Abstract
Background
To learn words and acquire language, children must be able to discriminate and correctly perceive phonemes. Although there has been much research on the general language outcomes of children with cochlear implants (CIs), little is known about the development of speech perception with regard to specific speech processes, such as speech discrimination.
Purpose
The purpose of this study was to investigate the development of speech discrimination in infants with CIs and identify factors that might correlate with speech discrimination skills.
Research Design
Using a Hybrid Visual Habituation procedure, we tested infants with CIs on their ability to discriminate the vowel contrast /i/-/u/. We also gathered demographic and audiological information about each infant.
Study Sample
Children who had received CIs before 2 yr of age served as participants. We tested the children at two post cochlear implantation intervals: 2–4 weeks post CI stimulation (N = 17) and 6–9 mo post CI stimulation (N = 10).
Data Collection and Analysis
The infants’ mean looking times during the novel versus old trials of the experiment were measured. A linear regression model was used to evaluate the relationship between the normalized looking time difference and the following variables: chronological age, age at CI stimulation, gender, communication mode, and best unaided pure-tone average.
Results
We found that the best unaided pure-tone average predicted speech discrimination at the early interval. In contrast to some previous speech perception studies that included children implanted before 3 yr of age, age at CI stimulation did not predict speech discrimination performance.
Conclusions
The results suggest that residual acoustic hearing before implantation might facilitate speech discrimination during the early period post cochlear implantation; with more hearing experience, communication mode might have a greater influence on the ability to discriminate speech. This and other studies on age at cochlear implantation suggest that earlier implantation might not have as large an effect on speech perception as it does on other language skills.
Keywords: cochlear implants, speech perception
INTRODUCTION
A primary reason that cochlear implants (CIs) are provided to deaf and hard-of-hearing infants is so that they may acquire spoken language. To learn words and acquire language, children must be able to discriminate and correctly perceive phonemes. Consequently, the success of CIs depends in large part on how well the devices are able to enhance the user’s speech perception skills and, specifically, their ability to correctly perceive phonemes.
Phoneme perception depends on several factors. The most obvious is the audibility of the acoustic–phonetic information in speech (i.e., hearing thresholds and dynamic range across the frequency range of the human voice). But phoneme perception also involves perceptual processes to interpret the raw acoustic–phonetic information into phonemes. The ability to process various levels of auditory information is crucial to understanding speech (Aslin and Smith, 1988; Holt, 2011). In their review of perceptual development, Aslin and Smith (1988) described three structural levels of perception that can be applied when examining the development of speech perception. The first level is the “sensory primitives” stage where sensory stimulation, such as an auditory stimulus, is detected. Development from the initial stage involves the restructuring of sensory primitives at the second level, the “perceptual representations” stage. At this level, stimulation is converted into a neural code that translates into meaningful objects or events. Although these elements can be discriminated from other elements, they do not carry semantic meaning until the final stage. The final level is the “higher order representations” stage where semantic meaning is provided to the events. Although there most certainly are top-down effects in speech perception, this perceptual framework suggests that one must first be able to detect speech to discriminate one sound from another. Subsequently, one must be able to discriminate among or between speech sounds to recognize words correctly (Holt, 2011). These higher level perceptual processes can be affected by several demographic variables related to the infant’s early experience with sound (e.g., age of deafness onset, amount of residual hearing before implantation, age at implantation). Determining the role of early auditory experience on the development of fundamental linguistic skills such as speech perception can help inform intervention strategies and, thus, help mitigate the effects of hearing loss on language development.
One demographic variable related to early auditory experience that might affect phoneme perception is age at cochlear implantation because age at implantation has been found to broadly affect language acquisition across several studies using a variety of measures. Research on the general language development of children with CIs suggests that earlier implantation leads to better vocabulary and receptive and expressive language (Kirk et al, 2002; Svirsky et al, 2004; Dettman et al, 2007; Geers et al, 2007; Nicholas and Geers, 2007; Holt and Svirsky, 2008; Miyamoto et al, 2008; Colletti, 2009; Colletti et al, 2011).
Despite these age-at-implantation findings for general language development, studies that have investigated the effects of early implantation on speech perception have been limited and have yielded mixed results. In children with CIs, investigators usually evaluate speech perception using either closed- or open-set tests of word recognition. In closed-set testing, children are asked to identify a word or select a response based on a limited number of items. In open-set testing, children are presented with words or sentences and asked to repeat back what they have heard. These measures are limited in the sense that they do not provide precise information regarding which phonemes children with CIs can and cannot discriminate and/or identify. Nonetheless, they do provide a general measure of speech perception. With these methods, some studies have found that children implanted before 3 yr of age perform better than children implanted later (Nikolopoulos et al, 2004; Svirsky et al, 2004; Zwolan et al, 2004), whereas others have not revealed age-at-implantation effects in children implanted before 2 yr of age (Holt and Svirsky, 2008; Houston and Miyamoto, 2010; Leigh et al, 2013).
