Abstract
Cardiac MR is appealing to guide complex cardiac procedures because it is ionizing radiation free and offers flexible soft tissue contrast. Interventional cardiac MR promises to improve existing procedures and enable new ones for complex arrhythmias, as well as congenital and structural heart disease.
Guiding invasive procedures demands faster image acquisition, reconstruction and analysis, as well as intuitive intra-procedural display of imaging data. Standard cardiac MR techniques such as 3D anatomical imaging, cardiac function and flow, parameter mapping and late-gadolinium enhancement can be used to gather valuable clinical data at various procedural stages. Rapid intraprocedural image analysis can extract and highlight critical information about interventional targets and outcomes. In some cases, real-time interactive imaging is used to provide a continuous stream of images displayed to interventionalists for dynamic device navigation. Alternatively, devices are navigated relative to a roadmap of major cardiac structures generated through fast segmentation and registration. Interventional devices can be visualized and tracked throughout a procedure with specialized imaging methods.
In a clinical setting, advanced imaging must be integrated with other clinical tools and patient data. In order to perform these complex procedures, interventional cardiac MR relies on customized equipment, such as interactive imaging environments, in-room image display, audio communication, hemodynamic monitoring and recording systems, and electroanatomical mapping and ablation systems. Operating in this sophisticated environment requires coordination and planning.
This review will provide an overview of the imaging technology used in MRI-guided cardiac interventions. Specifically, this review will outline clinical targets, standard image acquisition and analysis tools, and the integration of these tools into clinical workflow.
Keywords: Cardiac, Real-Time, Intervention, Fast acquisition, Image Analysis
Introduction
There is a growing interest in guiding complex cardiac procedures using magnetic resonance imaging (MRI). Advances in cardiac imaging technologies and integration of imaging data have already enabled procedural guidance with MRI in both the preclinical and clinical research environment.
The field of interventional cardiology has relied on fluoroscopic x-ray imaging for the last 60 years to guide cardiac catheterization. X-ray provides projection images that depict devices well, but provide poor anatomical soft tissue contrast which is often inadequate for increasingly complex interventions. X-ray fluoroscopy also irradiates both the patient directly and the staff indirectly. This is exacerbated when procedure time is increased in line with complexity. Procedures in children are a particular concern due to their high sensitivity to ionizing radiation1, 2. For staff and interventionalists, heavy lead aprons provide partial protection but prolonged use can result in orthopedic complications.3 Adjunctive ultrasound imaging may also be used for guidance of cardiac interventions; however, this modality suffers from limited soft tissue contrast, acoustic shadowing effects, and limited field of view.
As cardiac interventions aim to address more complex pathologies, the need for improved image guidance has become clear. MRI provides improved soft tissue contrast, allows 3D visualization and localization, and is not a source of ionizing radiation. MRI offers the potential to visualize infarcted,4, 5 ischemic and arrhythmogenic tissue,6 ablation lesions,7 hemorrhages, and detailed information about soft tissue deformation and morphology during device/soft-tissue contact. MRI also offers the ability to analyze local flow patterns, perfusion and myocardial function.8 Furthermore, interactive real-time cardiac MRI has become widely available for device navigation.9 With these added benefits, there is a great incentive to transition to MRI guidance for cardiac interventions.
Over the past two decades, interventional MR, has developed significantly as a research focus. Multiple review papers have summarized clinical translation of interventional MR,10, 11 electrophysiology applications,10 valve and stent applications,12 pediatric applications,13 and technical requirements.14, 15
While technologies continue to evolve, perhaps the most pressing focus is translation toward clinical application, with emphasis on efficient imaging, analysis, and integration of MR information across a range of contrasts, resolutions and time scales, tailored to address specific clinical challenges. In this context, the current review provides an overview of existing and future clinical interventional cardiac MR applications, surveys recent technological developments in imaging and image analysis for interventional guidance, and discusses the integration of these MRI tools into the workflow of cardiac interventions.
Clinical Needs
The capacity to visualize 3D anatomy to facilitate device and catheter navigation and the capacity to assess myocardial tissue as well as local hemodynamics before and immediately after procedures will drive the adoption of interventional MR for congenital, ischemic and structural heart disease, as well as complex arrhythmias.
Diagnostic Cardiac Catheterization
In the catheterization laboratory, hemodynamic assessment relies on imperfect measures, such as thermodilution and the Fick principle, to quantify cardiac output and intracardiac shunt size (Qp:Qs) plus pressure measurements to calculate pulmonary vascular resistance and gradients across valves or vessel stenosis.
Noninvasive MR can accurately quantify intracardiac shunts using phase contrast flow data.16 MRI can also provide additional hemodynamic and volumetric data that is more quantifiable, for example valve regurgitant fractions to replace descriptive angiography ratings of “mild” to “severe”.
Currently, interventional cardiac MR assessment of pulmonary hypertension is performed routinely at some centers with invasive pulmonary artery pressure measurement and MR derived flow measurements to calculate pulmonary vascular resistance.17 Multiple centers have reported real-time MRI-guided catheter navigation for diagnostic catheterization in children and adults, which may be especially valuable in complex congenital or post-surgical cardiac anatomy.18, 19
Diagnostic endomyocardial biopsy, used for unexplained heart failure and monitoring for heart transplant rejection, would also greatly benefit from the ability to target focal pathology and improve diagnostic yield by interventional cardiac MR.20
Large Vessel Stenotic Lesions
Pulmonary artery stenosis, coarctation of the aorta, and surgical conduit stenosis such as right ventricle to pulmonary artery conduits are currently routinely treated with balloon and stent angioplasty under fluoroscopic guidance, though MR soft tissue imaging of vessels and surrounding structures is extremely helpful in planning interventions. The potential for real-time assessment of an intervention's impact on structures, such as compression of the bronchus or coronary arteries, makes interventional MRI-guided procedures particularly appealing. Reports from preclinical studies include real-time MRI-guided stenting of aortic coarctation21 and stent angioplasty of pulmonary artery stenosis.22 There have also been reports of interventions on large vessel stenoses in humans including balloon angioplasty of coarctation of the aorta.23
Clinically, the majority of cardiac catheterization procedures performed in adults are for the evaluation and treatment of coronary artery disease, using angiography to identify coronary artery lesions with limited associated tissue perfusion data. Unfortunately, the spatial and temporal resolution required for accurate MR visualization of the coronary arteries, with typical diameters less than 5 mm, have not yet been met so this application is beyond the scope of the current discussion.
Valvular Disease
The simplicity of pulmonary valvuloplasty requiring only a guidewire and balloon positioned in the stenotic valve facilitated the first report of MRI-guided pulmonary valvuloplasty using a commercially available balloon.24 More complex valve interventions for stenosis or regurgitation such as mitral and aortic valve replacement are generally performed only in adult populations and currently rely on fluoroscopic guidance with adjunct imaging, usually transesophageal echocardiography, for visualization of the soft tissues and immediate functional assessments of the prosthetic valve and impact on surrounding structures. MR can provide this soft tissue visualization in addition to procedural guidance. In comparison to transesophageal or intracardiac echocardiography, MR provides excellent visualization of anterior cardiac structures such as the pulmonary valve. Importantly, real-time MRI guidance may also allow for novel approaches to facilitate valve implantation. While preclinical work is promising, including reports of real-time MRI-guided aortic valve replacement,25 the lack of MR-compatible complex valve delivery systems is an ongoing challenge to translation.
Intracardiac Shunt Device Closure
Intracardiac shunts such as atrial septal defects and ventricular septal defects can be closed in the catheterization laboratory with fluoroscopic guidance. Preclinical studies have demonstrated feasibility of real-time MRI-guided intracardiac shunt closure using modified devices and delivery systems.26 MR guidance offers far superior visualization of intracardiac defects, as well as impact of devices on surrounding structures and functional assessment post device placement.
Shunt Creation
Shunt creation, such as an atrial septostomy, may be required to increase mixing between intracardiac chambers and has been reported under real-time MRI-guidance in an animal model.27 Shunts may also be required to direct flow of blood from one structure to another such as a Blalock-Taussig shunt and usually require cardiac surgery. Real-time MRI guidance with superior soft tissue visualization compared to x-ray has facilitated multiple novel procedures in preclinical studies including a percutaneous cavopulmonary shunt; 28 ultimately, the ability to perform such novel procedures in patients, avoiding the need for surgery, will drive clinical adoption of interventional cardiac MR techniques.
Electrophysiology
Electrophysiology (EP) procedures are conventionally guided using fluoroscopy or electroanatomical mapping (EAM) systems. Many of these procedures are ‘complex’ ablations, treating atrial fibrillation (AF), atypical macro-re-entrant atrial tachycardias (AT) and ventricular tachycardia (VT) and success rates may be as low as 40% in some patient groups.29 There is therefore a broad scope for the improvement of complex procedures with the appropriate use of novel technology. Interventional MR offers significant advantages in three main areas: arrhythmia substrate identification, detailed procedural guidance and ablation lesion evaluation.
Both atrial and ventricular arrhythmogenic substrates have been identified on MR imaging,30, 31 and imaging may conventionally be fused with EAM-acquired anatomy. However, critical MR-derived targets for VT ablation are typically as small as 2-4mm wide32 and even smaller for atrial ablation.33 Inevitable errors in registration between modalities mean that either a very broad region must be ablated or critical targets are left untouched, with consequent impact on safety, time and efficacy. In contrast, MRI-guided EP may use imaging acquired at the same procedure and hemodynamic conditions, or even in real-time, to improve the outcome of imaging-guided ablation.
