Abstract
Clefts of the palate and/or lip are among the most common human craniofacial malformations and involve multiple genetic and environmental factors. Defects can only be corrected surgically and require complex life-long treatments. Our studies utilized the well-characterized Pax9−/− mouse model with a consistent cleft palate phenotype to test small-molecule Wnt agonist therapies. We show that the absence of Pax9 alters the expression of Wnt pathway genes including Dkk1 and Dkk2, proven antagonists of Wnt signaling. The functional interactions between Pax9 and Dkk1 are shown by the genetic rescue of secondary palate clefts in Pax9−/−Dkk1f/+;Wnt1Cre embryos. The controlled intravenous delivery of small-molecule Wnt agonists (Dkk inhibitors) into pregnant Pax9+/− mice restored Wnt signaling and led to the growth and fusion of palatal shelves, as marked by an increase in cell proliferation and osteogenesis in utero, while other organ defects were not corrected. This work underscores the importance of Pax9-dependent Wnt signaling in palatogenesis and suggests that this functional upstream molecular relationship can be exploited for the development of therapies for human cleft palates that arise from single-gene disorders.
KEY WORDS: Pax9, Wnt, Palatogenesis, Cleft palate, Mouse, Wnt agonist, Small molecule
Summary: Pax9-dependent Wnt signaling via Dkk1/2 expression in the posterior palatal mesenchyme enhances cell proliferation along the buccal-lingual axis, palatal shelf outgrowth and fusion.
INTRODUCTION
Failures in palatogenesis occur during the outgrowth, elevation, migration and fusion of palatal shelves, providing the best evidence that these developmental processes are under strict molecular control (Mossey et al., 2009; Greene and Pisano, 2010). Since the sequence of events in murine palatogenesis closely resembles that seen in humans, mouse genetic models with secondary palate defects have been valuable in dissecting the signaling pathways that drive palatogenesis (Funato et al., 2015). Gene ontology reveals that more than half of the genes associated with palatal clefts in mice are transcription factors, including Msx1 (Satokata and Maas, 1994; Zhang et al., 2002), Pax9 (Peters et al., 1998; Zhou et al., 2011), Osr2 (Lan et al., 2004), Gbx2 (Byrd and Meyers, 2005), Tbx22 (Pauws et al., 2009), Tfap2a (Brewer et al., 2004) and Hoxa2 (Gendron-Maguire et al., 1993). Moreover, growth factor families such as TGFβ, Shh, Fgf and Wnt, along with membranous molecules and extracellular matrix proteins are also implicated as playing important roles in palate formation (Greene and Pisano, 2010; Funato et al., 2015). Taken together, these studies reveal that a tight interplay of gene networks and environmental risk factors regulates key molecular, cellular and tissue interactions during palate development. However, much remains to be learned about how these molecules function and how such knowledge can be translated into new treatment strategies for preventing or reversing cleft conditions in humans.
Among the mouse genetic models of cleft palate that are available for study, the Pax9−/− model has provided valuable insights into the mechanisms that lead to the failure of palatogenesis. Pax9 belongs to the family of paired-box DNA-binding domain-containing transcription factors, which play key roles in organogenesis (Stapleton et al., 1993). Pax9−/− mice consistently exhibit clefts of the secondary palate and die shortly after birth. Other phenotypic defects include agenesis of the thymus, parathyroid glands, ultimobranchial bodies and teeth (Peters et al., 1998; Zhou et al., 2011). It has been proposed that Pax9 is involved in molecular signaling events that control the patterning of the anterior-posterior (AP) axis during palatal shelf outgrowth. Its control of cell proliferation and its interactions with the Bmp, Fgf and Shh pathways are hence important for expansion of the palatal shelves and their growth along the AP axis and towards the midline (Zhou et al., 2013). However, little is known about the morphogenetic gradient that controls patterning of the palatal shelves along the buccal-lingual axis. This information is crucial as the closure of palatal shelves towards the midline relies on the precise actions and interactions of genes along this axis.
In these studies we demonstrate the functionality of the upstream molecular relationship of Pax9 with Wnt signaling during palatogenesis. Our unbiased genome-wide analyses show that the inhibitors of Wnt, namely Dkk1 and Dkk2, are significantly upregulated in Pax9−/− palatal mesenchyme, while Wnt genes are downregulated. Definitive evidence of the importance of the relationship of Pax9 to Dkk1 is shown by the genetic rescue of secondary palate clefts in mice that are deficient in both Dkk1 and Pax9. Furthermore, the controlled intravenous delivery of a small-molecule Wnt agonist, which specifically inhibits Dkk1, into pregnant Pax9+/− mice corrects 100% of cleft palate defects in mutant progeny. These studies underscore the importance of Pax9-dependent Wnt signaling during palatogenesis and suggest that Wnt agonist therapies can be exploited to correct cleft palate conditions in humans that arise from single-gene defects.
RESULTS
Pax9 is upstream of the Wnt signaling pathway during murine palatogenesis
We first examined the molecular consequences of Pax9 deficiency in palatal shelves at E13.5 by RNASeq analyses. Our data indicate that the complete lack of Pax9 led to more than a 1.5-fold (P<0.01) change in expression of 1368 genes, among which the Wnt pathway genes appear strongly enriched (Table 1, left column). Significant is the higher expression of dickkopf 1 (Dkk1) and Dkk2, which encode extracellular proteins that block the binding of Wnt ligands to the Lrp5/6 receptor complex. The expression of Wnt ligands Wnt3, Wnt7a and Wnt9b was also altered, along with genes involved in Bmp, ectodysplasin (Eda), Fgf and Shh signaling (Table 1, right column). Quantitative RT-PCR analysis (Fig. 1) confirmed that the Wnt signaling pathway inhibitors Dkk1 and Dkk2 had increased 1.7-fold. By contrast, Lef1, Gbx2, Hand2 and Fgf4, which are involved in Wnt signaling, and Msx1 and Msx2, which are proved partner genes of Pax9 (Zhou et al. 2011), were significantly downregulated in the absence of Pax9. Moreover, Tfap2b, a member of the Ap2 family that interacts with Cited1 during craniofacial development (Bragança et al., 2002; Knight et al., 2005), was reduced more than 2-fold in E13.5 Pax9−/− palates.
Table 1.
Expression profiles of Pax9-affected genes with functional enrichment analysis in E13.5 palatal shelves
Fig. 1.
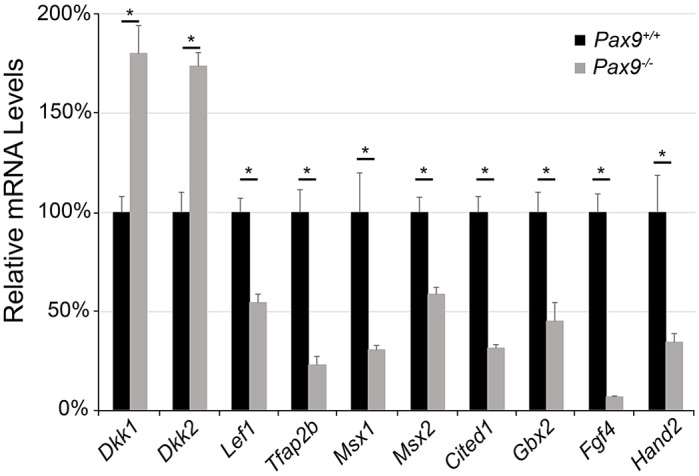
Quantitative RT-PCR analysis of gene expression in E13.5 Pax9−/− palatal shelves. The mRNA levels of a select group of differentially expressed genes were verified in microdissected palatal shelves of Pax9+/+ and Pax9−/− mouse embryos by quantitative RT-PCR (n=5 per group). Pax9 deficiency affects the expression of Wnt signaling pathway genes and known partner genes of Pax9. The expression levels of Dkk1 and Dkk2 are significantly upregulated in the absence of Pax9. By contrast, Wnt signaling pathway downstream targets, such as Lef1, Gbx2, Fgf4 and Hand2, as well as the Pax9 partner genes Msx1 and Msx2, were significantly downregulated in Pax9−/− samples. Results are shown as percentage of the mean±s.e.m. of the Pax9+/+ group. *P<0.01.
