Abstract
Background
Although hepatitis B virus (HBV) transmission in dental settings is rare, in 2009 a cluster of acute HBV infections was reported among attendees of a two-day portable dental clinic in West Virginia.
Methods
The authors conducted a retrospective investigation by using treatment records and volunteer logs, interviews of patients and volunteers with acute HBV infection as well as of other clinic volunteers, and molecular sequencing of the virus from those acutely infected.
Results
The clinic was held under the auspices of a charitable organization in a gymnasium staffed by 750 volunteers, including dental care providers who treated 1,137 adults. Five acute HBV infections—involving three patients and two volunteers—were identified by the local and state health departments. Of four viral isolates available for testing, all were genotype D. Three case patients underwent extractions; one received restorations and one a dental prophylaxis. None shared a treatment provider with any of the others. One case volunteer worked in maintenance; the other directed patients from triage to the treatment waiting area. Case patients reported no behavioral risk factors for HBV infection. The investigation revealed numerous infection control breaches.
Conclusions
Transmission of HBV to three patients and two volunteers is likely to have occurred at a portable dental clinic. Specific breaches in infection control could not be linked to these HBV transmissions.
Practical Implications
All dental settings should adhere to recommended infection control practices, including oversight; training in prevention of bloodborne pathogens transmission; receipt of HBV vaccination for staff who may come into contact with blood or body fluids; use of appropriate personal protective equipment, sterilization and disinfection procedures; and use of measures, such as high-volume suction, to minimize the spread of blood.
Keywords: Dentistry, hepatitis B virus, outbreak, infection control, portable dental equipment
Hepatitis B virus (HBV) is a highly infectious and environmentally stable bloodborne pathogen 1 that can lead to serious long-term liver disease in people who develop chronic infection. Improving the early identification and management of the care of people with HBV infection can help prevent morbidity and mortality and can complement immunization strategies to eliminate HBV transmission in the United States.2
During periods of high viral replication activity, an infected person’s blood can contain 106 to 1010 international units per milliliter of HBV DNA, and thus parenteral or mucosal exposure to even a small amount of blood or body fluid may result in infection in susceptible (not immune) people.2,3 Because the virus can be transmitted by means of contaminated dental equipment, instruments and environmental surfaces, meticulous compliance with dental infection control recommendations is crucial.4 However, HBV transmission in a dental health care setting has been rare, particularly since universal precautions and routine HBV vaccination of dental workers were recommended (1985 and 1987, respectively).4–6 Since 1987, the only reported transmission in a dental setting was a single episode, from one patient to another. 7
METHODS
Setting
During a two-day period—June 26 and 27, 2009—a temporary dental clinic involving portable equipment was set up in a high school gymnasium in West Virginia, held under the auspices of a charitable organization. More than 750 staff volunteers from surrounding communities, consisting of licensed dental health care providers, dental students and nonprofessional volunteer staff, provided dental services to an estimated 1,137 adult patients in West Virginia. Patients received, at no charge, dental examinations, radiographs when indicated and treatment services limited to dental cleanings, extractions and restorations. Most dental equipment—such as portable dental units, radiographic units, steam autoclaves, dental handpieces, hand instruments and supplies— were provided by a state dental association and one individual supplier. Some practitioners used their own equipment and supplies. Alcohol-based hand sanitizers, examination gloves and environmental surface disinfectants were available.
Operational section supervisors included a nurse responsible for central sterilization and licensed dental professionals responsible for providing local anesthetic at a “numbing station,” as well as care providers responsible for surgical dental services and restorative dental services. Supervisors provided dental treatment in addition to performing their supervisory duties. A safety officer oversaw waste management and follow-up of any reported needlestick injuries. No one was designated to oversee infection control practices.
In November 2009, approximately four months after the dental clinic, a local health department notified the West Virginia Department of Health and Human Resources (DHHR) of several cases of acute HBV infection among people who had visited the dental clinic. The initial report to the local health department originated from a concerned patient who reportedly had been told by his physician that several cases of acute HBV infection among dental clinic participants had been seen in the physician’s practice. Owing to concern about possible health care–associated HBV transmission, staff of the West Virginia DHHR initiated an epidemiologic investigation. During the course of the investigation, DHHR personnel notified the state dental director and the Centers for Disease Control and Prevention (CDC) and provided a final report to the West Virginia state dental board. Because our report is purely a description of the outbreak investigation, and all data collection was conducted during the routine course of investigation, an institutional review board approval was not required.