The procedural guidance provided by conventional EAM systems is generally excellent, but is limited to the chamber cavity with no information regarding adjacent structures. Newer commercial EAM solutions have enabled fusion with fluoroscopic or ultrasound imaging, but MR provides a vastly superior soft tissue contrast and depth of field. Detailed information on the chamber of interest and the surrounding structures such as esophagus, coronary arteries and adjacent chambers may assist the performance of many procedures, particularly those in patients with complex congenital heart disease.
The failure to create durable transmural lesions has been held largely responsible for the high recurrence rates following many complex ablations, particularly VT and AF.29, 33, 34 MR may be used to assess acute ablation lesions, 7, 33, 35, 36 and consequently enable targeted ‘top-up’ ablation at selected locations. More recently, interest has focused on real-time imaging of lesion formation, and with further developments it may even be possible to titrate energy delivery to ensure the creation of adequate ablation lesions. However, capacity to delineate acutely the extent of irreversible electrical block remains unclear. Furthermore, while the thicker ventricular myocardium is particularly amenable to imaging, and resistant to transmural ablation, the increased risks of ventricular ablation have meant that clinical implementation has not yet been achieved.
Imaging Tools
Interventional MR differs from standard diagnostic MR because it demands faster image acquisition, reconstruction and processing for clinical decision making during an invasive procedure. Additionally, dynamic procedural guidance uses real-time imaging with interactive parameter control and requires simultaneous visualization of interventional devices and tissue. This section will discuss the typical imaging methods used during MRI-guided cardiac procedures.
Cardiac MR Techniques in the Interventional Environment
Cardiac MR can produce valuable anatomical and functional data at various stages of an intervention. In some cases, roadmap images may be acquired at the beginning of a study. For example, magnetic resonance angiography37 or 3D self-navigated acquisitions of the cardiac anatomy38 may generate volumes which are subsequently used for navigation of catheters during MRI-guided EP procedures. Similarly, relevant 2D views along the predicted trajectory of the device may be planned before an MRI-guided diagnostic catheterization or structural intervention to later be used during the real-time image guidance.39
The measurement of cardiac function and vascular flow are the backbone of cardiac MR exams and are used during pre- and post-procedural assessment of valvular disease, large vessel stenotic lesions and intracardiac shunts. Moreover, in the context of a diagnostic catheterization study, measurements of function and flow may be repeated multiple times to assess hemodynamics under a variety of physiological provocation states (e.g. exercise, nitric oxide gas or IV fluid challenge).40
Breath-held ECG-gated cine images can require many minutes of acquisition and are not suitable for repeated measurement in the interventional environment. Recently, retrospectively gated and motion corrected free-breathing cine sequences with fast reconstructions have been developed41 to generate a full short axis stack that is acquired and reconstructed in <1 minutes,42 making repeated cardiac function exams feasible during a procedure.
ECG-gated phase contrast imaging provides quantitative flow measurements, preferred over Fick or thermodilution traditionally used in the cath-lab.17 Real-time phase contrast imaging using undersampled spiral acquisitions can now achieve <40 ms temporal resolution. Real-time flow measurements have been used during physiological exercise stress and to generate beat-to-beat cardiac output measurements.43 Acquisition and processing times of 4D flow are currently long for intraprocedural use, but can be used for assessing flow patterns before or after an intervention.
The ability to perform tissue characterization is a unique strength of MRI over other imaging modalities. Mapping fibrotic tissue with high resolution late-gadolinium enhancement (LGE) T1-weighted acquisitions is critical to the identification of arrhythmia substrates.4, 6 Recent advances include improvements in resolution and coverage with 3D acquisitions44 and improved specificity with multi-contrast late enhancement (MCLE) acquisitions facilitating T1-like mapping.5 Perhaps of greatest importance to EP interventions is the capacity of MRI to characterize ablation lesion extent to determine procedural success.45 Specifically, the ability to distinguish between acute edema and permanent necrosis is powerful. Typically, edema is visualized using T2 weighted fast spin echo imaging45 or T2 mapping methods. Necrosis has been historically visualized using LGE imaging45; however, the application of LGE is limited in the context of a lengthy MRI-guided procedures by the time-dependent nature of the contrast agent distribution throughout a procedure. Instead, native T1-weighted imaging, using inversion recovery gradient echo sequences, has emerged as an alternate method for necrosis imaging and provides superb sensitivity to tissue lesions7 (Figure 1).
Figure 1.
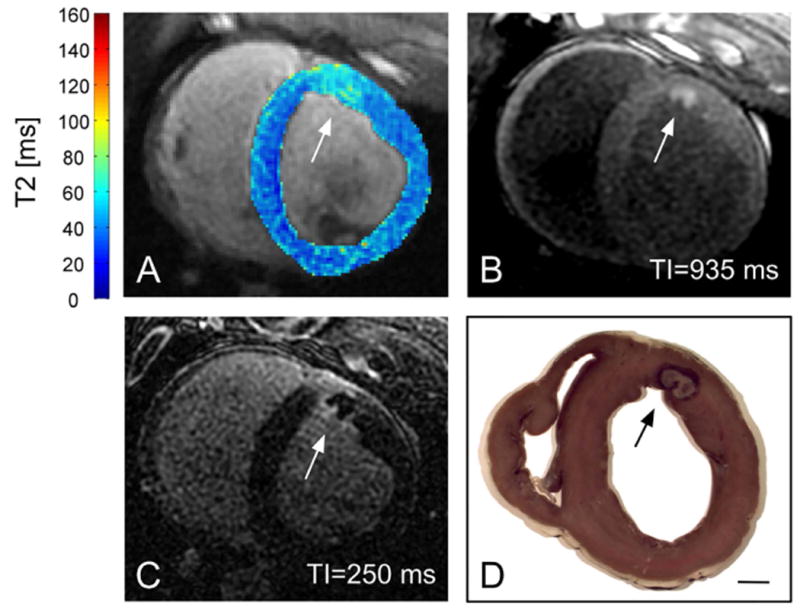
RF lesion on the anterior left ventricular wall of a swine. Elevated T2 surrounding the ablation site in (A) (T2 overlaid on anatomical T2-weighted image) demonstrated the development of edema. The more focal hyperenhancement in T1-weighted IR-SSFP (B), consistent with prior studies,7 likely reflected necrosis. (C) 3D late enhancement acquired 17 min after gadolinium injection. (D) Gross pathology. The scale bar indicates 1 cm (Figure courtesy to Philippa Krahn, Sunnybrook).
Real-time interactive imaging
During MRI-guided interventions, real-time imaging can be used for dynamic procedural guidance.46, 9 High frame rate imaging and display with minimal delay is essential for MRI to perform well compared to established interventional modalities such as x-ray and ultrasound. Real-time MRI produces a continuous stream of images that are not gated or breath held (Figure 2). Often, multiple slices are updated in rapid succession. Interactive control of slice geometry, image contrast and frame rate is used throughout the procedure. Balanced steady state free precession (bSSFP) sequences are commonly chosen for their good blood-myocardium contrast and fast repetition time. With standard Cartesian bSSFP, a frame rate of up to 10 frames/s is achieved with parallel imaging. Interactive modification of the parallel imaging acceleration factor is useful to trade-off frame-rate and image quality as needed during a procedure. 1.5T scanners are recommended for real-time imaging to reduce SAR safety concerns, bSSFP imaging artifacts, and device artifacts.
Figure 2.
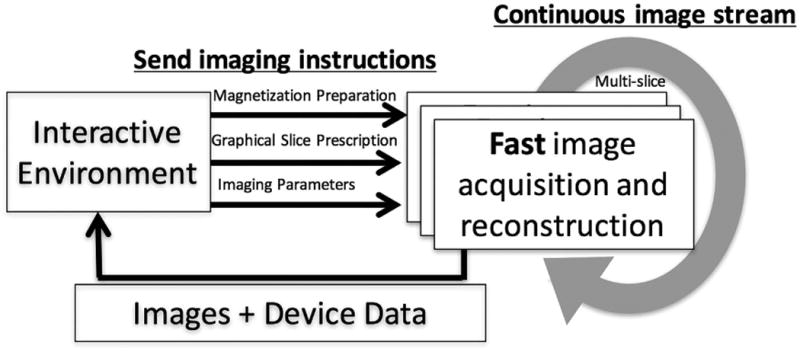
Schematic diagram of real-time imaging with interactive control. High frame rate images are continuously acquired. The image acquisition and reconstruction scheme adapts to real-time instructions from the graphical interactive environment. Image and device data returns to interactive environment for display with low-latency.
Typically, changes in imaging contrast are controlled by the addition of a magnetization preparation module before each single shot image that can be toggled on/off interactively. For example, saturation pulses are used to highlight T1 contrast of a gadolinium filled device, flow sensitive saturation pulses can produce dark blood/bright tissue images for improved anatomical context during device navigation,47 and real-time inversion recovery can be used for pseudo-LGE of lesions during real-time imaging.48 The ability to interactively modify imaging contrast throughout the procedure is a key advantage of MRI-guided interventions.
