Abstract
Purpose
We report outcome of linear accelerator (Linac)-based stereotactic radiosurgery (SRS) for trigeminal neuralgia (TGN) utilizing rigid head frame (RF) and facemask (FM) immobilization.
Method: From November 2008 to October 2012, 48 patients with idiopathic TGN underwent primary SRS by a dedicated Linac. RF immobilization was utilized for 34 patients, and frameless image-guided radiosurgery (IGRS) with FM immobilization was performed in 14 patients. Treatment outcome was assessed by patient interviews with a 7-item questionnaire.
Results
Sub-millimeter targeting accuracy (0.71±0.31 mm) was recorded for frameless IGRS by a novel hidden-target phantom method. With a follow-up of 26 months, significant pain relief was recorded in 43 (89%) patients, including 26 (54%) complete and 17 (35%) partial responses; with a significant reduction of 2.4±1.3 points (p < 0.01) on the 5-point Barrow Neurological Institute pain scale. No significant pain relief difference (p = 0.23) was detected between patients immobilized by RF and FM. Notable pin site problems were reported in 9 (26%) of 34 patients immobilized by RF.
Conclusion
Frameless IGRS with FM immobilization is more patient friendly and can achieve as excellent treatment outcome as with RF immobilization for idiopathic TGN.
Keywords: trigeminal neuralgia, radiosurgery, linear accelerator, targeting accuracy, frameless image-guided radiosurgery.
1. INTRODUCTION
Idiopathic Trigeminal neuralgia (TGN; “Tic douloureux”) is a chronic, frequently disabling, facial pain syndrome that affects approximately 5 per 100,000 people annually, with a slight female predominance (1.74 to 1) [1]. With its exact pathophysiology remains unclear, ephaptic transmission of impulses from light touch to pain fibers is hypothesized to be a mechanism for TGN [2-4].
Treatment options for TGN generally include pharmacological therapy, surgeries and stereotactic radiosurgery (SRS) [5]. Pharmacological therapy is usually the appropriate initial treatment modality for TGN [6]. Surgical procedures for TGN include percutaneous procedures and open brain surgery [7]. Percutaneous procedures generally have a shorter duration of pain relief and are associated with a rather high incidence of sensory dysesthesia [7]. Craniotomy with microvascular decompression (MVD) is a major procedure that has been reported to have a high 64% durable pain control at 10 years [7,8]. Nevertheless, MVD requires general anesthesia and is associated with risks of serious complications such as death and stroke [8].
Stereotactic radiosurgery (SRS) has emerged as an effective treatment for TGN. SRS can be delivered by Gamma Knife (GK) and linear accelerators (Linac)-based SRS machines [5,9]. While rigid head frame (RF) immobilization is required for GK SRS, frameless facemask (FM) immobilization can be used for Linac-based SRS [10]. Many professionals remain apprehensive on the accuracy of frameless image-guided radiosurgery (IGRS) for TGN [11]. The current study reports unique clinical operation and treatment outcome of idiopathic TGN patients treated by a dedicated Linac-based SRS machine. Particularly, we compared treatment outcome between frameless IGRS with FM immobilization and frame-based SRS with RF immobilization.
2. METHODS AND MATERIALS
2.1 Patient Population Characteristics
Between November 2008 and October 2012, 48 patients (27 women and 21 men) with idiopathic TGN underwent primary SRS at our institution. The patient characteristics are listed in Table 1. The median age at SRS was 66 years (range, 49-86). All patients were evaluated and treated by a multidisciplinary SRS team. All patients had failed pharmacological therapy. Among the three divisions of trigeminal nerve, the V2 division, followed by V3 division is the most affected. Most patients had facial pain involving either one or two divisions. Three patients (6%) had atypical facial pain such as dull and constant ache, and burning in contrast to the classic episodic, lancinating, electric shock-like pain. Four patients (8%) had previous invasive procedure, including MVD, glycerol or radiofrequency rhizotomy and balloon compression. Two patients (4%) had previous Botox injections. Four patients (8%) had MS. RF (BrainLAB stereotactic frame) immobilization was utilized for the first 34 patients (71%), and FM (BrainLAB facemask) immobilization was performed in 14 patients (29%) since March 2011 due to a change of institutional policy.
