Abstract
The majority of inherited retinal degenerations converge on the phenotype of photoreceptor cell death. Second- and third-order neurons are spared in these diseases, making it possible to restore retinal light responses using optogenetics. Viral expression of channelrhodopsin in the third-order neurons under ubiquitous promoters was previously shown to restore visual function, albeit at light intensities above illumination safety thresholds. Here, we report (to our knowledge, for the first time) activation of macaque retinas, up to 6 months post-injection, using channelrhodopsin-Ca2+-permeable channelrhodopsin (CatCh) at safe light intensities. High-level CatCh expression was achieved due to a new promoter based on the regulatory region of the gamma-synuclein gene (SNCG) allowing strong expression in ganglion cells across species. Our promoter, in combination with clinically proven adeno-associated virus 2 (AAV2), provides CatCh expression in peri-foveolar ganglion cells responding robustly to light under the illumination safety thresholds for the human eye. On the contrary, the threshold of activation and the proportion of unresponsive cells were much higher when a ubiquitous promoter (cytomegalovirus [CMV]) was used to express CatCh. The results of our study suggest that the inclusion of optimized promoters is key in the path to clinical translation of optogenetics.
Keywords: retinal gene therapy, visual restoration, retinitis pigmentosa, optogenetics, AAV
Vision restoration using microbial opsins has substantial clinical potential; however, it requires high-level expression of a foreign protein in the patient’s eyes. Our study shows the feasibility of obtaining safe and functional expression in primates using a cell-specific promoter and provides the basis for further clinical development of this optogenetic strategy.
Introduction
Inherited retinal degenerations (IRDs) affect around 1 in 3,000 people.1 IRDs are a diverse group of conditions that result from mutations in any one of over 250 different genes, with the most common form being retinitis pigmentosa (RP).2 Despite the great diversity of mutations, RP converges on a phenotype of photoreceptor cell loss in later stages of the disease. Studies of post-mortem retinas from patients with RP have shown that a large percentage of inner retinal neurons remain present even after photoreceptor degeneration.3 In the absence of functional photoreceptors, electrical stimulation of these inner retinal neurons was shown to enable patients to recover some visual perception and even perform some reading tasks.4 These results collectively demonstrated that RGCs remain able to transmit information to the brain in late-stage RP.5 However, prosthetic approaches do not yet allow sufficient resolution for face recognition or locomotion in an unknown environment.6
As an alternative to retinal prosthesis, optogenetics can be used to restore vision by expressing optical neuromodulators such as channelrhodopsins or photochemically modified mammalian ion channels in residual retinal neurons.7, 8, 9, 10, 11, 12, 13, 14, 15 Expression of channelrhodopsin in RGCs might allow greater spatial resolution and acuity than that achieved with current prosthetic devices. This has motivated a large body of proof-of-concept studies in rodents and other small animals, which have shown the feasibility of the approach,7, 8, 9, 10, 16, 17, 18 and a first-in-human phase 1 clinical trial was initiated recently (ClinicalTrials.gov NCT02556736). However, the use of genetically encoded opsins for vision restoration has several setbacks in terms of clinical development. First, optogenetically equipped cells require very high-level opsin expression, as there is no amplification cascade behind microbial opsins.19 Although adeno-associated viruses (AAVs) have a favorable safety profile in clinical gene therapy,20, 21, 22, 23 their safety is dose dependent,24, 25 limiting the injected dose. This makes our ability to obtain functional-level microbial opsin expression with a safe viral dose uncertain. Second, microbial opsins require high-intensity light to induce action potential firing in neurons. High-intensity blue light, which is necessary to activate channelrhodopsin-2, might exceed the safety threshold of retinal illumination due to photochemical damage. The intensity of illumination necessary to trigger spikes depends on the number of opsin molecules expressed on the cell surface and the sensitivity of the microbial opsin. These parameters need to be considered in an animal model that most closely resembles humans. Thus far, studies assessing the feasibility of using microbial opsins for vision restoration have been carried out in small laboratory animals such as rodents, rabbits, and marmosets.7, 8, 9, 10, 26, 27 To our knowledge, the viral doses and construct optimizations necessary to obtain functional optogenetic readout have not yet been investigated in macaques.
