Abstract
The acquisition of high-quality, anatomic images is essential for the accurate delineation of tumor volumes and critical structures used for stereotactic radiosurgery (SRS) treatment planning. This study investigates the effect of CT slice thickness and field of view (FOV), i.e., longitudinal and axial CT resolution, on volume delineation and treatment planning in SRS and suggests optimal CT acquisition parameters for brain SRS simulation. Optimization of such parameters will maximize clinical efficacy, alter data storage requirements, reduce dosimetric uncertainties, and may ultimately facilitate more favorable clinical outcomes. Changes in the extent, shape and the absolute volume of the GTV were recorded when the longitudinal and axial CT resolution were modified. These changes ultimately impacted the PTV dose coverage. Reducing CT slice thickness from 2mm to 1mm resulted in an average decrease of 8.6%±13.9% (max=52.2%) and 3.0 %±4.3% (max=13.1%) in PTV Dmin and PTV D95, respectively. Increasing CT slice thickness from 2mm to 3mm resulted in an average decrease of 10%±9.9% (max=26.8%) and 5.8%±5.8% (max=17.4%) in PTV Dmin and PTV D95, respectively. Similarly, on average, PTV coverage decreased when FOV decreased. The average decrease in PTV Dmin and PTV D95 for a 350cm FOV was 5.2%±7.2% (max=21.4%) and 1.9%±3.2% (max=7.5%), respectively. Decreasing FOV to 250cm yielded similar results with the average decrease of 5.6%±5.0% (max=13.2%) and 1.6%±2.6% (max=6.3%) in PTV Dmin and PTV D95, respectively. These results suggest that the slice thickness and FOV of CT images affect target delineation and may potentially compromise the quality of the target coverage.
Keywords: stereotactic radiotherapy, simulation, CT resolution, slice thickness, pixel size, treatment planning
1. INTRODUCTION
The therapeutic utility of three dimensional (3D) imaging in radiation therapy depends on the acquisition of high–quality, anatomic images. Computed tomography (CT) is the most commonly used imaging modality for the acquisition of patient data required for complex treatment planning in modern radiotherapy treatments. Recent developments in CT technology have led to the production of multi-slice, high-resolution scanners capable of generating anatomical images of superior quality. Improvements in spatial resolution minimize the partial volume effect, enhance the quality of 3D images and improve the visualization of target and critical structure volumes.
Uncertainty in radiotherapy treatment planning rests largely in the interpretation of tumor volumes and critical structures on CT-based images. This uncertainty, coupled with continued increases in the complexity of radiotherapy treatment techniques, emphasizes the need for the most accurate anatomical information about the full extent of the tumor volume and surrounding critical structures. Such a need is particularly relevant in the case of stereotactic radiosurgery (SRS), which involves the delivery of high-dose, precisely targeted radiation. In the case of SRS treatments, even small inconsistencies in target volume delineation may result in large dosimetric consequences, which may ultimately compromise the probability of tumor control and increase the probability of normal tissue complications. Avoiding such consequences further emphasizes the need for accuracy and precision in gross tumor volume (GTV) delineation.
The dependence of tumor delineation on CT slice thickness affects both treatment planning and patient setup [1]. Murphy et al showed that the precision of head localization in frameless radiosurgery improves by a factor of two when the CT slice thickness is reduced from 3 to 1.5 mm [2]. Previous studies have devised recommendations on optimum CT acquisition parameters based on tumor size and treatment technique. For 3D conformal brain treatments, a CT slice thickness of 2.5 mm was recommended by Prabhakar et al for tumor volumes < 25 cc. For tumors larger than 25 cc, a slice thickness of 5 mm was shown to be sufficient [3]. For stereotactic brain irradiation, a slice thickness of 2 mm was suggested by Jacob et al [4]. The studies mentioned have concluded that these acquisition parameters reduce the effect of partial volume averaging and improve the uncertainty in target volume and critical structure delineation.
This study investigates the effect of longitudinal CT resolution and pixel size on target delineation and treatment planning in SRS and suggests optimal CT acquisition parameters for SRS simulation.
2. MATERIALS AND METHODS
Ten patients undergoing SRS for various malignancies were evaluated on this retrospective study, which was approved by the institutional review board. Because some patients had multiple treatment sites, a total of 14 intracranial lesions, ranging from 0.15-33.86 cubic centimeters (cc), were included in the analysis. Eight of these 14 lesions were studied to evaluate the influence of longitudinal CT resolution on the delineation of gross tumor volume (GTV). Six of the 14 total lesions were evaluated to determine the variation in GTV definition with changes in FOV, i.e., pixel sizes.
2.1 Patient Data Acquisition
All patient data was acquired at the time of CT simulation using a dedicated 16-slice wide-bore CT scanner (Brilliance Big Bore, Philips Healthcare, Bothell WA). CT scanning was performed in accordance with the standard clinical protocol for obtaining 3D anatomic data to be used for SRS treatment planning. The standard clinical CT acquisition parameters at our institution consist of 2 mm slice thickness and a standard FOV (450-600 mm). The finest collimator setting (16 x 0.75 mm) was selected to facilitate the reconstruction of the data using a range of various parameters of interest, including slice thickness and pixel size. Longitudinal (z-direction) CT resolution is defined by the reconstructed CT slice thickness. For this portion of the study, image sets were retrospectively reconstructed with 1 mm (CT_1mm), 2 mm (CT_2mm) and 3 mm (CT_3mm) slice thicknesses. Pixel size is determined by the magnitude of the selected scan field of view (FOV). For this part of the investigation, image sets were retrospectively reconstructed with a standard 450-600 mm (CT_std), 350 mm (CT_350) and 250 mm (CT_ 250) scan FOV. In this study, CT_2mm and CT_std represent the designated reference image sets, which are consistent with our standard clinical protocol.
2.2 Phantom Study
In addition to the patient component, a phantom component was also included in this investigation. Measurements were carried out using the Lucy® phantom (Lucy® 3-D QA Phantom, Standard Imaging, Middleton, WI) to provide a method to determine the imaging parameters best suited for the most accurate volume definition. The Lucy Phantom is a high-precision, spherical phantom intended for performing a multitude of quality assurance tests specific to SRS. It is designed to hold a variety of accessories and imaging markers used to comprehensively evaluate each step of the SRS imaging and treatment process. The CT volume accessory contains three irregularly shaped, air-filled targets. The volumes and sizes of the targets are known and are explicitly stated as 250, 750 and 1750 mm3 for the small, medium and large targets, respectively. Comparing contoured volumes (measured in our treatment planning system) against these known volumes provides a method to quantify how precisely the images represent the true dimensions of the structure.
