Abstract
Acute and chronic pain complaints, while very common, are generally poorly served by existing therapies. The unmet clinical need reflects the failure in developing novel classes of analgesics with superior efficacy, diminished adverse effects and a lower abuse liability than those currently available. Reasons for this include the heterogeneity of clinical pain conditions, the complexity and diversity of underlying pathophysiological mechanisms coupled with the unreliability of some preclinical pain models. However, recent advances in our understanding of the neurobiology of pain are beginning to offer opportunities to develop new therapeutic strategies and revisit existing targets, including modulating ion channels, enzymes and GPCRs.
Introduction
Pain is the primary reason why patients seek medical care, with over 40% of the US population affected by chronic pain 1. In the US alone in 2013, the overall cost of treating certain chronic pain conditions amounted to >$130 billion2. Available analgesics – NSAIDs, amine reuptake inhibitors, antiepileptic drugs and opioids - have varying, but typically low levels of analgesic efficacy, and are generally coupled with deleterious effects2. Indeed, opioids, which are the most commonly used (~240 million prescriptions in 2014)3 and often the most effective class of analgesics, produce tolerance, dependence, and constipation, and are associated with major abuse liabilities, while the respiratory depression associated with high doses has led to a catastrophic increase in the number of drug overdose (OD) deaths in North America3,4.
Diverse pathological situations at different anatomical sites can lead to pain. Causes of pain include cancer, inflammation or tissue injury, as well as injury or lesions of the nervous system5–9. Diverse chronic widespread pain syndromes may also occur due to abnormal amplification states within the CNS10–12. All of these may lead, via abnormal activity in nociceptive systems, to pain in the absence of a stimulus (spontaneous pain), exaggerated responses to noxious stimuli (hyperalgesia) and pain evoked by normally innocuous stimuli (allodynia).
The heterogeneity of clinical pain conditions and the complexity and multiplicity of underlying pathophysiological mechanisms has made it difficult to identify tractable targets with broad involvement – the blockbuster model of one treatment for all pain conditions is not tenable13. Poor predictability of preclinical “pain” models may result in candidates being selected that do not have activity in the conditions suffered by patients14 (Box 1). Conversely, difficulty in ensuring target engagement, lack of sensitivity of clinical trials, and placebo-induced distortions increase the risk that potentially effective compounds or targets may be prematurely abandoned15,16. These issues have led to most developmental efforts being devoted to reformulations of existing validated analgesic classes; opioids, NSAIDs, anti-epileptic agents and amine uptake inhibitors, in spite of their well know limitations17.
Box 1. The challenges of preclinical models of pain.
Preclinical rodent efficacy models are essential for analgesic development268,269, but their predictive validity has been questioned due to several high-profile programs where rodent behavioral readouts predicted analgesic effects that were absent in humans. For example, FAAH inhibitors were found to be antinociceptive in a range of animal models, but compounds such as PF-04457845 produced no analgesic effect in osteoarthritis patients in spite of lowering FAAH activity by >96%254. Similarly, NK1 (substance P) antagonists were shown to robustly reverse rodent nociceptive responses in the context of inflammation and nerve injury, but failed to produce analgesia in subsequent clinical trials270. Nonetheless, many clinically used analgesics, such as NSAIDS and opioids, produce antinociceptive effects in rodents269 albeit typically at much higher doses than those used in patients.
Exploiting pain models in model organisms to detect putative analgesics faces several challenges: 1) how do you measure pain, a conscious subjective report of an unpleasant sensory experience, when you have no access to how an animal feels? 2) are the models true surrogates for the conditions/diseases that commonly produce pain in patients? 3) you need to overcome the technical challenge of how to eliminate the confounders of bias, observer-induced changes and lack of reproducibility; and 4) drugs that target human proteins may not be active on their rodent homologues. The first is the most difficult since we can only measure outcomes that may correlate with some aspect of pain, such as withdrawal from a stimulus or learned avoidance from a situation that may be painful. For reflexive measures of pain typically a brief stimulus lasting for seconds is applied to a part of an animal’s body and a response measured5.6. This clearly bears little correspondence to the ongoing spontaneous pain that is the major complaint of most patients. Attempts have been made to develop outcome measures that may reflect the presence of discomfort but these require more effort and validation to make them robust and useful268. Just because some classes of analgesics like opiates can reduce at high doses nociceptive reflexes does not mean that such reflexes have predictive validity for all classes of analgesics. We need measures that come as close as possible to the persistent levels of discomfort an animal is in; we are not there yet.
Some disease surrogates, such as pain in response to an acute noxious stimulus, incision wound, or traumatic injury to a nerve, are easy to model5,6. Much more difficult are models of chronic widespread pain, such as fibromyalgia, osteoarthritis, and low back pain where there are no rodent models that phenocopy the human disease exactly. Finally, a major difficulty with preclinical models is that the human observer may introduce changes in the animal’s behavior that may distort the outcome measure through induction of fear and anxiety266. Recently, even the gender of the investigator has been shown to have a significant impact on pain-related behavior in mice271. Best lab practices can eliminate bias through thorough blinding and independent replication should be a standard21,22. In conclusion, while preclinical models are invaluable for the validation of targets and the measurement of drug action, they currently lack predictive power and need to be interpreted with caution. New technologies using quantitative measures of altered behavior in freely behaving animals for prolonged periods in an observer-independent way may transform the utility of preclinical models in the near future.
Given the prevalence of opioid diversion and overdose, there is clearly an urgent need to develop novel effective and safe analgesics without abuse liability. Work from a large number of laboratories has shed new light on the mechanisms and pathways that mediate specific aspects of the nociceptive nexus9,11,12,18–20 (Fig 1), revealing the potential for more fine-tuned targeting of acute and chronic pain in different clinical conditions. Completely new therapeutic strategies targeting ion channels, enzymes and GPCRs, often with human genetic validation, are emerging. In this review, we present these emerging mechanisms, and assess novel targets and agents in preclinical and early clinical stages of development that show promise for the development of innovative analgesics (Table 1 and 2).
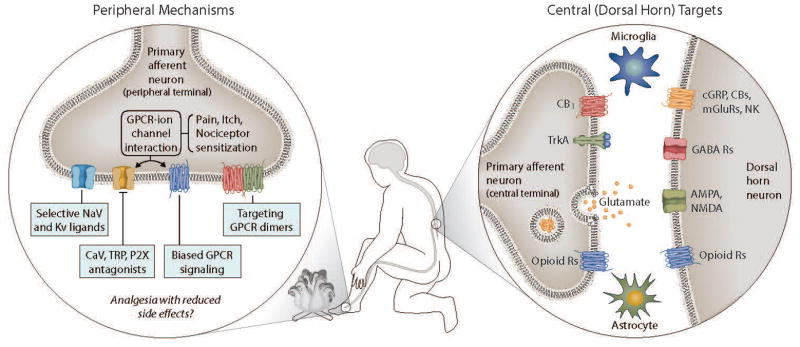
Figure 1. Mechanisms relevant for nociception and analgesia.
There are several targets in both peripheral neurons, and in the nociceptive synapse between glia, PNS and CNS neurons in the spinal dorsal horn, that provide interventional strategies for the development of analgesics. The figure summarizes some of the major targets that have been expanded upon in the text.
Table 1.
GPCR ligands at various stages of preclinical and clinical development
| Target/Category | Name | Mechanism | Preclinical data | Clinical status | References |
|---|---|---|---|---|---|
|
| |||||
| Abuse-deterrent formulations of opioids | MS-Contin OxyContin, etc |
Extended release opioids | Similar to opioid pharmacophores | Clinically used, controversial data suggesting increased misuse of opioids. More studies necessary. | 45,46,49,50 |
|
| |||||
| Peripheral GPCR ligand |
(Cara Tx) CR845 (i.v. and oral) |
Peripherally restricted kappa opioid agonist | Similar to other kappa agonists in rodent pain models (http://www.caratherapeutics.com/files/CARA_CR845_IASP_2008.pdf). Poor CNS penetration (http://www.caratherapeutics.com/cr845-other.shtml) |
Currently under clinical development Ph2a – oral Ph 3 - IV |
95, 278, 279 |
|
| |||||
| Heteromer GPCR ligands |
(Blue Tx) NNTA |
Selective mu- kappa opioid heteromer agonist (initial indication of greater potency and receptor expression in the spinal cord) | Analgesic, no dependence, no CPP | Preclinical IND development | 64 |
| MDAN-21 | Bivalent mu agonist-delta antagonist ligand | Analgesic, no tolerance or CPP Extremely potent analgesic in several rodent models |
No current information on clinical development | 58, 60, 62 | |
| MMG-22 | Bivalent mu agonist-mGluR5 antagonist ligand | Potent analgesia in inflammatory and neuropathic pain models | ND | 63 | |
| MCC-22 | Bivalent mu agonist-CCR5 antagonist ligand | ND | |||
|
| |||||
| Biased GPCR ligands | RB-64 (Trevena) |
Biased KOR ligand | Analgesic, CPA | ND | 72 |
| TRV130 TRV250 TRV734 PZM21 |
Biased mu opioid agonists | Varying levels of analgesic efficacy, no respiratory depression and reduced abuse potential and other side effects | Ph 1 or 2 trials completed, Ph 3 initiated for TRV130 | 84, 280 | |
|
| |||||
| Truncated GPCRs | IBNtxA | 6TM mu opioid truncated receptor. (Site of expression and extent of expression still to be determined) | Analgesic, no CPP | ND | 68–70,281 |
|
| |||||
| AT2R | Mycolactone (Spinifex Pharma) EMA401 |
Ang II R2 blockers (Mechanism still to be determined) | Analgesic, neuralgia indication | Under development for post-herpetic neuralgia. Phase 2 data showed increased efficacy when compared with placebo | 96,98,101 |
|
| |||||
| C5aR |
(Dompé Pharma) DF2593A |
C5aR allosteric antagonist | Allosteric C5aR inhibitor; effective in rodent models of inflammatory and neuropathic pain | ND | 282 |
|
| |||||
| Cannabinoid receptors |
(Various) APD371 LY2828360 S-777469 KHK6188 |
CB1 and 2 inhibitors (spinal and supraspinal mechanisms) | Antinociception, attenuate morphine tolerance | APD371 and KHK6188 in Phase 2 LY2828360 discontinued in phase 2 S-777469 has diverted to atopic dermatitis studies in the clinic |
85,86,283,284 |
|
| |||||
| GPR55 | ML-193 | GPR55 antagonist | Analgesia; agonists are pronociceptive | ND | 285 |
|
| |||||
| α2a-adrenergic receptor | Clonidine Tizanidine Dexmedetomidine |
α2a-adrenergic agonists | Some efficacy in neuropathic pain models in rodents. | Meta-analysis of 28 clinical trials suggests some clinical efficacy | 103–106,108,109 |
|
| |||||
| Chemokine receptors | Morphine + AMD3100 | Opioid+CCR antagonist | Reduced OIH and opioid tolerance in rodents, reduced neuropathic pain | ND | 121,132 |
| AZD2423 | CCR2 antagonist | Failed clinical trial | 133 | ||
| RAP-103 | Mixed CCR2- CCR5 antagonist | Attenuates neuropathic pain | ND | 134 | |
Key: ND – No data on clinical development, CPP – Conditioned Place Preference, CPA – Conditioned Place Aversion
Table 2.
Developmental status of ligands targeting ion channels involved in nociception
| Target/Category | Name | Mechanism | Preclinical data | Clinical status | References |
|---|---|---|---|---|---|
| TRPV1 | Several failures: AMG517; AZD1386; ABT- 102; MK-2295 Current in trial: JNJ-38893777 AZD1386 ABT NEO6860 |
agonists as patch/injectable Antagonists for pain relief |
Potent analgesia in inflammatory and neuropathic pain Especially beneficial for thermal nociceptive component |
Clinical trials failed due to hyperthermia and other side effects New ligands screened to not have the above side effects currently under clinical development |
144, 286–289 |
| VGCCs | Ziconotide Gabapentin Pregabalin Current trials: Z944 CNV2197944 TROX-1 Failed: Z160 |
Blockers for various pain indications | Efficacy for current approved ligands in models of neuropathic pain, but only moderate clinical efficacy (See box 2) New ligands have good potency in inflammatory, neuropathic and visceral pain models |
Z944 and CNV2197944 in Phase II | 290–292 |
| Nav 1.7 |
(Xenon and Teva) TV-45070 (Xenon and Genetech) GCD-0276 GCD-0310 (Pfizer) PF-05089771 |
Blockers for pain, human genetic data – mutations confer congenital pain insensitivity. Controversy that increased endogenous opioids in KOs are producing the insensitivity |
KOs are insensitive to nociceptive stimuli; loss of inflammatory pain | TV-45070 failed Ph II PF-05089771, GCD- 0276 and GCD-0310 in Ph I trials |
153, 160, 162, 293, 294 |
| Nav 1.8 |
(Pfizer) PF- 04531083 |
Target is widely expressed in nociceptors and blockers are proposed to be analgesic | PF-04531083 was effective in producing analgesia in neuropathic and inflammatory pain models | PF-04531083 failed in a clinical trial of dental pain | 162 |
Neurobiology of pain
The mechanisms that sustain and drive chronic pain (Fig 1) have been exhaustively reviewed elsewhere8,11,18,21–24. Pain is in physiological situations a necessary protective response initiated by high threshold peripheral sensory neurons to the risk of harm by tissue injury or infection. Indeed, the presence of pain acts as a homing beacon to alert the body to the presence of a noxious stimulus so that necessary corrective responses can be mounted. For tissue injury and infection, pain hypersensitivity accompanies the associated inflammation and persists for the duration of the inflammatory response18,25, helping healing to occur by avoiding use of the affected body part. However, pain can also be maladaptive and pathological where it arises due to dysfunction of the nociceptive system, due to genetic factors, for example paroxysmal extreme pain disorder due to gain-of-function mutations in Nav 1.726, injury to the nervous system that lead to neuropathic pain18,27 and abnormal central amplification syndromes. In all these cases pain is no longer the symptom but the disease itself.
Another driver for spontaneous pain in the absence of a peripheral stimulus is the ectopic activity of primary sensory neurons (Fig 1). Several voltage-gated sodium channels (Nav1.328,29, Nav 1.7, and Nav 1.830–35), potassium channels (KCNQ), calcium channels (Cav 2.2) and hyperpolarization-activated cyclic nucleotide-modulated channel (HCN) have been implicated in the modulation of ectopic activity and membrane excitability in sensory neurons especially after peripheral nerve injury36–39. However, a direct relationship between such ectopic activity of primary sensory neurons with spontaneous pain is yet to be proven, mainly due to inconsistent results and the paucity of reliable preclinical models, although live cell imaging with genetically encoded activity reporters should change this.