Faster Imaging
Imaging efficiency is crucial for real-time MRI and intra-procedural MRI. Parallel imaging can be used to improve acquisition times. Fast Cartesian GRAPPA and SENSE reconstructions are possible on standard vendor-supplied reconstruction hardware. Through-time parallel imaging techniques such as TSENSE and UNFOLD are specifically designed for high-frame rate imaging often employed in the interventional MR environment.49
Efficient k-space sampling such as echo planar imaging (EPI) and spiral imaging are very appealing to reduce acquisition time and generate high SNR images. Real-time distortion correction50 can improve image quality to make application of these techniques in the interventional environment feasible. Iterative parallel imaging reconstructions can be used to achieve fast reconstruction of undersampled non-Cartesian data.51
Compressed sensing algorithms show potential for further acceleration of image acquisitions in the future, but are currently incompatible with the interventional MRI environment because of long reconstruction times.52
Faster Reconstructions
Fast reconstruction is essential to produce images with low latency to be used for clinical decision making within the interventional environment. Fast reconstruction is especially important during real-time imaging where <200ms latency in image display is tolerable for device navigation. MRI manufacturers provide reconstruction software and hardware capable of achieving real-time Cartesian image reconstruction with parallel imaging. However, the reconstruction algorithms required for more complex imaging schemes often demand additional reconstruction tools for timely image generation.
Coil array compression is essential to reduce computational burden of reconstructions.53 Graphics processing units (GPUs) can accelerate computation and provide substantial improvements in reconstruction speed. To leverage additional GPU hardware, reconstructions must be performed on a computer with the required specifications. Inline reconstruction from external computers is made possible with specialized reconstruction packages, such as the Gadgetron.54 Furthermore, additional computational speed can be facilitated through distributed computing in local cluster or cloud environments.42
Passive Device Visualization
Passive devices are visualized using their inherent material properties (Figure 3). For example, metallic devices will produce a signal void in standard anatomical imaging induced by magnetic susceptibility artifacts that can be used for visualization. Stainless steel generates an overwhelming signal void that obstructs tissue visualization, whereas paramagnetic metal alloys, such as nitinol, produce a signal void that is often too subtle to use for reliable device navigation. Several alternate techniques have been proposed to improve the visualization of paramagnetic devices, including the addition dephasing gradients to produce positive contrast, off-resonance excitation and reconstruction techniques such as susceptibility gradient mapping.55 Imaging techniques that acquire a standard anatomical image and positive contrast device image can be combined with real-time image processing to isolate device signal and overlay this signal on the anatomy.56
Passive susceptibility markers, including iron oxide particles and stainless steel can be used to augment the visualization of passive devices. Susceptibility markers produce a signal void in a distinctive pattern to enable visual device tracking and have been used for MRI-guided electrophysiology procedures in humans.57
In the case of balloon-tipped pressure catheters, the balloon can be filled with a significant volume of contrast agent and this balloon contrast has been used for catheter navigation in patients.19 However, the small volume of the lumen limits the effectiveness of this method for visualization of the catheter shaft.
The primary limitation of passive device visualization is that devices are only visible within the imaging plane (5-15mm thickness), which can be challenging as a device is navigated through tortuous vasculature. Automatic slice plane prescription is not possible since the device location is being determined visually without any quantitative information on device position. Increasing slice thickness can help to relocate an out-of-plane device, but at the expense of image contrast. The primary advantage of passive device visualization is the ability to use many of the standard commercially available devices that are familiar to interventionists and effective for procedures, without the need to re-engineer an array of mechanical and physical properties.
Active Device Visualization
Active visualization utilizes electrical connection to the scanner (Figure 3). Active devices are manufactured to incorporate receiver electronics and therefore produce a very specific signal (received from surrounding tissue) that can precisely determine the position of the device. Receivers can be designed as microcoils, which are solenoids that act as point-source signals, or loopless antennas that provide signal along the entire length of the device.
The simplest visualization technique, called device profiling, isolates the signal received from the device channel and superimposes it on the image reconstructed from the other receivers. The device channel can also be colored to improve visualization.11 The signal received by the device coil drops off quickly away from the coil, creating some blurring to the signal.
Fast localization of microcoils can also be achieved by projecting the MR signal obtained by the solenoid onto three orthogonal planes.58 This is accomplished by a volume excitation and the acquisition of three gradient echoes in orthogonal directions. The microcoil appears as a signal peak in each projection. Device orientation can be determined using two microcoils in close proximity and assuming a linear relationship between them. High frame rate tracking can be used to navigate microcoil signal on a pre-acquired roadmap image or interleaved with real-time imaging.
The MRI signal received by the device channels can additionally be used for high resolution, small field-of-view intravascular imaging. The majority of intravascular imaging is targeted at the vessel wall and atherosclerotic plaques. Most active devices developed for this purpose are “side-looking” solenoid and loopless antenna designs. The loopless antenna design produces signal along the device length and slower signal drop off, making it much less sensitive to precise device positioning for intravascular imaging.59
Figure 3.
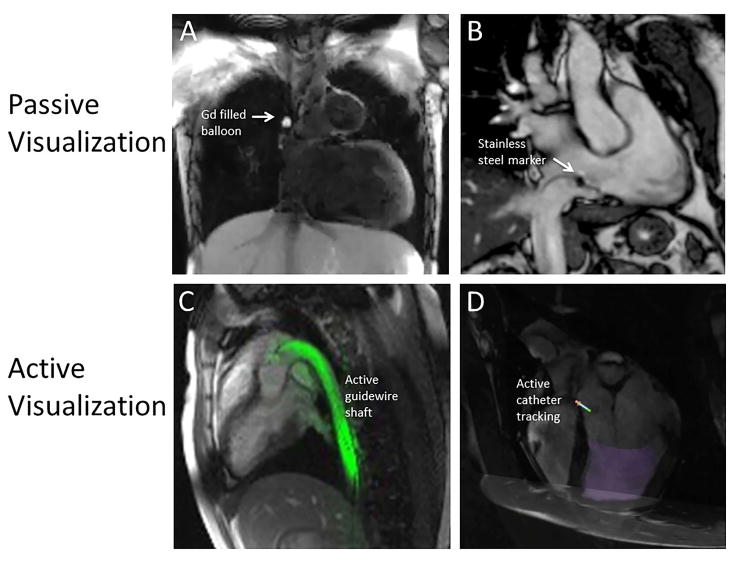
Examples of passive and active device visualization. A) Gadolinium-filled balloon tip catheter in the superior vena cava. Image contrast is created using a flow-sensitive dark blood saturation pulses during real-time bSSFP imaging. B) Stainless steel imaging marker on a passive catheter (Imricor Medical Systems, Burnsville, MN, USA). C) Color overlay of active guidewire (loopless antenna) depicting the entire device shaft in-plane. D) Active tracking coils used to overlay catheter tip position and orientation on a preacquired image (Imricor Medical Systems, Burnsville, MN, USA).
Hybrid Device Visualization Methods
Some methods exist to produce active-like visualization from a standard commercial device. Active-like visualization can also be generated using external devices which couple magnetically to the conductive guidewire which passes through it.60 In this configuration, a standard metallic guidewire acts like an antenna without any physical modification.
Inductively coupled markers are small solenoid coils that are tuned to the resonant frequency of the scanner and therefore are coupled by induction with the transmit coil. These inductively coupled markers produce an amplified signal in low flip angle imaging.61
Safety
The primary safety concern in MR imaging is heating of tissue due to RF energy absorption. Real-time bSSFP imaging for interventional cardiac MR applications has potentially high specific absorption rate (SAR). Software and hardware monitoring of SAR are in place to mitigate these risks. Pediatric applications require special attention, including use of coils specifically designed for this population. Importantly, during an interventional MR, the patient is generally sedated or anesthetized and won’t be able to report any burning or tingling sensations; therefore, extra care is required to minimize risk.
RF-induced heating is a major concern when using passive metallic guidewires and active devices that require a transmission line. The same issues exist for scanning patients with implanted pacemakers or defibrillators. These devices have long conductive structures that can couple to the electric component of the RF excitation field, creating current on the conductor and a large electric field and heating at the device tip.62 This effect is most severe at the edge of the bore where the electric field from the RF coil has higher amplitude. The temperature increase also depends on device length, orientation and insulation. Guidewires are essential for most catheterization and interventional procedures since they are used to navigate the vasculature and for device exchange. Thus, safety concerns about passive and active guidewires are an essential hurdle to overcome.
Device-based solutions to the problem of RF-induced heating aim to reduce the current formed on the device, for example using transformers in transmission lines63 or floating current traps64. The pulse sequence can also be altered to reduce RF duty cycle by moving to low flip-angle, long TR methods such as gradient echo spiral or EPI imaging, which have been demonstrated to substantially reduce RF-induced heating in passive guidewires.56 Parallel transmit with E-fields oriented in multiple directions has also been used to reduce the potential for coupling and, hence, heating on metallic devices.65
Gaining regulatory approval for an interventional MR device requires the demonstration of device safety with regards to potential of RF heating, the safe and proper operation of the device, as well as identification of any interference with the MRI system. The commonly used safety guideline to assess RF-induced heating of implantable passive devices is ASTM F2182. This ASTM guideline, which can be adapted for assessment of interventional MR devices, establishes a standardized gel phantom to be used for experiments as well as procedures for mapping the E-field and measuring local temperature near devices. The FDA has provided additional recommendations on RF safety of multi-configuration passive devices, which recommends simulations or experiments to establish a subset of device configurations that produce maximal heating for in-depth experimental testing. Aside from RF-induced heating, it is also important to note that the static magnetic field, can exert large forces and torques on any device that consists of ferromagnetic material. Also, conductive material may experience hazardous vibration because of induced eddy currents and Lorentz forces due to the switching of the gradient fields. The guideline ISO/TS 10974 (IEC) for MRI safety of implantable active devices covers this broader range of topics.