2.3 Patient and phantom volume delineation
Following reconstruction, all image sets were transferred via DICOM to the Eclipse treatment planning system (v8.6, Varian Medical Systems, Palo Alto, CA). The same physician contoured the target volumes on the reconstructed CT_1mm, CT_2mm and CT_3mm image sets and the CT_std, CT_350 and CT_250 image sets. In adherence with routine clinical practice, the CT image set was registered with pre-treatment magnetic resonance (MR) images and the initial gross tumor volume (GTV) was determined on MR image set. This initial GTV was copied to the reconstructed CT image sets. The GTV was then modified based on clinical judgment, taking into account the change in anatomical data on each reconstructed CT image set. In order to quantify intra-observer variation, on each image set, each GTV was contoured 3 times, on 3 separate days, without reference to the previously contoured GTV. The volume (in cc) was calculated in Eclipse for each GTV structure drawn.
The small, medium and large phantom targets were delineated on all scans by a single observer. For contouring purposes, the same window level setting was applied to all CT image sets. Each target was contoured a total of 3 times to evaluate intra-observer variability. The computation of target volume was carried out using the treatment planning system.
On each reconstructed CT image set, the average volume (n=3) was calculated for each GTV. For the CT slice thickness component of this study, the average volumes of GTV_1mm and GTV_3mm were compared with the standard GTV_2mm. Similarly, for the pixel size component of this study, the average volumes of the GTV_350 and GTV_250 were compared with the GTV_std. The percent difference was calculated between the GTV of interest, GTVx, and the reference GTV, GTVref:
(1).
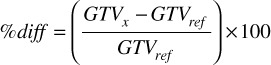
Additionally, the Dice similarity coefficient (DSC) was calculated to measure the spatial overlap between two GTVs. DSC takes into account how both the shape and the location of the GTV are altered when the resolution of the image is modified [5].
(2).

DSC values range from 0 to 1, where a value of 0 indicates lack of any overlap and 1 signifies complete overlap. For this study, the convention DSC_x&y is used to represent the DSC value calculated for GTVx and GTVy. Consistent with the literature, a threshold DSC value of 0.7 was used to represent two volumes with good overlap [5].
2.4 Variation in GTV extent
Geometric analysis of the GTV included an evaluation of the maximum extent of the tumor volume in the superior-inferior (S-I), anterior-posterior (A-P) and left-right (L-R) directions. The maximum extent was determined by using the Eclipse treatment planning system. This was achieved by centering an A-P field and a lateral field on the GTV and automatically fitting the X and Y jaws to the boundaries of the 3-D structure. The S-I, A-P and L-R dimensions were indicated by the Y and X-jaw settings for the lateral field and the X-jaw setting for the A-P field, respectively.
The mean extent in each dimension was calculated for each GTV on a single image set (n=3). The mean extent of the tumor volume was then averaged for all image sets. Maximum positive and negative deviations around this mean were calculated to determine any directional dependence.
2.5 Variation in GTV location
The centroid position of each GTV was determined and defined as an (x,y,z) coordinate within the Eclipse planning coordinate system. Because all GTVs were contoured 3 times per image set, the average centroid position was calculated for each GTV. The distance between the average centroid of interest (x0, y0, z0) and the average reference centroid (xr, yr, zr) was computed as follows:
Using Eq. 3, the distances from the GTV_2mm average centroid position to the GTV_3mm and GTV_1mm average centroid position were calculated. Similarly, for pixel size GTVs, the distances between the GTV_std average centroid position and the GTV_350 or GTV_250 average centroid positions were also calculated. This difference in average centroid position indicates a shift in isocenter coordinates.
(3).

2.6 Dosimetric analysis
Prior to planning, the GTVs were expanded with a 2 mm margin to generate a planning tumor volume (PTV). SRS treatment plans were created and optimized on the reference image sets using the Eclipse analytical anisotropic algorithm (AAA). The reference plans were then copied and superimposed onto CT_1mm and CT_3mm, for the CT slice thickness component of this study, and onto CT_250 and CT_350 for the pixel size component of this study. The dose was recalculated on the new image sets and a dosimetric evaluation of the GTV and PTV was performed. The dose volume histogram (DVH) was used to evaluate the impact of slice thickness and pixel value on dose coverage. The prescription dose covering 95% (D95) of the PTV_2mm and GTV_2mm was compared to the D95 of the PTV_3mm and PTV_1mm and GTV_3mm and GTV_1mm, respectively.
2.7 Assessment of DRR image quality
Digitally reconstructed radiographs (DRRs) were generated for each reconstructed image set. DRRs were exported from Eclipse via a DICOM filter to Image J software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997-2008) for analysis. The DRR image quality was qualitatively assessed based on the presence of image artifacts, the sharpness of anatomical edges and the ability to discern fine details in the vertebrae and skull. In order to define the regions of greatest difference in image quality, Image J was utilized to generate subtraction images. DRR_2mm was subtracted from DRR_1mm and DRR_3mm was subtracted from DRR_2mm, thereby providing a more quantitative comparison of image quality.
2.8 Statistical Analysis
In order to evaluate the clinical impact of the selection of SRS CT simulation parameters, the GTVs and PTVs contoured on each image set were compared to those drawn on images acquired with our institutional standard longitudinal and axial resolution, i.e., 2 mm slice thickness and 450-600 mm FOV. A two-tailed t-test was performed to test the statistical significance of the geometric and dosimetric differences in GTVs and PTVs. Statistical significance was indicated by p values < 0.05.
