Abstract
A causal role of hypercholesterolemia in non-ischemic heart failure has never been demonstrated. Adeno-associated viral serotype 8 (AAV8)-low-density lipoprotein receptor (AAV8-LDLr) gene transfer was performed in LDLr-deficient mice without and with pressure overload induced by transverse aortic constriction (TAC). AAV8-LDLr gene therapy resulted in an 82.8% (p < 0.0001) reduction of plasma cholesterol compared with controls. Mortality rate was lower (p < 0.05) in AAV8-LDLr TAC mice compared with control TAC mice (hazard ratio for mortality 0.457, 95% confidence interval [CI] 0.237–0.882) during 8 weeks of follow-up. AAV8-LDLr gene therapy attenuated cardiac hypertrophy, reduced interstitial and perivascular fibrosis, and decreased lung congestion in TAC mice. Cardiac function, quantified by invasive hemodynamic measurements and magnetic resonance imaging, was significantly improved 8 weeks after sham operation or after TAC in AAV8-LDLr mice compared with respective control groups. Myocardial protein levels of mammalian target of rapamycin and of acetyl-coenzyme A carboxylase were strikingly decreased following cholesterol lowering in mice without and with pressure overload. AAV8-LDLr therapy potently reduced cardiac glucose uptake and counteracted metabolic remodeling following pressure overload. Furthermore, oxidative stress and myocardial apoptosis were decreased following AAV8-LDLr therapy in mice with pressure overload. In conclusion, cholesterol-lowering gene therapy potently counteracts structural and metabolic remodeling, and enhances cardiac function.
Keywords: heart failure, gene transfer, metabolic remodeling, cardiac hypertrophy, hypercholesterolemia, gene therapy, low-density lipoprotein receptor, adeno-associated viral vectors, non-ischemic cardiomyopathy, cholesterol-lowering therapy
A causal role of hypercholesterolemia in non-ischemic heart failure has never been demonstrated. In this issue of Molecular Therapy, Muthuramu et al. demonstrate that AAV8-mediated cholesterol-lowering gene therapy potently counteracts structural and metabolic remodeling, and enhances cardiac function in mice with pressure overload induced by transverse aortic constriction.
Introduction
Epidemiological studies support a strong association between non-high-density lipoprotein (non-HDL) cholesterol levels and heart failure incidence. In Framingham Heart Study participants free of coronary heart disease at baseline, high non-HDL cholesterol levels were independently associated with heart failure incidence after adjustment for interim myocardial infarction and clinical covariates.1 The results of the controlled rosuvastatin multinational study in heart failure (CORONA) randomized clinical trial2 and the Gruppo Italiano per lo Studio della Sopravvivenza nella Insuffienza Cardiaca-heart failure (GISSI-HF) trial3 evaluating the effect of 10 mg of rosuvastatin in patients with established chronic heart failure do not support a role for cholesterol-lowering therapies in the management of these patients. However, statins not only inhibit the synthesis of cholesterol, but also interfere with isoprenoid synthesis and the synthesis of isopentenyl pyrophosphate,4, 5 which may result in untoward myocardial effects unrelated to cholesterol lowering.
It has previously been demonstrated that a high-fat/high-cholesterol diet in pigs results in hyperactive mammalian target of rapamycin (mTOR) signaling in the heart,6 which may have been directly related to hypercholesterolemia in these pigs. Because the mTOR complex 1 (mTORC1) promotes protein synthesis and cell growth, inhibits autophagy, and results in increased glucose oxidation and reduced fatty acid oxidation,7, 8 we hypothesized that cholesterol lowering per se may beneficially affect cardiac function via profound effects on myocardial metabolic remodeling. In addition, because oxidative stress may be critical for activation of apoptosis in the overloaded heart and cardiac cell death may contribute to deterioration of cardiac function,9 reduction of oxidative stress following cholesterol lowering may counteract the progression of heart failure. To investigate the effect of cholesterol-lowering gene therapy, we performed adeno-associated viral serotype 8 (AAV8)-low-density lipoprotein receptor (LDLr) gene transfer in LDLr-deficient mice. The objective of the current study was to evaluate the effect of cholesterol-lowering gene therapy on structural and metabolic remodeling and cardiac function in C57BL/6 LDLr−/− mice in the absence and presence of pressure overload induced by transverse aortic constriction (TAC).
Results
AAV8-LDLr Gene Transfer Potently Lowers Lipoprotein Cholesterol Levels in C57BL/6 LDLr−/− Mice
All female C57BL/6 LDLr−/− mice in this study were fed standard chow supplemented with 0.2% cholesterol 10% coconut oil from the age of 12 weeks to induce hypercholesterolemia, and this diet was continued for the entire duration of the experiment. Total and non-HDL, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), LDL, and HDL plasma cholesterol (mg/dL) at day 10 after saline injection or gene transfer are shown in Table S1. AAV8-LDLr gene transfer resulted in an 82.8% (p < 0.0001) reduction of plasma cholesterol levels compared with controls. Non-HDL, VLDL, IDL, and LDL cholesterol levels were 90.9% (p < 0.0001), 90.9% (p < 0.0001), 93.4% (p < 0.0001), and 87.6% (p < 0.0001) lower in AAV8-LDLr mice than in controls. HDL cholesterol levels were 35.8% (p < 0.001) decreased in AAV8-LDLr mice compared with controls. Cholesterol levels were stable for the entire duration of the experiment (data not shown). Plasma total cholesterol, non-HDL cholesterol, and HDL cholesterol values in AAV8-LDLr C57BL/6 LDLr−/− mice (Table S1) were very similar compared with reference C57BL/6 mice (n = 10) (total cholesterol 66.5 ± 3.3 mg/dL; non-HDL cholesterol 23.7 ± 2.6 mg/dL; HDL cholesterol 42.8 ± 3.5 mg/dL).
Murine LDLr expression in the liver of C57BL/6 mice, C57BL/6 LDLr−/− mice, and C57BL/6 LDLr−/− mice treated with 2 × 1012 genome copies/kg AAV8-LDLr is illustrated in Figure S1. Murine LDLr was undetectable in C57BL/6 LDLr−/− mice. Murine LDLr expression 10 weeks after gene transfer with AAV8-LDLr in C57BL/6 LDLr−/− mice was 1.56-fold (p < 0.01) higher compared with wild-type C57BL/6 mice.
Myocardial lipid levels 8 weeks after sham operation or after TAC in C57BL/6 LDLr−/− mice are shown in Table S2. No significant differences in total myocardial cholesterol levels were observed. Myocardial cholesteryl esters were reduced by 46.0% (p < 0.05) in AAV8-LDLr sham mice and by 76.4% (p < 0.05) in AAV8-LDLr TAC mice compared with respective control groups. Myocardial sphingomyelin was increased by 37.8% (p < 0.05) in control TAC mice compared with control sham mice (Table S2).
Cholesterol-Lowering Gene Transfer Reduces Mortality after TAC
TAC was performed at the age of 17 weeks to induce pressure overload. Comparison of Kaplan-Meier survival curves showed a significantly (p < 0.05) decreased mortality rate in AAV8-LDLr TAC mice compared with control TAC mice (hazard ratio for mortality 0.457, 95% confidence interval [CI] 0.237–0.882) during a follow-up period of 8 weeks (Figure S2). Sham operation did not result in any mortality (data not shown).