Recent research demonstrates that image-based current measurement techniques can be utilized to evaluate and characterize the safety of a device without the requirement of lengthy experimental testing.66 The benefit of this approach is that it permits labelling of the device based on induced RF current distributions which could permit many scans that may be otherwise forbidden with labelling that is based on global SAR levels.67 Furthermore, such measurements could be used to guide adjustments in acquisitions such as the use of transmit arrays described above.
Given these requirements, the design, testing and use of devices, for interventional MR applications, requires careful attention to ensure safety.
Image Analysis
Planning and guidance of some procedures requires roadmaps of major cardiac structures (i.e., heart and vessels) identified in MR images and registered to the real-time frame of reference of catheter location (to guide the device navigation), along with the identification of the target such as the arrhythmia substrate. These needs have driven the development of specific image analysis methods, including: a) fast segmentation methods; b) rapid registration of pre-, intra- and post–operative images; and c) clever encapsulation, fast updates, dynamic coupling and smooth integration of imaging features into user-friendly visualization platforms. The major challenge is to seamlessly integrate these components (i.e., ideally ‘on-the-fly’) into real-time interventional MR applications as illustrated in the diagram of workflow in Figure 4, without compromising accuracy and system performance during the image processing steps.
Figure 4.
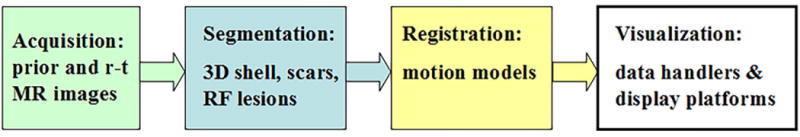
Diagram of workflow to integrate segmented MR images and registration steps into visualization platforms for MRI-guided interventions.
Segmentation
Following image acquisition, the first critical step is to deliver accurate 3D models (e.g., 3D shells with labelled anatomic zones and landmarks, often with major vessels delineated),68 like those used in conventional interventions.69 The most popular segmentation methods employed to delineate structures (e.g. chamber walls in cine images, infarcted areas in 2D/3D LGE images or RF ablation lesions) are typically based on manual or semi-/automated algorithms such as: classification (thresholding, k-means), deformable models (e.g. snake, level-set), atlases and probabilistic methods (e.g. Markov random field, graph cuts).70–72 Moreover, multi-atlas segmentation methods can use prior anatomical knowledge and combine intensity, gradient and contextual information into an augmented feature vector to guide the label fusion of cardiac structures by support vector machine classifiers, significantly improving the segmentation accuracy.73
Further developments are underway to implement optimization schemes that can speed up segmentation methods, to identify atrial/ventricular walls from surrounding structures and to generate intra-operative 3D whole heart (WH) models/shells. For example, a “near real time” heart segmentation was achieved using an efficient algorithm based on active geometric functions and implicit surface representation, enabling endocardial segmentation in a few milliseconds/frame.74 Others used the open-source Seg3D 2.0 software (www.sci.utah.edu/cibc-software/seg3d.html) to segment the right atrium (RA) geometry, and then employed SCIRun 4.6 (www.sci.utah.edu/cibc-software/seg3d.html) to generate 3D RA surface meshes and geometric models of RF lesions.75
Many of these segmentation tools are now being translated into the real-time interventional MR arena, where real-time MRI-guided RF ablation therapy is a prototypical application. For instance, Chubb et al.76 used non-contrast bSSFP-3DWH imaging prior to real-time MRI-guided RF ablation in patients with atrial flutter and derived the right atrium RA contour by employing an automated segmentation method based on shape-constrained deformable models. To help guide the RF catheter, they also manually delineated the coronary sinus CS and IVC using itk-SNAP (www.itksnap.org) and then integrated the results into a color-coded 3D shell model. Figure 5 shows a segmentation result obtained using SmartHeart segmentation software (Philips Research) for the navigation of RF catheters (see caption for details).
Figure 5.
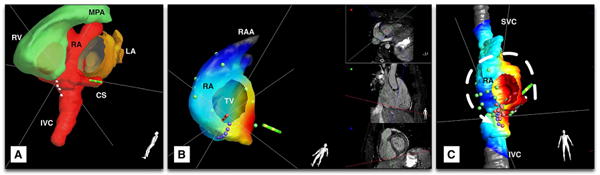
Segmentation of MR images for catheter navigation: (a) MRI-based segmented 3D whole heart, with left ventricle removed. Note the integration of automated (right ventricle (RV) and left atrium (LA)) segmentations and manual segmentation (right atrium (RA) with coronary sinus (CS) and inferior vena cava (IVC)); (b) reconstructed 3D shell of the RA with color-coded activation map recorded on pacing from the CS (green catheter icon) (red corresponds to early activation times); and c) ablation lesions (pink dots) relative to the anatomic model, with intended ablation line shown in purple dots. A line of conduction block has been created at the location of the ablation lesions, leading to the alteration of intracardiac conduction to take an anticlockwise path around the tricuspid valve (TV) annulus on pacing from the CS. RAA: right atrial appendage.
Manual segmentation of the WH is tedious and subject to bias; thus, future work will focus on refining deformable and atlas-based methods.77,78 Notably, deformable models are already computational effective (e.g. 4-30sec) with realistic results being granted by the shape constraint; however, the shape models need large databases for training and boundary detection is crucial in these cases. Recent advances demonstrate that non-rigid registration using GPU code for atlas-based propagation can be performed within 1 minute, opening up the possibility of intra-operative segmentation of WH using atlas-based methods.73
Furthermore, robust segmentation methods of real-time MR images are also being developed to quantify cardiac function and flow. These are complex problems because the periodicity of contraction and valvular movements is often compromised in pathologic cases. Real-time MR images can be segmented automatically to facilitate the calculation of EF, providing rapid feedback during diagnostic exams. For instance, a recent study79 used a context-based algorithm to demonstrate the feasibility of an automatic multi-cycle segmentation method for real-time images acquired with high temporal resolution (20ms), free breathing and no ECG synchronization. The method proved to be robust to inter-cycle variation of functional parameters, matching well expert manual segmentation. Others80 implemented in MATLAB a user-initialized active contour segmentation of real-time golden angle images (frame rate >89/sec), well suited for the rapid quantification of time-varying left ventricular function in patients with ectopic heart beats (i.e., data processing ∼3 minutes/slice).
Registration
For conventional X-ray guided procedures, several algorithms have been developed to deal with image alignment, registration, fusion and motion correction between pre-/intra-/post-operative images81, or between images acquired using different systems (e.g. MR images and EP maps). Most of these approaches involve registration via anatomical markers, which are labelled in the segmented 3D shells and provide pre-procedural road maps.78 For instance, most clinical centers perform RF ablations under X-ray guidance using electro-anatomical mapping systems which can upload and display segmented prior CT or MR images for fusion with electrical maps.82 For analysis purposes (e.g. correlation of voltage/activation time maps with MR-derived scar and substrate location), the EP surface maps are registered to segmented 3D shells via landmark registration algorithms, targeting a clinically accepted positional registration error <5mm.4, 83 Vendor tools (e.g. CARTOMERGE, Biosense Webster) can perform 3D registration between EAM maps and prior segmented MR images in <1 minutes, with some authors reporting registration errors of ∼4mm.84 One important source of errors is the respiratory motion (assuming that the EAM maps and MR datasets were recorded in a similar cardiac phase).
Real-time MR cannot provide the spatial resolution and 3D coverage needed to present updated roadmaps and target locations with sufficient temporal resolution to be concomitant with catheter manipulation.85 Therefore, alternative strategies have been developed to take advantage of the distinct prior roadmap images and real-time reference images (Figure 6); this is challenging due to their different resolutions, plane orientations and acquisition parameters (related to free breathing and no triggering during the real-time imaging) and the quest for rapid data processing. Furthermore, both cardiac and respiratory motion can cause discrepancies between the visualized prior volume and the underlying intra-procedural anatomy. Motion correction algorithms must compensate for both types of motion in real-time, with positional alignment accuracy <5mm, ideally with an execution speed of <1 heartbeat to adapt to changes in motion patterns.
Figure 6.
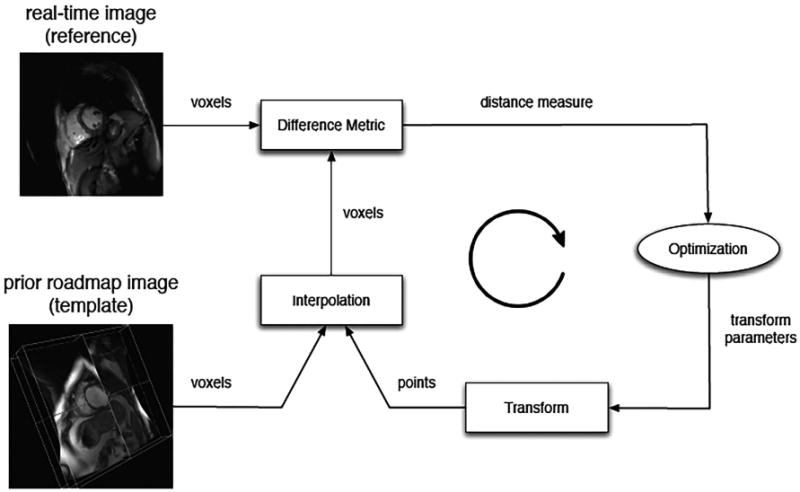
A general registration framework for aligning RT and prior images is shown. In each iteration, a difference measure reflecting misalignment between the reference and transformed template image is calculated and a spatial transformation is then applied to the template image to minimize the difference, with the process repeated until acceptable alignment is achieved (Figure courtesy Dr. Robert Xu, Sunnybrook).