One possible explanation for the lack of earlier age-at-implantation effects for speech perception is that the tests that are used might not be sensitive enough to detect differences in speech perception. Another possible explanation is that speech perception cannot be measured using these methods until the children are older for methodological/developmental reasons. Additionally, word recognition testing is influenced by vocabulary, and children with hearing loss have gaps and/or delays in their lexical development (e.g., Carney et al, 1993). Perhaps by the time the children are old enough to be tested using these conventional assessments, age-at-implantation effects among children implanted before 2 yr of age have become diminished by other factors, such as communication mode and social experience. One way to address this problem would be to test children when they are younger using materials that are not influenced by language development and methods that are developmentally appropriate. Unfortunately, the inability of very young children to participate in conventional testing batteries used in older children has largely limited their clinical assessment to parent-report questionnaires that assess general auditory attention, integration, and comprehension. With these parent assessments, some studies have found effects of age-at-implantation on the auditory skills of children implanted before their first birthday (Zwolan et al, 2004; Colletti et al, 2005; 2011).
These studies provide useful information regarding general listening skills and language outcomes in early-implanted children; however, because these studies use general language assessments, they do not provide us with information on the development of speech perception. To better assess speech perception in infants, a more sensitive and objective measure, such as speech discrimination, is needed.
To date, there have been few studies focusing on speech discrimination in early implanted children. Using a Visual Habituation (VH) procedure, Houston et al (2003) reported that prelingually deaf infants who received CIs before the age of 24 mo were able to discriminate the words “hop hop hop” and “ah” at 1 mo (n = 7), 3 mo (n = 8), and 6 mo (n = 8) post cochlear implantation. In another study, Horn et al (2007) tested ten prelingually deaf infants who were implanted before 24 mo of age and had a mean hearing age of 1.4 mo on their ability to discriminate two audiovisual nonwords, “seepug” and “boodup,” with a modified VH procedure. They found that the infants with CIs discriminated these audiovisual nonwords; however, there was no effect of age at implantation, age at test, length of CI use, or pre CI residual hearing on discrimination ability. It is important to note that one drawback to this study was that it could not be determined whether performance of the infants with CIs was due to auditory ability, visual ability, or both.
The purpose of the current study is to investigate the development of speech discrimination in infants with CIs and identify factors, such as age at implantation, that correlate with speech discrimination skills. Discovering relationships between these factors and speech perception will provide valuable information about the role of early auditory experience on the development of speech perception abilities in deaf children who use CIs.
Using a version of the Hybrid VH procedure, we tested prelingually deaf children who received CIs before 24 mo of age on their ability to discriminate the vowel contrasts /u/ and /i/. Our hypothesis is that if early auditory experience is important for speech discrimination, then age at implantation, length of CI use, and amount of residual hearing should predict performance on this speech discrimination task.
METHODS
Participants
Thirty-one children implanted at the Indiana University School of Medicine served as participants. Twenty-one participants (8 males, 13 females) were included in the final analyses; of those excluded, eight infants failed to complete the experiment and two went on to have a diagnosis of developmental delay. Although some studies have shown that children with inner ear malformations may demonstrate poorer speech perception outcomes (Rachovitsas et al, 2012; Black et al, 2014), we included two children with Mondini malformation in our study because they did not have any known developmental delay and performed within the range of the other participants. The children were from English-speaking homes. All participants had a hearing loss in the better ear of >86 dB HL, and they all received a CI before 24 mo of age. Seventeen children were tested on a speech discrimination task at 2–4 weeks post CI stimulation (early interval; N = 17, mean age = 16.4 mo, standard deviation [SD] = 3.6; mean hearing age = 0.8 mo, SD = 0.3) and ten children were tested at 6–9 mo post CI stimulation (later interval; N = 10, mean age = 23.2 mo, SD = 2.8; mean hearing age = 7.5 mo, SD = 1.8). Six children completed testing at both the early and later intervals. By caregiver report, participants did not have an ear infection at the time of testing and did not have >4 ear infections before 12 mo of age or six ear infections before 30 mo of age. All participants’ parents provided informed consent per the university Institutional Research Board policies. Parents were reimbursed $10 for their participation for each speech discrimination task.
