Abstract
Object
Stereotactic radiosurgery (SRS) is well established in the treatment of brain metastases, however it’s exact role remains unclear. A single metastasis at presentation raises additional challenges, however there is minimal outcome data within this subgroup. We sought to evaluate the outcomes of treatment in patients with a single brain metastasis, as well as factors impacting local control.
Methods
All patients treated with SRS for a single brain metastasis were evaluated. Data was collected regarding patient demographics, treatment characteristics, and treatment outcomes. Univariate analyses were performed to evaluate the impact of treatment and patient variables on these outcomes. Emphasis was placed on analyses of factors impacting LC.
Results
Between 1998 and 2011, a total of 141 patients underwent SRS for a single brain metastasis; in addition 31 had surgical resection, 15 received whole brain radiotherapy (WBRT), and 2 underwent both. There was no statistical impact on local control (LC) or distant intracranial control (DIC) with the addition of WBRT or surgery (LC 74%, 100%, and 58%, and DIC 37%, 67%, and 49% for SRS alone, SRS + WBRT, and SRS + surgery, respectively, smallest p = 0.17). Local control was decreased with larger tumors, doses <20Gy, and tight overtreatment ratios (i.e. conformity) (largest p = 0.02), although the independence of these factors could not be established. Long term freedom from requiring future whole brain radiotherapy was 73%.
Conclusions
SRS alone for patients with single brain metastases demonstrates acceptable intracranial outcomes. Further evaluation into factors impacting LC are warranted.
Keywords: radiosurgery, single, metastasis
1. Introduction
The role of stereotactic radiosurgery (SRS) in the treatment of brain metastases remains controversial. Its efficacy has been well established[1-5]; however, whether SRS is best utilized alone or in combination with either surgery or whole brain radiotherapy (WBRT) remains in debate[6]. There have been three randomized trials evaluating the efficacy of SRS alone; two randomized trials comparing the addition of WBRT to SRS[3,5], and one randomized trial comparing the addition of WBRT to either surgery or SRS[2]. The results of these studies have demonstrated an improvement in local control (LC) and distant intracranial control (DIC) with the addition of WBRT; however, significantly increased toxicity has also been demonstrated with combination therapy resulting in the early closure of one of the trials[3]. Of note, these studies included patients with up to 4 metastases, and patients who present with a single brain metastasis have been shown to have improved survival[7]. There is a relative paucity of data for patients with a single metastasis only evaluating these same outcomes.
The implementation of SRS alone for patients with single metastases poses an attractive clinical option, in order to avoid morbidity associated with either WBRT or surgery. However, this strategy places an emphasis on LC for these lesions, in order to maintain these benefits and avoid the need for salvage treatments. While the efficacy of SRS has certainly been shown, emphasis in the literature has been on factors impacting survival and intracranial control, with relatively few studies investigating factors impacting LC in detail.
The purpose of this study was to evaluate our experience in the treatment of patients with single brain metastases, and whether the addition of prior treatment with WBRT or surgery impacted either LC, DIC, or overall survival (OS). In addition, we wished to evaluate other patient and treatment variables which could impact these same outcomes.
2. Methods
At our institution, patients who present with a single metastasis are preferentially treated with radiosurgery upfront, unless symptoms or a need for pathologic confirmation necessitate surgery. Whole brain radiotherapy is not usually utilized for this patient subgroup unless they initially receive treatment at an outside facility, or require urgent treatment for clinical symptoms. Our institutional database was retrospectively reviewed, and all patients who presented with a single brain metastasis, and received treatment with radiosurgery as part of initial intracranial therapy during the period from October 1998 through December 2011 were included. IRB and institutional ethics committee approval was obtained from the University of Utah’s review committee. Patient, tumor, and treatment characteristics were collected for all patients. Follow up data, including available imaging studies were reviewed to determine LC, DIC, and OS. Local failure was defined as a persistent increase by >25% of the bidimensional product of contrast enhancement[5], new nodular enhancement, or the need for additional local therapy with surgery or SRS. When failure was in question, magnetic resonance spectroscopy or positron emission tomography (PET) were used to help determine radiation necrosis from lesion failure. When surgery was utilized for salvage, evidence of radiation necrosis did not change the local failure designation. Distant intracranial failure was defined as the presence of new brain metastases.
Radiosurgery was delivered via a linear accelerator based radiosurgical technique utilizing a single isocenter. Until the years 2008/2009, patients were treated with a Brainlab frame-based system, after which institutional planning transitioned to a Brainlab custom mask with ExacTrac image guidance. Lesions were outlined without a margin, and treatments were planned in most cases with 5 dynamic conformal arcs. Lesion diameter was determined as the diameter of a sphere that yielded the same volume as the measured lesion volume. In the majority of cases, dose was prescribed per RTOG 9005[1] guidelines, with lesions 3.01-4.00 cm in diameter treated to 15 Gy, lesions 2.01-3.00 cm treated to 18 Gy; however variation did occur with lesions ≤2 cm, where in the earlier years of the study these lesions were prescribed 20 Gy, and during the last year they were prescribed 24 Gy. Prescription isodose lines were chosen per our institutional standard[8], where the chosen isodose volume covers 95% of the tumor volume, and 95% of the dose covers 99% of the tumor volume. Conformity was calculated as described by Nakamura et al.[9], and the overtreatment ratio as the Radiation Therapy Oncology Group PITV ratio (PIV/TV, PIV – Prescription Isodose Volume, TV – Tumor Volume)[10] (referred to as the overtreatment ratio for conceptual clarity).