Thus, the goal is to dynamically register preprocedural 4D MR datasets with intra-procedural 2D real-time MR images. Gao et al.86 proposed ‘on-the-fly’ updating of a pre-operative 4D left ventricle model using real-time 2D images, realizing accelerated processing (ie., 26 frames/sec with 0.42mm accuracy) by matching common pre- and intra-operative adjunct data. Others have developed a robust multi-scale image-based registration framework to correct for respiratory motion between a 3D MR prior volume and 2D RT MR images. For this, a weighted total variation flow algorithm was used to extract coarse-to-fine features from the input images and subsequently register the corresponding scale images in a hierarchical manner.87 The registration method yielded a corresponding model that was used to characterize the respiratory motion during an MRI-guided ablation study.88 Further, accelerating the registration algorithm with a GPU-based implementation facilitated updating the respiratory motion model every heart beat to account for potential intraprocedural changes.89
Visualization
Platforms
Real-time MRI-guided cardiac procedures require advanced platforms for visualization, which have been continuously developed and refined, playing a vital role in supporting ongoing pre-clinical and clinical experimental projects. Currently, there are several well-established visualization platforms used by the main vendors for MR scanners (Siemens, Philips and GE), as summarized in Table 1. Briefly, Siemens offers a real-time interactive pulse sequence as a “work in progress” package, prototype software for procedural visualization and supports use of the Gadgetron open source reconstruction software (gadgetron.github.io).54 Similarly, iSuite by Philips provides a robust and versatile platform for interventional MR such as MRI-guided EP procedures.90 The RTHawk research platform, is based on the Heart Vista Cardiac Package (www.heartvista.com), and provides a comprehensive set of diagnostic applications combined with a simple and intuitive real-time interface for GE scanners. Lastly, the Vurtigo platform (www.vurtigo.ca) is a four dimensional (3D+time) real-time visualization application capable of displaying prior volume roadmaps, real-time MRI scan planes, electro-anatomical maps, segmented heart models and tracked catheters for GE scanners.91
Table 1.
Equipment that may be used for MRI-guided cardiovascular procedures.
| Equipment | Details and vendors |
|---|---|
| MRI scanner |
|
| Real-time scanner control |
|
| Visualization software |
|
| In-room display |
|
| Audio Communication | Noise cancelling headsets:
|
| Patient Monitoring |
|
| Hemodynamic Recording |
|
| Electrophysiology equipment | MRI equipment:
|
| MRI-compatible Anesthesia and ventilation cart |
|
| MRI safe tables and stands |
|
| In-suite cameras |
|
Integration of Image-Based Modelling in Real-Time Image-Guidance Platforms
In support of EP interventions, the 3D MR-based anatomical models can be coupled with mathematical formalisms to accurately simulate the propagation of electrical waves through the heart.92, 93 Such MR-based models can be used as an additional non-invasive tool to guide cardiac procedures and predict the therapy outcome via simulations.94 For example, in a recent study95 3D geometrical meshes were built from segmented 2D MCLE images obtained at 1×1×5mm spatial resolution prior to the real-time MRI-guided EP mapping studies. These were coupled with fast computational models and simulated the wave propagation through the left ventricle in <3 minutes using standard processors. The predicted activation times and patterns were comparable to the recorded EP maps and provided 3D transmural electrical information (Figure 7 a-b). This novel experimental-modelling framework could be integrated into interventional MR visualization platforms for use in guiding catheters to ablation targets derived from MR maps of VT substrate. Such a framework could also provide feedback regarding the therapy success by predicting subsequent electrical propagation patterns in ‘near’ real-time.
Figure 7.
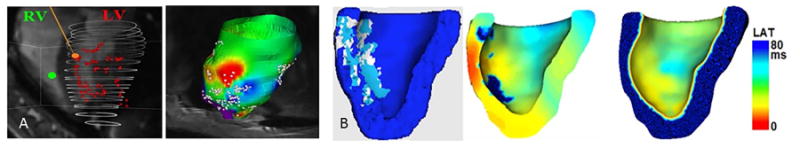
Integration of real time MRI-guided EP data with predictive computer models in an infarcted pig case: (a) real time EP mapping of the left ventricle (LV) under pacing from right ventricle (left) and associated late activation times (LAT) maps projected onto the LV surface mesh built from prior cine data (right); and (b) MCLE-derived mesh with scar (light blue), VT substrate (white) and healthy zones (dark blue) segmented and labelled correspondingly (left), output of the 3D predictive model (center); and measured LAT map projected onto the MCLE-derived mesh showing a pattern similar to the measured LAT map (right), where red corresponds to early depolarization times.
Integration with clinical workflow
In the clinical environment, the information provided by advanced MR imaging must be integrated with other critical patient information and communicated to a diverse team. A standard catheterization lab team includes invasive cardiologists, cath-lab technologists and nurses, and often respiratory therapists and anesthesia personnel. An MRI-guided catheterization team may add an interventional MR technologist, and academic teams may add MRI scientists and research coordinators. Staff training in MRI safety is essential before undertaking MRI-guided procedures, especially in clinical settings where staffing changes daily.
During a catheterization procedure, the interventionists and cath-lab technologists use multiple projectors or screens for in-room display of imaging sources (e.g. x-ray, echocardiography, MRI, electroanatomical mapping) and hemodynamics (e.g. ECG and invasive blood pressures). Furthermore, displays are mirrored throughout the suite such that other participating staff, including anesthesia personnel, technologists and nurses, can monitor the patient and follow the progress of the procedure.
Verbal communication between the interventionist inside the MRI suite and MRI technologist is commonly used for adjustment of MRI parameters and imaging planes and for interaction with visualization systems. Teams typically communicate using wireless headsets designed to eliminate MRI acoustic interference. Alternatively, MRI compatible accessories, such as gyroscopic computer mice, foot pedals,96 and gesture detection97, have been proposed for scan manipulation.
The MR environment also poses challenges for patient hemodynamic monitoring. Unexpected hemodynamic instability is common during cardiac procedures and therefore requires invasive blood pressure and ECG tracings, which are altered by the MR environment. Improved MRI hemodynamic recording is available with the NIH “PRiME” system (freely available from http://nhlbi-mr.github.io/PRiME/) and 12-lead ECG systems are also under development by researchers98 and industry (PinMed Inc.).
Once instrumented as above, interventional cardiac MR teams must be prepared to respond to life-threatening events such as arrhythmia, ventricular tachycardia or hypotension. Teams should prepare for emergency evacuation to a designated space for resuscitation with MRI-incompatible equipment.10 Mazal et al describes the evacuation roles and reports evacuation times of less than 1 minute.39 In-bore defibrillators99 and temporary pacemakers are also under development to address these contingencies.
Invasive procedures are performed in a sterile catheterization environment, which can be replicated by appropriate draping of the MRI equipment.39 When vascular access is obtained outside the scanner, cables and lines from the patient (e.g. ECG, pressure, IV, endotracheal tube) should be safely aligned before transfer into the bore. Vascular introducer sheaths may be sutured in place to prevent dislodgement during transfer.
MRI-safe anesthesia equipment and gas tanks (oxygen and nitric oxide) should be used throughout a procedure. Dedicated catheterization tables with non-ferromagnetic tools should also be prepared and checked before every procedure.39
As during conventional catheterization, technologists record key events during MRI catheterization procedures for offline review. Event records should be synchronized with MRI and hemodynamic recordings.
A list of the additional equipment required for MRI-guided interventions is provided in Table 1.
iMRI Suite configuration
Siting a new interventional MRI suite or retrofitting an existing suite requires careful planning. Although not strictly necessary, many suites are designed to adjoin catheterization labs since these procedures are not yet clinical standard. Co-location facilitates vascular access when undesirable in the MRI suite, as well as swift and safe bi-directional patient transfer and combined x-ray/MRI cases. In addition, the co-located catheterization lab can be used for case completion if the intervention cannot be completed under MRI. Combined suites are designed with RF-shielded sliding doors allowing direct patient transfer and independent use of facilities, which is economically appealing to hospitals.
A wide-bore MRI scanner is ergonomically preferred for interventional applications when interventionists need to reach into the scanner bore. While 1.5T yields a shorter bore, less concerns about device heating and reduced sensitivity to susceptibility effects, particularly for SSFP sequences, some sites are moving to 3T for the generally better image SNR these systems afford. Specifically designed interventional MRI scanners (e.g. open bore, C-shaped scanners) often do not have sufficient hardware performance to provide high quality cardiac images so have not generally been used for cardiac applications. To address the ergonomic challenges of patient access in conventional closed-bore scanners, Tavallaei et al. have proposed a remote catheter navigation system that allows the interventionalist to remotely manipulate a catheter with 3-degrees of freedom, in real-time, and under MRI guidance.100
Additional waveguides and penetration panels may be necessary to support additional interventional equipment (Table 1). Furthermore, a porthole for passing blood samples outside the room, for example for oximetry, can be installed. The MRI suite must be specifically designed with these requirements in mind given all the extra equipment that must be accommodated. A typical lay out of equipment in the scanner room and console area for an EP procedure is seen in Figure 8.
Figure 8.

The interventional MR setup, at the Sunnybrook Research Institute, for electrophysiology procedures is shown.
Concluding Remarks
This review has described the state-of-the-art technology for fast image acquisition, reconstruction, processing and display used for MRI-guided cardiac procedures. Additionally, we have outlined some key clinical targets. A major focus of recent developments is the need for seamless integration of MRI information and simplified workflows tailored to specific clinical applications.