2.2 Radiosurgery Targeting and Quality Assurance
All patients were treated using a dedicated Novalis Tx SRS system (BrainLab AG, Munich, Germany; Varian Medical Systems, Palo Alto, CA), which is equipped with the ExacTrac X-ray system with 6-degree of freedom (DOF) robotic couch for IGRS. Winston–Lutz tests were performed daily and beam alignment to isocenter of our Linac radiosurgery system was maintained to a linear error of less than 0.5 mm [12]. An in-house hidden-target phantom test (Fig. 1) was developed to assure targeting accuracy of frameless IGRS. Briefly, a Rando Head-and-Neck Phantom was modified to allow insertion of a Gafchromic film into its head and have an 18-gauge introducer needle embedded along its longitudinal central axis, leading up to the film (Fig. 1A). The introducer needle is used to guide a stylus to prick-marking a point target on the film. The phantom with the stylus tip served as the point target was immobilized by FM, under CT simulation and planned for a 120-degree single arc SRS treatment using HD120 MLC with a 0.5 cm planning margin. Gafchromic RTQA2 (Ashland Advanced Materials, Bridgewater, NJ, U.S.A) films were cut into dimension of 10 X 5 cm2 and inserted into the phantom daily. At each test, the inserted Gafchromic film was prick-marked a point target, and then received 5 Gy of frameless IGRS (Fig. 1B). After irradiation, each exposed film (see Fig. 1C for examples of exposed films) was visually checked by a physicist and scanned to image using a flatbed color scanner, Epson Expression 10000XL (Seiko Epson Corp, Nagano, Japan). The image was then imported into FilmQA Pro 3.0 software (Ashland Advanced Materials, Bridgewater, NJ, U.S.A) and converted to dose using the pre-established calibration curves. The delivered isocenter was determined by the geometrical center of the 70% isodose line. The distance between the delivered isocenter and planned isocenter (needle prick mark) was then measured. Recalibration was performed before patient treatment when the hidden-target phantom test showed > 1 mm deviation between the delivered isocenter and planned isocenter.
Table 1.
Pretreatment patient characteristics
| Characteristics | No. of patients (%) |
| No. of patients | 48 |
| Median | 66 |
| Range | 49 – 86 |
| Gender | |
| Male | 21 (42%) |
| Female | 27 (58%) |
| Side | |
| Right | 29 (60%) |
| Left | 19 (40%) |
| Involved Division (es) | |
| V1 | 16 (33%) |
| V2 | 39 (81%) |
| V3 | 27 (56%) |
| 1 division | 21 (44%) |
| 2 divisions | 20 (42%) |
| 3 divisions | 7 (14%) |
| Pain type | |
| Typical | 45 (94%) |
| Atypical | 3 (6%) |
| Previous invasive procedure | 4 (8%) |
| History of multiple sclerosis (MS) | 4 (8%) |
Figure 1.
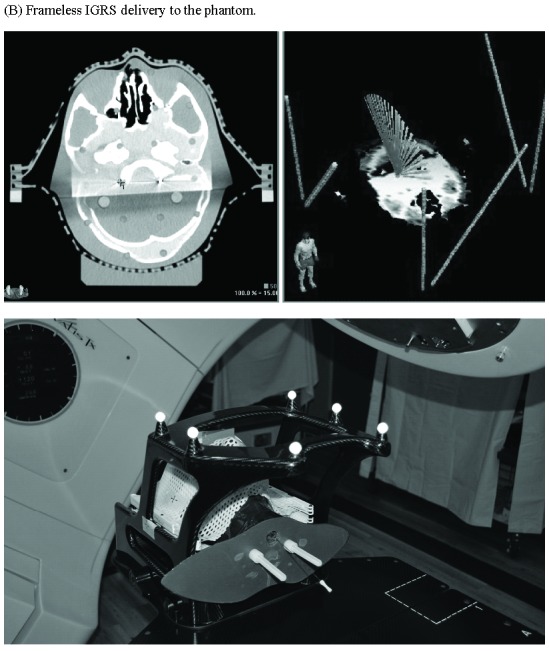

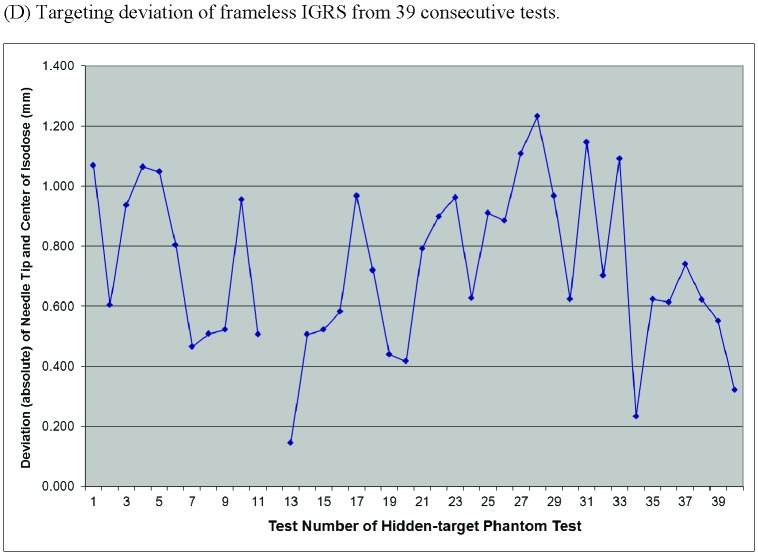
Targeting accuracy of frameless image-guided radiosurgery (IGRS) assessed by a hidden-target phantom test. (A) The modified Rando Head & Neck Phantom. (B) Frameless IGRS delivery to the phantom. (C) Examples of exposed Gafchromic films from the test. (D) Targeting deviations of 39 consecutive tests by measuring the absolute distance between planned isocenter (pricked point target) and delivered isocenter (center of the dark spot generated by radiation).