Case finding and investigation
Confirmed epidemiologic outbreak cases of HBV were defined by West Virginia DHHR and CDC investigators as patients or staff volunteers who attended the dental clinic, had an acute illness with a discrete onset of symptoms between Aug. 1 and Dec. 31, 2009, and had jaundice or elevated serum aminotransferase levels with laboratory evidence of HBV infection. Laboratory evidence of acute infection is defined by CDC and the Council of State and Territorial Epidemiologists (CSTE) as a positive immunoglobulin M antibody to hepatitis B core antigen (IgM anti-HBc) and a positive hepatitis B surface antigen (HBsAg).8 West Virginia DHHR and CDC investigators selected the period on the basis of the incubation period of hepatitis B, which is six weeks to six months between exposure and the onset of symptoms.2,3,9
In follow-up to the initial outbreak report, personnel in the local health department contacted the health care provider in whose office these cases reportedly had been seen to obtain information on all recent HBV cases. After identification of the initial cases, local health department staff reviewed information for patients with acute HBV reported to the health department since the dental clinic occurred. During routine case ascertainment for surveillance, West Virginia DHHR staff had interviewed these patients about HBV transmission risks by using questions from a standardized West Virginia DHHR viral hepatitis case report form. West Virginia DHHR staff reviewed information regarding receipt of dental care during the expected exposure period to look for additional patients with confirmed HBV who might have attended the dental clinic. Review of reported cases continued through at least six months after the date of the clinic.
Other active surveillance efforts included a national public health agency notification posted on Epi-X, the CDC’s secure communications network on which health care professionals can report health surveillance information; statewide health alerts sent to West Virginia health care providers; and notifications sent to the health departments of the surrounding states. Agents from the local health department notified people who had volunteered or received care at the clinic and offered bloodborne pathogen testing. West Virginia DHHR staff obtained the names of all patients treated at the dental clinic from clinic organizers and cross-matched them against the state’s HBV registry to identify additional people with acute HBV infection or people with preexisting chronic HBV infection who may have been a source of infection. West Virginia DHHR staff did not perform a match of dental staff volunteers to the state HBV registry because critical information, such as date of birth, was not available in the volunteer log. Personnel time was not available to collect this information from volunteers, and contact information was not available for all volunteers.
Laboratory testing
For all people who met the case definition, testing for antibodies to hepatitis C virus (HCV) and HIV was performed by health care providers through health insurance or by the West Virginia Office of Laboratory Services. West Virginia DHHR staff sent serum specimens from all available cases to CDC for testing of viral relatedness. One case patient was lost to follow-up before specimen collection. The CDC Division of Viral Hepatitis laboratory tested HBV genotypes and genetic relatedness among HBV strains by using phylogenetic analysis of the HBV S gene sequence (nucleotides222–656).10–12
Case questionnaires
To further characterize risk factors among outbreak cases, West Virginia DHHR staff developed two additional questionnaires and administered them to the acutely infected people. The first questionnaire was designed to assess risk factors for HBV transmission that could have occurred outside of the dental clinic. The second questionnaire was created to obtain more specific information regarding potential exposures at the dental clinic. West Virginia DHHR staff interviewed case patients about the type and extent of dental services they received and case staff volunteers about duties they performed.
Clinic infection control assessment
Because the clinic was no longer in operation and procedures could not be observed directly, West Virginia DHHR staff developed and used another questionnaire to obtain information from the clinic supervisors about their role and job duties at the clinic, including infection control practices and potential exposures to blood or body fluids. Owing to shortages of West Virginia DHHR investigative staff, only the clinic’s section supervisors and safety officer were interviewed.