Demographic Information
Demographic information and audiological history on each participant were collected and are summarized in Table 1. This information included age at the time of CI stimulation, chronological age at the time of speech discrimination testing, hearing age at the time of testing, gender, etiology of hearing loss, communication mode, and race. Audiological history also included the best unaided pure-tone average (PTA), obtained before implantation, as averaged across three frequencies (500, 1000, and 2000 Hz); a “no response” was coded as 120 dB HL for PTA calculation. The mean best unaided PTA for the group tested at the early interval was 108.7 dB HL (SD = 11.14, range = 86.7–120.0) and for the group tested at the later interval was 106.9 dB HL (SD = 11.35, range = 86.7–120.0). Detailed audiological information at the time of testing, including the device used in each ear (e.g., CI alone, bilateral CI, or CI with hearing aid), type of CI, and speech coding strategies, is summarized in Table 2. Of note, all children with bilateral CIs were implanted simultaneously. Using an independent samples t test, we found no significant difference in the best unaided PTA between the group tested at the early interval versus the group tested at the later interval.
Table 1.
Summary of Participant Demographics
| Later Interval (N = 10)
|
Early Interval (N = 17)
|
|||
|---|---|---|---|---|
| n | Mean (SD; Range) | n | Mean (SD; Range) | |
| Age at stimulation (mo) | 17 | 15.6 (3.5; 10.2–21.8) | 10 | 15.8 (2.0; 11.2–18.3) |
| Hearing age at test (mo) | 17 | 16.4 (3.6; 10.7–22.4) | 10 | 23.2 (2.8; 17.5–28.4) |
| Best unaided PTA (dB HL) | 17 | 0.8 (0.3; 0.5–1.2) 10 | 10 | 7.5 (1.8; 5.8–10.1) |
| Chronological age at test (mo) | 17 | 108.7 (11.1; 86.7–120) | 10 | 106.9 (11.4; 86.7–120) |
|
| ||||
| n | Percent | n | Percent | |
|
| ||||
| Gender | ||||
| Female | 9 | 52.9 | 7 | 70.0 |
| Male | 8 | 47.1 | 3 | 30.0 |
| Etiology of hearing loss | ||||
| Auditory neuropathy | 1 | 5.9 | 0 | 0 |
| CMV | 1 | 5.9 | 0 | 0 |
| Genetic | 3 | 17.6 | 1 | 10.0 |
| Mondini malformation | 1 | 5.9 | 1 | 10.0 |
| Unknown | 11 | 64.7 | 8 | 80.0 |
| Communication mode | ||||
| OC/cued | 13 | 76.5 | 8 | 80.0 |
| Sign/TC | 4 | 23.5 | 2 | 20.0 |
| Race | ||||
| White | 13 | 76.5 | 7 | 70.0 |
| Black | 3 | 17.6 | 3 | 30.0 |
| Other | 1 | 5.9 | 0 | 0 |
Notes: The column percentages show the percent of children with CIs in the early/later intervals with a specific characteristic. CMV = cytomegalovirus infection.
Table 2.
Participant Audiologic Information
| Participant | Right Ear Device Type | Left Ear Device Type | Right Ear Device System | Left Ear Device System | Right Ear Electrode Insertion | Left Ear Electrode Insertion | Right CI Speech Coding Strategy | Left CI Speech Coding Strategy | Right Unaided Preimplant PTA (dB HL) | Left Unaided Preimplant PTA (dB HL) | Right HA-Aided PTA (dB HL) | Left HA-Aided PTA (dB HL) | Right CI-Aide d PTA (dB HL) | Left CI-Aided PTA (dB HL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Interval | ||||||||||||||
|
| ||||||||||||||
| 1 | CI | CI | Nucleus System 5 | Nucleus System 5 | 22 | 22 | ACE | ACE | 108 | 95 | U | U | 35 | 40 |
| 2 | CI | CI | MedEl Concerto | MedEl Concerto | 19 | 19 | U | U | 120 | 120 | 120 | 120 | 40 | 35 |
| 3 | CI | CI | Nucleus System 5 | Nucleus System 5 | 22 | 22 | ACE | ACE | 108 | 107 | U | U | 33 | 35 |
| 4 | CI | None | Nucleus System 5 | NA | 22 | NA | ACE | NA | 120 | 120 | U | U | 35 | NA |
| 5 | CI | HA | Nucleus 512 | U | 22 | NA | ACE | NA | 100 | 92 | 87 | U | NA | 40 |
| 6 | CI | None | Nucleus Freedom | NA | 8 | NA | ACE | NA | 120 | 115 | U | U | 42 | NA |
| 7 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 90 | 107 | U | U | 22 | NA |
| 8 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 120 | 120 | U | U | 43 | NA |
| 9 | CI | None | Advance Bionics HiRes 90K | NA | U | NA | HiRes-P | NA | 120 | 120 | 120 | 120 | 32 | NA |
| 10 | None | CI | NA | Nucleus Freedom Contour | NA | 22 | NA | ACE | 105 | 120 | 88 | U | NA | 42 |
| 11 | CI | CI | Nucleus System 5 | Nucleus System 5 | 22 | 10 | ACE | ACE | 108 | 115 | U | U | 35 | 32 |
| 12 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 120 | 120 | U | U | 22 | NA |
| 13 | HA | CI | U | Nucleus Freedom | NA | 22 | NA | ACE | 86.7 | 93 | 90 | U | NA | 30 |
| 14 | CI | HA | Nucleus Freedom Contour | U | 22 | NA | ACE | NA | 98 | 95 | U | 70 | 27 | NA |