3. RESULTS
3.1 Phantom results
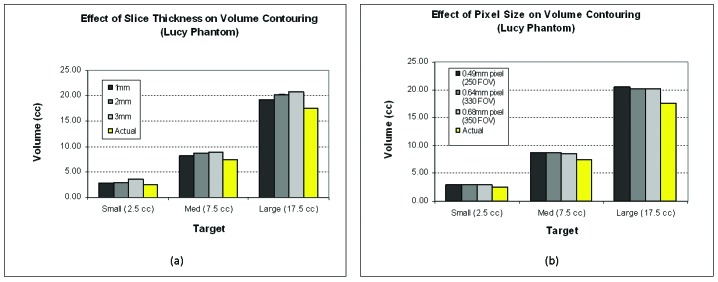
An overestimation in volume was observed for the small, medium and large phantom targets on the reconstructed 1, 2 and 3 mm CT image sets. The magnitude of overestimation was proportional to the reconstructed slice thickness, as displayed in Figure 1a. The overall average percent difference between the measured volumes and the true volumes was 11.4 ± 2.2%, 16.9 ± 2.1% and 26.1 ± 11.4% for CT_1mm, CT_2mm and CT_3mm, respectively. The discrepancy in measured versus true volume increased with decreasing target volume. The percent difference was approximately 15% for the medium and large volumes and 25% for the small volume.
Figure 1.
Phantom volume analysis. Phantom analysis of the effect of slice thickness (a) and pixel size (b) on target delineation. The average measured volumes (n=3) are displayed for the small (2.5 cc), medium (7.5 cc) and large targets (17.5 cc) and are compared to the true target volumes.
For the FOV analysis, the trend in percent difference in volume as a function of FOV was similar to that seen in the slice thickness analysis described above. Figure 1b, shows that all of the measured target volumes were larger than the true target volumes for each of the FOV sizes studied. The smaller the pixel size, the larger the difference in volume when compared to the actual target volume. Targets contoured on the CT_std, CT_350 and CT_250 image sets were overestimated by 16.9%, 16.5% and 18.0%, respectively. As in the slice thickness component of this study, the smallest target was observed to have the largest average percent difference in volume – 20% for the small structure and approximately 15% for both the medium and large structures.
3.2 Change in GTV volume with CT slice thickness
In the slice thickness portion of this study, GTV volumes ranged from 0.28 cc to 33.86 cc. Table 1 lists each of the GTVs and their corresponding volumes, as measured on CT image sets of various reconstructed slice thicknesses. The effect of slice thickness on GTV definition is represented visually in Figure 2. GTV_1mm is displayed in green, the GTV_2mm in blue and GTV_3mm in red. When the GTV_1mm and GTV_3mm are compared to the standard, the most notable variation in GTV contour can be seen in the superior and inferior direction.
Table 1.
A list of GTV volumes included in the slice thickness study. The average and SD of the GTV volume for each slice thickness (n=3) is a measure of intraobserver variability. The overall average and SD of the GTV volume is calculated over all slice thicknesses.
| GTV Volumes: Slice Thickness Study | ||||||||
| GTV # | Ave Vol GTV_1mm (cc) | SD GTV_1mm (n=3) | Ave Vol GTV_2mm (cc) | SD GTV_2mm (n=3) | Ave Vol GTV_3mm (cc) | SD GTV_3mm (n=3) | Overall Ave Vol (cc) | Overall SD |
| 1 | 39.73 | 1.61 | 29.69 | 3.09 | 32.18 | 3.29 | 33.86 | 5.23 |
| 2 | 8.93 | 1.19 | 8.57 | 0.17 | 8.95 | 0.56 | 8.82 | 0.22 |
| 3 | 13.61 | 0.92 | 13.35 | 0.75 | 14.66 | 0.47 | 13.87 | 0.70 |
| 4 | 2.54 | 0.19 | 2.60 | 0.14 | 3.44 | 0.33 | 2.86 | 0.50 |
| 5 | 0.39 | 0.08 | 0.42 | 0.15 | 0.61 | 0.25 | 0.47 | 0.12 |
| 6 | 0.28 | 0.05 | 0.28 | 0.08 | 0.30 | 0.11 | 0.28 | 0.01 |
| 7 | 0.30 | 0.02 | 0.38 | 0.10 | 0.43 | 0.06 | 0.37 | 0.07 |
| 8 | 2.29 | 0.12 | 2.38 | 0.27 | 2.57 | 0.97 | 2.41 | 0.14 |
| 9 | 1.50 | 0.05 | 1.32 | 0.05 | 1.35 | 0.35 | 1.39 | 0.10 |
| 10 | 4.11 | 0.35 | 4.69 | 0.48 | 4.17 | 0.37 | 4.32 | 0.32 |
| 11 | 9.30 | 0.29 | 9.94 | 0.25 | 9.46 | 0.34 | 9.57 | 0.33 |
| 12 | 12.49 | 0.50 | 11.73 | 0.36 | 15.09 | 0.49 | 13.10 | 1.76 |
Figure 2.
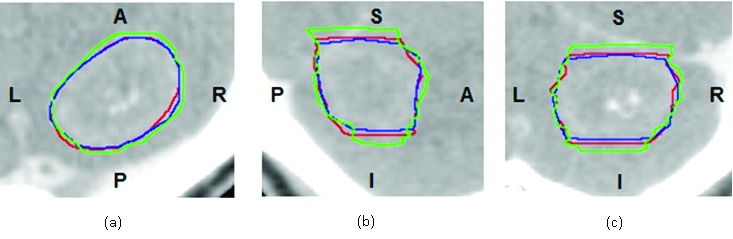
GTV_1mm, GTV_2mm and GTV_3mm contour comparison. Overlay of GTV_1mm (green), GTV_2mm (red) and GTV_3mm (blue) contours in transverse (a), sagittal (b) and coronal (c) planes. The variation in target delineation due to differing CT slice thicknesses is shown.
While no relationship was observed between change in absolute GTV volume and slice thickness, intra-observer variability was shown to be dependent on both the GTV volume and CT slice thickness. For instance, while the average standard deviations of the 1mm ( 0.44 cc) and 2mm (0.49 cc) volumes were comparable, the average standard deviation of the 3mm volumes was significantly larger (p=0.05) at 0.63 cc. In Figure 4a, the average GTV volume for all image sets is plotted as a function of the standard deviation in GTV volume for all image sets, displaying the increased slice thickness sensitivity for larger volumes. In other words, the inter-slice variability increases with larger CT slice thicknesses and larger GTV volumes.
Figure 4.

Slice thickness and pixel size sensitivity of contoured volumes. The overall standard deviation of the measured GTV volume for all slice thicknesses (a) and pixel sizes (b) is plotted as a function of volume. These figures represent the inter- slice and inter-pixel variability, which is shown to increase with increasing GTV volume.