Cholesterol-Lowering Gene Transfer Attenuates Cardiac Hypertrophy and Decreases Lung Congestion after TAC
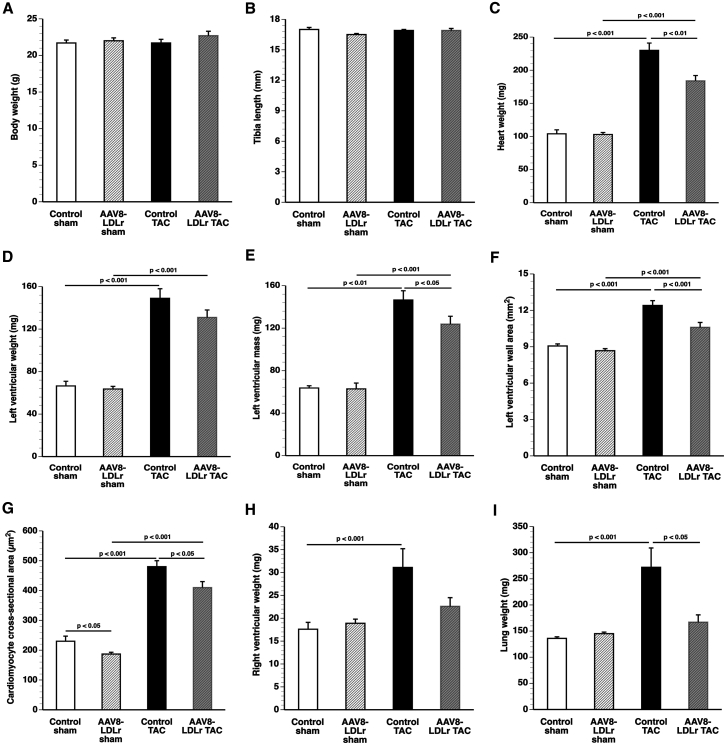
No significant differences in body weight (Figure 1A) and tibia length (Figure 1B) were observed between different groups. The heart weight was 2.21-fold (p < 0.001) higher in control TAC mice and 1.79-fold (p < 0.001) higher in AAV8-LDLr TAC mice compared with respective sham groups (Figure 1C). Cholesterol lowering attenuated cardiac hypertrophy as evidenced by a 20.0% (p < 0.01) lower heart weight in AAV8-LDLr TAC than in control TAC mice. Left ventricular weight at sacrifice (Figure 1D) and quantified by magnetic resonance imaging (Figure 1E) was significantly lower in AAV8-LDLr TAC mice than in control TAC mice. Histological analysis demonstrated a 14.5% (p < 0.001) smaller left ventricular wall area (Figure 1F) and a 15.5% (p < 0.05) reduction of cardiomyocyte cross-sectional area in AAV8-LDLr TAC mice than in control TAC mice (Figure 1G). Cholesterol-lowering gene therapy also significantly (p < 0.05) reduced cardiomyocyte cross-sectional area in sham mice (Figure 1G). Furthermore, right ventricular hypertrophy was observed in control TAC mice as evidenced by a 1.77-fold (p < 0.01) increase of right ventricular weight compared with the control sham group (Figure 1H). In contrast, no significant hypertrophy of the right ventricle was observed in AAV8-LDLr TAC mice (Figure 1H). Lung weight was 2.00-fold (p < 0.01) higher in the control TAC group compared with the control sham group (Figure 1I). Cholesterol-lowering gene transfer decreased lung congestion after TAC as evidenced by a 38.6% (p < 0.05) lower lung weight in AAV8-LDLr TAC mice than in control TAC mice (Figure 1I).
Figure 1.
Cholesterol-Lowering Gene Therapy Attenuates Cardiac Hypertrophy and Lung Congestion after TAC
(A–D) Bar graphs illustrating body weight (A), tibia length (B), heart weight (C), and left ventricular weight (D) in control sham (n = 10), AAV8-LDLr sham (n = 10), control TAC (n = 11), and AAV8-LDLr TAC (n = 11) mice 8 weeks after operation. (E) Left ventricular mass quantified by micro-MRI (n = 6 in each group). (F and G) Left ventricular wall area (F) and cardiomyocyte cross-sectional area (G) quantified by morphometric and histological analysis in control sham (n = 15), AAV8-LDLr sham (n = 22), control TAC (n = 23), and AAV8-LDLr TAC (n = 23) mice 8 weeks after operation. (H and I) Bar graphs showing right ventricular weight (H) and wet lung weight (I) in control sham (n = 10), AAV8-LDLr sham (n = 10), control TAC (n = 11), and AAV8-LDLr TAC (n = 11) mice 8 weeks after operation. Error bars represent SEM.
Representative Sirius-red-stained cross sections of sham hearts and TAC hearts are illustrated in Figure S3. An overview of morphometric and histological parameters is shown in Table S3. Representative photomicrographs of immunohistochemical analyses are illustrated in Figure S4.
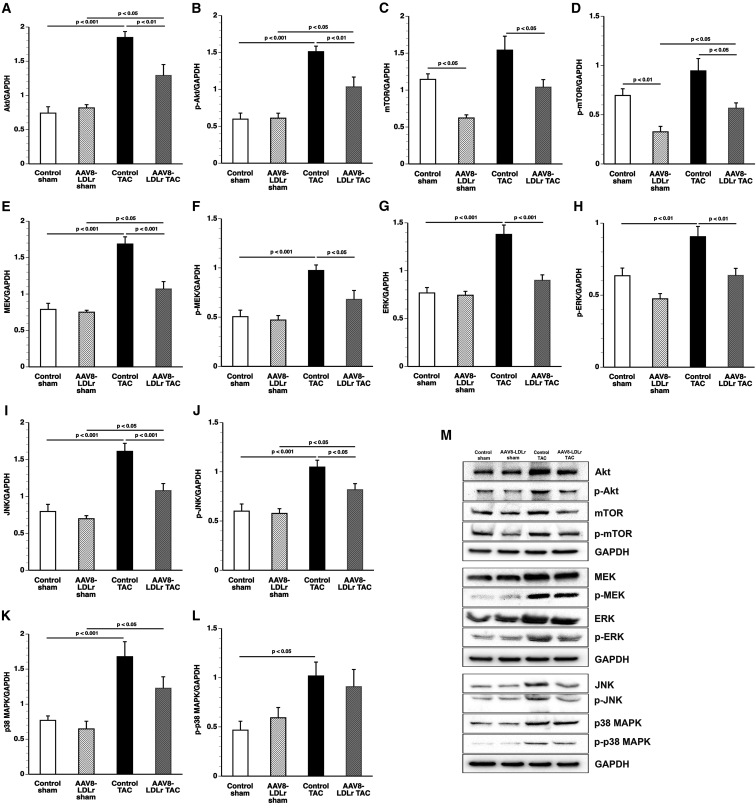
Sustained Akt activation induces cardiac hypertrophy, which may lead to heart failure. Myocardial Akt (Figure 2A) and p-Akt (Figure 2B) levels were reduced by 30.0% (p < 0.01) and 31.5% (p < 0.01) in AAV8-LDLr TAC mice compared with control TAC mice. Mammalian or mechanistic target of rapamycin (mTOR), involved in cell growth, autophagy, and metabolism, was reduced by 45.7% (p < 0.05) in AAV8-LDLr sham mice and by 32.5% (p < 0.05) in AAV8-LDLr TAC mice compared with respective control groups (Figure 2C). Myocardial levels of p-mTOR were 54.3% (p < 0.05) lower in AAV8-LDLr sham mice and 40.1% (p < 0.05) in AAV8-LDLr TAC mice than in respective control groups (Figure 2D). Mitogen-activated protein kinase (MAPK) kinase (MEK) protein levels (Figure 2E) and p-MEK levels (Figure 2F) were 36.6% (p < 0.001) and 30.2% (p < 0.05) lower in AAV8-LDLr TAC mice than in control TAC mice. Furthermore, cholesterol-lowering gene therapy decreased cardiac extracellular signal-regulated kinase (ERK) (Figure 2G), p-ERK (Figure 2H), c-Jun N-terminal kinase (JNK) (Figure 2I), and p-JNK (Figure 2J) levels after TAC compared with control TAC mice. In contrast, p38 MAPK (p38) (Figure 2K) and p-p38 MAPK levels (Figure 2L) were not significantly different between control TAC mice and AAV8-LDLr TAC mice. Representative images of western blots are shown in Figure 2M.
