Summary
Abnormal tumor vessels promote metastasis and impair chemotherapy. Hence, tumor vessel normalization (TVN) is emerging as anti-cancer treatment. Here, we show that tumor endothelial cells (ECs) have a hyper-glycolytic metabolism, shunting intermediates to nucleotide synthesis. EC haplo-deficiency or blockade of the glycolytic activator PFKFB3 did not affect tumor growth, but reduced cancer cell invasion, intravasation and metastasis by normalizing tumor vessels, which improved vessel maturation and perfusion. Mechanistically, PFKFB3 inhibition tightened the vascular barrier by reducing VE-cadherin endocytosis in ECs, and rendering pericytes more quiescent and adhesive (via upregulation of N-cadherin) through glycolysis reduction; it also lowered the expression of cancer cell adhesion molecules in ECs by decreasing NF-κB signaling. PFKFB3-blockade treatment also improved chemotherapy of primary and metastatic tumors.
Graphical Abstract

Introduction
Endothelial cell (EC) metabolism has gained attention as therapeutic target for inhibiting angiogenesis. We reported that blocking the glycolytic activator PFKFB3 or the fatty acid oxidation regulator CPT1a reduced angiogenesis in ocular and inflammatory disorders (De Bock et al., 2013a; Schoors et al., 2015; Schoors et al., 2014), but did not study the effect of the PFKFB3-blocker 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) on tumor vessels. Tumor endothelial cells (TECs) are activated cells (Jain, 2014), but it is unknown if they have an altered glycolytic metabolism, and if targeting glucose metabolism in TECs offers therapeutic benefit.
Tumor vessels are structurally and functionally abnormal (Jain, 2014). They are irregular in shape and size, tortuous and morphologically heterogeneous. This impairs perfusion, which deprives cancer cells from oxygen and nutrients, thus creating a hostile milieu from where cancer cells escape via invasion and metastasis (Carmeliet and Jain, 2011; Jain, 2014). Tumor vessels have a leaky EC barrier, facilitating intravasation and dissemination of cancer cells. They also have fewer, more detached pericytes, which further destabilizes vessels (Carmeliet and Jain, 2011). The perfusion defects also impair the delivery and efficacy of chemotherapy, as the latter often relies on conversion of oxygen to radicals, and oxygen supply in tumors is limited (Jain, 2014).
In contrast to traditional anti-angiogenic therapy that aims to inhibit tumor vessel growth, an emerging paradigm is to normalize tumor vessels in order to restore perfusion, and thereby to reduce metastasis while improving chemotherapy (Jain, 2014). This involves normalization of the endothelial layer, basement membrane and mural cells. However, all vessel normalization strategies focus on targeting angiogenic growth factors and downstream signaling. Here, we assessed if targeting PFKFB3 in ECs affects tumor vessels.
Results
Tumor endothelial cells (TECs) are highly glycolytic
We isolated TECs and characterized their metabolic profile. We injected B16-F10 melanoma cells in the portal vein (“p.v. B16 (liver)” model) of WT mice to induce tumor growth in the liver. After 14 days, we isolated ECs from the tumor-infested livers and, as controls, normal endothelial cells (NECs) from livers of healthy mice. As tumors represented 70–80% of the tissue volume in tumor-infested livers, the isolated EC population was highly enriched in TECs (“eTECs”), but still contained a minor fraction of NECs. Compared to NECs, eTECs proliferated and migrated more actively (Figures 1A and 1B). To assess which metabolic pathway was more active in eTECs versus NECs, we performed exploratory RNA-sequencing, which suggested that eTECs were hyperglycolytic, and then confirmed these findings with targeted metabolomics analysis.
Figure 1. Characterization of tumor endothelial cells.
(A) Proliferation of eTECs, expressed relative to NECs (n=3 biological repeats of pooled ECs isolated from 10–15 mice). (B) Migration of Mitomycin C-treated NECs and eTECs (n=5). (C) Correlation heatmap and hierarchical cluster analysis of transcript levels of 1,255 metabolic genes in NECs and eTECs (numbers in panel refer to individual samples n=4). Color scale: red, high correlation; blue, low correlation. Hierarchical clustering: color differences in dendrogram indicate significant clustering (p value < 0.05; multiscale bootstrap analysis). (D) Pathway map showing changes in transcript levels in eTECs (relative to NECs) of genes involved in glycolysis and side pathways (n=4; green: upregulated by at least 15%; gray: unchanged, fold change < 15%). (E) Heatmap and cluster analysis of transcript levels of genes in glycolysis and side pathways in eTECs versus NECs (numbers in panel refer to individual samples n=4). For color scale and clustering, see panel C. (F) Pathway map showing changes in transcript levels in eTECs (relative to NECs) of genes of nucleotide synthesis (n=4; green: upregulated by at least 15%; gray: unchanged, fold change < 15%). Ribonucleotides (red); deoxyribonucleotides (blue). (G) Correlation heatmap and cluster analysis of metabolites (shown in panel H) of glycolysis, PPP and nucleotide synthesis in eTECs versus NECs (numbers in panel refer to individual samples n=5). For color scale and clustering, see panel C. (H) Steady state metabolite levels of metabolites of glycolysis, PPP and nucleotide synthesis in eTECs, relative to NECs (n=10). Dotted line: expression level in NECs. (I) Glucose levels in medium of eTECs, relative to NECs (n=5). (J) Lactate levels in medium of eTECs, relative to NECs (n=5). All data are mean ± SEM. * p value < 0.05. For panel H, p values were calculated by one sample t-test. See also Figure S1, Tables S1–S4.
When performing RNA-sequencing of eTECs and NECs, we focused on the 1,255 metabolic genes, detectable in ECs. Correlation heatmap analysis and hierarchical clustering revealed that NECs and eTECs group into distinct metabolic clusters, indicating that their metabolic gene signatures differed (Figure 1C). Testing 10 focused self-contained gene sets in central carbon metabolism using the rotation gene set testing (ROAST) tool to assess the significance of changes in metabolic pathways as a unit (Wu et al., 2010) showed that glycolysis was upregulated and had the highest fraction of upregulated genes of all pathways in central metabolism (FDR-adjusted p value = 0.023) (Table S1).
Subsequent pathway mapping and heatmap analysis revealed that transcripts of most glycolytic genes were upregulated in eTECs (Figures 1D and 1E; Table S2), including PFKFB3, the glucose transporter GLUT1 (Slc2a1), and rate-limiting enzymes of glycolytic side-pathways, such as the pentose phosphate pathway (PPP) (glucose-6-phosphate dehydrogenase (G6pdx); hexose-6-phosphate dehydrogenase (H6pd)) and serine biosynthesis pathway (SBP) (phosphoglycerate dehydrogenase (Phgdh)), involved in biomass (nucleotide) synthesis (Figures 1D and 1E). A similar analysis revealed upregulation in eTECs of genes involved in nucleotide synthesis (Figure 1F; Table S3). RT-PCR confirmed mRNA upregulation in eTECs of enzymes involved in glycolysis (including PFKFB3) and nucleotide synthesis, as well as in cell proliferation (Figure S1A). As the data suggest that eTECs increase glycolysis to support proliferation and biomass production, we focused on glucose metabolism.
Using liquid chromatography-mass spectrometry (LC-MS), we measured steady state levels of a defined set of metabolites of glycolysis, PPP and nucleotide synthesis in eTECs to further establish the importance of these pathways. Correlation heatmap analysis, hierarchical clustering and principal component analysis showed that NECs and eTECs cluster in 2 separate groups (p value < 0.05), suggestive of a distinct metabolic profile. Comparing levels of measured metabolites by metabolite set analysis using ROAST confirmed that eTECs differed from NECs (FDR-adjusted p value = 0.0104) (Figures 1G and S1B). Levels of some metabolites of these pathways tended to be higher or were elevated in eTECs (Figure 1H; Table S4). Further characterization showed that glucose consumption and lactate excretion in the medium were increased (Figures 1I and 1J).
