Abstract
Linear accelerator (linac) based CNS stereotactic radiosurgery (SRS) requires significant time resources. We hypothesized that CNS SRS using a flattening filter free (FFF) linac would reduce treatment time and improve clinical efficiency. A FFF linac was recently commissioned for CNS radiosurgery at the University of Alabama at Birmingham. The efficiency of this linac for CNS SRS was retrospectively reviewed. Beam on time (BOT), time in room (TIR), and clinical dose rate (CDR) were calculated using an integrated treatment planning, record, and verification software platform and are proposed as surrogates for treatment efficiency. Twenty-seven eligible CNS SRS cases consisting of 1-5 fractions of 5 Gy or more per fraction were reviewed. Mean BOT was 1:21 (minutes:seconds; range: 00:36-2:52) and mean TIR was 10:42 (minutes:seconds; range: 6:05-22:56). The mean CDR was 1820 MU/ min (range: 872-2396). On regression analysis the number of alignment images, treatment arcs, targets, monitor units, and presence of intra-fraction imaging were factors significantly (p < 0.05) associated with prolonged TIR. Use of FFF mode in CNS SRS more than triples the CDR and results in shortened BOT and TIR compared to treatment at conventional dose rates. Reduction in clinical treatment times may translate to better target localization due to reduced opportunity for intrafraction motion. Linac-based CNS SRS can be completed in a normal time slot with a high output FFF linac.
Keywords: Radiosurgery, CNS radiation, SRS, SBRT, FFF, treatment efficiency
INTRODUCTION
Stereotactic radiosurgery (SRS) is a common technique for delivery of high dose, conformal radiation to localized lesions within the central nervous system (CNS). Despite its advantages over surgery and conventionally fractionated radiotherapy, a potential disadvantage of SRS is the lengthy treatment time required, particularly for lesions nearby critical structures or multiple target plans. In order to accurately deliver ablative doses of radiation to CNS lesions, relatively high doses of radiation are employed with strict image-guidance. When delivered at conventional dose rates of 400–600 monitor units per minute (MU/min), SRS can require upwards of one hour of dedicated time for proper immobilization, image-guidance and treatment. Length of treatment time is an important issue for patients and health care providers alike. First and foremost, long treatment time compounds patient discomfort related to uncomfortable positioning and immobilization devices (ie, face mask or head frame). Furthermore, evidence suggests that patients “immobilized” for long periods of time are actually more likely to experience intrafraction motion during radiation [1]. Secondarily, prolonged treatment time presents unique challenges to providers who must observe the entire treatment, and cancer centers which are hampered by diminished machine throughput and efficiency.
The primary factor acting to limit radiation dose rate in the range of 400–600 MU/min is the presence of the flattening filter, a beam modifying device used to create a more homogeneous cross beam profile. The device likely has very little impact on treatment time for conventional doses per fraction (ie, 180–300 cGy per fraction), but has profound impact on treatment time when higher doses used in SRS are employed. Preclinical reports indicate that beam time for SBRT plans can be reduced by more than 50% when the flattening filter is removed; however, these reports have not been verified in the clinical setting [2, 3]. The University of Alabama at Birmingham is one of the first clinical sites in the world to utilize a recently developed linear accelerator capable of operating in FFF mode (Varian TrueBeam). We hypothesized that clinical use of a FFF linear accelerator to deliver CNS SRS would significantly improve treatment time such that they could be completed in a standard 15-minute time slot.
MATERIALS AND METHODS
The medical records of all SRS patients treated using high intensity FFF mode at the University of Alabama-Birmingham were reviewed beginning with commissioning of the unit in July 2010. Treatments were completed on the TrueBeam STx (Varian Medical Systems) operating at up to 2400 monitor units/ minute (MU/min). Patients with localized CNS tumors in the brain or spine were eligible for inclusion. SRS was defined as 1–5 fractions of 5 Gy per fraction or higher with concomitant non-invasive immobilization and image guidance. At our institution, the standard immobilization procedure is aquaplast face mask and the standard image guidance protocol is paired orthogonal KV images followed by cone beam CT. Further imaging is only performed at the discretion of the treating physician when there is concern about potential intrafraction motion.
