Abstract
The nuclear receptor steroidogenic factor 1 (Sf1, Nr5a1, Ad4bp) is crucial for formation, development and function of steroidogenic tissues. A fetal adrenal enhancer (FAdE) in the Sf1 gene was previously identified to direct Sf1 expression exclusively in the fetal adrenal cortex and is bound by both Sf1 and Dax1. Here, we have examined the function of Sf1 SUMOylation and its interaction with Dax1 on FAdE function. A diffused prolonged pattern of FAdE expression and delayed regression of the postnatal fetal cortex (X-zone) were detected in both the SUMOylation-deficient-Sf12KR/2KR and Dax1 knockout mouse lines, with FAdE expression/activity retained in the postnatal 20αHSD-positive postnatal X-zone cells. In vitro studies indicated that Sf1 SUMOylation, although not directly influencing DNA binding, actually increased binding of Dax1 to Sf1 to further enhance transcriptional repression of FAdE. Taken together, these studies define a crucial repressor function of Sf1 SUMOylation and Dax1 in the physiological cessation of FAdE-mediated Sf1 expression and the resultant regression of the postnatal fetal cortex (X-zone).
KEY WORDS: Nuclear receptors, SUMOylation, X-zone, Adrenal gland
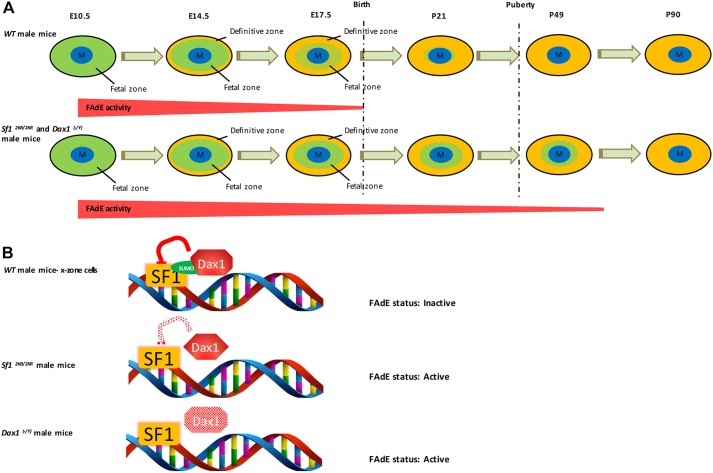
Summary: Lack of Sf1 SUMOylation and the genetic loss of Dax1 both cause extended activation of the fetal adrenoceptor enhancer of Sf1 and delayed regression of the postnatal fetal cortex (X-zone) in mouse adrenals.
INTRODUCTION
Although the fetal adrenal zone was originally described as a transient inner-most layer of cells within the adrenal gland of the mouse by Masui and Tamura (1926), recent developmental and linear-tracing studies define the adrenal fetal zone as the adrenal primordium, which is derived from the adrenogonadal primordia prior to separation into the adrenal cortex and bipotential gonad. Specifically, once encapsulated and infiltrated by neural crest cells, the catecholamine-producing cells of the adrenal medulla, the adrenal primordia, establish the stem/progenitor cell niche of the definitive cortex. Four events are needed for this process: (1) Sf1 expression must be extinguished in the fetal cortex; (2) capsular stem cells must be established from fetal cells (Wood and Hammer, 2011); (3) Sf1-expressing cells must be established in the definitive cortex from these capsular stem cells (Zubair et al., 2006, 2008; Morohashi and Zubair, 2011); and (4) the remnant fetal zone itself must regress and decrease in size. All the above events happen with strict timing to ensure proper development and normal function of the adrenal cortex.
Referred to postnatally as the X-zone in mice [defined by Howard-Miller (Howard-Miller, 1928)], the fetal cortex regresses at puberty in males or at first pregnancy in female mice. The first recognizable signs of the onset of degeneration are narrowing of the X-zone and development of a well-defined peripheral edge. At this stage, collagen fibers become evident between inner and outer borders of the zone, and gradually form a complete fiber layer around the medulla. The fibers become more evident with continued degeneration and narrowing (Holmes and Dickson, 1971; Tanaka and Matsuzawa, 1993). Although steroid hormones, including androgens, progesterone and oestradiol benzoate, have been shown to produce significant degenerative effects on the fetal cells (Ohno, 1962; Delost et al., 1971; Asari et al., 1979), little is known about how this zone is maintained after birth or what factors regulate the timing of its regression.
The nuclear receptor steroidogenic factor 1 or adrenal 4 binding protein (Sf1/Ad4bp/Nr5a1) is a member of the nuclear receptor superfamily. In mice, it is expressed in the pituitary, hypothalamus, gonads and adrenal glands (Ozisik et al., 2003; Kohler and Achermann, 2010). Sf1 is a crucial factor in the formation of the adrenal gland, and central to the expression of steroidogenic genes such as cholesterol side chain cleavage (Cyp11a1), aromatase (Cyp19a1), steroidogenic acute regulating protein (Star) and melanocortin 2 receptor (Mc2r) (Breckwoldt et al., 1996; Chung et al., 1997; Buaas et al., 2012). Given its functional importance, great emphasis has been placed on studying mechanisms that regulate Sf1 activity in adrenal development and steroidogenesis.
Importantly, the fetal and definitive adrenal cells use unique enhancer sequences that mediate restricted Sf1 expression in each cell type. Although the fetal adrenal enhancer (FAdE) of Sf1 has been defined, the definitive adrenal enhancer (DAdE) of Sf1 remains unknown. In fetal adrenal cells, Sf1 maintains its own expression through autoregulation of the FAdE (Zubair et al., 2006, 2009) until E14.5 when the definitive cells emerge below the adrenal capsule. However, mechanisms responsible for cessation of Sf1 expression (FAdE) in the fetal cells and re-establishment of Sf1 expression (DAdE) in the definitive cells with later regression of the fetal adrenal are unknown.
Post-translational modifications and the specificity of co-factor recruitment are important mechanisms that modify transcriptional activation and repression of Sf1. Moreover, the post-translational modifications, phosphorylation and SUMOylation of Sf1 play opposing roles in regulating Sf1 activity both in vivo and in vitro (Lee et al., 2011; Hammer et al., 1999; Sasaki et al., 2014; Yan et al., 2014). Of note, SUMOylation of Sf1 inhibits its activity on a subset of target genes, and mice with a SUMO-deficient form of Sf1 exhibit enhanced activation of a subset of target genes (Lee et al., 2011).
Dax1 (Nr0b1) is an unusual member of the nuclear receptor superfamily in that it lacks the conventional DBD (DNA-binding domain), modulator domain and hinge region. Instead, Dax1 contains a novel N-terminal structure consisting of 3.5 alanine/glycine-rich repeats, each 65-70 amino acids long (Iyer and McCabe, 2004). Dax1 has been shown to function as a nuclear receptor co-repressor that interacts with Sf1 to inhibit a number of genes involved in both adrenal development and steroidogenesis. The inhibition mechanism is thought to involve direct protein-protein interactions between Dax1 and Sf1, which lead to subsequent recruitment of co-repressors to the promoters of target genes. It is also possible that Dax1 competes with Sf1 transcriptional co-activators, including CREB-binding protein (CBP)/p300, glutamate receptor interacting protein 1 (Grip1) and tyrosine-protein kinase (Src), to promote transcriptional repression of Sf1 target genes (Zhou et al., 2008; Ferraz-de-Souza et al., 2009; Li et al., 2011). However, to date, it is unclear how Sf1 post-translational modulation might modify Dax1-mediated repression of Sf1 activity.