Metabolic pathway analysis further revealed that glycolytic flux was nearly 3-fold higher in eTECs (Figure 2A), while glucose oxidation and oxygen consumption linked to ATP production were not altered (Figures 2B and 2C). Also, eTECs incorporated more 14C-label from 14C-glucose into RNA and DNA (Figure 2D), implying that eTECs utilized more glucose carbons for biomass production. Hypoxia (omnipresent in tumors (Carmeliet and Jain, 2011)) increased glycolysis in NECs and eTECs (Figures 2E and 2F; gray bars). These data render glycolysis, and the glycolytic activator PFKFB3, in eTECs an attractive target.
Figure 2. Effect of genetic inhibition of PFKFB3 on B16-F10 tumor progression and metastasis.
(A) Glycolytic flux in eTECs, relative to NECs (n=3–5). (B) Glucose oxidation flux in eTECs, relative to NECs (n=10). (C) Basal and ATP-linked (oligomycin-sensitive) oxygen consumption (OCR) in NECs and eTECs (n=5). (D) Incorporation of 14C-glucose label in DNA and RNA in NECs and eTECs (n=4–5); dpm, disintegrations per minute. (E) Glycolytic flux in NECs from WT and Pfkfb3+/ΔEC mice, exposed to normoxia (21% oxygen) or hypoxia (0.5% oxygen) (n=5); values normalized to flux in normoxic WT cells. (F) Glycolytic flux in eTECs from WT and Pfkfb3+/ΔEC mice exposed to normoxia (21% oxygen) or hypoxia (0.5% oxygen) (n=5–10); values normalized to flux in normoxic WT cells. (G) Growth curve of s.c. B16-F10 tumors in WT and Pfkfb3+/ΔEC mice (n=10–20). (H) End-stage tumor weight of s.c. B16-F10 tumors in WT and Pfkfb3+/ΔEC mice (n=10–20). (I) Micrographs of H&E staining of necrotic areas (asterisks within dotted lines) in B16-F10 tumors in WT and Pfkfb3+/ΔEC mice; quantification of necrotic area is indicated (% of total tumor area; n=7–11). (J) Quantification of metastatic index (lung metastases / gram tumor) in s.c. B16-F10 tumor-bearing WT and Pfkfb3+/ΔEC mice (n=15–22). (K) Micrographs of H&E-stained B16-F10 tumor sections of cancer cell invasion in WT and Pfkfb3+/ΔEC mice. Dotted line: border between tumor and surrounding muscle; arrows: residual muscle tissue. (L) Micrographs of s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice, stained for CD31 and endoglin to assess intraluminal CD31− endoglin+ cancer cells (arrows). (M) Quantification of intraluminal cancer cells per vessel in (L) (n=12–16). (N) Quantification of % of vessels with intraluminal cancer cells in (L) (n=12–16). (O) Quantification of B16-F10 cancer cell colonies, upon isolation and culturing of circulating B16-F10 cancer cells from s.c. B16-F10 tumor bearing WT and Pfkfb3+/ΔEC mice (3 biological repeats of 4–6 pooled individual animals each). (P) Quantification of metastatic area in lungs (area of metastatic lesions, % of total lung area) upon tail vein injection of B16-F10 cancer cells in WT and Pfkfb3+/ΔEC mice (n=6–9). Bars: 75 µm (I), 50 µm (K,L). All data are mean ± SEM. * p value < 0.05. See also Figure S2.
Strategies for genetic and pharmacological inhibition of PFKFB3
To elucidate PFKFB3’s role in tumor angiogenesis, we used a genetic and pharmacological approach to inhibit PFKFB3. For the genetic approach, we crossed VE-cadherin(PAC)-CreERT2 mice (Benedito et al., 2009) with Pfkfb3+/lox mice (De Bock et al., 2013b), and treated 8 week old progenies with tamoxifen to obtain Pfkfb3+/ΔEC mice, haplodeficient for PFKFB3 in ECs. We then subcutaneously (s.c.) implanted in Pfkfb3+/ΔEC mice syngeneic B16-F10 melanoma (“s.c. B16” model) or Lewis lung carcinoma (LLC) cells (“s.c. LLC” model). PCR analysis showed that the Pfkfb3 allele was correctly recombined (Figure S2A), while RT-PCR confirmed that Pfkfb3 mRNA levels were reduced in NECs from PFKFB3+/ΔEC mice (Figure S2B). Pfkfb3+/ΔEC mice were healthy, fertile, gained normal body weight and had normal organ vessel densities (Figure S2C). Glycolysis was lower in NECs and TECs from Pfkfb3+/ΔEC mice, but hypoxia increased glycolysis in both genotypes (Figures 2E and 2F; blue bars). To assess the effects of pharmacological PFKFB3-blockade by 3PO treatment, we used 2 tumor models, i.e. orthotopic implantation of pancreatic Panc02 and the “s.c. B16” model (Mazzone et al., 2009).
PFKFB3 inhibition in ECs reduces tumor invasion and metastasis
B16-F10 tumor volume, end-stage tumor weight, cancer cell proliferation and apoptosis were comparable in WT and Pfkfb3+/ΔEC mice (Figure 2G, 2H, and S2D–S2F). In contrast, tumor necrosis, metastasis and invasion were reduced, and tumors had more sharply demarcated borders in Pfkfb3+/ΔEC mice (Figures 2I–2K, S2G, and S2,H). Similar results were obtained in the LLC tumor model (Figure S2I–S2M).
Double staining for CD31 and endoglin, labeling both ECs and B16-F10 cells, showed that the fraction of tumor vessels containing cancer cells in their lumen, and the number of cancer cells inside vessels were decreased in Pfkfb3+/ΔEC mice, suggesting impaired cancer cell intravasation (Figures 2L–2N), as confirmed by colony formation of circulating cancer cells (Figure 2O). We also explored if PFKFB3 inhibition affected later stages of metastasis. Upon tail vein injection of B16-F10 cells, metastasis in the lungs was reduced in Pfkfb3+/ΔEC mice (Figure 2P).
To mimic PFKFB3 haplodeficiency, we used a low dose of 3PO (25 mg/kg; 3x/week), which reduced glycolysis in vivo (Figure 3A). This dose did not affect cancer cells, as 3PO treatment did not alter tumor growth and cancer cell proliferation (Figures 3B and 3C. In line, cultured NECs and eTECs were more sensitive to PFKFB3-blockade than cancer cells (Figures 3D–3G and S3A–S3D). 3PO treatment phenocopied the genetic effects in both B16-F10 and Panc02 tumor models (Figures 3H–3N and S3E–S3J). 3PO treatment, initiated 3 days prior to cancer cell injection and continued thereafter, also reduced pulmonary metastases upon i.v. injection of B16-F10 cancer cells (Figure 3O).
Figure 3. Effect of PFKFB3 blockade on B16-F10 tumor progression and metastasis.
(A) Gas chromatography-mass spectrometry (GC-MS) analysis of blood [13C]-lactate levels upon i.v. injection of [U-13C]-glucose in control (ctrl) and 3PO-treated mice (n=7–8). (B) Growth of s.c. B16-F10 tumors in ctrl and 3PO-treated mice (n=7). (C) Micrographs of sections of B16-F10 tumors from ctrl or 3PO-treated mice, stained for proliferation marker Ki67. Nuclei are counterstained with DAPI. Quantification of Ki67+ cells (Ki67+ nuclei, % of total) is indicated (n=3). (D) Dose-response analysis of the effect of 3PO on proliferation of NECs (n=3 biological repeats of pooled ECs isolated from 10–15 individual animals each). (E) Dose-response analysis of the effect of 3PO on proliferation of eTECs (n=3 biological repeats of pooled ECs isolated from 10–15 individual animals each). (F) Dose-response analysis of the effect of 3PO on proliferation of B16-F10 (n=3). (G) Dose-response analysis of the effect of 3PO on proliferation of Panc02 cells (n=3). (H) Quantification of lung metastases in s.c. B16-F10 tumor-bearing mice treated with vehicle (ctrl) or 3PO (n=17). (I) Metastatic index (lung metastases / tumor weight) in s.c. B16-F10 tumor-bearing mice treated with vehicle (ctrl) or 3PO (n=17). (J) Micrographs of H&E-stained B16-F10 tumor sections in ctrl and 3PO-treated mice. Dotted line: border between tumor and surrounding muscle; arrows: residual muscle tissue. (K) Micrographs of s.c. B16-F10 tumor sections from ctrl and 3PO-treated mice, stained for CD31 and endoglin to assess intraluminal CD31− endoglin+ cancer cells (arrows). (L) Quantification of intraluminal cancer cells per vessel in (K) (n=8). (M) Quantification of % of vessels with intraluminal cancer cells in (K) (n=8). (N) Quantification of B16-F10 cancer cell colonies, obtained upon isolation and culturing of circulating B16-F10 cancer cells from s.c. tumor-bearing mice treated with vehicle (ctrl) or 3PO (n=3 biological repeats of 3–9 pooled individual animals each). (O) Quantification of metastatic area in lungs (area of metastatic lesions, % of total lung area) upon tail vein injection of B16-F10 cancer cells in mice pretreated 3 days before cancer cell injection with vehicle (ctrl) or 3PO (n=5). Bars: 100 µm (C), 50 µm (J,K). All data are mean ± SEM. * p value < 0.05. See also Figure S3.