One major consequence of peripheral inflammation is the hyperfunction of nociceptive transduction ion channels on the peripheral terminals of sensory neurons. These channels are responsible for converting noxious stimuli into inward currents. The peripheral sensitization of these transducers and ion channels involved in membrane excitability leads to a reduction in threshold for activation and hyperexcitability of nociceptor sensory neurons18. However, peripheral sensitization alone does not fully account for the prolonged presence of pain, dynamic tactile allodynia, temporal summation of pain and the spread of pain hypersensitivity to non-injured tissue (secondary hyperalgesia)27. These features of pain hypersensitivity are the consequence of augmented sensory signaling in the CNS due to a prolonged increase in excitability and synaptic efficacy of central nociceptive neurons and loss of inhibitory activity27. This phenomenon, called central sensitization, is common to all types of clinical pain, some of which is driven by activity in nociceptors as a form of activity-dependent plasticity, and others by autonomous changes in the central nervous system.
Several mechanisms contribute to central sensitization; increased synaptic strength due to an upregulation of presynaptic ion channels, increased presynaptic neurotransmitter release or enhanced post-synaptic neurotransmitter receptor activity18,27. Disinhibition, where inhibitory noradrenergic, opioid and GABAergic transmission is reduced via various synaptic changes and loss of spinal interneurons also play a major role, especially in neuropathic pain18,27. In addition, injury to the nervous system may produce structural changes in nociceptive circuits that lead to increased pain sensitivity. Depending on the underlying cause of pain, many of the above processes may also be acting in concert, further complicating treatment options8.
In addition to the above mechanisms, there are multiple local and distributed pain modulatory influences. Endocannabinoids, for example, target cannabinoid receptors in both GABAergic and glutamatergic synapses where they serve as retrograde transmitters and block the transmission of pain signals40. In addition, endogenous opioid peptides such as endorphins, enkephalins, dynorphin and endomorphins target the different opioid receptor subtypes (mu, kappa and delta) to provide analgesia in various contexts41–43.
Targeting the opioid system
Exogenous opioid ligands isolated from opium poppy seeds have been used as potent analgesics for over 3000 years42 and opioids such as hydrocodone and morphine still remain the analgesics of choice for many patients in the clinic. However, while targeting opioid receptors can lead to efficacious inhibition of pain especially in an acute injury or palliative setting, opioid receptors also induce deleterious effects, such as, tolerance, physical dependence and an addictive potential that has led to a crisis of prescription and recreational opioid abuse44. Numerous approaches are therefore being tested to mitigate the deleterious effects of opioid analgesics.
Abuse-deterrent opioids
Several companies have focused on modifying existing opioids to develop abuse-deterrent formulations (ADF) 45,46. For example, extended release (ER) oxycodone (OxyContin, Purdue Pharma) and Opana ER (Oxymorphone, Endo Pharmaceuticals) are commonly prescribed based on the assumption that abuse only occurs with drugs that have rapid kinetics, which increases euphoria. However, this may be a misconception. While clinical data is scant and mostly inconclusive, some data show that the incidence of abuse may actually increase with ADF opioids 45,46. Indeed, although formulated differently, the active agents are still euphoric opioids and the drugs retain the potential side effects of the class, including the potential to overdose on high dose exposure46. In addition, while ADF-Oxycontin has reduced the number of prescriptions for and abuse of Oxycontin47,48, many abusers have either learnt to tamper with the abuse deterrence or have moved to other prescription opioids, fentanyl or heroin49,50.
Peripherally-restricted kappa-selective ligands
All major opioid analgesics currently on the market are mu opioid receptor agonists. Early efforts were made to develop kappa or delta selective ligands as some of the initial selective compounds showed a lesser propensity for physical dependence and abuse41–43. Indeed, many kappa agonists produce mild or severe aversive behavior. However, such efforts have failed either due to ineffective analgesia (delta) or severe psychotomimetic and dysphoric effects (kappa). For instance, kappa ligands such as Enadoline and butorphanol were developed to have less abuse potential, but while they are still rewarding, they also induce fatigue, sedation, confusion, anxiety, visual and auditory distortions and depersonalization51,52.
Since many of the side effects of kappa agonists are generated in the CNS, Cara Therapeutics is currently developing a peripherally restricted kappa agonist CR845 to be administered either IV or orally. IV CR845 has been shown to successfully inhibit post-operative abdominal pain in Phase II trials53 and an oral formulation is currently being tested in patients with chronic knee pain in a Phase II trial.
These early clinical data are promising, but questions still remain as to the efficacy of peripherally restricted ligands in reducing most forms of chronic pain, especially those sustained by central sensitization, as described above.
Opioid receptor heteromers, bifunctional ligands and isoforms
Another approach to eliminate mu opioid-associated side effects is based on the burgeoning evidence that GPCRs, including opioid receptors, form both oligomers and higher order complexes54–56 (Fig 2). Homomers (complexes of similar receptors) and heteromers (complexes of different receptors) provide potential exciting new targets for pharmacological intervention.
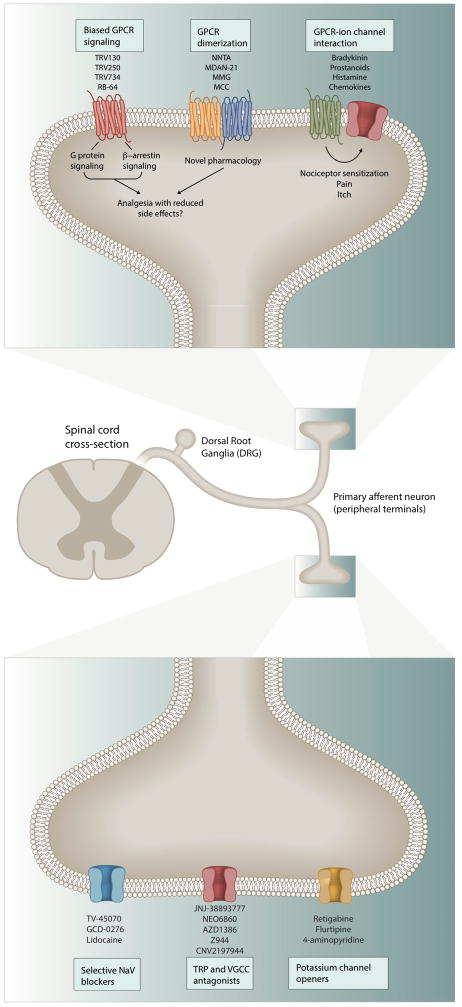
Figure 2. Peripheral analgesic drug discovery targets.
The peripheral terminals of primary afferent nociceptor neurons express GPCRs and various ion channels that mediate transduction of different sensory modalities, some of which have specific coupling and signaling features that offer new opportunities for novel drug classes. GPCRs can utilize both G-proteins and β-arrestins as secondary messengers and biased ligands can engage particular signaling to get increased efficacy and reduced adverse effects. GPCRs can also physically complex with other receptors leading to the formation of homomers or heteromers and also functionally interact with ion channels such as TRPV1, TRPA1, GIRKs, etc. Anti-NGF (nerve growth factor) antibodies have also been developed with the intent to alleviate certain pain modalities by blocking activation of the TrkA receptor. In addition, inhibitors for sodium and calcium channels (TRP and votage gated calcium channels) and potassium channel openers have been developed with intriguing pharmacology. We list ligands for some of these targets that are currently in analgesic clinical trials.
Several bivalent ligands that contain distinct pharmacophores for two receptors tethered together with a carbon atom linker of varying lengths have been generated that demonstrate some promising pharmacological profiles (Table 1). For example, MDAN-21 (μ agonist/δ antagonist)57–60, a series of mu agonist/CB1 antagonist ligands61, MMG22 (μ agonist/mGluR5 antagonist)62 and MCC22 (μ agonist/CCR5 antagonist)63 have demonstrated potent antinociceptive activity in various preclinical rodent models of pain and are also devoid of tolerance or place preference, a measure of abuse liability. However, these molecules are rather large, raising questions about their bioavailability. There is therefore interest in the development of small molecule ligands for heteromeric opioid receptors. NNTA, a selective agonist for mu-kappa opioid receptor heteromers, is an example64. Mu-kappa heteromeric opioid receptors have been found to form heteromers in the rat spinal cord and the presence of these receptors appears to be higher in female than male rats65. NNTA targets mu-kappa heteromers at sub-picomolar concentrations and is a potent antinociceptive agent that appears in rodents to be devoid of tolerance, physical dependence and drug-seeking behavior64. NNTA is currently being investigated by Blue Therapeutics via IND-enabling studies for safety with future plans for human clinical trials upon successful filing of the IND package. In addition, bifunctional ligands such as the newly described Buprenorphine small molecule analog, BU08028, for mu and nociceptin opioid receptors, is another potential drug candidate which has displayed potent antinociception devoid of respiratory depression or physical dependence in monkeys66.
A different, but complementary, approach has focused on targeting different isoforms of the mu opioid receptor (Table 1). The oprm1 gene has two independent promoters and can thus form several splice variants, including some unexpected 6TM domain receptors that are structurally quite different from 7TM domain GPCRs67,68. For instance, the splice variant MOR-1C results in a functionally active 6TM receptor and a ligand (IBNTxA) targeting this receptor produces antinociception without drug-seeking behavior in conditioned place preference studies or respiratory depression in mice 69,70. This suggests that the c-terminal transmembrane domain of the mu opioid receptor may not be necessary for the docking of certain ligands. Similarly, the modified c-terminus of the functional splice variant MOR-1D mediates its dimerization with GRPR to generate opioid-induced itch in mice, providing further evidence that the MOR c-terminus may play a key role in producing adverse effects of opioids71. Several questions remain, however, as to how these truncated receptors signal, the extent and level of tissue expression, and why targeting them alters mu opioid side effects. In addition, further research is warranted to determine the similarity between human and murine opioid receptor isoforms.
Biased ligands
While GPCRs were initially considered to signal only via the specific G proteins they couple with, the recent discovery of ligand bias has enhanced our understanding of GPCR signal transduction and provided new GPCR pharmacology72. GPCR coupling with β-arrestins can be promoted or avoided by ligands that restrict the receptor to particular conformations, and can thus lead to several distinct pharmacological outcomes from the same receptor73–80 (Fig 2).
Several biased agonists have been developed targeting opioid receptors that produce reduced opioid side effects with similar or better antinociception, at least in preclinical animal models72,81 (Table 1). A prominent example is TRV130, a mu opioid receptor biased agonist that activates G protein signaling, but not β-arrestin. Clinical trials with TRV130 have been largely positive, showing that it is well tolerated and has comparable analgesic efficacy to morphine but with a faster onset82,83. TRV130 also produces significantly reduced respiratory depression, nausea and vomiting in patients, however this seems to be dose-dependent82. The fact that respiratory depression is not completely eliminated with this approach is concerning, since the abuse liability of this compound has not yet been determined in humans.
The recent exciting discovery of PZM21 – a Gi biased mu opioid agonist - clearly illustrates that differential pharmacology can be achieved by selectively activating specific signaling messengers via the same receptor 84. The compound is the product of extensive in silico-assisted screening of >3 million ligands and is devoid of the common opioid side effects of constipation, respiratory depression and drug-seeking behaviors. However, some questions remain as to the analgesic efficacy of the molecule as it was found to be four times less potent than morphine in rodent studies73.
Non-opioid GPCRs
Outside of the opioid receptor system, a few other receptor systems have been found to be directly involved in pain pathways, including cannabinoid receptors, angiotensin type 2 receptor and α2 adrenergic receptors.
Cannabinoid receptors
The cannabinoid receptors, CB1 and CB2, have been intensively investigated as targets for potential pain therapeutics85. While CB1 receptor agonists produce severe psychotomimetic effects and have a large abuse potential, selective CB2 agonists produce analgesia in preclinical models without any major central side effects85,86. While initially thought of as a peripherally restricted target, CB2 receptors are expressed in the CNS (Fig 2 and Fig 3), especially by spinal microglia and interneurons. Many pharmaceutical companies have developed selective CB2 agonists, but initial efforts failed in clinical trials due to lack of efficacy87–90. However, Arena Pharmaceuticals has published positive Phase Ib clinical trial results on its selective CB2 agonist, APD371 and is planning for Phase II trials in 2016–201791. Several other candidates (Table 1), are also being evaluated in either Phase I or II trials85,92–95, the results of which are awaited.
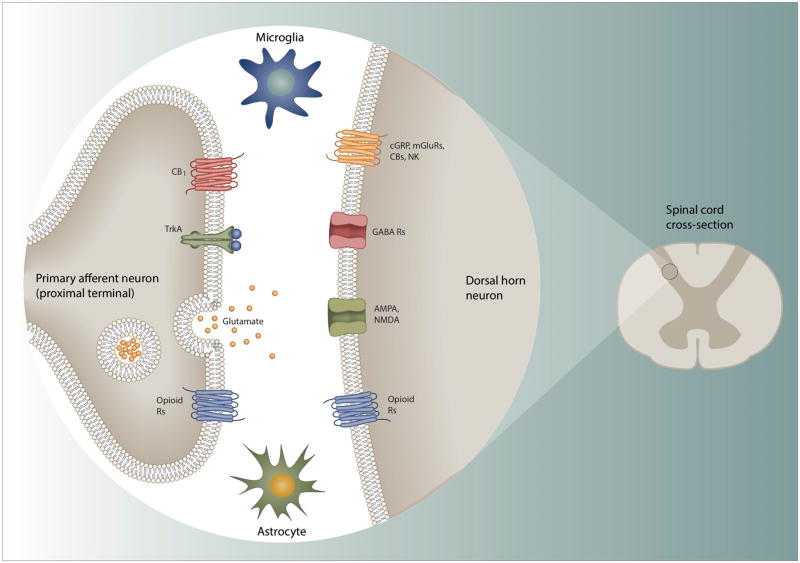
Figure 3. Targeting signaling between pre-synaptic nociceptors and post-synaptic neurons in the spinal dorsal horn.
The central terminals of nociceptor sensory neurons synapse on neurons in the dorsal horn of the spinal cord that after processing by local circuits relay the information to the brain. The pre-synaptic nociceptor terminals deliver input generated by noxious stimuli in the periphery, inflammation and peripheral nerve injury to the post-synaptic neurons. Several of the transmission/modulation mechanisms involved represent potential analgesic targets, either by reducing excitatory or augmenting inhibitory transmission. Some of the key players – opioid, cannabinoid, NK1 and other GPCRs, along with ion channels like NMDA, AMPA, and GABA are discussed throughout the article. Another key player is the TrkA receptor which is targeted by NGF – the blocking of which has shown to produce potent analgesia. However, lack of specificity for nociceptive pathways in many cases makes on-target undesirable effects problematic.