Figure 2.
Quantification of Pro-hypertrophic Myocardial Proteins by Western Blot
(A–L) Bar graphs illustrating Akt (A), p-Akt (B), mTOR (C), p-mTOR (D), MEK (E), p-MEK (F), ERK (G), p-ERK (H), JNK (I), p-JNK (J), p38 (K), and p-p38 (L) protein levels quantified by western blot in the myocardium of control sham (n = 10), AAV8-LDLr sham (n = 10), control TAC (n = 9), and AAV8-LDLr TAC (n = 10) mice 8 weeks after operation. All protein levels were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein level. (M) Representative images of western blots are shown. Error bars represent SEM.
To confirm that the housekeeping protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is an adequate reference for normalizing protein expression levels, we compared GAPDH and β-tubulin expression levels (Figure S5). GAPDH/β-tubulin ratios were highly similar between the four different groups, confirming that GAPDH is an adequate reference for normalizing protein expression levels (Figure S5).
Cholesterol-Lowering Gene Therapy Significantly Reduces Interstitial Fibrosis and Perivascular Fibrosis after TAC
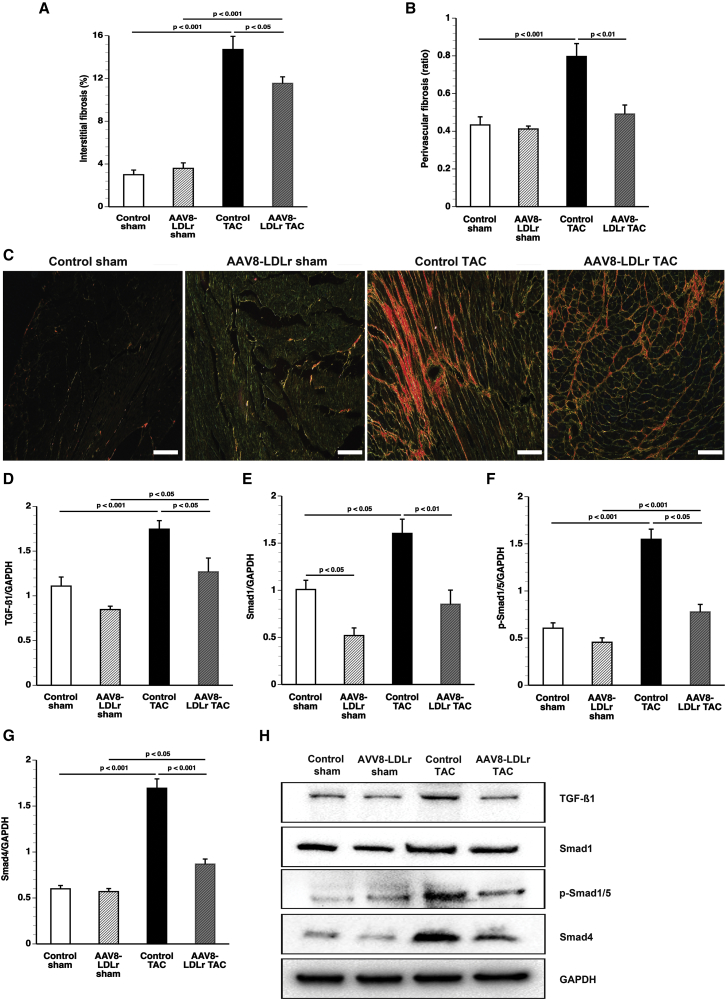
The degree of interstitial fibrosis (Figure 3A) and perivascular fibrosis (Figure 3B) was reduced by 21.8% (p < 0.05) and 38.3% (p < 0.01) in AAV8-LDLr TAC mice compared with control TAC mice. Representative photomicrographs of Sirius-red-stained interstitial collagen viewed under polarized light are shown in Figure 3C. The 12.5-kDa isoform of transforming growth factor β1 (TGF-β1) was reduced by 27.3% (p < 0.001) in AAV8-LDLr TAC mice compared with control TAC mice (Figure 3D). Cholesterol-lowering gene therapy reduced Smad1 (Figure 3E), p-Smad1/5 (Figure 3F), and Smad4 (Figure 3G) after TAC by 46.9% (p < 0.01), 49.7% (p < 0.001), and 48.7% (p < 0.001), respectively, compared with control TAC mice. Representative images of western blots are shown in Figure 3H.
Figure 3.
Cholesterol-Lowering Gene Transfer Significantly Reduces Interstitial Fibrosis and Perivascular Fibrosis after TAC
(A and B) Bar graphs illustrating the degree of interstitial fibrosis (A) and the degree of perivascular fibrosis (B) in control sham (n = 15), AAV8-LDLr sham (n = 22), control TAC (n = 23), and AAV8-LDLr TAC (n = 23) mice 8 weeks after operation. (C) Representative photomicrographs showing Sirius-red-stained interstitial collagen viewed under polarized light. Scale bars, 50 μm. (D–G) Bar graphs illustrating the 12.5-kDa isoform of TGF-β1 (D), Smad1 (E), p-Smad1/5 (F), and Smad4 (G) myocardial protein levels quantified by western blot in the myocardium of control sham (n = 10), AAV8-LDLr sham (n = 10), control TAC (n = 9), and AAV8-LDLr TAC (n = 10) mice 8 weeks after operation. All protein levels were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein level. (H) Representative images of western blots are shown. Error bars represent SEM.