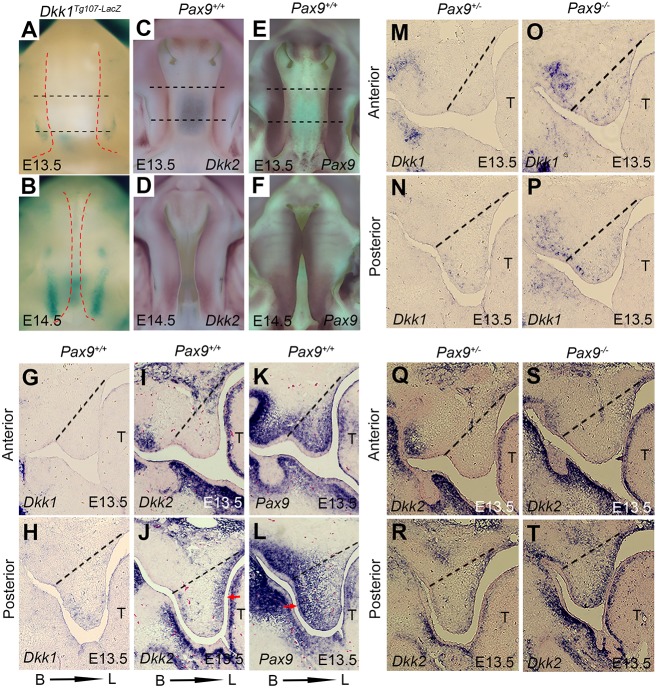
The relationship between Pax9 and the Wnt pathway was further assessed by studying the expression patterns of Dkk1, Dkk2 and Pax9 during normal palatogenesis. As shown in Fig. 2A-D, Dkk1 and Dkk2 expression levels increased from E13.5 to E14.5 and appeared restricted to posterior palatal mesenchyme, while Pax9 was expressed more strongly and present in both anterior and posterior palatal mesenchyme (Fig. 2E,F,K,L). Dkk1 transcripts are more visible in posterior palate compared with the anterior region (Fig. 2G,H) but appear less abundant than those of Dkk2 and Pax9 in the posterior region of E13.5 palate (Fig. 2H,J,L). The buccal-lingual gradient of expression for Pax9 and Dkk2 is inversely related, with Pax9 stronger on the buccal aspects of the palatal shelves (Fig. 2L) and Dkk2 transcripts more localized to the lingual region (Fig. 2J). Whereas Pax9 expression is broader and occupies the entire palatal mesenchyme in anterior palate (Fig. 2K), low levels of Dkk1/2 expression are visible in the anterior region (Fig. 2G,I,M,Q). Interestingly, these expression domains of Dkk1, Dkk2 and Pax9 in posterior palatal mesenchyme differ from that of Msx1, which localizes to the anterior region of the palatal mesenchyme (Zhang et al., 2002; Han et al., 2009; Fuchs et al., 2010).
Fig. 2.
Patterns of Pax9, Dkk1 and Dkk2 expression in E13.5 Pax9+/− and Pax9−/− palatal shelves suggest that Pax9 is upstream of Wnt signaling. (A,B) Whole-mount lacZ staining (blue) showing that Dkk1 expression is restricted to the posterior domain of palatal shelves in Dkk1Tg107−lacZ embryos at E13.5 and E14.5. Red dashed lines indicate the boundary of palate. (C,D) Whole-mount in situ hybridization with digoxigenin-labeled probes showing that Dkk2 transcripts (purple) are more visible at the medial edges of the palatal shelves in Pax9+/+ embryos at E13.5 and E14.5. (E,F) By contrast, Pax9 expression appears stronger and expands along the AP domain of palatal shelves at E13.5 and E14.5. Black dashed lines indicate the position of the sections in G-L. (G-L) In situ hybridizations on adjacent sections, presented in the coronal plane, confirm that Dkk1 (G,H) and Dkk2 (I,J) transcripts have inverse expression patterns with that of Pax9 (K,L) at E13.5 and E14.5. Pax9 expression is more intense along the buccal aspects of palatal mesenchyme (K,L), whereas Dkk2 transcripts localize on the lingual side (I,J). Arrows indicate the higher expression domain. (M-T) In the posterior regions of palatal mesenchyme, Dkk1 (M-P) and Dkk2 (Q-T) expression increased at E13.5 as a result of Pax9 deficiency. B, buccal; L, lingual; T, tongue. n=6 for whole-mount in situ hybridization, n=5 for section in situ hybridization.
In situ hybridizations showed that expression of both Wnt inhibitors increased in Pax9−/− palatal mesenchyme (Fig. 2M-T). At E13.5, Dkk1 expression was detected in Pax9+/+ posterior palatal mesenchyme (Fig. 2H,N), whereas its expression had increased in the posterior palate and extended into the anterior zone of Pax9−/− palatal mesenchyme (Fig. 2O,P). Dkk2 expression was also higher in the posterior palatal mesenchyme, with a lingual to buccal gradient of distribution (Fig. 2J,R) that intensified in Pax9−/− tissues (Fig. 2T).
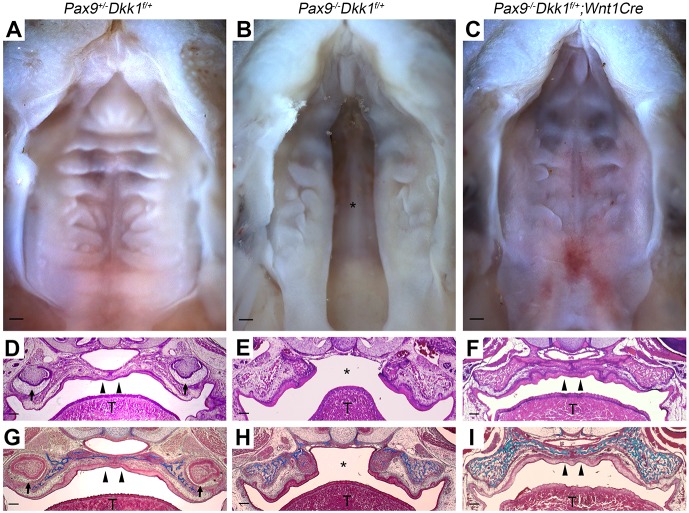
In order to assess whether the molecular upstream relationship between Pax9 and the Wnt pathway was functional, we generated compound mutant mice in which Dkk1 expression was reduced in a Pax9-deficient background. Pax9+/−Dkk1f/+ embryos showed normal secondary palates (Fig. 3A,D,G), whereas Pax9−/−Dkk1f/+ littermates showed clefts in this region (Fig. 3B,E,H). In all Pax9−/−Dkk1f/+;Wnt1Cre compound mutant mice retrieved (n=5), a complete rescue of secondary palate clefts was noted (Fig. 3C,F,I). All five Pax9−/−Dkk1f/+;Wnt1Cre compound mutant mice retained a supernumerary digit (Fig. S1L) and showed significant delay in tooth development (Fig. S1C), both features representing a consistent phenotype of Pax9−/−Dkk1f/+ mice (Fig. S1B,K). Furthermore, agenesis of the parathyroid glands and thymus was not rescued in Pax9−/−Dkk1f/+;Wnt1Cre compound mutant mice (Fig. S1F,I). Four of the five mice in this group were retrieved at E18.5 for analysis and the remaining pup that was born did not survive postnatally.
Fig. 3.
Genetic reduction of Dkk1 expression rescues secondary palate clefts in Pax9−/− mice. (A-C) Representative whole-mount oral views of palate formation in E18.5 embryos. (A) Normal palate closure in Pax9+/−Dkk1f/+ embryos. (B) The phenotype of cleft palate in Pax9−/−Dkk1f/+ embryos (n=4). (C) Intact palate with disordered rugae in Pax9−/−Dkk1f/+;Wnt1Cre embryos. n=5. (D-F) H&E staining of coronal sections. (D) Fusion of the palatal shelves in an Pax9+/−Dkk1f/+ embryo. (E) Failure of palatal shelf outgrowth and closure in a Pax9−/−Dkk1f/+ embryo. (F) The cleft defect is reversed genetically in a Pax9−/−Dkk1f/+;Wnt1Cre embryo. (G-I) Masson's trichrome staining of serial sections show the deposition of connective tissue (blue) within the palatal shelves of (G) Pax9+/−Dkk1f/+ and (I) Pax9−/−Dkk1f/+;Wnt1Cre, while only maxillary bone tissue was stained blue in Pax9−/−Dkk1f/+ (H) embryos. T, tongue; asterisk indicates cleft palate; arrowheads indicate intact palate shelves; arrows indicate molars. Scale bars: 200 µm.
Small-molecule Wnt agonists correct cleft palate defects in Pax9−/− mice
The small-molecule WAY-262611, which potentiates Wnt–β-catenin signaling by specifically inhibiting Dkk1 (Pelletier et al., 2009), was administered daily from E10.5 to E14.5, a critical developmental window during which palate initiation, shelf outgrowth and elevation occur (Fig. 4). The delivery of five doses of WAY-262611 (Fig. S2A), at either 12.5 or 25 mg/kg, into the tail veins of pregnant Pax9+/− mice resulted in fusion of the secondary palate in all Pax9−/− pups examined (n=18). Treatment from E10.5 to E13.5 rescued the cleft phenotype, but to a lesser extent than observed with the E10.5 to E14.5 regimen (Table 2). As shown in Table 2, 60% (11/18) additionally displayed complete fusion between the primary and secondary palates, while 7/18 showed minor residual defects in the zone of fusion of the primary and secondary palates around the third ruga (Fig. 2B). Since Pax9−/− pups die shortly after birth, E18.5 palatal shelves with residual defects were obtained from the group treated with WAY-262611 and cultured for 3 days. As shown in Fig. S3, all palates displayed complete fusion of residual defects. This suggests that the lack of complete fusion was due to a timing delay rather than to any differences in gene expression between the primary and secondary palates.
Fig. 4.
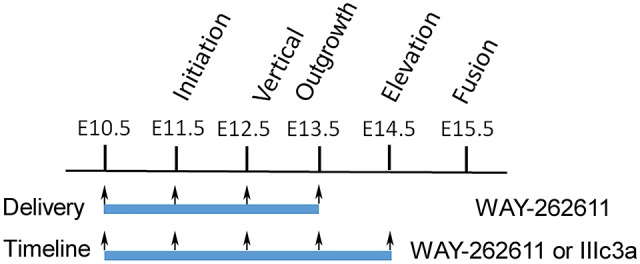
Controlled delivery schedule for small-molecule Wnt agonists. WAY-262611 (two timelines) or IIIc3a (one timeline, based on the high rate of palatal closure achieved with WAY-262611) was intravenously injected through the tail vein during the indicated periods (blue bars). Arrows indicate daily injection. See Table 2 for details.
Table 2.