Patients were scheduled for routine follow up both with the treating radiation oncologist and neurosurgeon, with contrast enhanced MRIs performed every three months. Local failure or new brain metastases were treated with either SRS, WBRT, or surgery depending upon size, symptoms, patient KPS, and disease status.
Patient outcomes were evaluated including local control, distant intracranial control, and overall survival. Univariate analysis was performed to assess factors impacting these outcomes, including prior treatment, histology, size, frameless or frame-based treatment, prescription isodose line, dose category, and overtreatment ratio. Additional analysis was performed to assess the impact of conformity index on local control; a loose conformity index can in effect create a de facto margin on the tumor volume, and this was investigated by evaluating the impact of the overtreatment ratio (PIV/TV) on local control. In order to identify independent prognostic factors for local control, a multivariate analysis was attempted both with the same factors as utilized in the univariate analysis as well as with statistically significant variables; however, there were inadequate events to identify associations with reasonable statistical power. Kaplan-Meier curves were generated for outcomes of interest, and log rank analysis was utilized for univariate analysis for comparisons between groups. Statistical analysis was performed using StatsDirect statistical software (version 2.7.9, Stats Direct Ltd., Altrincham, UK). Statistical significance was set at P ≤ 0.05.
3. Results
A total of 141 patients who presented with a single metastasis were identified. Patient demographics are listed in Table 1. Thirty one patients underwent surgical resection of the lesion prior to SRS (22%), 15 patients received WBRT prior to SRS (11%), and 2 patients underwent both (1%). Melanoma was the predominant histology, representing 61 patients (43%), with the second most common histology being non-small cell lung cancer (NSCLC) represented in 26 patients (18%). The majority were treated with a frame-based approach (67%). Lesions < 2 cm were the most common size, representing 62% of patients. Overall median dose and tumor diameter were 20 Gy and 1.7 cm, respectively.