Lowering of Cholesterol Improves Cardiac Function in the Absence and Presence of Pressure Overload
Systolic function and diastolic function 8 weeks after sham operation were significantly improved in AAV8-LDLr sham mice compared with control sham mice as evidenced by the increased peak rate of isovolumetric contraction (dP/dtmax), a more negative peak rate of isovolumetric relaxation (dP/dtmin), and a smaller time constant of left ventricular isovolumetric relaxation (τ [tau]) (Table 1). The peak rate of isovolumetric contraction (dP/dtmax) was increased by 20.0% (p < 0.05) in AAV8-LDLr TAC mice compared with control TAC mice. The time constant of left ventricular relaxation (τ) was 13.2% (p < 0.05) lower in AAV8-LDLr TAC mice than in control TAC mice. The pressure gradient over TAC was quantified by measuring the difference of the systolic pressure in the right carotid artery and the left carotid artery. This gradient was similar in control TAC mice (88.6 ± 9.7 mm Hg; n = 6) compared with AAV8-LDLr mice (91.2 ± 9.4 mm Hg; n = 6). Taken together, AAV8-LDLr gene transfer results in improved systolic and diastolic function in both in sham mice and TAC mice.
Table 1.
Hemodynamic Parameters in the Left Ventricle and in the Aorta 8 Weeks after Sham Operation and after TAC in C57BL/6 LDLr−/− Mice
| Control Sham | AAV8-LDLr Sham | Control TAC | AAV8-LDLr TAC | |
|---|---|---|---|---|
| Number of mice | 19 | 13 | 19 | 16 |
| Left Ventricle | ||||
| Peak systolic pressure (mm Hg) | 100 ± 1 | 103 ± 2 | 150 ± 8§§§ | 160 ± 9§§§ |
| End-diastolic pressure (mm Hg) | 2.15 ± 0.58 | 2.38 ± 0.35 | 3.64 ± 0.50 | 2.59 ± 0.84 |
| dP/dtmax (mm Hg/ms) | 9.76 ± 0.43 | 11.4 ± 0.3°° | 9.25 ± 0.47 | 11.1 ± 0.9* |
| dP/dtmin (mm Hg/ms) | −8.14 ± 0.31 | −10.2 ± 0.2°°° | −9.19 ± 0.55 | −10.5 ± 0.8 |
| τ (ms) | 5.32 ± 0.20 | 4.65 ± 0.13° | 5.70 ± 0.21 | 4.95 ± 0.21* |
| Heart rate (bpm) | 599 ± 11 | 610 ± 10 | 580 ± 20 | 622 ± 10 |
| Aorta | ||||
| Mean pressure (mm Hg) | 80.8 ± 1.5 | 82.1 ± 2.1 | 87.7 ± 3.8 | 97.9 ± 5.5§ |
| Systolic pressure (mm Hg) | 99.2 ± 1.6 | 101 ± 2 | 147 ± 8§§§ | 155 ± 11§§§ |
| Diastolic pressure (mm Hg) | 64.7 ± 2.0 | 64.0 ± 3.5 | 50.6 ± 4.4§ | 60.5 ± 7.0 |
Data are expressed as means ± SEM. °p < 0.05, °°p < 0.01, °°°p < 0.001 versus control sham; §p < 0.05, §§§p < 0.001 versus respective sham groups; *p < 0.05 versus control TAC.
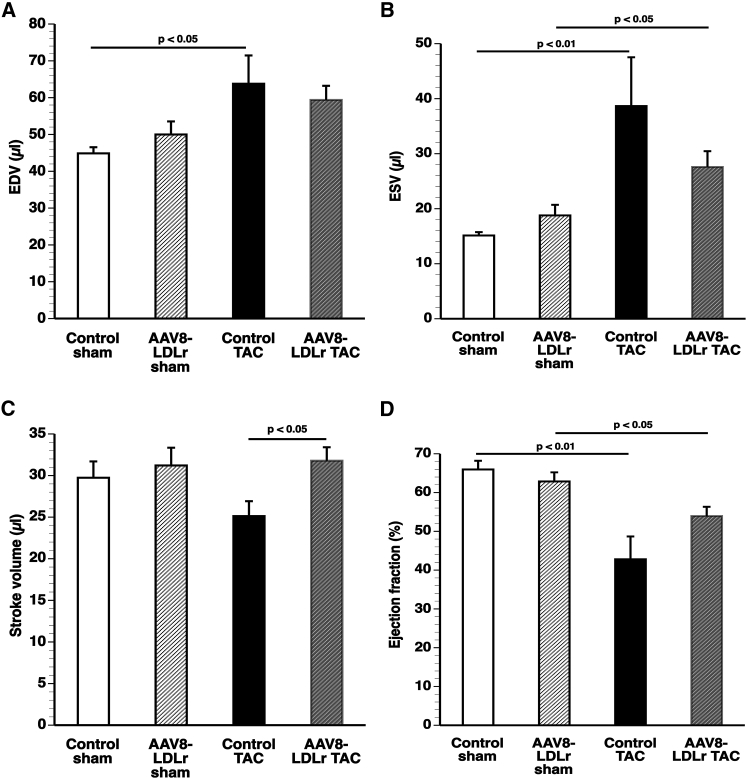
The quantification of end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume, and ejection fraction by micro-MRI is shown in Figure 4. The stroke volume was increased by 26.5% (p < 0.05) in AAV8-LDLr TAC mice compared with control TAC mice (Figure 4C). The ejection fraction was 1.54-fold (p < 0.01) lower in control TAC mice and 1.17-fold (p < 0.05) lower in AAV8-LDLr TAC mice compared with respective sham groups (Figure 4D).
Figure 4.
Cholesterol Lowering Increases Stroke Volume after TAC
(A–D) End-diastolic volume (EDV) (A), end-systolic volume (ESV) (B), stroke volume (C), and ejection fraction (D) in sham mice and in TAC mice (n = 6 for each group). Sham operation or TAC was performed at the age of 17 weeks. Micro-magnetic resonance imaging was performed 8 weeks later. Error bars represent SEM.
Reduction of Cholesterol Counteracts Metabolic Remodeling Induced by Pressure Overload
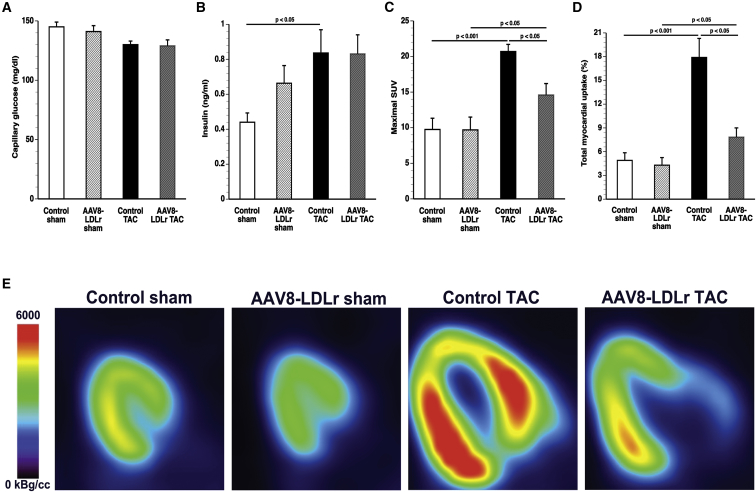
Capillary glucose levels did not differ between the different experimental groups (Figure 5A). Plasma insulin was 1.90-fold (p < 0.05) higher in control TAC mice than in control sham mice (Figure 5B). Parameters of glucose uptake in the myocardium and the left quadriceps quantified by micro-positron emission tomography (micro-PET) imaging using [18F]-fluorodeoxyglucose (FDG) as a tracer are shown in Figures 5C and 5D and in Table S4. TAC was associated with a significantly increased glucose uptake in the myocardium. The maximal standardized uptake value (SUV) was 2.12-fold (p < 0.001) higher in control TAC mice and 1.51-fold (p < 0.05) higher in AAV8-LDLr TAC mice than in respective sham groups. Maximal SUV was reduced by 29.5% (p < 0.05) in AAV8-LDLr TAC mice compared with control TAC mice. The total accumulation of glucose in the myocardium was 56.3% (p < 0.05) lower in AAV8-LDLr TAC mice than in control TAC mice. Taken together, increased glucose uptake in the myocardium after TAC is significantly attenuated following cholesterol-lowering gene therapy. Representative micro-PET images illustrating the myocardial uptake of [18F]-FDG are shown in Figure 5E.