The primary measure of treatment efficiency is the time a patient spends in the treatment room (time in room, TIR). TIR is composed of image-guidance time (IGT), beam on time (BOT), and intrafraction downtime (IDT). TIR is elapsed time measured from the first alignment image to last beam off, inclusive of all pre-treatment imaging studies and/or shifts. IGT is time spent performing image guidance procedures and subsequent shifts prior to first beam on. BOT is the actual time the radiation beam is on during a fraction. IDT accounts for time elapsed during any intrafraction interruptions after the first beam-on. The clinical dose rate (CDR) is the number of MU divided by the BOT. Treatment time parameters were collected from the electronic treatment record (Aria, Varian Medical Systems, Palo Alto, CA). The same software was also used to collect further information predicted to impact treatment time, including the number of planned or unplanned shifts as well as the frequency and type of pre-treatment and intrafraction imaging. Microsoft Excel (Microsoft Corp, Redmond, WA) was used to compile data and compute median and mean treatment times and SPSS v.19 (IBM, Armonk, NY) was used to perform regression analysis of factors predicted to influence length of treatment time.
RESULTS
Twenty-seven linac CNS radiosurgery cases treated from August 2010 to March 2011 were reviewed. Twenty-five cases were brain SRS and 2 cases were spinal SRS. There was a single target lesion in the majority of cases (19 cases, 70%), but multiple metastatic brain lesions were targeted in the remaining 8 cases (range 1–9 target lesions). The median prescription dose was 25 Gy in 5 fractions (range: 12–30 Gy, 1–5 fractions), but single fraction SRS was performed in 6 cases. Intensity modulated radiation therapy (IMRT) was used in all treatment plans and a single isocenter was used in all but one plan. The majority of plans (19; 70%) used volumetric modulated arc therapy (VMAT) (RapidArc, Varian Medical Systems) rather than static field IMRT for dose delivery. A mean of 2424 MUs (range: 873-6877) was delivered using 7 static fields or 1–4 volumetric arcs. Table 1 summarizes the treatment characteristics of all patients.
Table 1.
Treatment Characteristics
| Cases | |
| Brain | 25 |
| Spine | 2 |
| Number of lesions | |
| 1 | 19 |
| >1 | 8 |
| Number of Isocenters | |
| 1 | 26 |
| >1 | 1 |
| Type of IMRT | |
| Static field | 8 |
| Volumetric modulated arc | 19 |
| Dose Schedule | |
| 5 Gy × 5 fractions | 10 |
| 5.5 Gy × 5 fractions | 1 |
| 6 Gy × 4 fractions | 1 |
| 6 Gy × 5 fractions | 9 |
| 12 Gy × 1 fraction | 2 |
| 15 Gy × 1 fraction | 2 |
| 16 Gy × 1 fraction | 2 |
| Number of Monitor Units (MU) | |
| <2000 | 14 |
| 2000-3999 | 10 |
| ≥4000 | 3 |
Treatment details for 27 patients treated on FFF linac
Mean values and standard deviations for TIR, BOT, IGT, and IDT are presented in Table 2. Figure 1 graphically represents BOT, TIR and the relationship of the two factors. The mean CDR was 1820 MU/min (range: 872-2396; standard deviation 543). On regression analysis, factors significantly (p < 0.05) associated with prolonged TIR included increasing number of cone-beam CTs for alignment, number of treatment arcs, number of targets, number of MU’s, and the presence of intrafraction imaging. Number of MU delivered was the only factor associated with prolonged BOT (Table 3).
Table 2.