In the current study, we used two loss-of-function mouse models and in vitro assays to examine the contribution of SUMOylation of Sf1 and the recruitment of Dax1 to the inhibition of FAdE-mediated Sf1 expression in the fetal adrenal cortex. Our results demonstrate that the inability of Sf1 to be SUMOylated and the genetic loss of Dax1 both cause extended activation of the FAdE and delayed regression of the postnatal fetal cortex (X-zone) in mouse adrenals. Finally, we define the molecular mechanisms of a synergistic repression of FAdE by SUMOylated Sf1 and Dax1 that appear to be essential for repressing Sf1-mediated autoregulation of the FAdE enhancer and for ensuring proper timing of fetal adrenal (X-zone) regression.
RESULTS
Both Sf1 SUMO-deficiency and genetic Dax1 loss result in delayed fetal adrenal regression in vivo
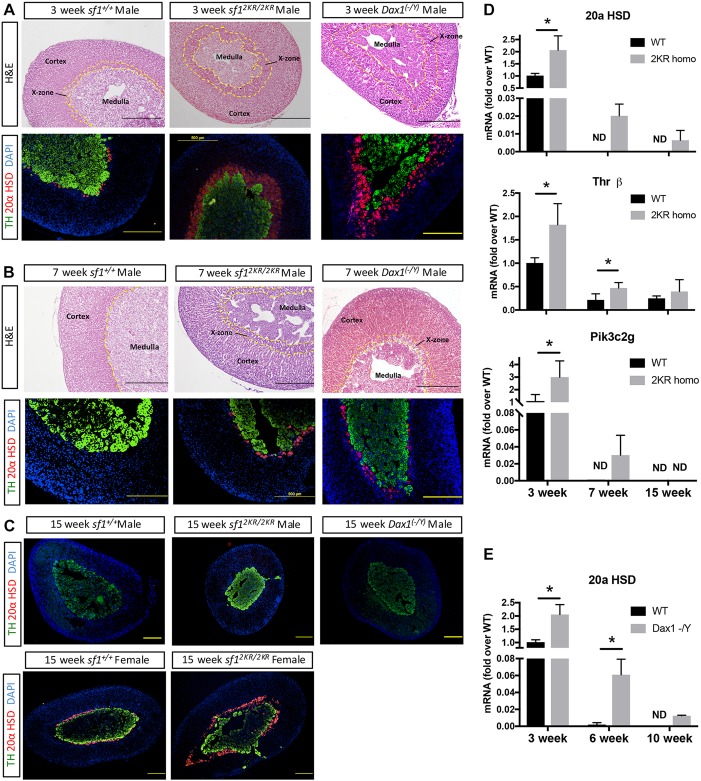
The fetal adrenal cortex is the inner-most zone of the adrenal cortex and is composed of remnant fetal cells. In mice, the remnant fetal zone cells (postnatally referred to as X-zone cells) regress at puberty in the male and during the first pregnancy in the female (Zubair et al., 2006, 2008, 2009; Morohashi and Zubair, 2011). In 3-week-old prepubertal Sf12KR/2KR mice, compared with wild-type mice, a markedly expanded X-zone with increased number of 20α HSD-expressing cells is observed (Fig. 1A). In addition, male Sf12KR/2KR mice continue to retain a distinct zone of 20α HSD-expressing fetal cells (X-zone) post-pubertally, at the age of 7 weeks (Fig. 1B). This zone only regresses after 15 weeks of age in the Sf12KR/2KR male mice (Fig. 1C). Although virgin wild-type females maintain some 20α HSD-expressing X-zone cells at 15 weeks, a much broader expression of 20α HSD is observed in 15-week-old Sf12KR/2KR mice (Fig. 1C). These data are in accordance with findings previously reported by some of us (Lee et al., 2011) and suggest that mutations that prevent Sf1 SUMOylation lead to expansion of prenatal fetal zone cells and delayed postnatal X-zone regression.
Fig. 1.
The adrenal X-zone is expanded in SUMO-deficient and Dax1 knockout male mice, and maintained after puberty. (A) Hematoxylin and Eosin (top panel) and immunostaining (bottom panel) of 3-week-old wild-type and Sf12KR/2KR male mouse adrenals. Staining with TH (green) marks the medulla and 20αHSD (red) marks X-zone cells. DAPI staining (nuclei) is in blue. (B) Hematoxylin and Eosin (top panel) and immunostaining (bottom panel) of 7-week-old wild-type and Sf 2KR/2KR male mice adrenals. The inner yellow dotted line marks the margin between cortex and medulla, whereas the area between two dotted lines represents X-zone. Sf12KR/2KR and Dax1−/y male mice have an expanded X-zone at a young age and this zone is retained after puberty. (C) Immunostaining of 15-week-old Sf12KR/2KR and Dax1−/y male mouse adrenals (upper panel) and 15-week-old wild-type and Sf12KR/2KR virgin female mouse adrenals. The X-zone in male Sf12KR/2KR mice regresses at a later age, whereas the X-zone and 20α HSD expression are retained in virgin females of both wild-type and Sf12KR/2KR mice, the latter having an expanded fetal zone with an expansion of 20α HSD-expressing cells. Scale bars: 500 µm. (D) X-zone marker gene expression in Sf12KR/2KR male mouse adrenals. RNA were isolated from paraffin wax-embedded sections of 3-, 7- and 15-week-old male Sf12KR/2KR adrenal glands. Gene expression was quantified using real-time qPCR with primers designed for individual genes. n=4-8 per group, *P<0.05. (E) X-zone marker 20αHSD gene expression in Dax1−/y male mouse adrenals. RNA were isolated from paraffin wax-embedded sections of 3-, 6- and 10-week-old male Dax1−/y adrenal glands. n=4-6 per group. *P<0.05. ND, not detectable.
Dax1 (NR0B1) is a unique nuclear receptor that functions primarily as a negative regulator of Sf1-mediated transcriptional regulation. Dax1 is expressed strongly in the prenatal fetal adrenal as early as E11.5 (Fig. S1) and postnatally in the outer zona glomerulosa with additional weaker scattered expression in the zona fasciculata of the definitive cortex (Mukai et al., 2002; Zubair et al., 2009). Genetic loss of Dax1 leads to adrenal failure in individuals with cytomegalic adrenal hypoplasia and in aging mice, highlighting the crucial role of Dax1 in homeostasis of the definitive cortex (Scheys et al., 2011). To delineate the role of Dax1 in the fetal adrenal cortex, we examined the fetal adrenal phenotype of Dax1 knockout mice (Dax1−/y or Dax1 KO) and found similar defects as observed in male Sf12KR/2KR mice. Not only were increased X-zone cell numbers observed in young male mice adrenals (Fig. 1A), but delayed X-zone regression was also detected in pubertal male mice (Fig. 1B). Real-time PCR was also used to quantify the expression levels of X-zone marker genes in those mice. In accordance with the observed expansion/retention of the X-zone in 2KR and Dax1−/y models, we observe both: (1) a significant increase in peak gene expression; and (2) a temporal delay in extinguishment of gene expression of the X-zone markers 20αHSD, Pik3c2g and Thrβ in 2KR and Dax1−/y adrenal glands compared with their wild-type littermates (Fig. 1D,E). Importantly however, loss of Sf1 SUMOylation or loss of Dax1 expression was insufficient to prevent the ultimate regression of the X-zone in the adult adrenal gland, suggesting that while additional intrinsic or compensatory mechanisms must contribute to elimination of remnant fetal adrenal cells (X-zone), Sf1 SUMOylation and Dax1 are both crucial for the proper timing of this event.