Table 1.
Patient Demographics (KPS – Karnofsky Performance Status, Gy – Gray, cm centimeter)
| Number | % | ||
|---|---|---|---|
| Patients | 141 | ||
| Mean Age (Range) | 61.3 (21.4 – 89.0) | ||
| Sex | Male | 101 | 72% |
| Female | 40 | 28% | |
| Mean KPS (Range) | 90 (40-100) | ||
| Prior Treatment | |||
| None | 93 | 66% | |
| WBRT | 15 | 11% | |
| Surgery | 31 | 22% | |
| Both | 2 | 1% | |
| Histology | Breast | 12 | 9% |
| NSCLC | 26 | 18% | |
| RCC | 21 | 15% | |
| Melanoma | 61 | 43% | |
| Other | 21 | 15% | |
| Frameless | Yes | 47 | 33% |
| No | 94 | 67% | |
| Dose (Gy) | ≤15 | 19 | 13% |
| 15.1-17.9 | 7 | 5% | |
| 18-19.9 | 37 | 26% | |
| 20 - 23.9 | 73 | 52% | |
| ≥ 24 | 5 | 4% | |
| RPA Class | 1 | 14 | 10% |
| 2 | 124 | 88% | |
| 3 | 3 | 2% | |
| Size (cm) | ≤2 | 88 | 62% |
| 2.01-3.0 | 42 | 30% | |
| > 3 | 11 | 8% |
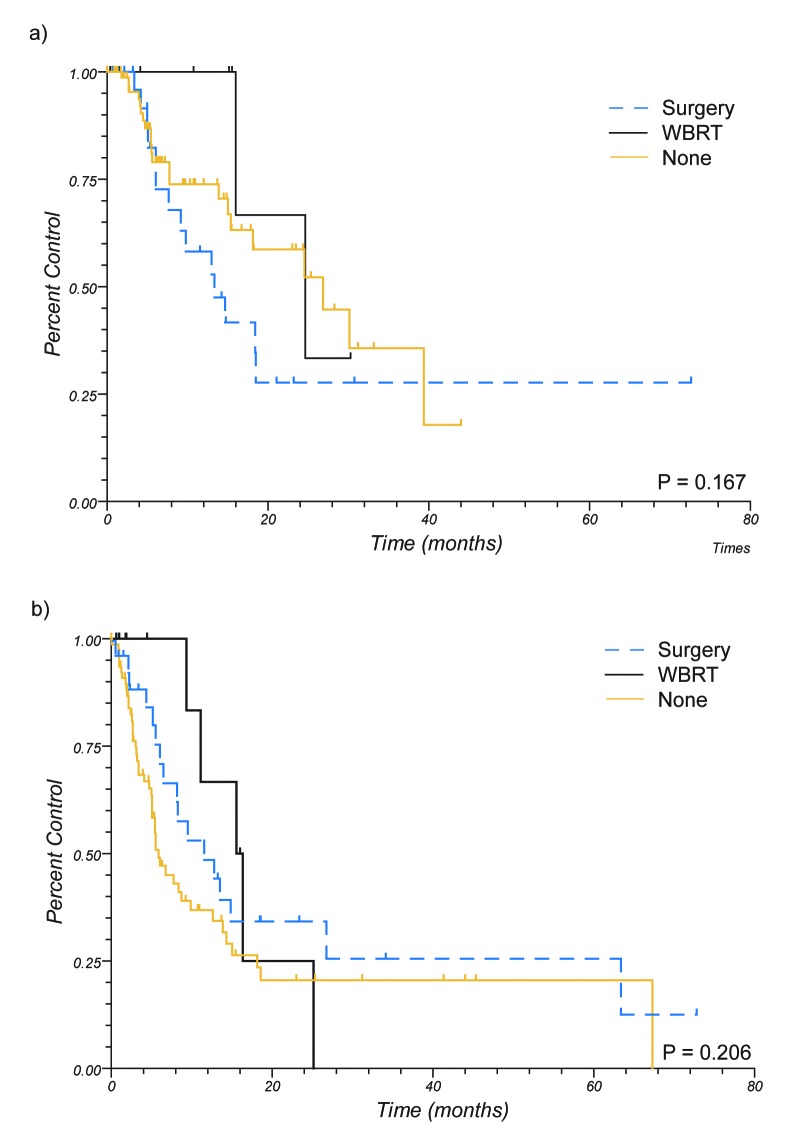
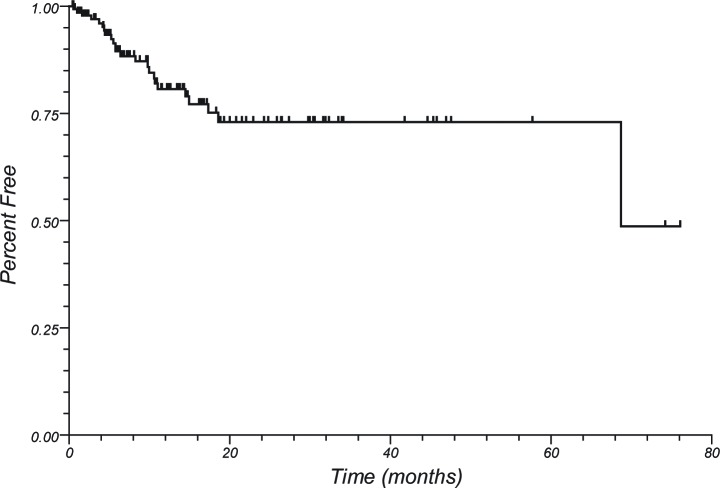
Median follow up of living patients was 30 months. 1 year OS for the entire group was 47%. Local control and DIC at 1 year were 71% and 42%, respectively. One year LC by treatment group was 74%, 100%, and 58% for patients treated with SRS alone, those receiving WBRT and SRS, and those undergoing surgical resection and SRS, respectively (p = 0.17) (see Figure 1). One year DIC was 37%, 67%, and 49% for the same treatment groups (p = 0.21). One year LC was 61%, 73%, 73%, 100%, and 68% for melanoma, breast, NSCLC, RCC, and other histologies, respectively (p = 0.31). One year DIC was 29%, 51%, 66%, 64%, and 29% for the same histologies (p = 0.01). Thirty two patients (23%) underwent salvage therapy for local failure, the majority of whom received salvage radiosurgery (15 patients, 47%), with the next most common salvage therapy being surgery (10 patients, 31%). At 1 year, 81% of patients were free from requiring WBRT, with long term freedom at 5 years being 73% (see Figure 2). Freedom from any further intracranial treatment, including further SRS, was 57% at 1 year.
Figure 1.
Local and Distant Intracranial Control by Prior Treatment. Upper: Local control. Lower: Distant Intracranial Control (WBRT – whole brain radiotherapy).
Figure 2.
Freedom from whole brain radiotherapy.