Secondary palate closure after treatment with Wnt agonists
Recent studies (Li et al., 2017) showed partial rescue of palatal defects in 64% of Pax9−/− embryos treated from E12.5 to E14.5 using an intraperitoneal route of delivery of another small-molecule Wnt agonist, IIIc3a. IIIc3a blocks the Wnt antagonists Dkk1, Dkk2 and Dkk4 (Li et al., 2012). Here, we tested the effects of daily intravenous delivery of IIIc3a to pregnant dams from E10.5 to E14.5. This schedule of IIIc3a intervention resulted in closure of the secondary palate defects in 80% (12/15) of embryos, of which six embryos showed residual fusion defects (Table 2). The sample size for each treatment group was confirmed by power analysis (using PASS version 11, NCSS; provided by University of Utah Study Design and Biostatistics Center) to reach power >80%.
H&E as well as Masson's trichrome staining revealed that both pharmacological interventions brought about a true correction of the secondary palatal defect and the deposition of collagen-enriched matrix in the area of the midline (Fig. 5A-P). The more detailed analyses of the molecular mechanisms underlying the correction of palatal defects was performed on tissues exposed to WAY-262611 treatment, as successful outcomes reached 100% with this experimental group.
Fig. 5.
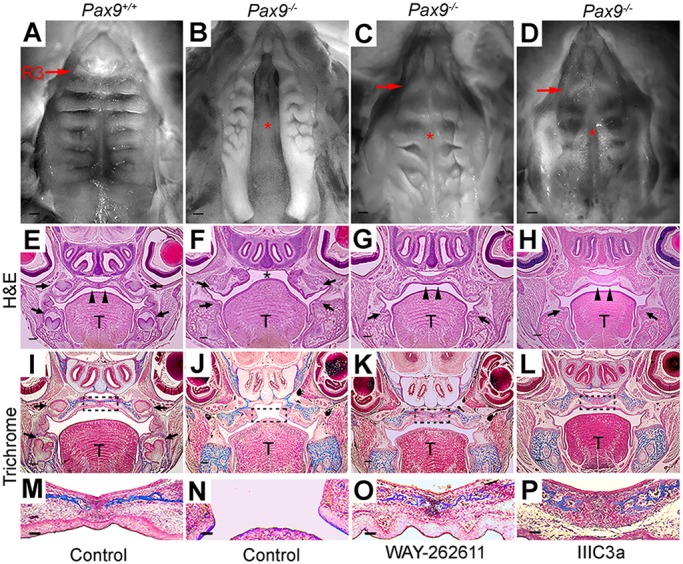
Fusion of the secondary palate in Pax9−/− embryos after intravenous delivery of small-molecule Wnt agonists. (A-D) Representative whole-mount oral views of palatal shelves. (A) In a Pax9+/+ embryo at E18.5, normal palatal fusion with parallel rugae arrangement is visible. (B) Cleft in the secondary palate was a consistent phenotype in control-treated Pax9−/− embryos at E18.5 (n=10). (C,D) Intravenous delivery of WAY-262611 (n=18) and IIIc3a (n=12) led to the closure of palatal shelves in the midline. However, rugae appear less organized compared with Pax9+/+ embryos. R3, ruga 3; arrows indicate the third ruga; asterisk indicates cleft palate or secondary palate fusion after treatment. (E-H) H&E staining of coronal sections through E18.5 heads, showing that the palatal shelves were fused in Pax9+/+ mice (E), whereas a cleft palate was visible in a mutant littermate (F). Note the arrest in tooth formation at the bud stage (arrows). Compared with those that received control injections (F), closure of the palatal shelves was clearly evident in WAY-262611-treated (G) or IIIc3a-treated (H) Pax9−/− mice. Note that in treated mice, tooth organs did not advance beyond the early cap stage (arrows). (I-P) Masson's trichrome staining of position-matched sections showing that the deposition of connective tissue (blue) within the palatal shelves of WAY-262611-treated (K,O) and IIIc3a-treated (L,P) Pax9−/− mice resembled that in control samples (I,J,M,N). The boxed region in I-L is enlarged in M-P. T, tongue; arrowheads indicate intact palate shelves; arrows indicate the first molar; asterisk indicates cleft palate. Scale bars: 200 µm.
Since Pax9 regulates Msx1, a transcription factor that also plays a role in palatogenesis, intravenous injections of WAY-262611 and IIIc3a at 25 mg/kg were given to pregnant Msx1+/− mice. Such interventions failed to rescue cleft palate defects in mutant pups (0/50; Fig. S4). Since Msx1 expression is spatially restricted to the anterior region of palatal mesenchyme, where Dkk1 and Dkk2 are expressed at low levels, it is likely that the reactivation of Wnt signaling only influenced downstream events in posterior regions of the palate where Pax9 is more dominantly expressed.
The in utero delivery of Wnt agonists restores growth of the palatal shelves
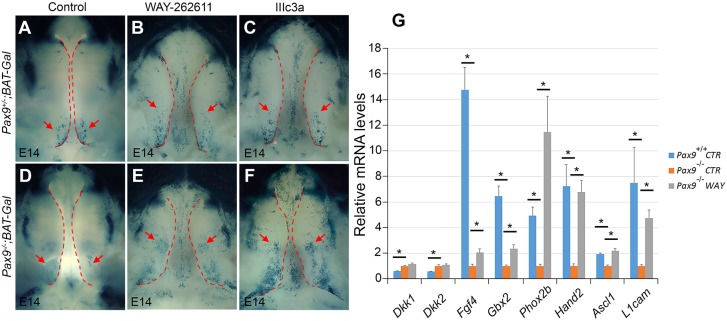
Evidence that these pharmacological interventions activated Wnt signaling in palatal mesenchyme comes from the increased levels of β-catenin (Ctnnb1) activity visible in Pax9−/− palatal mesenchyme after treatment with WAY-262611 or IIIc3a (Fig. 6A-F). RNASeq data showed increased levels of Wnt target gene expression in treated palates (Table 3). WAY-262611 had no effect on Dkk1 and Dkk2 mRNA levels as it acts at the protein level. Expression levels of Gbx2 and L1cam, which are downstream targets of Wnt signaling, are restored following Wnt agonist therapy (Fig. 6G). Notably, Gbx2 mutant mice have cleft palate defects, while L1cam-deficient mice show other craniofacial defects (Byrd and Meyers, 2005). Furthermore, quantitative RT-PCR analysis showed that the expression levels of endogenous Osr2, Msx1 and Bmp4, which were significantly downregulated in the absence of Pax9, were not restored after treatment with WAY-262611 (Fig. S5). This suggested that the signaling hierarchy involving Pax9 and the Wnt pathway, although crucial in posterior palatal mesenchyme, might not involve these molecules.
Fig. 6.
Wnt signaling activity and the expression of downstream targets were partially restored in Pax9−/− palate after treatments. (A-F) Whole-mount lacZ staining (blue) of Pax9−/−;BAT-Gal mice at E14.0 to assess Wnt signaling activity after Wnt agonist therapies. WAY-262611 and IIIc3a slightly increased Wnt activity in the posterior palatal region of Pax9+/−;BAT-Gal mice (compare A with B,C). However, a more significant and expanded level of Wnt activity is observed within posterior palatal shelves of Pax9−/−;BAT-Gal mice (compare D with E,F). Arrows point to the Wnt signaling activity in palate; dashed line indicates the boundary of palate. (G) The mRNA levels of selected differentially expressed genes were verified by quantitative RT-PCR using E13.5 microdissected control (CTR) or WAY-26261-treated (WAY) palatal shelves (n=5). Error bars indicate s.e.m. *P<0.01.
Table 3.