RESULTS
Case finding
The local health department, through contact with the health care provider, confirmed four acute HBV infections meeting the CDC/CSTE case definition: one in a staff volunteer and three in patients of the temporary portable dental clinic. Further prospective review of new HBV reports up to six months after the clinic was held identified an additional case of acute HBV in another staff volunteer at the dental clinic. Retrospective review of reports back to the date of the clinic revealed no additional reports meeting the case definition. Dates of symptom onset for all these people indicated that their clinic participation fell within their likely exposure periods. In total, five cases of acute hepatitis B were identified among three patients and two non–health care professional volunteers of the temporary portable dental clinic.
Through a mass mailing and a public announcement in June 2010, West Virginia DHHR staff notified patients and providers of the outbreak, and voluntary bloodborne pathogen testing was recommended. Of 389 people who were tested by the local health department, one additional patient was identified as positive for HBsAg. Because of the delay in notification, this positive result could not be linked to the clinic conclusively.
A cross-match of clinic patients against the state’s HBV registry with data available retrospectively to 2002 identified three patients who were positive for HBsAg before visiting the dental clinic and thus were possible source patients, but serologic specimens were not available from these patients for viral sequencing and comparison with outbreak cases. These three patients attended the clinic on the same day as did the three case patients. The last positive HBsAg test dates reported for two of these patients were June 2007 and July 2008. Therefore, it is uncertain if they still were infected one to two years later. For the third potential source patient, the last positive HBsAg test date was in April 2009, three months before the clinic.
Laboratory findings
All five outbreak cases had serologic evidence of acute HBV infection but were negative for antibodies to H HIV. On the basis of information from routine HBV surveillance reports, all five (100 percent) were considered confirmed HBV cases according to the CDC/CSTE case definition,8 with elevated serum alanine aminotransferase (ALT) levels (ranging from 1,322 to 2,057 units per liter) and positive test results for HBsAg and IgM anti-HBc. Four of the five (80 percent) had jaundice, indicating hyperbilirubinemia; the ALT level at diagnosis for the patient without jaundice was 1,664 U/L. HBV isolates from four patients (both of the case volunteers and two of the three case patients) were tested at CDC by means of viral molecular analysis. The HBV strain in all four cases belonged to genotype D, which is found in only 18 percent of acute HBV infections in the United States.13 Only a partial gene sequence (S gene) was available from all specimens, and it was identical among all four cases.
Case investigation
The types of dental procedures received by the case patients, but not the procedures’ time or location within the clinic, were recorded in the treatment record. Extractions were performed in a common surgical area. The table summarizes the dental procedures received, duties performed and reported blood exposures for HBV case patients and volunteers. All three case patients underwent extractions; one case patient also received restorations, and one received a dental prophylaxis. According to treatment records, none of the three case patients had a common provider. Of the two case volunteers, one set up supplies and performed maintenance of both clean and dirty equipment such as air compressors. The other case volunteer escorted patients from the dental triage section to the waiting area of the treatment section. All five outbreak cases either worked or received treatment during the first day of the dental clinic. The two case volunteers also worked during the second day of the dental clinic.
Table I.
Summary of dental procedures, duties performed by volunteers, and reported blood exposure for HBV cases associated with a two-day portable dental clinic — West Virginia, 2009
| Dental Clinic Participation |
Dental Procedure(s) Received |
Symptom Onset Date |
Duties Performed | Reported Blood Exposure During Dental Clinic |
Lost to Follow-Up |
|---|---|---|---|---|---|
| volunteer | none | Oct 2009 | Escorted patients to treatment waiting area | No | No |
| volunteer | none | Sept 2009 | Logistics; Maintenance of clean and dirty medical equipment | Yes | No |
| patient | extractions, dental cleaning | Oct 2009 | N/A | Unknown | Yes |
| patient | extractions, filling | Oct 2009 | N/A | Unknown | No |
| patient | extractions | Sept 2009 | N/A | Unknown | Yes |
The potential source patient with known HBsAg positivity in April 2009 received lidocaine injections at the numbing station and underwent a full-mouth extraction. The same provider administered lidocaine injections for this patient and one of the case patients. No other instances of common providers between potential source patients and case patients were identified. The other two potential source patients also received lidocaine at the numbing station and underwent restorative procedures performed by a common provider.