2.3 Radiosurgery Treatment Procedures
All patients were immobilized by either RF or FM and simulated with CT localizer utilizing a CT scan with 1.25-mm-thick sequential axial slices. All patients underwent a thin-slice, volumetric three-dimensional spoiled gradient (3D-SPGR) and a 3D-Fast Imaging Employing Steady-state Acquisition (FIESTA) magnetic resonance imaging (MRI) studies for target delineation. Imaging data were imported to the treatment planning station, fused and planned using the iPlan® RT computer software (BrainLAB AG, Feldkirchen, Germany). For patients immobilized by RF, a RF was first mounted with 4 metal pins with local anesthetics, followed by CT simulation, treatment planning and then SRS delivery continuously in 4 to 6 hours on the same day. For patients immobilized by FM, simulation, planning and SRS had the flexibility to be performed on the same day or in different days.
For FM immobilization, a non-invasive bivalve-style thermoplastic mask (BrainLAB facemask) was custom-molded to the patient’s face and head at CT simulation. At the time of treatment, each patient was repositioned in the immobilization FM, and an infrared tracking array was used to obtain the initial positioning of the patient. An orthogonal pair of stereoscopic x-rays was then taken and positioning offsets were calculated after image fusion with digitally reconstructed radiographs generated by the ExacTrac system. The calculated offsets were used to correct positioning errors under control of the infrared tracking system. X-ray imaging was repeated after patient positioning corrections until the observed translational offsets (X, Y and Z axes) were less than 0.5 mm and rotational offsets (pitch, yaw and raw angles) were less than 0.5 degree. At this point, treatment was begun. Imaging and repositioning cycles were repeated for each of six table angles. Our Novalis Tx Linac is capable of delivering a dose rate of 1000 MU per minute, and total treatment time was often within 45 minutes to deliver 90 Gy to the isocenter.
An “asymmetric” 7-arc technique (Fig. 2) was designed to provide optimal dose coverage for the trigeminal nerve root while minimizing dose to the brainstem. The 7 arcs, each utilizing a 4-mm circular collimator, consisted of 5 full arcs and a pair of “split-arcs” alone the longitudinal plane of brainstem at 90 degree couch position. The arcs were 20 degrees apart at 6 couch positions, with a total arc length of 700 degrees. A SRS target on the affected trigeminal nerve was determined by using MR images fused to the stereotactic CT scan for correction of MR image distortion and dose calculation. The point target on the trigeminal nerve, about 3-4 mm from the nerve entry to brainstem, was chosen to keep the 30% isodose line off the brainstem surface. An isocenter point dose of 90 Gy was delivered at 1000 monitor unit (MU) per minute to the target.
Figure 2.