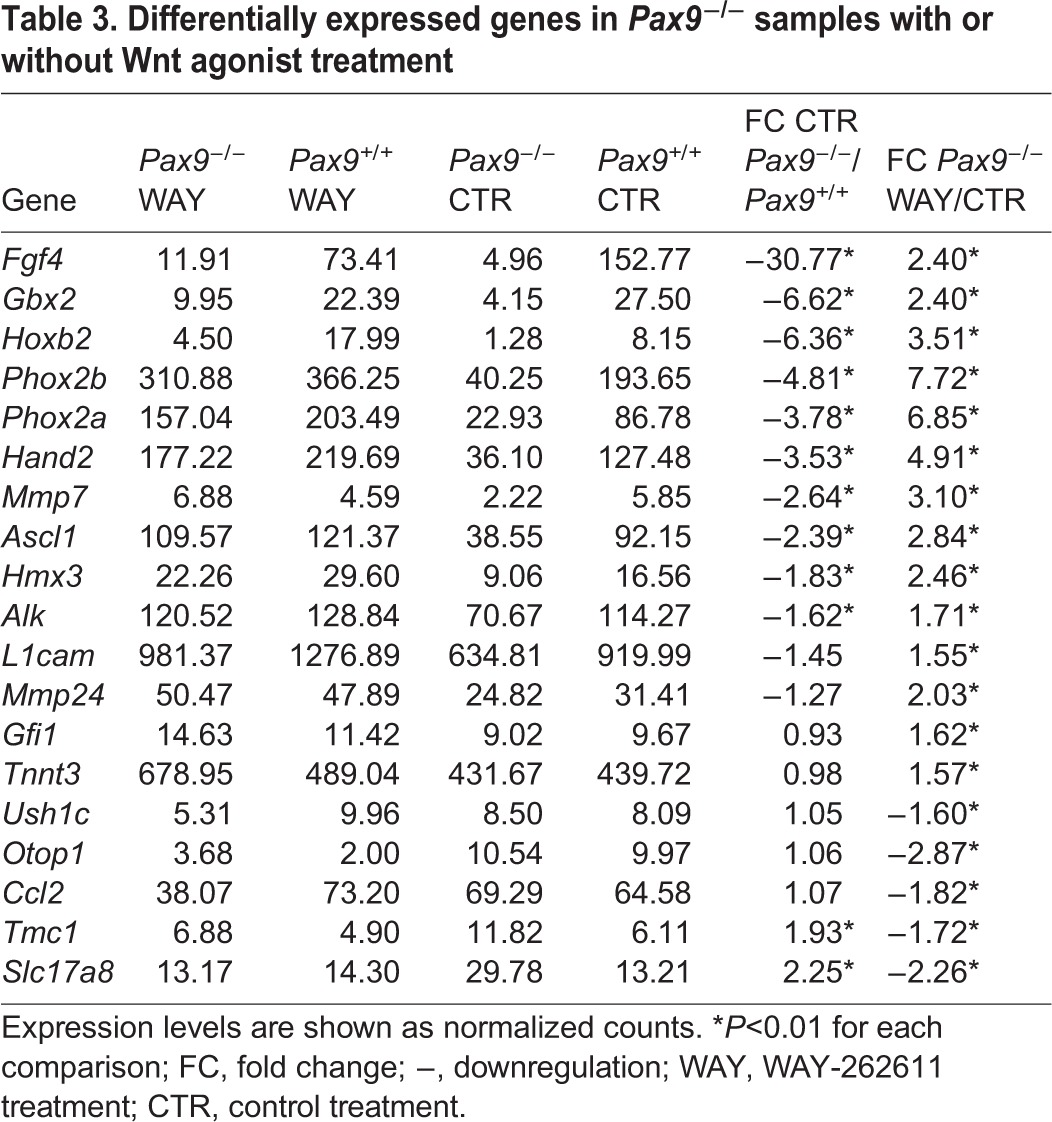
Differentially expressed genes in Pax9−/− samples with or without Wnt agonist treatment
As detailed by Zhou et al. (2011), the targeting vector used for the generation of Pax9−/− mice contained an FRT-flanked Neo expression cassette followed by a Myc-Osr2 cDNA inserted in intron 2 of Pax9. Pax9+/− mice were derived from crossings with EIIa (E2a)-Cre mice (Zhou et al., 2011). Studies of Myc-Osr2 expression are facilitated by further crossing of Pax9+/− mice with FLPeR mice, thus eliminating the Neo cassette and resulting in expression of Myc-Osr2 (Zhou et al., 2011). Here, we performed additional experiments to rule out the possibility that WAY-262611 treatments affected transgenic Myc-Osr2 protein and mRNA expression. Fig. S6A-D shows the absence of a translated protein product from exogenous Myc-Osr2 in both untreated and treated Pax9+/− and Pax9−/− palatal shelves. By contrast, Myc-Osr2 is clearly visible in a section through Pax9+/−;FLP palate and tooth mesenchyme, where Myc-Osr2 from the transgenic Osr2 allele is able to be translated (Fig. S6E). In situ hybridization using an Osr2-specific probe that detects both exogenous and endogenous Osr2 mRNA showed stronger Osr2 signals in Pax9−/− samples (Fig. S6G,I) as the extra knock-in Osr2 allele was transcribed into mRNA. However, no significant differences were noted in Osr2 mRNA expression levels when comparing untreated and treated Pax9+/− samples (Fig. S6F,H) or untreated and treated Pax9−/− samples (Fig. S6G,I). Thus, WAY-262611 treatment itself did not affect Osr2 expression and the closure of palatal shelves following Wnt interventions cannot be attributed to transgenic Myc-Osr2 expression.
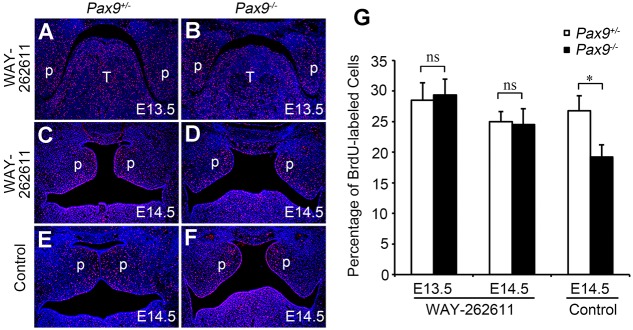
Pax9 deficiency lowers cell proliferation in E13.5 posterior palatal mesenchyme, as seen by the significant reduction in BrdU incorporation (Zhou et al., 2013). BrdU assays were performed in WAY-262611-treated embryos at E13.5 and E14.5, stages when palatal outgrowth is most visible. After treatment with WAY-262611, cell proliferation was restored to a level that appeared adequate for the outgrowth of palatal shelves (Fig. 7A-G). The BrdU labeling assay in posterior palate at E14.5 showed that the ratios of BrdU+ palatal mesenchymal cells were significantly reduced in Pax9−/− samples (Fig. 7E,F), confirming previous reports (Zhou et al., 2013). After Wnt signaling agonist treatments, the ratios of BrdU+ palatal mesenchymal cells were consistently restored in Pax9−/− samples at both E13.5 and E14.5 (Fig. 7B,D) as compared with Pax9+/− tissues (Fig. 7A,C,G).
Fig. 7.
Cell proliferation in the palate of WAY-262611-treated Pax9−/− mice. Representative images of sections through the posterior regions of the palate in E13.5 and E14.5 Pax9+/− (A,C,E) and Pax9−/− (B,D,F) embryos with (A-D) or without (E,F) WAY-262611 treatment. Red signals show the distribution of BrdU-labeled nuclei. Blue signals show total nuclei (Hoechst). T, tongue; p, palate shelf. (G) The percentage of BrdU-labeled cells in the palatal mesenchyme. n=5. Error bars represent s.d. *P<0.01; ns, not significant.
To better understand whether the closure of Pax9−/− palatal shelves following Wnt agonist treatment included palatine bone formation, we compared alkaline phosphatase activity and type I collagen (Col1a1) mRNA expression in treated and untreated Pax9+/+ and Pax9−/− maxillary and palatine processes. The robust levels of enzymatic activity observed in treated Pax9−/− tissues closely resembled that in Pax9+/+ tissues and showed the onset of osteogenesis in these areas (Fig. 8A-F). The alkaline phosphatase activity that marks early osteoblast differentiation was activated in areas of mesenchymal condensations at osteogenic fronts within palatal mesenchyme at E15.5 in Pax9+/+ embryos (Fig. 8A,D). However, alkaline phosphatase activity was not detected in stunted Pax9−/− palatal shelves (Fig. 8B,E). After Wnt agonist treatment, enzymatic activity was restored in the Pax9−/− palate (Fig. 8C,F). Col1a1, a marker of differentiated osteoblasts, appeared in osteoblasts in Pax9+/+ palatal mesenchyme. By contrast, Pax9−/− samples failed to show Col1a1 expression in putative mesenchyme. After Wnt agonist treatment, Col1a1 expression was restored along with the osteogenic activity within the palatal shelves (Fig. 8G-X).
Fig. 8.
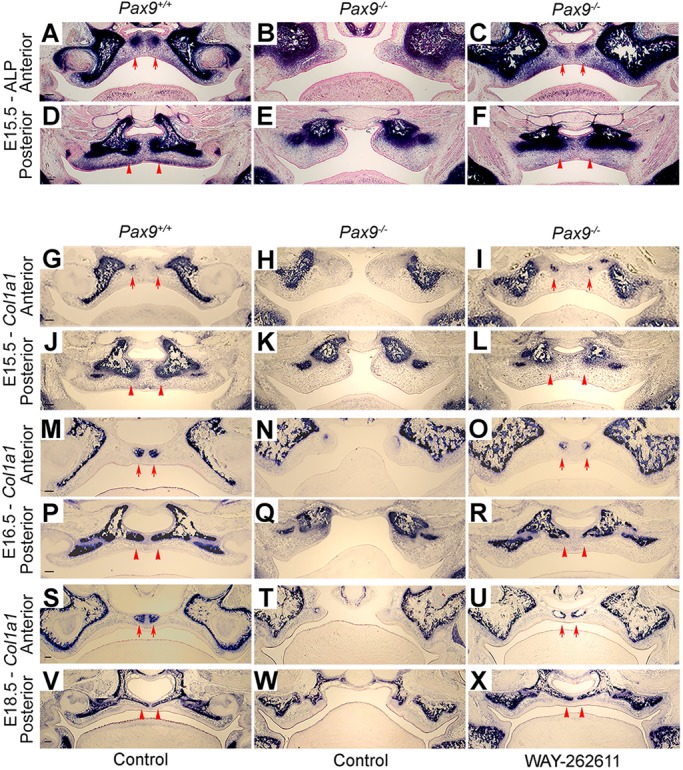
The restoration of osteogenesis in WAY-262611-treated Pax9−/− palatal shelves. (A-F) Detection of alkaline phosphatase activity in the palatal mesenchyme during palatal bone formation in Pax9+/+ and Pax9−/− embryos without or with WAY-262611 treatment. Position-matched coronal sections of E15.5 embryos through the anterior and posterior palates are shown. (A,D) Alkaline phosphatase activity (blue) was detected in the palate of Pax9+/+ embryos but not in Pax9−/− embryos (B,E). After Wnt agonist treatment, enzymatic activity was detected in the Pax9−/− palate (C,F). (G-X) Comparison of expression of Col1a1 mRNA (blue) in the palatal mesenchyme during palatal bone formation in Pax9+/+ and Pax9−/− embryos without or with WAY-262611 treatment. Position-matched coronal sections of E15.5 (G-L), E16.5 (M-R) and E18.5 (S-X) embryos through the anterior and posterior palates are shown. Expression of Col1a1 was detected within osteogenic zones as early as E15.5 (G,J) and increased from E16.5 (M,P) to E18.5 (S,V) in Pax9+/+ palates. In Pax9−/− palate, expression of Col1a1 was highly reduced (H,K,N,Q,T,W). After Wnt agonist treatment, Col1a1 expression was restored in the palate of Pax9−/− embryos (I,L,O,R,U,X). Arrows point to osteogenic centers of the developing palatal processes of the maxilla; arrowheads indicate prospective palatal processes of the palatine bone. n=5 for each assay. Scale bars: 200 µm.