Figure 5.
Cholesterol-Lowering Gene Transfer Counteracts Metabolic Remodeling
(A and B) Capillary glucose (A) and plasma insulin levels (B) in control sham (n = 10), AAV8-LDLr sham (n = 10), control TAC (n = 11), and AAV8-LDLr TAC (n = 11) mice 8 weeks after operation. (C and D) Quantification of glucose uptake in the myocardium determined by micro-PET as shown by the maximal standardized uptake (SUV) value (C) and total myocardial uptake (% of injected dose) (D) 8 weeks after sham operation or after TAC (n = 10 in each group). (E) Representative micro-PET images illustrating the uptake of [18F]-FDG in the myocardium of sham mice and TAC mice at day 56 after operation. Error bars represent SEM.
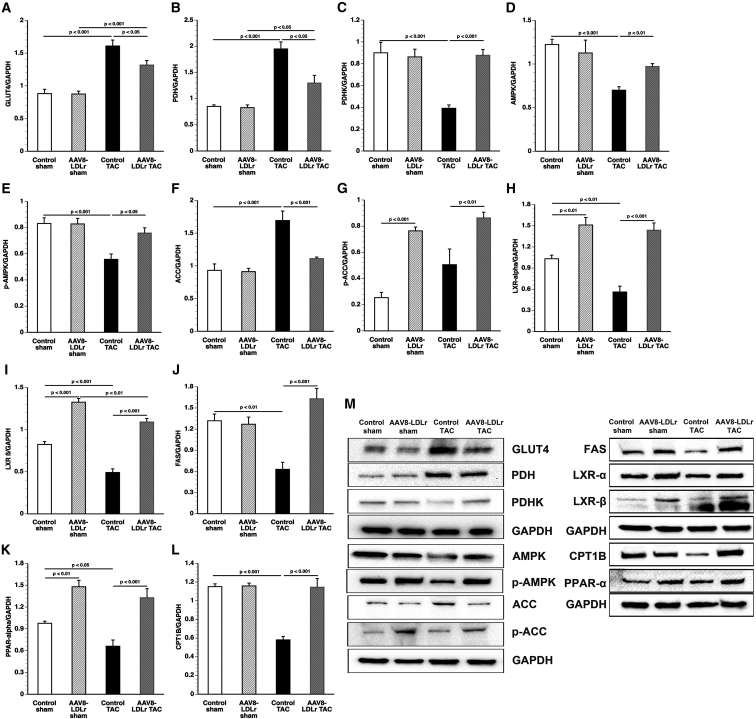
Pressure overload significantly increased myocardial GLUT4 protein levels (Figure 6A) and pyruvate dehydrogenase (PDH) levels (Figure 6B). Cholesterol-lowering gene transfer reduced GLUT4 protein levels by 18.0% (p < 0.05) and PDH levels by 33.3% (p < 0.001) compared with control TAC mice. Myocardial protein levels of PDH kinase (PDHK), which inactivates PDH, were reduced by 56.5% (p < 0.001) in control TAC mice compared with control sham mice and were 2.24-fold (p < 0.001) higher in AAV8-LDLr TAC mice than in control TAC mice (Figure 6C). Taken together, these data suggest that increased glucose uptake is accompanied by increased glucose oxidation in control TAC mice.
Figure 6.
Quantification of Metabolic Proteins by Western Blot
(A–L) Bar graphs illustrating GLUT4 (A), PDH (B), PDHK (C), AMPK (D), p-AMPK (E), AAC (F), p-ACC (G), PPAR-α (H), CPT1B (I), LXR-α (J), LXR-β (K), and FAS (L) protein levels quantified by western blot in the myocardium of control sham (n = 10), AAV8-LDLr sham (n = 10), control TAC (n = 9), and AAV8-LDLr TAC (n = 10) mice 8 weeks after operation. All protein levels were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein level. (M) Representative images of western blots are shown. Error bars represent SEM.
Myocardial AMP-activated protein kinase (AMPK) (Figure 6D) and p-AMPK (Figure 6E) protein levels were significantly lower in control TAC mice compared with control sham mice and AAV8-LDLr TAC mice. Levels of acetyl-coenzyme A (acetyl-CoA) carboxylase (ACC) (Figure 6F) were 34.4% lower in AAV8-LDLr TAC mice compared with control TAC mice. Levels of p-ACC (Figure 6G), which constitutes the inactive form of the enzyme, were significantly higher in AAV8-LDLr sham mice (p < 0.001) and AAV8-LDLr TAC mice (p < 0.01) compared with respective control groups. Myocardial protein levels of peroxisome proliferator-activated receptor (PPAR)-α, a key transcriptional factor regulating fatty acid oxidation, were 1.52-fold (p < 0.01) higher in AAV8-LDLr sham mice and 2.01-fold (p < 0.001) higher in AAV8-LDLr TAC mice compared with respective control groups (Figure 6H). PPAR-α levels were 32.3% (p < 0.05) lower in control TAC mice compared with control sham mice. Similar profound differences of expression levels were also observed for retinoid X receptor (RXR)-α, the heterodimerization partner of PPAR-α and of liver X receptor (LXR)-α and LXR-β (Figure S6A). Myocardial protein levels of carnitine palmitoyltransferase IB (CPT1B) were 1.97-fold (p < 0.001) higher in AAV8-LDLr TAC mice than in control TAC mice (Figure 6I). Similarly, protein levels of long-chain acyl-CoA synthetase, member 1 (ACSL1), and of long-chain acyl-CoA dehydrogenase (ACADL) were significantly (p < 0.05) higher in AAV8-LDLr TAC mice than in control TAC mice (Figures S6B and S6C). Moreover, ACADL levels were 1.61-fold (p < 0.001) higher in AAV8-LDLr sham mice than in control sham mice. Expression of CD36 or fatty acid translocase (FAT) was similar between different groups (Figure S6D). Myocardial protein levels of LXR-α and of LXR-β were significantly higher in AAV8-LDLr sham mice and in AAV8-LDLr TAC mice compared with respective control groups and were significantly lower in control TAC mice compared with control sham mice (Figures 6J and 6K). Levels of fatty acid synthase (FAS), an LXR target, were 2.59-fold (p < 0.001) higher in AAV8-LDLr TAC mice than in control TAC mice (Figure 6L). Representative images of western blots are shown in Figure 6M.