The GTV volumes measured on CT_1mm and CT_3mm image sets were compared to the designated standard, i.e., GTV_2mm volumes (contoured on the CT_2mm image sets). The percent difference in measured GTV volume, displayed graphically in Figure 5a, ranged from 20.9% to 33.8% for GTV_1mm (0.8% ± 13.8%) and 11.2% to 44.4% (11.9% ± 15.8%) for GTV_3mm. An increase in the average percent difference was observed when GTV_2mm was compared to GTV_3mm as opposed to GTV_1mm. The average percent difference in volume is 11% larger when GTV_2mm is compared to the GTV_3mm. The GTV_1mm percent differences fluctuate around 0%, with a majority (~67%) within ± 10%. However, the GTV_3mm percent differences are predominantly positive, implying that contouring on the CT_3mm leads to an overestimation in GTV.
Figure 5.

Percent difference in absolute GTV volume for the slice thickness and FOV investigations. The GTV_1mm and GTV_3mm volumes were compared to the GTV_2mm volume and a percent difference was calculated (a). Similarly, the GTV_350 and GTV_250 volumes were compared to the GTV_std volume and a percent difference was calculated (b). A negative percent difference implies that the volume was smaller in relation to the GTV_2mm or GTV_std volume.
DSC values for the 1 mm and 2 mm volumes (DSC_1&2) ranged from 0.69-0.94 (0.82 ± 0.08), and DSC values for 3mm and 2 mm volumes (DSC_3&2) from 0.71-0.9 (0.81 ± 0.07). As displayed in Table 2, only one of the DSC_1&2 and none of the DSC_ 3 &2 values fell below the 0.7 threshold. This means that the 1 mm and 2 mm volumes and the 3 mm and 2 mm volumes had good overlap, which implies that the spatial variation in GTVs with change in slice thickness is not substantial. The difference in average DSC_1&2 and DSC_3&2 was 0.1.
Table 2.
Dice similarity coefficient for slice thickness volumes and FOV volumes. DSC values range from 0 to1 and are a measure of special overlap between two GTVs. In this study, DSC ≥ 0.7 indicate two volumes with good overlap.
| DSC for Slice Thickness and FOV GTVs | ||
| GTV # | DSC_1&2 | DSC_3&2 |
| 1 | 0.84 | 0.87 |
| 2 | 0.89 | 0.93 |
| 3 | 0.94 | 0.91 |
| 4 | 0.69 | 0.76 |
| 5 | 0.69 | 0.71 |
| 6 | 0.80 | 0.74 |
| 7 | 0.73 | 0.72 |
| 8 | 0.88 | 0.79 |
| 9 | 0.86 | 0.78 |
| 10 | 0.85 | 0.86 |
| 11 | 0.88 | 0.87 |
| 12 | 0.84 | 0.81 |
| Average | 0.82 | 0.81 |
| SD | 0.08 | 0.07 |
| GTV # | DSC_std&350 | DSC_std&250 |
| 1 | 0.85 | 0.87 |
| 2 | 0.81 | 0.86 |
| 3 | 0.77 | 0.77 |
| 4 | 0.85 | 0.78 |
| 5 | 0.65 | 0.67 |
| 6 | 0.50 | 0.58 |
| 7 | 0.81 | 0.86 |
| 8 | 0.86 | 0.82 |
| Average | 0.76 | 0.78 |
| SD | 0.12 | 0.10 |
3.3 Change in GTV volume with pixel size
GTV volumes ranged from 0.15 to 15.39 cc in the field of view study. Figure 3 shows that decreasing the CT FOV, i.e., pixel size, resulted in noticeably different GTV contours. Qualitatively, it can be observed that the delineation of the lateral extent of the GTV caused the largest inconsistency in contouring. The variation in measured GTV volume across all 3 image sets (inter-pixel variation) increased with increasing GTV volume. This is displayed in Figure 4b, which depicts the influence of pixel size on target delineation, particularly for larger volumes. Intra-observer variability, on the other hand, was not shown to correlate with pixel size.
Figure 3.

GTV_350, GTV_250 and GTV_std contour comparison. Overlay of GTV_350 (green), GTV_std (red) and GTV_250 (blue) contours in transverse (a), sagittal (b) and coronal (c) planes. The variation in target delineation due to differing CT pixel size is shown.
The percent differences in volume when GTV_350mm and GTV_250mm are compared to the clinically-treated GTV_Std are shown in Figure 5b. The percent differences for GTV_350cm and GTV_250cm ranged from 42.11% to 25.22% (4.0% ±22.5%), and from 21.05% to 49.87% (20.1%±21.5%), respectively. These are listed in Table 3. A significant increase in the volume of the GTV was seen when the FOV was decreased from the standard FOV to 350 and 250 cm. The average increase in GTV_350 volume was 4.0% (p=0.004) and the average increase in GTV_250 volume was 20.1% (p~0.0).
Table 3.
A list of GTV volumes included in the FOV (pixel size) study. The average and SD of the GTV volume for each FOV (n=3) is a measure of intra-observer variability. The overall average and SD of the GTV volume is calculated over all FOVs.
| GTV Volumes: FOV Study | ||||||||
| GTV # | Ave Vol GTV_std (cc) | SD GTV_std (n=3) | Ave Vol GTV_350 (cc) | SD GTV_350 (n=3) | Ave Vol GTV_250 (cc) | SD GTV_250 (n=3) | Overall Ave Vol (cc) | Overall SD |
| 1 | 4.69 | 0.48 | 4.60 | 0.26 | 6.71 | 0.73 | 5.33 | 1.20 |
| 2 | 2.38 | 0.27 | 2.82 | 0.07 | 2.75 | 0.11 | 2.65 | 0.24 |
| 3 | 1.32 | 0.05 | 1.64 | 0.05 | 1.97 | 0.09 | 1.64 | 0.33 |
| 4 | 9.94 | 0.25 | 10.88 | 0.11 | 12.18 | 0.49 | 11.00 | 1.12 |
| 5 | 13.35 | 0.75 | 16.71 | 0.14 | 16.10 | 0.68 | 15.39 | 1.79 |
| 6 | 11.73 | 0.36 | 12.91 | 0.23 | 12.91 | 0.23 | 12.52 | 0.68 |
| 7 | 0.25 | 0.02 | 0.22 | 0.03 | 0.30 | 0.04 | 0.26 | 0.04 |
| 8 | 0.19 | 0.01 | 0.11 | 0.00 | 0.15 | 0.01 | 0.15 | 0.04 |
DSC_std&350 and DSC_std&250 ranged from 0.5-0.86 (0.76 ± 0.12) and from 0.58-0.87 (0.78 ± 0.10), respectively, as shown in Table 2. Because the average DSC values calculated for FOV volumes was > 0.7, these volumes are thought to have good overlap. Additionally, the small difference in average DSC_std&350 and DSC_std&250 implies that the degree of structural overlap was not affected by change in pixel size.
