Abstract
Purpose
Determine the risk of vertebral compression fracture (VCF) following stereotactic body radiotherapy (SBRT), specific to osteolytic renal cell carcinoma (RCC) spinal metastases, and associated predictive factors.
Methods
187 RCC osteolytic spinal tumor segments in 116 patients obtained from a multi-institutional pooled database were reviewed. Each segment was evaluated according to the Spinal Instability Neoplastic Score (SINS).
Results
The median follow-up was 8.0 months. 34 VCF (34/187, 18%) were observed and median time to VCF was 2.4 months. VCF was observed in 43% (10/23), 24% (4/17) and 14% (20/147) of segments treated with 24Gy/fraction (fx), 20-23Gy/fx and ≤19Gy/fx, respectively. Multivariate analysis identified dose per fx (p=0.005), baseline VCF (p<0.001) and spinal misalignment (p=0.002) as predictors of VCF. Prior conventional radiotherapy (p=0.029) was found to be protective.
Conclusions
18% of osteolytic RCC spinal metastases fractured post-SBRT. The presence of a baseline fracture, spinal mal-alignment and treatment with ≥20Gy/fx predicted for VCF.
Keywords: vertebral compression fracture, renal cell carcinoma, spine metastases, lytic bone metastases, spine stereotactic radiosurgery, spine stereotactic body radiotherapy, intensity-modulated radiation therapy, robotic radiosurgery, radiotherapy, side effects, spine instability neoplastic score
1. INTRODUCTION
Stereotactic body radiotherapy (SBRT) is a modern image-guided high dose radiation technique increasingly applied to spinal metastases16,18. The intent of SBRT is to ablate tumor rather than simply yield short-term palliation of symptoms associated with conventional palliative radiotherapy12; however, it is not without risk18. While in concept, the two most concerning toxicities specific to spine SBRT are radiation myelopathy17,19 and vertebral compression fracture (VCF)15,20, radiation myelopathy is a rare event and published guidelines have clarified safe practice17,19. This is in contrast to VCF which is increasingly being recognized as a relatively frequent SBRT-induced adverse event2, one that can significantly impair quality of life, impose a surgical procedure that the patient may not otherwise have required and potentially impact on survival by delaying oncologic therapy6,15,20.
Our prior multi-institutional analysis reported a 14% risk of VCF amongst a cohort of 410 spinal segments of mixed histologies15. The aim of the current report was to specifically investigate the renal cell cancer (RCC) osteolytic spinal metastases population within that previously published cohort, as SBRT for RCC is a major indication given its inherent radioresistance, hypervascular characteristic making surgery less attractive, and because of its tendency to form lytic disease which increases the risk of VCF. In addition, we investigated the predictive capacity of each criteria within the Spinal Instability Neoplastic Score (SINS) classification system, which is a newly developed and recently validated (amongst both surgeons and radiation oncologists) tool to identify mechanically unstable spine patients7-9.
2. MATERIALS AND METHODS
This multi-institutional retrospective cohort study was designed to evaluate the development of VCF following SBRT in patients with osteolytic spinal RCC metastases, and consisted of cases from the MD Anderson Cancer Center, Cleveland Clinic, and University of Toronto. The cases were obtained from our previously reported series15 and analyzed for this report. Vertebral segments with prior spine surgery, segments developing a local tumor recurrence prior to VCF, and those being surgerized or retreated with radiation before the occurrence of a VCF post-SBRT were excluded. Insufficient information to provide a baseline SINS score was also an exclusion factor. From the original 410 spinal segments and 252 patients15, we report on 187 spinal segments in 116 patients.
Each vertebral segment was scored according to the SINS system based on their pre-SBRT evaluation. In brief, SINS is based on 6 criteria to evaluate the spinal instability: location, pain, type of bone lesion (lytic, mixed, blastic), spinal alignment (normal, de novo kyphosis/scoliosis deformity, subluxation/translation), presence of a baseline fracture (collapse of ≥50% or <50%, no collapse but ≥50% tumor body involvement) and posterolateral elements tumor involvement7. As all tumors were lytic, each of the remaining five SINS criteria were assessed for their potential predictive value for VCF. Additional dosimetric and clinical factors evaluated for their predictive significance are listed in Tables 1 and 2.