Oxidative Stress and Myocardial Apoptosis Are Reduced by Cholesterol-Lowering Gene Therapy in Mice with Chronic Pressure Overload
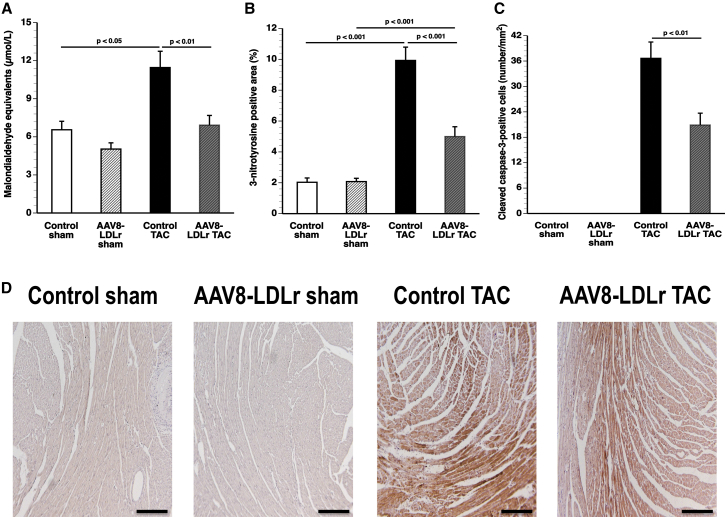
Plasma thiobarbituric acid reactive substances (TBARSs) and the 3-nitrotyrosine-positive area (%) in the myocardium are shown in Figures 7A and 7B, respectively. Plasma TBARSs were 74.7% (p < 0.05) higher in control TAC mice compared with control sham mice. A 39.6% (p < 0.01) reduction of plasma TBARSs was observed in AAV8-LDLr TAC mice compared with control TAC mice. Compared with respective sham groups, 3-nitrotyrosine-positive area (%) in the myocardium quantified by immunohistochemistry was increased 4.89-fold (p < 0.001) and 2.40-fold (p < 0.001) in control TAC mice and in AAV8-LDLr TAC mice, respectively. The 3-nitrotyrosine-positive area was 49.7% (p < 0.001) lower in AAV8-LDLr TAC mice than in control TAC mice, indicating decreased nitro-oxidative stress. Apoptosis in the myocardium was evaluated using immunohistochemical quantification of cleaved caspase-3. Cleaved caspase-3-positive cells were undetectable in the myocardium of sham mice (Figure 7C). Compared with control TAC mice, the number of cleaved caspase-3 positive cells was reduced by 43.0% (p < 0.01) in AAV8-LDLr TAC mice (Figure 7C). Representative myocardial sections immunostained for 3-nitrotyrosine are shown in Figure 7D.
Figure 7.
Oxidative Stress and Myocardial Apoptosis Are Reduced by Cholesterol-Lowering Gene Transfer in Mice with Chronic Pressure Overload
(A–C) Bar graphs illustrating plasma TBARSs expressed as plasma malondialdehyde equivalents (A), the percentage of 3-nitrotyrosine-positive area in the myocardium (B), and the number of cleaved caspase-3-positive cells (C) in control sham (n = 15), AAV8-LDLr sham (n = 22), control TAC (n = 23), and AAV8-LDLr TAC (n = 23) mice 8 weeks after operation. (D) Representative photomicrographs showing myocardial sections stained for 3-nitrotyrosine. Scale bars, 100 μm. Error bars represent SEM.
Discussion
The main findings of the current study are that cholesterol-lowering gene therapy attenuated cardiac hypertrophy, decreased interstitial fibrosis, counteracted metabolic remodeling, and lowered oxidative stress in mice with pressure overload. Systolic and diastolic cardiac functions were improved following AAV8-mediated LDLr gene transfer both in the absence and presence of pressure overload.
The impact of plasma cholesterol on cardiac function in the absence of coronary heart disease is not well established, and the effect of statins in the setting of heart failure is controversial. AAV8-LDLr gene transfer resulted in a very profound reduction of plasma cholesterol levels. Because cholesterol reduction in this study was achieved via AAV8-mediated LDLr gene transfer (and not, e.g., as a result of dietary manipulations that may result in non-selective effects), this strategy is appropriate to study the effect of cholesterol levels per se. In contrast, statins do not specifically lower plasma cholesterol, and pleiotropic effects of statins might be detrimental in the setting of heart failure. Endogenous coenzyme Q10 synthesis is blocked by 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins).4 Coenzyme Q10 is a component of the electron transport chain and participates in aerobic cellular respiration. Low plasma coenzyme Q10 levels are a risk factor for worsened outcomes in heart failure.10 In addition, blocking the mevalonate pathway by statins also inhibits the synthesis of isopentenyl pyrophosphate. This molecule is not only a building block for cholesterol, but is also required for the post-transcriptional enzymatic isopentenylation of selenocysteine tRNA and its maturation to a functional tRNA molecule. Inhibition of isopentenyl pyrophosphate synthesis by statins results in a decrease in available selenoproteins,5 including glutathione peroxidases. A reduction of myocardial glutathione peroxidases may impair cardiac function via increased oxidative stress.11
The attenuation of cardiac hypertrophy induced by pressure overload in mice treated with AAV8-LDLr gene transfer is a striking observation in the current study. The mTOR pathway plays a key role in sensing and integrating multiple environmental signals.12 The mTORC1 promotes protein synthesis and cell growth, inhibits autophagy, and results in increased glucose oxidation and reduced fatty acid oxidation.7, 8 Whereas complete genetic disruption of mTORC1 impairs the capacity of the heart to respond to pressure overload and potentiates the development of dilated cardiomyopathy, partial inhibition of mTORC1 decreases cardiac hypertrophy and improves cardiac function in the presence of pressure overload.13, 14, 15 Cholesterol trafficking has been shown to be required for mTOR activation.12 An obvious upregulation of mTOR and p-mTOR protein in macrophage-derived foam cells generated using Cu2+-oxidized LDL was observed by Wang et al.16 and suggests a link between cholesteryl ester accumulation and mTOR expression. The decrease of myocardial cholesteryl ester levels following cholesterol-lowering gene transfer is consistent with altered intracellular cholesterol dynamics. This may directly underlie the fact that protein levels of mTOR and p-mTOR were strikingly decreased following cholesterol-lowering gene transfer both in the absence and presence of pressure overload. In mice with pressure overload, differential Akt and AMPK activity may also have contributed to differences in mTORC1 activity. Activated Akt can phosphorylate and activate mTOR or inhibit PRAS40, an endogenous mTORC1 inhibitor.7 The AMPK pathway leads to inhibition of mTORC1.17 However, at least part of the effect of cholesterol-lowering gene therapy on p-mTOR is independent of Akt and AMPK because no effect on Akt and AMPK was observed in the absence of pressure overload.