FAdE expression is retained in postnatal X-zone (remnant fetal zone) cells of Sf12KR/2KR and Dax1 KO mice
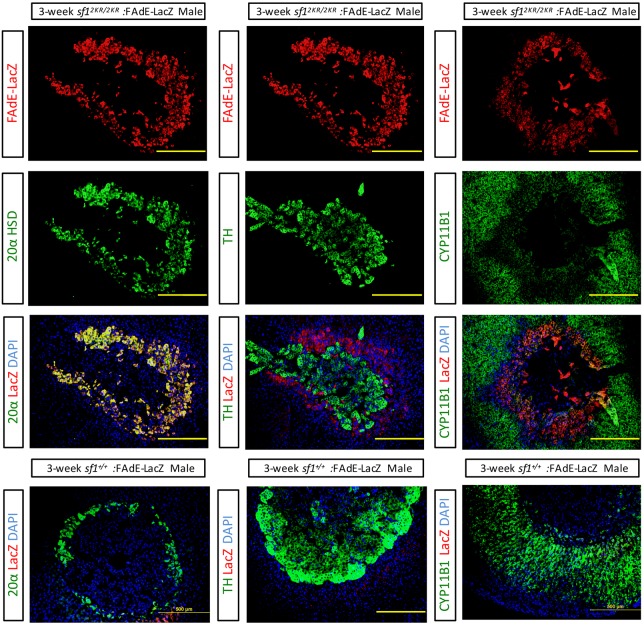
As reported previously, expression of Sf1 in the fetal adrenal is regulated by a fetal adrenal enhancer (FAdE) in the Sf1 gene locus and this enhancer is autoregulated by Sf1 (Fig. 4). At early developmental stages, the FAdE is active in all fetal zone cells but is gradually repressed in cells located in the outer part of the cortical region of the adrenal after E14.5. Following birth, FAdE-positive cells can be found only in the innermost 20alpha-positive X-zone (remnant fetal zone) cells. FAdE-mediated transcription ceases coincident with X-zone regression during puberty in the male mouse and during first pregnancy in the female mouse. Our observation of an expanded and retained X-zone in both Sf12KR/2KR and Dax1 KO mouse lines led us to investigate whether elevated/retained FAdE activity might contribute to this phenotype. First, Sf12KR/2KR mouse lines were crossed into the FAdE-Ad-LacZ line, and FAdE activity was assessed by LacZ expression. As expected, a broad expression of LacZ was detected in adrenals of young (3-week-old) Sf12KR/2KR mice, colocalizing with the same cells that express the X-zone marker 20α HSD (Fig. 2). Few LacZ- or 20α HSD-positive cells were observed in wild-type males at this age. This result indicates that disturbing the SUMOylation cycle of Sf1, FAdE remains active in both prenatal fetal zone cells and corresponding postnatal X-zone cells. Moreover, the prolonged FAdE expression correlates with retention of the X-zone in the pubertal adrenal cortex. Last, the FAdE-positive X-zone cells do not express the ZF marker, Cyp11B1 (Fig. 2), supporting the conclusion that those cells are descendants of fetal zone cells rather than peripheral adult cortical cells. Although it remains possible that FAdE-LacZ expression observed in 20αHSD-expressing cells reflects ectopic expression in a cell not derived from the FAdE-activated fetal cell, this is extremely unlikely based on previous lineage-tracing studies (Zubair et al., 2006; Freedman et al., 2013). Prior lineage data from Zubair et al. (2008) that used a fetal zone-specific driver (FAdE-Ad4BP-Cre-ERT2) reveal retained LacZ expression in the X-zone after 2 months of tracing, indicating that the postnatal X-zone is (derived from) the prenatal fetal zone. In addition, lineage data from Freedman et al. (2013) that used a peripheral zona glomerulosa driver (AS-Cre) indicate that X-zone cells do not originate from the peripheral cortex of the adult zone. These data indicate that the X-zone precedes the development of the adult cortex and is the postnatal fetal zone.
Fig. 4.
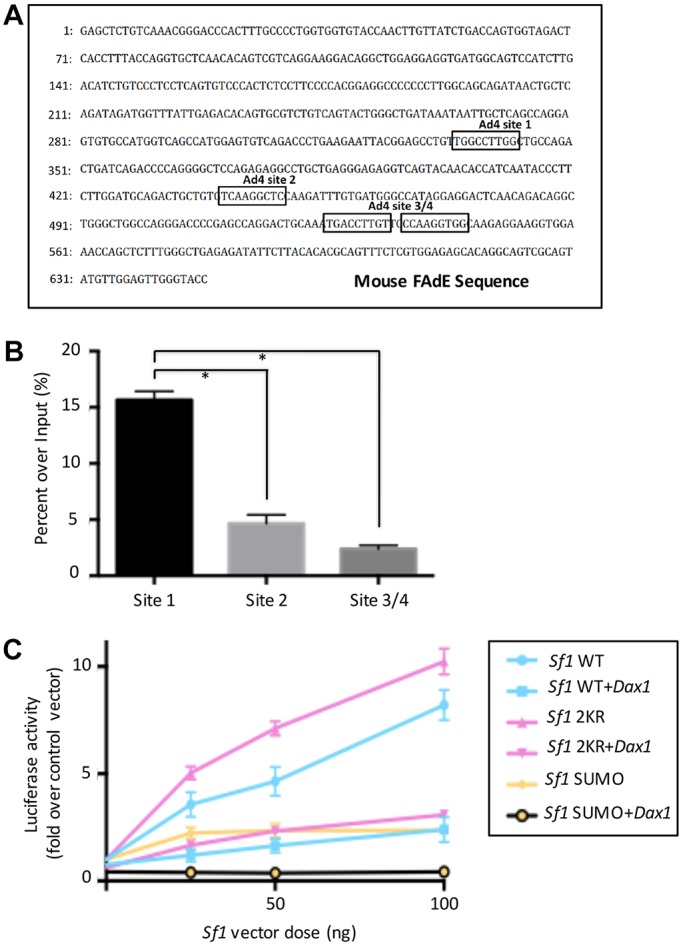
Sf1 SUMOylation and Dax1 modify Sf1 activity on the FAdE enhancer in vitro. (A) Ad4 (Sf1) binding sites in the mouse FAdE region (Zubair et al., 2002). (B) Binding capacity of Sf1 on the different Ad4 sites in the FAdE region. ChIP assays were performed on Y1 cell lines using anti-Sf1 antibodies. Immunoprecipitates were analyzed by quantitative PCR using primers designed for each individual site. The data were normalized to values obtained for 1% input controls, and the results are presented as percentage of input. Data are mean±s.e.m. (C) SUMOylation and Dax regulate Sf1 activity on the FAdE enhancer. HEK293T cells were plated at 105 cells/well in 24-well plates 24 h before transfection and were transfected in triplicate with FAdE Luc (100 ng/well), with Sf1 as indicated, and with or without Dax1 (50 ng/well). Luciferase assays were carried out and the data were normalized to renilla level and shown as fold change over control vectors. n=6. *P<0.05. Error bars indicate s.e.m.
Fig. 2.
FAdE expression is retained in the X-zone cells of Sf12KR/2KR mice. Immunostaining of 3-week-old Sf12KR/2KR: FAdE-LacZ male mice adrenals. Expression of LacZ is overlaid with either the fetal zone marker 20α HSD (left panels), the medulla marker TH (middle panels) or zona fasciculata cell marker CYP11B1 (right panels). FAdE activity colocalizes with fetal zone cells. Scale bars: 500 µm.