Table 1.
Baseline patient, tumor and treatment characteristic
| Factor | VCF Cohort (N=34) | No VCF Cohort (N=153) | Percent VCF Based on Total Cohort (N=187) |
| Spine level: | |||
| Cervical | 3 | 12 | 3/15 (20%) |
| Thoracic | 15 | 74 | 15/89 (16.85%) |
| Lumbar | 16 | 50 | 16/66 (24.24%) |
| Sacrum | 0 | 17 | 0/17 (0%) |
| Paraspinal/Epidural Disease: | |||
| Present | 26 | 96 | 26/122 (21.31%) |
| Absent | 8 | 57 | 8/65 (12.31%) |
| On targeted systemic therapies | 22 | 122 | 22/144 (15.28%) |
| On bisphosphonate therapy | 11 | 44 | 11/55 (20%) |
| Prior Radiation: | |||
| Present | 3 | 31 | 3/34 (8.82%) |
| Absent | 31 | 122 | 31/153 (20.26%) |
| Mean Age (range) in years | 59.38 | 60.39 | 60.20 (33-87.67) |
| (33-87.67) | (41-81) | ||
| Segment | |||
| Single | 28 | 66 | 28/94 (29.79%) |
| Multiple within a single field | 6 | 87 | 6/93 (6.45%) |
| Total Dose/Fraction: | |||
| 10-18Gy/1frx | 14 | 87 | 14/101 (13.86%) |
| 20-24Gy/1frx | 14 | 26 | 14/40 (35%) |
| 18-24 Gy/2frx | 2 | 16 | 2/20 (10%) |
| 18-30Gy/3frx | 2 | 1 | 2/18 (11.11%) |
| 25-30Gy/4frx | 1 | 5 | 1/2 (50%) |
| 25-30Gy/5frx | 1 | 1/6 (20%) | |
| Dose/Fraction: | |||
| <=19Gy | 20 | 127 | 20/147 (13.61%) |
| 20-23 Gy | 4 | 13 | 4/17 (23.53%) |
| ≥24 Gy | 10 | 13 | 10/23 (43.48%) |
| Median Follow-Up to Fracture (range) in months | 2.35 | 10.22 | 8.02 (0.03-75.99) |
| (0.03-43.01) | (1.05-75.99) |
Table 2.
Baseline classification of each treated vertebral segment according to the 6 SINS components and global SINS, grouped by segments with and without a radiation-induced fracture
| Factor | VCF Cohort (N=34) | No VCF Cohort (N=153) | Percent VCF Based on Total Cohort (N=187) |
| Location | |||
| Junctional (occiput-C2, C7-T2, T11-L1, L5-S1) | 16 | 66 | 16/82 (19.51%) |
| Mobile spine (C3-C6, L2-L4) | 12 | 34 | 12/46 (26.09%) |
| Semi-rigid (T3-T10) | 6 | 43 | 6/49 (12.24%) |
| Rigid (S2-S5) | 0 | 10 | 0/10 (0%) |
| Pain | |||
| Mechanical | 18 | 51 | 18/69 (26.09%) |
| Non-mechanical | 10 | 60 | 10/70 (14.29%) |
| Pain free | 6 | 42 | 6/48 (12.50%) |
| Bone Lesion Type | |||
| Lytic | 34 | 153 | 34/187 (18.18%) |
| Alignment | |||
| Kyphosis/Scoliosis | 4 | 7 | 4/11 (36.36%) |
| Normal | 30 | 146 | 30/176 (17.05%) |
| Vertebral Body Collapse | |||
| >50% | 3 | 2 | 3/5 (60%) |
| <50% | 16 | 19 | 16/35 (45.71%) |
| No collapse but >50% body involved by tumor | 8 | 17 | 8/25 (32%) |
| None of the above | 7 | 115 | 7/122 (5.74%) |
| Posterior Element Involvement | |||
| Bilateral | 3 | 20 | 3/23 (13.04%) |
| Unilateral | 20 | 68 | 20/88 (22.73%) |
| Not involved | 11 | 65 | 11/76 (14.47%) |
| SINS Classification | |||
| Stable | 5 | 64 | 5/69 (7.25%) |
| Indeterminant Instability | 27 | 88 | 27/115 (23.48%) |
| Unstable | 2 | 1 | 2/3 (66.67%) |
The occurrence of a VCF following SBRT was defined as the development of a new fracture (de novo) or progression of an existing fracture, and this definition is consistent with the prior literature4,6,14. The SBRT treatment planning computed tomography (CT) scan and/or magnetic resonance imaging (MRI) were compared to follow-up images obtained at regular intervals, typically spanning every 2 to 4 months, to determine if a VCF had occurred. Each institution’s spine SBRT technique has been previously described3,5,10,21.