Angiotensin type 2 receptor (ATR2)
Another intriguing GPCR, AT2R, belongs to the renin-angiotensin system that is now recognized as contributing to neuropathic pain in preclinical rodent models96,97. While AT1R plays a major role in hypertension, AT2R is expressed in human sensory neurons and, when activated, sensitizes TRPV1 ion channels that mediate thermal pain98,99. While the mechanism by which AT2R inhibitors reduce neuropathic pain remains unknown, inhibition of p38 and ERK MAPK pathways have been implicated96,100. Spinifex Pharmaceuticals has developed a highly selective AT2R inhibitor, EMA401, that inhibits capsaicin-induced calcium fluxes in human and rat DRGs, and which produces dose-dependent antinociception in mouse neuropathic pain models, effects that are abolished in AT2R null mice96(Table 1). Phase I and II clinical trials show that EMA401 is well tolerated and produces significant analgesia with daily dosing in patients suffering from post-herpetic neuralgia98. However, in contrast, a mycobacterial polyketide mycolactone has been shown to activate AT2R to induce analgesia in mice with buruli ulcer 101. The fact that both activators and antagonists for the same receptor produce analgesia complicates the delineation of the mechanism of action of AT2R in pain.
α 2 adrenergic receptors
Studies on the role of the α2 adrenergic system in pain are conflicting. From pharmacological studies and null mice, α2 receptors seem to play a role in diverse painful syndromes due to an inhibition of presynaptic release of neuropeptides in the spinal cord and action on microglia102–106. However, pontine α2 adrenergic activation increases pain by attenuating descending inhibition107. Meta-analysis of 28 clinical trials found that while intraoperative administration of the α2 adrenergic receptor agonist dexmedetomidine (DEX) reduced postoperative pain, opioid consumption, and lowered risk of opioid-related adverse effects, some patients suffered from intraoperative bradycardia, and the postoperative administration of DEX did not have significant effects on pain management108,109. Recro Pharma is developing an intranasal (IN) DEX formulation that is administered at 1/8 to 1/10 the intravenous recommended dose, which could reduce adverse effects. In Phase II trials, DEX-IN produced positive efficacy results for treatment of postoperative (bunionectomy) pain, but likewise generated adverse effects characteristic of α2 adrenergic agonists, including reduced blood pressure and bradycardia, and plans for Phase III trials of DEX-IN for postoperative pain are pending FDA input110,111
Chemokine receptors
Pro-inflammatory chemokines contribute to the maintainance of chronic inflammatory pain112. Chemokines modulate nociceptors via the activation and sensitization of sodium and TRPV1 channels and also communicate between immune, neuronal and glial cells113–120. Injection of certain chemokines leads to pain114,117, while chemokine antagonists produce antinociception121. However, given the numbers of these signaling molecules and their importance in immune function122, chemokines are challenging therapeutic targets.
There appears to be some crosstalk between opioid and chemokine pathways – especially in the context of opioid tolerance, opioid withdrawal-induced hyperalgesia and opioid-induced hyperalgesia – that may reflect hetero-desensitization and heterooligomerization between opioid and chemokine receptors123–126. Co-administration of morphine with either CXCL12, CX3CL1 or CCL5 leads to reduced opioid analgesia in rodents127–131. On the other hand, administration of antibodies to CCL2 or a CXCR4 inhibitor (AMD3100) leads to reduced opioid-induced hyperalgesia and tolerance in rodents128,132. Bivalent ligands for mu-CCR5 heterooligomers show potent antinociception without any tolerance, dependence or abuse potential in mice 63. However, clinical trials with chemokine antagonists have not been successful. AZD2423, a selective CCR2 antagonist, effectively reduced neuropathic pain in rodent models but did not produce analgesia in patients with post-herpetic neuralgia133. While species differences or poor CNS activity could have led to this failure, dual targeting antagonists like RAP-103 (a CCR2/CCR5 dual antagonist) are under development with the aim of achieving a better efficacy profile134.
Ion Channels as Drug Targets
Ion channels underlie all electrical activity by neurons. In principle, targeting ion channels to disrupt pain signaling could be done at all stages, from inhibiting firing of primary nociceptors to inhibiting second- or higher-order neurons in the spinal cord and brain. The most obvious way of selectively targeting pain signaling is to target ion channels that have preferential expression in primary nociceptors. This is the strategy embodied in drugs targeted to TRPV1 channels, which are strongly expressed in primary nociceptors and have little or no expression in most other neuronal types135. Several other potential drug targets, including Nav1.8 and Nav1.9 voltage-dependent sodium channels, are also highly expressed in primary nociceptors with little expression in other neuronal types, while Nav1.7 sodium channels are expressed in a wider variety of peripheral sensory and sympathetic neurons but have little expression in the central nervous system136,137.
While current strategies have focused on channels in primary sensory neurons, the potential of targeting ion channels in higher order neurons is also emerging. In this case, it is likely difficult or impossible to confine drug effects only to nociceptive pathways. However, this is not necessarily an insurmountable problem. Many existing ion channel-based therapies for other conditions are also targeted to channels with wide expression in the nervous system, including anti-epileptic drugs targeted broadly to sodium channels and anxiolytic drugs targeted to GABAA channels17. In general, our knowledge of the organization of neurons and neuronal circuits is too primitive to understand how such drugs targeted to widely-expressed ion channels can achieve clinical efficacy without debilitating side effects. As we gain more detailed knowledge about the patterns of expression, regulation and function of various channels in higher-order pain signaling neurons, it may be possible to target activity of these neurons in a rational manner.
TRPV1 channels
The molecular cloning of TRPV1 channels and identification as a receptor in primary sensory neurons mediating thermal pain in 1997138 (Fig 3) led to an avalanche of studies implicating TRPV1 activation in a variety of preclinical pain models. Drug development programs targeting TRPV1 channels followed quickly, with patents filed for over 1,000 compounds by approximately 50 companies139. So far, the results have been disappointing. In general, efficacy of TRPV1 antagonists in clinically-relevant pain conditions in humans has been modest, and there are two problematic adverse on-target effects seen with many of the compounds: reduced sensitivity to noxious heat, potentially leading to accidental burns, and an increase in body temperature, likely reflecting participation by TRPV1 channels in normal temperature regulation139–141. These issues, along with modest efficacy, have resulted in discontinuation of most programs.
However, recent work suggests that it may be possible to develop compounds that retain anti-nociceptive activity while avoiding adverse effects, by finding agents that can inhibit activation of TRPV1 channels by the endogenous activators mediating pain without inhibiting activation by increases in temperature or low pH (which has been suggested to mediate control of body temperature142). Such “modality-specific antagonists” are based on evidence that vanilloid ligands like capsaicin (and perhaps endogenous agents mediating pain) activate TRPV1 in a different way than temperature or pH and that these modalities of activation can be differentially inhibited141–143. NEO6860 (Neomed) is one such modality-specific agent144 and is currently in Phase II trials for osteoarthritis. New technology enabling high resolution structures of ligand-bound TRPV1 channels using cryo-electron microscopy145,146 should greatly facilitate further development of this approach141.
Sodium channels
Voltage-dependent sodium channels underlie electrical signaling in all neurons. There are nine distinct types of sodium channels encoded by different genes, of which three (Nav1.1, Nav1.2, and Nav1.6) are widely expressed in both the CNS and PNS (Fig 2 and 3) and three more (Nav1.7, Nav1.8, and Nav1.9) are expressed mainly in subsets of peripheral neurons. Currently used sodium channel blockers, including lidocaine, amitriptyline, and mexiletine, display relatively little selectivity among different sodium channels, and their therapeutic window is limited by unwanted side-effects, mainly from actions on the CNS. This has stimulated efforts to develop compounds selective for the sodium channels with little expression in the CNS.
Nav1.7
Nav1.7 channels are expressed in nociceptors and sympathetic neurons, with only low expression in the CNS136,147. They emerged as prime candidates for novel analgesics following the identification of rare human mutations in which loss of function in Nav1.7 channels resulted in congenital insensitivity to pain148,149. In addition, a number of gain-of-function mutations in Nav1.7 have been identified, in which patients experience pain, sometimes severe, either spontaneously or triggered by normally innocuous stimuli137. Similarly, mouse knockout models of Nav1.7 show nearly complete insensitivity to nociceptive stimuli and loss of inflammatory pain, while responding normally to a variety of non-painful stimuli150–152.
Nav1.7 is currently being actively pursued by the pharmaceutical industry. Potent small-molecule inhibitors of Nav1.7 channels have been found153–155, most notably a class of sulfonamide compounds that can be made highly selective for Nav1.7 channels over other sodium channels, and even selective for human Nav1.7 channels over those from other species156. Clinical trials of several of these compounds are underway (Table 2), including the Icagen/Pfizer compound PF-05089771 which completed Phase II trials for wisdom tooth removal and primary erythromelalgia 250, and two compounds (GDC-276 and GDC-0310) developed by Xenon Pharmaceuticals which are being tested in Phase I trials in collaboration with Genentech251.
The binding site for sulfonamide inhibitors has recently been determined and is completely different to that of classic small molecule sodium channels blockers such as lidocaine, carbamazepine, and other local anesthetics and anti-epileptic agents, which interact with the pore region of the Na channels and generally have little selectivity among different sodium channel sub-types 167–168. The high specificity of sulfonamide compounds and the unusually detailed knowledge of its binding site should allow design of even more potent and selective small molecule Nav1.7 inhibitors.
Several non-sulfonamide class compounds that are targeted primarily to Nav1.7 channels, but are generally less selective, are also being tested. Raxatrigine (Convergence/Biogen) has been tested in Phase II trials in trigeminal neuralgia157, and funamide (TV-45070, Xenon/Teva), is in Phase IIb trials for post-herpetic neuralgia249.
In addition, peptide inhibitors of Na1v.7 channels have been developed by cleverly modifying naturally-occurring peptides from spider venom. This approach exploits amino-acid differences among different sodium channel types in extracellular loops of the channel proteins and has yielded peptide inhibitors with very high affinity for blocking Nav1.7 channels and a high degree of subtype selectivity 158. However, delivery of these large peptide compounds may present a significant challenge for clinical translation.
A potential problem with the clinical use of Nav1.7 blockers is that while the channels are minimally expressed in the CNS, they are expressed in primary olfactory neurons159, meaning that a potent Nav1.7 blocker may well produce anosmia, a side-effect that has been found intolerable by a substantial fraction of patients in clinical trials of drugs with other targets.
Another potential issue relates to the recent finding that sensitivity to pain can be restored by naloxone in both Nav1.7-null mice and in a human lacking Nav1.7160. This raises the possibility that the loss of pain sensation in null mutants is more complicated than simple functional removal of Nav1.7 channels and may result from upregulation of opioid pathways inhibiting pain. However, it is possible that such upregulation reflects complex compensatory events during development that would not be mimicked by acute inhibition.
Nav1.8
Nav1.8 channels are tetrodotoxin-resistant voltage-gated sodium channels that are expressed in small diameter primary nociceptive neurons, with little or no expression in other neuronal types. In principle, their selective expression and dominant contribution to the overall sodium current in small diameter DRG neurons (at least in the soma) in principle make Nav1.8 channels attractive as drug targets. Fairly selective Nav1.8 inhibitors have been developed and have exhibited anti-nociceptive properties in rodent models31,161. There have been efforts to develop Nav1.8 inhibitors for clinical use in humans153. However, the only reported clinical test of a Nav1.8-selective inhibitor, the Pfizer compound PF-04531083162, resulted in a terminated trial on dental pain and there is no evidence of other efforts120. Although Nav1.8 channels are prominent in the cell bodies of nociceptive DRG neurons, axonal propagation in these neurons generally depends on TTX-sensitive channels33,163. In a rat model of neuropathic pain, expression of Nav1.8 in axons is sufficiently up-regulated to allow TTX-resistant propagation of action potentials in some C-fibers33, but it remains unclear under what conditions Nav1.8 inhibition can inhibit generation or propagation of action potentials in human pain conditions.
Nav1.9
Like Nav1.8 channels, Nav1.9 channels are expressed in small diameter primary nociceptive neurons. Nav1.9 channels activate and inactivate very slowly and under normal physiological conditions appear to be mostly inactivated. Their normal physiological function may be to provide a small current to help depolarize the resting potential and amplify slow subthreshold depolarizations between the resting potential and spike threshold164. Nav1.9 knockout mice exhibit normal sensitivity to acute pain but reduced hyperalgesia in response to inflammation, suggesting that these channels may represent therapeutic targets165. However it is not evident whether Nav1.9 is currently being clinically pursued. Screening for blockers of Nav1.9 has been hampered by the inability to express Nav1.9 in heterologous systems although an expressible chimeric Nav1.9/Nav1.4 channel has recently been reported166.
Targeted Delivery of Charged Sodium Channel Blockers
For selectively inhibiting electrical activity of primary pain-initiating nociceptor neurons, an alternative strategy to targeting Nav1.7 or Nav1.8 channels is to use large-pore channels selectively expressed by the neurons as portals for delivering drugs (Fig 2). Many primary nociceptors express either TRPV1 channels or TRPA1 channels or both. These channels are unusual in that they form a pore that allows entry of very large cations167, including N-methyl-D-glucamine (MW 195 Daltons)168 and the cationic dye FM1-43 (MW 452 Daltons)169. This property can be exploited to deliver cationic drug molecules into the neurons. The lidocaine derivative QX-314 (N-ethyl-lidocaine) is a cationic molecule (MW 263 Daltons) that is ineffective in inhibiting neuronal sodium channels when presented on the extracellular side of the channel, because it is too large to enter the sodium channel pore from the outside, but it can enter and inhibit channels when presented on the intracellular side. Such entry occurs only when channels are open and the QX-314 molecule can be trapped inside the channels when the channel closes again, so block accumulates in a use-dependent manner. The pores of both TRPV1 and TRPA1 channels are large enough for QX-314 to permeate into the cell170–172, with entry of the cationic molecule driven not only by the concentration gradient but also by the negative intracellular membrane voltage, promoting higher concentrations inside the cell. Thus, externally applied QX-314 can enter and inhibit sodium channels in neurons when TRPV1 or TRPA1 channels are activated170,172. Some P2X family channels, including P2X2, P2X4, and P2X7 also have large pores and can likely admit QX-314 and similar molecules173–175.
In initial experiments studying perineural application to sciatic nerves in normal animals, QX-314 co-applied with capsaicin produced long-lasting inhibition of pain signaling with little effect on motor function mediated by the same nerve trunk170,176,177. In later experiments, QX-314 was co-applied with lidocaine, which acts as an agonist at both TRPV1 and TRPA1 channels at mM concentrations178,179. Lidocaine blocks all nerve function but only transiently (~ 1hr), while co-application with QX-314 produced nociceptive block lasting up to 9 hours or more176,177, likely reflecting long residency of QX-314 once it has entered TRPV1- and TRPA1-containing fibers.