For these reasons, we studied (to our knowledge, for the first time) the safety and light-intensity requirements of optogenetic vision restoration in cynomolgus macaques. AAV2 was chosen for gene delivery due to its efficacy in targeting RGCs and to the large body of knowledge using this serotype in non-human primate (NHP) studies24, 28, 29 and in human clinical trials.20, 21, 22, 23, 30 To obtain functional responses at lower light intensities, we optimized several parameters. First, we used a human codon-optimized Ca2+-permeable channelrhodopsin (CatCh), shown to be 70 times more sensitive than channelrhodopsin.31 Second, as we are limited in the number of AAV particles we can inject into the eye, we searched for a promoter sequence to increase the efficiency of transgene expression. The promoter sequence we designed is derived from the regulatory region of the human gamma-synuclein gene (SNCG) and leads to robust and specific transgene expression in RGCs in both mice and in NHPs when combined with AAV2 capsid. After the vector dosing studies in blind rd1 mice, we examined the efficiency of CatCh expression in RGCs of 20 macaque eyes over a period of 3–6 months. Our study shows (to our knowledge, for the first time) CatCh expression in macaque RGCs and CatCh-mediated light responses with light intensities below illumination safety limits. Results from our study will be highly valuable in guiding viral dose and promoter choice in further clinical development of this approach and other optogenetic approaches aimed at vision restoration. Furthermore, the retinal ganglion cell (RGC) promoter we describe here will benefit both basic research32 and clinical gene therapies targeting RGCs.33
Results
Strong, RGC-Specific Expression in the Mouse Retina by a New Promoter Sequence Based on the Regulatory Region of Human SNCG
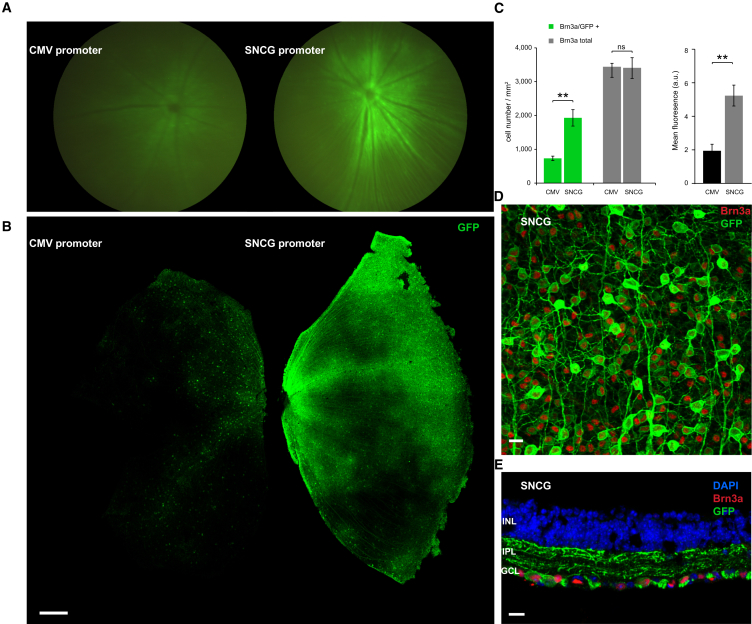
To identify a promoter sequence that can drive higher-level gene expression in RGCs than the previously used ubiquitous promoters such as cytomegalovirus (CMV), we searched for genes that are highly and exclusively expressed in RGCs.34 Data from the literature show that SNCG is expressed in both rodent and human ganglion cells independently of the RGC type.34 The promoter sequence of the gene, however, had not yet been described. We thus amplified a human genomic DNA fragment of 1 kB between the +1 of the SNCG gene and the untranslated 5′ region downstream (Figure S1). This amplified region contains the promoter, but we did not attempt any additional promoter characterization within this region (i.e., promoter bashing). We cloned this fragment upstream of humanized CatCh (human codon-optimized channelrhodopsin bearing the L132C mutation) in an AAV backbone. AAV2 vectors were produced with either a CMV or SNCG promoter driving the expression of CatCh in fusion with GFP. Five rd1 mouse eyes were intravitreally injected with AAV encoding CatCh-GFP under either CMV or SNCG promoter. Fluorescent fundus images showed higher fluorescence in all eyes injected with SNCG with respect to eyes injected with CMV (Figure 1A). Eyes were enucleated 8 weeks after injection and retinal flat mounts corroborated higher-intensity fluorescence