Treatment Time Components
| Component | Mean | Standard deviation |
|---|---|---|
| Time in room (TIR) | 10:42 | 3:49 |
| Beam on time (BOT) | 1:21 | 0:33 |
| Image-guidance time (IGT) | 7:26 | 1:54 |
| Intrafraction downtime (IDT) | 1:55 | 2:37 |
Mean time and standard deviation for 4 treatment time variables indentified. TIR is elapsed time measured from the first alignment image to last beam off, inclusive of all pretreatment imaging studies and/or shifts. IGT is time spent performing image guidance procedures and subsequent shifts prior to first beam on. BOT is the actual time the radiation beam is on during a fraction. IDT accounts for time elapsed during any intrafraction interruptions after the first beam-on.
Figure 1.
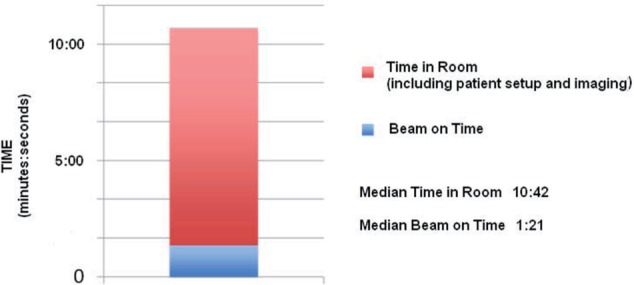
Graphical representation of treatment time for CNS SRS using FFF linac. The median radiation-beam on time was 1:21 while the median time the patient spent in the treatment room, including treatment setup and imaging, was 10:42.
Table 3.
Regression Analysis
| Factor | p-value | |
|---|---|---|
| BOT | TIR | |
| Number of intrafraction images | NS | 0.006 |
| Number of KV†intrafraction images | NS | NS |
| Number of CBCT‡intrafraction images | NS | <0.001 |
| Number of shifts made | NS | NS |
| Number of MU delivered | <0.001 | 0.01 |
| Number of isocenters | NS | NS |
| Number of arcs or fields | NS | 0.014 |
| Number of targets | NS | 0.006 |
† Kilovoltage orthogonal X-Ray
‡ Cone-beam computed tomography
Statistical regression analysis describing the interaction of BOT and TIR with eight treatment-related factors.
(Abbreviations: BOT: beam on time, TIR: time in room, NS: non-statistically significant)
DISCUSSION
Our early results indicate that use of FFF mode in CNS SRS increases the clinically observed dose rate (CDR) by a factor of 3–4 and results in dramatically reduced BOT and TIR when compared to conventional dose rate treatment (400-600 MU/min). These results are not surprising. Preclinical reports indicate that beam time for SBRT plans is reduced by more than 50% when the flattening filter is removed [2,3]. The current study corroborates these previously published findings, but to our knowledge, is the first to report efficiency parameters for FFF beams in the clinical setting.
The mean BOT observed in this SRS cohort is comparable to the typical BOT for conventionally fractionated daily radiotherapy, and is much shorter than typical linac-based SRS. The only factor identified which leads to longer BOT was number of MU’s, which is itself the primary determinate of beam time. It is notable that TIR is significantly longer than BOT. Regression analysis identified several factors related to longer TIR, underscoring the time intensive process of imaging and positioning that occurs prior to and sometimes during SRS for CNS malignancies. Nevertheless, the total TIR was an average of just over 10 minutes, indicating that these plans can routinely be scheduled for standard 15 minute time slots when using our institution’s image guidance procedures. Although the majority of these cases were hypofractionated and not single fraction treatments, our experience suggests that single fraction doses of 15–24 Gy can be administered within the same time frame. It is important to note that although treatment efficiency is markedly improved, the unit did not operate at maximum output (2400 MU/min) during the entire treatment for the majority of cases due primarily to treatment geometry. For VMAT plans treatment efficiency is limited by the gantry rotation speed of 1 revolution per minute. This is particularly true if more than one arc is selected for therapy. Similarly, treatment efficiency is improved if multiple targets are treated with a single isocenter.