PFKFB3 inhibition improves perfusion
We explored if PFKFB3 haplodeficiency altered structural and functional properties of tumor vessels by staining for CD31. Compared to WT mice, B16-F10 tumors in Pfkfb3+/ΔEC mice had a comparable vessel density (Figures 4A and 4B), however the vessel lumen size and total perfusable area (sum of lumen area of all vessels) were increased (Figures 4C and 4D). This was surprising given that EC proliferation was reduced in tumor vessels of Pfkfb3+/ΔEC mice (Figure 4E). However, EC apoptosis was also decreased (Figure 4F), thus balancing off impaired EC growth. Moreover, ECs were arranged / positioned differently in Pfkfb3+/ΔEC tumor vessels, which could further explain the vessel enlargement. Indeed, CD31 staining of thick tumor sections revealed that the vascular architecture in tumors of Pfkfb3+/ΔEC mice was less irregular, tortuous and disorganized (Figures 4G and 4H). When analyzing cross-sectional vessel profiles, the EC layer in WT mice appeared thick, irregular, packed and protruded extensions into the lumen, while the EC layer in Pfkfb3+/ΔEC mice was thinner, more elongated and stretched, appearing more regular and flattened with a smoother surface (Figures 4I). As a result of this morphogenic rearrangement, tumor vessels in Pfkfb3+/ΔEC mice contained fewer EC nuclei per µm cross-sectional vessel length (Figure 4J).
Figure 4. Effect of PFKFB3 haplodeficiency on tumor vessels, perfusion and oxygenation.
(A) Micrographs of B16-F10 tumor sections from WT and Pfkfb3+/ΔEC mice, stained for CD31. (B) Quantification of tumor vessel density in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice (n=10–20). (C) Quantification of vessel lumen size in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice (n=6) (D) Quantification of total perfusable area in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice (n=6). (E) Quantification of the % of proliferating PHH3+ CD31+ ECs in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice (n=5). (F) Quantification of the % of apoptotic TUNEL+ CD31+ ECs in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice (n=11). (G) Micrographs of confocal images of CD31-stained thick sections of s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice. (H) Quantification of vessel tortuosity in (G) (n=10–12). (I) Micrographs of confocal images of CD31+ s.c. B16-F10 tumor sections from WT and Pfkfb3+/ΔEC mice. Nuclei are counterstained with DAPI. (J) Quantification of EC nuclei per cross-sectional tumor vessel length in (I) (n=11). (K) Micrographs of lectin-FITC perfused and CD31-stained vessels in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice. (L) Quantification of vessel perfusion in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice (n=9–12). (M) Micrographs of H&E and pimonidazole (PIMO) staining (brown zones within dotted lines) of hypoxic zones in s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice. (N) Quantification of PIMO+ area (n=14–17). Bars: 75 µm (A,K), 200 µm (G), 20 µm (I), 100 µm (M). All data are mean ± SEM. * p value < 0.05. A.U.: arbitrary units.
As hypoxia promotes cancer cell invasion and metastasis (De Bock et al., 2011), we investigated tumor perfusion and oxygenation. Injection of FITC-conjugated lectin (labeling perfused vessels) and staining for CD31 to identify all tumor vessels showed that the perfused tumor vessel area was increased in Pfkfb3+/ΔEC mice (Figures 4K and 4L). Staining for the hypoxia marker pimonidazole revealed reduced hypoxic B16-F10 tumor area in Pfkfb3+/ΔEC mice (Figures 4M and 4N).
Comparable results were obtained when using LLC tumors in Pfkfb3+/ΔEC mice and treating B16-F10 or Panc02 tumor bearing mice with 3PO, though we adapted the vascular analysis according to how vessels formed. Indeed, in LLC tumors, we observed a contiguous network of tiny vessels, such that individual vessel profiles could not be detected, precluding us from counting vessel densities; we therefore quantified total CD31+ vessel area. In LLC and Panc02 tumors, the vessel lumen was too small to reliably quantify lumen size; hence, as alternative of the perfusable area, we quantified the lectin+ vessel area. Regardless, qualitatively similar results of comparable vessel density, vascular enlargement, reduced EC proliferation matched by reduced EC death, fewer ECs per vessel length, increased perfusion and reduced hypoxia were obtained for LLC tumors in Pfkfb3+/ΔEC mice (Figure S4A–S4F), and for B16-F10 or Panc02 tumors upon 3PO treatment (Figures 5A–5L and S4G–SN). Notably, 3PO treatment also induced TVN in metastatic liver tumors upon portal vein injection of B16-F10 cancer cells (Figure S4O–S4Q).
Figure 5. Effect of PFKFB3 inhibition on vessel characteristics in s.c. B16-F10 tumors.
(A) Micrographs of CD31-stained sections of s.c. B16-F10 tumors from ctrl and 3PO-treated mice. (B) Quantification of tumor vessel density in s.c. B16-F10 tumors from ctrl and 3PO-treated mice (n=5). (C) Quantification of vessel lumen size in s.c. B16-F10 tumors from ctrl and 3PO-treated mice (n=5). (D) Quantification of total perfusable area (sum of lumen area of all vessels, % of tumor area) in s.c. B16-F10 tumors from ctrl and 3PO-treated mice (n=5). (E) Quantification of % of proliferating PHH3+ CD31+ ECs in s.c. B16-F10 tumors from ctrl and 3PO-treated mice (n=5–7). (F) Quantification of % of apoptotic TUNEL+ CD31+ ECs in s.c. B16-F10 tumors from ctrl and 3PO-treated mice (n=7–8). (G) Micrographs of confocal images of CD31-stained sections of s.c. B16-F10 tumor from ctrl and 3PO-treated mice. Nuclei are counterstained with DAPI. (H) Quantification of EC nuclei per cross-sectional tumor vessel length (n=11). (I) Micrographs of lectin-FITC perfused and CD31-stained vessels in s.c. B16-F10 tumors from ctrl and 3PO-treated mice. (J) Quantification of vessel perfusion in s.c. B16-F10 tumors from ctrl and 3PO-treated mice (n=8). (K) Micrographs of pimonidazole (PIMO) staining (brown zones within dotted lines) of hypoxic zones in s.c. B16-F10 tumors from ctrl and 3PO-treated mice. (L) Quantification of PIMO+ area in (K) (n=10). Bars: 50 µm (A), 20 µm (G), 75 µm (I), 100 µm (K). All data are mean ± SEM. * p value < 0.05. See also Figure S4.
