Introduction
Hepatitis C Virus (HCV) infection is the most common blood-borne infection in the U.S. and is of concern in older adults. Although the rate of new HCV infections has declined over the last two decades due to implementation of HCV screening of donated blood and harm reduction programs, the proportion of HCV-infected patients that are of older age and that have had infection for a prolonged duration of time has increased. The growing burden of older HCV-infected patients poses challenges for clinicians who care for these patients and for the health care system because of the increased utilization of health care resources to treat the long-term sequelae of HCV-associated liver disease including cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation.
Prior to the advent of all oral direct acting antiviral (DAA) agents, HCV treatment was associated with poor response and increased adverse side effects with some studies showing worse outcomes in the older patient. Since the introduction of DAA agents in 2011 to treat HCV infection, clinicians are now able to successfully treat the growing number of HCV-infected patients of older age. However, information regarding the effect of HCV treatment on short and long term clinical outcomes in the older HCV-infected patient is limited.
We summarize the literature regarding the epidemiology, natural history and clinical course of HCV infection, the impact of age on clinical outcomes in persons with HCV infection, and current knowledge regarding the safety and efficacy of newer HCV treatment regimens in the older HCV-infected patient.
Epidemiology and Screening of HCV infection in the U.S
An estimated 4.1 million persons in the U.S. (or 1.6% of the U.S. population) have been exposed to HCV.1 About 70% of these persons were born between 1945 and 1964, and most were infected between 1970 and 1990 when the incidence of new HCV infections peaked.2 Since the identification of HCV as the main cause of non-A/non-B chronic hepatitis in 1989, HCV incidence has declined due to the implementation of donor blood screening and increased availability of harm reduction programs for persons who inject drugs. However, recent data suggests that there may be an emerging epidemic of HCV infection among young nonurban persons mainly of white race; prescription opioid use has been implicated as a factor.3
In 2012, the U.S. Centers for Disease Control and Prevention revised their recommendations to include one-time HCV serologic testing of all adults born from 1945 through 1965 regardless of HCV risk status.4 The prevalence of HCV antibodies in persons born between 1945 and 1964 (also known as the Baby Boomer cohort in the U.S.) has been estimated to be about 3.5%, which is more than double the reported HCV prevalence in the U.S. population. The revised recommendations were based upon findings from the Chronic Hepatitis Cohort Study of 4,689 HCV-infected persons who completed a survey regarding the reason for their HCV testing.5 That study found that <25% of the HCV-infected persons had an identifiable risk factor for HCV infection; rather 78% were born during the period between 1945 and 1965. With implementation of this new recommendation, an increase in incident HCV infections is expected.
Recent studies6,7 have also suggested that older patients in long term care facilities should be targeted for HCV screening and confirmatory HCV RNA screening, and that there should be a shared commitment by all healthcare facilities to adhere to basic infection control procedures. These studies have largely been borne out of reports of viral hepatitis outbreaks resulting from lapses in infection control practices, particularly in ambulatory care settings. A systematic review and meta-analysis of HCV infection prevalence in long term care facilities found a pooled prevalence of 3.3% (95% CI: 1.5 – 7.2%) compared to the 0.9 – 1.0% prevalence reported in non-institutionalized elders (generally defined as older than 65 years of age).6 In that study, it was unclear if adults were previously infected or were exposed to HCV in the long term care setting. Another case-control study7 examined the association of healthcare exposures with acute hepatitis B and C from 2006 to 2008 in 71 (mostly acute hepatitis B infection) cases who were 55 years and older and found that 37% of new infections were likely attributable to injections of parenteral medications and 8% to hemodialysis. There is concern that as the Baby Boomer cohort of HCV-infected persons seeks more health care in ambulatory settings and residency in long term care facilities, there could be a growing reservoir of infected persons who could serve as a source of transmission. Therefore, studies advocate for greater adherence to HCV screening recommendations (particularly in institutionalized settings), basic infection control precautions and safe injection practices.