| 15 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 120 | 120 | U | U | 73 | NA |
| 16 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 120 | 120 | 120 | 107 | 23 | NA |
| 17 | CI | CI | Nucleus Freedom Contour | Nucleus Freedom Contour | 22 | 22 | ACE | ACE | 107 | 112 | U | U | U | U |
|
| ||||||||||||||
| Later Interval | ||||||||||||||
|
| ||||||||||||||
| 1 | CI | CI | Nucleus 512 | Nucleus 512 | 22 | 22 | ACE | ACE | 113 | 105 | U | U | 22 | 33 |
| 2 | CI | CI | Nucleus 512 | Nucleus 512 | 22 | 22 | ACE | ACE | 110 | 108 | U | U | 35 | 30 |
| 3 | CI | CI | Nucleus 512 | Nucleus 512 | 22 | 22 | ACE | ACE | 120 | 120 | U | U | 27 | 35 |
| 4 | HA | CI | U | Nucleus Freedom | NA | 22 | NA | ACE | 86.7 | 93 | 90 | U | NA | 30 |
| 5 | CI | HA | Nucleus Freedom Contour | U | 22 | NA | ACE | NA | 98 | 95 | U | 70 | 27 | NA |
| 6 | CI | HA | Nucleus 512 | U | 22 | NA | ACE | NA | 100 | 92 | 87 | U | NA | 40 |
| 7 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 90 | 107 | U | U | 22 | NA |
| 8 | CI | None | Nucleus Freedom Contour | NA | 22 | NA | ACE | NA | 120 | 120 | U | U | 43 | NA |
| 9 | CI | None | Advance Bionics HiRes 90K | NA | U | NA | HiRes-P | NA | 120 | 120 | 120 | 120 | 32 | NA |
| 10 | CI | None | Nucleus Freedom | NA | 11 | NA | ACE | NA | 120 | 120 | U | U | U | RA |
Note: HA = hearing aid; U = unknown; NA = not applicable.
Speech Discrimination
Apparatus
The experiment was conducted in a sound booth. A 55″ wide-screen television monitor was located inside the sound booth with a wooden façade built around it so that only the screen was visible to the participants. A camera was used to watch and record infants’ looking behavior and was placed above the television monitor behind a small hole in the façade. The camera was connected via closed circuit to an observation monitor located in a control room adjacent to the sound booth. During testing, an experimenter observed from the control room and controlled the experiment using a G4 Macintosh computer running Habit software (Cohen et al, 2004), which contained the audio and image files used to test the infants.
Auditory Stimuli
Auditory stimuli consisted of the vowel contrast /i/-/u/. A single, synthetically produced token of each vowel was created using the KLSYN speech synthesis program (Klatt, 1980) and a Pentium III computer. The duration of each token was 410 msec, and the interstimulus interval was 400 msec. The formant values were based on Hillenbrand et al (1995). F1 for /i/ was 270 Hz and for /u/ was 300 Hz. F2 for /i/ was 2300 Hz and for /u/ was 850 Hz. The total root-mean-square power was equalized between the two vowels. These vowels were chosen based on previous studies showing that 8- to 12-mo-old normal-hearing infants could easily discriminate this contrast (Trehub, 1973; Tsao et al, 2004). In a preliminary study, 9-mo-old children with normal hearing (n = 40) who were tested in our laboratory could also discriminate this contrast easily (p < 0.0001). Auditory stimuli were presented above the children’s aided CI thresholds at an average root-mean-square of 65 dBA.
Visual Stimuli
A single image of a checkerboard pattern was displayed concurrently with all auditory stimuli during each trial of the experiment. Additionally, a silent video of a smiling baby was used as an attention-getter before each trial, and a computer-graphic animation consisting of a looming geometric shape paired with a sequence of short, frequency-varying tones was used to gauge the infants’ general attention level before and after the experiment.
Procedure
The experiments were conducted using a version of the Hybrid VH procedure (Houston et al, 2007). Figure 1 illustrates the experiment set-up. Infants were seated on their caregiver’s lap in front of the television monitor. At the beginning of each trial, a video of a smiling baby (the attention-getter) was presented in the center of the screen until the infant oriented to the center. Then, the attention-getter turned off and a checkerboard pattern appeared concurrently with repeated presentations of the auditory stimuli. Each trial continued until the infant looked away for one second or until the infant looked for a maximum of 30 sec. The amount of time the infant looked at the checkerboard while the stimuli were presented was recorded for each trial in real time by the experimenter.