with the SNCG promoter compared to CMV (Figure 1B). We evaluated the strength and efficacy of gene expression in RGCs by investigating localization of expression by co-labeling with Brn3a (Figure 1C). Brn3a is specifically expressed in RGCs, and antibodies against this transcription factor are considered a reliable marker to identify and quantify RGCs.35 Confocal microscopy images of retinal flat mounts (Figure 1D) and cross-sections (Figure 1E) from SNCG-CatCh-GFP retinas showed strong GFP expression in RGCs and this expression was highly co-localized with the Brn3a labeling. No expression was noted in deeper retinal layers and in ChAT-positive amacrine cells of the RGC layer (Figure S2). In cell quantification of such images, we showed that the Brn3a antibody labels an equal number of RGCs on retinas transfected with either the SNCG or CMV promoter. In SNCG retinas, 57% of these Brn3a-positive RGCs also expressed GFP, whereas this proportion decreased to 21% in CMV retinas (Figure 1C, left). Furthermore, GFP intensity, as quantified by area mean immunofluorescence (Figure 1C, right), was higher in retinas expressing GFP under the SNCG promoter. The differences between SNCG and CMV retinas were statistically significant (p < 0.01 for both Brn3a/GFP+ co-labeling and mean fluorescence values, unpaired t tests). The greater number of GFP-expressing Brn3a-positive RGCs and the intensity of GFP expression demonstrated the SNCG promoter’s efficacy in driving high-level gene expression in mouse RGCs (Figures 1D and 1E). The minor percentage of Brn3a-negative but GFP-positive cells might belong to a class of RGCs of the accessory optic, pretectal, or hypothalamic pathways that do not stain with Brn3a.35
Figure 1.
The SNCG Promoter Drives Higher-Level hCatCh-GFP Expression than the CMV Promoter in Mouse RGCs
(A) Fundus image of representative rd1 mouse retinae injected with 5 × 109 vg of either AAV2-CMV-hCatCh-GFP (left) or AAV2-SNCG-hCatCh-GFP (right). (B) Retinal flat mounts obtained from the same injection series showing CatCh-GFP fluorescence obtained under the CMV promoter (left) and the SNCG promoter (right). The scale bar represents 300 μm. (C) Quantification of Brn3a-positive, GFP-positive, and double-labeled cells in rd1 mouse retina injected with either AAV2-SNCG-hCatCh-GFP or AAV2-CMV-hCatCh-GFP. Confocal stack projections across the ganglion cell layer for cell counts over chosen fields in the central and peripheral regions of the retina. Regions were chosen in each quadrant and cell counts were averaged to obtain Brn3a-positive, GFP-positive, and co-labeled cells per square millimeter. Mean fluorescence values were measured over the same areas using the same number of z stacks. Error bars represent the SEM. (D) Representative confocal stack projection across the RGC layer of rd1 mouse retina transduced with SNCG-CatCh-GFP, co-labeled with Brn3a (red) and anti-GFP (green) antibodies. (E) Cross-sections obtained from one representative retinal flat mount in the SNCG-CatCh-GFP injected retinas co-labeled with Bnr3a (red) and anti-GFP (green) and nuclei were labeled with DAPI (blue). Scale bars in (D) and (E) represent 10 μm. **p < 0.01, statistically significant.