Natural History and Clinical Course of HCV infection and the Impact of Age
Studies estimate that between 55% and 75% of newly infected persons develop chronic HCV infection as determined by detectable HCV RNA in the blood.8 Patients of older age at time of infection and impaired immune system are at increased risk of developing chronic HCV infection.8
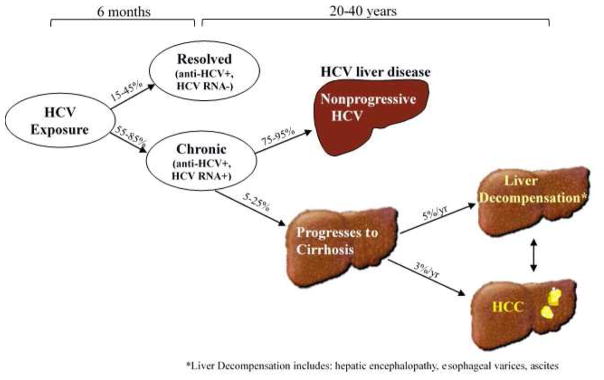
A large proportion of chronically HCV-infected persons in the U.S. are now about 50 – 70 years old and have lived with HCV infection for about 25 – 45 years.9 The increased duration of HCV infection has been accompanied by an increased incidence of liver disease and related sequelae. Over the natural course of HCV infection, it is expected that at least one third of HCV-infected persons progress to advanced fibrosis and cirrhosis, and among those with cirrhosis, about 3 – 5% per year develop decompensated cirrhosis (i.e. ascites, hepatic encephalopathy, esophageal varices) and/or HCC (Figure).10
Figure.
Natural history and clinical course of HCV infection
Because several decades can elapse from incident HCV infection until the peak prevalence of cirrhosis, it has been estimated that the proportion of liver-related deaths and patients diagnosed with HCV-related cirrhosis and HCC is fast approaching its peak.11 This increase is largely driven by the burden of HCV in the Baby Boomer cohort and will be associated with increased health care utilization and hospitalizations for end stage liver disease and the subsequent need for liver transplantation.12
Because the clinical sequelae of HCV disease is expected to increase in the older patient, some studies have specifically examined the association of older age (defined as 65 years or older) with clinical outcomes in HCV-infected persons. One retrospective cohort study13 of 161,744 HCV-infected patients in the U.S. Veterans Health Administration Hepatitis C Clinical Case Registry compared HCV-infected veterans aged >65 and with those aged 20–49 years. They found that even after adjusting for several metabolic factors, including diabetes and obesity, age ≥ 65 years remained associated with a 1.14, 2.44, and 2.09 greater risk of cirrhosis, HCC, and death from all causes, respectively. Longer duration of HCV infection is likely a primary reason for the increased risk in older HCV-infected persons. Prolonged duration of HCV infection has been shown to predict faster progression to cirrhosis 14 and has been associated with increased risk of HCC. 15
However, studies suggest that age-related mechanisms may also play a role. In one study of patients who acquired HCV infection during transfusion, the median time to development of cirrhosis was reported to decrease from 33 years in patients who acquired the infection between the ages of 21 to 30 years to 16 years in patients who acquired the infection when they 40 years or older.16 Another study of patients who acquired HCV infection during transfusion found that the mean time to development of HCC was 15 years in persons 50 years or older compared with 32 years in those infected <50 years of age.15 While Poynard et al14 established that duration of HCV infection predicted faster progression to cirrhosis, they also demonstrated that in those over 50 years at the time of infection, the progression of fibrosis was substantially greater when compared to those less than 50 years at the time of infection. Finally, recurrent HCV infection after liver transplantation is nearly universal among patients with HCV viremia at the time of transplant, and in this context, older donor age has consistently been associated with accelerated graft loss.17 These studies indicate that older age independent of duration of HCV infection may also play a role in the progression of HCV-associated liver disease.