2.1 Statistical analysis
Descriptive and categorical variables were used to assess the selected patient demographics, tumor characteristics and dose parameters. The primary endpoint was the occurrence of a VCF following SBRT. Each treated spinal segment was considered independent, even in the case of multiple adjacent segments included within a single target volume. The time to event was calculated in months from the start of SBRT to the event date, or to the last follow-up imaging study if VCF-free. Fine and Gray’s method for competing risk was used for the univariate predictive model. Statistically significant (p<0.05) and borderline significant variables (p<0.125), as described in Tables 1 and 2, from the univariate analysis were used to create the multivariate model. Overall survival was calculated using the Kaplan-Meier method, based on each patient treated. Statistical analyses were performed using SAS system (SAS Institute, Inc., Cary, NC) version 9. All p values were two-sided.
3. RESULTS
187 segments with osteolytic RCC metastases received SBRT with a median total dose, dose per fraction, and number of fractions of 16 Gy (range, 10-30 Gy), 15 Gy (range, 6-24 Gy), and 1 fraction (range, 1-5 fractions), respectively. Table 1 summarizes the patient, tumor and treatment baseline characteristics, Table 2 summarizes the applied SINS criteria for each treated spinal segment, categorized according to those segments that developed a VCF and those that did not. According to the total SINS score, 36.9% (69/187) of segments were stable, 61.5% (115/187) potentially unstable, and 1.6% (3/187) unstable.
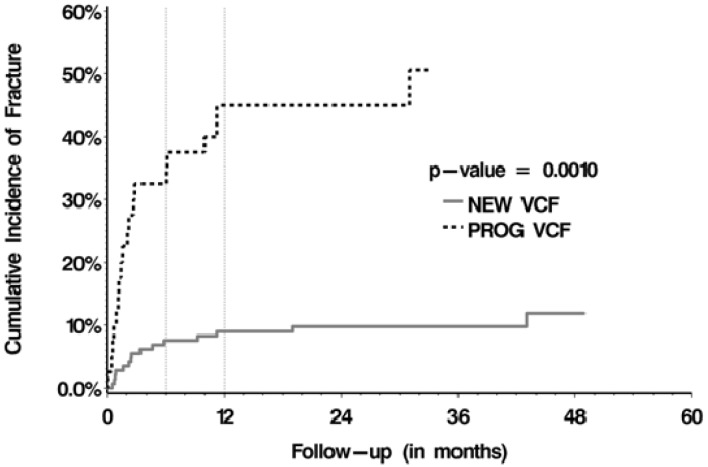
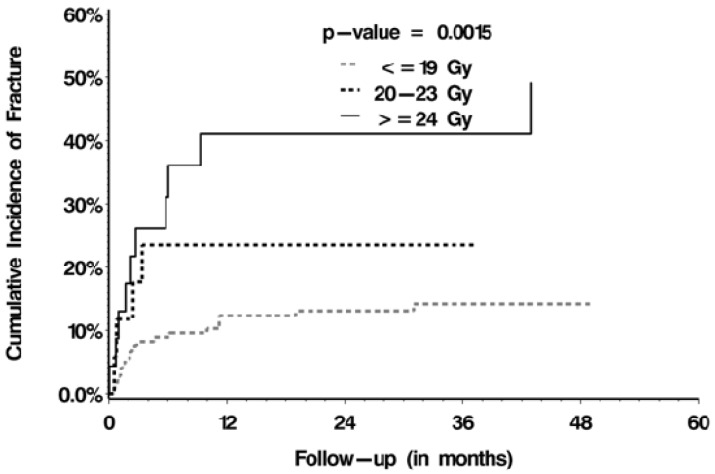
The median follow-up after SBRT was 8.02 months (range, 0.03-75.99 months). The median OS was 11.0 months (range, 8.8-13.2 months). 34 VCFs (34/187, 18.2%) were observed, 15 were de novo fractures (15/147, 10.2%) and 19 were fracture progression (19/40, 47.5%). The cumulative incidence rates for the de novo and fracture progression cohorts were 7.5% and 32.5% at 6 months, and 9.0% and 45.0% at 12 months, respectively (p =0.001; Figure 1). The median time to fracture was 2.35 months (range, 0.03-43.01 months). With respect to predictors of VCF post-SBRT, multivariate analyses (Table 3) confirmed baseline fracture (p<0.001), dose per fraction of ≥20 Gy (p=0.005, Figure 2), and spinal misalignment (p=0.002) as significant predictors. Prior conventional radiation was found to be significant and protective (p=0.029).