Case questionnaires
West Virginia DHHR staff administered the questionnaire regarding HBV risk factors outside of the dental clinic to the two case volunteers and two case patients. One of the three case patients became lost to follow-up before the interview was conducted. None of the four people interviewed reported use of injected drugs or recent multiple sex partners (known behavioral risks for HBV infection) or common health care exposures during the exposure period other than attendance at the dental clinic. None had been vaccinated against HBV.
West Virginia DHHR staff administered the questionnaire regarding exposures at the dental clinic to three outbreak cases (one case patient and two case volunteers). One other case patient was available for the initial questionnaire but became lost to follow-up before administration of the second survey. The single case patient who completed this questionnaire reported spending seven hours at the clinic, visiting CV and five separate dental stations (medical triage, dental triage, numbing station, restorative dental services and surgical dental services), receiving a finger-stick to monitor blood glucose (although this was not noted on the patient’s dental clinic record) and receiving local anesthetic multiple times. This case patient reported having observed his dentist pick up a composite placement instrument, a dental bur and a curing light from what he believed to be another patient’s previously used bracket tray and subsequently use the instruments during his own procedure.
Neither of the case volunteers had received HBV vaccine nor received infection control training in preparation for working at the dental clinic. None of the case patients or volunteers reported having experienced a sharps injury. The case volunteer responsible for equipment maintenance reported on the questionnaire that during this clinic, he had contact with blood-contaminated equipment (an air compressor) while wearing gloves, and that he wiped sweat from his brow and blew his nose frequently during the clinic without removing his gloves. Temperatures during clinic operation were higher than 100°F because there was no air conditioning in the gymnasium. This case volunteer also reported another exposure, at a similar temporary dental clinic in the preceding month (May 2009), when blood-contaminated saliva from a full suction container blew back into his face while he was wearing no mask or eye protection. The timing of this exposure was within his possible incubation period.
Clinic infection control assessment
Patients of the dental clinic visited multiple stations, including those for medical triage, dental triage for examination and treatment planning, radiology, local anesthesia and dental operations (treatment areas). A finger-stick blood glucose test or blood clotting test was completed if deemed necessary by triage staff. In dental triage, oral examinations and radiographs helped determine necessary dental services. Local anesthetic was administered with nondisposable aspirating syringes, disposable needles and single-dose anesthetic carpules.
There were no reports of reuse of anesthetic carpules or disposable needles in more than one patient. Intravenous or conscious sedation was not conducted in the clinic. With the exception of the anesthetic carpule, no single-dose or multiple-dose medication or saline vials were used. However, when interviewed, one case patient reported that he and other patients were allowed to carry their partially used anesthetic carpules in the metal syringes on a tray to other stations if needed for later reuse; this report was corroborated by interviews with clinic volunteers. It is unknown whether or how the trays were cleaned between patients.
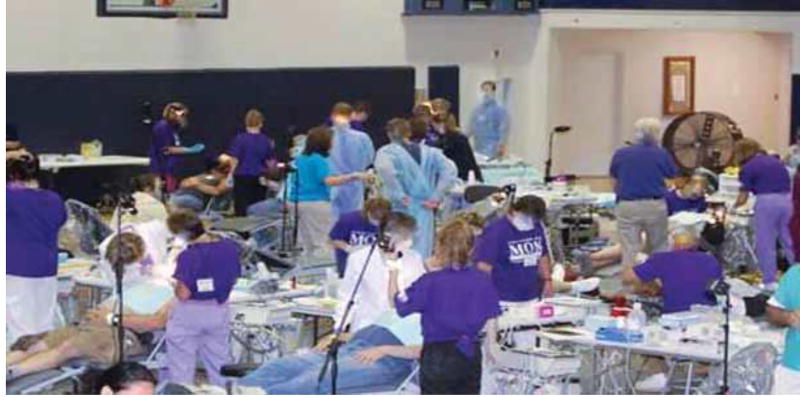
Set up in the middle of the gymnasium, the surgical and restorative stations were the largest area. There were no barriers, such as curtains, to separate any of the operations areas or portable dental units. Patients, staff, instruments and equipment—clean and contaminated— were in close proximity, similar to another temporary clinic conducted in the gymnasium the previous year (Figure). When interviewed, supervisors reported that they had not observed any infection control breaches. However, the supervisors for extractions, restorative care and numbing had multiple responsibilities, including direct patient care, troubleshooting and media interviews. Assistants were stationed at most chairs to help the dentists who were performing procedures. The dentists used portable dental units that included low-volume and possibly high-volume evacuation lines connected