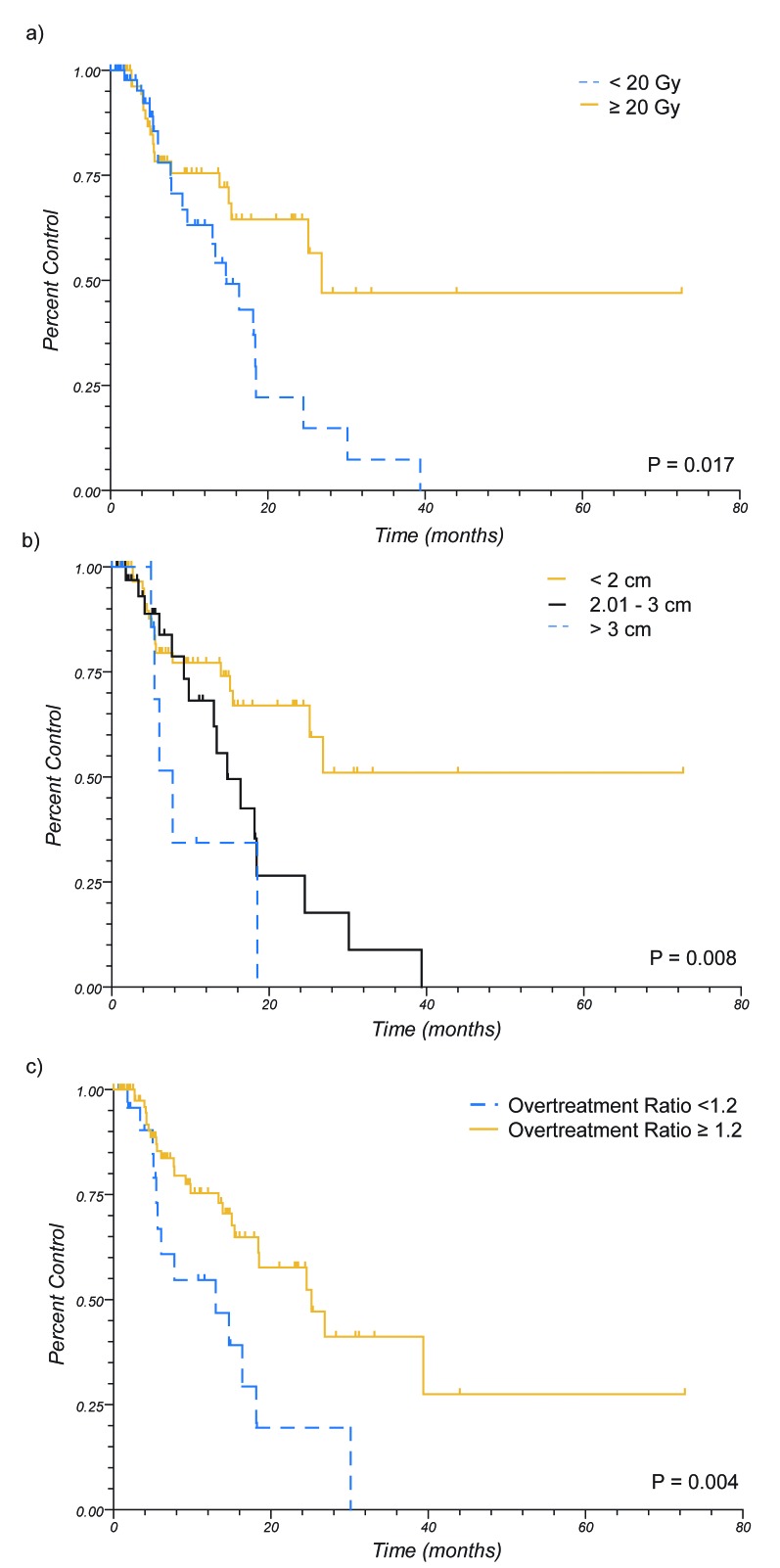
Univariate analysis for factors predicting for LC are shown in Table 2. When patients were evaluated by treatment group, there was no significant impact on LC. Positively prognostic factors for LC were renal cell histology (RCC) (HR = 0.30, 95% C.I. 0.13 – 0.70, p = 0.04), and negatively prognostic factors were tumor size of 2.01 – 3 cm (HR 2.29, 95% C.I. 1.11 – 4.70, p = 0.01), dose of < 20 Gy (HR 2.13, 95% C.I. 1.10 – 4.11, p = 0.02), and an overtreatment ratio of <1.2 (HR 2.58, 95%. C.I. 1.10 – 6.03, p < 0.01). However, it was noted that these variables were not independent. Patients receiving ≥ 20 Gy had smaller tumors ≤ 2.0 cm, had looser conformity indices, and higher overtreatment ratios (see Figure 3). Further attempt was made at investigating which of these factors was driving the improved local control, including multivariate analysis and stratified subgroup analyses, however due to co linearity and the low incidence of events statistical power was not adequate for further conclusions to be drawn.
Table 2.
