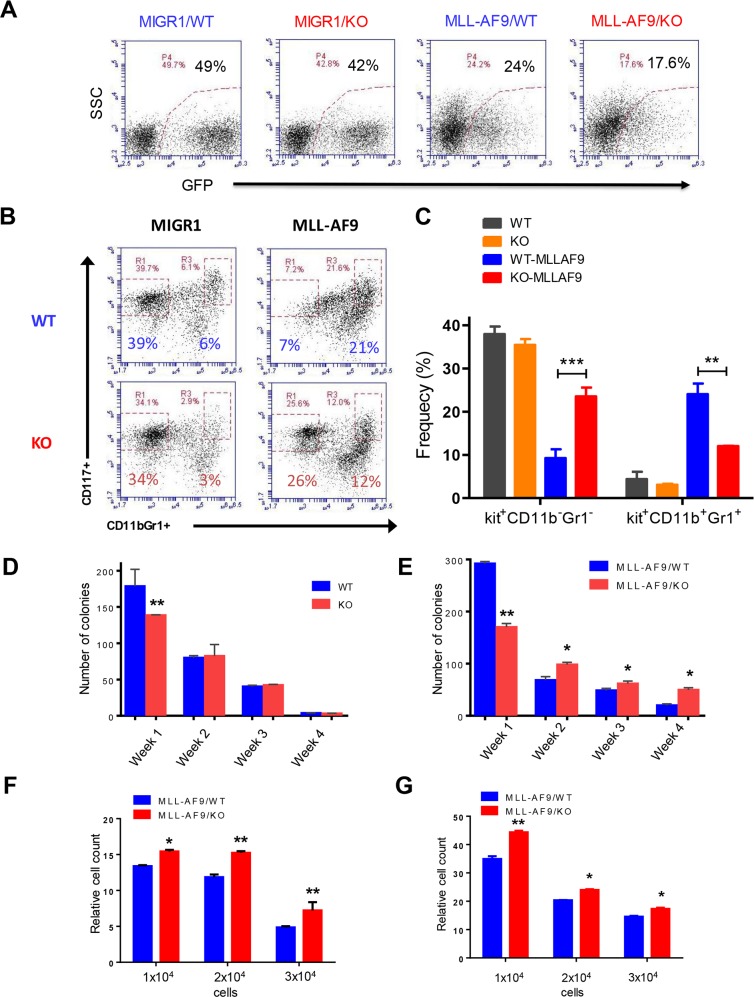
Figure 1. Necdin deficiency enhances the proliferation of hematopoietic progenitor cells expressing MLL-AF9.
(A) Fetal liver cells isolated from wild-type (WT) or Necdin knock-out (KO) mice were transduced with retroviruses expressing GFP (MIGR1) or MLL-AF9. Representative flow cytometry plots show the frequency of transduced cells (GFP+) 72 hours following transduction. (B) Transduced wild type and Necdin null fetal liver cells (GFP+) were cultured in serum free medium in the presence of cytokines for seven days. The frequency of hematopoietic stem and progenitor cells was determined by flow cytometry analysis. Representative flow cytometry plots show the frequency of Kit+CD11b−Gr1− and Kit+CD11b+Gr1+ cells at 7 days in liquid culture. (C) The frequency of Kit+CD11b−Gr1− and Kit+CD11b+Gr1+ cells in the liquid culture (**p<0.01, ***p<0.001, n=2). (D) Serial replating studies. CFUs were quantified by methylcellulose culture using WT and Necdin null fetal liver cells. The methylcellulose cultures were serially replated, weekly, for 4 weeks. Mean values (± SD) were shown (**p<0.01, n=3). (E) Necdin null fetal liver cells expressing MLL-AF9 show enhanced replating potential compared to WT cells (*p<0.05, **p<0.01, n=3). (F) and (G) Liquid culture of WT and Necdin null fetal liver cells expressing MLL-AF9. 48 (F) and 72 (G) hours later, cell proliferation was determined by cell counting. Cell growth was presented relative to the number of input cells in each group, set as 1 (*p<0.05, **p<0.01, n=3).