The standard imaging methodology for MRI-guided cardiac interventions is readily available. Specifically, real-time MR imaging on interactive environments is a mature technology and techniques for device visualization have been rigorously tested in preclinical settings. Rapid approaches to image segmentation, registration and display are also in advanced stages of development. Ongoing technical improvements will focus on the customization of imaging and visualization methods designed for individual procedural needs.
Clinical adoption will be driven by procedural efficiency and/or clear patient benefit relative to existing methods. Importantly, clinical sites can start adopting these methods immediately by doing MRI-guided right heart catheterization with passive catheters and straightforward diagnostic EP procedures under institutional ethics approval. Although these procedures are simple compared to the lofty promises of interventional cardiac MR, they are an essential starting point to gain operator familiarity with MRI guidance and to gain industry support.
The primary roadblock for interventional cardiac MR procedures is still the unavailability of devices for the MRI environment. Currently, short needles and plastic catheters are available, and many MRI devices are in various stages of development. However, to achieve complex cardiac interventions under MRI guidance, MRI-safe guidewires, delivery systems and additional catheters are essential. Careful device design and engineering is required to maintain the mechanical properties and workflow that interventionists rely on. Primary regulatory concerns include electrical safety (active devices), appropriate coatings for human use and RF safety. Significant engineering and regulatory challenges must be overcome to achieve clinical MRI-guided interventions with these specialized devices.
Industry partners will be critical in moving this technology forward. As new devices and systems are developed and integrated, the responsibility will be on the imaging vendor to validate the imaging methods and to ensure precise imaging feedback and on the device vendor to validate use of these imaging methods and associated interfaces as they directly apply to the operation of their interventional technology (e.g. visualization of device location relative to overlaid anatomy).
Real-time MRI guidance of cardiac procedures relies on sophisticated MR imaging techniques, integrated into an interventional environment. Interventional cardiac MR will enable radiation-sparing procedures, and the soft tissue contrast of MRI will facilitate improvements to existing procedures and the development of novel catheter-based procedures. Real-time MRI guidance will continue to push the limits of MR imaging technology as these procedures evolve, and therefore will remain an active area of technological development.
Acknowledgments
The authors would like to thank Ms. Philippa Krahn for her great help in preparing some of the figures. The authors also acknowledge the kind assistance of Dr. Martin Janich for providing the 3D MDE (3D LGE) sequence that was used to acquire some of the images presented in this paper.
Grant Support: ACW's research is supported by the National Heart, Lung, and Blood Institute Division of Intramural Research (ZIA-HL006039, ZIA-HL005062, ZIA-HL006213, ZIA-HL006214). EKG's research is supported by the National Heart, Lung, and Blood Institute (HHSN268201500001C). GAW, MP, and MAT acknowledge related grant support from the Canadian Institutes of Health Research (MOP-93531) and the Federal Development Agency of Canada, as well as Imricor Medical, Innovere Medical, HeartVista, and GE Healthcare.
References
- 1.Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–2708. doi: 10.1093/eurheartj/ehl014. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JN, Hornik CP, Li JS, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;130:161–167. doi: 10.1161/CIRCULATIONAHA.113.005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreassi MG, Piccaluga E, Guagliumi G, Del Greco M, Gaita F, Picano E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ Cardiovasc Interv. 2016;9:e003273. doi: 10.1161/CIRCINTERVENTIONS.115.003273. [DOI] [PubMed] [Google Scholar]
- 4.Wijnmaalen AP, Van Der Geest RJ, Van Huls Van Taxis CFB, et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: Real-time image integration and reversed registration. Eur Heart J. 2011;32:104–114. doi: 10.1093/eurheartj/ehq345. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Connelly KA, Zeidan-Shwiri T, et al. Multi-contrast late enhancement CMR determined gray zone and papillary muscle involvement predict appropriate ICD therapy in patients with ischemic heart disease. J Cardiovasc Magn Reson. 2013;15:57. doi: 10.1186/1532-429X-15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt A, Azevedo CF, Cheng A, et al. Infarct Tissue Heterogeneity by Magnetic Resonance Imaging Identifies Enhanced Cardiac Arrhythmia Susceptibility in Patients With Left Ventricular Dysfunction. Circulation. 2007;115 doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celik H, Ramanan V, Barry J, et al. Intrinsic Contrast for Characterization of Acute Radiofrequency Ablation Lesions. Circ Arrhythmia Electrophysiol. 2014;7:718–727. doi: 10.1161/CIRCEP.113.001163. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy R, Cheong B, Muthupillai R. Tools for cardiovascular magnetic resonance imaging. Cardiovasc Diagn Ther. 2014;4:104–25. doi: 10.3978/j.issn.2223-3652.2014.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guttman MA, Ozturk C, Raval AN, et al. Interventional cardiovascular procedures guided by real-time MR imaging: An interactive interface using multiple slices, adaptive projection modes and live 3D renderings. J Magn Reson Imaging. 2007;26:1429–1435. doi: 10.1002/jmri.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhagirath P, van der Graaf M, Karim R, et al. Interventional cardiac magnetic resonance imaging in electrophysiology: advances toward clinical translation. Circ Arrhythm Electrophysiol. 2015;8:203–11. doi: 10.1161/CIRCEP.114.002371. [DOI] [PubMed] [Google Scholar]
- 11.Saikus CE, Lederman RJ. Interventional Cardiovascular Magnetic Resonance Imaging. JACC Cardiovasc Imaging. 2009;2:1321–1331. doi: 10.1016/j.jcmg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath KA, Li M, Mazilu D, Guttman MA, McVeigh ER. Real-time magnetic resonance imaging guidance for cardiovascular procedures. Semin Thorac Cardiovasc Surg. 2007;19:330–5. doi: 10.1053/j.semtcvs.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnayaka K, Lederman RJ. Interventional cardiovascular MR—The next stage in pediatric cardiology. Prog Pediatr Cardiol. 2010;28:59–67. [Google Scholar]
- 14.McVeigh ER, Guttman MA, Kellman P, Raval AN, Lederman RJ. Real-time, interactive MRI for cardiovascular interventions. Acad Radiol. 2005;12:1121–1127. doi: 10.1016/j.acra.2005.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lardo AC. Real-time magnetic resonance imaging: Diagnostic and interventional applications. Pediatr Cardiol. 2000;21:80–98. doi: 10.1007/s002469910010. [DOI] [PubMed] [Google Scholar]
- 16.Brenner LD, Caputo GR, Mostbeck G, et al. Quantification of left to right atrial shunts with velocity-encoded cine nuclear magnetic resonance imaging. J Am Coll Cardiol. 1992;20:1246–1250. doi: 10.1016/0735-1097(92)90384-y. [DOI] [PubMed] [Google Scholar]
- 17.Muthurangu V, Taylor A, Andriantsimiavona R, et al. Novel Method of Quantifying Pulmonary Vascular Resistance by Use of Simultaneous Invasive Pressure Monitoring and Phase-Contrast Magnetic Resonance Flow. Circulation. 2004;110 doi: 10.1161/01.CIR.0000138741.72946.84. [DOI] [PubMed] [Google Scholar]
- 18.Razavi R, Hill DLG, Keevil SF, Miquel ME. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003 doi: 10.1016/S0140-6736(03)14956-2. [DOI] [PubMed] [Google Scholar]
- 19.Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34:380–389. doi: 10.1093/eurheartj/ehs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers T, Ratnayaka K, Karmarkar P, et al. Real-Time Magnetic Resonance Imaging Guidance Improves the Diagnostic Yield of Endomyocardial Biopsy. JACC Basic to Transl Sci. 2016;1:376–383. doi: 10.1016/j.jacbts.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raval AN, Telep JD, Guttman MA, et al. Real-Time Magnetic Resonance Imaging–Guided Stenting of Aortic Coarctation With Commercially Available Catheter Devices in Swine. Circulation. 2005;112 doi: 10.1161/CIRCULATIONAHA.105.542647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehne T, Saeed M, Higgins CB, et al. Endovascular Stents in Pulmonary Valve and Artery in Swine: Feasibility Study of MR Imaging–guided Deployment and Postinterventional Assessment. Radiology. 2003;226:475–481. doi: 10.1148/radiol.2262011639. [DOI] [PubMed] [Google Scholar]
- 23.Krueger JJ, Ewert P, Yilmaz S, et al. Magnetic Resonance Imaging–Guided Balloon Angioplasty of Coarctation of the Aorta. Circulation. 2006;113 doi: 10.1161/CIRCULATIONAHA.105.578112. [DOI] [PubMed] [Google Scholar]