Univariate Analysis on Local Control (C.I. – confidence interval, WBRT – whole brain radiotherapy, NSCLC – non small cell lung cancer, RCC – renal cell carcinoma, PIV – prescription isodose volume, TV – tumor volume, Gy – Gray, cm – centimeter) (significant findings in bold)
| HR | 95% C.I. | ||
|---|---|---|---|
| Prior Treatment | |||
| None | referent | ||
| WBRT | 0.58 | 0.18 - 1.86 | |
| Surgery | 1.56 | 0.76 - 3.21 | |
| Histology | |||
| Melanoma | referent | ||
| Breast | 0.74 | 0.26 - 2.12 | |
| NSCLC | 1 | 0.40 - 2.49 | |
| RCC | 0.3 | 0.13 - 0.70 | |
| Other | 1.01 | 0.31 - 3.36 | |
| Size (cm) | |||
| ≤2 | referent | ||
| 2.01-3 | 2.29 | 1.11 - 4.70 | |
| >3 | 3.45 | 0.86 - 13.84 | |
| Frameless | |||
| No | referent | ||
| Yes | 0.99 | 0.49 - 2.00 | |
| Prescription Isodose Line | |||
| < 90 | referent | ||
| ≥ 90 | 1.39 | 0.52 3.73 | |
| Dose (Gy) | |||
| < 15 | 0.46 | .04 - 5.77 | |
| 15.1 - 17.9 | referent | ||
| 18 - 19.9 | 0.52 | 0.04 - 6.36 | |
| 20 - 23.9 | 0.25 | 0.02 - 2.87 | |
| 24 | *** | ||
| Dose (Gy) | |||
| ≥ 20 | referent | ||
| < 20 | 2.13 | 1.10 - 4.11 | |
| Overtreatment Ratio (PIV/TV) | |||
| <1.2 | 2.58 | 1.10 - 6.03 | |
| ≥1.2 | referent | ||
Figure 3.
Local Control by Dose, Size, and Overtreatment Ratio (PIV/TV). A) Control by Dose. B) Control by Lesion Size. C) Control by Overtreatment Ratio. (PIV – prescription isodose volume, TV – tumor volume)
Factors impacting DIC were then investigated (Table 3). Additional treatment with surgery or WBRT demonstrated no significant impact on DIC. Breast histologies, NSCLC, and RCC demonstrated improved intracranial control compared to melanoma (HR 0.45, 0.50, and 0.28, 95% C.I. 0.22 – 0.95, 0.26 – 1.0, and 0.15 – 0.55, respectively, greatest p = 0.05). Further analysis was performed when stratified by prior WBRT, and statistical significance was maintained for improved intracranial control for RCC, but was lost for other histologies.
Table 3.
Univariate Analysis on Distant Intracranial Control and Overall Survival (C.I. – confidence interval, WBRT – whole brain radiotherapy, NSCLC – non small cell lung cancer, RCC – renal cell carcinoma, RPA – recursive partitioning analysis, Gy – Gray, cm - centimeter)
| Distant Intracranial Failure | Overall Survival | ||||
| HR | 95% C.I. | HR | 95% C.I | ||
| Prior Treatment | |||||
| None | referent | referent | |||
| WBRT | 0.6 | 0.28 1.28 | 1.5 | 0.82 2.75 | |
| Surgery | 0.69 | 0.41 1.15 | 0.58 | 0.380.88 | |
| Histology | |||||
| Melanoma | referent | referent | |||
| Breast | 0.45 | 0.220.95 | 0.64 | 0.35 1.19 | |
| NSCLC | 0.5 | 0.261.0 | 1.19 | 0.70 2.00 | |
| RCC | 0.28 | 0.150.55 | 0.95 | 0.56 1.63 | |
| Other | 0.57 | 0.24 1.35 | 1.24 | 0.67 2.27 | |
| Size (cm) | |||||
| ≤2 | referent | referent | |||
| 2.01-3 | 0.8 | 0.47 1.36 | 0.91 | 0.61 1.37 | |
| >3 | 0.84 | 0.37 1.92 | 0.87 | 0.45 1.68 | |
| RPA Class | |||||
| 1 | referent | referent | |||
| 2 | 1.01 | 0.52 1.97 | 1.47 | 0.86 2.51 | |
| 3 | ** (no events) | 2.58 | 0.37 17.89 | ||
| Dose (Gy) | |||||
| < 15 | 0.54 | 0.15 1.87 | 0.88 | 0.34 2.28 | |
| 15.1 17.9 | referent | referent | |||
| 18 19.9 | 0.71 | 0.22 2.36 | 1.1 | 0.45 2.71 | |
| 20 23.9 | 0.93 | 0.30 2.91 | 0.99 | 0.42 2.34 | |
| 24 | 0.6 | 0.12 3.05 | 0.17 | .05.52 | |
Prognostic factors for OS were also evaluated on univariate analysis (see Table 3). The addition of upfront surgery (HR 0.58, 95% C.I. 0.38 – 0.88, p = 0.02) and a SRS prescription dose of 24 Gy (HR 0.17, 95% C.I. 0.05 – 0.52, p = 0.04) were significant variables on univariate analysis. While RPA class was not statistically prognostic due to low number of patients in classes I and III, graphical trends in survival were seen (data not shown).