PFKFB3 inhibition alters EC properties
We explored if a tightened EC barrier could contribute to the reduced cancer cell intravasation in Pfkfb3+/ΔEC mice. Disorganized tumor vessels have lower levels of the junctional protein VE-cadherin, while normalized tumor vessels tighten their EC barrier by upregulating VE-cadherin (Mazzone et al., 2009). RT-PCR showed that VE-cadherin (Cdh5) mRNA levels were lower in eTECs (Figure S1A). Whole-mount staining of B16-F10 tumor sections for CD31 and VE-cadherin showed that VE-cadherin+ adherens junctions were more abundant and more strongly stained in Pfkfb3+/ΔEC mice (Figure 6A). Also, scanning electron microscopy (SEM) showed a regular, smooth, orderly formed, flat surface of a monolayer of ECs in tumor vessels of Pfkfb3+/ΔEC mice, in contrast to the more irregular, chaotically organized and often discontinuous EC lining in WT mice (Figure 6B). Similar results were obtained in LLC tumors in Pfkfb3+/ΔEC mice (Figure S5A and S5B) and upon 3PO treatment of s.c. B16-F10 tumors (Figure S5C and S5D) and Panc02 tumors (Figure S5E), as well as in B16-F10 liver metastases (Figure S5F).
Figure 6. Effect of PFKFB3 inhibition on EC Barrier Properties.
(A) Micrographs of thick sections of s.c. B16-F10 tumors from WT or Pfkfb3+/ΔEC mice, double stained for VE-cadherin (VE-cadh) and CD31. (B) Micrographs of SEM images of B16-F10 tumor vessels in WT and Pfkfb3+/ΔEC mice. (C) Images of ctrl and 3PO-treated EC monolayers, stained for VE-cadherin (Arrows: intercellular gaps). (D) Quantification of continuous versus discontinuous junctions (junctional length, % of total junctional length) in control (ctrl) and 3PO-treated ECs (n=3). (E) Quantification of gap index in ctrl and 3PO-treated ECs (n=3). (F) Quantification of transendothelial electrical resistance (TEER) of ECs upon treatment with vehicle (ctrl), 3PO (5 µM), VEGF (100 ng/ml), or VEGF plus 3PO. Data are presented as cumulative change over 4 hr in monolayer resistance normalized to TEER value of control (n=4). (G) Micrographs of ECs showing internalized phRodo-dextran (red) upon treatment with vehicle (ctrl), 3PO or endocytosis blocker dynasore. Cells were visualized in blue by cyan fluorescent protein. (H) Quantification of phRodo-dextran fluorescence intensity in (G) (n= 16–48 cells; 3 independent measurements). (I) Micrographs of ECs labeled with FITC conjugated anti-VE-cadherin antibody for ctrl, VEGF, 3PO and VEGF + 3PO pretreatment. (J) Quantification of internalized VE-cadherin in (I) (n=3). Bars: 10 mm (A,B; G), 20 mm (C; I). A.U.: arbitrary units. Data in panels D,E,F,H,J are mean ± SEM. * p value < 0.05. A.U., arbitrary units. See also Figure S5.
We assessed how PFKFB3 inhibition regulated VE-cadherin+ adherens junctions (AJs) in cultured NECs and human umbilical venous ECs (HUVECs, abbreviated as ECs). Two types of AJs can be distinguished: (i) continuous stable AJs in a quiescent EC network, associated with parallel cortical actin bundles and in which VE-cadherin is localized linearly along cell-cell borders; and (ii) discontinuous fragmented AJs in ECs with reduced network integrity, attached to radial stress fibers and in which VE-cadherin is distributed in short linear structures perpendicular to cell–cell borders (Fraccaroli et al., 2015). PFKFB3 blockade increased the length of continuous VE-cadherin+ AJs, while reducing the fraction of discontinuous VE-cadherin+ AJs, thus indicating that 3PO promoted vascular integrity and EC inter-connectivity (Figures 6C and 6D). PFKFB3 blockade also reduced the number of intercellular gaps, further evidence for a tighter, more connected monolayer with increased integrity (Figure 6E). Also, 3PO increased the trans-EC electrical resistance (TEER), a measure of barrier tightness, and largely abrogated the permeability increase induced by VEGF (Figure 6F).
We then explored how PFKFB3 blockade increased plasma membrane VE-cadherin levels. Given that VE-cadherin is internalized via clathrin-mediated endocytosis (Giannotta et al., 2013), and this process relies on ATP-consuming actin-myosin remodeling (Boulant et al., 2011), we hypothesized that PFKFB3-driven glycolysis was necessary for VE-cadherin endocytosis. Indeed, using phRodo-dextran, a pH-sensitive fluorescent reporter, the intensity of which intensifies with increasing acidity (as in acidified endocytic vesicles), we observed that PFKFB3-blockade reduced internalization of phRodo-dextran in ECs (Figures 6G and 6H). Similarly, incubation of ECs with a fluorescently labeled anti-VE-cadherin antibody, followed by mild acid wash to remove cell surface bound antibody, revealed cytosolic accumulation of fluorescent label in response to VEGF stimulation of NECs (Figures 6I and 6J). Notably, 3PO, which alone did not have an effect, largely abrogated the increase in VE-cadherin endocytosis induced by VEGF in NECs (Figures 6I and 6J).
We also explored if PFKFB3 inhibition changed other EC characteristics that could reduce cancer cell intravasation. Of note, the finding that metastasis was reduced only when 3PO was initiated before – not after (see below) – systemic cancer cell injection suggests that PFKFB3 inhibition also impaired cancer cell extravasation. We considered if PFKFB3 inhibition rendered ECs less adhesive for cancer cells. Indeed, PFKFB3-blockade reduced the number of cancer cells adhering to and migrating across ECs, pre-activated with IL-1β or TNF-α (Figures 7A–7C, S6A, and S6B). Notably, the reduced adhesion was due to an altered EC pro-inflammatory signature. Indeed, upon activation with IL-1β, PFKFB3-inhibited ECs expressed lower levels of VCAM-1, ICAM-1 and E-selectin (Figures 7D and S6C–S6E), adhesive molecules involved in cancer cell intra/extravasation (Eichbaum et al., 2011). In accordance, ICAM-1 immunoreactive levels in TECs were lower in tumors of Pfkfb3+/ΔEC mice (Figure 7E). We then assessed if PFKFB3 inhibition decreased NF-κB signaling in ECs, because of several reasons: (i) IL-1β induces signaling partly via NF-κB; (ii) the glycolytic product lactate stimulates NF-κB signaling in ECs (Vegran et al., 2011); (iii) PFKFB3 inhibition lowers lactate levels in ECs (see above and (De Bock et al., 2011; Schoors et al., 2014)); and (iv) IL-1β and TNF-α treatment of ECs increased glycolysis and, in accordance, PFKFB3 levels (Figures S6F and S6G), while PFKFB3 blockade counteracted this increase and lowered the elevated levels of glycolysis (Figure S6F). Indeed, PFKFB3 inhibition reduced NF-κB signaling (Figure 7F). In response to cytokine activation, IκBα, a chaperone that keeps the NF-κB subunits p65 and p50 inactive in the cytosol, becomes phosphorylated before degradation, resulting in release and activation (phosphorylation) of p65 and p50. In agreement, IL-1β stimulation increased levels of phospho-p65 and phospho-IκBα (Figures 7G–7I). Notably however, upon PFKFB3 blockade, levels of phospho-p65 and phospho-IκBα were only minimally elevated (Figures 7G–7I). In agreement, phospho-p65 immunoreactive levels appeared lower in ECs of tumors in 3PO-treated mice (Figure 7J).
Figure 7. Effect of PFKFB3 inhibition on expression of adhesion molecules and vessel maturation.