Figure 1.
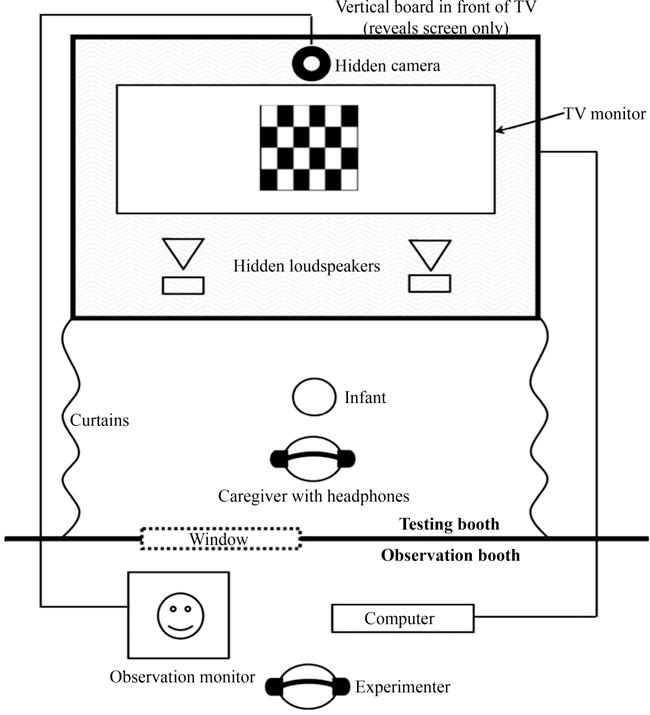
Hybrid VH procedure.
The experiment consisted of two phases: habituation and test. During the habituation phase, the infants were presented with repetitions of one of the vowels (e.g., /u/). The habituation phase continued until a maximum of 15 trials was reached or until the habituation criterion was met. The habituation criterion was defined as three consecutive trials in which the infant’s mean looking time to the video was ≤50% of the infant’s mean looking time during the first three trials. After the habituation criterion was reached, the test phase began.
During the test phase, infants were presented with ten “old” trials and four “novel” trials in pseudorandom order. The old trials consisted of repetitions of the vowel presented during habituation (i.e., /u/-/u/-/u/…). The novel trials consisted of repetitions of the vowel presented during habituation alternating with repetitions of the novel vowel (i.e., /i/-/u/-/i/…). The first two test trials consisted of a novel trial and an old trial, the order of which was counterbalanced across participants. The remaining 12 test trials were grouped into three blocks of four trials. Within each block, there were three old trials and one novel trial presented in random order. However, if one block ended with a novel trial then the subsequent block did not begin with a novel trial, which prevented the occurrence of two consecutive novel trials. After the experiment was completed, the infant’s mean looking times to the checkerboard pattern during the novel versus old trials were measured.
RESULTS
The normalized looking time difference between the novel and old trials was calculated using the following equation:
A linear regression model was then used to evaluate the relationship between the normalized looking time difference and the following variables: chronological age, age at CI stimulation, gender, communication mode, and the best unaided PTA. Early and late intervals were analyzed with separate linear regression models, which predicted 32% of the variance in looking time difference at the early interval and 52% of the variance at the later interval. At the early interval, the results revealed that the best unaided PTA was the only factor that accounted for a significant amount of variance in looking time differences. The best unaided PTA significantly predicted speech discrimination based on normalized looking time difference values at the early interval [b = −0.57, t(17) = −2.65, p = 0.018], but not at the later interval. These results are displayed in Table 3. Figure 2 illustrates the normalized looking time difference versus the best unaided PTA at the early interval on a scatterplot. Among the early group, approximately half of the participants (9 out of 17) had the best unaided PTAs >110 dB HL; these participants significantly discriminated between the novel versus the old stimuli [t(8) = 2.089, p = 0.035 (one-tailed)].
Table 3.
Predictors of Performance on Discrimination Task at 2–4 Weeks Post CI Stimulation (Early Interval)
| B | SE B | β | |
|---|---|---|---|
| Constant | 3.42 | 1.27 | |
| Best unaided PTA | −0.03 | 0.01 | −0.57* |
| Chronological age | 0.11 | 0.70 | 0.62 |
| Age at CI stimulation | −0.12 | 0.72 | −0.68 |
| Gender | 0.09 | 0.40 | 0.08 |
| Communication mode | 0.30 | 0.36 | 0.21 |
Notes: R2 = 0.319.
p < 0.05. B = the unstandardized regression coefficient; SE B = the standard error of that unstandardized coefficient.
Figure 2.