Aging-related mechanisms that have been postulated to increase the risk of liver disease outcomes in the setting of HCV infection include a greater vulnerability to environmental factors such as oxidative stress with increasing age, reduction in the rate of hepatic flow, reduced mitochondrial capacity, impaired immunity, and increased carcinogenic potential due to a reduced ability to repair DNA.18,19 There are also limited data that HCV infection may be associated with increased markers of immune-senescence, which has been shown to occur in the setting of HIV infection and is thought to play a role in the earlier onset of aging-related comorbidities in HIV infection. HCV infection itself might be associated with loss of early-differentiated T cells and progressive accumulation of chronically-activated, late-differentiated senescent T cells. One small study20 comparing HCV-infected individuals with healthy controls, all of whom were less than 54 years of age, found that the CD4 and CD8 T cells from HCV-infected individuals showed a significant increase in the T cell immunosenescent phenotype that is more commonly associated with advancing age. Whether or not this increase is associated with the premature onset of not only liver but also non-liver clinical outcomes related to aging in HCV-infected persons is unclear.21
HCV infection and extra-hepatic clinical outcomes
HCV infection is also associated with extra-hepatic disorders (Table), likely because in addition to being a hepatotropic virus, it is also lymphotropic leading to immune-system dysregulation. Thus, a variety of autoimmune disorders have been associated with HCV infection including systemic disorders such as mixed cryoglobulinemia and less commonly arthritis, sicca syndrome, and porphyria cutanea tarda, or organ-specific disorders such as glomerulonephritis, diabetes, or thyroiditis. Apart from diabetes, these disorders are thought to be uncommon, so few studies have been able to adequately examine the effect of age on these disorders in the HCV-infected patient.
Table.
Common extrahepatic outcomes associated with both HCV infection and aging
|
By contrast, HCV infection is a chronic inflammatory process leading to not only hepatic inflammation but also persistent systemic inflammation which has been associated with extrahepatic outcomes that are also common with aging including extrahepatic malignancies, cardio-metabolic complications, and neurocognitive disturbances. The complex interplay between aging outcomes and HCV-induced immune dysregulation and systemic inflammation could partly explain why some but not all studies show an association of HCV infection with these outcomes.
HCV infection and malignancy
Few studies have examined the association of HCV infection with non-HCC malignancy in the older patient. A recent registry-based case-control study22 using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database in U.S. adults aged ≥65 years from 1993 to 2011 found that as expected, HCV infection was strongly associated with cancers of the liver compared to those without HCV infection. Interestingly, however, HCV infection was also associated with higher odds of intrahepatic (adjusted odds ratio (aOR): 3.40) and extrahepatic (aOR: 1.90) bile duct cancer; pancreatic cancer (aOR: 1.23); anal cancer (aOR: 1.97); nonmelanoma nonepithelial skin cancer (aOR: 1.53); myelodysplastic syndrome (aOR: 1.56), and diffuse large B-cell lymphoma (aOR: 1.57).
The increased risk for non-HCC cancers could indicate that HCV infection directly promotes oncogenesis. As a lymphotropic virus, HCV infection is thought to trigger B cell proliferation and thus, has been associated with a greater risk of lymphoproliferative disorders such as B-cell lymphoma. 23,24 Alternatively, the increased risk for non-HCC cancers could also be explained as confounding by shared risk factors. Risk factors such as high-risk sexual behaviors and injection drug use could explain the association with anal cancer and skin cancer, but were not accounted for in the analysis. These findings suggest that in addition to HCC, providers should be vigilant to the fact that HCV-infected patients who are 65 years or older could have an increased risk of non-HCC malignancies compared to HCV-uninfected patients who are 65 years or older.
HCV infection and kidney disease
HCV infection has been associated with an earlier onset of kidney disease and progression to chronic kidney disease (CKD)25–27 and end stage renal disease 28,25 when compared to those without HCV infection. Among those with HIV infection, HCV coinfection has also been associated with a greater risk of developing CKD, and increasing age is associated with more advanced CKD in HIV/HCV-coinfected persons compared to HIV-monoinfected persons.29 Kidney dysfunction is often multifactorial in older HCV-infected patients. Besides the less common immune-mediated renal damage secondary to cryoglobulinemia, lifestyle factors, such as substance abuse30,31 and co-morbid diseases common with aging, such as diabetes and hypertension31 are also important determinants of worsening renal function in HCV-infected persons.