Retinal and Cortical Responses following CatCh Expression in RGCs of the rd1 Retina under the SNCG Promoter
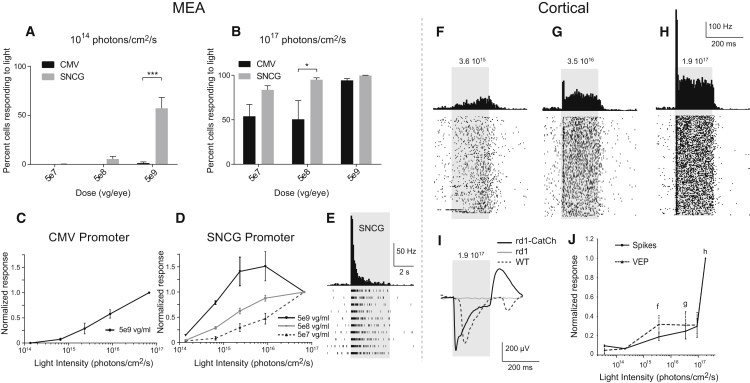
To demonstrate that selective RGC targeting of CatCh-GFP can restore visual function in blind rd1 retinas (age >12 weeks), we recorded spiking activity from RGCs using a multi-electrode array (MEA) (252 electrodes) 8–12 weeks after injection (Figures 2A–2E). rd1 mice were injected at 4–8 weeks after birth with three doses of AAV2 encoding CatCh-GFP either under the CMV or SNCG promoter. Wild-type (c57bl6) mice and uninjected rd1 mice served as controls. All rd1 retinas were recorded in presence of l-(+)-2-amino-4-phosphonobutyric acid (L-AP4). Only the wild-type controls were recorded without L-AP4. A variety of response profiles were observed in wild-type retinal ganglion cells (Figure S3) at an intensity of 1014 photons/cm2/s. Ganglion cells from rd1 mice expressing CatCh displayed only ON responses, between 1014 to 1017 photons/cm2/s. Light-evoked spiking activity was observed with 2-s full-field flashes (Figures 2A–2E), whereas control rd1 retinas did not show any light-elicited increase in spiking activity (data not shown). The percentage of cells responding to light was dependent on the viral dose (Figures 2A and 2B). Greater numbers of recorded cells responded in rd1 animals injected with the SNCG promoter at the highest AAV dose at 1014 photons/cm2/s (Figure 2A; p < 0.001, two-way ANOVA followed by Holm-Sidak multiple comparisons). This improved sensitivity corroborates that SNCG drives higher-level CatCh expression in RGCs, ensuring light responses with a lower viral load. Firing rate frequency was intensity dependent, giving rise to robust light responses at 1014 photons/cm2/s for CatCh expressed under the SNCG promoter at a viral dose of 5 × 109 viral genome (vg) per eye (Figure 2A). The normalized firing rate increased with rising light intensities (Figures 2C and 2D), with a representative raster plot and peri-stimulus time histogram showing an increase in spike frequency during full-field flashes at 480 nm in a retina expressing CatCh under control of the SNCG promoter (Figure 2E). To demonstrate the functional efficacy in live animals, we recorded local field potentials and spiking activity in the visual cortex in vivo. For this experiment, another series of rd1 mice were injected with AAV2-SNCG-hCatCh-GFP, and electrophysiological recordings were performed in the visual cortex in response to increasing light intensities (Figures 2F–2J). Visually evoked potentials (VEPs) were recorded in the contralateral hemisphere when the treated eye was stimulated with 200-ms pulses of blue light (with light intensities up to 1.7 1017 photons/cm2/s) repeated 200 times at 1 Hz (Figures 2F–2H). Due to the use of blue light, a small percentage of recorded RGCs in MEA experiments and some of the cortical responses could be mediated by intrinsically light sensitive retinal ganglion cells (ipRGCs)36 depolarized with the same wavelength. No VEPs were visible (flat traces) on recordings from untreated rd1 mice. CatCh-driven VEPs had shorter latencies compared to VEPs measured in wild-type mice (Figure 2I) as described previously.12, 13 A shorter latency is expected because the phototransduction cascade and subsequent retinal computation done by the circuit are bypassed. Figures 2F–2H and 2J illustrate the intensity dependence of the cortical spiking responses, showing that sizeable light responses are observed at light intensities as low as 1015 photons/cm2/s. The latency of the light-elicited spikes in CatCh-treated rd1 mice was shorter (10.1 ± 2 ms, n = 3) than the mean ON latency in wild-type mice (52.98 ± 3.83 ms, n = 3) as expected. These functional results clearly demonstrate that AAV-mediated expression of CatCh under the SNCG promoter can activate RGCs in the blind mouse retina and restore light sensitivity up to the visual cortex.