to a receptacle for patient materials, a contained water system and an air compressor. Saliva ejectors were single use and discarded between patients. Volunteers handling contaminated dental instruments in the sterilization area reportedly wore heavy-duty gloves for sharps protection as recommended. Volunteer staff cleaned and sterilized instruments in the concession area of the gym by using ultrasonic cleaners and steam autoclaves. Investigators did not ascertain whether dirty and clean areas were clearly delineated. Instruments were sterilized unwrapped, and no chemical indicators reportedly were used. We do not know whether steam autoclaves underwent biological monitoring before they were used. Sterilized instruments were placed unwrapped on a clean table to dry in the dental operations area for dental providers to retrieve. Assigned staff members monitored the instrument table to ensure that instruments were picked up with clean gloves. The outer surfaces of dental handpieces and disposable mirrors were cleaned with disinfectant wipes and returned to service without being heat sterilized between patient uses.
Figure.
Photograph of a 2008 temporary dental clinic operations area in a West Virginia high school gymnasium, a setup similar to that used in the same gymnasium in the 2009 clinic discussed in this article
Plastic barriers were used to cover dental chairs and were changed between patients, except when the supply became scarce toward the end of the two-day clinic, and barriers were not always changed between patients. Chairs and other clinical contact surfaces were wiped with disinfectant between patients. Suctioned fluids were emptied into buckets by waste removal volunteers and disposed of in bathroom toilets. Sharps containers for used needles were located between chairs throughout the dental operations area and the numbing station, and they were replaced when they became full.
Providers reportedly changed gloves between patients and used alcohol-based hand sanitizers, which were available at each dental unit. Sinks were not available for hand washing within the gymnasium, but they were available in the building’s two bathrooms, located in the hallway outside the gymnasium. The investigators did not ascertain dental care providers’ use of masks, protective eyewear and gowns. The more than 750 participating volunteers included general volunteers, dental students and licensed dental professionals. General volunteers, such as non–health care professionals, received training relevant to the areas to which they were assigned by the area supervisors.
Licensed dentists and dental students were assumed to have received previous training in prevention of bloodborne pathogens transmission, as well as hepatitis B vaccination. Other volunteers, including predental undergraduate students, may not have received bloodborne pathogens training or HBV vaccination before participating at the clinic. A policy for reporting and follow-up of needlestick injuries was available. There were no written policies, standard operating procedures, or training in or oversight for infection control.
DISCUSSION
In this article, we have identified the likely transmission of HBV to five participants (three patients and two volunteers) in a large temporary dental clinic in West Virginia in 2009. None of those infected had been vaccinated against HBV, reported typical risk factors associated with the transmission of HBV or had other known common health care exposures. This outbreak occurred during a time of increasing reports of acute HBV infection in the region, with a more than 700 percent increase in acute HBV reports in West Virginia during the years 1997 through 2012 (B. Kupronis, Viral Hepatitis Surveillance Team, Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta, written communication, Feb.11, 2013). This increase in infections may have led to a larger pool of potential source patients and thus greater likelihood of an outbreak in a setting in which recommended infection control practices were not fully implemented. Although it is likely that transmissions occurred in the clinic, in light of regional increases, we cannot rule out the possibility that the infections were acquired in the community. Laboratory results from partial HBV gene sequences provided some limited evidence that these five outbreak cases could have been exposed to blood from a common source, although no source could be identified. Two case volunteers did not receive treatment at the dental clinic; one reported two probable blood exposures, one in this clinic and one in a clinic held the month before, and the other staff volunteer did not.