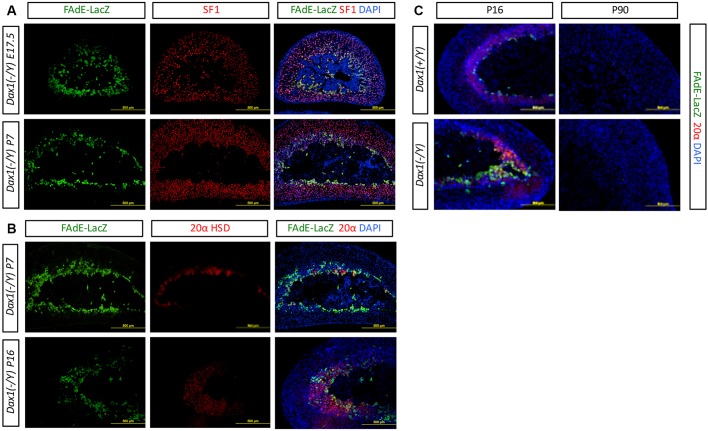
Next, we investigated FAdE activity in the Dax1 KO male mice that express FAdE-LacZ. In contrast to adrenals from wild-type mice in which FAdE activity is largely extinguished by E17.5 (Zubair et al., 2009) (Fig. S2), LacZ expression was detected at as late as E17.5, P7 and P16 in Dax1 KO mice (Fig. 3A,B). Interestingly, co-immunostaining with 20αHSD demonstrated that LacZ-positive cells are present in the inner X-zone, but not observed in the peripheral X-zone or differentiated adult cortex. These data indicate that Dax1 suppresses FAdE activity in fetal zone/X-zone cells, perhaps by repressing the activity of Sf1. Furthermore, similar to the LacZ-positive cells of the Sf12KR/2KR adrenals, those FAdE-expressing cells also expressed the X-zone marker 20αHSD in young pre-pubertal Dax1 KO mice (P7 and P16) (Fig. 3B). The Dax1-mediated effect on FAdE activity is sustained until at least P16 whereas in wild-type mice LacZ-positive cells are only present as a thin rim of remaining X-zone cells in the inner adrenal; Dax1 KO mice exhibit increased numbers of LacZ-positive cells that are arranged in a broader and more diffused pattern (Fig. 3C). However, by P90, LacZ-positive cells disappeared in Dax1 KO mice as in their wild-type littermates, supporting the hypothesis that additional inherent or compensatory mechanisms are used to fully extinguish FAdE activity and ultimately induce X-zone regression in male mice.
Fig. 3.
Expansion and delayed regression of FAdE-expressing cells in adrenals of young Dax1 knockout mice compared with wild type. (A) FAdE activity is maintained in Dax1 KO mice during early developmental stages as shown by immunostaining for β-galactosidase to detect LacZ expression (upper panel, E17.5; lower panel, P7). (B) LacZ expression colocalizes with the fetal zone marker 20αHSD and is found in the inner rim of the 20αHSD-expressing zone at the age of P16 (lower panel). (C) Immunostaining with LacZ and 20αHSD in wild-type and Dax1 KO male mice at P16 (left panel) and P90 (right panel). Although Dax1 KO mice have more FAdE-positive cells at a young age, those cells and the fetal zone regress by 3 months of age. Scale bars: 500 µm.
When comparing the two genetic models, 2KR adrenals exhibit a more profound but qualitatively similar phenotype when compared with the Dax1−/y adrenals. Specifically, LacZ expression (reflecting FAdE-LacZ activity) is maintained for a longer period of time and in a larger number of cells of the X-zone of the 2KR adrenals when compared to the adrenals of Dax1−/y mice. This is consistent with a dominant role of Sf1 as a transcription factor at the FAdE locus. By contrast, Dax1 has indeed classically been defined as a transcriptional co-factor: a co-regulator of Sf1-mediated transcription that requires Sf1 for its activity. More specifically, our data are consistent with the SUMOylation of Sf1 playing a dominant role in the extinguishment of FAdE expression and the correlative regression of the X-zone. The data are also consistent with Dax1 being a co-regulator of Sf1-mediated transcriptional regulation of FAdE.
Although the spatial and temporal extent of FAdE expression is correlated with the timing of X-zone regression in both 2KR and Dax1−/y mice, whether or not the delay in X-zone regression in both 2KR and Dax1−/y mice is mediated directly by prolonged FAdE activity in the X-zone cells of these mice is not known. It is feasible that Sf1 SUMOylation and Dax1 have transcriptional effects that influence X-zone regression independent of FAdE activity.
Besides its above-shown function in later adrenal development, Dax1 has also been predicted to play an important role in early establishment of the adrenal gland. Consistent with previous studies, immunohistochemistry in E11.5 mouse embryo showed that both Sf1 and Dax1 are expressed in the gonadal ridge and adrenal. Moreover, Dax1 is also detected in wider areas, including medial ceolomic bay located in the posterior of adrenal region, in where Sf1 cannot be detected (Fig. S1A). As shown by Zubair et al. (2009), Sf1 is expressed weakly and instantly in a wider region in E11.5 embryos; however, this weak expression is immediately canceled by an unknown mechanism, and Sf1 can be detected only in the genital ridge and adrenal region at later stages. Studies using transgenic assay revealed that fusion of Sf1 promoter and FAdE drives reporters such as EGFP or Cre expression in the fetal adrenal, and instantly in the wider region of several lines of transgenic mice (Zubair et al., 2009), indicating that FAdE controls embryonic Sf1 expression patterns. When Dax1 KO mice are crossed with FAdE-Ad-LacZ mice and analyzed at different development stages, whole-mount LacZ staining indicated modified FAdE function in embryos from E11.5 and E13.5 (Fig. S1B,C). In the wild-type embryo at E11.5, LacZ-positive cells were detected clearly to be present in the fetal adrenal region. In contrast, in the Dax1 KO embryo, LacZ-positive cells were observed in a larger area, and sporadically unorganized in the surrounding region (arrows). This shows that the number of LacZ-positive cells increase in the absence of Dax1 at E11.5, supporting the notion that Dax1 inhibits FAdE expression. However, at later stages (E13.5), LacZ expression becomes restricted to the adrenal gland and is almost indistinguishable in both genotypes. These data suggest that Dax1 is the key repressor of the FAdE activity at E11.5.
SUMOylation of Sf1 leads to increased recruitment of Dax1 and an inhibition of FAdE-mediated transcriptional activity in vitro
To examine the molecular mechanisms of increased FAdE activity in Sf12KR/2KR and Dax1 KO mice, we investigated the contributions of Sf1 SUMOylation and Dax1 to the inhibition of FAdE-mediated transcriptional activation in an adrenocortical cell culture system. As shown previously, there are four potential Sf1-binding sites in the FAdE region (Fig. 4A). Using ChIP assay, we confirmed that Ad4BP site #1 (Zubair et al., 2002) is the major binding site of Sf1 in the FAdE region, with more than threefold higher affinity than site #2 and site #3/4 (Fig. 4B). Based on these data, we mainly focused on the Ad4 sites #1 and #2 for the remainder of our studies. Promoter reporter constructs were then engineered by fusing the -88 to +467 bp of the enhancer sequence with the minimal promoter of thymidine kinase of herpes simplex virus into pGL3-Basic (FAdE-Luc construct) and tested in HEK293K cells, used as a heterologous cell line not expressing endogenous Sf1 or Dax1. Cells were transfected with FAdE-Luc and different constructs of SUMOylation-modified Sf1 with or without pcDNA Dax1. Analysis of luciferase activity revealed that Sf1-2KR induced the highest stimulating activity on the FAdE-Luc construct. That SUMOylation of Sf1 decreases the ability of Sf1 to activate FAdE is shown most dramatically using a construct in which SUMO2 protein is fused to the N-terminal of Sf1 (Sf1-SUMO). Sf1-SUMO has only 20% stimulating activity of FAdE-Luc as compared with Sf1-2KR (Fig. 4C). In order to exclude the possibility that Sf1 with SUMO2 protein fused to the N-terminus engages the transcriptional machinery differently from the natural SUMO-conjugated Sf1, we also performed FAdE activation experiments in the Y1 adrenocortical cell line in the presence or absence of overexpressed Senp1 (sentrin-specific protease 1) or SUMO-activating enzyme E1/Ubc9 (ubiquitin conjugating enzyme E2)/SUMO to manipulate the SUMOylation status of endogenous Sf1. Congruent with the inhibition of the FAdE enhancer observed with SUMO2-conjugated Sf1 in comparison with wild type, SENP1-mediated inhibition of endogenous Sf1 SUMOylation enhanced the Sf1-mediated activation of the FAdE enhancer (Fig. S3). Furthermore, Dax1 dramatically blunted the stimulating effect of all forms of Sf1. These data indicate that both SUMOylation modification and Dax1 decrease the stimulating effects of Sf1 on the FAdE. Of note, when Sf1-SUMO and Dax1 are co-transfected in this experiment, we observe a complete inhibition (lack of activation) of FAdE activity, indicating that Sf1 SUMOylation and Dax1 act synergistically to repress Sf1 activity.