(A) Micrographs of B16-F10 cancer cells (green) adhering to an EC monolayer upon single or combined treatment with vehicle (ctrl), 3PO (10 µM), IL-1β (1 ng/ml), and 3PO plus IL-1β. (B) Quantification of number of adherent cancer cells per well in (A) (n=4). (C) Quantification of transendothelial cancer cell migration through EC monolayer, stimulated with IL-1b (1 ng/ml) or TNF-a (10 ng/ml) without or with 3PO (10 µM) (n=5). (D) RT-PCR of mRNA expression levels of VCAM-1, ICAM-1 and E-selectin in ECs upon treatment with IL-1β alone (1 ng/ml) or together with 3PO (20 µM). Values are expressed relative to IL-1b stimulated cells (n=4–5). (E) Representative micrographs of B16-F10 tumor sections from ctrl and 3PO-treated mice co-stained for CD31 and ICAM1 with quantification of ICAM+ area (% of total vessel area) (n=5–6). Right panels: ICAM1 signal channel only. (F) Quantification of NF-κB luciferase reporter activity in ECs upon treatment with vehicle (ctrl), 3PO (20 µM), IL-1β (1 ng/ml) alone and together with 3PO (n=4). (G) Immunoblot of protein levels of phosphorylated p65 (p-p65) and total p65 (upper blot), and of phosphorylated IκBα (p-IκBα) and total IκBα (bottom blot) in ctrl and 3PO-treated ECs upon treatment with vehicle, 3PO (20 µM), IL-1b (1 ng/ml) alone and together with 3PO (20 µM). b-tubulin was used as loading control. (H) Densitometric quantifications of p-p65 (relative to total p65) in G (n=3). (I) Densitometric quantifications of p-IκBα (relative to total IκBα) in G (n=3). ND, not detectable. (J) Representative micrographs of p-p65 staining of tumor vessels in s.c. B16-F10 tumors of ctrl versus 3PO-treated mice and quantification of p-p65+ signal (average pixel intensity expressed in arbitrary units) (n=5). Arrows: ECs. The top and the bottom small images at the right show the p-p65 + DAPI channels only, with a higher magnification of the boxed areas. Dotted lines: vessel wall. (K) Micrographs of sections of s.c. B16-F10 tumors from WT and Pfkfb3+/ΔEC mice, stained for CD31 and NG-2. Nuclei are counterstained with DAPI. (L) Quantification of the % of pericyte-covered vessels in (K) (n=10–11). (M) Micrographs of B16-F10 tumor sections from WT and PFKFB3+/ΔEC mice, stained for CD31 and laminin to visualize the basement membrane. Quantification of % of laminin+ vessels is indicated (n=10–19). (N) Micrographs of sections of s.c. B16-F10 tumors from ctrl or 3PO-treated mice stained for CD31 and NG-2. Nuclei are counterstained with DAPI. (O) Quantification of the % of pericyte covered vessels in N (n=4–6). Bars: 75 µm (A; E; K; N), 10µm (J), 200 µm (M). All data are mean ± SEM. * p value < 0.05. See also Figure S6.
PFKFB3 inhibition improves vessel maturation
In healthy microvessels, quiescent pericytes stay put and cover ECs, but in tumor vessels, pericytes are activated and proliferate more actively, yet become detached from tumor ECs (resulting in reduced pericyte coverage), partly because of reduced adhesive properties (due to lower levels of N-cadherin, mediating adhesion between ECs and pericytes) and increased motility (Francescone et al., 2014). In addition, unstable tumor vessels are continuously remodeled so that their basement membrane (deposited partly by quiescent pericytes) is incomplete or even absent. Double staining for CD31 and pericyte marker NG-2 showed that more pericytes covered ECs in B16-F10 tumors of Pfkfb3+/ΔEC mice (Figures 7K and 7L). A substantial fraction of tumor vessels in WT mice consisted of naked ECs without or with little laminin-positive basement membrane, while tumors in Pfkfb3+/ΔEC mice contained more mature CD31+ laminin+ vessels (Figure 7M). Similar results of improved vessel maturation were obtained when analyzing LLC tumors in Pfkfb3+/ΔEC mice (Figure S6H and S6I) and B16-F10 or Panc02 tumors upon treatment with 3PO (Figures 7N, 7O, S6J, and S6K). PFKFB3 blockade by 3PO also increased maturation of tumor vessels in liver metastases upon portal vein injection of B16-F10 cancer cells (Figure S6L and S6M). Mechanistically, pre-treatment of ECs with 3PO increased adhesion of pericytes to ECs (Figure S6N) and elevated N-cadherin mRNA levels in these cells (Figure S6O), contributing to pericyte recruitment upon cell autonomous endothelial PFKFB3 inhibition.
Pharmacological PFKFB3 blockade promotes mural coverage by lowering pericyte glycolysis
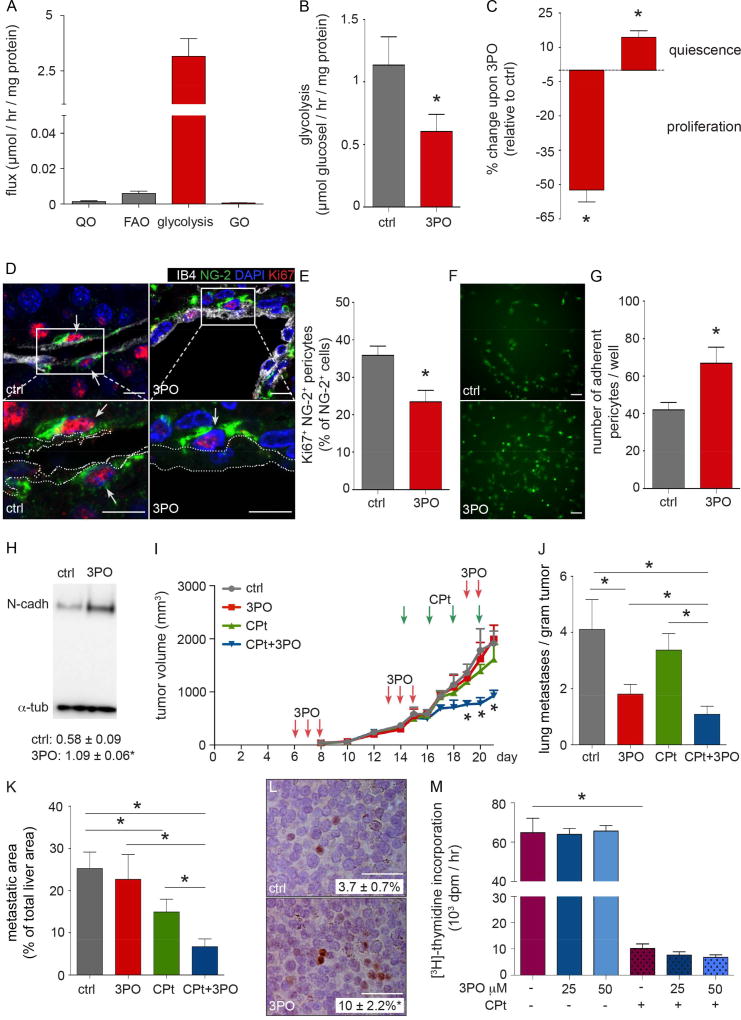
Given that 3PO treatment improved pericyte coverage more than EC deficiency of PFKFB3 (Figure 7O versus Figure 7L), we explored if pericytes were also a target of 3PO. We first characterized its glucose metabolism, and observed that pericytes are highly glycolytic (Figure 8A). When calculating the theoretical amount of ATP generated by glycolysis, glucose oxidation, glutamine oxidation and fatty acid oxidation (De Bock et al., 2013b) in pericytes, glycolysis contributed for up to 85%, with minimal contribution by other pathways (Figure S7A). Treatment of pericytes with 3PO reduced glycolysis (Figure 8B), and impaired proliferation and migration, while increasing quiescence (Figures 8C and Figure S7B). In line, there were fewer proliferating NG-2+ pericytes around tumor vessels in 3PO-treated tumors (Figures 8D and 8E), indicating they were less activated. Pericytes, pretreated with 3PO, also adhered better to control ECs (Figures 8F and 8G), which can be explained by findings that 3PO upregulated N-cadherin levels in pericytes (Figure 8H). These effects can explain why the more sedentary, quiescent and adhesive pericytes stay put and cover ECs in tumors better upon 3PO treatment.
Figure 8. Effect of PFKFB3 inhibition on pericytes and delivery and efficacy of chemotherapy.