Taken together, these data establish that the intravenous delivery of small-molecule Wnt agonists to pregnant Pax9+/− females within a critical gestational window is effective in bringing about true closure of the palatal shelves in Pax9−/− embryos (Figs 5 and 8). Although Wnt signaling was restored in treated palates, Wnt agonist therapies did not correct the developmental arrest of tooth organs, parathyroid glands, thymus and hind limb defects in mutants (Fig. S7), and nor did the Wnt therapy lead to prolonged survival of treated Pax9−/− neonates, suggesting that the cleft palate was not the only defect causing Pax9−/− neonate lethality after birth. MRI imaging showed that Pax9+/− mothers injected with WAY-262611, along with their wild-type and heterozygous progeny, showed no toxic effects or tumor development up to 18 months following the last tail vein injection (data not shown).
DISCUSSION
In these studies, we provide definitive evidence that Pax9 deficiency alters Wnt activity in palatal shelves and that this upstream relationship, when genetically restored, leads to rescue of the cleft palate phenotype in Pax9−/− embryos. Of significance is the high fidelity correction of palatal defects in Pax9−/− embryos after the controlled intravenous delivery of small-molecule Dkk inhibitors (Wnt agonists) to pregnant Pax9+/− mice during a critical developmental window for palatogenesis. Such interventions restored Wnt signaling in palatal mesenchyme and specifically increased cell proliferation within posterior palatal mesenchyme, where Pax9 and Dkk1/2 have inverse expression patterns. Wnt activation in Pax9−/− palates led to a true bony closure of palatal shelves through osteogenesis. Collectively, these data demonstrate that the Pax9/Wnt pathway is crucial for the formation of the secondary palate and that restoring this molecular equilibrium in Pax9−/− mice in utero leads to the complete fusion of palatal shelves.
Pax9-dependent Wnt signaling along the buccal-lingual axis is important for palate development
Our studies demonstrate that Pax9-dependent Wnt signaling in the posterior domain of palatal mesenchyme influences growth along the buccal-lingual axis, thus providing new insights into the molecular mechanisms underlying Pax9 actions in palatogenesis. The inverse or complementary patterns of Pax9 and Dkk1/2 gene expression in posterior mesenchyme suggest that Pax9 modulates Wnt signaling in this region of the palate. Our unbiased studies of genome-wide alterations in whole Pax9−/− palatal shelves showed a significant increase in Dkk1 and Dkk2 expression. Genes involved in other signaling pathways, such as Msx1 and Bmp4, were also altered. Since these genes influence signaling events in anterior palatal mesenchyme this explains why Wnt agonist therapy did not result in closure of palatal defects in Msx1−/− mice. We also conclude that Pax9-dependent Wnt signaling through the control of Dkk1 expression is key for posterior palate formation. Our expression analyses support the results of studies by Li et al. (2017) that show the restriction of Axin2 and Ctnnb1 to the posterior region of the palate and that these genes are downregulated in the absence of Pax9, while Dkk2 levels increase in posterior palatal mesenchyme. Notably, the expression levels of selective Wnt ligands were reduced in the absence of Pax9 and were not normalized after activation of Wnt signaling. Earlier studies have shown that Dkk1 expression can be regulated by Wnt-induced TCF activation through a negative-feedback mechanism (Niida et al., 2004). Hence, it will be interesting to explore whether Pax9 directly regulates the expression of Wnt ligands.
Notably, the role of Pax9 in regulating the Wnt signaling pathway through its inhibition of Dkk1 suggests that it serves dual transcriptional functions in palatogenesis. As an activator of Bmp and Fgf signaling in anterior palatal mesenchyme and as a likely repressor of Dkk1 in posterior mesenchyme, Pax9 influences patterning morphogenesis throughout the forming palate. The literature underscores the role of Pax family members as transcriptional regulators that act as activators and repressors during development. For example, Pax6 has dual roles in eye development (Duncan et al., 1998), as it serves as an activator of the αA-, αB- and δ1-crystallin genes while repressing expression of the β-crystallin gene in the lens. Pax4 also acts as a transcriptional repressor in the pancreas (Fujitani et al., 1999). It is well established that Pax9 is involved in the formation of pharyngeal organs (thymus, parathyroid) as well as tooth and palate development. Pax9 activates the Bmp and Fgf signaling pathways (Mensah et al., 2004) during tooth development but is strongly synergistic with BF-1 (brain factor 1, also known as FOXG1) and the potent breast cancer-associated transcriptional repressor PLU-1 (also known as KDM5B) (Tan et al., 2003). Hence, the functions of Pax9 in the development of craniofacial and pharyngeal pouch derivatives are the result of fine-tuned modulations of its multiple transcriptional properties.
Although it can be argued that relief of Dkk1 inhibition could overcome reduced Wnt signaling activity at some other point in the pathway, the rescue of the cleft palate defect in Pax9−/−Dkk1f/+;Wnt1Cre compound mutant mice, as shown here, suggests that the functional relationship of Pax9 with Dkk1 is both necessary and sufficient for palate shelf closure. Li et al. (2017) indicate that secondary palate clefts are rescued in 14 out of 20 double mutants that lack Wise (also known as Sostdc1), which encodes another secreted Wnt ligand-receptor antagonist that also modulates Bmp signaling. A confounding observation is that, in the absence of Pax9, expression levels of Wise are downregulated in palatal shelves. Our studies provide definitive evidence that the role of Pax9 in regulating the Wnt signaling pathway through its inhibition of Dkk1 is one means by which it exerts modulatory functions in patterning morphogenesis. Clearly, the relationship with other Wnt signaling molecules and pathways, such as Bmp and Fgf signaling, also plays a role in processes that drive palatogenesis.
The correction of Pax9 mutant palatal defects with Wnt agonists underscores the importance of this molecular pathway in secondary palate formation
The surprising fidelity with which palatal defects are corrected in Pax9−/− embryos through the controlled intravenous delivery of Wnt agonists can be explained by the fact that Wnt signaling genes are potent downstream effectors of Pax9 functions in palatal mesenchyme. Interestingly, such interventions could not restore the development of the thymus, parathyroid glands and ultimobranchial bodies, or correct other defects such as supernumerary preaxial digits. It is likely that organs, other than the secondary palate, that are derived from the pharyngeal pouches may require essential contributions from other downstream effector pathways such as Bmp, Fgf and Shh. Alternatively, Wnt agonist delivery and dosage schedules that proved optimal for palatogenesis would have to be modified to achieve the correction of other organ defects in Pax9−/− mice.
The interventions with small-molecule Wnt agonists failed to prolong the survival of Pax9−/− neonates from treated pregnant dams as they succumb at birth. While earlier studies have noted that Pax9−/− newborn pups die shortly after birth, most likely as a consequence of secondary palate clefts (Peters et al., 1998), our data suggest that systemic alterations caused by the absence of parathyroids and thymus glands might lead to early death.
It is intriguing that Wnt agonists when delivered intravenously during a critical developmental window for odontogenesis only advanced tooth organs to the early cap stage. In humans, mutations in PAX9 are mainly associated with congenitally missing posterior teeth (Goldenberg et al., 2000; Stockton et al., 2000). Pax9 also influences epithelial-mesenchymal signaling events that drive murine tooth morphogenesis (Peters et al., 1998; Nakatomi et al., 2010). Mutations in WNT10A account for the majority of human tooth agenesis cases (Bohring et al., 2009; van den Boogaard et al., 2012; Mues et al., 2014) and canonical Wnts are key drivers of tooth signaling interactions that are likely to be downstream of Pax9 in dental mesenchyme (O'Connell et al., 2012; Jia et al., 2016). Pax9 also integrates dental mesenchymal signaling events that involve Bmp4, Fgf3 or Fgf10 (Peters et al., 1998; Nakatomi et al., 2010). Hence, combinatorial therapies involving Wnt agonists and these growth factors might prove more effective in advancing tooth morphogenesis and should be the focus of future studies.
We also observed that the intravenous delivery of small-molecule Wnt agonists failed to rescue cleft defects in any of 50 Msx1−/− mice examined. Msx1 is a partner gene of Pax9 that also plays a role in palatogenesis and is more dominantly expressed in the anterior zone of palatal mesenchyme. By contrast, Pax9 and Dkk1/2 are more restricted to the posterior region. We hypothesize that a morphogenetic gradient of differential gene expression along the AP axis controls the patterning and closure of palatal shelves (Hilliard et al., 2005; Zhou et al., 2013) and that downstream effector genes other than the Wnts are likely to mediate Msx1 actions in anterior palatal mesenchyme.