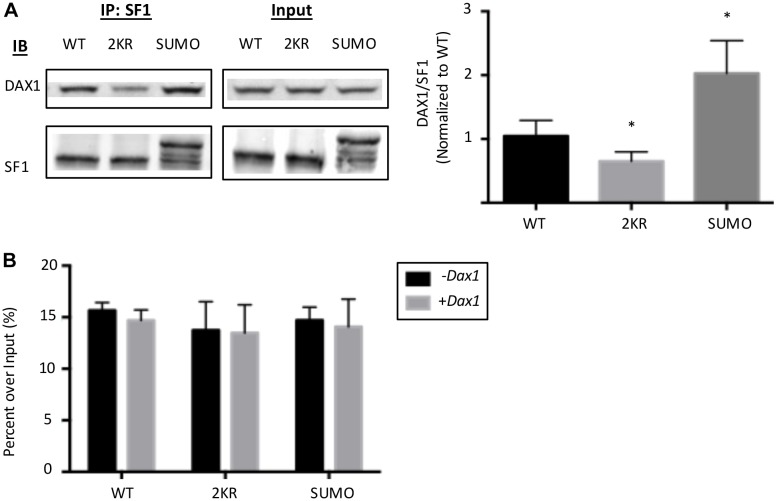
To explore the mechanisms related to the repressive effects of SUMOylation and Dax1 on Sf1 stimulation of the FAdE, Co-IP assays were performed using Sf1 antibody to pull down the protein complex of different SUMOylation forms of Sf1 and Dax1 in transfected HEK293T cells. The SUMO2-conjugated Sf1 significantly increases the binding of Sf1 with Dax1 (2.2-fold increase), while mutation of the SUMOylation site decreases binding (Fig. 5A). The current data are in contrast to the previously published study by Campbell et al. (2008), which clearly demonstrated a modest but consistent reduction of DAX1 interaction with SUMOylated Sf1 LBD (Campbell et al., 2008). However, as the previous experiments were conducted in a cell-free system using only the C-terminal region of Sf1 (the ligand-binding domain of Sf1 protein and a small part of the hinge region containing only one of the SUMOylation sites, K194), the two systems are not directly comparable.
Fig. 5.
SUMOylation enhances Sf1 binding to Dax1, but does not influence its binding affinity to DNA. (A) SUMOylation increases the binding of Sf1 to Dax1. HEK293T cells were plated at 106 cells/dish in 10 mm cell culture dishes 24 h before transfection and were transfected with 1 µg pcDNA-Sf1 and 1 µg pcDNA-Dax1 vectors. After 48 h, cells were lysed and co-immunoprecipitation assays performed. Lysates were precipitated using anti-Sf1 antibody and immunoblotted with anti-HA (for Dax1) or -Sf1 antibodies. The data were normalized to input and shown as fold change over wild-type Sf1. *P<0.05. Error bars indicate s.e.m. (B) SUMOylation or Dax1 do not change the DNA-binding capacity of Sf1. HEK293T cells were plated at 106 cells/dish in 10 mm cell culture dishes 24 h before transfection and were transfected with 1 µg pcDNA-Sf1 and 1 µg pcDNA-Dax1 vectors with 25 ng of linearized pGL3-FAdE construct. After 48 h, cells were fixed and processed for ChIP assay using anti-Sf1 antibody. The data were normalized to values obtained for 1% input controls, and the results are presented as percentage of input (n=5). Error bars indicate s.e.m.
Interestingly, neither SUMOylation nor the presence of Dax1 changes Sf1 DNA-binding affinity to the FAdE sites. ChIP assay using different SUMOylation forms of Sf1 together with Dax1 and vectors containing the DNA sequence of the FAdE Ad4 site #1 in HEK293T cell lines showed no changes in Sf1 DNA-binding capacity with or without SUMOylation modification (Fig. 5B). Similar effects were confirmed in Y1 cells by examining the DNA-binding capacity of different forms of endogenous Sf1. No significant changes in all four Ad4-binding sites were observed (Fig. S4B). The data indicate that differential DNA binding of Sf1 to FAdE in the context of SUMOylated Sf1 and Dax1 does not occur, and therefore does not significantly contribute to changes in transcriptional activation of FAdE-mediated gene expression.
DISCUSSION
Effective Sf1 dose has been shown to be a critical determinant of Sf1 transcriptional activity. Effective Sf1 dose is influenced by genetic dose (derived from studies in patients and in engineered mice with Sf1 haplo-insufficiency manifesting with adrenal defects), mRNA/protein level, DNA binding, SF-1 ligand, post-translational modifications and co-factor recruitment – all of which ultimately influence Sf1 transcriptional activity on Sf1 genomic targets (Bland et al., 2004; Blind et al., 2014; Chen et al., 2005; Ozisik et al., 2002; Urs et al., 2007; Yang et al., 2009; Zubair et al., 2009).
Developmental studies in mice have determined that Sf1 dose (Sf1 mRNA/protein level) is a critical determinant of the specification/formation of the adrenal primordium (fetal zone) as it buds off of the shared adrenogonadal primordia (Bland et al., 2004). The expression of Sf1 in the adrenal primordia (fetal zone) is driven by the fetal zone-restricted Sf1 enhancer FAdE. The activation of FAdE in the fetal zone is maintained by Sf1 itself, which binds to and activates FAdE – hence maintaining its own expression (Zubair et al., 2006). Moreover, the forced expression of Sf1 under the control of a transgene driven by the fetal zone restricted Sf1 enhancer FAdE results in both: (1) an expansion of ectopic adrenal tissue that persists postnatally, expresses FAdE-LacZ and hence is consistent with a fetal zone identity; and (2) a persistent expanded postnatal FAdE-LacZ population in the bona fide adrenal cortex consistent with a retained X-zone (Zubair et al., 2009). Both studies define an essential role of Sf1 dose in the extent of FAdE activation and the extent of X-zone retention. Moreover, the work indicates that FAdE-mediated Sf1 dose is a critical determinant of the extent of X-zone retention.
Two of the most well-studied physiological mediators of effective Sf1 dose are the post-translational modification of SUMOylation of the Sf1 protein and the recruitment of the transcriptional co-factor Dax-1 to the Sf1 complex on DNA. Previous studies have shown that SUMOylation at K194/K119 inhibits Sf1 transcriptional activity on a number of target genes active in the definitive adrenal cortex, including StAR and MC2R (Yang et al., 2009). When those two SUMOylation sites are eliminated in mice, defects in the adult differentiated cortex include decreased steroidogenesis and an enhancement in expression/signaling of progenitor cell sonic hedgehog (Shh) expression (Lee et al., 2011). In the present study, we examined the roles of Sf1 SUMOylation specifically in the fetal adrenal cortex. We demonstrate that both SUMOylation-deficient Sf1 and knockout of the Sf1 repressor Dax1 result in retention of FAdE expression (consistent with persistent FAdE-mediated Sf1 expression) and a prolonged retention (delayed regression) of the X-zone (fetal adrenal remnant) in the mouse adrenal gland (Fig. 6A). Furthermore, we determine that in cell culture, SUMOylation of Sf1 increases the interaction between Sf1 and Dax1, resulting in a near-complete inactivation of FAdE activity (Fig. 6B), providing a potential molecular mechanism for the active extinguishing of FAdE-mediated Sf1 transcription, which ultimately leads to postnatal regression of the remnant fetal cortex – the X-zone.