In many clinical situations, TRPV1 and TRPA1 channels may be sufficiently activated endogenously that there is no need to apply an exogenous TRP channel activator along with the cationic sodium channel blockers. For example, during itch, TRPV1 or TRPA1 channels are activated by second messengers whose release is triggered by a variety of G-protein coupled receptors180. Activation of these receptors is sufficient to facilitate QX-314 inhibition of experimentally-induced itch180. During inflammation, TRPV1 and TRPA1 channels are activated by endogenous agents, and in this circumstance it is likely also unnecessary to co-apply an exogenous TRP channel activator. Moreover, because activation of C-fibers and release of neuropeptides is often part of the inflammatory process, inhibiting C-fiber electrical activity can actually reduce inflammation, not just block the pain or itch resulting from the inflammation. C-fiber silencing by entry of charged sodium channel blockers can reduce airway inflammation in animal models of asthma181
A limitation of this strategy is that the cationic sodium channel blockers must be applied in close proximity to the nerve fibers being silenced, since as cationic molecules they have limited tissue penetration. Suitable application methods include direct instillation into wounds, local injections, and delivery by aerosol to affect lung inflammation. Application to broken skin in dermatitis or similar conditions could also be effective. Notably, this limited tissue penetration means that there is little or no systemic exposure of the cationic agents, greatly minimizing chances of unwanted effects.
Calcium channels
Voltage-dependent calcium channels are calcium-selective channels that are closed at normal resting potentials and opened by depolarization. They underlie many neuronal functions including helping regulate cellular excitability and mediating release of synaptic vesicles. Of ten voltage-dependent calcium channels encoded by the mammalian genome, eight have wide expression in neurons.
The most widely used current therapeutic agents targeting voltage-dependent calcium channels are gabapentin (Neurontin) and pregabalin (Lyrica), which are believed to act by affecting trafficking and recycling of calcium channel proteins182,183. For treating hyperalgesia and neuropathic pain, gabapentin and pregabalin are effective in only a minority of patients, and the reasons for this are still unknown. It has been proposed that improved therapeutic selectivity might be attained by agents selective for alpha2delta-1 subunits, as this subunit appears to mediate the antihyperalgesic effects of gabapentin and pregabalin, while the drugs also bind to alpha2delta-2 subunits, which may mediate side effects183. In addition, recent advances in our understanding of a couple of calcium channels have opened up some additional avenues for analgesic drug design.
Cav2.2 splice variants
Synaptic transmission is triggered by calcium entry through presynaptic calcium channels. Most synaptic transmission in the mammalian brain and spinal cord is mediated by Cav2.1 (P/Q-type) and Cav2.2 (N-type) calcium channels183,184. Interestingly, transmission at the first synapses in the pain pathway, glutamatergic synapses from primary sensory neurons to second-order neurons in the dorsal horn, appears to rely nearly exclusively on presynaptic Cav2.2 channels. Cav 2.2 knockout mice have reduced sensitivity to some but not all types of acute, inflammatory, and neuropathic pain (reviewed by38). Cav2.2 channels can be blocked selectively by several peptides from the venom of cone snails and one of these,ω-conotoxin MVIIA (ziconitide, Prialt), is currently used to treat pain, with application by intrathecal pump. However, its utility is limited by a small therapeutic window, likely reflecting participation of Cav2.2 channels in other functions of the spinal cord and perhaps spillover of peptide into higher regions of the nervous system.
The efficacy of Prialt has stimulated efforts to find small-molecule inhibitors of Cav2.2 channels that could be effective against pain. Several such inhibitors have been reported, including TROX-1185,186. Like most small-molecule sodium channel inhibitors, these are state-dependent inhibitors, which bind more tightly to activated states of the channel than resting closed states. Therefore, state-dependent inhibition results in greater inhibition of channels that are repetitively cycling quickly through activated states, potentially conferring at least some selectivity for neurons that are hyperactive. Although several such state-dependent calcium channel inhibitors have demonstrated effective pain relief in several rodent models185–187, efficacy in human trials remains to be demonstrated.
As Cav2.2 channels are widely expressed in the brain, a systemically available channel blocker that crosses the blood-brain barrier would likely exhibit serious unwanted effects. However, the Cav2.2 channels in primary sensory neurons have different splice variants than the predominant channels in the brain (reviewed by188), raising the possibility of developing small molecules with sufficient selectivity to reduce spinal cord transmission without deleterious central effects.
Cav3.2
Cav3.2 channels are low-threshold T-type channels that in various central neurons can mediate burst firing following hyperpolarization (which removes resting inactivation of the channels). Their normal physiological function in sensory neurons is enigmatic, but knockout of Cav3.2 channels or knockdown by antisense RNA has an impressive analgesic effect for mechanical and thermal pain in rats189,190, raising the possibility that they could be targeted therapeutically. The Abbvie compound ABT-639, an orally available Cav3.2 inhibitor with reasonable selectivity produced dose-dependent antinociception in a rat model of knee joint pain and reduced tactile allodynia in several rodent models of neuropathic pain, including spinal nerve ligation and CCI191. However, subsequent clinical trials of ABT-639 with human volunteers showed no effect in an acute intradermal capsaicin pain model in which pregabalin was effective192. Similarly, ABT-639 did not reduce pain in patients with diabetic neuropathy193. Other Cav3.2 inhibitors have been described to be effective in pain models, including a rat model of pain from irritable bowel syndrome.194
Several molecules that have state-dependent blocking activity at both Cav2.2 and Cav3.2 channels (and variable potency for blocking other calcium channels such as Cav2.1) have been described and reported to be effective in rat models of neuropathic pain, inflammatory pain, and mechanical allodynia.195–197. However, whether related compounds are being pursued for possible clinical use is unclear.
Potassium channels
Mammalian neurons express many different types of potassium channels, with significantly different expression patterns among various types of neurons. A number of different potassium channels are down-regulated in primary sensory neurons in animal models of hyperalgesia and neuropathic pain39,198–203, likely contributing to consequent hyperexcitability of pain-signaling neurons. In principle, enhancing current through potassium channels could be an effective way of inhibiting neuronal activity, and the differential expression patterns of various types of potassium channels offers the possibility of inhibiting the activity of a specific neuronal population, like nociceptors, without excessive disruption of other neuronal functions204. The search for enhancers of potassium currents to inhibit pain signaling is relatively recent, but already it is evident that the general strategy may be promising205,206. So far, the most attention for this approach has been on Kv7 (KCNQ) family channels36,207–209, and several members of the “two-pore” (K2P) family of channels198,210. An alternative approach to small molecule enhancers of channel function is to attempt to correct the changes in potassium channel gene expression associated with pain201.
Kv7 Channels
Kv7 channels are voltage-dependent channels that typically activate and deactivate relatively slowly and often can be activated by small subthreshold depolarizations. The original description of a neuronal Kv7 current was “M-current”, named for its ability to be inhibited by muscarinic stimulation in sympathetic neurons and later shown to be mediated by Kv7.2/Kv7.3 heteromeric channels211. By activating slowly with small depolarizations, Kv7 current produces strong adaptation of firing evoked by steady depolarization. Kv7.2/Kv7.3 channels appear to be widely expressed in the nervous system, often with strong expression at the axon initial segment, where they are well positioned to modulate action potential firing212,213. A number of compounds have been found that enhance the activation of Kv7 family channels by shifting the voltage-dependence of activation to more negative voltages. The best-known of these is retigabine, introduced for use as an anti-epileptic drug. Retigabine inhibits nociceptive behaviors in rodent models of neuropathic pain214,215 and hyperalgesia37. Whether the effects are mediated by central or peripheral neurons is unclear. Disappointingly, however, a clinical study on human neuropathic pain failed to meet its primary efficacy endpoint216, adding retigabine to the long list of compounds with efficacy in rodent models of pain that seem to have much less dramatic effects in human patients.
Another compound that enhances Kv7 channel-mediated current by shifting activation to more negative voltages is flupirtine, which is in the same chemical class as retigabine (differing only by substitution of a pyridine ring for a phenyl ring). Interestingly, flupirtine was introduced to clinical practice as a centrally acting non-opioid analgesic in Europe in 1984. The effects of flupirtine may be mediated centrally and/or peripherally. Small-diameter DRG neurons likely corresponding to C-fibers express Kv7.2 subunits and a component of their potassium current is inhibited by the Kv7 blocker XE991217. Knockout of Kv7.2 in all somatic sensory neurons produces a modest enhancement of withdrawal from painful mechanical and thermal stimuli, mediated primarily by loss of Kv7.2 from A-delta fibers218, perhaps reflecting the prominent expression of Kv2 channels in nodes of Ranvier of many myelinated nerves. Of particular interest, the primary Kv7 subunit expressed by small-diameter C-fiber nociceptors is Kv7.5219, raising the possibility that screening for selective enhancers of this subunit might identify novel peripherally-acting analgesics. However, Kv7.5 is also prominently expressed in vascular smooth muscle, so such agents may also decrease blood pressure. However, if channels in C-fiber nociceptors are Kv7.5 homomers and those in vascular muscle are heteromers with other Kv7 subunits, there may be an opportunity for selective targeting.
K2P Channels
Two-pore-domain (K2P) family channels constitute a diverse group of 15 gene products with each protein containing four transmembrane segments and two pore domains, with channels formed by dimers of subunits220. K2P channels are widely expressed in both neurons and non-neuronal cells. Many K2P channels are constitutively open, and K2P channels underlie the main resting potassium conductance responsible for the negative resting potential of cells. Because of their regulation of both resting potential and input conductance of neurons, K2P channels can exert strong control over neuronal excitability. For that reason, compounds that enhance opening of K2P channels (or perhaps upregulate their expression) are an attractive possibility for drug development. Compared to many other channels, there is relatively little known about the functional contributions of specific K2P channels in various neuronal types, largely because there are currently few specific pharmacological tools. Nevertheless, recent findings suggest that several K2P channels could be attractive clinical targets for pain relief. A number of K2P channels expressed in DRG neurons are down-regulated after nerve injury and various other animal models of neuropathic pain associated with neuronal hypersensitivity221. Of particular interest for a role in nociceptors is TREK1, based on the striking finding that TREK1 can be activated by morphine via mu opioid receptors, with analgesic action of morphine in TREK1 knockout animals being reduced by about half222. Currently, too little is known about relative expression of various K2P channels in different neuronal (and non-neuronal) types to predict which might be the best targets for pain relief, while minimizing disturbance of other functions. Development of specific pharmacological tools, which is underway in several labs223,224, should be a key step.
Kv2/Kv9 Channels
Kv2 channels are voltage-activated delayed rectifier potassium currents widely expressed in both peripheral and central neurons. Kv2.1 subunits have especially wide and high expression in many neuronal types. Of particular interest for possible drug development, Kv2 subunits can make heteromeric channels with subunits from other gene families, including Kv5, Kv6, Kv8, and Kv9 subunits, so-called “silent” subunits that seem not to make functional channels on their own225,226. In the context of pain, attention has focused particularly on Kv9.1 subunits, for which a common human allelic variant is strongly associated with increased susceptibility to neuropathic pain7. Subsequent animal studies showed that Kv9.1 subunits are strongly down-regulated in primary sensory neurons after nerve injury, in parallel with development of pain behaviors227. The reduction of Kv9.1 expression is hypothesized to produce spontaneous firing of Adelta fibers221,227,228. Thus, a plausible pharmacological strategy would be to find small molecule enhancers of Kv2.1-containing channels, either targeted to remaining Kv2.1/Kv9.1 channels or to whatever heteromeric combinations are favored after down-regulation of Kv9.1. Kv2.1 channels are also strongly expressed in small diameter C-fiber DRG neurons, where they are potentially associated with a variety of other silent subunits, including Kv6.1, Kv8.1, Kv9.2, and Kv9.3229. The potential for Kv2-based channels made by highly diverse combinations of subunits in different neuronal types, raises the possibility of finding small molecules with differential selectivity for particular subunit combinations that could preferentially reduce excitability of pain-signaling neurons. The possibility of pharmacologic selectivity targeted to different subunit combinations is validated by differential sensitivity to the broad-spectrum inhibitor 4-aminopyridine230, but this has not yet been systematically explored for enhancers of function.
Targeting ion channels in higher-order neurons
In neuropathic pain, there is very likely altered excitability and circuitry of higher-order nociceptive neurons in the spinal cord and brain. Little is known about the ion channels involved in controlling changes in excitability of high-order neurons, but as understanding increases, it may be possible to selectively target such activity. Agents that reduce sodium channel activity broadly without subtype selectivity, like amitryptiline, could well act in pain relief by modifying activity of higher-order neurons as well as primary sensory neurons (Fig 3).
Discussion of the mechanism of action of gabapentinoids has generally focused on synaptic transmission at first-order synapses in the spinal cord, since effects on trafficking of calcium channels has been studied in DRG neurons182, but such effects could also occur at higher-order synapses. Positive allosteric modulators of GABAA chloride channels could have potential as pain relievers231,232, but generally also produce strong sedation. It was recently found that flupirtine acts to enhance GABA-mediated currents at concentrations comparable to those enhancing Kv7 activity233. Because GABAA channels are highly diverse in terms of subunit structure (being pentameric combinations of 19 different potential subunits) with different combinations in different neurons and sub-cellular regions, which is still very incompletely understood, it may be possible to find agents with selectivity for specific subunit combinations that enhance pain-relief properties relative to sedation234. A better understanding of expression patterns of specific GABAA receptor subunit combinations in the higher-order neuronal pain-mediating circuitry would be useful for a rational approach in this direction.
Enzymes as analgesic drug targets
NSAIDs, the most widely used analgesics, target the enzyme cyclooxygenase and there is growing interest in targeting other enzymes in the development of novel analgesics (Table 3).
Table 3.