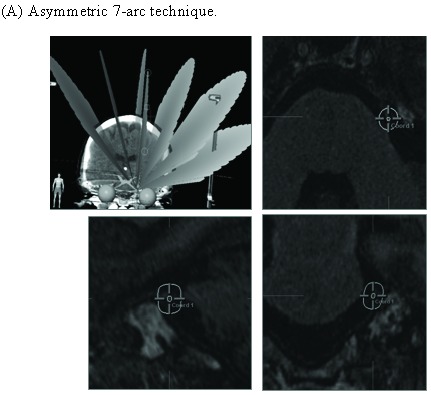
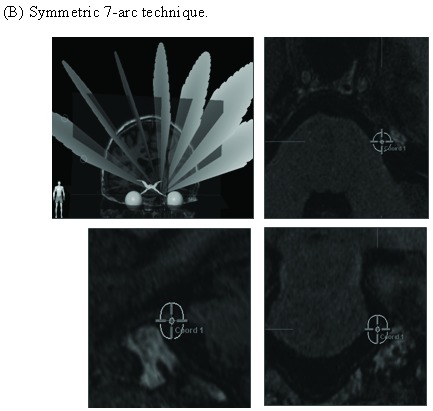
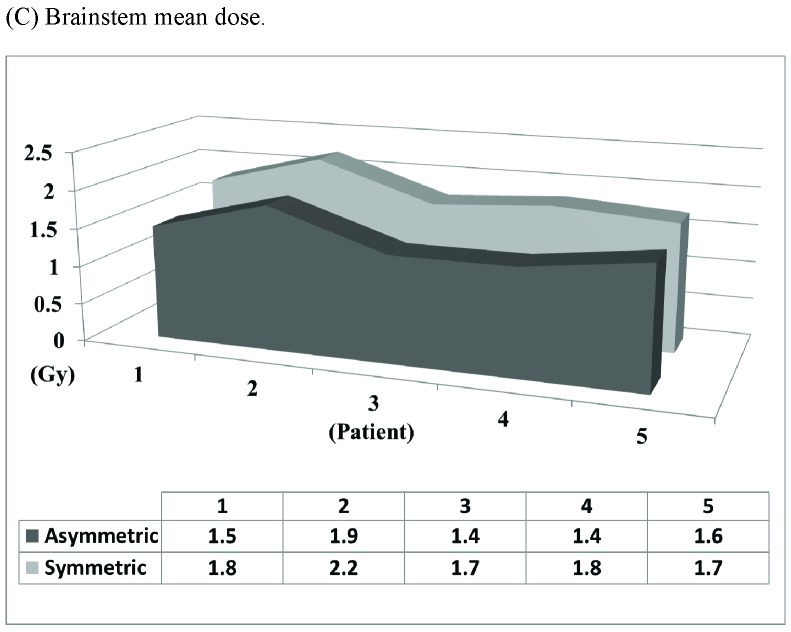
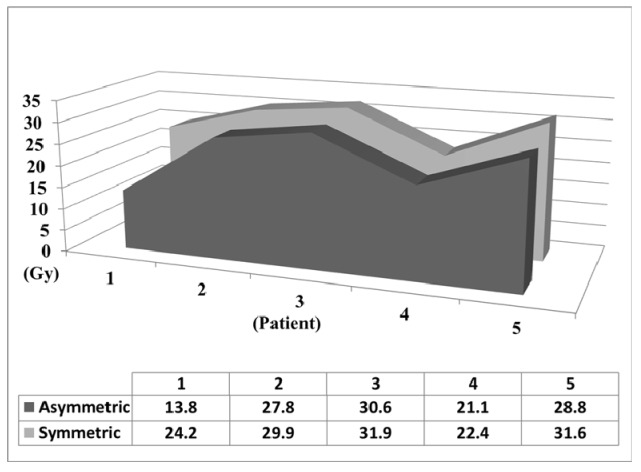
Asymmetric 7-arc technique reduces brainstem dose. (A) Asymmetric 7-arc technique. (B) Symmetric 7-arc technique. Brainstem mean dose (C) and maximum dose (D) are based on simulated plans for 5 patients.
After SRS and removal of immobilization device, the patient was discharged to home. The patient was advised to continue pain medications until pain alleviation is achieved.
2.4 Treatment Response Evaluation and Statistical Analysis
Treatment outcomes were assessed by patient interviews with a 7-item TGN treatment questionnaire (Table 2) conducted during initial consultation before SRS and at follow-up appointments. The major contents of the questionnaire, including the Barrow Neurological Institute (BNI) pain scale, House-Brackmann Scale for facial weakness and assessment for facial numbness have been previously used and validated in many studies for trigeminal neuralgia [13-17 ]. Any patient who did not reach a treatment response within 6 months from SRS was counted as a non-responder. Time to pain recurrence was defined as the time from SRS until a patient’s categorical pain response increased.
Table 2.