4. Discussion
Outcomes after SRS have been well established[1-5], although its exact role in the management of brain metastases is still controversial[6]. Multiple randomized controlled trials have been performed, investigating the roles of SRS, WBRT, and surgery, and while it has been established that WBRT in addition to surgery is helpful in preventing local progression and in delaying neurological death[11,12], the ability of SRS to replace either WBRT or surgery, and potentially be utilized as monotherapy is still contentious[9]. At the same time, single brain metastases have been shown to have improved clinical behavior[7], and are an area where the implementation of SRS has a great potential for benefit; however, reported clinical outcomes for a single metastasis alone are quite limited[13-15], and appropriate upfront treatment for a single metastasis is still unclear[6].
In our study, when evaluating the addition of either WBRT or surgery to SRS, there was no difference in either local or distant intracranial control. These findings conflict with literature results. There have been two randomized controlled trials that have compared SRS alone to SRS with WBRT, both of which demonstrated that the addition of WBRT statistically improved both local and intracranial control[3,5]. In another randomized trial, the addition of WBRT to either surgical resection or SRS has demonstrated similar findings[2]. The difference seen in our study is likely mostly attributable to the lower number of patients and events resulting in inadequate statistical power, as graphical trends could be seen. However, the previous studies included patients with up to either 3 or 4 metastases; patients presenting with a single metastasis could be hypothesized to demonstrate a different clinical course.
There have been relatively few studies investigating the addition of other treatments to SRS for patients presenting with a single brain metastasis. A randomized trial was attempted, TROG 9805[14], comparing the addition of WBRT to either surgery or SRS, however it closed due to slow accrual after 19 patients were enrolled. No differences were found between the groups, although there was a trend towards decreased CNS relapse with the addition of WBRT (30 vs 78%, p = 0.12). In another study, Clarke et al.[15] evaluated the addition of WBRT to SRS for radioresistant histologies with a single metastasis. The addition of WBRT was not found to affect local control, progression free survival, or overall survival, although none of the five patients receiving WBRT developed distant brain failure. More recently, Rades et al.[13] evaluated the addition of WBRT to SRS for patients with a single lesion, and found improved local control (49% vs 77%, p = 0.04), and trends towards improved distant control (70% vs 90%, p = 0.08). The overall outcomes of our study compare favorably to these results, with 1 yr LC 74%, 100%, and 58% for patients receiving SRS alone ,WBRT plus SRS, and surgery plus SRS. 1 yr DIC was 37%, 67%, and 49% for the same groups. However there was no statistical difference found between treatment groups (smallest p = 0.17). Nevertheless, these results taken with the above findings seem to demonstrate that in patients with single metastases, similar to multiple metastases, WBRT is likely to improve both local and intracranial control, although this has not been shown definitively.
The importance of improved local control and distant intracranial control could be questioned. As shown in the study by Chang et al.[3], worse neurocognitive function resulted with the addition of WBRT (mean posterior probability of decline increased from 24% to 52% at 4 months), resulting in the trial being stopped prematurely. Utilizing an upfront SRS approach in our study with “as needed” salvage resulted in long term freedom from WBRT of 73% at 5 years (see Figure 2). In addition, when salvage was required for local recurrence, repeat SRS was able to be utilized in 47% of patients, and freedom from any further intracranial treatment was 57% at 1 year. Given the morbidity that can be attributed to the addition of WBRT[3] or surgical resection, this has potential advantages.
The overall 1 yr LC for our entire cohort was 71%, and when analyzed for patients receiving SRS alone was 74%. Literature results for local control from radiosurgery vary, with most numbers ranging from 49% 100%[6,13,16]. However, it should be noted that local failure definitions vary widely, and without stringent criteria are susceptible to significant bias, especially in histologies prone to hemorrhage. When looking at the previously noted randomized trials with consistent definitions and quality control, 1 yr local control rates for SRS alone were 72.5%[5] and 67%[3]. In addition, in our study the most common histology was melanoma, traditionally thought of as a radioresistant tumor[17-19]. While melanoma radiosurgical outcomes have been found to be promising[20,21], it does make our patient population somewhat unique.
We performed an analysis to evaluate factors predicting for local control within our study population. On univariate analysis, patients with RCC demonstrate statistically improved local control. The excellent response of this histology has been noted in other series as well[22-24], and in our series demonstrated the best overall response, with 1 yr LC of 100%. In addition, worse LC was noted for patients with tumors 2.01 – 3 cm, for those receiving doses of < 20 Gy, and for those with relatively tight overtreatment ratios of <1.2. This indicates that small tumors, higher prescription doses, and loose conformity indices have improved control. In reality, these are all the same patients, as dose is prescribed based upon lesion size per RTOG 9005 recommendations, and due to technical considerations with planning, smaller lesions are frequently the lesions with higher conformity indices due to higher overtreatment ratios. While this group of patients demonstrated improved control (see Figure 3), we were unable to identify which factor/factors were driving the improved control, as when either stratified univariate analysis or multivariate analysis was performed there was inadequate statistical power.