Ligands targeting various enzymes relevant to pain
| Target/Category | Name | Mechanism | Preclinical | Clinical status | References |
|---|---|---|---|---|---|
| SPR |
(Quartet Medicine) Sulfasalazine SPRi3 |
SPE inhibitors, BH4 pathway | Analgesia – CFA, CCI, SNI | ND | 236 |
| FAAH and monogycerol lipase |
(Various) PF-04457845 BIA 10-2474 |
endocannabinoid pathway | Analgesia in several models | several clinical trials failed | 254, 295–298 |
| mPGE synthase 1 |
(Pfizer) PF 4693627 (Eli Lily) LY3023703 |
PGE pathway | Inflammatory pain | LY3023703 in phase 2 study of post-dental surgical pain | 239, 299–301, 302 |
| iNOS |
(Pfizer) Cindunistat |
iNOS inhibitor | Significant reduction on OA lesions in rodent and canine models | No clinical efficacy in patients with OA | 303 |
| Glutamate carboxypeptidase II | E2072 2-MPPA |
inhibitors | Neuropathy, neuropathic pain | ND | 304,305 |
| D-amino acid oxidase | SUN | inhibitor | Neuropathic pain, bone cancer pain | ND | 306 |
| Leukocyte elastase |
(Ono Pharma) Sivelestat |
inhibitor | Neuropathic pain | Approved for use during acute respiratory distress, no clinical trials yet for neuropathic pain | 248 |
| p38 |
(GSK) Losmapimod |
inhibitor | Inhibition of neuropathic pain | No differentiation from placebo | 251 |
Key: OA – Osteoarthritis; ND – no data on clinical development
BH4 synthesis - sepiapterin reductase
Axonal injury increases the production of tetrahydrobiopterin (BH4) from GTP in injured neurons, an essential cofactor for aromatic amine hydroxylases, nitric oxide synthases and alkylglycerol monooxygenase, due to increased expression of GTP cyclohydrolase 1 (GCH1) the rate limiting enzyme in the BH4 pathway in the injured cells235. Nerve injury is also accompanied by increased BH4 synthesis in the macrophages that infiltrate proximal to the injury236. Human genetic studies reveal an association between GCH1 polymorphisms which decrease BH4 levels, and reduced pain in patients, and decreasing BH4 levels by inhibiting or knocking out GCH1 in sensory neurons produces analgesia in rodents235,236. Inhibition of GCH1 does however create a risk of adverse effects in the nervous and cardiovascular systems due to decreased synthesis of biogenic amines and NO if BH4 falls excessively below basal levels. An alternative strategy to minimize the risk of side effects is targeting sepiapterin reductase (SPR), the terminal enzyme in the BH4 synthetic pathway, whose blockade allows enough BH4 production through the BH4 salvage pathway to prevent adverse effects, while nevertheless reducing the elevated levels that contribute to neuropathic and inflammatory pain. SPR inhibition produces analgesia in multiple acute and chronic neuropathic and inflammatory pain models with no tolerance and no effect on acute nociception236. One attractive feature of SPR inhibition for drug development is that target engagement can be determined by measuring sepiapterin levels in plasma and biomarker of efficacy by a reduction in BH4 levels236. Interestingly, a yeast three hybrid screen using drugs with clinical activity but no known target led to the discovery that sulfasalazine, an FDA approved drug used to reduce inflammation and its associated pain in rheumatoid arthritis and inflammatory bowel disease, is an SPR inhibitor, although with very low potency237. Quartet Medicine, through a strategic partnership with Merck, are developing novel SPR inhibitors for the treatment of neuropathic and inflammatory pain.
Microsomal prostaglandin E2 synthase-1
Microsomal prostaglandin E2 synthase-1 is responsible for PGE2 production during inflammation as the terminal enzyme in the prostanoid pathway, acting downstream of cyclooxygenase 2238. PGE2 is a major inflammatory sensitizer of nociceptors, and mPGES1 inhibition using MF-63 [2-(6-chloro-1H-phenanthro-[9,10-d]imidazol-2-yl)isophthalonitrile] and two novel 2-Acylaminoimidazole inhibitors has been shown to reduce inducible PGE synthesis without suppressing prostacyclin generation. The two novel mPGES1 inhibitors produced analgesia equivalent to diclofenac in a guinea pig model of knee joint pain (mono iodoacetate model of arthritis). mPGES1 inhibition therefore represents a novel approach for the management of inflammatory pain, likely without the adverse cardiovascular (myocardial infarction) and gastrointestinal bleeding adverse effects of COX2 inhibitors239.
Soluble epoxide hydrolase
Epoxyeicosatrienoic acids (EETs) are cytochrome P450-epoxygenase-derived metabolites of arachidonic acid that act as endogenous signaling molecules in multiple biological systems. Soluble epoxide hydrolase (sEH) is the enzyme that degrades EETs, whose actions involve analgesia. An inhibitor of sEH, trans-4-[4-(3-trifluoromethoxyphenyl-1-ureido)-cyclohexyloxy]-benzoic acid (t-TUCB) stabilizes bioactive mediators, epoxy fatty acids (EpFAs) that have low-abundance, but are highly potent bioactive lipids that maintain homeostasis and have analgesic action in a mouse model of diabetic neuropathy240. EpFAs act as upstream modulators of ER stress pathways, which turn out to be engaged in diabetic neuropathy and whose reduction leads to analgesia 241. However as some EETs sensitize nociceptors242, sEH inhibition is likely to increase inflammatory pain243. This would likely complicate development of these inhibitors as analgesics, as inflammatory and neuropathic pain may coexist.
Nascent emerging mechanisms
Proteases
Several proteases – serine proteases (trypsin), matrix metalloproteinases (MMPs), cysteine proteases (cathepsin S), caspases and protease-activated receptor PAR2 have been implicated in pain modulation. Elevated levels of serine proteases and PAR2 have been observed in mice with cancer pain, which is attenuated by inhibition of protease levels or completely eliminated in mice deficient in PAR2244. Transient elevation of MMP9 and delayed, but persistent elevation of MMP2 levels have been shown to induce and maintain neuropathic pain in rodents245. The same study also showed that MMP inhibitors can produce antinociception, suggesting that MMPs may be a target for developing therapeutics. It should be noted however that MMPs are also involved in several other pathways and broad spectrum inhibitors can cause toxic side effects. Indeed, clinical trials with MMP inhibitors for cancer have failed due to toxicity and lack of efficacy. Similarly, both caspase 6 and cathepsin S have been shown to regulate neuron-glial interactions during neuropathic pain and potentially lead to central sensitization246,247.
SerpinA3N, an endogenous serine protease inhibitor, is upregulated in injured sensory neurons after axonal injury and appears to protect against neuropathic pain in those animals where the degree of upregulation is high. Mice lacking SerpinA3N develop more mechanical allodynia after nerve injury than wild-type mice, and exogenous delivery of SerpinA3N attenuates mechanical hypersensitivity248. SerpinA3N inhibits leukocyte elastase (LE) which is released by the T lymphocytes that infiltrate the DRG after nerve injury. Either genetic loss of LE or an LE inhibitor (Sivelestat) has been shown to attenuate mechanical allodynia in neuropathic models248. These findings reveal complex cross talk between injured sensory neurons and immune cells and identifies a novel immune cell target for further evaluation as an analgesic for neuropathic pain.
Challenges and considerations in the clinical development of novel analgesics
The assessment of failure in proof-of-principle efficacy clinical trials for novel analgesics is commonly beset by insufficient reporting by industry and in consequence it is often uncertain if the wrong target, drug, outcome measure or patient was selected, if there was a failure of target engagement, or too strong a placebo response. If nothing is learned by these Phase II trials it is cumulatively an enormous waste of resources and some drugs may be prematurely abandoned.
Particularly worrying is the possibility that preclinical models are not true surrogates of human disease and that reflexive stimulus-evoked “pain” measurement may not be sensitive predictors of efficacy in patients (Box 1). In many cases, adverse effects mean that it is simply not possible to achieve in humans the drug exposure levels required in animals to show efficacy. In addition, a large placebo effect and emotional control of pain, amongst other reasons, further complicate analgesic clinical trial design (Box 2).
Box 2. Pain clinical trial design.
Phase 2 proof of concept clinical trials for novel analgesics are beset by a number of major problems; how to measure the pain, how to differentiate placebo from drug effect, and which patients to select. Pain is a subjective experience influenced not only by heritable traits but also by psychosocial processes, culture, experience, social and biological context and mood272. Translating this dynamic complex internal experience into verbal descriptors, numerical or analog scales of intensity, may not capture comparable levels of sensation between or within subjects, even with training273, and certainly does not reflect the degree or nature of the precipitating initiator or maintainer of the pain. In addition, the high level of placebo and nocebo responders in chronic pain trials is a major confounder274. Attempts are being made to deal with bias and unreliability, most notably by ACTTION16, the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks a public-private partnership with the United States Food and Drug Administration (FDA). Trial design can be improved by better diagnostics, blinding and choice of primary outcome measure, adaptive designs, crossovers, and random withdrawal, use of adequate power and responder analyses275. However, there remains the problem that lack of predictive biomarkers either of pain or of target engagement makes it difficult to understand the underlying pathophysiology that leads to various clinical presentations of pain and if a drug is having any action, although new imaging technology may change this276. While preclinical pain research has evolved into classifying different types of pain based on mechanism, little of this has translated into pain diagnosis in the clinic13. Recent studies have proposed several novel ways to approach this issue: a) a successive classification methodology can be used to identify the pain state, pain mechanism and molecular target in sequence - emphasizing the development of individualized therapy or precision medicine13, b) identifying clinically relevant patient clusters based on pathophysiological mechanisms267, and in a similar vein c) matching patients based on phenotypic characteristics predictive of individual variation277. These efforts are a first effort to classify clinical pain in a fashion that lends itself to improve clinical trial design and to meaningful pharmacological intervention and where efficacy can be readily identified and quantified. This will be critical because if a drug is acting on a target that plays a role only in certain mechanisms that operate to produce pain, then clinical trials will be more successful if the presence of those mechanisms can be identified in the patient inclusion criteria to enrich for potential responders.
Illustrations of the challenges in assessing novel analgesic action can be gleaned from some recent pain trials exploring novel “pain” targets:
Predictive failure of preclinical models
Rodents, especially mice, remain the preclinical model organism of choice for validation of targets and then testing their ligands, typically using pain-related behavioral measures. Since many neurobiological and disease mechanisms and pathways are evolutionarily conserved, much useful information can be gleaned by utilizing wildtype or genetically modified animals. However major problems remain, first there is considerable debate as to the relevance of reflexively evoked responses to noxious stimuli to clinical pain conditions, especially spontaneous pain and changes in cognitive function and mood. Secondly many of the pain models are not good surrogates of human disease (Box 1 and Box 2). Inflaming or damaging a joint, for example, does not make a good model of osteoarthritis. Below are a few examples where preclinical efficacy could not be replicated in human clinical trials.
Propentofylline
The glial modulating agent propentofylline failed in a study conducted by Solace Pharmaceuticals to decrease pain in patients with post-herpetic neuralgia, in spite of some activity in rodent peripheral neuropathic models. The likely explanation for this failure could be a major species difference between rodents and humans, both in microglial activation and in propentofylline action249. The involvement of microglia and astrocytes in pain in humans remains then to be tested and the failure of propentofylline indicates the importance both of human target validation, most commonly by genetics, and of drug action on human cells.
p38 pathway inhibition
The p38 MAP kinase inhibitor losmapimod exhibited no efficacy in patients with neuropathic pain from lumbosacral radiculopathy, despite preclinical studies showing involvement of p38 signaling pathways in the development of persistent pain after peripheral nerve injury250. It is uncertain if the trial failed because of a lack of adequate target engagement or differences between chronic (years) lumbosacral radiculopathy and much more acute animal models of neuropathic pain (days/weeks)251. Because the preclinical data was not aligned with the patient cohort tested, the significance of the failed trial for other pain types is uncertain, which is true for most neuropathic pain trials.. However, p38 activation is downstream of NGF, a validated target for novel analgesics (see below) and for angiotensin, which appears to act in the periphery to block TRPV1 sensitization96. Notably, an angiotensin type 2 receptor (AT2R) antagonist EMA401 has efficacy in postherpetic neuralgia98, representing the first drug acting on a novel target to show efficacy in neuropathic pain for some time. At the time that EMA401 was tested its analgesic mechanism of action was uncertain100,252. TRPV1 antagonists however, have had to be abandoned because of on-target hyperthermia253.
Achieving CNS penetration
Many of the targets relevant to pain are expressed in the central nervous system (CNS). However, the blood brain barrier is a barrier for ligands of certain chemical structures to effectively penetrate into the CNS and reach their targets or are actively pumped out of the brain even if they do enter. This is difficult to predict and can lead to failed clinical trials.
The endocannabinoids anandamide and 2-arachidonoylglycerol, acting at CB receptors, are metabolized by hydrolytic enzymes (fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL)) and by oxygenating enzymes (cyclooxygenase-2, COX-2). An irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, failed to reduce pain from osteoarthritis of the knee, in spite of decreasing FAAH activity by >96% and substantially increasing fatty acid amide endogenous substrates, and many preclinical studies showing efficacy in models of inflammatory pain254. In spite of this FAAH failure, others are entering clinical trials255,256 and there is preliminary human genetic support for FAAH involvement in cold pain sensitivity257. Was the failure of the Pfizer compound a failure to access FAAH in the CNS? Certainly, cannabinoid agonists (CB1) have major actions in humans including claims of analgesia85,86. Clinical use of CB1 agonists or cannabis is complicated by the drug abuse liability from psychoactive effects, hence the theoretical attraction of elevating endocannabinoids.
Adverse effects when targeting protective pain mechanisms
While chronic pain can be very debilitating and reduce quality of life, in several clinical conditions ongoing pain protects damaged tissue from further insult. For example, Anti-NGF antibodies (tanezumab, fulranumab and fasinumab) have proven in a very unusual twist to be more efficacious in patients with osteoarthritis (OA) than the preclinical data would have expected258, with greater signals than gold standard NSAIDs and opioids for chronic low back pain – sometimes thought of as the graveyard of analgesic trials – as well as in OA and diabetic neuropathy259–261. Conversely, tenezumab has been found to produce rapid progression of OA particularly when administered at high doses and with NSAIDs262. While the cause of this is uncertain, one possibility is that overuse of damaged joints because of high analgesia coverage may lead to accelerating joint destruction – pain is after all a protective mechanism as well as a disease burden. Additional concern comes from the growth factor role of NGF in development. In pregnant cynomolgus monkeys intravenous tenezumab increased stillbirth and infant mortality, decreased infant growth with changes in the peripheral sympathetic and sensory nervous system including developmental neurotoxicity263,264. This risk, as well as the possibility of some neurotoxicity in adults will need to be carefully monitored and excluded.
Conclusions – the road ahead
There are several promising emerging targets for the treatment of acute and chronic pain. However, the key question is still whether preclinical efficacy data will translate to humans, and if not, why not. While high-throughput screening (HTS) in heterologous cell lines generates selective ligands, the lack of normal pathways that affect the ‘target of interest’ in its native cellular environment may lead to the selection of suboptimal ligands. In this context, phenotypic screens performed directly in human neurons differentiated from patient iPSC lines may help in greatly enhancing the quality of patient-relevant data146,265. A similar argument exists with many of the commonly used reflexive-avoidance pain behavioral models in rodents (Box 1). Given that rodents are prey animals, their behavior can be highly influenced by environment and investigator266. Together, these issues suggest that we need to make a significant effort to develop assays that can measure spontaneous pain automatically, without the presence of any investigator and model human disease conditions as closely as possible (Box 1).
Finally, focusing on targets that already have human genetic data supporting involvement in pain, like Nav1.7, may provide less risky opportunities to develop new effective analgesics than reliance only on rodent based models of pain. However, it may be a mistake to study only targets with human genetic data, since many targets of the most widely clinically used drugs would not have been selected based on genetic variants producing clear disease related phenotypes. In addition, large rare phenotypes such as insensitivity to pain may not be useful for identifying targets engaged in clinical conditions, such as after damage to the nervous system or in response to chronic inflammation.