Figure 1.
Cumulative incidence rates stratified by de novo versus progressive fractures
Table 3.
Significant predictors of VCF on univariate and multivariable analysis
| Factor | Univariate p-value | Multivariable Proportional Hazards Model, p-value/Hazard Ratio (95%CI) |
| Baseline vertebral body collapse | <0.0001 | Global p-value < 0.0001: |
| >50% VCF: <0.0001/47.70 (10.817-210.342) | ||
| <50% VCF: <0.0001/10.60 (3.942-28.477) | ||
| No VCF but >50% of VB involved : | ||
| <0.0001/9.94 (3.470-28.457) | ||
| Dose per fraction | 0.0015 | Global p-value=0.0045: |
| ≥24 Gy: 0.0013/7.60 (2.202-26.220) | ||
| 20-23 Gy: 0.0142/6.401 (1.450-28.247) | ||
| Prior radiotherapy | 0.0981 | 0.0292/0.26 (0.078-0.873) |
| Spinal Misalignment | 0.1235 | 0.0015/5.805 (1.964-17.154) |
| SINS Classification | 0.0001 | Non-significant |
Figure 2.
Cumulative incidence of fracture stratified according to the dose per fraction delivered
Of the 34 lytic RCC segments that fractured post-SBRT, 41% (14/34) were symptomatic and 32% (11/34) were stabilized surgically. More specifically, 17% (6/34) underwent a minimally invasive cement augmentation procedure, 3% (1/34) a minimally invasive percutaneous instrumented stabilization procedure, and 12% (4/34) an open invasive instrumented stabilization procedure.
4. DISCUSSION
Spine SBRT is designed to deliver ablative doses of radiation with extreme precision to the involved vertebral segment. Several reports have confirmed high rates of efficacy in patients with RCC spinal metastases, which have traditionally been considered radio-resistant3,13. The adverse effects of ablative doses on the vertebral body bone are increasingly being recognized as a major issue, manifesting as radiation-induced VCF2,20. The mechanism has been postulated as the induction of osteoradionecrosis which damages the bone quality and ability to withstand the physiologic load; hence, leading to fracture20.
Our initial multi-institutional analysis of 410 spinal segments treated with SBRT reported a 14% risk of VCF15; however, the challenge lies in generalizing those results to specific histologies and clinical situations. Therefore, this report was aimed at clarifying the risk of VCF and predictive factors specific to this sub-group of 187 osteolytic RCC spinal metastases. Furthermore, these data are applicable to osteolytic RCC spinal metastases who scored as SINS stable (36.9%) or potentially unstable (61.5%), given that only 1.6% were SINS unstable. Importantly, these are the metastases where the decision for surgery is based largely on clinical judgment, and outcome-based data are lacking.
We observed a crude risk of VCF of 18% (34/187) and this is consistent with the limited and non-site-specific spine SBRT literature reporting rates ranging from 11-40%15,20. Specific to RCC, there is even more limited literature. Zelefsky et al. reported a 4% (4/105) incidence of fracture in extra-cranial RCC metastases treated with SBRT, amongst 59 spine and 46 non-spine sites22. However, they did not specify the incidence of fracture specific to spinal sites. Balagamwala et al. reported a dedicated spine SBRT series for RCC, and VCF was observed in 14% of the 88 sites treated3. However, the authors did not stipulate the proportion of tumors that were lytic, nor analyzed for potential predictors. Therefore, this study provides VCF outcome data specific to osteolytic RCC spinal metastases and much needed given that RCC is a major indication for SBRT due to its inherent radio-resistance, its tendency to manifest as osteolytic tumor and the inherent hypervascularity that makes surgery unattractive.