The reappearance of a fetal metabolic pattern characterized by decreased fatty acid oxidation, increased glycolysis, and increased anaplerosis has been repeatedly demonstrated in models of pathological hypertrophy.18, 19, 20, 21, 22 Cholesterol lowering had a profound effect on metabolic remodeling induced by pressure overload. Glucose uptake in the myocardium was strikingly lower in AAV8-LDLr TAC mice compared with control TAC mice. Although this does not obligatory imply a difference in glucose oxidation, several lines of evidence suggest that cholesterol lowering interfered with a switch of fatty acids to glucose as metabolic substrate in mice with pressure overload. First of all, cholesterol-lowering gene therapy in TAC mice decreased myocardial protein levels of PDH, which catalyzes oxidative decarboxylation of pyruvate to form acetyl-CoA and increased protein levels of PDHK, which inactivates PDH. These data suggest that changes in glucose oxidation may parallel shifts in glucose uptake. Second, data on myocardial ACC protein levels and on p-AAC protein levels, representing the inactive form of the enzyme, suggest lower activity of this enzyme following cholesterol-lowering gene transfer. Because malonyl-CoA production via ACC inhibits fatty acid transport across the mitochondrial membrane via carnitine palmitoyl transferase I,23 reduced ACC activity following cholesterol lowering would maintain fatty acid oxidation. Finally, the potent effect of cholesterol lowering on fatty acid oxidation in TAC mice is indicated by the significantly increased PPAR-α levels in AAV8-LDLr TAC mice compared with control TAC mice. PPAR-α is a key transcriptional factor regulating substrate metabolism that induces CPT1B expression24 and expression of PDHK.25
The question of whether the metabolic phenotype is the cause or consequence of cardiac dysfunction has been debated. Cardiac-specific deletion of ACC 2, which induced a significant reduction of cardiac malonyl-CoA levels and led to a maintenance of fatty acid oxidation, attenuated cardiac hypertrophy and significantly reduced cardiac fibrosis following pressure overload.22 Therefore, the observations on ACC may be of particular importance to understand the effect of cholesterol lowering on cardiac structure and function. The observed effects of cholesterol lowering on ACC protein levels may reflect differences in p-mTOR levels. Sterol regulatory element binding protein (SREBP-1) activation may be regulated via mTORC-1,26, 27 and the mTOR inhibitor rapamycin has been shown to reduce the expression of many SREBP-1 target genes, including ACC.28
MicroRNAs play an important role in regulating lipoprotein metabolism,29 and these microRNAs also contribute to the regulation of fatty acid metabolism.30 The effect of plasma cholesterol levels on several microRNAs has been demonstrated.31 In the current study, we are forcing the system by drastically reducing plasma cholesterol levels via AAV8-mediated LDLr expression in hepatocytes. AAV8-mediated LDLr expression is expected to affect the levels of several microRNAs and may therefore affect mRNA stability or translation of transcription factors and enzymes involved in substrate metabolism. This may explain why the predominant effect in our experiments is at the level of protein expression and not, e.g., at the level of the degree of phosphorylation in case of proteins that are activated by phosphorylation.
The current study was designed as a prevention study because cholesterol-lowering gene therapy was performed before TAC. Therefore, the study is not the equivalent of a clinical intervention study in patients with established heart failure. The prevention study design allowed us to demonstrate a potent effect of cholesterol-lowering gene therapy on mortality after TAC but represents at the same time a limitation of the current study. Whether cholesterol-lowering gene therapy improves established heart failure remains to be established.
In conclusion, cholesterol-lowering gene therapy in mice improves survival, attenuates left ventricular hypertrophy, mitigates metabolic remodeling, and enhances cardiac function in a model of pressure-overload-induced cardiomyopathy. The effect of cholesterol lowering on p-mTOR and ACC may be a critical mediator of the observed beneficial effects. The profound impact of plasma cholesterol on myocardial biology suggests that targeted cholesterol-lowering therapies may be beneficial for prevention of heart failure.
Materials and Methods
For detailed methodology, see the Supplemental Materials and Methods.
Construction, Generation, and Production of Gene Transfer Vectors
Cholesterol-lowering gene therapy was performed using an adeno-associated viral (AAV) serotype 8 vector containing a hepatocyte-specific expression cassette32 to induce expression of the murine LDLr (AAV8-LDLr). The expression cassette of this vector consists of the 1,272 bp DC172 promoter, comprising an 890 bp α1-antitrypsin promoter fused together with two copies of the 160 bp α1-microglobulin enhancer,32 upstream of the human A-I 5′UTR containing the first intron (247 bp) followed by the murine LDLr cDNA sequence (2,598 bp), one copy of the 774 bp hepatic control region-1, and the rabbit β-globin polyadenylation signal (127 bp). AAV vector production was performed as described previously.33
In Vivo Experiments on the Effect of Cholesterol-Lowering Gene Therapy on Cardiac Remodeling
All experimental procedures in animals were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee of the Catholic University of Leuven (approval no. P154/2013). At the age of 12 weeks, female C57BL/6 LDLr−/− mice, originally purchased from Jackson Laboratories, were fed standard chow diet (Sniff Spezialdiäten) supplemented with 0.2% cholesterol 10% coconut oil to induce pronounced hypercholesterolemia. Gene transfer in C57BL/6 LDLr−/− mice was performed at the age of 15 weeks by tail-vein injection of 2 × 1012 genome copies/kg AAV8-LDLr. Control mice were untreated. To induce pressure overload, we performed TAC 2 weeks later.34, 35 TAC initially leads to compensatory hypertrophy of the heart, but over time the response to chronic hemodynamic overload becomes maladaptive and results in cardiac dilatation and heart failure. The sham procedure was identical except that no constriction on the aorta was applied. Anesthesia was performed with a single intraperitoneal injection of sodium pentobarbital (Nembutal; Ceva Sante Animale) at a dose of 40–70 mg/kg. All randomized mice were included in the analyses. Morphometric and hemodynamic quantifications were performed in a blinded fashion.
In Vivo Hemodynamic Measurements
Invasive hemodynamic measurements were performed 8 weeks after TAC or after sham operation as described previously.36 Mice were anesthetized by intraperitoneal administration of 1.4 g/kg urethane (Sigma).
Evaluation of Cardiac Glucose Metabolism by Micro-PET
Glucose uptake in the myocardium and in the skeletal muscle was quantified by micro-PET using [18F]-fluorodeoxyglucose (FDG) as a tracer (309 ± 22 μCi). Imaging was performed 60 min after tracer administration. Animals were anesthetized by inhalation of 2% isoflurane in 100% oxygen and underwent static imaging for 10 min on a micro-PET Focus 220 scanner (Concorde Microsystems). Images were reconstructed with ordered subset expectation maximization algorithm with six iterations (OSEM3D 6i) and analyzed with PMOD v.3.4 (Pmod Technologies).
Statistical Analysis
All data are expressed as means ± SEM. Parameters between four groups were compared by one-way analysis of variance followed by Bonferroni multiple comparisons post-test for comparing sham groups, TAC groups, and sham versus respective TAC groups using GraphPad Instat (GraphPad Software). When indicated, a logarithmic transformation or a square root transformation or a non-parametric test was performed. Parameters between two groups were compared using Student’s t test. When indicated, a logarithmic transformation, a square root transformation, or a non-parametric Mann-Whitney test was performed. The assumption of Gaussian distribution was tested using the Kolmogorov and Smirnov method. Kaplan-Meier survival curves were analyzed by log rank test using Prism4 (GraphPad Software). A two-sided p value of less than 0.05 was considered statistically significant.