3.4 Directional dependence in GTV delineation
Table 4 exhibits the variation in maximum GTV extent in the left-right (L-R), superior-inferior (S-I) and anterior-posterior (A-P) dimensions. The deviation in volume extent is expressed as a coefficient of variation (COV). For the slice thickness component of this study, the average COV was largest in the superior-inferior dimension. The average COV in the S-I dimension was 0.13, as compared to 0.07 and 0.08, the average COV in the L-R and A-P dimensions, respectively.
Directional dependence in GTV delineation was not observed in the FOV component of this study. The magnitude of variation in maximum GTV extent was similar in all three dimensions. The coefficient of variation in the L-R, A-P and S-I dimensions was 0.06, 0.08 and 0.07, respectively.
3.5 Isocenter shift with slice thickness and pixel size
Modifications in the shape and location of the target volume due to differing imaging parameters resulted generally in sub-millimeter variations in the coordinates of the treatment isocenter. In the slice thickness component of this study, the calculated shifts between the treated isocenter (located at centroid of GTV_2mm) and the isocenter associated with GTV_1mm and GTV_3mm ranged from 0.1 to 2.5 mm (0.8±0.7 mm) and from 0.2 to 1.9 mm (0.7±0.5 mm), respectively. For the FOV component of this study, the range of GTV_1mm shifts was 0.2 to 1.5 mm (mean 0.6 mm), and similarly, the range of GTV_3mm shifts was 0.1 to 1.6 mm (mean 0.6 mm). Our results indicate that neither reconstructed CT slice thickness variation (p=0.65) nor pixel size variation (p=0.44) had a statistically significant impact on isocenter location. However, in the context of SRS treatment delivery, even submillimeter isocenter may be of importance.
Table 4.
The coefficient of variation of GTV extent in each dimension.
| Variation in GTV dimensions | |||
| SLICE THICKNESS | |||
| GTV# | Coefficient of Variation | ||
| Left-Right | Ant-Post | Sup-Inf | |
| 1 | 0.12 | 0.03 | 0.08 |
| 2 | 0.04 | 0.07 | 0.11 |
| 3 | 0.03 | 0.02 | 0.04 |
| 4 | 0.03 | 0.08 | 0.19 |
| 5 | 0.06 | 0.16 | 0.25 |
| 6 | 0.10 | 0.13 | 0.19 |
| 7 | 0.09 | 0.11 | 0.19 |
| 8 | 0.07 | 0.05 | 0.16 |
| 9 | 0.14 | 0.06 | 0.15 |
| 10 | 0.15 | 0.04 | 0.07 |
| 11 | 0.03 | 0.06 | 0.08 |
| 12 | 0.02 | 0.07 | 0.05 |
| Average | 0.07 | 0.08 | 0.13 |
| FIELD OF VIEW | |||
| GTV# | Coefficient of Variation | ||
| Left-Right | Ant-Post | Sup-Inf | |
| 1 | 0.07 | 0.03 | 0.01 |
| 2 | 0.07 | 0.07 | 0.07 |
| 3 | 0.05 | 0.07 | 0.07 |
| 4 | 0.10 | 0.05 | 0.03 |
| 5 | 0.04 | 0.10 | 0.19 |
| 6 | 0.08 | 0.16 | 0.06 |
| 7 | 0.06 | 0.06 | 0.07 |
| 8 | 0.04 | 0.07 | 0.02 |
| Average | 0.06 | 0.08 | 0.07 |
3.6 Change in GTV and PTV dose coverage with CT slice thickness
The influence of CT slice thickness on SRS treatment planning can be observed in Figure 6. The dose volume histograms (DVHs) for GTV_1mm, GTV_2mm and GTV_3mm (Figure 6a) and for PTV_1mm, PTV_2mm and PTV_3mm (Figure 6b) for GTV # 9 were overlaid. Qualitative analysis shows a high degree of similarity between the GTV_1mm and GTV_2mm DVHs, and also between the PTV_1mm and PTV_2mm DVHs. However, a decrease in both GTV and PTV coverage was observed as the slice thickness was increased from 2 mm to 3mm. In general, the dosimetric consequences in GTV coverage were exacerbated in the case of PTV coverage.
Figure 6.
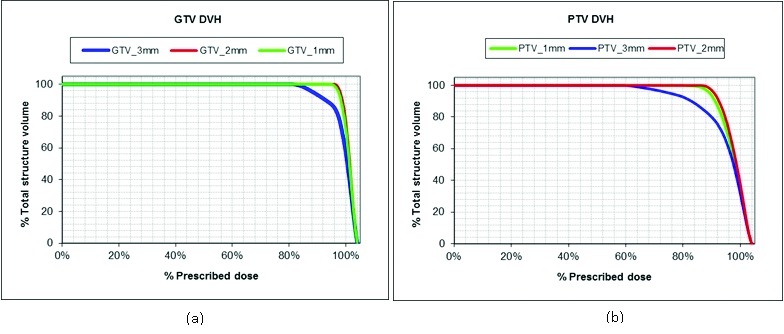
DVH comparison of slice thickness volumes. The overlay of dose volume histograms (DVHs) for GTV_1mm, GTV_2mm and GTV_3mm (a) and for PTV_1mm, PTV_2mm and PTV_3mm (b) for GTV # 9. The difference in DVHs show the dosimetric impact of slice thickness.
A comparative analysis was performed on the minimum target dose and the dose to 95% of the target volume (D95). The percent difference was calculated between the GTV_1mm and the GTV_2mm and between the GTV_3mm and GTV_2mm. In a similar manner, the percent difference was calculated for the PTVs. These results are presented in Table 5. The magnitude of the percent difference in minimum GTV (-4.5% ± 7.5%) and PTV (-10% ± 9.9%) dose was larger when the 3 mm volumes were compared to the 2 mm volumes, versus when the 1mm volumes were compared to the 2mm volumes: average minimum GTV_1mm dose (-3.6% ± 13.8%) and min PTV_1mm dose (8.6% ± 13.9%). When the delivered treatment plan was superimposed onto the 3mm CT image set, a significant decrease in the minimum PTV dose was observed (p = 0.004). While not statistically significant, a similar decrease in minimum GTV dose on the CT_3mm plan was observed (p=0.07). On the other hand, the difference in minimum GTV (p = 0.402) and PTV (p = 0.056) dose was not as pronounced on the CT_1mm plans, therefore suggesting that structures delineated on the 1mm and 2mm image sets are similar.