Our analysis identified dose per fraction, presence of baseline fracture, and spinal mis-alignment as significant predictors of VCF specific to osteolytic RCC. The strongest predictor was the presence of a baseline VCF. Relative to segments with no baseline VCF and <50% body involvement, the HR for further collapse in segments with a baseline VCF and degree of collapse being ≥50% and <50% was 47.7 and 10.6, respectively (Table 3). This finding makes physiologic sense as lytic tumor compromises the vertebral bone architecture, and a baseline fracture further compromises the mechanical integrity of the vertebrae to accommodate the physiological load20. Furthermore, the greater degree of collapse the greater was the risk of subsequent VCF (i.e. ≥50% collapse vs. <50% collapse). Therefore, after the effect of SBRT in weakening the bone further by inducing an inflammatory/necrotic reaction, the increase in relative risk is not unexpected and now confirmed.
With respect to spinal mis-alignment predicting for radiation-induced VCF (Table 3), a similar rationale based on anatomy and spinal integrity can be applied. A kyphotic or scoliotic deformity noticed at baseline, in a patient already at risk of VCF due to lytic disease, can further compromise the ability of the spine to handle the physiologic load20. Further weakening of the vertebral segment with SBRT would only serve to increase the risk of VCF. Importantly, in this series there were no translation or subluxation spinal deformities as these are rare in the tumor patient, as opposed to the trauma patient, and this clinical situation usually requires urgent surgical stabilization.
We also confirm, as per our prior report15, that SBRT dose per fraction is a predictive factor for VCF. With reference to treatments with ≤19 Gy/fraction, the HR was 7.6 if treating with ≥24 Gy/fraction and 6.4 if treating with 20-23 Gy/fraction. Therefore, caution must be applied when treating with high dose single fraction SBRT as there are no data that conclusively support superior efficacy as compared to lower dose single fraction SBRT or fractionated SBRT. We also conclude that the risk is prohibitive with 24 Gy/fraction at 43.5% risk (Table 1). A recent review of the pathomechanism of VCF in the SBRT patient provides a detailed analysis and suggested for the interested reader15.
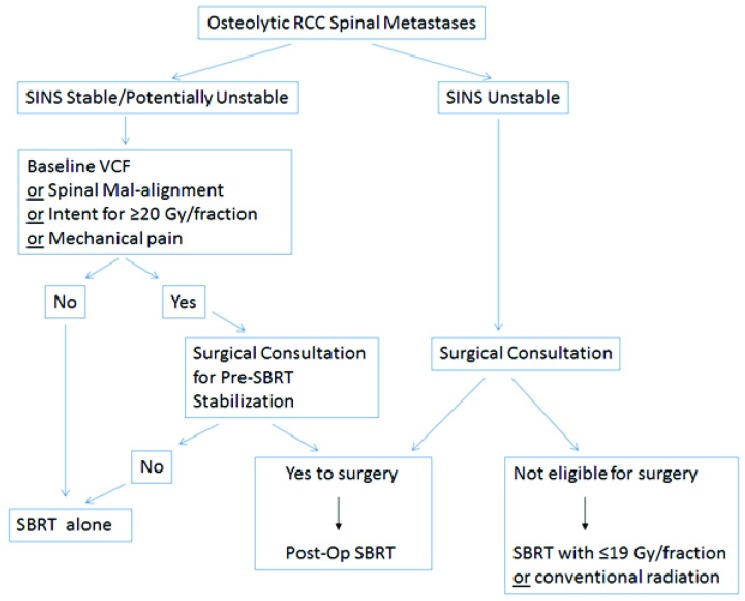
Given the literature, and our extensive experience in treating spinal metastases with SBRT, we propose an approach to patients with osteolytic RCC spinal metastases considered for SBRT with respect to VCF in Figure 3. We propose SINS as the backbone of the decision making given that it has been validated amongst both radiation oncologists and spine surgical oncologists8,9, and we have validated 2 of 5 relevant criteria (all patients were lytic hence not the full 6 criteria) in this analysis. We did include mechanical pain (the 3rd SINS criteria) as a risk factor, as it is an important and established symptom to consider as a surgical indication. The absence of its statistical significance as a predictor in this analysis may reflect selection bias, as those with frank mechanical pain may have been triaged to surgery upfront and not included in this study. Therefore, although 37% of our study population had mechanical pain, it may be that those patients with more “severe” mechanical pain underwent upfront surgical treatment, and hence in the current study we have captured more “borderline” cases. Moreover, the issue of mechanical pain is a major challenge with respect to definition. Of note, it is the only “symptom” within SINS as opposed to the other criteria which are based on objective anatomical findings. The inherent subjectivity in the assessment, as there is no known scale as to severity of mechanical pain and the inherent mechanism of the pain is unknown and may reflect other pathological features not otherwise captured