Fig. 6.
The synergistic interaction between Sf1 SUMOylation and Dax1 controls the timing of X-zone regression. (A) Genetic depletion of Sf1 SUMOylation or Dax1 expression in mouse adrenal cortex delays the timing of X-zone regression by activating FAdE in postnatal adrenal glands. (B) SUMOylation of Sf1 enhances Dax1 binding to Sf1 protein and subsequent inhibition of FAdE activity in adrenocortical cells.
SUMOylation is emerging as a versatile modification and has been shown to modify protein function through regulation of protein–protein interactions, subcellular nuclear localization, protein–DNA interactions or enzymatic activity (Wilson and Rangasamy, 2001; Melchior and Hengst, 2002; Pichler and Melchior, 2002; Freiman and Tjian, 2003; Verger et al., 2003; Müller et al., 2004). Consequently, the SUMOylation process can influence a variety of biological processes, including apoptosis, cell cycle regulation, cell growth and differentiation (Andreou and Tavernarakis, 2009; Bettermann et al., 2012; Krumova and Weishaupt, 2013). Previous studies have indicated that SUMOylation of Sf1 does not alter its nuclear localization or DNA interaction (Chen et al., 2004; Lee et al., 2005), both of which are confirmed in the current study (data not shown). In vitro studies using the Y1 adrenocortical cell line have shown that SUMOylation inhibits Sf1 activity by reducing phosphorylation at S203, suggesting the interplay between SUMOylation and phosphorylation (Yang et al., 2009). However, no changes in the phosphorylation level of Sf1 were detected in whole organ lysates of adrenal glands from Sf12KR/2KR mice (data not shown), suggesting the phenotypes we observed are unlikely to be mediated by an increase in phosphorylation of Sf1 alone. Importantly, this observation does not preclude the possibility that the deficiency in SUMOylation only enhances CDK7-mediated phosphorylation of Sf1, as shown in a previous study (Yang et al., 2009), without elevating the total phosphorylation levels. An in vitro CoIP assay failed to detect CDK7 protein in the Sf1/Dax1 complex (data not shown).
In the current system, the auto-activation of FAdE by Sf1 itself is almost completely inhibited by SUMOylation of Sf1 and an enhanced recruitment of the co-repressor Dax1, resulting in a complete repression of FAdE-mediated transcription of the Sf1 gene. It is expected that SUMOylation can alter the surface of the target protein and cause either general conformational changes or specific changes at crucial interfaces, which consequently may modify the interaction of the protein with co-activators or co-repressors. This change in transcriptional complexes leads to regulation of target gene expression. Based on the high sequence and 3D-structure homology with mLRH-1, Sf1 is proposed to have two Dax1-binding sites: one at AF2 region and one at the hormone pocket entrance region. Owing to the proximity of SUMOylation sites K119 and K194 with LBD (ligand-binding domain), it is possible that SUMOylation at the hinge region changes the conformation/ligand-binding property of the ligand-binding pocket, which in turn enhances Dax1 binding with Sf1. No traditional co-partners (i.e. SRC or P300) were detected in the Sf1/Dax1 complex (data not shown), but it is reasonable to speculate SUMOylation/binding with Dax1 may lead to exchange of other co-activators or co-repressors.
MATERIALS AND METHODS
Experimental animals
All animal experiments were carried out in accordance with protocols approved by the University Committee on Use and Care of Animals at the University of Michigan. The Sf12KR/2KR mouse line were generated as described and maintained on a C57BL/6J background (Lee et al., 2011). Dax1-deficient mice were obtained previously (Scheyset al., 2011) and maintained on a 129S1/SvImJ background. To obtain Dax1−/y mice, wild-type males were mated with heterozygous females (Babu et al., 2002; Yu et al., 1998). Both lines were crossed with FAdE-LacZ reporter mice (Zubair et al., 2006) for experimental purposes.
LacZ (β-galactosidase) staining, histology and immunohistochemistry
LacZ activity in fetal tissues was examined as described previously (Zubair et al., 2009). After being stained, the tissue samples were fixed with 4% paraformaldehyde in phosphate-buffered saline (PFA-PBS) for 6 h at 4°C, embedded in optimal-cutting-temperature compound, and sectioned at 16-18 µm. Hematoxylin and Eosin and immunofluorescence staining was performed following a previously published protocol (Kim et al., 2008). Images were captured using a Nikon Optiphot microscope and color Nikon digital camera. Images were processed using Photoshop software (Adobe System). Primary antibodies used for immunohistochemistry were anti-β-galactosidase (1:1000; Abcam, ab9361), anti-tyrosine hydroxylase (TH) (1:500; Cell Signaling Technology, 2792), anti-Sf1 (1:2000, Lab custom antibody) (Walczak et al., 2014), anti-Dax1 [1:1000; generously provided by Enzo Lalli (Centre National de la Recherche Scientifique, France) (Tamai et al., 1996)] and anti-20α hydroxysteroid dehydrogenase (20αHSD) (1:2000; Dr Yacob Weinstein, Ben Gurion University, Israel; Hershkovitz et al., 2007). Secondary antibodies used were goat anti-mouse Alexa 488 and goat anti-rabbit 549 (1:1000; Molecular Probes, A-10680 and A-27039).
RNA isolation and real-time qPCR
RNA was isolated from paraffin wax-embedded adrenal tissue section of wild-type, Dax1−/y and Sf12KR/2KR male mice using the RNeasy FFPE kit from Qiagen following the manufacturer's manual. cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific). Sequences of primers used for qPCR amplification of x-zone markers were: 20αHSD F, 5′-TGGTCACTCCATTCCTGTGG-3′; R, 5′-TGAGATGCTCTTCAGTTGCA-3′; Thrβ F, 5′-GCTGGTAGGAATGTCTGAAGC-3′; R, 5′-AGTCTGGAAAGTCTGGGCAC-3′; Pik3c2g-F, 5′-GTGGACCCAGGTGAGAACT-3′; R, 5′-GGAACACACTTTGTTTTCTTT CTC -3′.
Construct engineering
Mouse Sf1 cDNA was amplified by PCR using the forward primer 5′-TCGTGGATCCATGGACTACTCGTACGACGAG-3′ and the reverse primer 5′-ACGAAAGCTTTCAAGTCTGCTTGGCCTGCAG-3′, and integrated into pcDNA3 construct. The pcDNA3-HIS-FLAG-SF1 2KR, pcDNA3-HA-SUMO2-SF1 plasmids were derived from the wild-type pcDNA3-HIS-FLAG-SF1 vector using the QuikChange site-directed mutagenesis approach (Strategene). The reporter plasmid was constructed by inserting the FAdE fragment (Zubair et al., 2006) together with the minimal promoter of thymidine kinase of herpes simplex virus into the KpnI-HindIII sites of pGL3-Basic (Promega).
Cell culture and transient transfection
Mouse adrenal Y1 cells and human embryonic kidney HEK293T cells were cultured in DME/F-12 medium (Invitrogen Life Technologies) supplemented with 10% Cosmic Calf Serum (ThermoFisher Scientific) and antibiotics in humidified air containing 5% CO2 at 37°C. Twenty-four hours before transfection, HEK293T cells were plated at 105 cells/well in 24-well plates. Transfections were carried out in triplicate with a combination of plasmids and using FuGENE 6 (Roche, Basel, Switzerland) according to the manufacturer's protocol. Plasmids used include pGL3-FAdE luciferase reporter plasmid at 100 ng/well; pcDNA-Sf1 (wild type, SUMO2 or 2KR) in varying amounts, pcDNA-Dax1, Senp1, Ubc9 and pSA2 (Addgene); cytomegalovirus-β-galactosidase (40 ng/well) as a control for transfection efficiency; pcDNA to bring the total to 265 ng/well. The cells were harvested 48 h after transfection, and activities in cell lysates were determined using the dual luciferase reporter assay system (Promega). The luciferase values were normalized to the β-galactosidase activity.