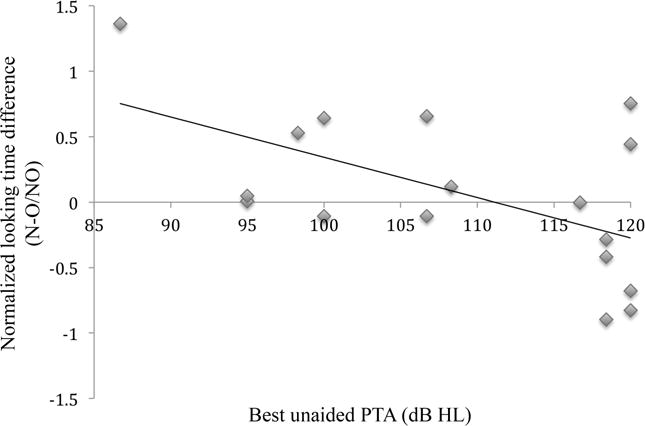
Scatterplot and regression line of the regression analyses, plotting normalized looking time difference versus best unaided PTA at 2–4 weeks post CI stimulation (early interval). N = average looking time to novel trials; O = average looking time to old trials; NO = average looking time to novel and old trials.
At the later interval, communication mode was the only factor that significantly influenced normalized looking time difference values [β = 0.87, t(10) = 2.698, p = 0.031]. Children who use an oral mode of communication (OC) generally performed better than children who use total communication (TC). However, only two participants used TC, so the results as shown in Table 4 should be interpreted with much caution.
Table 4.
Predictors of Performance on Discrimination Task at 6–9 Months Post CI Stimulation (Later Interval)
| B | SE B | β | |
|---|---|---|---|
| Constant | −2.66 | 1.26 | |
| Best unaided PTA | 0.02 | 0.01 | 0.60 |
| Chronological age | −0.03 | 0.08 | −0.22 |
| Age at CI stimulation | 0.14 | 0.09 | −0.76 |
| Gender | 0.17 | 0.20 | 0.22 |
| Communication mode | 0.76 | 0.28 | 0.87* |
Notes: R2 = 0.52.
p < 0.05. B = the unstandardized regression coefficient; SE B = the standard error of that unstandardized coefficient.
Age at CI stimulation, chronological age, and gender were not significant predictors of speech discrimination at either testing interval.
DISCUSSION
The goal of the present study was to investigate the development of speech discrimination in infants with CIs and identify factors that might correlate with speech discrimination skills. We found that residual hearing (as measured by the best unaided PTA) at 2–4 weeks post cochlear implantation was significantly correlated with performance on a vowel discrimination task. However, there was no significant influence of chronological age, age at CI stimulation, or gender.
The results suggest that some amount of residual acoustic hearing before implantation may facilitate the development of speech discrimination early on. At the early interval, children with comparatively more residual hearing discriminated the contrasts better than children with less hearing before cochlear implantation, although these results should be interpreted cautiously given the small sample size. One possible explanation for these findings may be related to the fact that children with more residual hearing have had more access to auditory information from birth. Having more access to hearing, even nonoptimal, limited amounts that is amplified through hearing aids or that is unamplified (and naturally intense), from the very beginning of development might be more valuable for speech perception than receiving more robust access to sound via a CI at an earlier age. The access, albeit limited, to sound from birth might, for example, lead to these infants to attend more to speech (Houston and Bergeson, 2014). Another possibility is that the residual hearing provides infants with complementary acoustic hearing to their electric hearing. This may be especially important during the early post cochlear implantation periods before the infants adapt to the electric hearing.
In the later interval of testing, we found that communication mode was a significant predictor of performance on this speech discrimination task. Children who employed an OC had better vowel discrimination than children who used TC. However, these results should be interpreted conservatively as there were only two children in the later interval group who used TC. Additionally, it is possible that the difference in performance between children using OC versus TC could be influenced by factors that were not accounted for in this study, such as cognitive ability and socioeconomic status. Nevertheless, other studies have similarly demonstrated that, with device experience, children who use OC often perform better than children who use TC on some speech perception tasks (Meyer et al, 1998; Osberger et al, 1998; O’Donoghue et al, 2000; Young et al, 2000; Kirk et al, 2002). In the current study, the auditory modality was the only process tested. Thus, one possible explanation for this finding is that children who use OC may rely more heavily on auditory cues in their daily interactions than children who use TC, who also employ signing and other visual aids when communicating. As a result, children who use OC may be able to perform better on an auditory-only discrimination task.