Seven-item trigeminal neuralgia questionnaire
| 1. Mark an X next to the location of your trigeminal pain. Mark all that apply. | |||
| ______ Right | ______Left | ||
| ______ | Forehead/eyebrow (V1) | ||
| ______ | Nose/upper lip/upper cheek (V2) | ||
| ______ | Chin/lower lip/lower cheek (V3) | ||
| 2. Mark an X next to the sentence that best describes your trigeminal pain. | |||
| BNI Scale | |||
| ______ | No trigeminal pain, no medication | ||
| ______ | Occasional pain, not requiring medication | ||
| ______ | Some pain, adequately controlled with medication | ||
| ______ | Some pain, not adequately controlled with medication | ||
| ______ | Severe pain/no pain relief | ||
| 3. Mark an X next to the medication you are taking/have taken for your trigeminal pain. Mark all that apply. | |||
| Presently | Previously | ||
| ______ | Tegretol | (carbamazepine) | |
| ______ | ______ | Neurontin | (gabapentin) |
| ______ | ______ | Trileptal | (oxcarbazepine) |
| ______ | ______ | Baclofen | (lioresal) |
| ______ | ______ | Dilatin | (phenytoin) |
| ______ | ______ | Other ___________________________________ | |
| 4. Mark an X next to sentence that best describes your facial numbness. | |||
| ______ | No facial numbness | ||
| ______ | Mild facial numbness | ||
| ______ | Facial numbness, somewhat bothersome | ||
| ______ | Facial numbness, very bothersome | ||
| 5. Mark an X next to the statement that best describes your facial weakness on the affected side. | |||
| House-Brackmann Scale | |||
| ________ | Normal | ||
| ________ | Slight weakness, noticeable on close inspection | ||
| ________ | Obvious weakness, but not disfiguring | ||
| ________ | Obvious disfiguring weakness | ||
| ________ | Motion barely perceptible | ||
| ________ | No movement, loss of tone | ||
| 6. Mark an X next to the phrase that best describes how long your trigeminal pain took to go away after treatment. | |||
| ______ | I have not been treated yet | ||
| ______ | Within two weeks | ||
| ______ | Within 1 month | ||
| ______ | 1-2 months | ||
| ______ | 3-4 months | ||
| ______ | 5-6 months | ||
| ______ | No pain relief | ||
| 7. Please mark an X next to any of the following you experienced after treatment. Mark all that apply. | |||
| ______ | I have not been treated yet | ||
| ______ | Pin site pain lasting more the 30 days | ||
| ______ | Pin site numbness lasting more that 30 days | ||
| ______ | Unacceptable pin site scarring (small amount is expected) | ||
| Comments: | |||
Statistical analyses of facial pain outcome were carried out with a 2-tailed paired Student’s t tests as appropriate. Values of p < 0.01 were considered statistically significant.
3. RESULTS
3.1 Sub-millimeter Targeting Accuracy of Frameless Image-guided Radiosurgery (IGRS)
Targeting accuracy of our frameless IGRS system was assessed by a hidden-target phantom test (Fig. 1). The absolute distance between planned isocenter (the stylus pricked point target) and delivered isocenter (define by symmetrical isodose line of the dark spot generated by radiation) was measured on the exposed Gafchromic film (see Fig. 1C for examples of exposed films). Based on an analysis of 39 consecutive exposed films of the hidden-target phantom test performed during a 2-months’ time span, our frameless IGRS system demonstrated a sub-millimeter mean targeting accuracy of 0.71 ± 0.27 mm (Fig. 1D). As shown in Fig. 1D, the deviation of frameless IGRS was within 1.0 mm in 32 out of 39 tests (82%) and within 1.2 mm in 38 out of 39 tests (97%). The quantitative data not only confirm high accuracy of our frameless IGRS treatment delivery, but also demonstrate the Rando Head-and-Neck phantom-based hidden-target as an efficient and reliable tool for frameless IGRS isocenter validation.
Clinical localization data were recorded for FM immobilization patients regarding positioning deviations detected by stereoscopic x-ray imaging during the procedure. Overall, 85% of the initial positioning corrections were within 2 mm and 2 degrees in the translational and rotational shifts, respectively. And, 95% of the intra-fraction corrections between imaging cycles were within 0.7 mm in translational directions and 0.7 degrees along rotational axes.
3.2 “Asymmetric” 7-arc Technique Reduces Brainstem Radiation
Different techniques have been utilized for Linac-based SRS for TGN [9,14-16]. An “asymmetric” 7-arc technique was designed to provide optimal dose coverage of the trigeminal nerve root (Fig. 2). By “tilting” the arc set toward the target side, the “asymmetric” technique elongates the dose distribution in the vertical axis and moves it away from the superior part of the brainstem. Based on simulated plans for 5 patients, the “asymmetric” technique substantially reduces brainstem radiation (14.7% in mean dose, 12.8% in max dose) than a “symmetric” technique (Fig. 2C and 2D).
3.3 Treatment Response From Stereotactic Radiosurgery
With a median follow-up of 26 months (range 4–47 months), pain relief was recorded in 43 of 48 patients (89%; Fig. 3A), including 31 of 34 patients (91%; Fig. 3B) immobilized by rigid frame (RF) and 12 of 14 patients (86%; Fig. 3C) immobilized by face mask (FM). A total of 26 patients (54%) achieved a complete response and 17 patients (35%) achieved a partial response (Fig. 3). The median time to pain relief was 1.6 months (range 0.5 to 5.5 months). Among the 5 patients with no recorded pain relief within 6 months, 3 patients were immobilized by RF and 2 patients were immobilized by FM.
Figure 3.