Table 5.
The difference in GTV and PTV dose coverage with change in slice thickness. The percent difference in minimum GTV and PTV dose and D95 was calculated.
| Change in GTV and PTV dose coverage with varying slice thickness | |||||||||
| GTV # | 1mm % Diff GTV min | 3mm % Diff GTV min | 1mm % Diff GTV D95 | 3mm % Diff GTV D95 | 1mm % Diff PTV min | 3mm % Diff PTV min | 1mm % Diff PTV D95 | 3mm % Diff PTV D95 | |
| 1 | -45.0% | -2.0% | -1.5% | -0.3% | -52.2% | -1.6% | -5.3% | -0.6% | |
| 2 | -3.0% | 0.7% | -0.5% | 0.0% | -5.7% | 2.0% | -2.6% | 0.4% | |
| 3 | -0.3% | -0.3% | 0.0% | -0.6% | -1.1% | -0.4% | 0.1% | -1.1% | |
| 4 | 19.7% | 13.2% | -1.9% | -1.3% | -5.7% | -22.2% | -8.1% | -17.4% | |
| 5 | -1.2% | -10.5% | -0.5% | -3.5% | -5.0% | -18.9% | -2.5% | -6.8% | |
| 6 | -2.0% | -2.4% | -0.5% | -1.5% | -5.8% | -5.4% | -2.7% | -3.2% | |
| 7 | -1.6% | -10.4% | -0.5% | -3.5% | -5.9% | -14.9% | -2.5% | -7.1% | |
| 8 | -0.3% | -9.5% | 0.0% | -3.9% | -3.2% | -18.6% | 0.0% | -8.5% | |
| 9 | -2.4% | -15.2% | -0.5% | -9.5% | -6.7% | -26.8% | -13.1% | -15.4% | |
| 10 | -2.0% | 0.2% | 0.0% | -0.5% | -3.6% | 0.0% | -0.2% | -0.2% | |
| 11 | -4.6% | -4.1% | -0.5% | -2.0% | -8.5% | -4.7% | -2.2% | -5.6% | |
| 12 | -0.8% | -13.1% | 2.8% | 0.0% | 0.4% | -8.6% | 3.2% | -3.8% | |
| AVERAGE | -3.6% | -4.5% | -0.3% | -2.2% | -8.6% | -10.0% | -3.0% | -5.8% | |
| SD | 13.8% | 7.5% | 1.1% | 2.6% | 13.9% | 9.9% | 4.3% | 5.8% | |
D95 was shown to be highly sensitive to the longitudinal resolution of the treatment planning CT. A significant decrease in D95 was observed for the PTV on both the 1mm (p=0.056) and 3mm (p=0.0052) treatment plans. While the GTV target coverage was compromised for both the 1mm and 3mm volumes, this decrease was only significantly different for GTV_3mm (p=0.016)
3.7 Change in GTV and PTV dose coverage with pixel size
Two comparison DVHs (for GTV # 7), shown in Figure 7, illustrate the difference in GTV and PTV dose coverage with change in pixel size. The SRS plans yielded inferior target coverage when superimposed on image sets with decreased FOV as indicated by the more rounded shoulder of the GTV_350 and GTV_250 DVHs. The dosimetric results indicate a similarity between the GTV_250 and GTV_350 structures.
Figure 7.
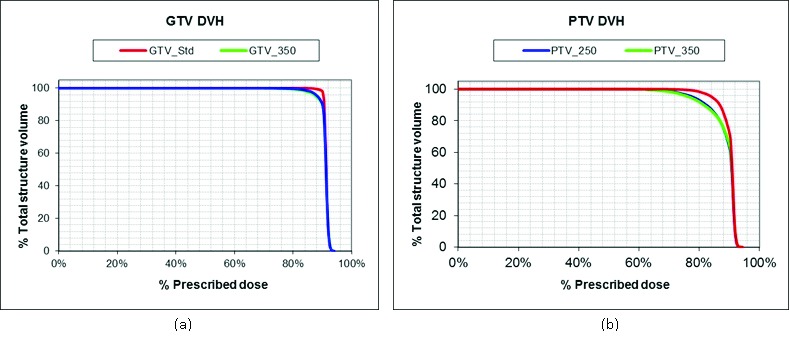
DVH comparison of FOV volumes. The overlay of dose volume histograms (DVHs) for GTV_std, GTV_250 and GTV_350 (a) and for PTV_std, PTV_250 and PTV_350 (Fig. 5b) for GTV # 7. The difference in DVH represents the dosimetric effect of field of view.
The percent differences in the minimum target dose and D95 were calculated (Table 6). The average percent differences in minimum PTV dose were found to be similar for the 350 FOV (5.2% ± 7.2%) and 250 FOV (5.6% ± 5.0%) treatment plans, when compared to the minimum dose for PTV_std plan. Assessment of minimum GTV dose showed similar findings; however, only the decrease in minimum GTV_350 dose was significant (p=0.02).
Table 6.
The difference in GTV and PTV dose coverage with change in FOV. The percent difference in minimum GTV and PTV dose and D95 was calculated.