Our findings are consistent with other studies indicating that the age of implantation at <2 yr of age does not have an effect on speech perception skills (Harrison et al, 2005; Horn et al, 2007). However, studies that have investigated broad measures of language development (e.g., vocabulary, word learning) have demonstrated that earlier implantation leads to better language outcomes (Kirk et al, 2002; Svirsky et al, 2004; Dettman et al, 2007; Geers et al, 2007; Nicholas and Geers, 2007; Holt and Svirsky, 2008; Miyamoto et al, 2008; Colletti, 2009; Colletti et al, 2011; Houston et al, 2012). One possible reason to explain these seemingly opposing findings is that very early implantation (i.e., <1 yr) may not necessarily help deaf children to hear or discriminate phonemes better, but it may aid in the development of other processes related to language development, such as the ability to learn associations between spoken words and their referents (i.e., word learning).
Houston et al (2012) investigated the effects of early cochlear implantation on word-learning skills and found that toddlers who had their CIs activated between 7 and 14 mo of age were significantly better at a novel word-learning task than toddlers who had their CIs activated between 16 and 22 mo of age. Their findings suggest that early access to sound via a CI may facilitate the ability to learn novel words, which would put these children on a course for better language outcomes. Early access to speech may improve other skills as well, such as working memory and sensory integration, which would allow these children to better learn language.
It is important to mention several limitations of this study. First, the sample sizes for this study were small (n = 17 at the early interval and n = 10 at the later interval). Analyses with such a small sample size should be interpreted conservatively. Further, there were fewer children overall who used TC (n = 4 at the early interval and n = 2 at the later interval) versus OC. Another limitation of this study was that speech discrimination was assessed using one point-vowel contrast, /u/ vs. /i/. It may be useful to determine whether CI children perform similarly with other contrasts, such as those involving consonant changes, vowels with more similar formant values, or rhythmic differences, and whether the same factors influence discrimination of these contrasts. Lastly, the current study only evaluated five factors that might influence speech discrimination skills: chronological age, age at CI stimulation, best unaided PTA, gender, and communication mode. To perform a more thorough assessment, it may be helpful to obtain information on other potential factors that might impact speech discrimination, such unilateral versus bilateral CI at the time of testing, cognitive assessment scores, and information on socioeconomic status or maternal education.
The present study adds to our knowledge of the role of early auditory experience on the development of speech perception abilities in deaf children who use CIs. It is among the few studies that have assessed speech perception in young children using direct behaviorally based assessment rather than parental report. Moreover, it is the only study we know of to date to investigate the effects of residual hearing on speech discrimination. Future studies involving a larger sample size, a number of varied speech contrasts, and a more comprehensive evaluation of potential factors influencing speech discrimination are needed to strengthen the results of this study.
Acknowledgments
This work was supported by the NIH/NIDCD Research Grant (R01 DC006235) and by the NIH/NIDCD Training Grant (T32 DC0012).
Abbreviations
- CI
cochlear implant
- OC
oral mode of communication
- PTA
pure-tone average
- SD
standard deviation
- TC
total communication
- VH
Visual Habituation
References
- Aslin RN, Smith LB. Perceptual development. Annu Rev Psychol. 1988;39:435–473. doi: 10.1146/annurev.ps.39.020188.002251. [DOI] [PubMed] [Google Scholar]
- Black J, Hickson L, Black B, Khan A. Paediatric cochlear implantation: adverse prognostic factors and trends from a review of 174 cases. Cochlear Implants Int. 2014;15(2):62–77. doi: 10.1179/1754762813Y.0000000045. [DOI] [PubMed] [Google Scholar]
- Carney AE, Osberger MJ, Carney E, Robbins AM, Renshaw J, Miyamoto RT. A comparison of speech discrimination with cochlear implants and tactile aids. J Acoust Soc Am. 1993;94(4):2036–2049. doi: 10.1121/1.407477. [DOI] [PubMed] [Google Scholar]
- Cohen LB, Atkinson DJ, Chaput HH. Habit X: A new Program for Obtaining and Organizing Data in Infant Perception and Cognition Studies (Version 1.0) Austin, TX: University of Texas; 2004. [Google Scholar]
- Colletti L. Long-term follow-up of infants (4–11 months) fitted with cochlear implants. Acta Otolaryngol. 2009;129(4):361–366. doi: 10.1080/00016480802495453. [DOI] [PubMed] [Google Scholar]