HCV infection and diabetes
An association between HCV infection and diabetes mellitus (DM) has been demonstrated in several studies.32–35 In one longitudinal study, the development of DM was found to be eleven times more common in HCV-infected than HCV-uninfected persons.36 In persons older than 39 years of age, HCV infection increased the risk of DM by almost four times.37 While a direct effect of HCV on the hepatocyte insulin-signaling cascade 38–40 and pancreatic beta-cell function41 has been postulated as a cause of insulin resistance, the etiology of DM is invariably multifactorial. In older HCV-infected persons, DM onset may be a result of the direct effects of HCV and increasing visceral adiposity that occurs with older age.
HCV infection and cardiovascular disease
Similarly, there is growing evidence that HCV infection is associated with an increased risk of cardiovascular disease (CVD) and heart failure.42 The mechanisms by which HCV infection might be associated with cardiovascular disease include an HCV-induced pro-inflammatory state43 and possible direct effects of the virus on the myocardium and endothelium.44 HCV infection is also associated with a higher prevalence of DM, a well-known risk factor for CVD. However, LDL-cholesterol and total cholesterol are reported to be lower in HCV-infected persons compared to those without HCV infection.45 Lower circulating levels of LDL-C are commonly observed in primates and humans in response to infection and inflammation, but other changes in LDL-C metabolism (i.e. increased small LDL particle size) may occur that could promote atherogenesis.46 There may also be direct effects of HCV infection that lower LDL levels by lowering VLDL secretion independent of liver fibrosis severity47 which could potentially decrease the risk of CVD. The contribution of aging to lipid levels and thus CVD in the setting of direct effects of HCV infection and HCV-associated systemic inflammation add some uncertainty as to whether HCV infection is associated with an increased risk of CVD compared to those without HCV infection.
HCV and neuropsychological and neurocognitive effects
Up to 30% of HCV-infected persons report neuropsychological disorders, not limited to depression, and up to two thirds complain of fatigue; the older HCV-infected patient may be at particular risk.48 While the presence of depressive symptoms might be related to the psychological burden of chronic HCV infection, some studies suggest that HCV infection directly affects the central nervous system (CNS) through alterations in serotoninergic and dopaminergic neurotransmission, with resultant depressive symptoms.49 This mechanism might also explain other CNS symptoms seen in HCV infection, such as fatigue, although a causal link has not been established.50,51
HCV infection is also associated with increased cognitive impairment when compared to those without HCV infection.52 Between 33% and 50% of all HCV-infected persons report some degree of impaired neurocognition.53,54 Whether this impairment is directly attributable to HCV infection, advancing age, progressive liver disease, and/or other comorbid conditions is often difficult to elucidate. Studies have demonstrated HCV RNA in brain tissue and cerebrospinal fluid suggesting active HCV replication in the CNS.55 There is also a growing body of evidence that HCV directly affects the brain and nerves independently of hepatic mediated processes.56,57
HCV treatment in the older patient with HCV infection
Because patients between the ages of 50 and 70 will constitute a large proportion of the patients being treated in the next decade, understanding the impact of age on HCV treatment outcomes in the era of all oral DAA regimens is important. Prior to the advent of DAA agents, some 58,59 but not all studies 13,60 found that HCV-infected patients that were of older age had worse sustained virologic response (SVR) rates than those that were of younger age. Some attributed the worse response to the more frequent treatment discontinuation and/or dose reductions in the older patient resulting from treatment with an interferon-based regimen plus ribavirin, which are often accompanied by adverse effects including cytopenia, flu-like symptoms, and central nervous system effects.