Clinical implications for the use of small-molecule replacement therapies for the treatment of developmental disorders of the craniofacial complex
The involvement of the Wnt–β-catenin signaling pathway in many developmental processes and in adult tissue homeostasis has encouraged the development of pharmacological modulators of this pathway (Clevers and Nusse, 2012; Baron and Kneissel, 2013; Kahn, 2014). Previously, a small-molecule-based chemical genetic approach restored the stability and function of GSK3β in Gsk3bFRB*/FRB* mice such that, after rapamycin treatment, cleft defects were rescued in 6/9 mutant pups (Liu et al., 2007). New therapies that block the function of the Wnt antagonist sclerostin restored bone mass and strength in humans at risk of fractures (Recker et al., 2015). Several preclinical studies also report that other therapies targeting Dkk1 inhibition induce bone gain (Baron and Kneissel, 2013).
Our data indicate that the intravenous administration of small-molecule Dkk inhibitors increases Wnt signaling in palatal mesenchyme and enhances cell proliferation, palatal shelf outgrowth and fusion. Restoration of cell proliferation during a critical developmental window, when expansion of the cranium can force shelves apart, appears crucial for palate formation. In humans, submucosal clefting occurs as commonly as overt cleft palate and is the result of apposition of overlying soft tissue with a lack of palatal bone formation (Garcia Velasco et al., 1988). For this reason, it was important for us to first demonstrate that Wnt agonist therapies positively affect palate morphogenesis (as shown by increased cell proliferation) and that this was sufficient for osteogenesis to ensue within the palatal shelves. Further studies are needed to delineate stage-specific effects of Wnt agonist therapies and the role of Pax9-dependent Wnt signaling in palatal shelf growth and bone formation.
Questions naturally arise about how such approaches can be translated into therapies for human cleft palate disorders. Since Wnt signaling is key to the development of several organs, activation of the pathway through exogenous Wnt agonist therapies could lead to abnormalities in other organ systems. Our histopathologic and MRI imaging analyses of pregnant Pax9+/− females that received Wnt agonist injections and surviving Pax9+/+ or Pax9+/− littermates showed no toxic effects or tumor development up to 18 months following the last tail vein injection. Hence, small doses of Wnt agonists that target inhibitors of receptor binding, when delivered in a controlled manner and during a specific developmental window, only affect cells such as those in the palatal mesenchyme that have not reached the threshold levels needed for the hierarchy of Wnt signaling.
Although ultrasound technologies can diagnose cleft palate conditions as early as 13 weeks of gestation, small-molecule therapies delivered in low doses to pregnant mothers are likely to be fraught with difficulties and will require further experimentation on risk-to-benefit ratios. Alternate strategies to reduce exposure of the mother could include delivery into the amniotic fluid, as shown to be effective for the delivery of recombinant ectodysplasin (Hermes et al., 2014). Alternatively, early postnatal interventions that utilize the well-timed local delivery of Wnt agonist small molecules or proteins during surgical correction procedures might prove effective in bringing about the timely closure of palatal shelves.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Utah (Protocol #17-02004). Pax9+/− and Msx1+/− mice were provided by Dr Rulang Jiang (Cincinnati Children's Hospital) (Zhou et al., 2011) and Dr Yiping Chen (Tulane University). Whole heads of E13.5 and E14.5 Dkk1Tg107−lacZ embryos were provided by Dr Ulrich Rüther (Heinrich-Heine University) (Lieven et al., 2010). BAT-Gal mice for studies on Wnt activity (Maretto et al., 2003) and FLP male mice for the generation of positive control Myc-Osr2 (JAX 016226) were purchased from Jackson Laboratories. Pax9+/−, Msx1+/− and BAT-Gal mice were maintained in a C57BL/6 background. Females at 2-8 months of age were used for intercross matings and adult mice, over 18 months of age, were used for the histopathologic and magnetic resonance imaging (MRI) analysis of potential side effects and toxicity following treatments. We were diligent in including litter-matched controls that were subject to the same Wnt agonist therapy in utero. However, since some litters failed to yield Pax9+/+ progeny, Pax9+/− littermates were used instead as it is well documented that Pax9 haploinsufficiency does not compromise the development or function of any organ system (Peters et al., 1998; Zhou et al., 2011).
Pax9−/−Dkk1f/+;Wnt1Cre compound mutant mice were generated through serial matings. Pax9+/− mice were first mated with Dkk1f/+ mice [EM: 09872 from the European Mouse Mutant Archive (EMMA) repository] to generate Pax9+/−Dkk1f/+ progeny. We next mated Pax9+/−Dkk1f/+ with Wnt1-Cre (JAX 022501, Jackson Laboratory) to generate Pax9+/−Dkk1f/+;Wnt1Cre males. Pax9−/−Dkk1f/+;Wnt1Cre embryos were then obtained from the mating of Pax9+/−Dkk1f/+;Wnt1Cre males with Pax9+/−Dkk1f/+ females.
Palatal dissections, RNASeq and quantitative RT-PCR
The embryos were harvested at E13.5 and heads collected in cold PBS. After the lower jaw and brain were removed under a dissecting microscope, the status of the palatal shelves was evaluated. Palatal tissues were carefully dissected using fine forceps, quickly frozen in liquid nitrogen and stored individually at −80°C for total RNA extraction (Zhou et al., 2013; Kim et al., 2017).
Whole-transcriptome profiling of developing palatal shelves was performed by RNASeq as previously described (Jia et al., 2013). Sequenced reads were mapped to the reference mouse genome (mm10) using NovoAlign (v2.08.03, Novocraft). Read counts were generated using the USeq (http://useq.sourceforge.net) Defined Region Differential Seq application and normalized counts were used in DESeq2 (Bioconductor) to measure differential expression. The RNASeq raw data are available at GEO under accession numbers GSE89603 and GSE101825. The candidate genes were screened from a comparison of five sets of data as follows: first, the normalized counts were cut-off at 10 in at least one of the samples, then the fold change was chosen at 1.5-fold or higher and P<0.01 from the Audic-Claverie test with Benjamini-Hochberg false-discovery rate (FDR) multiple testing correction; next, the gene list was sorted by P-value and lists of genes were submitted to DAVID (https://david.ncifcrf.gov) or ToppGene (https://toppgene.cchmc.org) for functional enrichment analysis.
Palatal shelves were microdissected from E13.5 embryos. After genotyping, total RNA was extracted from individual samples using the RNeasy Micro Kit (Qiagen). First-strand cDNA was synthesized using the SuperScript First-Strand Synthesis System IV (Thermo Fisher Scientific). Quantitative reverse-transcription PCR (RT-PCR) was performed in a StepOnePlus Real-Time PCR System (Applied Biosystems) using the SYBR GreenER qPCR Supermix (Thermo Fisher Scientific). A list of gene-specific primers is provided in Table S1. For each sample, the relative levels of target mRNAs were normalized to Gapdh using the standard curve method. Five sets of replicates were analyzed for each gene.
Delivery of small-molecule Wnt pathway agonists
Two small-molecule agonists (Millipore) were used to treat timed pregnant female mice. WAY-262611, {1-[4-(naphthalen-2-yl)pyrimidin-2-yl]piperidin-4-yl}methanamine, is a 2-aminopyrimidine compound (Fig. S2A) that antagonizes the effect of Dkk1 by preventing the formation of the Dkk1-LRP5-Kr2 complex. It has a half-life (t1/2) of 6-8 h in plasma (Pelletier et al., 2009) and specifically inhibits Dkk1 at EC50=0.63 µM, based on a TCF-luciferase assay. IIIc3a, 9-carboxy-3-(dimethyliminio)-6,7-dihydroxy-10-methyl-3H-phenoxazin-10-ium iodide, is an enhanced in-solution stable gallocyanine analog (Fig. S2B) that disrupts the interaction of LRP5/6 with Dkk1, Dkk2 and Dkk4 in a competitive manner at EC50=5 µM in a LEF-luciferase assay using NIH3T3 cells (Li et al., 2012). Stock solutions of WAY-262611 and IIIc3a were prepared at 25 mg/ml in DMSO. Dilutions at 12.5 and 25 mg/kg per mouse body weight were prepared prior to injection into the tail vein as previously described (Kowalczyk et al., 2011). Control treatments consisted of tail vein injections of 10% DMSO in PBS. WAY-262611 or IIIc3a was delivered separately on a daily basis from E10.5 to E14.5, while an additional group of mice received WAY-262611 treatments from E10.5 to E13.5 as detailed in Table 2. Mice were closely monitored for any discomfort or side effects.
Microscopic evaluations and in situ hybridizations
Embryos retrieved from treated and untreated litters were immediately fixed in 4% paraformaldehyde (PFA) overnight. Paraffin sections (7 μm thick) were prepared and stained with Hematoxylin and Eosin (H&E) for microscopic evaluation. In situ hybridizations were performed using digoxigenin-labeled RNA probes to Dkk1, Dkk2, Pax9, Osr2 and Col1a1 as described previously (D'Souza et al., 1993; Zhang et al., 1999). 1 µg/ml antisense RNA probe was loaded on each section and anti-digoxigenin-AP antibody (11093274910, Roche; 1:1000) was used to detect the labeled probe. BM-purple (11442074001, Roche) was used for color development. Comparable images were captured on a digital microscope (EVOS). For whole-mount in situ hybridizations, 2 µg/ml antisense RNA probe was loaded on each sample and the anti-digoxigenin-AP antibody (1:2000) used to detect the labeled probe. NTMT buffer with 4.5 μl/ml nitroblue tetrazolium (NBT) and 3.5 μl/ml 5-bromo-4-chloro-3-indolyl phosphate (BCIP) was used for color development. Adding both NBT and BCIP helped enhance the development of the reaction. For each assay, at least five replicates were performed.