| Change in GTV and PTV dose coverage with varying FOV | |||||||||
| GTV # | 350 % Diff GTV min | 250 % Diff GTV min | 350 % Diff GTV D95 | 250 % Diff GTV D95 | 350 % Diff PTV min | 250 % Diff PTV min | 350 % Diff PTV D95 | 250 % Diff PTV D95 | |
| 1 | -2.1% | -7.7% | -1.1% | -1.1% | -2.8% | -13.2% | -1.4% | -1.6% | |
| 2 | -3.2% | -0.7% | -0.6% | 0.0% | -4.1% | -0.9% | -0.8% | 0.4% | |
| 3 | -12.5% | -3.8% | -2.5% | -1.5% | -21.4% | -5.1% | -5.2% | -3.1% | |
| 4 | -2.4% | -3.7% | 0.0% | -0.5% | -2.2% | -5.2% | 0.3% | -1.4% | |
| 5 | -4.8% | -18.5% | -2.3% | -10.7% | -5.6% | -7.3% | -2.3% | -3.4% | |
| 6 | 1.1% | 0.8% | 1.1% | 1.1% | 3.7% | 2.5% | 2.5% | 1.6% | |
| 7 | -15.2% | -9.9% | -2.5% | -2.0% | -6.2% | -10.6% | -7.5% | -6.3% | |
| 8 | -3.7% | -5.0% | 0.0% | 2.8% | -3.0% | -5.0% | -0.7% | 0.6% | |
| AVERAGE | -5.3% | -6.1% | -1.0% | -1.5% | -5.2% | -5.6% | -1.9% | -1.6% | |
| SD | 5.6% | 6.1% | 1.4% | 4.0% | 7.2% | 5.0% | 3.2% | 2.6% | |
D95 for the PTV and GTV did not change significantly with pixel size, which contrasts with the change found with slice thickness. The percent differences in D95 for the PTV and GTV were negative and positive—ranging from 7.5% to 2.5% and 6.3% to 1.6% for PTV_350 and PTV_250, respectively and from 2.5% to 1.1% and 10.7% to 2.8% for GTV_350 and GTV_250, respectively. These results suggest that decreasing the FOV improved target coverage for approximately half of the patients. The average percent difference in GTV and PTV D95 did not exceed ± 2%.
3.8 DRR image quality evaluation
The image quality of the DRR is dependent on the spatial resolution of the reference CT image. Figures 8a-c display the DRRs generated from the reconstructed CT image sets of each slice thicknesses (CT_1mm, CT_2mm and CT_3mm). There was a noticeable increase in image artifact with increasing CT slice thickness demonstrated by the presence of a ring artifact at the top of the skull in the DRR_3mm (Figure 8c) which was not observed in the DRR_1mm (Figure 8a). As the longitudinal CT resolution decreased, the ability to discern fine details in bony anatomy decreased, which is most evident in the vertebrae. There was minimal difference in DRR image quality with pixel size, however. A qualitative comparison failed to discern obvious differences between the images.
Figure 8.

DRR image quality comparison. . Figures (a) through (c) display the DRRs generated from the CT_1mm, CT_2mm and CT_3mm image sets, respectively. The DRR image quality is shown to degrade with increase in CT slice thickness.
Subtraction images were generated to identify the regions of greatest difference between the DRRs. Figure 9a shows the difference between the 1 mm DRR and the 2 mm DRR and Figure 9b is the subtraction of the 3mm DRR from the 2 mm DRR. The brighter the pixel value in the subtraction image, the larger the discrepancy between the two images. It can be observed that the magnitude of difference between the 1mm and 3 mm DRR was larger as compared to the difference between the 2mm and 1mm DRR.
Figure 9.
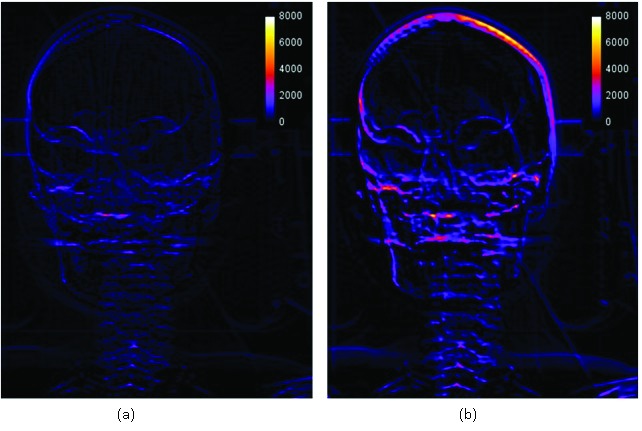
DRR subtraction images. (a) is the DRR_2mm-3mm subtraction image and (b) is the DRR_1mm-2mm subtraction image. Subtraction images provide a quantitative evaluation of the difference in DRR_3mm and DRR_1mm image quality when compared to the DRR_2mm. The presence of brighter pixels indicates regions of largest difference. DRR_2mm and DRR_3mm are of comparable image quality, whereas DRR_2mm and DRR_1mm are notably dissimilar especially at the top of the skull and in the facial bones.
4. DISCUSSION
4.1 General
Other investigators have previously presented the effect of reconstructed CT slice thickness on CTV and organ contouring and dose calculation for 3D conformal and stereotactic treatment planning [1] [3] [4]. However, these prior studies did not investigate slice thicknesses <2mm and failed to include a full dosimetric evaluation. The present study evaluates the influence of longitudinal CT resolution on target delineation and is further strengthened by the inclusion of varying pixel size data. Additionally, this work provides a comprehensive assessment of the dosimetric effect of employing different imaging parameters for SRS simulation.
As the complexity of radiotherapy treatments like SRS continues to grow, high accuracy in target volume delineation becomes most critical. As such, it is essential to determine which imaging techniques facilitate the most accurate target delineation. Based on our findings in the phantom portion of the study, the accuracy of volume measurements was highly dependent upon both the size of the object imaged and the CT slice thickness selected. Our results showed that the discrepancy in measured versus true volume increased with decreasing target volume and the magnitude of overestimation was proportional to the reconstructed slice thickness. Jacob, et. al concluded, based on a preliminary analysis, that a slice thickness of 2mm was appropriate for SRS brain cases [4]. AAPM Report 54, Task Group 42, states that the net uncertainty in target localization and treatment delivery can be improved by 35% by reducing the CT slice thickness from 3mm to 1mm [6]. Therefore, considering the small size of typical SRS target volumes, is it not only necessary but practical to utilize the full potential of modern scanners and acquire CT images with the thinnest slices available?
4.2 Variation in contoured target volume
The results of this study suggest that the imaging parameters selected at the time of CT simulation can influence the clinical interpretation of GTV extent. For both the phantom and patient components of this investigation, more substantial variations in GTV definition resulted from modifying the longitudinal resolution than from modifying the FOV. Partial volume effect contributed in large part to this variation in GTV definition [1]. The larger the slice thickness, the more challenging it was to discern the extent of the GTV, primarily in the cephalad-caudad direction. A slightly larger or smaller cross sectional area of the volume will be depicted on the reconstructed image depending on how much of the target volume is included in the scan thickness [7]. In the phantom study, it was shown that CT_1mm produced the smallest and most accurate volumes across all 3 targets, supporting the idea that the larger the slice thickness, the larger the volume averaging effect. However, a similar trend was not observed in the patient study. This can be attributed to the increased difficulty of differentiating tumor boundaries due to confounding factors such as the location and size of the tumor, the inherent image contrast, and the window-level thresholding--all of which influence the visibility of the tumor boundary. The full extent of the target was much more distinguishable in the phantom scans as the contrast of target medium was sufficiently different than the surrounding medium.