In spite of this, combinations of phenotypic screens in patient-derived neurons, human genetics, genome editing in mice and human cell lines, as well as improvements in drug design and kinetics, identification of biomarkers and in clinical trial design (Box 2), together all offer an increasing probability of success, something “sorely” needed. As new therapeutic opportunities arise from such efforts, they will add to the armamentarium of pain physicians creating opportunity for a Precision Medicine approach, where treatment can be targeted at the particular mechanisms driving the pain present in individual patients267.
References
- 1.Dubois MY, Gallagher RM, Lippe PM. Pain medicine position paper. Pain Med. 2009;10:972–1000. doi: 10.1111/j.1526-4637.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 2.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Opioid overdose deaths. Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 5.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 6.Honore P, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 7.Costigan M, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain: a journal of neurology. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardeh D, Mannion RJ, Woolf CJ. Toward a Mechanism-Based Approach to Pain Diagnosis. J Pain. 2016;17:T50–69. doi: 10.1016/j.jpain.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 15.Andrews NA, et al. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain. 2016;157:901–909. doi: 10.1097/j.pain.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singla N, et al. Assay sensitivity of pain intensity versus pain relief in acute pain clinical trials: ACTTION systematic review and meta-analysis. J Pain. 2015;16:683–691. doi: 10.1016/j.jpain.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolf CJ. Pain: morphine, metabolites, mambas, and mutations. Lancet Neurol. 2013;12:18–20. doi: 10.1016/S1474-4422(12)70287-9. [DOI] [PubMed] [Google Scholar]
- 21.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 22.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 25.Michaud K, Bombardier C, Emery P. Quality of life in patients with rheumatoid arthritis: does abatacept make a difference? Clin Exp Rheumatol. 2007;25:S35–45. [PubMed] [Google Scholar]
- 26.Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007;117:3603–3609. doi: 10.1172/JCI33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hains BC, et al. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nassar MA, et al. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol Pain. 2006;2:33. doi: 10.1186/1744-8069-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong XW, et al. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146:812–821. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis MF, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekberg J, et al. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc Natl Acad Sci U S A. 2006;103:17030–17035. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold MS, et al. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi SK, et al. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123:75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550:921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritch PC, et al. Novel KCNQ2/Q3 agonists as potential therapeutics for epilepsy and neuropathic pain. Journal of medicinal chemistry. 2010;53:887–896. doi: 10.1021/jm901497b. [DOI] [PubMed] [Google Scholar]
- 37.Dost R, Rostock A, Rundfeldt C. The anti-hyperalgesic activity of retigabine is mediated by KCNQ potassium channel activation. Naunyn-Schmiedeberg’s archives of pharmacology. 2004;369:382–390. doi: 10.1007/s00210-004-0881-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee S. Pharmacological Inhibition of Voltage-gated Ca(2+) Channels for Chronic Pain Relief. Current neuropharmacology. 2013;11:606–620. doi: 10.2174/1570159X11311060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stemkowski PL, Noh MC, Chen Y, Smith PA. Increased excitability of medium-sized dorsal root ganglion neurons by prolonged interleukin-1beta exposure is K(+) channel dependent and reversible. The Journal of physiology. 2015;593:3739–3755. doi: 10.1113/JP270905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol. 2013;27:64–80. doi: 10.1111/fcp.12008. [DOI] [PubMed] [Google Scholar]
- 41.Gutenstein HaAH. Goodman and Gilman’s Pharmacological basis of therapeutics. Ch. 21. The McGraw Hill companies, Inc; 2006. [Google Scholar]
- 42.Fries DS. In: Principles of Medicinal Chemsitry. Foye WO, Lemke TL, Williams DA, editors. Ch. 14. William & Wilkins; 1995. pp. 247–269. [Google Scholar]
- 43.Lesniak A, Lipkowski AW. Opioid peptides in peripheral pain control. Acta Neurobiol Exp (Wars) 2011;71:129–138. doi: 10.55782/ane-2011-1829. [DOI] [PubMed] [Google Scholar]
- 44.Kolodny A, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 36:559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 45.Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med. 2014;15:440–451. doi: 10.1111/pme.12295. [DOI] [PubMed] [Google Scholar]
- 46.Kunins HV. Abuse-deterrent opioid formulations: part of a public health strategy to reverse the opioid epidemic. JAMA Intern Med. 2015;175:987–988. doi: 10.1001/jamainternmed.2015.0939. [DOI] [PubMed] [Google Scholar]
- 47.Chilcoat HD, Coplan PM, Harikrishnan V, Alexander L. Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin) Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Coplan PM, et al. The Effect of an Abuse-Deterrent Opioid Formulation on Opioid Abuse-Related Outcomes in the Post-Marketing Setting. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012;367:187–189. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- 50.Cicero TJ, Ellis MS, Kasper ZA. A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. Pain. 2016;157:1232–1238. doi: 10.1097/j.pain.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 51.Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- 52.Pallasch TJ, Gill CJ. Butorphanol and nalbuphine: a pharmacologic comparison. Oral Surg Oral Med Oral Pathol. 1985;59:15–20. doi: 10.1016/0030-4220(85)90108-2. [DOI] [PubMed] [Google Scholar]
- 53.Webster L, Menzaghi F, Spencer R. CR845, a novel peripherally-acting kappa opioid receptor agonist, has low abuse potential compared with pentazocine. Journal of Pain. 2015;16:S81. doi: http://dx.doi.org/10.1016/j.jpain.2015.01.340. [Google Scholar]
- 54.Milligan G. The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization. Mol Pharmacol. 2013;84:158–169. doi: 10.1124/mol.113.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yekkirala AS. Two to tango: GPCR oligomers and GPCR-TRP channel interactions in nociception. Life Sci. 2013;92:438–445. doi: 10.1016/j.lfs.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem Biol. 2013;8:1412–1416. doi: 10.1021/cb400113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 59.Aceto MD, et al. MDAN-21: A Bivalent Opioid Ligand Containing mu-Agonist and Delta-Antagonist Pharmacophores and Its Effects in Rhesus Monkeys. Int J Med Chem. 2012;2012:327257. doi: 10.1155/2012/327257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniels DJ, et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Naour M, et al. Bivalent ligands that target mu opioid (MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance. J Med Chem. 2013;56:5505–5513. doi: 10.1021/jm4005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akgun E, et al. Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proc Natl Acad Sci U S A. 2013;110:11595–11599. doi: 10.1073/pnas.1305461110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akgun E, et al. Inhibition of Inflammatory and Neuropathic Pain by Targeting a Mu Opioid Receptor/Chemokine Receptor5 Heteromer (MOR-CCR5) J Med Chem. 2015;58:8647–8657. doi: 10.1021/acs.jmedchem.5b01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yekkirala AS, et al. N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc Natl Acad Sci U S A. 2011;108:5098–5103. doi: 10.1073/pnas.1016277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakrabarti S, Liu NJ, Gintzler AR. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A. 2010;107:20115–20119. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding H, et al. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1605295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marrone GF, et al. Truncated mu opioid GPCR variant involvement in opioid-dependent and opioid-independent pain modulatory systems within the CNS. Proc Natl Acad Sci U S A. 2016;113:3663–3668. doi: 10.1073/pnas.1523894113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Majumdar S, et al. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci U S A. 2011;108:19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wieskopf JS, et al. Broad-spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene. Pain. 2014;155:2063–2070. doi: 10.1016/j.pain.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu XY, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White KL, et al. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric modulation of beta-arrestin-biased angiotensin II type 1 receptor signaling by membrane stretch. J Biol Chem. 2014;289:28271–28283. doi: 10.1074/jbc.M114.585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drake MT, et al. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 75.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukla AK, et al. Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci U S A. 2008;105:9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gesty-Palmer D, et al. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strachan RT, et al. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR) J Biol Chem. 2014;289:14211–14224. doi: 10.1074/jbc.M114.548131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci. 2014;35:308–316. doi: 10.1016/j.tips.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Soergel DG, et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155:1829–1835. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 83.Soergel DG, et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54:351–357. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]
- 84.Manglik A, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tabrizi MA, Baraldi PG, Borea PA, Varani K. Medicinal Chemistry, Pharmacology, and Potential Therapeutic Benefits of Cannabinoid CB2 Receptor Agonists. Chemical Reviews. 2016;116:519–560. doi: 10.1021/acs.chemrev.5b00411. [DOI] [PubMed] [Google Scholar]
- 86.Han S, Thatte J, Buzard DJ, Jones RM. Therapeutic utility of cannabinoid receptor type 2 (CB(2)) selective agonists. J Med Chem. 2013;56:8224–8256. doi: 10.1021/jm4005626. [DOI] [PubMed] [Google Scholar]
- 87.Ostenfeld T, et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27:668–676. doi: 10.1097/AJP.0b013e318219799a. [DOI] [PubMed] [Google Scholar]
- 88.
- 89.Kalliomäki J, et al. Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scandanavian Journal of Pain. 2013;4:17–22. doi: 10.1016/j.sjpain.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Nevalainen T. Recent development of CB2 selective and peripheral CB1/CB2 cannabinoid receptor ligands. Curr Med Chem. 2014;21:187–203. doi: 10.2174/09298673113206660296. [DOI] [PubMed] [Google Scholar]
- 91.Arena Pharmaceuticals Reports Favorable Results from Phase 1b Multiple-Ascending Dose Clinical Trial of APD37. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=964598.
- 92.Eli Lilly and Company. ClinicalTrials.gov. US National Library of Medicine; A Study of LY2828360 in Patients With Osteoarthritic Knee Pain. https://clinicaltrials.gov/ct2/show/NCT01319929. Identifier: NCT01319929. [Google Scholar]
- 93.Shionogi Inc. ClinicalTrials.gov. US National Library of Medicine; A Phase Ib/IIa, Double-Blind, Randomized Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of S-777469 in Subjects With Atopic Dermatitis. https://clinicaltrials.gov/ct2/show/NCT00697710. Identifier: NCT0069771. [Google Scholar]
- 94.Kyowa Hakko Kirin Company. ClinicalTrials.gov. US National Library of Medicine; A Comparative Study of KHK6188. https://clinicaltrials.gov/ct2/show/NCT01544296. Identifier: NCT01544296. [Google Scholar]
- 95.Cara Therapeutics, Inc. ClinicalTrials.gov. US National Library of Medicine; A Phase 2 Study to Evaluate Analgesic Effect of IV CR845 For Pain Following Bunionectomy Surgery. https://clinicaltrials.gov/ct2/show/NCT01789476. Identifier: NCT01789476. [Google Scholar]
- 96.Anand U, et al. Mechanisms underlying clinical efficacy of Angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: clinical tissue and in vitro studies. Mol Pain. 2015;11:38. doi: 10.1186/s12990-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Danser AH, Anand P. The angiotensin II type 2 receptor for pain control. Cell. 2014;157:1504–1506. doi: 10.1016/j.cell.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 98.Rice AS, et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–1647. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- 99.Anand U, et al. Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain. 2013;17:1012–1026. doi: 10.1002/j.1532-2149.2012.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith MT, Woodruff TM, Wyse BD, Muralidharan A, Walther T. A small molecule angiotensin II type 2 receptor (AT(2)R) antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen-activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. Pain Med. 2013;14:1557–1568. doi: 10.1111/pme.12157. [DOI] [PubMed] [Google Scholar]
- 101.Marion E, et al. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell. 2014;157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 102.Lemmens S, Brone B, Dooley D, Hendrix S, Geurts N. Alpha-adrenoceptor modulation in central nervous system trauma: pain, spasms, and paralysis--an unlucky triad. Med Res Rev. 2015;35:653–677. doi: 10.1002/med.21337. [DOI] [PubMed] [Google Scholar]
- 103.Giovannitti JA, Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62:31–39. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mori K, et al. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43:1026–1034. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 105.Lavand’homme PM, Eisenach JC. Perioperative administration of the alpha2-adrenoceptor agonist clonidine at the site of nerve injury reduces the development of mechanical hypersensitivity and modulates local cytokine expression. Pain. 2003;105:247–254. doi: 10.1016/s0304-3959(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 106.Feng X, et al. Intrathecal administration of clonidine attenuates spinal neuroimmune activation in a rat model of neuropathic pain with existing hyperalgesia. Eur J Pharmacol. 2009;614:38–43. doi: 10.1016/j.ejphar.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 107.Wei H, Pertovaara A. Spinal and pontine alpha2-adrenoceptors have opposite effects on pain-related behavior in the neuropathic rat. Eur J Pharmacol. 2006;551:41–49. doi: 10.1016/j.ejphar.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 108.Schnabel A, Meyer-Friessem CH, Reichl SU, Zahn PK, Pogatzki-Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154:1140–1149. doi: 10.1016/j.pain.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 109.Schnabel A, et al. Efficacy and safety of intraoperative dexmedetomidine for acute postoperative pain in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2013;23:170–179. doi: 10.1111/pan.12030. [DOI] [PubMed] [Google Scholar]
- 110.DEX-IN PhII efficacy press release. http://ir.recropharma.com/press-releases/detail/27/recro-pharma-announces-positive-top-line-results-for-phase.
- 111.DEX-IN PhII side effects press release. http://ir.recropharma.com/press-releases/detail/29/recro-pharma-announces-additional-information-for-phase-ii.