CoIP and western analysis
Protein extracts were prepared by homogenization of cells and tissues in RIPA buffer [50 mM Tris HCl (pH 8), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS] containing protease inhibitor cocktail (Sigma). Soluble protein was collected and immunoprecipitated with the Sf1 antibody overnight. Protein G-dynabeads were added to the protein lysates and incubated for 2 h at 4°C. The beads were precipitated and washed with buffers of increasing stringency. The proteins were eluted by boiling in 30 μl of sample buffer, resolved by 10% SDS-PAGE gel, and transferred to a nitrocellulose membrane. Immunoblot was performed using the Odyssey system for protein detection. Primary antibodies used were rabbit polyclonal anti-Ad4BP/SF1 [1:1500; custom antibody (Walczak et al., 2014)] and mouse anti-HA antibody (1:300, Covance).
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed using the ChIP-Easy kit (Upstate) following the manufacturer's protocol. Antibodies used for ChIP were non-immune rabbit IgG (Santa Cruz Biotechnology) and anti-Ad4BP/Sf1 (Custom antibody). Sequences of primers used for qPCR amplification of immunoprecipitated chromatin were as follows: FAdE site 1 F, CATGGTCAGCCATGGAGTG; FAdE site 1 R, CTGGGGTCTGATCAGTCTG; FAdE site 2 F, CTGCTGAGGGAGAGGTCAGT, FAdE site 2 R, TCCTCCTATGGCCCATCACA; FAdE site 3/4 F, TGTGATGGGCCATAGGAGGA; FAdE site 3/4 R, ACCTTCCTCTTGCCACCTTG.
Statistical analyses
Data are expressed as means±s.e.m. A one-way ANOVA was applied to compare means among the groups. Appropriate post-hoc pair-wise multiple comparisons were performed. Prism was used for the statistical analysis. P<0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Dr J. Larry Jameson (University of Pennsylvania, Philadelphia, PA, USA) for providing Dax1−/y mice, Dr C. Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS, USA) for providing anti-Cyp11b1 and anti-Cyp11b2 antibody, and Dr William Rainey for editing the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.X., G.D.H.; Methodology: Y.X., K.M.; Formal analysis: Y.X.; Investigation: Y.X.; Resources: H.A.I., K.M.; Data curation: Y.X.; Writing - original draft: Y.X.; Writing - review & editing: H.A.I., K.M., G.D.H.; Supervision: G.D.H.; Funding acquisition: H.I., G.D.H.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases National Institutes of Health research grants (2R01-DK062027 to G.D.H. and R01DK099722 to H.A.I.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.150516.supplemental
References
- Andreou A. M. and Tavernarakis N. (2009). SUMOylation and cell signalling. Biotechnol. J. 4, 1740-1752. 10.1002/biot.200900219 [DOI] [PubMed] [Google Scholar]
- Asari M., Fukaya K., Eguchi Y., Nishida S. and Kano Y. (1979). [Effect of testosterone and progesterone on the adrenal X-zone in female mice (author's transl)]. Nihon Juigaku Zasshi 41, 61-67. 10.1292/jvms1939.41.61 [DOI] [PubMed] [Google Scholar]
- Babu P. S., Bavers D. L., Beuschlein F., Shah S., Jeffs B., Jameson J. L. and Hammer G. D. (2002). Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143, 665-673. 10.1210/endo.143.2.8658 [DOI] [PubMed] [Google Scholar]
- Bettermann K., Benesch M., Weis S. and Haybaeck J. (2012). SUMOylation in carcinogenesis. Cancer Lett. 316, 113-125. 10.1016/j.canlet.2011.10.036 [DOI] [PubMed] [Google Scholar]
- Bland M. L., Fowkes R. C. and Ingraham H. A. (2004). Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol. Endocrinol. 18, 941-952. 10.1210/me.2003-0333 [DOI] [PubMed] [Google Scholar]
- Blind R. D., Sablin E. P., Kuchenbecker K. M., Chiu H.-J., Deacon A. M., Das D., Fletterick R. J. and Ingraham H. A. (2014). The signaling phospholipid PIP3 creates a new interaction surface on the nuclear receptor SF-1. Proc. Natl. Acad. Sci. USA 111, 15054-15059. 10.1073/pnas.1416740111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckwoldt M., Selvaraj N., Aharoni D., Barash A., Segal I., Insler V. and Amsterdam A. (1996). Expression of Ad4-BP/cytochrome P450 side chain cleavage enzyme and induction of cell death in long-term cultures of human granulosa cells. Mol. Hum. Reprod. 2, 391-400. 10.1093/molehr/2.6.391 [DOI] [PubMed] [Google Scholar]
- Buaas F. W., Gardiner J. R., Clayton S., Val P. and Swain A. (2012). In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development 139, 4561-4570. 10.1242/dev.087247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Faivre E. J., Show M. D., Ingraham J. G., Flinders J., Gross J. D. and Ingraham H. A. (2008). Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1). Mol. Cell Biol. 28, 7476-7486. 10.1128/MCB.00103-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-Y., Lee W.-C., Hsu N.-C., Huang F. and Chung B. C. (2004). SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J. Biol. Chem. 279, 38730-38735. 10.1074/jbc.M405006200 [DOI] [PubMed] [Google Scholar]
- Chen W.-Y., Juan L.-J. and Chung B.-C. (2005). SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol. Cell Biol. 25, 10442-10453. 10.1128/MCB.25.23.10442-10453.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B.-C., Guo I.-C. and Chou S.-J. (1997). Transcriptional regulation of the CYP11A1 and ferredoxin genes. Steroids 62, 37-42. 10.1016/S0039-128X(96)00156-0 [DOI] [PubMed] [Google Scholar]
- Delost P., Dalle M., Tournaire C. and Delost H. (1971). [Androgens and adrenal X-zone]. J. Physiol. (Paris) 63, 197a. [PubMed] [Google Scholar]
- Ferraz-de-Souza B., Martin F., Mallet D., Hudson-Davies R. E., Cogram P., Lin L., Gerrelli D., Beuschlein F., Morel Y., Huebner A. et al. (2009). CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2, and pre-B-cell leukemia transcription factor 1 in human adrenal development and disease. J. Clin. Endocrinol. Metab. 94, 678-683. 10.1210/jc.2008-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B. D., Kempna P. B., Carlone D. L., Shah M. S., Guagliardo N. A., Barrett P. Q., Gomez-Sanchez C. E., Majzoub J. A. and Breault D. T. (2013). Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 26, 666-673. 10.1016/j.devcel.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman R. N. and Tjian R. (2003). Regulating the regulators: lysine modifications make their mark. Cell 112, 11-17. 10.1016/S0092-8674(02)01278-3 [DOI] [PubMed] [Google Scholar]
- Hammer G. D., Krylova I., Zhang Y., Darimont B. D., Simpson K., Weigel N. L. and Ingraham H. A. (1999). Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell 3, 521-526. 10.1016/S1097-2765(00)80480-3 [DOI] [PubMed] [Google Scholar]
- Hershkovitz L., Beuschlein F., Klammer S., Krup M. and Weinstein Y. (2007). Adrenal 20α-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-Zone. Endocrinology 148, 976-988. 10.1210/en.2006-1100 [DOI] [PubMed] [Google Scholar]
- Holmes P. V. and Dickson A. D. (1971). X-zone degeneration in the adrenal glands of adult and immature female mice. J. Anat. 108, 159-168. [PMC free article] [PubMed] [Google Scholar]
- Howard-Miller E. (1928). A transitory zone in the adrenal cortex which shows age and sex relationships. Am. J. Anat. 40, 43. [Google Scholar]