- 24.Tzifa A, Krombach GA, Kramer N, et al. Magnetic Resonance-Guided Cardiac Interventions Using Magnetic Resonance-Compatible Devices: A Preclinical Study and First-in-Man Congenital Interventions. Circ Cardiovasc Interv. 2010;3:585–592. doi: 10.1161/CIRCINTERVENTIONS.110.957209. [DOI] [PubMed] [Google Scholar]
- 25.Kahlert P, Parohl N, Albert J, et al. Real-Time Magnetic Resonance Imaging–Guided Transarterial Aortic Valve Implantation. J Am Coll Cardiol. 2012;59 doi: 10.1016/j.jacc.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Rickers C, Jerosch-Herold M, Hu X, et al. Magnetic resonance image-guided transcatheter closure of atrial septal defects. Circulation. 2003;107:132–138. doi: 10.1161/01.cir.0000039343.95540.cf. [DOI] [PubMed] [Google Scholar]
- 27.Raval AN, Karmarkar PV, Guttman MA, et al. Real-time MRI guided atrial septal puncture and balloon septostomy in swine. Catheter Cardiovasc Interv. 2006;67:637–643. doi: 10.1002/ccd.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratnayaka K, Rogers T, Schenke WH, et al. Magnetic Resonance Imaging–Guided Transcatheter Cavopulmonary Shunt. JACC Cardiovasc Interv. 2016;9:959–970. doi: 10.1016/j.jcin.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design. Europace. 2012;14 doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 30.Marrouche NF, Wilber D, Hindricks G, et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA. 2014;311:498. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 31.Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic Resonance Assessment of the Substrate for Inducible Ventricular Tachycardia in Nonischemic Cardiomyopathy. Circulation. 2005;112 doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreu D, Berruezo A, Ortiz-Pérez JT, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythmia Electrophysiol. 2011;4:674–683. doi: 10.1161/CIRCEP.111.961946. [DOI] [PubMed] [Google Scholar]
- 33.Ranjan R, Kholmovski EG, Blauer J, et al. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circ Arrhythmia Electrophysiol. 2012;5:1130–1135. doi: 10.1161/CIRCEP.112.973164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias. Hear Rhythm. 2009;6:886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Rogers T, Mahapatra S, Kim S, et al. Transcatheter Myocardial Needle Chemoablation During Real-Time Magnetic Resonance Imaging. Circ Arrhythmia Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttman M, Kolandaivelu A, Fink S, Halperin H, Herzka DA. Towards MRI-guided cardiac ablation procedures with no contrast agent: safety and efficacy considerations. J Cardiovasc Magn Reson. 2016;18(Suppl 1):213. [Google Scholar]
- 37.Saybasili H, Faranesh AZ, Saikus CE, Ozturk C, Lederman RJ, Guttman MA. Interventional MRI using multiple 3D angiography roadmaps with real-time imaging. J Magn Reson Imaging. 2010;31:1015–9. doi: 10.1002/jmri.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccini D, Monney P, Sierro C, et al. Respiratory self-navigated postcontrast whole-heart coronary MR angiography: initial experience in patients. Radiology. 2014;270:378–86. doi: 10.1148/radiol.13132045. [DOI] [PubMed] [Google Scholar]
- 39.Mazal JR, Rogers T, Schenke WH, et al. Interventional-Cardiovascular MR: Role of the Interventional MR Technologist. Radiol Technol. 2016;87:261–70. [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers T, Ratnayaka K, Lederman RJ. MRI Catheterization in Cardiopulmonary Disease. Chest. 2014;145:30–36. doi: 10.1378/chest.13-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue H, Kellman P, Larocca G, Arai AE, Hansen MS. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J Cardiovasc Magn Reson. 2013;15:102. doi: 10.1186/1532-429X-15-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue H, Inati S, Sørensen TS, Kellman P, Hansen MS. Distributed MRI reconstruction using gadgetron-based cloud computing. Magn Reson Med. 2015;73:1015–1025. doi: 10.1002/mrm.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steeden JA, Atkinson D, Taylor AM, Muthurangu V. Assessing vascular response to exercise using a combination of real-time spiral phase contrast MR and noninvasive blood pressure measurements. J Magn Reson Imaging. 2010;31:997–1003. doi: 10.1002/jmri.22105. [DOI] [PubMed] [Google Scholar]
- 44.Peters DC, Appelbaum EA, Nezafat R, et al. Left ventricular infarct size, peri-infarct zone, and papillary scar measurements: A comparison of high-resolution 3D and conventional 2D late gadolinium enhancement cardiac MR. J Magn Reson Imaging. 2009;30:794–800. doi: 10.1002/jmri.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lardo AC, McVeigh ER, Jumrussirikul P, et al. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation. 2000;102:698–705. doi: 10.1161/01.cir.102.6.698. [DOI] [PubMed] [Google Scholar]
- 46.Santos JM, Wright GA, Pauly JM. 26th Annu Int Conf IEEE Eng Med Biol Soc. Vol. 3. IEEE; 2004. Flexible real-time magnetic resonance imaging framework; pp. 1048–1051. [DOI] [PubMed] [Google Scholar]
- 47.Faranesh AZ, Hansen M, Rogers T, Lederman RJ. Interactive black blood preparation for interventional cardiovascular MRI. J Cardiovasc Magn Reson. 2014;16(Suppl 1):32. [Google Scholar]
- 48.Guttman MA, Dick AJ, Raman VK, Arai AE, Lederman RJ, McVeigh ER. Imaging of myocardial infarction for diagnosis and intervention using real-time interactive MRI without ECG-gating or breath-holding. Magn Reson Med. 2004;52:354–61. doi: 10.1002/mrm.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging. 2012;36:55–72. doi: 10.1002/jmri.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell-Washburn AE, Xue H, Lederman RJ, Faranesh AZ, Hansen MS. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med. 2016;75:2278–2285. doi: 10.1002/mrm.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright KL, Hamilton JI, Griswold MA, Gulani V, Seiberlich N. Non-Cartesian parallel imaging reconstruction. J Magn Reson Imaging. 2014;40:1022–40. doi: 10.1002/jmri.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 53.Huang F, Vijayakumar S, Li Y, Hertel S, Duensing GR. A software channel compression technique for faster reconstruction with many channels. Magn Reson Imaging. 2008;26:133–141. doi: 10.1016/j.mri.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 54.Hansen MS, Sørensen TS. Gadgetron: An open source framework for medical image reconstruction. Magn Reson Med. 2013;69:1768–1776. doi: 10.1002/mrm.24389. [DOI] [PubMed] [Google Scholar]
- 55.Seppenwoolde JH, Viergever MA, Bakker CJG. Passive tracking exploiting local signal conservation: The white marker phenomenon. Magn Reson Med. 2003;50:784–790. doi: 10.1002/mrm.10574. [DOI] [PubMed] [Google Scholar]
- 56.Campbell-Washburn AE, Rogers T, Basar B, et al. Positive contrast spiral imaging for visualization of commercial nitinol guidewires with reduced heating. J Cardiovasc Magn Reson. 2015;17:114. doi: 10.1186/s12968-015-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommer P, Grothoff M, Eitel C, et al. Feasibility of real-time magnetic resonance imaging-guided electrophysiology studies in humans. Europace. 2013;15:101–108. doi: 10.1093/europace/eus230. [DOI] [PubMed] [Google Scholar]
- 58.Dumoulin CL, Souza SP, Darrow RD. Real-time position monitoring of invasive devices using magnetic resonance. Magn Reson Med. 1993;29:411–415. doi: 10.1002/mrm.1910290322. [DOI] [PubMed] [Google Scholar]
- 59.Ocali O, Atalar E. Intravascular magnetic resonance imaging using a loopless catheter antenna. Magn Reson Med. 1997;37:112–118. doi: 10.1002/mrm.1910370116. [DOI] [PubMed] [Google Scholar]
- 60.Etezadi-Amoli M, Stang P, Kerr A, Pauly J, Scott G. Interventional device visualization with toroidal transceiver and optically coupled current sensor for radiofrequency safety monitoring. Magn Reson Med. 2015;73:1315–1327. doi: 10.1002/mrm.25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quick HH, Zenge MO, Kuehl H, et al. Interventional magnetic resonance angiography with no strings attached: Wireless active catheter visualization. Magn Reson Med. 2005;53:446–455. doi: 10.1002/mrm.20347. [DOI] [PubMed] [Google Scholar]
- 62.Konings MK, Bartels LW, Smits HF, Bakker CJ. Heating around intravascular guidewires by resonating RF waves. J Magn Reson Imaging. 2000;12:79–85. doi: 10.1002/1522-2586(200007)12:1<79::aid-jmri9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 63.Weiss S, Vernickel P, Schaeffter T, Schulz V, Gleich B. Transmission line for improved RF safety of interventional devices. Magn Reson Med. 2005;54:182–189. doi: 10.1002/mrm.20543. [DOI] [PubMed] [Google Scholar]
- 64.Griffin G, Anderson K, Wright G. Miniaturizing Floating Traps to Increase RF Safety of Magnetic Resonance Guided Percutaneous Procedures. IEEE Trans Biomed Eng. 2016:1–1. doi: 10.1109/TBME.2016.2553680. [DOI] [PubMed] [Google Scholar]
- 65.Etezadi-Amoli M, Stang P, Kerr A, Pauly J, Scott G. Controlling radiofrequency-induced currents in guidewires using parallel transmit. Magn Reson Med. 2015;74:1790–1802. doi: 10.1002/mrm.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park SM, Kamondetdacha R, Nyenhuis JA. Calculation of MRI-induced heating of an implanted medical lead wire with an electric field transfer function. J Magn Reson Imaging. 2007;26:1278–1285. doi: 10.1002/jmri.21159. [DOI] [PubMed] [Google Scholar]