Figure 2.
Functional CatCh Responses in the Retina and Cortex of rd1 Mice
(A and B) Percentages of cells with spontaneous activity showing a light response at 480 nm under either 1014 photons/cm2/s (A) or 1017 photons/cm2/s (B) in retinas expressing CatCh under control of CMV (black) or SNCG (gray) at 5 × 107, 5 × 108, and 5 × 109 vg per eye (n = 4). (C) Response amplitude (normalized to the response obtained at maximum luminance) as a function of light intensity in retinas expressing CatCh under control of CMV promoter at 5 × 109 viral particle dose (n = 4, 155 cells). (D) Response amplitude (normalized to the response obtained at maximum luminance) as a function of light intensity in retinas expressing CatCh under control of the SNCG promoter at a dose of 5 × 107 (light gray; n = 4 retinas, 158 cells), 5 × 108 (dark gray; n = 4 retinas, 221 cells), and 5 × 109 (black, n = 4 retinas, 261 cells) viral particles (vg) per eye. (E) Raster plots and peri-stimulus time histogram (PSTH) showing the light response or increase in spike frequency during full-field flashes at 480 nm in a retina expressing CatCh under control of the SNCG promoter. Note that with the SNCG promoter, the curve reaches a plateau at the maximum AAV vector dose. (F–H) PSTHs (top) and corresponding raster plots (bottom) of visual cortex neurons of rd1 mice expressing SNCG-CatCh in response to 475-nm full-field flashes at three different increasing light intensities of 1015 (F), 1016 (G), and 1017 (H) photons/cm2/s, respectively. (I) Comparison of visually evoked potentials (VEPs) recorded in rd1 retinas treated with the 5 × 109 vg dose of SNCG-CatCh, compared to untreated rd1 retinas and wild-type mouse retinas. (J) Normalized cortical activity (spikes and VEP) as a function of light intensities at 475 nm (n = 3 mice). Error bars represent SEM. ***p < 0.001, statistically significant.