(A) Analysis of glutamine oxidation (QO), fatty acid oxidation (FAO), glycolysis and glucose oxidation (GO) in pericytes (n=3). (B) Effect of 3PO on pericyte glycolysis flux (n=3). (C) Effect of 3PO on pericyte proliferation (left bar; n=6) and quiescence (flow cytometric analysis upon staining for EdU, right bar; n=3). (D) Micrographs of sections of s.c. B16-F10 tumors from ctrl and 3PO-treated mice stained for EC marker isolectin B4 (IB4), pericyte marker NG-2 and proliferation marker Ki67. Nuclei are counterstained with DAPI. Arrows: Ki67+ pericyte nuclei. Dotted lines in zoom-in panels: vessel wall. (E) Quantification of proliferating tumor vessel pericytes in (D) (n=4–5). (F) Micrographs of adherent ctrl and 3PO-pretreated pericytes (green) to an EC monolayer. (G) Quantification of adherent pericytes in (F) (n=5). (H) Immunoblot of protein levels of N-cadherin (N-cadh) in ctrl and 3PO-treated pericytes. Densitometric quantification is indicated (normalized to α-tubulin; n=3). (I) Growth curve of s.c. B16-F10 tumor upon treatment with vehicle (ctrl), 3PO (25 mg/kg; 3x/week initiated when tumors reached a volume of 100 mm3) and a sub-maximal dose of CPt (2.5 mg/kg administered every other day during the last week before the termination of the experiment), alone or together with 3PO (n=20–24). (J) Metastatic index of s.c. implanted B16-F10 cancer cells disseminating to the lungs from mice in (I). (K) Quantification of metastatic liver area upon portal vein injection of B16-F10 cancer cells in mice treated with vehicle (ctrl), 3PO (25 mg/kg daily for 5 consecutive days, initiated on the 3rd day after cancer cell injection, followed by 2 day drug holiday and 5 days of daily treatment), and a standard (maximal) dose of CPt (10 mg/kg administered on day 5 and 11 after cancer cell injection), alone and together with 3PO (n=10–11). (L) Images of B16-F10 tumor sections, stained for CPt-DNA adducts from tumors treated with vehicle (ctrl) or 3PO upon administration of a single dose of Cpt (10 mg/kg). Quantification of Cpt-DNA adducts is indicated (n=19). (M) Proliferation of cultured B16-F10 cancer cells upon treatment with 25 µM CPt in the presence of increasing concentrations of 3PO (n=4). Bars: 10 µm (D), 75 µm (F), 100 µm (L). All data are mean ± SEM. * p value < 0.05. For panels B,C and H, p values were calculated by mixed model statistics (Kenward-Roger Test). In panel I, the p value refers to CPt +3PO vs ctrl. See also Figure S7.
PFKFB3 blockade enhances the delivery and efficacy of chemotherapy
We then explored if treatment of B16-F10 tumor-bearing mice with 3PO enhanced the anti-tumor growth effect of a dose of cisplatin (CPt) (2.5 mg/kg body weight; 3x/week), which affected tumor growth only minimally (Figure 8I). When mice were pretreated with 3PO to induce TVN before administration of CPt and then continued 3PO treatment, CPt inhibited tumor growth more substantially (Figure 8I). Of note, CPt monotherapy did not significantly reduce metastatic burden in control mice, but nearly completely prevented metastasis in combination with 3PO (Figure 8J). 3PO treatment also enhanced the cytotoxic effect of a clinically relevant standard (maximal) dose of CPt in liver and lung metastasis models upon, respectively, injection of B16-F10 cancer cells into the portal or tail vein (Figure 8K and Figure S7C). 3PO monotherapy only tended to reduce metastasis, presumably because 3PO treatment was initiated only 3 days after cancer cell injection, thus phenocopying the lack of an effect of 3PO on primary tumor growth (see above). However, the finding that 3PO treatment reduced metastasis only when 3PO was administered 3 days before – not after – systemic cancer cell injection further supports an effect of 3PO on cancer cell extravasation.
The improved chemotherapeutic effect was accompanied by more efficient delivery of CPt. Indeed, when treating B16-F10 tumor-bearing mice with 3PO before administering a single dose of cisplatin (CPt) (10 mg/kg), we observed more CPt-DNA adducts in cancer cells treated with 3PO compared to controls (Figure 8L), indicating that CPt delivery was increased. Similar results were observed in Pfkfb3+/ΔEC mice (Figure S7D and S7E. The increased anti-tumor effect of the combination of 3PO plus CPt in vivo was not due to an increase in chemosensitivity of cancer cells to CPt in the presence of 3PO (Figure 8M).
Discussion
The key findings of our study are: (i) TECs are hyperglycolytic and targeting TEC glycolysis induces tumor vessel normalization (TVN), thereby reducing cancer cell invasion, intravasation and dissemination; (ii) pharmacological blockade of PFKFB3 at a dose that does not affect cancer cell proliferation, promotes TVN and improves delivery of and response to a standard dose of chemotherapy; and (iii) mechanistically, PFKFB3 inhibition induces these effects partly by impairing VE-cadherin endocytosis and inflammation in ECs, and by rendering pericytes more quiescent and adhesive via glycolysis reduction.
Transcriptomic and metabolic analysis showed that eTECs have a hyperglycolytic metabolism, which they need in part for high ATP demanding processes (increased motility, active proliferation) (De Bock et al., 2013a). Proliferating cells use glucose carbons, partly via the PPP and SBP, for nucleotide synthesis (Fan et al., 2012). In agreement, eTECs, which proliferated more actively, expressed higher transcript levels of enzymes involved in glycolysis, PPP and SBP, and increased 14C-glucose incorporation into RNA and DNA. Hence, eTECs divert more glucose carbons into these pathways for enhanced nucleotide synthesis to sustain rapid proliferation, though glucose carbons can also contribute to nucleotide synthesis via other pathways. In agreement, eTECs expressed higher mRNA levels of enzymes involved in nucleotide synthesis. Hypoxia, angiogenic factors such as VEGF and bFGF, known to upregulate PFKFB3 levels (De Bock et al., 2013a), and cytokines contribute to hyperglycolysis of eTECs. Since ECs heavily rely on glycolysis to meet bioenergetics needs, even more so than certain cancer cells (De Bock et al., 2013b), they are very sensitive to changes in PFKFB3 gene dosage or activity.
All previous TVN strategies focused on targeting angiogenic signals, but targeting EC metabolism as an approach for promoting TVN remained uncharted territory. Our data show that lowering of TEC glycolysis can induce TVN. As TECs are hyperactivated (Jain, 2014; Mazzone et al., 2009), rendering them more quiescent by lowering glycolysis counteracts their hyper-proliferative and -migratory behavior (De Bock et al., 2013b) and contributes to TVN. PFKFB3 inhibition did not affect tumor vessel density, but enlarged tumor vessel lumen, smoothened EC surfaces, and stabilized tumor vessels by depositing a more prominent basement membrane and increasing coverage with pericytes. While TVN can be accompanied by pruning of immature vessels (f.i. when using anti-VEGF agents), other studies report that tumor vessel densities remain unchanged during TVN (Carmeliet and Jain, 2011; Jain, 2014; Mazzone et al., 2009).
Notably, even though PFKFB3 inhibition reduced eTEC proliferation, tumor vessels were enlarged. This is likely due to several reasons: (i) the reduced TEC proliferation was balanced off by reduced TEC death; and (ii) TEC alignment and arrangement were different, i.e. TECs appeared more stretched and aligned more uniformly, forming a thinner EC layer with fewer cells per vessel length. It is tempting to speculate that the latter phenomenon relates to the higher expression of VE-cadherin in TECs. Indeed, according to Laplace’s law, vessel wall tension is proportional to the radius of the vessel. Hence, stronger expression of VE-cadherin, functionally reflected by increased electrical resistance, in PFKFB3-inhibited ECs may allow these cells to cope with a greater wall tension, thus allowing them to enlarge their lumen, a hypothesis requiring confirmation.