According to the published literature, interest in the clinical use of FFF photon beams dates back to 1991 [4]. Its use, however, has been limited to pre-clinical projects at research institutions due to the lack of a commercially available treatment unit. Dosimetric data suggest that radiation plans delivered without the flattening filter are equivalent to conventionally flattened beam plans. Researchers at the University of Texas compared plans from prostate, lung, brain, and head and neck and found no measureable differences between the two methods [5]. With the recent introduction of linacs designed to treat in FFF-mode, clinical use for a broad variety of applications will likely become prevalent in coming years.
Despite its importance in conventional radiotherapy, the flattening filter is less important in the modern era, particularly in the setting of SRS. The basic function of the filter is to make beam intensity homogenous across the beam profile, in order to prevent the area surrounding the central ray from being many times more intense than the field edge (see Figure 2). However, with the small fields utilized in SRS this issue is rarely encountered, and some have argued that this may present an advantage whereby the higher-dosed central region receives a simultaneously integrated boost [3]. Furthermore–and perhaps more importantly–the flattening filter presents several dosimetric disadvantages in the setting of IMRT. Beam homogeneity in IMRT is accomplished by modulation of beamlet fluence, not the physical properties of the flattening filter. Data suggests that the filter is the primary factor in beam attenuation, accounting for loss of approximately 50–75% of the beam intensity at the central ray. Simply removing the device from the path of the beam can more than double the dose rate. Therefore, in the setting of IMRT, the flattening filter serves primarily to reduce dose rate and not to modulate beam intensity, which was the original intent. Apart from its detrimental effect on dose rate, data suggest that the FF imparts several other dosimetric disadvantages. Preclinical research suggests FFF beams generate less neutron contamination, lower doses outside the field edge, and less MLC leakage than comparable beam-flattened plans [3,6,7]. In a recent study comparing dosimetry between flattened and unflattened radiation plans, Cashmore et al observed up to a 70% decrease in scattered photon dose to critical organs. The investigators posit that reduced doses to organs such as the thyroid, ovaries, and testes could potentially lead to lower rates of secondary malignancies in children treated with IMRT [8]. However, despite promising dosimetric and preclinical data demonstrating the equivalence of unflattened photons, there is no published data to confirm these assumptions with clinical outcomes.
Figure 2.
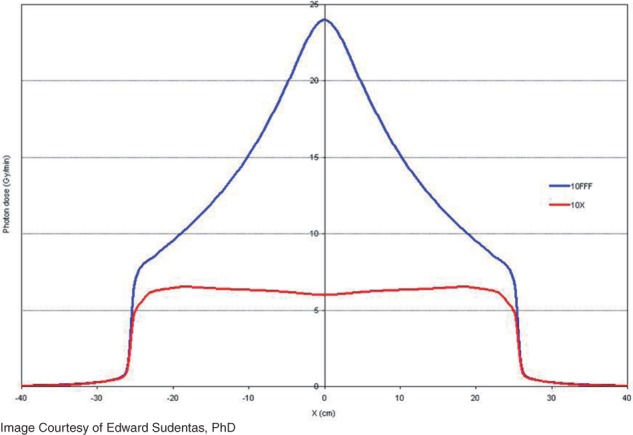
The cross beam profile of a conventional (with flattening filter) 10 megavolt photon beam (red) is compared to the cross beam profile of a unflattened photon beam (blue) of equivalent energy. The unflattened beam has approximately four times higher dose rate at central axis.
Our findings are important as reduction in clinical treatment times can be expected to improve the patient’s treatment experience and may translate to better target localization due to reduced opportunity for intrafraction motion. Prior studies have suggested that longer treatment time yields decreased target accuracy [9]. In a study of 32 cranial SRS patients, Hoogeman, et al demonstrated a linear correlation between increasing treatment time and intrafraction motion. The authors conclude that intrafraction motion will significantly affect accuracy for high precision treatments taking 15 minutes or longer to deliver [10]. Due to treatment efficiency advantages with an unflattened beam, none of the patients in this cohort had treatment times exceeding 15 minutes. Although care should always be taken to assure proper target localization, the issue of a marginal miss that may be associated with prolonged treatment times is magnified in the setting of CNS malignancies, where radiosenstive tissues such as the optic nerves or retina may be adjacent to the target.