Few studies have examined the association of older age with SVR rates using all oral DAA regimens partly because elderly patients are often excluded from clinical trials. A recent study of four open-label phase 3 clinical trials was able to examine the safety and efficacy of ledipasvir/sofosbuvir for the treatment of Genotype 1 HCV in subjects 65 years or older.61 Of the 2293 subjects in the four trials, 264 (12%) were ≥65 years of age (and 24 of those were ≥75). That study found little difference in SVR rates (97% in those <65 years vs. 98% in those ≥65 years) despite subjects ≥65 years being more likely to have cirrhosis. Furthermore, there was little difference by age in the proportion reporting at least one adverse effect (78% in those <65 years vs. 80% in those ≥65 years).
The most common adverse effects were fatigue and headache in both groups, but in subjects who were also on ribavirin, the rate of study drug modification or interruption was double in the older group (6% in those <65 years vs. 13% in those ≥65 years). That study suggests that age is not a barrier to achieving SVR in patients taking a ledipasvir/sofosbuvir, but ribavirin-free regimens should be considered for the treatment of elderly patients. If ribavirin must be used, then close monitoring is needed for the development of anemia.
Furthermore, because sofosbuvir and ribavirin are renally eliminated, safe and effective doses of sofosbuvir in those with an estimated glomerular filtration rate less than 30 mL/min have not been established. In the HCV-infected patient with severely compromised renal function, other HCV regimens such as grazoprevir plus elbasvir have been shown to be safe and effective.
Another cohort-based retrospective study62 of 17,487 HCV-infected patients grouped into 6 age categories (<55 years, 55 – 59, 60 – 64, 65 – 69, 70 – 74, and 75 years or older) in the Veterans Affairs Healthcare System who started an all oral HCV regimen between 2014 and 2015 also found that DAAs were associated with high SVR rates (from 90% to 94%) even in the oldest age cohort (≥75 years) and that advanced age was not a negative predictor for SVR. While the SVR rates were lower in those with cirrhosis compared to those without cirrhosis, the SVR rates were similar between age groups among those with cirrhosis. Similarly, SVR rates were lower in treatment-experienced patients compared to treatment naïve patients but similar between age groups among those who were treatment-experienced.
Another question of tremendous interest to clinicians is whether HCV treatment will be associated with improvement in long-term outcomes, especially in the older patient. A study of U.S. veterans 13 prior to the advent of DAA therapy found that successful HCV treatment is associated with significant reductions in HCC and overall mortality. That study found mortality benefit in all age categories including those 65 – 85 years. The same group also reported in another publication63 that while achievement of SVR was associated with decreased HCC risk, the annual risk of HCC among those who cleared the virus was not negligible ranging from 0.1% to 1.55% (overall 0.33%) with the highest residual risk in those diagnosed with cirrhosis followed by those who achieved SVR after age 65 irrespective of cirrhosis. They concluded that there remains a risk of HCC post-SVR, and the risk may be greater in those with cirrhosis or in the elderly, supporting HCV treatment before the development of cirrhosis and continued surveillance even after SVR in those who already developed cirrhosis.
Finally, whether HCV treatment improves long-term non-liver disease outcomes is unclear. A recent small study of HIV/HCV-coinfected persons demonstrated that even after SVR, older patients treated with DAA agents did not experience any change in neuropsychological assessments.64 By contrast, a small study from the interferon era of 34 HCV-infected adults with a median age <40 years found that successful HCV eradication can lead to improvements in cognitive function, at least for individuals with mild deficits.65 These apparently contradictory findings from the pre and post-DAA area may reflect how older patients, with more advanced neurocognitive deficits who may not have been candidates for treatment are now being treated more readily. Additional studies examining the impact of HCV cure in the era of DAA agents on long term outcomes are needed.