- Colletti L, Mandalà M, Zoccante L, Shannon RV, Colletti V. Infants versus older children fitted with cochlear implants: performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011;75(4):504–509. doi: 10.1016/j.ijporl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino FG. Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope. 2005;115(3):445–449. doi: 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- Dettman SJ, Pinder D, Briggs RJ, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28(2 Suppl):11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG, Moog JS. Estimating the influence of cochlear implantation on language development in children. Audiol Med. 2007;5(4):262–273. doi: 10.1080/16513860701659404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–261. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- Hillenbrand J, Getty LA, Clark MJ, Wheeler K. Acoustic characteristics of American English vowels. J Acoust Soc Am. 1995;97(5, Pt 1):3099–3111. doi: 10.1121/1.411872. [DOI] [PubMed] [Google Scholar]
- Holt RF. Enhancing speech discrimination through stimulus repetition. J Speech Lang Hear Res. 2011;54(5):1431–1447. doi: 10.1044/1092-4388(2011/09-0242). [DOI] [PubMed] [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29(4):492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Houston DM, Miyamoto RT. Speech discrimination skills in deaf infants before and after cochlear implantation. Audiol Med. 2007;5:232–241. [Google Scholar]
- Houston DM, Horn DL, Qi R, Ting JY, Gao S. Assessing speech discrimination in individual infants. Infancy. 2007;12(2):119–145. doi: 10.1111/j.1532-7078.2007.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Houston DM, Miyamoto RT. Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants: implications for sensitive periods of language development. Otol Neurotol. 2010;31(8):1248–1253. doi: 10.1097/MAO.0b013e3181f1cc6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DM, Stewart J, Moberly A, Hollich G, Miyamoto RT. Word learning in deaf children with cochlear implants: effects of early auditory experience. Dev Sci. 2012;15(3):448–461. doi: 10.1111/j.1467-7687.2012.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DM, Bergeson TR. Hearing versus listening: attention to speech and its role in language acquisition in deaf infants with cochlear implants. Lingua. 2014;139:10–25. doi: 10.1016/j.lingua.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DM, Pisoni DB, Kirk KI, Ying EA, Miyamoto RT. Speech perception skills of deaf infants following cochlear implantation: a first report. Int J Pediatr Otorhinolaryngol. 2003;67(5):479–495. doi: 10.1016/s0165-5876(03)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI, Miyamoto RT, Ying EA, Perdew AE, Zuganelis H. Cochlear implantation in young children: effects of age at implantation and communication mode. Volta Review. 2002;102:127–144. monograph. [Google Scholar]
- Klatt DH. Software for a cascade/parallel formant synthe-sizer. J Acoust Soc Am. 1980;67:971–995. [Google Scholar]
- Leigh J, Dettman S, Dowell R, Briggs R. Communication development in children who receive a cochlear implant by 12 months of age. Otol Neurotol. 2013;34(3):443–450. doi: 10.1097/MAO.0b013e3182814d2c. [DOI] [PubMed] [Google Scholar]
- Meyer TA, Svirsky MA, Kirk KI, Miyamoto RT. Improvements in speech perception by children with profound prelingual hearing loss: effects of device, communication mode, and chronological age. J Speech Lang Hear Res. 1998;41(4):846–858. doi: 10.1044/jslhr.4104.846. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Hay-McCutcheon MJ, Kirk KI, Houston DM, Bergeson-Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Otolaryngol. 2008;128(4):373–377. doi: 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res. 2007;50(4):1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos TP, Gibbin KP, Dyar D. Predicting speech perception outcomes following cochlear implantation using Not-tingham children’s implant profile (NChIP) Int J Pediatr Otorhi-nolaryngol. 2004;68(2):137–141. doi: 10.1016/j.ijporl.2003.09.020. [DOI] [PubMed] [Google Scholar]
- O’Donoghue GM, Nikolopoulos TP, Archbold SM. Determinants of speech perception in children after cochlear implantation. Lancet. 2000;356(9228):466–468. doi: 10.1016/S0140-6736(00)02555-1. [DOI] [PubMed] [Google Scholar]
- Osberger MJ, Fisher L, Zimmerman-Phillips S, Geier L, Barker MJ. Speech recognition performance of older children with cochlear implants. Am J Otol. 1998;19(2):152–157. [PubMed] [Google Scholar]
- Rachovitsas D, Psillas G, Chatzigiannakidou V, Triaridis S, Constantinidis J, Vital V. Speech perception and production in children with inner ear malformations after cochlear implantation. Int J Pediatr Otorhinolaryngol. 2012;76(9):1370–1374. doi: 10.1016/j.ijporl.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9(4):224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Trehub SE. Infants’ sensitivity to vowel and tonal contrasts. Dev Psychol. 1973;9:91–96. [Google Scholar]
- Tsao FM, Liu HM, Kuhl PK. Speech perception in infancy predicts language development in the second year of life: a longitudinal study. Child Dev. 2004;75(4):1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Young NM, Grohne KM, Carrasco VN, Brown CJ. Speech perception in young children using nucleus or Clarion Cochlear Implants: effect of communication mode. Ann Otol Rhinol Lar-yngol Suppl. 2000;185:77–79. doi: 10.1177/0003489400109s1233. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Ashbaugh CM, Alarfaj A, Kileny PR, Arts HA, El-Kashlan HK, Telian SA. Pediatric cochlear implant patient performance as a function of age at implantation. Otol Neurotol. 2004;25(2):112–120. doi: 10.1097/00129492-200403000-00006. [DOI] [PubMed] [Google Scholar]