Finger-stick monitoring of blood glucose has been identified as a source of patient-to-patient HBV transmission in long-term care facility settings with inappropriate reuse of testing devices.14 In this clinic, a finger-stick check of blood glucose was provided to only a small proportion of dental clinic patients, including only one of the case patients. We could not conduct a full retrospective assessment of infection control practices during this procedure. We identified opportunities for potential exposure of patients to HBV and other bloodborne pathogens, such as reusing unsterilized handpieces, leaving sterilized instruments unwrapped and allowing patients to transport. Dental handpieces were not heat sterilized between patients and were only wiped externally with a disinfectant. Contamination of internal and external surfaces could have resulted in exposure of a subsequent patient to blood and potentially infectious materials.15,16 There is concern that expulsion of these materials from one patient to another could have occurred during subsequent uses of the handpieces.17
The physical design of the temporary clinic, with multiple treatment areas in close proximity to those in which clinicians provided invasive surgical procedures, increased the potential for spray and spatter to contaminate unwrapped instruments, supplies and equipment in adjacent areas. Another concern involved practices in the anesthetic station, where anesthetized patients were instructed to carry their own partially used anesthetic carpules in the syringe on a tray to the areas where treatment was provided. This practice could have resulted in contaminated carpules’ being misplaced and used on a subsequent patient. One case patient reported seeing his provider take and use instruments from what the patient thought was another patient’s tray, although this could not be confirmed. Despite the identification of infection control breaches, it was not possible to link specific practices to transmission of HBV in this outbreak. The majority of staff at temporary portable dental clinics are volunteers, including licensed dental health care providers, as well as administrative staff and community volunteers. All dental health care providers should receive annual bloodborne pathogens training.18 In portable dental clinics, all volunteers should receive this training, given the potential for exposure to blood and other potentially infectious materials.
Clinic organizers should check records of HBV immunization status for all dental health care providers. In addition, clinic organizers should discuss the importance of HBV immunization with community volunteers, some of whom are exposed to potentially infectious materials when handling used medical equipment or waste. Guidelines from the Advisory Committee on Immunization Practice 19 recommend hepatitis B immunization for all health care personnel, who are defined as “all paid and unpaid persons working in health-care settings who have the potential for exposure to patients and/or to infectious materials, including body substances, contaminated medical supplies and equipment, contaminated environmental surfaces, or contaminated air.”
Large temporary clinics, such as the one in this report, often operate in settings in which compliance with recommended infection control procedures can be challenging. For example, gymnasiums and auditoriums may be the only large spaces available for dental screenings or treatment. In these settings, certain conditions (such as limited sources of running water and the logistics of transporting contaminated instruments for processing) demand careful planning to ensure safety. In addition, treatment commonly includes invasive surgical procedures that place staff and patients at risk of coming into contact with bloodborne pathogens. Barriers such as curtains should be used to separate operational areas, particularly when patients are in close proximity.
CDC recommends that all dental care settings have written policies, procedures and guidelines for infection control, including education and training, immunizations and postexposure management.4 An infection control coordinator (such as a dentist or another dental professional) should be responsible for coordinating the program. On the basis of the anticipated number of patients, each dental care setting should have adequate quantities of instruments, hand hygiene products, personal protective equipment (PPE) and supplies to ensure that recommended cleaning, disinfection and sterilization procedures are followed and that PPE is worn and changed as recommended. State-specific laws, regulations and registration requirements governing licensure, certification or staffing for dentistry—including mobile or portable dental programs—should be followed.4
This outbreak placed a high burden on the state and local health departments, which supported the extensive personnel and monetary costs of the investigation, notification of potentially infected people and the recommended bloodborne pathogen testing. Notification activities included letters in English and Spanish sent to clinic participants, a statewide health alert for physicians, a press release, a call-in hotline, and a website with frequently asked questions and guidelines for submitting screening specimens to the state public health laboratory.