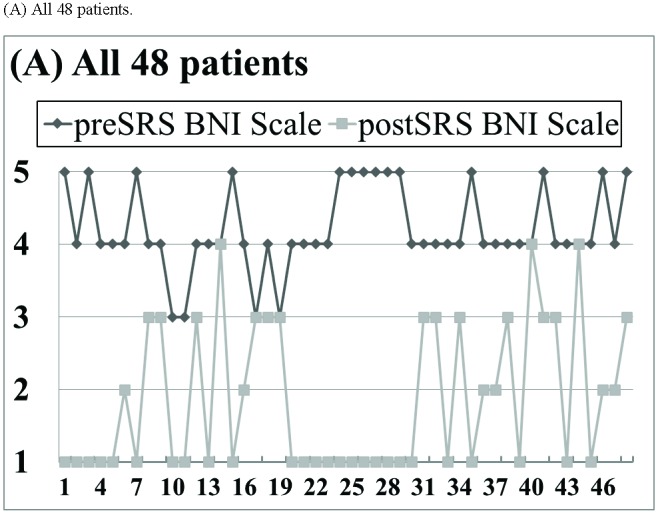
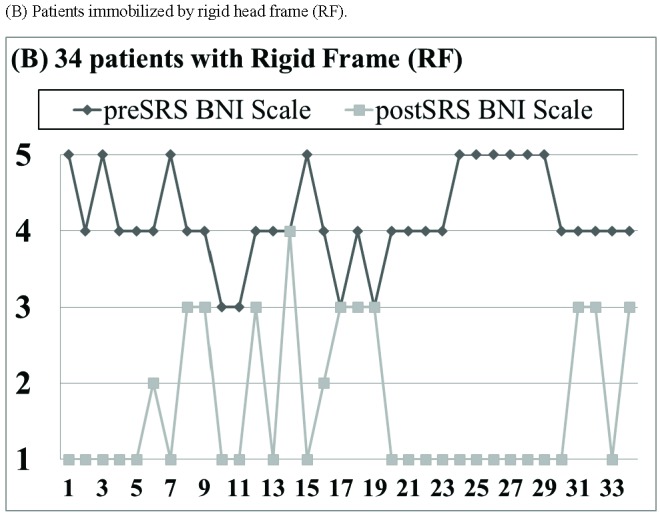
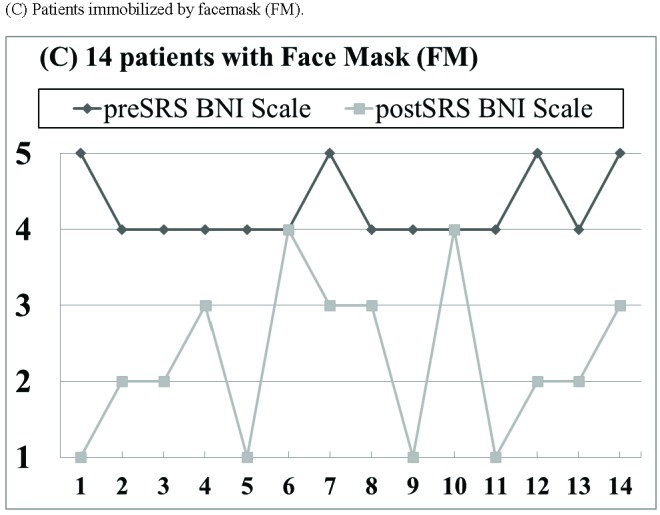
Pain relief outcome based on the 5-point Barrow Neurological Institute (BNI) pain scale. (A) All 48 patients. (B) Patients immobilized by rigid head frame (RF). (C) Patients immobilized by facemask (FM).
A significant average reduction of 2.4±1.3 points (p < 0.01) on the 5-point BNI scale was achieved for all 48 patients (Table 3). Particularly, there was no pain relief difference on the BNI scale between patients immobilized by RF and patients immobilized by FM (RF: 2.2±1.4 versus FM: 2.0±1.2; p = 0.23).
A total of 7 patients (17%), including 4 immobilized by RF and 3 immobilized by FM, reported pain recurrence in an average time to onset of 18 months (range 2-58 months). Three patients (7%) noted bothersome facial numbness or paresthesia with median time to onset of 8 months (range 1-19 months). Only one patients (2%) immobilized by RF reported obvious facial weakness.
All 34 patients immobilized by RF reported certain degree of “rebound” headache after removal of the RF. In addition, 9 patients (26%) reported noteworthy pin site issues, including long duration of severe pin site pain in 4 patients, scar and indentation at pin sites in 2 patients, numbness at pin site area in 2 patients and chronic itchiness at pin sites due to infection in 1 patient.
4. DISCUSSIONS
The current study demonstrates that frameless IGRS can treat TGN as effective as frame-based SRS. The sub-millimeter targeting accuracy demonstrated by a novel hidden-target phantom test (Fig. 1) for frameless IGRS system is comparable to frame-based SRS. The “asymmetric” 7-arc technique (Fig. 2) provides optimal dose coverage for the trigeminal nerve root while minimizing dose to the brainstem.