- Iyer A. K. and McCabe E. R. B. (2004). Molecular mechanisms of DAX1 action. Mol. Genet. Metab. 83, 60-73. 10.1016/j.ymgme.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Kim A. C., Reuter A. L., Zubair M., Else T., Serecky K., Bingham N. C., Lavery G. G., Parker K. L. and Hammer G. D. (2008). Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135, 2593-2602. 10.1242/dev.021493 [DOI] [PubMed] [Google Scholar]
- Kohler B. and Achermann J. C. (2010). Update--steroidogenic factor 1 (SF-1, NR5A1). Minerva Endocrinol. 35, 73-86. [PubMed] [Google Scholar]
- Krumova P. and Weishaupt J. H. (2013). Sumoylation in neurodegenerative diseases. Cell. Mol. Life Sci. 70, 2123-2138. 10.1007/s00018-012-1158-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. B., Lebedeva L. A., Suzawa M., Wadekar S. A., Desclozeaux M. and Ingraham H. A. (2005). The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol. Cell. Biol. 25, 1879-1890. 10.1128/MCB.25.5.1879-1890.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. Y., Faivre E. J., Suzawa M., Lontok E., Ebert D., Cai F., Belsham D. D. and Ingraham H. A. (2011). Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev. Cell 21, 315-327. 10.1016/j.devcel.2011.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lu Y., Liu R., Xiong X., Zhang Z., Zhang X., Ning G. and Li X. (2011). DAX1 suppresses FXR transactivity as a novel co-repressor. Biochem. Biophys. Res. Commun. 412, 660-666. 10.1016/j.bbrc.2011.08.020 [DOI] [PubMed] [Google Scholar]
- Masui K. and Tamura Y. (1926). The effect of gonadectomy on the structure of the suprarenal glands of mice, with special reference to the functional relation between this gland and the sex gland of the female. J. Coll. Agric. Tokyo. [Google Scholar]
- Melchior F. and Hengst L. (2002). SUMO-1 and p53. Cell Cycle 1, 245-249. 10.4161/cc.1.4.131 [DOI] [PubMed] [Google Scholar]
- Morohashi K. and Zubair M. (2011). The fetal and adult adrenal cortex. Mol. Cell. Endocrinol. 336, 193-197. 10.1016/j.mce.2010.11.026 [DOI] [PubMed] [Google Scholar]
- Mukai T., Kusaka M., Kawabe K., Goto K., Nawata H., Fujieda K. and Morohashi K. (2002). Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells 7, 717-729. 10.1046/j.1365-2443.2002.00556.x [DOI] [PubMed] [Google Scholar]
- Müller S., Ledl A. and Schmidt D. (2004). SUMO: a regulator of gene expression and genome integrity. Oncogene 23, 1998-2008. 10.1038/sj.onc.1207415 [DOI] [PubMed] [Google Scholar]
- Ohno T. (1962). The effects of stress and ACTH-stimulus on the X-zone of the mouse adrenals with and without hypophysectomy. Tohoku J. Exp. Med. 77, 195-203. 10.1620/tjem.77.195 [DOI] [PubMed] [Google Scholar]
- Ozisik G., Achermann J. C. and Jameson J. L. (2002). The role of SF1 in adrenal and reproductive function: insight from naturally occurring mutations in humans. Mol. Genet. Metab. 76, 85-91. 10.1016/S1096-7192(02)00032-X [DOI] [PubMed] [Google Scholar]
- Ozisik G., Achermann J. C., Meeks J. J. and Jameson J. L. (2003). SF1 in the development of the adrenal gland and gonads. Horm. Res. 59 Suppl. 1, 94-98. [DOI] [PubMed] [Google Scholar]
- Pichler A. and Melchior F. (2002). Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic 3, 381-387. 10.1034/j.1600-0854.2002.30601.x [DOI] [PubMed] [Google Scholar]
- Sasaki G., Zubair M., Ishii T., Mitsui T., Hasegawa T. and Auchus R. J. (2014). The contribution of serine 194 phosphorylation to steroidogenic acute regulatory protein function. Mol. Endocrinol. 28, 1088-1096. 10.1210/me.2014-1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheys J. O., Heaton J. H. and Hammer G. D. (2011). Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology 152, 3430-3439. 10.1210/en.2010-0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K. T., Monaco L., Alastalo T. P., Lalli E., Parvinen M. and Sassone-Corsi P. (1996). Hormonal and developmental regulation of DAX-1 expression in Sertoli cells. Mol. Endocrinol. 10, 1561-1569. 10.1210/mend.10.12.8961266 [DOI] [PubMed] [Google Scholar]
- Tanaka S. and Matsuzawa A. (1993). [What mouse contributed the first representation of the adrenal cortex X-zone?]. Jikken Dobutsu 42, 305-316. 10.1538/expanim1978.42.3_305 [DOI] [PubMed] [Google Scholar]
- Urs A. N., Dammer E., Kelly S., Wang E., Merrill A. H. Jr and Sewer M. B. (2007). Steroidogenic factor-1 is a sphingolipid binding protein. Mol. Cell. Endocrinol. 265-266, 174-178. 10.1016/j.mce.2006.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A., Perdomo J. and Crossley M. (2003). Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 4, 137-142. 10.1038/sj.embor.embor738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak E. M., Kuick R., Finco I., Bohin N., Hrycaj S. M., Wellik D. M. and Hammer G. D. (2014). Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol. Endocrinol. 28, 1471-1486. 10.1210/me.2014-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. G. and Rangasamy D. (2001). Intracellular targeting of proteins by sumoylation. Exp. Cell Res. 271, 57-65. 10.1006/excr.2001.5366 [DOI] [PubMed] [Google Scholar]
- Wood M. A. and Hammer G. D. (2011). Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol. Cell. Endocrinol. 336, 206-212. 10.1016/j.mce.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. E., Liu L., Wang J. F., Liu F., Li X. H., Qin H. Q. and Wang H. (2014). Prenatal nicotinic exposure suppresses fetal adrenal steroidogenesis via steroidogenic factor 1 (SF-1) deacetylation. Toxicol. Appl. Pharmacol. 277, 231-241. 10.1016/j.taap.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Yang W.-H., Heaton J. H., Brevig H., Mukherjee S., Iniguez-Lluhi J. A. and Hammer G. D. (2009). SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol. Cell Biol. 29, 613-625. 10.1128/MCB.00295-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. N., Ito M., Saunders T. L., Camper S. A. and Jameson J. L. (1998). Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 20, 353-357. 10.1038/3822 [DOI] [PubMed] [Google Scholar]
- Zhou J., Oakley R. H. and Cidlowski J. A. (2008). DAX-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X-chromosome, gene 1) selectively inhibits transactivation but not transrepression mediated by the glucocorticoid receptor in a LXXLL-dependent manner. Mol. Endocrinol. 22, 1521-1534. 10.1210/me.2007-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M., Oka S., Ishihara S. and Morohashi K. (2002). Analysis of Ad4BP/SF-1 gene regulatory region. Endocr. Res. 28, 535 10.1081/ERC-120016834 [DOI] [PubMed] [Google Scholar]
- Zubair M., Ishihara S., Oka S., Okumura K. and Morohashi K. (2006). Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol. Cell. Biol. 26, 4111-4121. 10.1128/MCB.00222-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M., Parker K. L. and Morohashi K. (2008). Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol. Cell. Biol. 28, 7030-7040. 10.1128/MCB.00900-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M., Oka S., Parker K. L. and Morohashi K. (2009). Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol. Endocrinol. 23, 1657-1667. 10.1210/me.2009-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.