Reduced metastasis is a hallmark of tumor vessel normalization (Carmeliet and Jain, 2011; Jain, 2014). Lowering glycolysis in TECs impaired initial and late steps of metastasis by at least 4 mechanisms. First, the observed vascular changes improved perfusion and lowered tumor hypoxia, a stimulus for cancer cell invasion, intravasasion and metastasis (De Bock et al., 2011). Second, PFKFB3 inhibition tightened the EC barrier by upregulating plasma membrane VE-cadherin levels, which impairs cancer cell intravasation (Mazzone et al., 2009). Third, by reducing migration and activation, but increasing adhesion of pericytes, PFKFB3 inhibition improved vessel maturation. Fourth, PFKFB3-inhibition lowered EC expression of cancer cell adhesion molecules (VCAM-1, E-selectin, ICAM-1), and reduced adhesion and transmigration of cancer cells through EC monolayers. While the latter mechanism is expected to impair cancer cell extravasation (Kobayashi et al., 2007), VCAM-1 and ICAM-1 are also expressed at the basolateral surface of activated ECs and regulate cancer cell intravasation (Bogetto et al., 2000; Viola et al., 2013). Mechanistically, the effect of PFKFB3 inhibition on reducing cancer cell adhesion may relate to reduced NF-κB signaling, as the glycolytic product lactate stimulates NF-κB signaling in ECs (Vegran et al., 2011) and PFKFB3 inhibition lowered lactate levels (this study and (De Bock et al., 2011; Schoors et al., 2014)).
PFKFB3 inhibition in ECs promoted the formation of stable, continuous VE-cadherin+ adherens junctions and elevated VE-cadherin protein levels at the plasma membrane, and consistent herewith, reduced the number of intercellular gaps, while increasing the trans-EC electrical resistance, all signs of barrier tightening and monolayer integrity. Tightening of EC junctions contributes to vessel integrity, vascular barrier tightness, induction of an EC “phalanx” phenotype (smooth, streamlined cobblestone monolayer of interconnected adherent ECs aligned in the direction of flow), possibly also vessel enlargement, and reduced vessel tortuosity – all processes that improve tumor vessel perfusion and oxygenation. Mechanistically, PFKFB3 inhibition impaired VE-cadherin endocytosis induced by VEGF. Given that VE-cadherin is internalized via clathrin-mediated endocytosis (Xiao et al., 2005), and this actin-myosin contraction dependent process requires ATP (Boulant et al., 2011), our findings suggest that ECs use glycolytic ATP for VE-cadherin endocytosis. This could explain why a reduction of glycolysis elevated VE-cadherin levels at the cell surface, and hence tightened EC junctions.
Compared to healthy vessels, tumor vessels are more devoid of pericytes, which makes them more leaky, and facilitates cancer cell intravasation and dissemination (Armulik et al., 2011). Immature tumor vessels without pericytes are also poorly perfused (Carmeliet and Jain, 2011; Jain, 2014). Hence, promoting pericyte coverage to stabilize tumor vessels not only improves perfusion, but also increases barrier tightness, which impairs cancer cell intravasation and metastasis (Mazzone et al., 2009). Contrary to quiescent pericytes in healthy vessels, pericytes in tumors are activated and proliferate more actively, yet are detached from tumor vessels, partly because of reduced N-cadherin-dependent adhesive properties and increased motility (Francescone et al., 2014). Thus, although pericyte proliferation is increased, pericyte numbers around tumor vessels are reduced, indicating that their reduced adhesiveness and increased motility rather than their proliferation rate determine pericyte coverage.
Our data indicate that pericytes are highly glycolytic. Hence, inhibition of PFKFB3 in pericytes (as occurs during 3PO treatment) rendered these cells less motile and more quiescent and sedentary, and increased their adhesion to ECs, all changes that improve pericyte coverage of ECs. The elevated expression of N-cadherin, mediating adhesion between ECs and pericytes (Tillet et al., 2005), might be due to the fact that pericytes proliferate less and are more quiescent, conditions known to elevate N-cadherin expression (Blindt et al., 2004). Hence, by rendering pericytes more quiescent and adhesive, glycolysis reduction in these cells (upon 3PO treatment) improves pericyte coverage, and hence TVN.
Cell autonomous endothelial deletion of PFKFB3 also promoted pericyte coverage. This is likely the result of a cross-talk from the ECs (normalized by PFKFB3 inhibition) towards the pericytes. Indeed, PFKFB3 inactivation in ECs promotes EC quiescence (Schoors et al., 2014), which is known to favor pericyte recruitment via enhanced expression of N-cadherin (as observed in this study) (Marx et al., 1994). Further, PFKFB3 inhibition reduces NF-κB signaling, a pathway known to inhibit pericyte coverage (Caporali et al., 2015). Also, the improved oxygenation and enhanced blood flow, resulting from EC layer normalization, can stimulate pericyte coverage (Hutter-Schmid and Humpel, 2016). The improved pericyte coverage then further stabilizes the normalized EC layer to promote normalization of the whole tumor vessel wall. Overall, while PFKFB3 inhibition in pericytes directly promotes TVN, cell autonomous endothelial inhibition of PFKFB3 can also indirectly promote TVN through an EC ➔ pericyte cross-talk.
TVN is receiving increasing attention as an alternative therapeutic strategy to reduce metastasis and improve chemo- and immunotherapy (Carmeliet and Jain, 2011; Huang et al., 2013). PFKFB3 inhibition improved chemotherapy with a standard cytotoxic dose, reducing growth of both primary and metastatic tumors, and impaired cancer cell dissemination. These findings might merit further consideration of targeting TEC glycolysis as anti-cancer therapeutic strategy.
Experimental procedures
Detailed methods are described in Supplemental Experimental Procedures.
Cell culture and in vitro assays
Mouse ECs were isolated from livers of WT or Pfkfb3+/ΔEC mice as described in the Supplement. Murine cancer cells (B16-F10, Lewis lung carcinoma, Panc02), human umbilical vein endothelial cells and human placental pericytes were cultured and used for in vitro functional assays and in vivo experiments (Supplement).
Transcriptomic analysis
RNA extraction and libraries were obtained as described (Supplement). The raw sequencing reads were mapped to the mouse reference transcriptome and genome (GRCm38/mm10) using the Bowtie TopHat pipeline (Langmead and Salzberg, 2012).
Metabolic analysis
Metabolite profiling of murine ECs was done by mass-spectrometry as described (Supplement). Glycolysis and glucose, glutamine or fatty acid oxidation were determined using radiolabeled tracers; oxygen consumption using a Seahorse XF24 analyzer.
Mouse models
Animal procedures were approved by the Institutional Animal Care and Research Advisory Committee (KU Leuven) (ECD207/2014) and were performed in accordance with the institutional and national guidelines and regulations. B16-F10 cancer cells were injected intravenously, via the portal vein or tail vein in syngeneic WT or transgenic mice to obtain liver or lung tumors, respectively. To assess subcutaneous tumor growth, 150 × 103 B16-F10 murine melanoma or 1.5 × 106 Lewis lung carcinoma (LLC) cells were injected s.c. into the right flank. Tumor volumes were measured 3x/week with a caliper using the formula V = π × [d2 × D]/6, where d is the minor tumor axis and D is the major tumor axis. For orthotopic pancreatic tumor growth, 1 × 106 Panc02 cancer cells were injected into the head of the pancreas.
In vivo treatments
In tumor experiments using conditional knock-out mice, tamoxifen treatment was given over one week before cancer cell injection. 3PO treatment was started when tumors reached 100 mm3. In B16-F10 melanoma, mice received i.p. injections of 25 mg/kg 3PO or dimethyl sulfoxide (DMSO) as control, 3x/week. In orthotopic pancreatic model, mice received daily i.p. injections of 25 mg/kg 3PO or DMSO for 7 days starting on the 3rd day after cancer cell injection. In the metastatic models, mice received i.p. injections of 25 mg/kg 3PO or DMSO for 5 days starting on the 3rd day after cancer cell injection followed by an interval of 2 days and additional 5 days of treatment. A submaximal dose of cisplatin (CPt) (2.5 mg/kg in s.c. B16 model) or a standard dose of cisplatin (10 mg/kg in p.v. liver and i.v. lung B16 models) was administered every other day by i.p. injections during the last week before termination of the experiment (s.c. B16 model), and on days 5 and 11 after cancer cell injection (p.v. and i.v. B16 model). To assess tumor drug delivery, a single dose of cisplatin (10 mg/kg) was injected i.p. 6–8 hr before euthanizing the mice.
Cellular ELISA and Immunohistochemistry
was performed using methods and antibodies listed in the Supplement.