The overall standard deviation of absolute GTV volume for all slice thicknesses and all FOVs represents the inter-slice variation and inter-pixel variation, respectively. The inter-slice and inter-pixel variation significantly exceeded the intra-observer variation with the exception of the GTV_3mm intra-observer variation. These results would suggest that the selection of CT acquisition parameters has a greater impact on delineation of GTV volume than the uncertainty associated with the actual process of contouring the GTV. However, one notable shortcoming of this study is that it does not include the effect of inter-observer variability on GTV delineation, which has been shown to be a considerable factor in the overall uncertainty in radiation treatment planning. In addition, these results may have been influenced by memory bias. Despite the fact that GTV delineation was repeated on separate days, it is possible that the physician retained memory of previous GTV interpretations. Moreover, because of the specific cohort of patients included in this study, a degree of selection bias also undoubtedly plays a role in the data presented herewith.
4.3 GTV Characteristics
Evaluating the change in GTV solely in terms of absolute volume does not provide adequate spatial information about the tumor volume, such as its shape and location. For instance, two objects may have the same absolute volume but radically different shapes. However, the shape of an object largely determines the influence of partial volume effect on that object. Objects, such as spheres, that possess a shape which changes rapidly along the longitudinal axis (Z-direction), will be most altered by the partial volume effect. Objects more cylindrical in nature, however, won’t be as significantly affected [3].
The Dice similarity index afforded a metric to quantify the change in the shape of the GTV and address the effects of morphology. However, a correlation did not exist between DSC and target coverage—which implies that other morphologic aspects of the GTV may have influenced our results. In other words, the unique morphology of the GTV can affect how distinguishable a lesion is on an image.
4.4 Clinical Significance
The evaluation of GTV and PTV dose volume histograms revealed significant dosimetric consequences resulting from variations in GTV. Our dosimetric analysis included superimposing the 2 mm and standard reference plans onto different image sets, essentially defining a new GTV as the target. Our expectation was that those GTVs most dissimilar in volume and shape would have the largest dose difference; however, a direct correlation was not found between tumor coverage and neither DSC nor percent difference in volume. Weltons et.al investigated the impact of inter-observer variations in GTV delineation of brain tumors on 3D conformal treatment planning [8]. While they measured large GTV variations, they found little clinical impact of such variations on the accuracy of treatment due to the 3cm margin used to create the PTV. Therefore, even when the smallest GTV was used for treatment planning, the largest GTV was still covered by the field. However, in our investigation, not only were the applied PTV margins considerably smaller (2 mm) but the treatment plan involved significantly sharper dose gradients—indicating that even small variations in GTV were substantial enough to severely compromise the plan integrity.
Also of clinical relevance was the noticable improvement in DRR image quality achieved by reducing the CT slice thickness to 1 mm. DRRs play a critical role in the verification of patient setup in SRS. Thus, optimizing DRRs by increasing the sharpness of the edges and improving the visibility of specific anatomic structures have the potential to allow for better accuracy in patient positioning.
5. Conclusion
This study emphasizes the critical importance of accurate tumor delineation in stereotactic radiotherapy. CT acquisition parameters, such as slice thickness and field of view, may contribute to uncertainties in target definition. Such uncertainties may ultimately increase the possibility of a geographical miss during treatment delivery, especially in the context of SRS planning, where planning margins are traditionally only a few millimeters and treatments are hypofractionated or only single fraction. Moreover, such effects may have an effect on treatment outcome. While the findings of this study support the need for the highest resolution (longitudinal and axial dimension) scans in SRS planning, further investigation incorporating larger patient populations, is necessary to establish conclusive recommendations on optimal CT simulation parameters used for SRS treatment planning.
REFERENCES
- 1. Berthelet E, Liu M, Truong P, Czaykowski P, Kalach N, C Yu, Patterson K, Currie T, Kristensen W, Kwan W, Moravan V. CT slice index and thickness: Impact on organ contouring in radiation treatment planning for prostate cancer. Journal of Applied Clinical Medical Physics, [S.I.], p. 365-373; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy MJ. The importance of computed tomography slice thickness in radiographic patient positioning for radiosurgery. Med Phys 1999; 26(2): 171–175. [DOI] [PubMed] [Google Scholar]
- 3. Prabhakar R, Ganesh T, Rath G, Julka P, Sridhar P, Joshi R, Thulkar S. Impact of different CT slice thickness on clinical target volume for 3D conformal radiation therapy. Med Dosim 2009; 34(1): 36–41. [DOI] [PubMed] [Google Scholar]
- 4. Jacob V, Kneschaurek P, Dössel O, Schlegel WC, Magjarevic R. Influence of the CT slice thickness on the dose calculation for stereotactic treatment planning. In: World Congress on Medical Physics and Biomedical Engineering, September 7 12, 2009, Munich, Germany. Springer Berlin Heidelberg; 2009: 204-206. [Google Scholar]
- 5. Zou KH, Warfield SK, Bharatha A, Tempany CM, Kraus MR, Haker SJ, Wells III WM, Ferenc JA, Kikinis R. Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol 2004; 11:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schell MC, Bova FJ, Larson DA, Leavitt DD, Lutz WR, Podgorsak EB, A Wu. AAPM Report No. 54: Stereotactic radiosurgery. Report of task group No. 42 of the American Association of Physicists in Medicine 1995.
- 7. Bushberg JT, Seibert A, Leidholdt EM, Boone JM. The essential physics of medical imaging. 2nd ed. Philadelphia (PA): Lippincott Williams and Wilkins; 2001. 371-372. [Google Scholar]
- 8. Weltons C, Menten J, Feron M, Bellon E, Demaerel P, Maes F, Van den Bogaert W, Van der Schueren E. Interobserver variations in gross tumor volume delineation of brain tumors on computed tomography and the impact of magnetic resonance imaging. Radiother Oncol 2001; 60: 49-59. [DOI] [PubMed] [Google Scholar]