Author Contributions
Conceptualization, I.M., B.D.G.; Methodology, I.M., A.P., T.D., U.H., P.P.V.V., O.G., F.J.; Formal Analysis, I.M., A.P., T.D., P.P.V.V., B.D.G.; Investigation, I.M., R.A., M.M., J.P.A.; Writing – Original Draft, I.M., B.D.G.; Writing – Review & Editing, R.A., M.M., J.P.A., T.D., U.H., P.P.V.V., O.G., F.J.; Funding Acquisition, B.D.G.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
I.M. is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. This work was supported by Onderzoekstoelagen grant OT/13/090 of the KU Leuven and grant G0A3114N of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, six figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.07.017.
Supplemental Information
References
- 1.Velagaleti R.S., Massaro J., Vasan R.S., Robins S.J., Kannel W.B., Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009;120:2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjekshus J., Apetrei E., Barrios V., Böhm M., Cleland J.G., Cornel J.H., Dunselman P., Fonseca C., Goudev A., Grande P., CORONA Group Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi L., Maggioni A.P., Marchioli R., Barlera S., Franzosi M.G., Latini R., Lucci D., Nicolosi G.L., Porcu M., Tognoni G., Gissi-HF Investigators Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 4.Felker G.M. Coenzyme Q10 and statins in heart failure: the dog that didn’t bark. J. Am. Coll. Cardiol. 2010;56:1205–1206. doi: 10.1016/j.jacc.2010.03.088. [DOI] [PubMed] [Google Scholar]
- 5.Moosmann B., Behl C. Selenoprotein synthesis and side-effects of statins. Lancet. 2004;363:892–894. doi: 10.1016/S0140-6736(04)15739-5. [DOI] [PubMed] [Google Scholar]
- 6.Glazer H.P., Osipov R.M., Clements R.T., Sellke F.W., Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle. 2009;8:1738–1746. doi: 10.4161/cc.8.11.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciarretta S., Volpe M., Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 2014;114:549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y., Soto J., Anderson B., Riehle C., Zhang Y.C., Wende A.R., Jones D., McClain D.A., Abel E.D. Regulation of fatty acid metabolism by mTOR in adult murine hearts occurs independently of changes in PGC-1α. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H41–H51. doi: 10.1152/ajpheart.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesselli D., Jakoniuk I., Barlucchi L., Beltrami A.P., Hintze T.H., Nadal-Ginard B., Kajstura J., Leri A., Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res. 2001;89:279–286. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux S.L., Florkowski C.M., George P.M., Pilbrow A.P., Frampton C.M., Lever M., Richards A.M. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008;52:1435–1441. doi: 10.1016/j.jacc.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N. Engl. J. Med. 2014;370:1756–1760. doi: 10.1056/NEJMcibr1402199. [DOI] [PubMed] [Google Scholar]
- 12.Xu J., Dang Y., Ren Y.R., Liu J.O. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc. Natl. Acad. Sci. USA. 2010;107:4764–4769. doi: 10.1073/pnas.0910872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shioi T., McMullen J.R., Tarnavski O., Converso K., Sherwood M.C., Manning W.J., Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 14.McMullen J.R., Sherwood M.C., Tarnavski O., Zhang L., Dorfman A.L., Shioi T., Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 15.Völkers M., Toko H., Doroudgar S., Din S., Quijada P., Joyo A.Y., Ornelas L., Joyo E., Thuerauf D.J., Konstandin M.H. Pathological hypertrophy amelioration by PRAS40-mediated inhibition of mTORC1. Proc. Natl. Acad. Sci. USA. 2013;110:12661–12666. doi: 10.1073/pnas.1301455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Li L., Niu X., Dang X., Li P., Qu L., Bi X., Gao Y., Hu Y., Li M. mTOR enhances foam cell formation by suppressing the autophagy pathway. DNA Cell Biol. 2014;33:198–204. doi: 10.1089/dna.2013.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 18.Akki A., Smith K., Seymour A.M. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol. Cell. Biochem. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 19.Nascimben L., Ingwall J.S., Lorell B.H., Pinz I., Schultz V., Tornheim K., Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 20.Sorokina N., O’Donnell J.M., McKinney R.D., Pound K.M., Woldegiorgis G., LaNoue K.F., Ballal K., Taegtmeyer H., Buttrick P.M., Lewandowski E.D. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 21.Pound K.M., Sorokina N., Ballal K., Berkich D.A., Fasano M., Lanoue K.F., Taegtmeyer H., O’Donnell J.M., Lewandowski E.D. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ. Res. 2009;104:805–812. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolwicz S.C., Jr., Olson D.P., Marney L.C., Garcia-Menendez L., Synovec R.E., Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ. Res. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saddik M., Gamble J., Witters L.A., Lopaschuk G.D. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 24.Mascaró C., Acosta E., Ortiz J.A., Marrero P.F., Hegardt F.G., Haro D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 1998;273:8560–8563. doi: 10.1074/jbc.273.15.8560. [DOI] [PubMed] [Google Scholar]
- 25.Huang B., Wu P., Bowker-Kinley M.M., Harris R.A. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes. 2002;51:276–283. doi: 10.2337/diabetes.51.2.276. [DOI] [PubMed] [Google Scholar]
- 26.Porstmann T., Santos C.R., Griffiths B., Cully M., Wu M., Leevers S., Griffiths J.R., Chung Y.L., Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laplante M., Sabatini D.M. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown N.F., Stefanovic-Racic M., Sipula I.J., Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism. 2007;56:1500–1507. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Aryal B., Singh A.K., Rotllan N., Price N., Fernández-Hernando C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017;28:273–280. doi: 10.1097/MOL.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dávalos A., Goedeke L., Smibert P., Ramírez C.M., Warrier N.P., Andreo U., Cirera-Salinas D., Rayner K., Suresh U., Pastor-Pareja J.C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desgagné V., Bouchard L., Guérin R. microRNAs in lipoprotein and lipid metabolism: from biological function to clinical application. Clin. Chem. Lab. Med. 2017;55:667–686. doi: 10.1515/cclm-2016-0575. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs F., Snoeys J., Feng Y., Van Craeyveld E., Lievens J., Armentano D., Cheng S.H., De Geest B. Direct comparison of hepatocyte-specific expression cassettes following adenoviral and nonviral hydrodynamic gene transfer. Gene Ther. 2008;15:594–603. doi: 10.1038/sj.gt.3303096. [DOI] [PubMed] [Google Scholar]
- 33.Lock M., Alvira M., Vandenberghe L.H., Samanta A., Toelen J., Debyser Z., Wilson J.M. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buys E.S., Raher M.J., Blake S.L., Neilan T.G., Graveline A.R., Passeri J.J., Llano M., Perez-Sanz T.M., Ichinose F., Janssens S. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H620–H627. doi: 10.1152/ajpheart.01236.2006. [DOI] [PubMed] [Google Scholar]
- 35.Hu P., Zhang D., Swenson L., Chakrabarti G., Abel E.D., Litwin S.E. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 36.Muthuramu I., Singh N., Amin R., Nefyodova E., Debasse M., Van Horenbeeck I., Jacobs F., De Geest B. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. J. Mol. Med. (Berl.) 2015;93:609–618. doi: 10.1007/s00109-015-1281-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.