In evaluating the prognostic effect of these variables, literature results are relatively sparse. The prognostic effect of dose and size have been seen, and in a study by Vogelbaum et al.[25], it was noted that LC varied by dose level utilized on RTOG 9005, with control varying from 85% for lesions treated with 24 Gy, to 45% for lesions treated with 15 Gy. However, as dose was prescribed per RTOG 9005, dose was directly correlated to size, and as a result which factor drove the improved local control is not known. While relatively few studies have directly evaluated factors predicting for local control, dose and tumor size have been identified as being prognostic in other institutional studies as well[26,27]. There are no studies to date to the authors’ knowledge that have directly evaluated the effect of conformity indices on local control, although minimum tumor dose has been identified as prognostic[27]. However, the effect of a loose overtreatment ratio is essentially similar to a tumor margin, and studies evaluating the addition of a margin have showed mixed results[28-30]. In addition, the effect of a margin frequently results in dose escalation in the central tumor volume, and as a result, while relationships between local control and dose, size, and margins have been established, which factor is driving improved control is unclear. This interplay can also be demonstrated in trials which have shown benefits in local control with the addition of WBRT [3, 5], which could be argued to provide central dose escalation as well as creating an effective margin.
It should also be noted that while the potential for increased control exists with either an intended or de facto margin, it also has the potential to increase toxicity. A loose overtreatment ratio results in increased dose to normal brain tissue outside of the metastasis, and this margin has the potential to increase parenchymal complications and toxicity, as has been seen on at least one trial investigating the use of a margin [30]. While toxicity was not quantitatively evaluated within this study, there was no qualitative increase in patients treated with a relatively loose overtreatment ratio, likely due to the high conformality of treatments, however this still needs to be considerd.
Univariate analyses were also performed on factors predicting for DIC and OS. Factors which were prognostic for improved DIC included histologic subtypes of breast, NSCLC and RCC when compared to melanoma. These findings also held true when only patients who did not undergo prior WBRT were evaluated. Treatment group did not statistically affect DIC, although graphical differences were seen. There were two factors predictive of improved OS: upfront surgery and SRS dose of 24 Gy. However, as neither factor demonstrated improved LC, it is unlikely that either factor is independently prognostic for survival. In addition, patients receiving upfront surgery were likely susceptible to selection bias, with only healthier patients, with less extracranial tumor burden chosen to undergo this procedure, likely contributing to their improved survival.
As a retrospective institutional study, limitations to the current study exist. As noted above, the majority of our patients had melanoma metastases, and the behavior of melanoma as a more radioresistant tumor has been shown[17-19]. As a result, demonstrating improvements in either LC or IC with WBRT becomes more difficult with the majority of patients demonstrating this histology. In addition, while it was attempted to establish a uniform LC definition, with the review of previous scans, assessment bias certainly exists within a single institutional setting. A larger cohort of patients would also likely be required to truly investigate all factors which predict for LC, IC, and OS, where a multivariate analysis could be performed with adequate statistical power. In addition, to adequately demonstrate superiority or equivalence in any treatment approach a prospective randomized trial with adequate power would be required. Nevertheless, it serves as the largest study that we are aware of to date evaluating outcomes for patients treated for a single brain metastasis.
Overall, the role of SRS in the treatment of brain metastases is still being actively defined. Patients with single metastases are a unique population where it seems an ideal situation to implement SRS alone. However clinical courses and outcomes within this very narrow population are not well studied. The above study is the largest study to date that we are aware of to evaluate this population of patients, and demonstrates that upfront SRS alone is an excellent treatment approach, and utilization of this paradigm allows for long term freedom from WBRT in more than 70% of patients. Improved LC can certainly be seen in a subgroup of patients, and further investigation into whether dose, size, margin, or all three are the driving factor for control would be warranted.