Conclusion
Understanding the impact of older age and HCV infection on liver and non-liver outcomes is critical. The advent of potent all oral DAA agents for HCV infection has ushered in a new era where declines in HCV-associated liver disease are tangible; yet whether there will be an effect on longer term outcomes in other organ tissues besides the liver is unclear and needs study. Examination of limited published studies of the safety and efficacy of DAAs in the older HCV-infected patient suggests that age should not be a barrier to treatment. Given that the proportion of older patients with HCV is increasing, clinical trials of DAA agents should include older HCV-infected patients.
Hepatitis C Virus (HCV) infection is of concern in older adults, because the proportion of HCV-infected patients that are of older age and that have had infection for a prolonged duration of time has increased.
Older age has been associated with an increased risk of HCV-associated liver disease including cirrhosis and hepatocellular carcinoma in those with HCV infection likely due to not only a longer duration of HCV infection but also possibly aging-related mechanisms.
HCV infection has also been associated with an increased risk of extrahepatic comorbidities common to the aging patient including malignancy, kidney disease, diabetes, cardiovascular disease, and neurocognitive impairment.
The impact of new direct acting antiviral agents for the treatment of HCV on HCV-associated comorbidities is unclear.
Examination of limited published studies of the safety and efficacy of DAAs in the older HCV-infected patient suggests that age should not be a barrier to treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156(4):263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59(10):1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 5.Smith BD, Beckett GA, Yartel A, Holtzman D, Patel N, Ward JW. Previous exposure to HCV among persons born during 1945–1965: prevalence and predictors, United States, 1999–2008. Am J Public Health. 2014;104(3):474–481. doi: 10.2105/AJPH.2013.301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez KJ, Smaldone A, Larson EL. Burden of Hepatitis C Virus Infection Among Older Adults in Long-Term Care Settings: a Systematic Review of the Literature and Meta-Analysis. Current infectious disease reports. 2016;18(4):13. doi: 10.1007/s11908-016-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perz JF, Grytdal S, Beck S, et al. Case-control study of hepatitis B and hepatitis C in older adults: Do healthcare exposures contribute to burden of new infections? Hepatology. 2013;57(3):917–924. doi: 10.1002/hep.25688. [DOI] [PubMed] [Google Scholar]
- 8.Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20(1):1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- 9.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 11.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9(4):331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 12.Pessoa MG, Wright TL. Hepatitis C infection in transplantation. Clin Liver Dis. 1997;1(3):663–690. doi: 10.1016/s1089-3261(05)70328-7. [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB, Kramer J, Duan Z, Kanwal F. Epidemiology and outcomes of hepatitis C infection in elderly US Veterans. J Viral Hepat. 2016;23(9):687–696. doi: 10.1111/jvh.12533. [DOI] [PubMed] [Google Scholar]
- 14.Pradat P, Voirin N, Tillmann HL, Chevallier M, Trepo C. Progression to cirrhosis in hepatitis C patients: an age-dependent process. Liver international: official journal of the International Association for the Study of the Liver. 2007;27(3):335–339. doi: 10.1111/j.1478-3231.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 15.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 16.Minola E, Prati D, Suter F, et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99(12):4588–4591. doi: 10.1182/blood-2001-12-0192. [DOI] [PubMed] [Google Scholar]
- 17.Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(3):549–557. doi: 10.1111/j.1600-6143.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. Journal of hepatology. 2001;34(5):730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 19.Horiike N, Masumoto T, Nakanishi K, et al. Interferon therapy for patients more than 60 years of age with chronic hepatitis C. J Gastroenterol Hepatol. 1995;10(3):246–249. doi: 10.1111/j.1440-1746.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 20.Barathan M, Mohamed R, Saeidi A, et al. Increased frequency of late-senescent T cells lacking CD127 in chronic hepatitis C disease. European journal of clinical investigation. 2015;45(5):466–474. doi: 10.1111/eci.12429. [DOI] [PubMed] [Google Scholar]