The local health department held a clinic in June 2010 to offer bloodborne pathogen screening for patients and volunteers, covering the costs of staff and testing. An estimated 45 million adults younger than 65 years are without access to dental care in the United States.20 Temporary clinics play a critical role in providing episodic dental care in areas of particularly high need, such as West Virginia.21 Because temporary clinics cannot provide routine preventive or follow-up care, however, additional strategies are needed to fulfill unmet dental care needs. Organizers invited local and state lawmakers to observe the 2009 temporary dental clinic and the level of unmet dental care need among attendees. After this temporary clinic was held, public and private grant funds were raised to provide seed money and the basis for a community fund-raising campaign, which enabled the establishment of the Healthy Smiles Clinic, a permanent dental clinic in Martinsburg, W.Va. In the initial two years of its operation, the clinic provided services to more than 5,000 adults and children, with more than 45,000 procedures performed. Reimbursement came mainly from Medicaid (48 percent), sliding fee payment (19.2 percent) or the state Children’s Healthcare Insurance Program (6.7 percent) (D. Gaviria, written communication, April 19, 2012).
Limitations
This investigation was subject to several limitations. Because the dental clinic was temporary, investigators could not access the clinic or staff and observe infection control practices directly; thus, the investigation relied on retrospective review. Whereas most of the information obtained about the clinic was provided by clinic supervisors, who were licensed dental professionals, these reports were provided months after the dental clinic occurred, limiting the reporters’ ability to recall details. One case volunteer reported no potential exposures at the clinic; the other case volunteer had a significant exposure and also had been exposed at a temporary dental clinic held one month earlier. It is possible their infections were acquired through different means than those of the case patients. It is often difficult to identify asymptomatic patients with acute HBV in the community, and typically 50 to 70 percent of adults with acute HBV do not have symptoms.2 Thus, it is possible that in addition to the five symptomatic people with HBV, there may have been additional people with asymptomatic acute HBV infection associated with the clinic who were not identified. Multiple factors limited our ability to search for possible source patients: specimens for genotyping were unavailable from clinic patients identified in the HBV registry cross-match, records did not contain any information about time and location of services received by patients, dental care providers were not assigned to use a specific dental chair for treatment but used any chair that was open, and registry cross-match could not be performed for clinic volunteers. In addition, notification of clinic attendees did not occur until almost one year after the clinic was held, limiting the ability to link any hepatitis B–positive patients with the clinic. The laboratory investigation was limited by the lack of availability of a specimen from one case patient. Low viral titers among cases allowed only for comparison of a partial HBV genetic sequence. Because of these limitations and the small number of patients involved, we were unable to identify the exact source and mode of HBV transmission that likely occurred during the dental clinic.
CONCLUSIONS
Temporary dental clinics can and do provide needed dental care to many patients. However, because of the types of settings and volume of patients treated, consistently maintaining recommended infection control practices in these clinics can be challenging.22 Coordinators of temporary dental clinics should inform volunteers about these challenges and measures to prevent infection control lapses, and they should designate an infection control coordinator to plan for and oversee infection control and volunteer safety during clinic operation. An important step in developing an effective infection control program in temporary dental clinics, where portable dental equipment is used, is for the infection control coordinator to visit and assess the site before initial preparations are made.23 Site assessment allows identification of available space, facilities and resources. A sample site assessment and a checklist developed by the Organization for Safety, Asepsis, and Prevention 23 may be useful in developing practical protocols for these settings. By following infection control recommendations that include operational oversight of infection control practices, training in prevention of bloodborne pathogen transmission, dental health care providers’ receipt of HBV vaccination, clinical record documentation, use of appropriate PPE with hand hygiene, sterilization and disinfection procedures, and measures that minimize the spread of blood, patients and providers can be assured that oral health care in temporary dental settings can be safely delivered and safely received.
References
- 1.Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;317(8219):550–551. doi: 10.1016/s0140-6736(81)92877-4. [DOI] [PubMed] [Google Scholar]
- 2.Weinbaum CM, Williams I, Mast EE, et al. Centers for Disease Control and Prevention. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 3.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28(1):112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 4.Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings: 2003. MMWR Recomm Rep. 2003;52(RR-17):1–61. [PubMed] [Google Scholar]
- 5.Cleveland JL, Cardo DM. Occupational exposures to human immunodeficiency virus, hepatitis B virus, and hepatitis C virus: risk, prevention, and management. Dent Clin North Am. 2003;47(4):681–696. doi: 10.1016/s0011-8532(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 6.Younai FS. Health care–associated transmission of hepatitis B and C viruses in dental care (dentistry) Clin Liver Dis. 2010;14(1):93–104. doi: 10.1016/j.cld.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Redd JT, Baumbach J, Kohn W, Nainen O, Khristova M, Williams I. Patient-to-patient transmission of hepatitis B virus associated with oral surgery. J Infect Dis. 2007;195(9):1311–1314. doi: 10.1086/513435. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Notifiable Disease Surveillance System: Hepatitis B, Acute. [Accessed Aug. 28, 2013]; wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=711&DatePub=1/1/2012%2012:00:00%20AM.