4.1 TGN Radiosurgery Utilizing Rigid Head Frame
SRS for TGN is mostly performed with RF immobilization. The success of frame-based SRS for TGN is illustrated by the University of Pittsburgh experience [17]. In their 220 patients treated by GK-SRS (including 7% atypical TGN patients), pain relief was recorded in 82% of patients, including 48% of patients with complete response. While 5-years durable pain relief was maintained in 57% of patients, facial paresthesia occurred in 7.7% of patients [17]. Successful frame-based Linac-based SRS for TGN has also been reported [14-16]. In a study of 41 patients by Smith et al., 87% with classic TGN achieved good pain relief. However, with a median follow-up of 23 months, 28% of patients developed pain recurrence [16]. Overall, up to 10-25% of facial numbness has been reported with Linac-based SRS [14-16].
Table 3.
Pain relief comparisons in patients mmobilized with rigid head frame (RF) versus facemask (FM)
| Immobilization method | Barrow Neurological Institute (BNI) pain scale | ||
| Pre-SRS | Post-SRS | Improvement | |
| All Patients<br/>(n = 48) | 4.2±0.6 | 1.9±1.0 | 2.4±1.3 (p< 0.01) |
| Rigid head frame (RF)<br/>(n = 34) | 4.2±0.6 | 1.7±1.1 | 2.5±1.4 (p< 0.01) |
| Frameless facemask (FM)<br/>(n = 14) | 4.3±0.5 | 2.3±1.1 | 2.0±1.2 (p< 0.01) |
| P value between RF and FM | p = 0.23 | ||
In frame-based SRS, a RF provides a reproducible patient setup between simulation and treatment. Nevertheless, mounting the RF to the patient causes pain, swelling, risks for bleeding, infection and scarring at pin sites. In contrast, frameless SRS is non-invasive and allowing imaging, treatment planning and SRS delivery to be performed in different time, which is beneficial to both patients and clinicians. Lately, good setup accuracy of stereotactic immobilization and targeting systems has been demonstrated, and frameless IGRS has been successfully used for different indications [18,19].
4.2 TGN Image-guided Radiosurgery Utilizing Frameless Facemask
Recently, advanced image-guided radiotherapy systems have been developed [20]. The process of utilizing real-time image-guidance for positioning-alignment has further enhanced targeting accuracy for frameless IGRS [21]. In a pilot study by Chen, et al., 40 of 44 patients with typical TGN treated by frameless IGRS achieved at least partial pain relief [22]. With a short mean follow up of 15 months, they observed treatment associated hypoesthesia in only 5 (11%) patients, but rather high rate of pain recurrence in 11 of 40 (27.5%) patients [22].
With a median follow-up of 26 months, our study demonstrated that significant pain relief was achieved in 89% of patients, with a low 8% rate of facial numbness and 14% rate of pain recurrence. Our treatment outcome compares favorably to most reported series, including GK-SRS and Linac-based SRS [9,13,17,22]. The favorable outcome in our series indicates accurate treatment deliver of frameless IGRS. It may also partly attributed to the low number of our patients with atypical TGN (6%) and previous invasive procedures (8%); both are known negative predictors for treatment response [9].
One limitation of our study is the relatively short follow-up time of 26 months. This should not impact the response data significantly, since most patients achieved pain response within 4 months of SRS. However, patients susceptible to late facial pain recurrence were not captured. Shortcomings of our study also include the relatively small number of patients treated with FM versus RF immobilization, and our study being a retrospective analysis instead of a randomize study.
5. CONCLUSIONS
Our results showed Linac-based SRS can achieve excellent outcomes with a low complication rate for drug-refractory TGN patients. Our study is the first to compare and demonstrate that frameless IGRS can achieve as good treatment outcome as frame-based SRS, but without pin site issues. Our study provides support for usage of more patient-friendly frameless IGRS for TGN.
6. Acknowledgements
The authors would like to thank the patients who responded to the telephone questionnaire for their time and effort in helping to evaluate the treatments they received.