- 21.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19(9):1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahale P, Torres HA, Kramer JR, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer. 2017;123(7):1202–1211. doi: 10.1002/cncr.30559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zignego AL, Macchia D, Monti M, et al. Infection of peripheral mononuclear blood cells by hepatitis C virus. Journal of hepatology. 1992;15(3):382–386. doi: 10.1016/0168-8278(92)90073-x. [DOI] [PubMed] [Google Scholar]
- 24.Zignego AL, Giannini C, Gragnani L. HCV and lymphoproliferation. Clin Dev Immunol. 2012;2012:980942. doi: 10.1155/2012/980942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61(5):1495–1502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z. A meta-analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. Journal of viral hepatitis. 2015;22(11):897–905. doi: 10.1111/jvh.12413. [DOI] [PubMed] [Google Scholar]
- 27.Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4(1):207–220. doi: 10.2215/CJN.03710708. [DOI] [PubMed] [Google Scholar]
- 28.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney international. 2014;85(5):1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 29.Fischer MJ, Wyatt CM, Gordon K, et al. Hepatitis C and the risk of kidney disease and mortality in veterans with HIV. J Acquir Immune Defic Syndr. 2010;53(2):222–226. doi: 10.1097/QAI.0b013e3181b980d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buettner M, Toennes SW, Buettner S, et al. Nephropathy in illicit drug abusers: a postmortem analysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014;63(6):945–953. doi: 10.1053/j.ajkd.2014.01.428. [DOI] [PubMed] [Google Scholar]
- 31.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60(6):941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stepanova M, Lam B, Younossi Y, Srishord MK, Younossi ZM. Association of hepatitis C with insulin resistance and type 2 diabetes in US general population: the impact of the epidemic of obesity. Journal of viral hepatitis. 2012;19(5):341–345. doi: 10.1111/j.1365-2893.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 33.Mangia A, Ripoli M. Insulin resistance, steatosis and hepatitis C virus. Hepatol Int. 2013;7(Suppl 2):782–789. doi: 10.1007/s12072-013-9460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647–652. doi: 10.1111/apt.12234. [DOI] [PubMed] [Google Scholar]
- 35.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology. 2001;33(6):1554. doi: 10.1053/jhep.2001.0103306le01. [DOI] [PubMed] [Google Scholar]
- 36.Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38(1):50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 37.Mehta SH, Strathdee SA, Thomas DL. Association between hepatitis C virus infection and diabetes mellitus. Epidemiologic reviews. 2001;23(2):302–312. doi: 10.1093/oxfordjournals.epirev.a000808. [DOI] [PubMed] [Google Scholar]
- 38.Kawaguchi Y, Mizuta T. Interaction between hepatitis C virus and metabolic factors. World journal of gastroenterology. 2014;20(11):2888–2901. doi: 10.3748/wjg.v20.i11.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38(6):1384–1392. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. The American journal of pathology. 2004;165(5):1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. The American journal of gastroenterology. 2007;102(3):570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 42.Fibach E, Gatt S, Rachmilewitz EA. Selective photosensitization of human leukemic cells by a pyrene-containing fatty acid. Exp Hematol. 1990;18(2):89–93. [PubMed] [Google Scholar]
- 43.Oliveira CP, Kappel CR, Siqueira ER, et al. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. International journal of cardiology. 2013;164(2):221–226. doi: 10.1016/j.ijcard.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Boddi M, Abbate R, Chellini B, et al. Hepatitis C virus RNA localization in human carotid plaques. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2010;47(1):72–75. doi: 10.1016/j.jcv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Fabris C, Federico E, Soardo G, Falleti E, Pirisi M. Blood lipids of patients with chronic hepatitis: differences related to viral etiology. Clin Chim Acta. 1997;261(2):159–165. doi: 10.1016/s0009-8981(97)06532-7. [DOI] [PubMed] [Google Scholar]