within SINS, makes it unreliable as a factor to consistently report upon. Therefore, it is an area of research and development and at this point we advocate to maintain this factor in the decision making process despite lack of significance in the analysis. It is included as a risk factor in Figure 3. The dose per fraction is also included as a risk factor in our approach, as if the intent is to treat with high dose single fraction SBRT, ≥20 Gy, then a surgical consultation is wise. The clear dose-complication relationship is described in Figure 2, and the lowest risk of VCF was observed in those patients treated with ≤19 Gy per fraction.
Figure 3.
A decision making approach with respect to fracture risk and surgical consultation prior to SBRT for osteolytic renal cell carcinoma (RCC) spinal metastases.
With respect to the radiation approach recommended (conventional vs. SBRT), if a patient is SINS unstable and the patient is not operated upon then conventional radiation may be a safer option as compared to SBRT. At this time we do not have sufficient data to understand the implications of SBRT in this population, and although an option we simply propose caution and consideration of conventional radiation. Ultimately, multidisciplinary discussion is required and will guide the treatment approach. If insufficient risk factors for surgery, and the patient is SINS stable or potentially unstable, then SBRT alone is reasonable and we recommend a dose per fraction under 20 Gy if the aim is to be conservative with respect to VCF risk. Our data also support close observation is required to monitor for VCF as the median time to fracture is short (median, 2.4 months). If the patient is operated upon, then we recommend post-operative SBRT as the emerging data show both efficacy and safety. Of note, if surgery is to be performed, we recommend decompression to downgrade any epidural disease given the report from Al-Omair et al. that show therapeutic benefit to epidural disease debulking1.
As to protective factors, we identified those spinal segments exposed to previous radiation as a cohort with a lower risk of subsequent VCF. It may be that the prior radiation induced successful remineralization, and despite tumor progression the vertebral body was still stronger than de novo counterparts. The ability of conventional radiation to induce remineralization has been reported upon by by Koswig et al.11 They observed that the greatest improvement in remineralization occurred in patients treated with low dose per fraction radiation at 3 Gy/fraction, as opposed to those treated with higher dose per fraction delivered in a single treatment (8 Gy in 1 fraction). This observation is powerful in that it may also explain our dose-complication relationship with a greater risk of VCF when SBRT is delivered with 20 Gy or more in a single fraction, and add to our understanding of potential mechanisms of action beyond the induction of osteoradionecrosis2. We also hypothesize, that those lytic tumors previously radiated and did not undergo remineralization, may have destabilized upon tumor progression and operated upon prior to consideration of SBRT. Therefore, this group would not have been included in this study, and selection bias explains the result. Further study is required to determine the physiologic and metabolic characteristics of this patient population, and it is premature to conclude prior radiation as a protective factor for VCF. Therefore, we excluded this finding from our summary of recommendations in Figure 3.
5. Conclusions
We observed an 18 % crude risk of VCF in osteolytic RCC spinal metastases treated with SBRT. Predictive factors included presence of a baseline VCF, spinal misalignment and treatment with 20-24 Gy/fraction. We propose an approach to the patient with osteolytic RCC spinal metastases being considered for SBRT with respect to fracture risk using SINS as the backbone.