- 112.Melik Parsadaniantz S, Rivat C, Rostene W, Reaux-Le Goazigo A. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci. 2015;16:69–78. doi: 10.1038/nrn3858. [DOI] [PubMed] [Google Scholar]
- 113.Abbadie C, et al. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reaux-Le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Rostene W, et al. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem. 2011;118:680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- 116.Van Steenwinckel J, et al. Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun. 2015;45:198–210. doi: 10.1016/j.bbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 117.Van Steenwinckel J, et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31:5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oh SB, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie F, Wang Y, Li X, Chao YC, Yue Y. Early Repeated Administration of CXCR4 Antagonist AMD3100 Dose-Dependently Improves Neuropathic Pain in Rats After L5 Spinal Nerve Ligation. Neurochem Res. 2016;41:2289–2299. doi: 10.1007/s11064-016-1943-8. [DOI] [PubMed] [Google Scholar]
- 122.Talbot S, Foster SL, Woolf CJ. Neuroimmunity: Physiology and Pathology. Annu Rev Immunol. 2016;34:421–447. doi: 10.1146/annurev-immunol-041015-055340. [DOI] [PubMed] [Google Scholar]
- 123.Szabo I, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99:10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szabo I, et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol. 2003;74:1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- 125.Grimm MC, et al. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pello OM, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–549. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- 127.Rivat C, et al. Src family kinases involved in CXCL12-induced loss of acute morphine analgesia. Brain Behav Immun. 2014;38:38–52. doi: 10.1016/j.bbi.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 128.Zhao CM, et al. Spinal MCP-1 contributes to the development of morphine antinociceptive tolerance in rats. Am J Med Sci. 2012;344:473–479. doi: 10.1097/MAJ.0b013e31826a82ce. [DOI] [PubMed] [Google Scholar]
- 129.Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J Immunol. 2004;173:594–599. doi: 10.4049/jimmunol.173.1.594. [DOI] [PubMed] [Google Scholar]
- 130.Ye D, et al. Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: involvement of Gi protein. J Mol Neurosci. 2014;53:571–579. doi: 10.1007/s12031-013-0223-1. [DOI] [PubMed] [Google Scholar]
- 131.Rittner HL, et al. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J. 2006;20:2627–2629. doi: 10.1096/fj.06-6077fje. [DOI] [PubMed] [Google Scholar]
- 132.Wilson NM, Jung H, Ripsch MS, Miller RJ, White FA. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun. 2011;25:565–573. doi: 10.1016/j.bbi.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kalliomaki J, et al. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain. 2013;154:761–767. doi: 10.1016/j.pain.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 134.Padi SS, et al. Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain. 2012;153:95–106. doi: 10.1016/j.pain.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 135.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. nrd2280 [pii] [DOI] [PubMed] [Google Scholar]
- 136.Habib AM, Wood JN, Cox JJ. Sodium channels and pain. Handbook of Experimental Pharmacology. 2015;227:39–56. doi: 10.1007/978-3-662-46450-2_3. [DOI] [PubMed] [Google Scholar]
- 137.Waxman SG, et al. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. The Lancet. Neurology. 2014;13:1152–1160. doi: 10.1016/S1474-4422(14)70150-4. [DOI] [PubMed] [Google Scholar]
- 138.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 139.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- 140.Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 141.Carnevale V, Rohacs T. TRPV1: A Target for Rational Drug Design. Pharmaceuticals (Basel) 2016;9 doi: 10.3390/ph9030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lehto SG, et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluorom ethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326:218–229. doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]
- 143.Watabiki T, et al. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther. 2011;336:743–750. doi: 10.1124/jpet.110.175570. [DOI] [PubMed] [Google Scholar]
- 144.Chiche D, Brown W, Walker P. NEO6860, a novel modality selective TRPV1 antagonist: results from a phase I, double-blind, placebo-controlled study in healthy subjects. Journal of Pain. 2016;17:S79. doi: http://dx.doi.org/10.1016/j.jpain.2016.01.397. [Google Scholar]
- 145.Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534:347–351. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cao L, et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med. 2016;8:335ra356. doi: 10.1126/scitranslmed.aad7653. [DOI] [PubMed] [Google Scholar]
- 147.Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nature reviews. Neuroscience. 2013;14:49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- 148.Cox JJ, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. nature05413 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cox JJ, et al. Congenital insensitivity to pain: novel SCN9A missense and in-frame deletion mutations. Human mutation. 2010;31:E1670–1686. doi: 10.1002/humu.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nassar MA, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Minett MS, et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nature communications. 2012;3:791. doi: 10.1038/ncomms1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gingras J, et al. Global Nav1.7 knockout mice recapitulate the phenotype of human congenital indifference to pain. PloS one. 2014;9:e105895. doi: 10.1371/journal.pone.0105895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bagal SK, Marron BE, Owen RM, Storer RI, Swain NA. Voltage gated sodium channels as drug discovery targets. Channels (Austin, Tex) 2015;9:360–366. doi: 10.1080/19336950.2015.1079674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun S, Cohen CJ, Dehnhardt CM. Inhibitors of voltage-gated sodium channel Nav1.7: patent applications since 2010. Pharmaceutical patent analyst. 2014;3:509–521. doi: 10.4155/ppa.14.39. [DOI] [PubMed] [Google Scholar]
- 155.Focken T, et al. Discovery of Aryl Sulfonamides as Isoform-Selective Inhibitors of NaV1.7 with Efficacy in Rodent Pain Models. ACS medicinal chemistry letters. 2016;7:277–282. doi: 10.1021/acsmedchemlett.5b00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McCormack K, et al. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2724–2732. doi: 10.1073/pnas.1220844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raxatrigine press release. http://www.pharmaceutical-journal.com/news-and-analysis/features/a-new-channel-for-pain-treatment/20200841.article.
- 158.Murray JK, et al. Single Residue Substitutions That Confer Voltage-Gated Sodium Ion Channel Subtype Selectivity in the NaV1.7 Inhibitory Peptide GpTx- 1. Journal of medicinal chemistry. 2016;59:2704–2717. doi: 10.1021/acs.jmedchem.5b01947. [DOI] [PubMed] [Google Scholar]
- 159.Weiss J, et al. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature. 2011;472:186–190. doi: 10.1038/nature09975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Minett MS, et al. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nature communications. 2015;6:8967. doi: 10.1038/ncomms9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kort ME, et al. Subtype-selective Na(v)1.7 sodium channel blockers: identification of potent, orally active nicotinamide derivatives. Bioorganic & medicinal chemistry letters. 2010;20:6812–6815. doi: 10.1016/j.bmcl.2010.08.121. [DOI] [PubMed] [Google Scholar]
- 162.Bagal SK, et al. Recent progress in sodium channel modulators for pain. Bioorg Med Chem Lett. 2014;24:3690–3699. doi: 10.1016/j.bmcl.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 163.Wilson MJ, et al. mu-Conotoxins that differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10302–10307. doi: 10.1073/pnas.1107027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. The Journal of physiology. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. jphysiol.2006.121483 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dib-Hajj SD, Black JA, Waxman SG. NaV1.9: a sodium channel linked to human pain. Nature reviews. Neuroscience. 2015;16:511–519. doi: 10.1038/nrn3977. [DOI] [PubMed] [Google Scholar]
- 166.Goral RO, Leipold E, Nematian-Ardestani E, Heinemann SH. Heterologous expression of NaV1.9 chimeras in various cell systems. Pflugers Arch. 2015;467:2423–2435. doi: 10.1007/s00424-015-1709-1. [DOI] [PubMed] [Google Scholar]
- 167.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annual Review of Physiology. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 168.Hellwig N, et al. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. The Journal of biological chemistry. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- 169.Meyers JR, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. 23/10/4054 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. nature06191 [pii] [DOI] [PubMed] [Google Scholar]
- 171.Puopolo M, et al. Permeation and block of TRPV1 channels by the cationic lidocaine derivative QX-314. Journal of neurophysiology. 2013;109:1704–1712. doi: 10.1152/jn.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brenneis C, et al. Bupivacaine-induced cellular entry of QX-314 and its contribution to differential nerve block. British journal of pharmacology. 2014;171:438–451. doi: 10.1111/bph.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nature neuroscience. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 174.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nature neuroscience. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 175.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. The Journal of general physiology. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Binshtok AM, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. British journal of pharmacology. 2011;164:48–58. doi: 10.1111/j.1476-5381.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leffler A, et al. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. The Journal of clinical investigation. 2008;118:763–776. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Leffler A, Lattrell A, Kronewald S, Niedermirtl F, Nau C. Activation of TRPA1 by membrane permeable local anesthetics. Molecular pain. 2011;7:62-8069-8067-8062. doi: 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roberson DP, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nature neuroscience. 2013;16:910–918. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Talbot S, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bauer CS, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacological reviews. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nature reviews. Drug discovery. 2016;15:19–34. doi: 10.1038/nrd.2015.5. [DOI] [PubMed] [Google Scholar]
- 185.Abbadie C, et al. Analgesic effects of a substituted N-triazole oxindole (TROX-1), a state-dependent, voltage-gated calcium channel 2 blocker. J Pharmacol Exp Ther. 2010;334:545–555. doi: 10.1124/jpet.110.166363. [DOI] [PubMed] [Google Scholar]
- 186.Patel R, et al. Electrophysiological characterization of activation state-dependent Ca(v)2 channel antagonist TROX-1 in spinal nerve injured rats. Neuroscience. 2015;297:47–57. doi: 10.1016/j.neuroscience.2015.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shao PP, et al. Aminopiperidine sulfonamide Cav2.2 channel inhibitors for the treatment of chronic pain. J Med Chem. 2012;55:9847–9855. doi: 10.1021/jm301056k. [DOI] [PubMed] [Google Scholar]
- 188.Lipscombe D, Andrade A. Calcium Channel CaValpha(1) Splice Isoforms - Tissue Specificity and Drug Action. Current molecular pharmacology. 2015;8:22–31. doi: 10.2174/1874467208666150507103215. CMP-EPUB-67163 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bourinet E, et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. The EMBO journal. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. 7600515 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Choi S, et al. Attenuated pain responses in mice lacking Ca(V)3.2 T-type channels. Genes, brain, and behavior. 2007;6:425–431. doi: 10.1111/j.1601-183X.2006.00268.x. GBB268 [pii] [DOI] [PubMed] [Google Scholar]
- 191.Jarvis MF, et al. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochemical pharmacology. 2014;89:536–544. doi: 10.1016/j.bcp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 192.Wallace M, Duan R, Liu W, Locke C, Nothaft W. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study of the T-Type Calcium Channel Blocker ABT-639 in an Intradermal Capsaicin Experimental Pain Model in Healthy Adults. Pain medicine (Malden, Mass) 2015 doi: 10.1093/pm/pnv068. pnv068 [pii] [DOI] [PubMed] [Google Scholar]
- 193.Ziegler D, Duan WR, An G, Thomas JW, Nothaft W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain. 2015;156:2013–2020. doi: 10.1097/j.pain.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Francois A, et al. State-dependent properties of a new T-type calcium channel blocker enhance Ca(V)3.2 selectivity and support analgesic effects. Pain. 2013;154:283–293. doi: 10.1016/j.pain.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 195.Xu J, et al. A mixed Ca2+ channel blocker, A-1264087, utilizes peripheral and spinal mechanisms to inhibit spinal nociceptive transmission in a rat model of neuropathic pain. J Neurophysiol. 2014;111:394–404. doi: 10.1152/jn.00463.2013. [DOI] [PubMed] [Google Scholar]
- 196.Zhu CZ, et al. Mechanistic insights into the analgesic efficacy of A-1264087, a novel neuronal Ca(2+) channel blocker that reduces nociception in rat preclinical pain models. J Pain. 2014;15:387 e381–314. doi: 10.1016/j.jpain.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 197.Scott VE, et al. A-1048400 is a novel, orally active, state-dependent neuronal calcium channel blocker that produces dose-dependent antinociception without altering hemodynamic function in rats. Biochem Pharmacol. 2012;83:406–418. doi: 10.1016/j.bcp.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 198.Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K(+) channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Molecular and cellular neurosciences. 2012;49:375–386. doi: 10.1016/j.mcn.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pollema-Mays SL, Centeno MV, Ashford CJ, Apkarian AV, Martina M. Expression of background potassium channels in rat DRG is cell-specific and down-regulated in a neuropathic pain model. Molecular and cellular neurosciences. 2013;57:1–9. doi: 10.1016/j.mcn.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zheng Q, et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 201.Laumet G, et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nature neuroscience. 2015;18:1746–1755. doi: 10.1038/nn.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu R, et al. Slack channels expressed in sensory neurons control neuropathic pain in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:1125–1135. doi: 10.1523/JNEUROSCI.2423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lyu C, et al. G protein-gated inwardly rectifying potassium channel subunits 1 and 2 are down-regulated in rat dorsal root ganglion neurons and spinal cord after peripheral axotomy. Molecular pain. 2015;11 doi: 10.1186/s12990-015-0044-z. 44-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maljevic S, Lerche H. Potassium channels: a review of broadening therapeutic possibilities for neurological diseases. Journal of neurology. 2013;260:2201–2211. doi: 10.1007/s00415-012-6727-8. [DOI] [PubMed] [Google Scholar]
- 205.Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends in neurosciences. 2014;37:146–158. doi: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsantoulas C. Emerging potassium channel targets for the treatment of pain. Current opinion in supportive and palliative care. 2015;9:147–154. doi: 10.1097/SPC.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 207.Wickenden AD, McNaughton-Smith G. Kv7 channels as targets for the treatment of pain. Current pharmaceutical design. 2009;15:1773–1798. doi: 10.2174/138161209788186326. [DOI] [PubMed] [Google Scholar]
- 208.Wu YJ, et al. Discovery of (S,E)-3-(2-fluorophenyl)-N-(1-(3-(pyridin-3-yloxy)phenyl)ethyl)-acrylamide as a potent and efficacious KCNQ2 (Kv7.2) opener for the treatment of neuropathic pain. Bioorganic & medicinal chemistry letters. 2013;23:6188–6191. doi: 10.1016/j.bmcl.2013.08.092. [DOI] [PubMed] [Google Scholar]
- 209.Zheng Y, et al. Activation of peripheral KCNQ channels relieves gout pain. Pain. 2015;156:1025–1035. doi: 10.1097/j.pain.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mathie A, Veale EL. Two-pore domain potassium channels: potential therapeutic targets for the treatment of pain. Pflugers Archiv: European journal of physiology. 2015;467:931–943. doi: 10.1007/s00424-014-1655-3. [DOI] [PubMed] [Google Scholar]
- 211.Wang HS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science (New York, NY) 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 212.Pan Z, et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. 26/10/2599 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cooper EC. Made for “anchorin”: Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons. Seminars in cell & developmental biology. 2011;22:185–192. doi: 10.1016/j.semcdb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. European journal of pharmacology. 2003;460:109–116. doi: 10.1016/s0014-2999(02)02924-2. S0014299902029242 [pii] [DOI] [PubMed] [Google Scholar]
- 215.Li H, et al. Antinociceptive Efficacy of Retigabine in the Monosodium Lodoacetate Rat Model for Osteoarthritis Pain. Pharmacology. 2015;95:251–257. doi: 10.1159/000381721. [DOI] [PubMed] [Google Scholar]
- 216.Retigabine Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT00612105.