- 67.Griffin GH, Anderson KJT, Celik H, Wright GA. Safely assessing radiofrequency heating potential of conductive devices using image-based current measurements. Magn Reson Med. 2015;73:427–441. doi: 10.1002/mrm.25103. [DOI] [PubMed] [Google Scholar]
- 68.Peng P, Lekadir K, Gooya A, Shao L, Petersen SE, Frangi AF. A review of heart chamber segmentation for structural and functional analysis using cardiac magnetic resonance imaging. Magn Reson Mater Physics, Biol Med. 2016;29:155–195. doi: 10.1007/s10334-015-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linte CA, Moore J, Wedlake C, et al. Inside the beating heart: An in vivo feasibility study on fusing pre- and intra-operative imaging for minimally invasive therapy. Int J Comput Assist Radiol Surg. 2009;4:113–123. doi: 10.1007/s11548-008-0278-6. [DOI] [PubMed] [Google Scholar]
- 70.Karim R, Bhagirath P, Claus P, et al. Evaluation of state-of-the-art segmentation algorithms for left ventricle infarct from late Gadolinium enhancement MR images. Med Image Anal. 2016;30:95–107. doi: 10.1016/j.media.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Knowles BR, Caulfield D, Cooklin M, et al. 3-D visualization of acute RF ablation lesions using MRI for the simultaneous determination of the patterns of necrosis and edema. IEEE Trans Biomed Eng. 2010;57:1467–1475. doi: 10.1109/TBME.2009.2038791. [DOI] [PubMed] [Google Scholar]
- 72.Pop M, Ghugre NR, Ramanan V, et al. Quantification of fibrosis in infarcted swine hearts by ex vivo late gadolinium-enhancement and diffusion-weighted MRI methods. Phys Med Biol. 2013;58:5009–5028. doi: 10.1088/0031-9155/58/15/5009. [DOI] [PubMed] [Google Scholar]
- 73.Zhuang X, Shen J. Multi-scale patch and multi-modality atlases for whole heart segmentation of MRI. Med Image Anal. 2016;31:77–87. doi: 10.1016/j.media.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Duan Q, Angelini ED, Laine AF. Real-time segmentation by Active Geometric Functions. Comput Methods Programs Biomed. 2011;98:223–230. doi: 10.1016/j.cmpb.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vergara GR, Vijayakumar S, Kholmovski EG, et al. Real-time magnetic resonance imaging–guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Hear Rhythm. 2011;8:295–303. doi: 10.1016/j.hrthm.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chubb H, Harrison JL, Weiss S, et al. Development, Pre-Clinical Validation, and Clinical Translation of a Cardiac Magnetic Resonance–Electrophysiology System With Active Catheter Tracking for Ablation of Cardiac Arrhythmia. JACC Clin Electrophysiol. 2016 doi: 10.1016/j.jacep.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Zhuang X, Xiahai Challenges and methodologies of fully automatic whole heart segmentation: a review. J Healthc Eng. 2013;4:371–408. doi: 10.1260/2040-2295.4.3.371. [DOI] [PubMed] [Google Scholar]
- 78.Zhuang X, Rhode KS, Razavi RS, Hawkes DJ, Ourselin S. A registration-based propagation framework for automatic whole heart segmentation of cardiac MRI. IEEE Trans Med Imaging. 2010;29:1612–1625. doi: 10.1109/TMI.2010.2047112. [DOI] [PubMed] [Google Scholar]
- 79.Chitiboi T, Hennemuth A, Tautz L, et al. 2014 IEEE 11th Int Symp Biomed Imaging. IEEE; 2014. Context-based segmentation and analysis of multi-cycle real-time cardiac MRI; pp. 943–946. [Google Scholar]
- 80.Contijoch F, Witschey WRT, Rogers K, et al. User-initialized active contour segmentation and golden-angle real-time cardiovascular magnetic resonance enable accurate assessment of LV function in patients with sinus rhythm and arrhythmias. J Cardiovasc Magn Reson. 2015 May;17:37. doi: 10.1186/s12968-015-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smolikova R, Wachowiak MP, Drangova M. Registration of fast cine cardiac MR slices to 3D preprocedural images: toward real-time registration for MRI-guided procedures. In: Fitzpatrick JM, Sonka M, editors. Med Imaging 2004 Int Soc Opt Photonics. Vol. 5370. International Society for Optics and Photonics; 2004. p. 1195. [Google Scholar]
- 82.Reddy VY, Malchano ZJ, Holmvang G, et al. Integration of cardiac magnetic resonance imaging with three-dimensional electroanatomic mapping to guide left ventricular catheter manipulation: Feasibility in a porcine model of healed myocardial infarction. J Am Coll Cardiol. 2004;44:2202–2213. doi: 10.1016/j.jacc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 83.Pop M, Oduneye S, Ghugre N, et al. Lect Notes Comput Sci. Springer Berlin Heidelberg; 2013. A Pre-clinical Framework to Characterize Peri-infarct Remodelling Using in vivo T1 Maps and CARTO Data; pp. 326–335. [Google Scholar]
- 84.Gupta S, Desjardins B, Baman T, et al. Delayed-Enhanced MR Scar Imaging and Intraprocedural Registration Into an Electroanatomical Mapping System in Post-Infarction Patients. JACC Cardiovasc Imaging. 2012;5:207–210. doi: 10.1016/j.jcmg.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oduneye SO, Pop M, Shurrab M, et al. Distribution of abnormal potentials in chronic myocardial infarction using a real time magnetic resonance guided electrophysiology system. J Cardiovasc Magn Reson. 2015;17:27. doi: 10.1186/s12968-015-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao X, Navkar NV, Shah DJ, Tsekos NV, Deng Z. Intraoperative registration of preoperative 4D cardiac anatomy with real-time MR images. IEEE 12th Int Conf Bioinforma Bioeng BIBE 2012. 2012;(i):583–588. [Google Scholar]
- 87.Xu R, Athavale P, Nachman A, Wright GA. Multiscale Registration of Real-Time and Prior MRI Data for Image-Guided Cardiac Interventions. IEEE Trans Biomed Eng. 2014;61:2621–2632. doi: 10.1109/TBME.2014.2324998. [DOI] [PubMed] [Google Scholar]
- 88.Xu R, Athavale P, Krahn P, et al. Feasibility Study of Respiratory Motion Modeling Based Correction for MRI-Guided Intracardiac Interventional Procedures. 2015;62:2899–2910. doi: 10.1109/TBME.2015.2451517. [DOI] [PubMed] [Google Scholar]
- 89.Xu R, Wright GA. GPU accelerated dynamic respiratory motion model correction for MRI-guided cardiac interventions. Comput Methods Programs Biomed. 2016;136:31–43. doi: 10.1016/j.cmpb.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Chubb H, Harrison JL, Weiss S, et al. Development, Preclinical Validation, and Clinical Translation of a Cardiac Magnetic Resonance - Electrophysiology System With Active Catheter Tracking for Ablation of Cardiac Arrhythmia. JACC Clin Electrophysiol. 2017;3:89–103. doi: 10.1016/j.jacep.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Radau PE, Pintilie S, Flor R, et al. Stat Atlases Comput Model Hear Imaging Model Challenges. Springer; 2012. VURTIGO: Visualization Platform for Real-Time, MRI-Guided Cardiac Electroanatomic Mapping; pp. 244–253. [Google Scholar]
- 92.Sermesant M, Delingette H, Ayache N. An electromechanical model of the heart for image analysis and simulation. IEEE Trans Med Imaging. 2006;25:612–625. doi: 10.1109/TMI.2006.872746. [DOI] [PubMed] [Google Scholar]
- 93.Pop M, Sermesant M, Mansi T, et al. Correspondence Between Simple 3-D MRI-Based Computer Models and In-Vivo EP Measurements in Swine With Chronic Infarctions. IEEE Trans Biomed Eng. 2011;58:3483–3486. doi: 10.1109/TBME.2011.2168395. [DOI] [PubMed] [Google Scholar]
- 94.Pernod E, Sermesant M, Relan J, Delingette H. Interactive real time simulation of cardiac radio-frequency ablation. Proc Eurographics Work Vis Comput Biol Med. 2010:91–98. [Google Scholar]
- 95.Ferguson S, Sermesant M, Oduneye S, Ayache N, Wright G, Pop M. Novel framework to augment real-time cardiac MR image-guided EP studies with T1 mapping-based computational heart models. Lect Notes Comput Sci. 2017;10124:11–20. [Google Scholar]
- 96.Mazilu MT, Faranesh AZ, Derbyshire JA, Lederman RJ, Hansen MS. Low-Cost MRI Compatible Interface Device for Interactive Scan Plane Control. Proc 19th Sci Meet ISMRM, Montr Quebec, Canada. 2011;19:3752. [Google Scholar]
- 97.Güttler F, Heinrich A, Hench T, Teichgräber U. Controlling Real Time Interventional MRI Using Camera-Based Computer Vision and Gesture Recognition. 2016 Interv MRI Symp. 2016 [Google Scholar]
- 98.Zhang SH, Tse ZTH, Dumoulin CL, et al. Gradient-induced voltages on 12-lead ECGs during high duty-cycle MRI sequences and a method for their removal considering linear and concomitant gradient terms. Magn Reson Med. 2016;75:2204–2216. doi: 10.1002/mrm.25810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmidt EJ, Watkins RD, Zviman MM, Guttman MA, Wang W, Halperin HA. A Magnetic Resonance Imaging–Conditional External Cardiac Defibrillator for Resuscitation Within the Magnetic Resonance Imaging Scanner BoreCLINICAL PERSPECTIVE. Circ Cardiovasc Imaging. 2016;9:e005091. doi: 10.1161/CIRCIMAGING.116.005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tavallaei MA, Lavdas MK, Gelman D, Drangova M. Magnetic resonance imaging compatible remote catheter navigation system with 3 degrees of freedom. Int J Comput Assist Radiol Surg. 2016;11:1537–1545. doi: 10.1007/s11548-015-1337-4. [DOI] [PubMed] [Google Scholar]