- 9.Mast EE, Weinbaum CM, Fiore AE, et al. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), part II—immunization of adults (published correction appears in MMWR Morb Mortal Wkly Rep 2007;56[42]:1144) MMWR Recomm Rep. 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- 10.Forbi JC, Vaughan G, Purdy MA, et al. Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria (published online July 19, 2010) PLoS One. 2010;5(7):e11615. doi: 10.1371/journal.pone.0011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran S, Zhai X, Thai H, et al. Evaluation of intrahost variants of the entire hepatitis B virus genome (published online Sept. 20, 2011) PLoS One. 2011;6(9):e25232. doi: 10.1371/journal.pone.0025232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teshale EH, Ramachandran S, Xia GL, et al. Genotypic distribution of hepatitis B virus (HBV) among acute cases of HBV infection, selected United States counties, 1999–2005. Clin Infect Dis. 2011;53(8):751–756. doi: 10.1093/cid/cir495. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Multiple outbreaks of hepatitis B virus infection related to assisted monitoring of blood glucose among residents of assisted living facilities: Virginia, 2009–2011. MMWR Morb Mortal Wkly Rep. 2012;61(19):339–343. [PubMed] [Google Scholar]
- 15.Lewis DL, Arens M, Appleton SS, et al. Cross-contamination potential with dental equipment. Lancet. 1992;340(8830):1252–1254. doi: 10.1016/0140-6736(92)92950-k. [DOI] [PubMed] [Google Scholar]
- 16.Herd S, Chin J, Palenik CJ, Ofner S. The in vivo contamination of air-driven low-speed handpieces with prophylaxis angles. JADA. 2007;138(10):1360–1365. doi: 10.14219/jada.archive.2007.0053. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Recommended infection-control practices for dentistry, 1993. MMWR Recomm Rep. 1993;42(RR-8):1–12. [PubMed] [Google Scholar]
- 18.Occupational Safety and Health Administration, Department of Labor. Occupational exposure to bloodborne pathogens; needlestick and other sharps injuries; final rule. [Accessed Aug. 12, 2013];Fed Regist. 2001 66:5317–5325. To be codified at 29 CFR §1910. www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=16265&p_table=FEDERAL_REGISTER. [PubMed] [Google Scholar]
- 19.Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60(RR-7):1–45. [PubMed] [Google Scholar]
- 20.Bloom B, Cohen RA. Dental insurance for persons under age 65 years with private health insurance: United States, 2008. Hyattsville, Md.: National Center for Health Statistics; 2010. [Accessed Aug. 12, 2013]. NCHS data brief no. 40. www.cdc.gov/nchs/data/databriefs/db40.htm. [PubMed] [Google Scholar]
- 21.Hughes E, Kilmer G, Li Y Centers for Disease Control and Prevention. Surveillance for certain health behaviors among states and selected local areas: United States, 2008. MMWR Surveill Summ. 2010;59(10):1–221. [PubMed] [Google Scholar]
- 22.November-Rider D, Bray KK, Eklund K, Williams KB, Mitchell TV. Massachusetts dental public health program directors practice behaviors and perceptions of infection control. J Dent Hyg. 2012;86(3):248–255. [PubMed] [Google Scholar]
- 23.Organization for Safety, Asepsis, and Prevention. Infection control checklist for dental settings using mobile vans or portable dental equipment. 2010–2011 http://c.ymcdn.com/sites/www.osap.org/resource/resmgr/Checklists/OSAP.checklist.portabledenta.