Gross morphological examinations, histopathology and MRI analyses
All mouse embryos, neonates and adults were first evaluated through full-body visual examination. Whole embryos were fixed in 10% neutral-buffered formalin, and 7 μm-thick transverse paraffin sections were stained with H&E for microscopic evaluation. Pax9−/− embryos from treated and control litters were easily identifiable by the supernumerary hind limb digit. However, genotyping was performed on all animals. Adult mice were anaesthetized with isoflurane and fixed by whole-body perfusion prior to magnetic resonance imaging (MRI) that was performed using a 7 Tesla Bruker BioSpec MRI scanner under the following parameters: a T2 weighted RARE scan was acquired using a 7.2 cm diameter quadrature radiofrequency transmitter-receiver (Bruker Biospec), with 125 µm isotropic resolution, echo train length of 4, matrix size of 768×256×256 and TE/TR=41/1200 ms on a Bruker Biospec 70/30 instrument (Bruker Biospin).
Histochemical and immunofluorescent staining procedures
For whole-mount lacZ staining, embryos were fixed in 1% PFA then treated as previously described (Lan et al., 2004). In addition, serial coronal sections were stained with Masson's trichrome (Chen et al., 2009) to evaluate connective tissue deposition. For alkaline phosphatase (AP) staining, embryos were fixed in 4% PFA overnight. The frozen sections were incubated in NTMT buffer (100 mM NaCl, 100 mM Tris-HCl pH 9.5, 50 mM MgCl2, 0.1% Tween 20) containing 4.5 μl/ml NBT and 3.5 μl/ml BCIP for 20 min at room temperature (Baek et al., 2011). Comparable images were taken with a digital microscope (EVOS) or a stereomicroscope (Zeiss Stemi 508). For each assay, at least five replicates were used to establish reproducibility of results.
For immunofluorescent staining, embryos were fixed in 4% PFA overnight at 4°C and processed for paraffin sections (7 µm). Indirect immunofluorescent staining was performed as described (Zhou et al., 2011). After blocking with PBS containing 10% goat serum, 2% BSA and 0.1% Tween 20, overnight incubation with monoclonal anti-Myc antibody (Millipore, 05-724; 1:100 in blocking solution) was carried out at 4°C. The secondary antibody used was goat anti-mouse IgG, Alexa Fluor 594 (Thermo Fisher Scientific, R37121; 1:1000 in blocking solution).
BrdU labeling and cell proliferation assay
As reported, palatal cell proliferation is decreased in Pax9−/− embryos as early as E13.5 (Zhou et al., 2013) and contributes to the failure of shelf outgrowth. Daily delivery of WAY-262611 from E10.5 to E14.5 (5 days) resulted in 100% palate closure in Pax9−/− embryos, whereas the incidence of palatal shelf fusion was reduced when drug delivery was stopped a day earlier at E13.5. Hence, we analyzed E13.5 palatal shelves along with E14.5 palatal shelves to assess the effects of Wnt agonist therapy on cell proliferation at a critical phase of palatogenesis.
Timed pregnant female mice were injected once intraperitoneally at E13.5 and E14.5 with BrdU Labeling Reagent (Roche; 15 μl/g body weight). Embryos were harvested 2 h later. Paraffin sections (7 µm) were prepared in the coronal plane and spanning the entire posterior region of the palatal shelves. After rehydration, proteinase K digestion was followed by HCl treatment and incubation in a blocking solution (2% BSA, 10% goat serum, 0.1% Tween 20 in PBS). Samples were treated with Alexa Fluor 594-conjugated anti-BrdU antibody (Thermo Fisher Scientific, B35132; 1:50 in blocking solution) overnight. After counterstaining with Hoechst, images were obtained from 20 sections per tissue sample using a Nikon A1R confocal microscope and analyzed with Imaris software (Bitplane). The cell proliferation data were recorded and analyzed from five independent control and mutant littermate pairs.
Whole palate shelf organ culture
The WAY-262611-treated palates were dissected on ice by removing the lower jaw and brain, and samples with residual fusion defects were marked and cultured as previously described (Almaidhan et al., 2014). Pax9+/− and untreated Pax9−/− embryos were cultured as controls. The dissected E18.5 palate shelves were placed in 2 ml medium [CO2 Independent Medium (Thermo Fisher Scientific, 18045-088), 20% FBS, 1× antimycotic-antibiotic (Thermo Fisher Scientific, 15240-096)]. Culture tubes were rotated 12 rpm at an angle of 20° in a 37°C incubator for 3 days with daily changes of medium. After images were taken with a stereomicroscope, tissues were fixed and embedded for histologic analyses. At least five replicates of each treatment and genotype were utilized for culture experiments.
Supplementary Material
Acknowledgements
We are grateful to Dr Sarah Millar for her feedback and advice on the manuscript and to Dr Jeffrey Pelletier for sharing insights on the use of WAY-262611. The technical assistance of Mr Greg Pratt is appreciated.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.J., J.B., G.M., R.N.D.; Methodology: S.J., J.Z., Y.W., P.S., R.N.D.; Validation: J.Z.; Formal analysis: J.Z.; Investigation: S.J., J.Z., C.F., Y.W., J.B.; Data curation: S.J.; Writing - original draft: S.J., R.N.D.; Writing - review & editing: S.J., J.Z., P.S., R.N.D.; Visualization: S.J., J.Z., C.F., J.B.; Supervision: P.S., G.M., R.N.D.; Project administration: S.J., R.N.D.; Funding acquisition: G.M., R.N.D.
Funding
The following grants from the National Institutes of Health have supported this research: DE019471, DE019471-ARRA supplement and DE027255 to R.N.D., DE019554 to G.M., and a training stipend from DE018380 (Principal Investigator R.N.D.) to J.B. P.S. is supported by grants from the Swiss National Science Foundation (Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung). Deposited in PMC for release after 12 months.
Data availability
The RNASeq raw data have been deposited at NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo) under accession numbers GSE89603 and GSE101825.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.157750.supplemental
References
- Almaidhan A., Cesario J., Landin Malt A., Zhao Y., Sharma N., Choi V. and Jeong J. (2014). Neural crest-specific deletion of Ldb1 leads to cleft secondary palate with impaired palatal shelf elevation. BMC Dev. Biol. 14, 3 10.1186/1471-213X-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J.-A., Lan Y., Liu H., Maltby K. M., Mishina Y. and Jiang R. (2011). Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev. Biol. 350, 520-531. 10.1016/j.ydbio.2010.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. and Kneissel M. (2013). WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179-192. 10.1038/nm.3074 [DOI] [PubMed] [Google Scholar]
- Bohring A., Stamm T., Spaich C., Haase C., Spree K., Hehr U., Hoffmann M., Ledig S., Sel S., Wieacker P. et al. (2009). WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am. J. Hum. Genet. 85, 97-105. 10.1016/j.ajhg.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragança J., Swingler T., Marques F. I. R., Jones T., Eloranta J. J., Hurst H. C., Shioda T. and Bhattacharya S. (2002). Human CREB-binding protein/p300-interacting transactivator with ED-rich tail (CITED) 4, a new member of the CITED family, functions as a co-activator for transcription factor AP-2. J. Biol. Chem. 277, 8559-8565. 10.1074/jbc.M110850200 [DOI] [PubMed] [Google Scholar]
- Brewer S., Feng W., Huang J., Sullivan S. and Williams T. (2004). Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev. Biol. 267, 135-152. 10.1016/j.ydbio.2003.10.039 [DOI] [PubMed] [Google Scholar]
- Byrd N. A. and Meyers E. N. (2005). Loss of Gbx2 results in neural crest cell patterning and pharyngeal arch artery defects in the mouse embryo. Dev. Biol. 284, 233-245. 10.1016/j.ydbio.2005.05.023 [DOI] [PubMed] [Google Scholar]
- Chen J., Lan Y., Baek J.-A., Gao Y. and Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev. Biol. 334, 174-185. 10.1016/j.ydbio.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. and Nusse R. (2012). Wnt/beta-catenin signaling and disease. Cell 149, 1192-1205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- D'Souza R. N., Niederreither K. and de Crombrugghe B. (1993). Osteoblast-specific expression of the alpha 2(I) collagen promoter in transgenic mice: correlation with the distribution of TGF-beta 1. J. Bone Miner. Res. 8, 1127-1136. 10.1002/jbmr.5650080914 [DOI] [PubMed] [Google Scholar]
- Duncan M. K., Haynes J. I. II, Cvekl A. and Piatigorsky J. (1998). Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol. Cell. Biol. 18, 5579-5586. 10.1128/MCB.18.9.5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A., Inthal A., Herrmann D., Cheng S., Nakatomi M., Peters H. and Neubüser A. (2010). Regulation of Tbx22 during facial and palatal development. Dev. Dyn. 239, 2860-2874. 10.1002/dvdy.22421 [DOI] [PubMed] [Google Scholar]
- Fujitani Y., Kajimoto Y., Yasuda T., Matsuoka T.-A., Kaneto H., Umayahara Y., Fujita N., Watada H., Miyazaki J.-I., Yamasaki Y. et al. (1999). Identification of a portable repression domain and an E1A-responsive activation domain in Pax4: a possible role of Pax4 as a transcriptional repressor in the pancreas. Mol. Cell. Biol. 19, 8281-8291. 10.1128/MCB.19.12.8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato N., Nakamura M. and Yanagisawa H. (2015). Molecular basis of cleft palates in mice. World J. Biol. Chem. 6, 121-138. 10.4331/wjbc.v6.i3.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Velasco M., Ysunza A., Hernandez X. and Marquez C. (1988). Diagnosis and treatment of submucous cleft palate: a review of 108 cases. Cleft Palate J. 25, 171-173. [PubMed] [Google Scholar]