- 217.Rose K, et al. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 2011;152:742–754. doi: 10.1016/j.pain.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.King CH, Lancaster E, Salomon D, Peles E, Scherer SS. Kv7.2 regulates the function of peripheral sensory neurons. The Journal of comparative neurology. 2014;522:3262–3280. doi: 10.1002/cne.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.King CH, Scherer SS. Kv7.5 is the primary Kv7 subunit expressed in C-fibers. The Journal of comparative neurology. 2012;520:1940–1950. doi: 10.1002/cne.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Feliciangeli S, Chatelain FC, Bichet D, Lesage F. The family of K2P channels: salient structural and functional properties. The Journal of physiology. 2015;593:2587–2603. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Busserolles J, Tsantoulas C, Eschalier A, Lopez Garcia JA. Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain. 2016;157(Suppl 1):S7–14. doi: 10.1097/j.pain.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 222.Devilliers M, et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nature communications. 2013;4:2941. doi: 10.1038/ncomms3941. [DOI] [PubMed] [Google Scholar]
- 223.Bagriantsev SN, et al. A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. ACS chemical biology. 2013;8:1841–1851. doi: 10.1021/cb400289x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rodrigues N, et al. Synthesis and structure-activity relationship study of substituted caffeate esters as antinociceptive agents modulating the TREK-1 channel. European journal of medicinal chemistry. 2014;75:391–402. doi: 10.1016/j.ejmech.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 225.Bocksteins E, Snyders DJ. Electrically silent Kv subunits: their molecular and functional characteristics. Physiology (Bethesda, Md) 2012;27:73–84. doi: 10.1152/physiol.00023.2011. [DOI] [PubMed] [Google Scholar]
- 226.Bocksteins E. Kv5, Kv6, Kv8, and Kv9 subunits: No simple silent bystanders. The Journal of general physiology. 2016;147:105–125. doi: 10.1085/jgp.201511507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsantoulas C, et al. Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:17502–17513. doi: 10.1523/JNEUROSCI.3561-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tsantoulas C, et al. Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Experimental neurology. 2014;251:115–126. doi: 10.1016/j.expneurol.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bocksteins E, et al. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. American journal of physiology. Cell physiology. 2009;296:C1271–1278. doi: 10.1152/ajpcell.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stas JI, Bocksteins E, Labro AJ, Snyders DJ. Modulation of Closed-State Inactivation in Kv2.1/Kv6.4 Heterotetramers as Mechanism for 4-AP Induced Potentiation. PloS one. 2015;10:e0141349. doi: 10.1371/journal.pone.0141349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Knabl J, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 232.Bonin RP, De Koninck Y. Restoring ionotropic inhibition as an analgesic strategy. Neuroscience letters. 2013;557(Pt A):43–51. doi: 10.1016/j.neulet.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 233.Klinger F, et al. delta Subunit-containing GABAA receptors are preferred targets for the centrally acting analgesic flupirtine. British journal of pharmacology. 2015;172:4946–4958. doi: 10.1111/bph.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zeilhofer HU, Ralvenius WT, Acuna MA. Restoring the spinal pain gate: GABA(A) receptors as targets for novel analgesics. Advances in Pharmacology (San Diego, Calif) 2015;73:71–96. doi: 10.1016/bs.apha.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 235.Tegeder I, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 236.Latremoliere A, et al. Reduction of Neuropathic and Inflammatory Pain through Inhibition of the Tetrahydrobiopterin Pathway. Neuron. 2015;86:1393–1406. doi: 10.1016/j.neuron.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chidley C, Haruki H, Pedersen MG, Muller E, Johnsson K. A yeast-based screen reveals that sulfasalazine inhibits tetrahydrobiopterin biosynthesis. Nat Chem Biol. 2011;7:375–383. doi: 10.1038/nchembio.557. [DOI] [PubMed] [Google Scholar]
- 238.Chandrasekhar S, et al. Identification and Characterization of Novel Microsomal Prostaglandin E Synthase-1 Inhibitors for Analgesia. J Pharmacol Exp Ther. 2016;356:635–644. doi: 10.1124/jpet.115.228932. [DOI] [PubMed] [Google Scholar]
- 239.Jin Y, et al. Pharmacodynamic comparison of LY3023703, a novel microsomal prostaglandin e synthase 1 inhibitor, with celecoxib. Clin Pharmacol Ther. 2016;99:274–284. doi: 10.1002/cpt.260. [DOI] [PubMed] [Google Scholar]
- 240.Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain. 2014;15:907–914. doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Inceoglu B, et al. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sisignano M, et al. 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J Neurosci. 2012;32:6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brenneis C, et al. Soluble epoxide hydrolase limits mechanical hyperalgesia during inflammation. Mol Pain. 2011;7:78. doi: 10.1186/1744-8069-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lam DK, Dang D, Zhang J, Dolan JC, Schmidt BL. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. J Neurosci. 2012;32:14178–14183. doi: 10.1523/JNEUROSCI.2399-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Christianson CA, et al. Spinal matrix metalloproteinase 3 mediates inflammatory hyperalgesia via a tumor necrosis factor-dependent mechanism. Neuroscience. 2012;200:199–210. doi: 10.1016/j.neuroscience.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Berta T, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest. 2014;124:1173–1186. doi: 10.1172/JCI72230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clark AK, Grist J, Al-Kashi A, Perretti M, Malcangio M. Spinal cathepsin S and fractalkine contribute to chronic pain in the collagen-induced arthritis model. Arthritis Rheum. 2012;64:2038–2047. doi: 10.1002/art.34351. [DOI] [PubMed] [Google Scholar]
- 248.Vicuna L, et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med. 2015;21:518–523. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Landry RP, Jacobs VL, Romero-Sandoval EA, DeLeo JA. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol. 2012;234:340–350. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 250.Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs. 2004;5:71–75. [PubMed] [Google Scholar]
- 251.Ostenfeld T, et al. A randomized, placebo-controlled trial of the analgesic efficacy and safety of the p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain from lumbosacral radiculopathy. Clin J Pain. 2015;31:283–293. doi: 10.1097/AJP.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 252.Smith MT, Wyse BD, Edwards SR. Small molecule angiotensin II type 2 receptor (AT(2)R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding, and efficacy in rats. Pain Med. 2013;14:692–705. doi: 10.1111/pme.12063. [DOI] [PubMed] [Google Scholar]
- 253.Bevan S, Quallo T, Andersson DA. Trpv1. Handb Exp Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 254.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 255.Keith JM, et al. Preclinical Characterization of the FAAH Inhibitor JNJ-42165279. ACS Med Chem Lett. 2015;6:1204–1208. doi: 10.1021/acsmedchemlett.5b00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pawsey S, et al. Safety, Tolerability and Pharmacokinetics of FAAH Inhibitor V158866: A Double-Blind, Randomised, Placebo-Controlled Phase I Study in Healthy Volunteers. Drugs R D. 2016;16:181–191. doi: 10.1007/s40268-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cajanus K, et al. Effect of endocannabinoid degradation on pain: role of FAAH polymorphisms in experimental and postoperative pain in women treated for breast cancer. Pain. 2016;157:361–369. doi: 10.1097/j.pain.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 258.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 259.Gimbel JS, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014;155:1793–1801. doi: 10.1016/j.pain.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 260.Bramson C, et al. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015;16:1163–1176. doi: 10.1111/pme.12677. [DOI] [PubMed] [Google Scholar]
- 261.Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage. 2015;23(Suppl 1):S8–17. doi: 10.1016/j.joca.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 262.Hochberg MC, et al. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016;68:382–391. doi: 10.1002/art.39492. [DOI] [PubMed] [Google Scholar]
- 263.Bowman CJ, et al. Developmental toxicity assessment of tanezumab, an anti-nerve growth factor monoclonal antibody, in cynomolgus monkeys (Macaca fascicularis) Reprod Toxicol. 2015;53:105–118. doi: 10.1016/j.reprotox.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 264.Butt M, et al. Morphologic, stereologic, and morphometric evaluation of the nervous system in young cynomolgus monkeys (Macaca fascicularis) following maternal administration of tanezumab, a monoclonal antibody to nerve growth factor. Toxicol Sci. 2014;142:463–476. doi: 10.1093/toxsci/kfu192. [DOI] [PubMed] [Google Scholar]
- 265.Wainger BJ, et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat Neurosci. 2015;18:17–24. doi: 10.1038/nn.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sorge RE, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 267.Baron R, et al. Peripheral Neuropathic Pain: A mechanism-related organizing principle based on sensory profiles. Pain. 2016 doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 269.Whiteside GT, Pomonis JD, Kennedy JD. An industry perspective on the role and utility of animal models of pain in drug discovery. Neurosci Lett. 2013;557(Pt A):65–72. doi: 10.1016/j.neulet.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 270.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 271.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain. 2016;17:T70–92. doi: 10.1016/j.jpain.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Smith SM, et al. Pain intensity rating training: results from an exploratory study of the ACTTION PROTECCT system. Pain. 2016;157:1056–1064. doi: 10.1097/j.pain.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 274.Dodd S, Dean OM, Vian J, Berk M. A Review of the Theoretical and Biological Understanding of the Nocebo and Placebo Phenomena. Clin Ther. 2017 doi: 10.1016/j.clinthera.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 275.Paice JA, et al. AAPT Diagnostic Criteria for Chronic Cancer Pain Conditions. J Pain. 2017;18:233–246. doi: 10.1016/j.jpain.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Smith SM, et al. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain. 2017 doi: 10.1016/j.jpain.2017.02.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Edwards RR, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Cara Therapeutics Announces Positive Top-Line Results From Phase 2a Trial of Oral CR845 in Chronic Pain Patients With Osteoarthritis of the Knee or Hip. http://ir.caratherapeutics.com/releasedetail.cfm?ReleaseID=946233.
- 279.Cara Therapeutics Resumes Patient Recruitment for Adaptive Phase 3 Trial of I.V. CR845 in Postoperative Pain. http://ir.caratherapeutics.com/releasedetail.cfm?ReleaseID=974348.
- 280.Viscusi ER, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 281.Lu Z, et al. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest. 2015;125:2626–2630. doi: 10.1172/JCI81070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Moriconi A, et al. Targeting the minor pocket of C5aR for the rational design of an oral allosteric inhibitor for inflammatory and neuropathic pain relief. Proc Natl Acad Sci U S A. 2014;111:16937–16942. doi: 10.1073/pnas.1417365111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Altun A, et al. Attenuation of morphine antinociceptive tolerance by cannabinoid CB1 and CB2 receptor antagonists. J Physiol Sci. 2015;65:407–415. doi: 10.1007/s12576-015-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Haugh O, Penman J, Irving AJ, Campbell VA. The emerging role of the cannabinoid receptor family in peripheral and neuro-immune interactions. Curr Drug Targets. 2016 doi: 10.2174/1389450117666160112113703. [DOI] [PubMed] [Google Scholar]
- 285.Deliu E, et al. The Lysophosphatidylinositol Receptor GPR55 Modulates Pain Perception in the Periaqueductal Gray. Mol Pharmacol. 2015;88:265–272. doi: 10.1124/mol.115.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kort ME, Kym PR. TRPV1 antagonists: clinical setbacks and prospects for future development. Prog Med Chem. 2012;51:57–70. doi: 10.1016/B978-0-12-396493-9.00002-9. [DOI] [PubMed] [Google Scholar]
- 287.Manitpisitkul P, et al. Safety, Tolerability and Pharmacokinetic and Pharmacodynamic Learnings from a Double-Blind, Randomized, Placebo-Controlled, Sequential Group First-in-Human Study of the TRPV1 Antagonist, JNJ-38893777, in Healthy Men. Clin Drug Investig. 2015;35:353–363. doi: 10.1007/s40261-015-0285-7. [DOI] [PubMed] [Google Scholar]
- 288.Quiding H, et al. TRPV1 antagonistic analgesic effect: a randomized study of AZD1386 in pain after third molar extraction. Pain. 2013;154:808–812. doi: 10.1016/j.pain.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 289.De Petrocellis L, Moriello AS. Modulation of the TRPV1 channel: current clinical trials and recent patents with focus on neurological conditions. Recent Pat CNS Drug Discov. 2013;8:180–204. doi: 10.2174/1574889808666131209124012. [DOI] [PubMed] [Google Scholar]
- 290.Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov. 2016;15:19–34. doi: 10.1038/nrd.2015.5. [DOI] [PubMed] [Google Scholar]
- 291.HPR Dr. Schaffler GmbH in cooperation with Zalicus announces positive results of Z944 Phase 1B Clinical Study in Pain. Efficacy Signals in Inflammatory and Neuropathic Pain Observed. http://hpr-cro.com/wp-content/uploads/2014/06/Press_release_HPR_170614.pdf.
- 292.Convergence Pharmaceuticals Announces Start of Phase II Study for Cav2.2 Selective Blocker, CNV2197944 in Pain Associated with Diabetic Peripheral Neuropathy (DPN) http://www.convergencepharma.com/userfiles/file/944_PhaseII_DPNFINAL.pdf.
- 293.Xenon still committed to TV-45070 for neuropathic pain. http://biotuesdays.com/2015/07/21/xenon-still-committed-to-tv-45070-for-neuropathic-pain/
- 294.Xenon and Genentech Extend Collaborations Focused on Pain. https://globenewswire.com/news-release/2016/03/15/819870/0/en/Xenon-and-Genentech-Extend-Collaborations-Focused-on-Pain.html.
- 295.Ghosh S, et al. Full Fatty Acid Amide Hydrolase Inhibition Combined with Partial Monoacylglycerol Lipase Inhibition: Augmented and Sustained Antinociceptive Effects with Reduced Cannabimimetic Side Effects in Mice. J Pharmacol Exp Ther. 2015;354:111–120. doi: 10.1124/jpet.115.222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Grim TW, et al. Combined inhibition of FAAH and COX produces enhanced anti-allodynic effects in mouse neuropathic and inflammatory pain models. Pharmacol Biochem Behav. 2014;124:405–411. doi: 10.1016/j.pbb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Starowicz K, et al. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS One. 2013;8:e60040. doi: 10.1371/journal.pone.0060040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Schlosburg JE, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Luz JG, et al. Crystal Structures of mPGES-1 Inhibitor Complexes Form a Basis for the Rational Design of Potent Analgesic and Anti-Inflammatory Therapeutics. J Med Chem. 2015;58:4727–4737. doi: 10.1021/acs.jmedchem.5b00330. [DOI] [PubMed] [Google Scholar]
- 300.Leclerc P, et al. Characterization of a human and murine mPGES-1 inhibitor and comparison to mPGES-1 genetic deletion in mouse models of inflammation. Prostaglandins Other Lipid Mediat. 2013;107:26–34. doi: 10.1016/j.prostaglandins.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 301.Sjogren T, et al. Crystal structure of microsomal prostaglandin E2 synthase provides insight into diversity in the MAPEG superfamily. Proc Natl Acad Sci U S A. 2013;110:3806–3811. doi: 10.1073/pnas.1218504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.LY3023703 Clinical trial Phase 2. https://clinicaltrials.gov/ct2/show/NCT01872910 Identifier: NCT01872910.
- 303.Hellio le Graverand MP, et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2013;72:187–195. doi: 10.1136/annrheumdis-2012-202239. [DOI] [PubMed] [Google Scholar]
- 304.Wozniak KM, et al. The orally active glutamate carboxypeptidase II inhibitor E2072 exhibits sustained nerve exposure and attenuates peripheral neuropathy. J Pharmacol Exp Ther. 2012;343:746–754. doi: 10.1124/jpet.112.197665. [DOI] [PubMed] [Google Scholar]
- 305.Vornov JJ, et al. Pharmacokinetics and pharmacodynamics of the glutamate carboxypeptidase II inhibitor 2-MPPA show prolonged alleviation of neuropathic pain through an indirect mechanism. J Pharmacol Exp Ther. 2013;346:406–413. doi: 10.1124/jpet.113.205039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Huang JL, Chen XL, Guo C, Wang YX. Contributions of spinal D-amino acid oxidase to bone cancer pain. Amino Acids. 2012;43:1905–1918. doi: 10.1007/s00726-012-1390-z. [DOI] [PubMed] [Google Scholar]