Statistics
For in vitro experiments, the data in the figures and text represent mean ± SEM of at least 3 independent experiments involving at least 3 technical replicates. For in vivo experiments, the data represent mean ± SEM of n individual mice pooled from 3 to 4 independent experiments for primary tumor and metastasis growth experiments. For staining of in vivo material, at least 5 mice were used unless otherwise stated. Unless otherwise stated, statistical significance was calculated by ANOVA or standard two-sided t-test with F-testing to confirm equality of variance (Prism v6.0f); in case of unequal variance, two-sided t-test with Welch’s correction was used (Prism v6.0f). When inter-experimental variability was large in in vitro experiments with cells from different donors, mixed model statistics (R version 3.2.4 using Kenward-Roger approximation) was used with experiment (i.e. donor) as random factor to correct for variation between individual experiments. For hierarchical clustering of transcriptomic data, significant clusters were calculated via multiscale bootstrap analysis (R, Pvclust package). Self-contained gene set analysis was performed via rotation gene set analysis (R, Limma package). P value < 0.05 was considered statistically significant.
Accession Number
RNA sequencing data are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-4842.
Supplementary Material
Highlights.
Tumor endothelial cells (ECs) have a hyperglycolytic metabolism
Endothelial PFKFB3 haplodeficiency/blockade induces tumor vessel normalization
PFKFB3 inhibition tightens EC barrier and promotes pericyte quiescence
PFKFB3 blockade reduces metastasis and promotes chemotherapy
Cantelmo et al. show that tumor endothelial cells have hyper-glycolytic metabolism. Inactivation of the glycolytic activator PFKFB3 normalizes tumor vessels and improves vessel perfusion via tightening the vascular barrier, which reduces cancer cell intravasation and metastasis and improves chemotherapy response.
Significance.
Anti-angiogenic drugs blocking VEGF signaling are used for anti-cancer treatment, but their success is limited. Tumor vessel normalization (TVN) is emerging as a therapeutic anti-cancer approach, capable of reducing metastasis while improving delivery and response to chemotherapy, but strategies promoting TVN by targeting tumor endothelial cell (TEC) metabolism have not been tested. Here, we report that reduction of EC glycolysis by haplodeficiency of the glycolytic activator PFKFB3 in ECs decreased metastasis at least in part by promoting TVN. Treatment with a low dose of the pharmacological PFKFB3-blocker 3PO, which did not affect cancer cell proliferation, induced similar therapeutic benefit and increased the delivery and response to chemotherapy. These findings merit further consideration of blocking TEC glycolysis for anti-cancer therapy.
Acknowledgments
We acknowledge M. Mazzone, E. Dejana and H. Gerhardt for scientific discussions and advice; B. Floot (NKI, Amsterdam, the Netherlands) for providing NKI-A59; J. Chesney and S. Telang (University of Louisville, KY, USA) for providing Pfkfb3lox/lox mice. ARC, JK, JG and KV are supported by the Research Foundation Flanders (FWO); AB by Erasmus Mundus, ERAWEB fellowship; LCC by the Else Kroener-Fresenius-Stiftung; AP by the Austrian Science Fund FWF (J3730-B26); BC by the Agency for Innovation by Science and Technology in Flanders (IWT); LT by the Bettencourt Schueller Foundation. The work of PC is supported by a Belgian Science Policy grant (IUAP7/03), long-term structural Methusalem funding by the Flemish Government, grants from the FWO, the Foundation Leducq Transatlantic Network (ARTEMIS), Foundation against Cancer, an European Research Council (ERC) Advanced Research Grant (EUERC269073) and an AXA Research grant. PC declares to be named as inventor on patent applications, claiming subject matter related to the results described in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: PCa conceptualized and supervised the study; ARC, LCC and AB executed most experiments; JG, BT, BB, DL and BG analysed the metabolomics and transcriptomic data; JK, AP, LAT and LT isolated endothelial cells; AK, KV, BC and KK helped with morphometry; SS, LF, FB, PS and ID helped with pericyte experiments; PCh, JR, and ABM performed and analysed the VE-cadherin endocytosis and permeability in vitro assays; JH, TESN and GH performed and analysed the in vitro NF-κB-related experiments; JV helped with the phRodo-dextran endocytosis experiments; KDB, LS, GE, TR and MD helped to analyze the experiments and/or revise the text; ARC, LCC, GE, MD, JG and PCa wrote the paper. All authors discussed results and commented on the manuscript.
The other authors declare no conflict of interests.
References
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Blindt R, Bosserhoff AK, Dammers J, Krott N, Demircan L, Hoffmann R, Hanrath P, Weber C, Vogt F. Downregulation of N-cadherin in the neointima stimulates migration of smooth muscle cells by RhoA deactivation. Cardiovasc Res. 2004;62:212–222. doi: 10.1016/j.cardiores.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bogetto L, Gabriele E, Cariati R, Dolcetti R, Spessotto P, Doglioni C, Boiocchi M, Perris R, Colombatti A. Bidirectional induction of the cognate receptor-ligand alpha4/VCAM-1 pair defines a novel mechanism of tumor intravasation. Blood. 2000;95:2397–2406. [PubMed] [Google Scholar]
- Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13:1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali A, Meloni M, Nailor A, Mitic T, Shantikumar S, Riu F, Sala-Newby GB, Rose L, Besnier M, Katare R, et al. p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015;6:8024. doi: 10.1038/ncomms9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nature reviews. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013a;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell. 2013b;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- Eichbaum C, Meyer AS, Wang N, Bischofs E, Steinborn A, Bruckner T, Brodt P, Sohn C, Eichbaum MH. Breast cancer cell-derived cytokines, macrophages and cell adhesion: implications for metastasis. Anticancer research. 2011;31:3219–3227. [PubMed] [Google Scholar]
- Fan TW, Tan J, McKinney MM, Lane AN. Stable Isotope Resolved Metabolomics Analysis of Ribonucleotide and RNA Metabolism in Human Lung Cancer Cells. Metabolomics : Official journal of the Metabolomic Society. 2012;8:517–527. doi: 10.1007/s11306-011-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraccaroli A, Pitter B, Taha AA, Seebach J, Huveneers S, Kirsch J, Casaroli-Marano RP, Zahler S, Pohl U, Gerhardt H, et al. Endothelial alpha-parvin controls integrity of developing vasculature and is required for maintenance of cell-cell junctions. Circ Res. 2015;117:29–40. doi: 10.1161/CIRCRESAHA.117.305818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescone R, Ngernyuang N, Yan W, Bentley B, Shao R. Tumor-derived mural-like cells coordinate with endothelial cells: role of YKL-40 in mural cell-mediated angiogenesis. Oncogene. 2014;33:2110–2122. doi: 10.1038/onc.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Developmental cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer research. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter-Schmid B, Humpel C. Platelet-derived Growth Factor Receptor-beta is Differentially Regulated in Primary Mouse Pericytes and Brain Slices. Curr Neurovasc Res. 2016;13:127–134. doi: 10.2174/1567202613666160219120411. [DOI] [PubMed] [Google Scholar]
- Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Current medicinal chemistry. 2007;14:377–386. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx M, Perlmutter RA, Madri JA. Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. J Clin Invest. 1994;93:131–139. doi: 10.1172/JCI116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de, Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, et al. Partial and Transient Reduction of Glycolysis by PFKFB3 Blockade Reduces Pathological Angiogenesis. Cell metabolism. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Tillet E, Vittet D, Feraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400. doi: 10.1016/j.yexcr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer research. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Viola K, Kopf S, Rarova L, Jarukamjorn K, Kretschy N, Teichmann M, Vonach C, Atanasov AG, Giessrigl B, Huttary N, et al. Xanthohumol attenuates tumour cell-mediated breaching of the lymphendothelial barrier and prevents intravasation and metastasis. Arch Toxicol. 2013;87:1301–1312. doi: 10.1007/s00204-013-1028-2. [DOI] [PubMed] [Google Scholar]
- Wu D, Lim E, Vaillant F, Asselin-Labat ML, Visvader JE, Smyth GK. ROAST: rotation gene set tests for complex microarray experiments. Bioinformatics. 2010;26:2176–2182. doi: 10.1093/bioinformatics/btq401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.