5. Acknowledgements
None
6. Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000; 47(2): 291–298. [DOI] [PubMed] [Google Scholar]
- 2. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011; 29(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009; 10(11): 1037–1044. [DOI] [PubMed] [Google Scholar]
- 4. Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, Regine WF, Weltman E, King VJ, Breneman JC, Sperduto PW, Mehta MP. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002; 53(3): 519–526. [DOI] [PubMed] [Google Scholar]
- 5. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006; 295(21): 2483–2491. [DOI] [PubMed] [Google Scholar]
- 6. Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Kalkanis SN. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96(1): 45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008; 70(2): 510–514. [DOI] [PubMed] [Google Scholar]
- 8. Hazard LJ, Wang B, Skidmore TB, Chern SS, Salter BJ, Jensen RL, Shrieve DC. Conformity of LINAC-based stereotactic radiosurgery using dynamic conformal arcs and micro-multileaf collimator. Int J Radiat Oncol Biol Phys. 2009; 73(2): 562–570. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura JL, Verhey LJ, Smith V, Petti PL, Lamborn KR, Larson DA, Wara WM, McDermott MW, Sneed PK. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys. 2001; 51(5): 1313–1319. [DOI] [PubMed] [Google Scholar]
- 10. Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993; 27(5): 1231–1239. [DOI] [PubMed] [Google Scholar]
- 11. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322(8): 494–500. [DOI] [PubMed] [Google Scholar]
- 12. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998; 280(17): 1485–1489. [DOI] [PubMed] [Google Scholar]
- 13. Rades D, Hornung D, Veninga T, Schild SE, Gliemroth J. Single brain metastasis: radiosurgery alone compared with radiosurgery plus up-front whole-brain radiotherapy. Cancer. 2012; 118(11): 2980–2985. [DOI] [PubMed] [Google Scholar]
- 14. Roos DE, Wirth A, Burmeister BH, Spry NA, Drummond KJ, Beresford JA, McClure BE. Whole brain irradiation following surgery or radiosurgery for solitary brain metastases: mature results of a prematurely closed randomized Trans-Tasman Radiation Oncology Group trial (TROG 98.05). Radiother Oncol. 2006; 80(3): 318–322. [DOI] [PubMed] [Google Scholar]
- 15. Clarke JW, Register S, McGregor JM, Grecula JC, Mayr NA, Wang JZ, Li K, Gupta N, Kendra KL, Olencki TE, Cavaliere R, Sarkar A, Lo SS. Stereotactic radiosurgery with or without whole brain radiotherapy for patients with a single radioresistant brain metastasis. Am J Clin Oncol. 2010; 33(1): 70–74. [DOI] [PubMed] [Google Scholar]
- 16. B Li, J Yu, Suntharalingam M, Kennedy AS, Amin PP, Chen Z, Yin R, Guo S, Han T, Wang Y, Yu N, Song G, Wang L. Comparison of three treatment options for single brain metastasis from lung cancer. Int J Cancer. 2000; 90(1): 37–45. [PubMed] [Google Scholar]
- 17. Harwood AR, Cummings BJ. Radiotherapy for malignant melanoma: a re-appraisal. Cancer Treat Rev. 1981; 8(4): 271–282. [DOI] [PubMed] [Google Scholar]
- 18. Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983; 1(4): 313–317. [DOI] [PubMed] [Google Scholar]
- 19. Choi KN, Withers HR, Rotman M. Intracranial metastases from melanoma. Clinical features and treatment by accelerated fractionation. Cancer. 1985; 56(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 20. Chang EL, Selek U, Hassenbusch SJ, 3rd, Maor MH, Allen PK, Mahajan A, Sawaya R, Woo SY. Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery. 2005; 56(5): 936-945 [PubMed] [Google Scholar]
- 21. Gaudy-Marqueste C, Regis JM, Muracciole X, Laurans R, Richard MA, Bonerandi JJ, Grob JJ. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006; 65(3): 809–816. [DOI] [PubMed] [Google Scholar]
- 22. Noel G, Valery CA, Boisserie G, Cornu P, Hasboun D, Marc Simon J, Tep B, Ledu D, Delattre JY, Marsault C, Baillet F, Mazeron JJ. LINAC radiosurgery for brain metastasis of renal cell carcinoma. Urol Oncol. 2004; 22(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 23. Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003; 98(2): 342–349. [DOI] [PubMed] [Google Scholar]
- 24. Mori Y, Kondziolka D, Flickinger JC, Logan T, Lunsford LD. Stereotactic radiosurgery for brain metastasis from renal cell carcinoma. Cancer. 1998; 83(2): 344–353. [DOI] [PubMed] [Google Scholar]
- 25. Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006; 104(6): 907–912. [DOI] [PubMed] [Google Scholar]
- 26. Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009; 23(2): 170–178. [DOI] [PubMed] [Google Scholar]
- 27. Schomas DA, Roeske JC, MacDonald RL, Sweeney PJ, Mehta N, Mundt AJ. Predictors of tumor control in patients treated with linac-based stereotactic radiosurgery for metastatic disease to the brain. Am J Clin Oncol. 2005; 28(2): 180–187. [DOI] [PubMed] [Google Scholar]
- 28. Noël G, Simon JM, Valery CA, Cornu P, Boisserie G, Hasboun D. Radiosurgery for brain metastasis: impact of CTV on local control. Radiother Oncol. 2003; 68(1): 15–21. [DOI] [PubMed] [Google Scholar]
- 29. Baumert BG, Rutten I, Dehing-Oberije C, Twijnstra A, Dirx MJ, Debougnoux-Huppertz RM, Lambin P, Kubat B. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2006; 66(1): 187–194. [DOI] [PubMed] [Google Scholar]
- 30. Nataf F, Schlienger M, Liu Z, Foulquier JN, Grès B, Orthuon A, Vannetzel JM, Escudier B, Meder JF, Roux FX, Touboul E. Radiosurgery with or without A 2-mm margin for 93 single brain metastases. Int J Radiat Oncol Biol Phys. 2008; 70(3): 766–772. [DOI] [PubMed] [Google Scholar]