R.M. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 2017;35:1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster S.J., Svoboda J., Nasta S.D., Porter D.L., Chong E.A., Landsburg D.J., Mato A.R., Lacey S.F., Melenhorst J.J., Chew A. Sustained remissions following chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. Blood. 2015;126:183. [Google Scholar]
- 11.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzinger A., Barden M., Abken H. The growing world of CAR T cell trials: a systematic review. Cancer Immunol. Immunother. 2016;65:1433–1450. doi: 10.1007/s00262-016-1895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maus M.V., Haas A.R., Beatty G.L., Albelda S.M., Levine B.L., Liu X., Zhao Y., Kalos M., June C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen M.C., Popplewell L., Cooper L.J., DiGiusto D., Kalos M., Ostberg J.R., Forman S.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamers C.H., Willemsen R., van Elzakker P., van Steenbergen-Langeveld S., Broertjes M., Oosterwijk-Wakka J., Oosterwijk E., Sleijfer S., Debets R., Gratama J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 19.Wang W., Singh S., Zeng D.L., King K., Nema S. Antibody structure, instability, and formulation. J. Pharm. Sci. 2007;96:1–26. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- 20.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koide A., Bailey C.W., Huang X., Koide S. The fibronectin type III domain as a scaffold for novel binding proteins. J. Mol. Biol. 1998;284:1141–1151. doi: 10.1006/jmbi.1998.2238. [DOI] [PubMed] [Google Scholar]
- 22.Koide A., Koide S. Monobodies: antibody mimics based on the scaffold of the fibronectin type III domain. Methods Mol. Biol. 2007;352:95–109. doi: 10.1385/1-59745-187-8:95. [DOI] [PubMed] [Google Scholar]
- 23.Hackel B.J., Ackerman M.E., Howland S.W., Wittrup K.D. Stability and CDR composition biases enrich binder functionality landscapes. J. Mol. Biol. 2010;401:84–96. doi: 10.1016/j.jmb.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidle U.H., Auer J., Brinkmann U., Georges G., Tiefenthaler G. The emerging role of new protein scaffold-based agents for treatment of cancer. Cancer Genomics Proteomics. 2013;10:155–168. [PubMed] [Google Scholar]
- 25.Martinelli E., De Palma R., Orditura M., De Vita F., Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson R.I., Gee J.M., Harper M.E. EGFR and cancer prognosis. Eur. J. Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S.V., Bell D.W., Settleman J., Haber D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 28.Galizia G., Lieto E., De Vita F., Orditura M., Castellano P., Troiani T., Imperatore V., Ciardiello F. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654–3660. doi: 10.1038/sj.onc.1210381. [DOI] [PubMed] [Google Scholar]
- 29.Emanuel S.L., Engle L.J., Chao G., Zhu R.R., Cao C., Lin Z., Yamniuk A.P., Hosbach J., Brown J., Fitzpatrick E. A fibronectin scaffold approach to bispecific inhibitors of epidermal growth factor receptor and insulin-like growth factor-I receptor. MAbs. 2011;3:38–48. doi: 10.4161/mabs.3.1.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subik K., Lee J.F., Baxter L., Strzepek T., Costello D., Crowley P., Xing L., Hung M.C., Bonfiglio T., Hicks D.G. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl.) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 31.Kim G.P., Grothey A. Targeting colorectal cancer with human anti-EGFR monoclonocal antibodies: focus on panitumumab. Biologics. 2008;2:223–228. doi: 10.2147/btt.s1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., Jiang S., Fang C., Yang S., Olalere D., Pequignot E.C., Cogdill A.P., Li N., Ramones M., Granda B. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caruso H.G., Hurton L.V., Najjar A., Rushworth D., Ang S., Olivares S., Mi T., Switzer K., Singh H., Huls H. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan H.K., Walker F., Burgess A.W., Rigopoulos A., Scott A.M., Johns T.G. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 increases the formation of inactive untethered EGFR dimers. Implications for combination therapy with monoclonal antibody 806. J. Biol. Chem. 2007;282:2840–2850. doi: 10.1074/jbc.M605136200. [DOI] [PubMed] [Google Scholar]
- 35.Chmielewski M., Hombach A., Heuser C., Adams G.P., Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 36.Hudecek M., Lupo-Stanghellini M.T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C., Riddell S.R. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone J.D., Aggen D.H., Schietinger A., Schreiber H., Kranz D.M. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs) OncoImmunology. 2012;1:863–873. doi: 10.4161/onci.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim W.A., June C.H. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T., Barber A., Sentman C.L. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–11036. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 41.Niederman T.M., Ghogawala Z., Carter B.S., Tompkins H.S., Russell M.M., Mulligan R.C. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc. Natl. Acad. Sci. USA. 2002;99:7009–7014. doi: 10.1073/pnas.092562399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.C., Naranjo A., Starr R., Wagner J., Wright C. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voigt M., Braig F., Göthel M., Schulte A., Lamszus K., Bokemeyer C., Binder M. Functional dissection of the epidermal growth factor receptor epitopes targeted by panitumumab and cetuximab. Neoplasia. 2012;14:1023–1031. doi: 10.1593/neo.121242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yewale C., Baradia D., Vhora I., Patil S., Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34:8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 46.Xu L., Aha P., Gu K., Kuimelis R.G., Kurz M., Lam T., Lim A.C., Liu H., Lohse P.A., Sun L. Directed evolution of high-affinity antibody mimics using mRNA display. Chem. Biol. 2002;9:933–942. doi: 10.1016/s1074-5521(02)00187-4. [DOI] [PubMed] [Google Scholar]
- 47.Getmanova E.V., Chen Y., Bloom L., Gokemeijer J., Shamah S., Warikoo V., Wang J., Ling V., Sun L. Antagonists to human and mouse vascular endothelial growth factor receptor 2 generated by directed protein evolution in vitro. Chem. Biol. 2006;13:549–556. doi: 10.1016/j.chembiol.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Parker M.H., Chen Y., Danehy F., Dufu K., Ekstrom J., Getmanova E., Gokemeijer J., Xu L., Lipovsek D. Antibody mimics based on human fibronectin type three domain engineered for thermostability and high-affinity binding to vascular endothelial growth factor receptor two. Protein Eng. Des. Sel. 2005;18:435–444. doi: 10.1093/protein/gzi050. [DOI] [PubMed] [Google Scholar]
- 49.Ramamurthy V., Krystek S.R., Jr., Bush A., Wei A., Emanuel S.L., Das Gupta R., Janjua A., Cheng L., Murdock M., Abramczyk B. Structures of adnectin/protein complexes reveal an expanded binding footprint. Structure. 2012;20:259–269. doi: 10.1016/j.str.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Han X., Bryson P.D., Zhao Y., Cinay G.E., Li S., Guo Y., Siriwon N., Wang P. Masked chimeric antigen receptor for tumor-specific activation. Mol. Ther. 2017;25:274–284. doi: 10.1016/j.ymthe.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C., Hu B., Xiao L., Liu Y., Wang P. Pseudotyping lentiviral vectors with lymphocytic choriomeningitis virus glycoproteins for transduction of dendritic cells and in vivo immunization. Hum. Gene Ther. Methods. 2014;25:328–338. doi: 10.1089/hgtb.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.