- 46.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Siagris D, Christofidou M, Theocharis GJ, et al. Serum lipid pattern in chronic hepatitis C: histological and virological correlations. Journal of viral hepatitis. 2006;13(1):56–61. doi: 10.1111/j.1365-2893.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 48.Hassoun Z, Willems B, Deslauriers J, Nguyen BN, Huet PM. Assessment of fatigue in patients with chronic hepatitis C using the Fatigue Impact Scale. Digestive diseases and sciences. 2002;47(12):2674–2681. doi: 10.1023/a:1021040702370. [DOI] [PubMed] [Google Scholar]
- 49.Forton DM. Altered monoaminergic transporter binding in hepatitis C related cerebral dysfunction: a neuroimmunologial condition? Gut. 2006;55(11):1535–1537. doi: 10.1136/gut.2006.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casato M, Saadoun D, Marchetti A, et al. Central nervous system involvement in hepatitis C virus cryoglobulinemia vasculitis: a multicenter case-control study using magnetic resonance imaging and neuropsychological tests. J Rheumatol. 2005;32(3):484–488. [PubMed] [Google Scholar]
- 51.Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624–1630. doi: 10.1136/gut.2005.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Digestive diseases and sciences. 2008;53(2):307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- 53.Kramer L, Bauer E, Funk G, et al. Subclinical impairment of brain function in chronic hepatitis C infection. Journal of hepatology. 2002;37(3):349–354. doi: 10.1016/s0168-8278(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 54.Fontana RJ, Bieliauskas LA, Back-Madruga C, et al. Cognitive function in hepatitis C patients with advanced fibrosis enrolled in the HALT-C trial. Journal of hepatology. 2005;43(4):614–622. doi: 10.1016/j.jhep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Laskus T, Radkowski M, Bednarska A, et al. Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. Journal of virology. 2002;76(19):10064–10068. doi: 10.1128/JVI.76.19.10064-10068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paulino AD, Ubhi K, Rockenstein E, et al. Neurotoxic effects of the HCV core protein are mediated by sustained activation of ERK via TLR2 signaling. Journal of neurovirology. 2011;17(4):327–340. doi: 10.1007/s13365-011-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Letendre S, Paulino AD, Rockenstein E, et al. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196(3):361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- 58.Sato I, Shimbo T, Kawasaki Y, Mizokami M, Masaki N. Efficacy and safety of interferon treatment in elderly patients with chronic hepatitis C in Japan: A retrospective study using the Japanese Interferon Database. Hepatol Res. 2015;45(8):829–386. doi: 10.1111/hepr.12419. [DOI] [PubMed] [Google Scholar]
- 59.Tsui JI, Currie S, Shen H, et al. Treatment eligibility and outcomes in elderly patients with chronic hepatitis C: results from the VA HCV-001 Study. Dig Dis Sci. 2008;53(3):809–814. doi: 10.1007/s10620-007-9926-x. [DOI] [PubMed] [Google Scholar]
- 60.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151(3):457–471. e455. doi: 10.1053/j.gastro.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saab S, Park SH, Mizokami M, et al. Safety and efficacy of ledipasvir/sofosbuvir for the treatment of genotype 1 hepatitis C in subjects aged 65 years or older. Hepatology. 2016;63(4):1112–1119. doi: 10.1002/hep.28425. [DOI] [PubMed] [Google Scholar]
- 62.Su F, Beste LA, Green PK, Berry K, Ioannou GN. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17–487 patients. Eur J Gastroenterol Hepatol. 2017;29(6):686–693. doi: 10.1097/MEG.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64(1):130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mastrorosa I, Lorenzini Patrizia, Ricottini Martina, Fabbri Gabriele, Balestra Pietro, Vergori Alessandra, Pinnetti Carmela, Zaccarelli Mauro, Ammassari Adriana, Antinori Andrea. DAAs IMPROVE VACS BUT DO NOT INFLUENCE COGNITIVE IMPAIRMENT IN HIV/HCV COINFECTED. CROI; 2017; Seattle, WA. [Google Scholar]
- 65.Thein HH, Maruff P, Krahn MD, et al. Improved cognitive function as a consequence of hepatitis C virus treatment. HIV medicine. 2007;8(8):520–528. doi: 10.1111/j.1468-1293.2007.00505.x. [DOI] [PubMed] [Google Scholar]