REFERENCES
- 1. Al-Omair A, Masucci L, Masson-Cote L, Campbell M, Atenafu EG, Parent A, et al. : Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol 15: 1413-1419, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Omair A, Smith R, Kiehl TR, Lao L, E Yu, Massicotte EM, et al. : Radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: clinicopathological correlation. J Neurosurg Spine 18: 430-435, 2013 [DOI] [PubMed] [Google Scholar]
- 3. Balagamwala EH, Angelov L, Koyfman SA, Suh JH, Reddy CA, Djemil T, et al. : Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma. J Neurosurg Spine 17: 556-564, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Boehling NS, Grosshans DR, Allen PK, McAleer MF, Burton AW, Azeem S, et al. : Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine 16: 379-386, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, et al. : Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 7: 151-160, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cunha MV, Al-Omair A, Atenafu EG, Masucci GL, Letourneau D, Korol R, et al. : Vertebral Compression Fracture (VCF) After Spine Stereotactic Body Radiation Therapy (SBRT): Analysis of Predictive Factors. Int J Radiat Oncol Biol Phys 84: e343-349, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, et al. : A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 35: E1221-1229, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Fisher CG, Schouten R, Versteeg AL, Boriani S, Varga PP, Rhines LD, et al. : Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol 9: 69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fourney DR, Frangou EM, Ryken TC, Dipaola CP, Shaffrey CI, Berven SH, et al. : Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol 29: 3072-3077, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Hyde D, Lochray F, Korol R, Davidson M, Wong CS, L Ma, et al. : Spine Stereotactic Body Radiotherapy Utilizing Cone-Beam CT Image-Guidance With a Robotic Couch: Intrafraction Motion Analysis Accounting for all Six Degrees of Freedom. Int J Radiat Oncol Biol Phys 82: e555-562, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Koswig S, Budach V: [Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study]. Strahlenther Onkol 175: 500-508, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen J, Chow E, Zeng L, Zhang L, Culleton S, Holden L, et al. : Palliative response and functional interference outcomes using the Brief Pain Inventory for spinal bony metastases treated with conventional radiotherapy. Clin Oncol (R Coll Radiol) 23: 485-491, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Nguyen QN, Shiu AS, Rhines LD, Wang H, Allen PK, Wang XS, et al. : Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 76: 1185-1192, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Rose PS, Laufer I, Boland PJ, Hanover A, Bilsky MH, Yamada J, et al. : Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 27: 5075-5079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahgal A, Atenafu EG, Chao S, Al-Omair A, Boehling N, Balagamwala EH, et al. : Vertebral Compression Fracture After Spine Stereotactic Body Radiotherapy: A Multi-Institutional Analysis With a Focus on Radiation Dose and the Spinal Instability Neoplastic Score. J Clin Oncol 31: 3426-3431, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahgal A, Bilsky M, Chang EL, L Ma, Yamada Y, Rhines LD, et al. : Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 14: 151-166, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Sahgal A, L Ma, Weinberg V, Gibbs IC, Chao S, Chang UK, et al. : Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 82: 107-116, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Sahgal A, Roberge D, Schellenberg D, Purdie TG, Swaminath A, Pantarotto J, et al. : The Canadian Association of Radiation Oncology Scope of Practice Guidelines for Lung, Liver and Spine Stereotactic Body Radiotherapy. Clin Oncol (R Coll Radiol) 24: 629-639, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Sahgal A, Weinberg V, L Ma, Chang E, Chao S, Muacevic A, et al. : Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys 85: 341-347, 2013 [DOI] [PubMed] [Google Scholar]
- 20. Sahgal A, Whyne CM, L Ma, Larson DA, Fehlings MG: Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol 14: e310-320, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Sangha A, Korol R, Sahgal A: Stereotactic body radiotherapy for the treatment of spinal metastases: An overview of the University of Toronto, Sunnybrook Health Sciences Odette Cancer Centre, Technique. Journal of Medical Imaging and Radiation Sciences 44: 126-133, 2013 [DOI] [PubMed] [Google Scholar]
- 22. Zelefsky MJ, Greco C, Motzer R, Magsanoc JM, Pei X, Lovelock M, et al. : Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 82: 1744-1748, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]