This study has several inherent weaknesses based on the retrospective design and small patient numbers. Additionally, due to the recent installation and clinical use of this technology, follow up is short and clinical outcomes are lacking. Future studies are planned to assess clinical outcomes, which are predicted to be comparable to conventional therapy. Likewise, future studies are needed to assess if improved treatment efficiency will improve patient quality of life and reduce operations costs.
CONCLUSIONS
The use of FFF linacs for CNS SRS improves clinical efficiency, both in terms of treatment time and dose rate delivery. More frequent in-room imaging, more treatment arcs, more target lesions, and more MU’s prescribed are associated with prolonged TIR, which may negatively impact a patient’s treatment experience and could result in intrafraction motion if invasive immobilization is not utilized. Based on these results, both single fraction and hypofractionated CNS SRS can be accomplished within a standard 15-minute radiotherapy time slot.
REFERENCES
- 1. Purdie TG, Bissonnette JP, Bezjak A Franks, Payne D, Sie F, Sharpe MB, Jaffray DA. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys. 2007; 68(1): 243–52. [DOI] [PubMed] [Google Scholar]
- 2. Vassiliev ON, Kry SF, Chang JY, Balter PA, Titt U, Mohan R. Stereotactic radiotherapy for lung cancer using a flattening filter free Clinac. J Appl Clin Med Phys. 2009; 10(1): 2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cashmore J. The characterization of unflattened photon beams from a 6 MV linear accelerator. Phys Med Biol. 2009; 53(7): 1933–46. [DOI] [PubMed] [Google Scholar]
- 4. O’Brien PF, Gillies BA, Schwartz M, Young C, Davey P. Radiosurgery with unflattened 6-MV photon beams. Med Phys. 1992; 18(3): 519–21. [DOI] [PubMed] [Google Scholar]
- 5. Stathakis S, Esquivel C, Gutierrez A, Buckey CR, Papanikolaou N. Treatment planning and delivery of IMRT using 6 and 18MV photon beams without flattening filter. Appl Radiat Isot. 2009; 67(9): 1629–37. [DOI] [PubMed] [Google Scholar]
- 6. Kragl G, Baier F, Lutz S, Albrich D, Dalaryd M, Kroupa B, Wiezorek T, Knöös T, Georg D. Flattening filter free beams in SBRT and IMRT: dosimetric assessment of peripheral doses. Z Med Phys. 2011; 21(2): 91–101. [DOI] [PubMed] [Google Scholar]
- 7. Kry SF, Titt U, Pönisch F, Vassiliev ON, Salehpour M, Gillin M, Mohan R. Reduced neutron production through use of a flattening-filter-free accelerator. Int J Radiat Oncol Biol Phys. 2007; 68(4): 1260–4. [DOI] [PubMed] [Google Scholar]
- 8. Cashmore J, Ramtohul M, Ford D. Lowering Whole-Body Radiation Doses in Pediatric Intensity-Modulated Radiotherapy Through the Use of Unflattened Photon Beams. Int J Radiat Oncol Biol Phys. 2011; 80(4): 1220–7. [DOI] [PubMed] [Google Scholar]
- 9. Murphy MJ, Chang SD, Gibbs IC, Le QT, Hai J, Kim D, Martin DP, Adler JR., Jr. Patterns of patient movement during frameless image-guided radiosurgery. Int J Radiat Oncol Biol Phys. 2003; 55(5): 1400–8. [DOI] [PubMed] [Google Scholar]
- 10. Hoogeman MS, Nuyttens JJ, Levendag PC, Heijmen BJ. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008; 70(2): 609–18. [DOI] [PubMed] [Google Scholar]