- Gendron-Maguire M., Mallo M., Zhang M. and Gridley T. (1993). Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75, 1317-1331. 10.1016/0092-8674(93)90619-2 [DOI] [PubMed] [Google Scholar]
- Goldenberg M., Das P., Messersmith M., Stockton D. W., Patel P. I. and D'Souza R. N. (2000). Clinical, radiographic, and genetic evaluation of a novel form of autosomal-dominant oligodontia. J. Dent. Res. 79, 1469-1475. 10.1177/00220345000790070701 [DOI] [PubMed] [Google Scholar]
- Greene R. M. and Pisano M. M. (2010). Palate morphogenesis: current understanding and future directions. Birth Defects Res. C Embryo Today 90, 133-154. 10.1002/bdrc.20180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Mayo J., Xu X., Li J., Bringas P. Jr, Maas R. L., Rubenstein J. L. R. and Chai Y. (2009). Indirect modulation of Shh signaling by Dlx5 affects the oral-nasal patterning of palate and rescues cleft palate in Msx1-null mice. Development 136, 4225-4233. 10.1242/dev.036723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes K., Schneider P., Krieg P., Dang A. T., Huttner K. and Schneider H. (2014). Prenatal therapy in developmental disorders: drug targeting via intra-amniotic injection to treat X-linked hypohidrotic ectodermal dysplasia. J. Invest. Dermatol. 134, 2985-2987. 10.1038/jid.2014.264 [DOI] [PubMed] [Google Scholar]
- Hilliard S. A., Yu L., Gu S., Zhang Z. and Chen Y. P. (2005). Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J. Anat. 207, 655-667. 10.1111/j.1469-7580.2005.00474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Zhou J., Gao Y., Baek J.-A., Martin J. F., Lan Y. and Jiang R. (2013). Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development 140, 423-432. 10.1242/dev.081927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Kwon H.-J. E., Lan Y., Zhou J., Liu H. and Jiang R. (2016). Bmp4-Msx1 signaling and Osr2 control tooth organogenesis through antagonistic regulation of secreted Wnt antagonists. Dev. Biol. 420, 110-119. 10.1016/j.ydbio.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. (2014). Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 13, 513-532. 10.1038/nrd4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Prochazka J. and Bush J. O. (2017). Live imaging of mouse secondary palate fusion. J. Vis. Exp. 125, e56041 10.3791/56041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. D., Javidan Y., Zhang T., Nelson S. and Schilling T. F. (2005). AP2-dependent signals from the ectoderm regulate craniofacial development in the zebrafish embryo. Development 132, 3127-3138. 10.1242/dev.01879 [DOI] [PubMed] [Google Scholar]
- Kowalczyk C., Dunkel N., Willen L., Casal M. L., Mauldin E. A., Gaide O., Tardivel A., Badic G., Etter A.-L., Favre M. et al. (2011). Molecular and therapeutic characterization of anti-ectodysplasin A receptor (EDAR) agonist monoclonal antibodies. J. Biol. Chem. 286, 30769-30779. 10.1074/jbc.M111.267997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Ovitt C. E., Cho E. S., Maltby K. M., Wang Q. and Jiang R. (2004). Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 131, 3207-3216. 10.1242/dev.01175 [DOI] [PubMed] [Google Scholar]
- Li X., Shan J., Chang W., Kim I., Bao J., Lee H.-J., Zhang X., Samuel V. T., Shulman G. I., Liu D. et al. (2012). Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc. Natl. Acad. Sci. USA 109, 11402-11407. 10.1073/pnas.1205015109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lan Y., Krumlauf R. and Jiang R. (2017). Modulating Wnt signaling rescues palate morphogenesis in Pax9 mutant mice. J. Dent. Res. 22034517719865 10.1177/0022034517719865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieven O., Knobloch J. and Rüther U. (2010). The regulation of Dkk1 expression during embryonic development. Dev. Biol. 340, 256-268. 10.1016/j.ydbio.2010.01.037 [DOI] [PubMed] [Google Scholar]
- Liu K. J., Arron J. R., Stankunas K., Crabtree G. R. and Longaker M. T. (2007). Chemical rescue of cleft palate and midline defects in conditional GSK-3beta mice. Nature 446, 79-82. 10.1038/nature05557 [DOI] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M. and Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299-3304. 10.1073/pnas.0434590100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah J. K., Ogawa T., Kapadia H., Cavender A. C. and D'Souza R. N. (2004). Functional analysis of a mutation in PAX9 associated with familial tooth agenesis in humans. J. Biol. Chem. 279, 5924-5933. 10.1074/jbc.M305648200 [DOI] [PubMed] [Google Scholar]
- Mossey P. A., Little J., Munger R. G., Dixon M. J. and Shaw W. C. (2009). Cleft lip and palate. Lancet 374, 1773-1785. 10.1016/S0140-6736(09)60695-4 [DOI] [PubMed] [Google Scholar]
- Mues G., Bonds J., Xiang L., Vieira A. R., Seymen F., Klein O. and D'Souza R. N. (2014). The WNT10A gene in ectodermal dysplasias and selective tooth agenesis. Am. J. Med. Genet. A 164A, 2455-2460. 10.1002/ajmg.a.36520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi M., Wang X.-P., Key D., Lund J. J., Turbe-Doan A., Kist R., Aw A., Chen Y., Maas R. L. and Peters H. (2010). Genetic interactions between Pax9 and Msx1 regulate lip development and several stages of tooth morphogenesis. Dev. Biol. 340, 438-449. 10.1016/j.ydbio.2010.01.031 [DOI] [PubMed] [Google Scholar]
- Niida A., Hiroko T., Kasai M., Furukawa Y., Nakamura Y., Suzuki Y., Sugano S. and Akiyama T. (2004). DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 23, 8520-8526. 10.1038/sj.onc.1207892 [DOI] [PubMed] [Google Scholar]
- O'Connell D. J., Ho J. W. K., Mammoto T., Turbe-Doan A., O'Connell J. T., Haseley P. S., Koo S., Kamiya N., Ingber D. E., Park P. J. et al. (2012). A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci. Signal. 5, ra4 10.1126/scisignal.2002414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauws E., Hoshino A., Bentley L., Prajapati S., Keller C., Hammond P., Martinez-Barbera J.-P., Moore G. E. and Stanier P. (2009). Tbx22null mice have a submucous cleft palate due to reduced palatal bone formation and also display ankyloglossia and choanal atresia phenotypes. Hum. Mol. Genet. 18, 4171-4179. 10.1093/hmg/ddp368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. C., Lundquist J. T., Gilbert A. M., Alon N., Bex F. J., Bhat B. M., Bursavich M. G., Coleburn V. E., Felix L. A., Green D. M. et al. (2009). (1-(4-(Naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine: a wingless beta-catenin agonist that increases bone formation rate. J. Med. Chem. 52, 6962-6965. 10.1021/jm9014197 [DOI] [PubMed] [Google Scholar]
- Peters H., Neubuser A., Kratochwil K. and Balling R. (1998). Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, 2735-2747. 10.1101/gad.12.17.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recker R. R., Benson C. T., Matsumoto T., Bolognese M. A., Robins D. A., Alam J., Chiang A. Y., Hu L., Krege J. H., Sowa H. et al. (2015). A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J. Bone Miner. Res. 30, 216-224. 10.1002/jbmr.2351 [DOI] [PubMed] [Google Scholar]
- Satokata I. and Maas R. (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6, 348-356. 10.1038/ng0494-348 [DOI] [PubMed] [Google Scholar]
- Stapleton P., Weith A., Urbánek P., Kozmik Z. and Busslinger M. (1993). Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat. Genet. 3, 292-298. 10.1038/ng0493-292 [DOI] [PubMed] [Google Scholar]
- Stockton D. W., Das P., Goldenberg M., D'Souza R. N. and Patel P. I. (2000). Mutation of PAX9 is associated with oligodontia. Nat. Genet. 24, 18-19. 10.1038/71634 [DOI] [PubMed] [Google Scholar]
- Tan K., Shaw A. L., Madsen B., Jensen K., Taylor-Papadimitriou J. and Freemont P. S. (2003). Human PLU-1 has transcriptional repression properties and interacts with the developmental transcription factors BF-1 and PAX9. J. Biol. Chem. 278, 20507-20513. 10.1074/jbc.M301994200 [DOI] [PubMed] [Google Scholar]
- van den Boogaard M.-J., Créton M., Bronkhorst Y., van der Hout A., Hennekam E., Lindhout D., Cune M. and Ploos van Amstel H. K. (2012). Mutations in WNT10A are present in more than half of isolated hypodontia cases. J. Med. Genet. 49, 327-331. 10.1136/jmedgenet-2012-100750 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhao X., Hu Y., St Amand T., Zhang M., Ramamurthy R., Qiu M. and Chen Y. (1999). Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 215, 45-53. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Song Y., Zhao X., Zhang X., Fermin C. and Chen Y. (2002). Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129, 4135-4146. [DOI] [PubMed] [Google Scholar]
- Zhou J., Gao Y., Zhang Z., Zhang Y., Maltby K. M., Liu Z., Lan Y. and Jiang R. (2011). Osr2 acts downstream of Pax9 and interacts with both Msx1 and Pax9 to pattern the tooth developmental field. Dev. Biol. 353, 344-353. 10.1016/j.ydbio.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gao Y., Lan Y., Jia S. and Jiang R. (2013). Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development 140, 4709-4718. 10.1242/dev.099028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.