References
- 1. Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol 1990; 27(1):89–95. [DOI] [PubMed] [Google Scholar]
- 2. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain 2001; 124:2347. [DOI] [PubMed] [Google Scholar]
- 3. Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 2. Neurovascular compression of the trigeminal nerve in cadaveric controls and patients with trigeminal neuralgia: quantification and influence of method. Clin Anat 1997; 10(6):380-8. [DOI] [PubMed] [Google Scholar]
- 4. Brisman R. Trigeminal neuralgia and multiple sclerosis. Arch Neurol 1987; 44:379-381. [DOI] [PubMed] [Google Scholar]
- 5. Obermann M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord 2010; 3(2):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiffen PJ, McQuay HJ, Moore RA. Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev 2005; (3):CD005451. [DOI] [PubMed] [Google Scholar]
- 7. van Loveren H, Tew JM, Jr, Keller JT, Nurre MA. A 10-year experience in the treatment of trigeminal neuralgia. Comparison of percutaneous stereotaxic rhizotomy and posterior fossa exploration. J Neurosurg 1982; 57:757-764. [DOI] [PubMed] [Google Scholar]
- 8. Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 1996; 334:1077-1083. [DOI] [PubMed] [Google Scholar]
- 9. Stereotactic radiosurgery for patients with intractable typical trigeminal neuralgia who have failed medical management, the IRSA® (International RadioSurgery Association) Radiosurgery Practice Guideline Report #1-03, 2009; from http://www.irsa.org/guidelines.html
- 10. Gevaert T, Verellen D, Engels B, Depuydt T, Heuninckx K, Tournel K, Duchateau M, Reynders T, De Ridder M. Clinical Evaluation of a Robotic 6-Degree of Freedom Treatment Couch for Frameless Radiosurgery. Int J Radiation Oncol Biol Phys 2012; 83(1):467-474. [DOI] [PubMed] [Google Scholar]
- 11. Lamba M, Breneman J, Warnick R. Evaluation of image guided positioning for frameless intracranial radiosurgery. Int J Radiat Oncol Biol Phys 2009; 74:913-919. [DOI] [PubMed] [Google Scholar]
- 12. Winston KR, Lutz W. Linear accelerator as a neurosurgical tool for stereotactic radiosurgery. Neurosurgery 1988; 22:454–464. [DOI] [PubMed] [Google Scholar]
- 13. Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys 2000; 47(4):1013-9. [DOI] [PubMed] [Google Scholar]
- 14. Chen JC, Girvigian M, Greathouse H, Miller M, Rahimian J. Treatment of trigeminal neuralgia with linear accelerator radiosurgery: Initial results. J Neurosurg 2004; 101(Suppl 3):346–350. [PubMed] [Google Scholar]
- 15. Richards GM, Bradley KA, Tomé WA, Bentzen SM, Resnick DK, Mehta MP. Linear Accelerator radiosurgery for trigeminal neuralgia. Neurosurgery 2005; 57:1193-1200. [DOI] [PubMed] [Google Scholar]
- 16. Smith ZA, Gorgulho AA, Bezrukiy N, McArthur D, Agazaryan N, Selch MT, De Salles AA. Dedicated linear accelerator radiosurgery for trigeminal neuralgia: a single-center experience in 179 patients with varied dose prescriptions and treatment plans. Int J Radiat Oncol Biol Phys 2011; 81(1):225-31. [DOI] [PubMed] [Google Scholar]
- 17. Maesawa S, Salame C, Flickinger JC, Pirris S, Kondziolka D, Lunsford LD. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg 2001; 94:14–20. [DOI] [PubMed] [Google Scholar]
- 18. van Santvoort J, Wiggenraad R, Bos P. Positioning accuracy in stereotactic radiotherapy using a mask system with added vacuum mouth piece and stereoscopic X-ray positioning. Int J Radiat Oncol Biol Phys 2008; 72(1):261–67. [DOI] [PubMed] [Google Scholar]
- 19. Breneman JC, Steinmetz R, Smith A, Lamba M, Warnick RE. Frameless image-guided intracranial stereotactic radiosurgery; clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys 2009; 74:702-706. [DOI] [PubMed] [Google Scholar]
- 20. Takakura T, Mizowaki T, Nakata M, Yano S, Fujimoto T, Miyabe Y, Nakamura M, Hiraoka M. The geometric accuracy of frameless stereotactic radiosurgery using a 6D robotic couch system. Phys Med Biol 2010; 55:1-10. [DOI] [PubMed] [Google Scholar]
- 21. Gevaert T, Verellen D, Tournel K, Linthout N, Bral S, Engels B, Collen C, Depuydt T, Duchateau M, Reynders T, Storme G, De Ridder M. Setup accuracy of the Novalis ExacTrac 6DOF system for frameless radiosurgery. Int J Radiat Oncol Biol Phys 2011; 82(5):1627-35. [DOI] [PubMed] [Google Scholar]
- 22. Chen JC, Rahimian J, Rahimian R, Arellano A, Miller MJ, Girvigian MR. Frameless Image-Guided Radiosurgery for Initial Treatment of Typical Trigeminal Neuralgia. World Neurosurg 2010; 74:538-543. [DOI] [PubMed] [Google Scholar]