In Vivo Inflammatory Responses in NHP Eyes
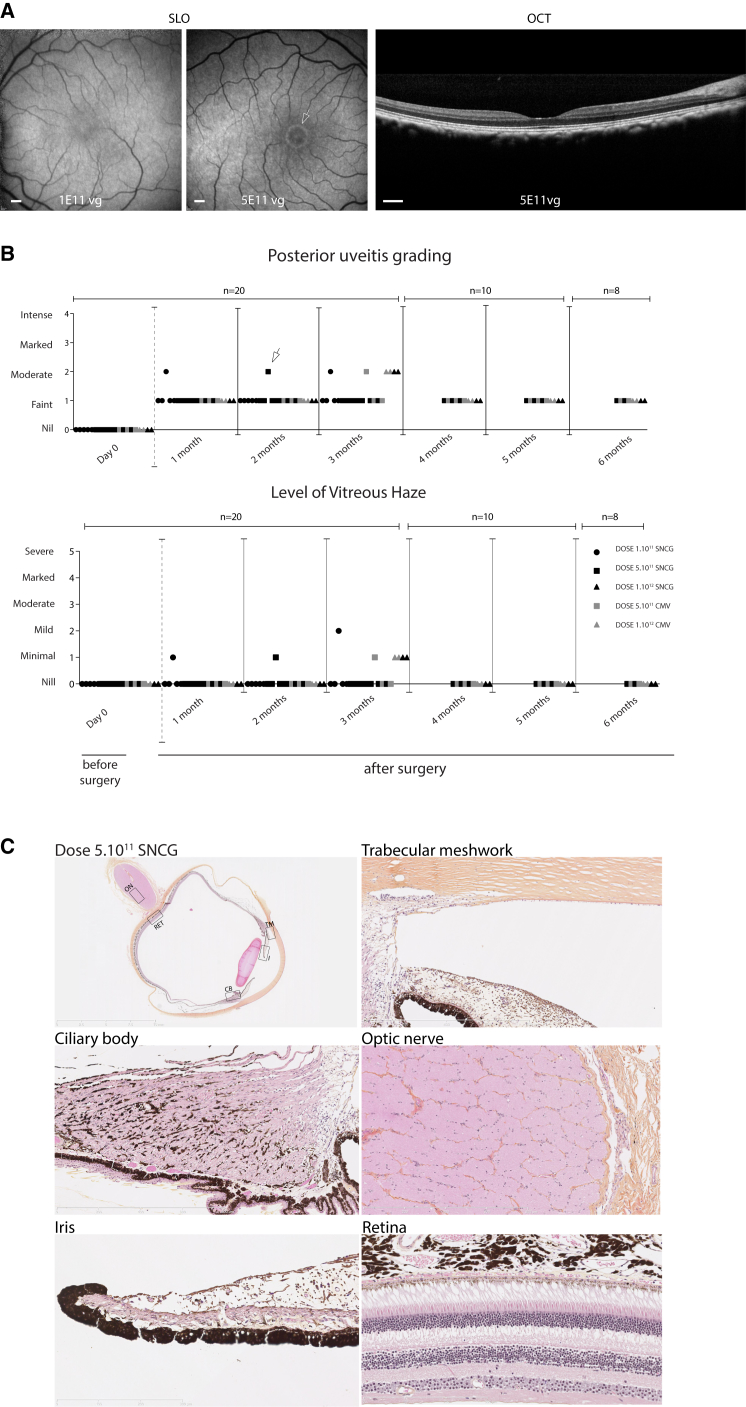
In mouse studies, we used doses ranging from 5 × 107 to 5 × 109 vg per eye. We observed that using low light intensities required for minimizing phototoxicity in humans (range, 1014–1015 photons/cm2/s), the particle numbers necessary to obtain light responses on MEA and at the visual cortex were in the range of 5 × 108 to 5 × 109 vg per eye. Since the volume of the macaque vitreous is about 100 times greater than the vitreous volume of a mouse, we decided to use the pharmacological equivalent of this dose range in our NHP experiments. Ten NHP were chosen based on the absence of neutralizing antibody titers against AAV2 in their blood sera.37 One primate was injected intravitreally with two vector doses (one eye with 1 × 1011 and the other with 5 × 1011 vg), with CatCh expressed under the SNCG in fusion with GFP to monitor gene expression in vivo (NHP1). As GFP can be immunogenic,24 we used CatCh without a fluorescent tag for the remaining nine primates. 18 macaque eyes were injected intravitreally with either 1 × 1011 (n = 5 eyes) or 5 × 1011 (n = 5 eyes) or 1 × 1012 AAV2 particles encoding CatCh under the SNCG or CMV promoter (n = 4 eyes) (Table 1). NHPs were followed for 3–6 months with regular ophthalmic exams (Figures 3A and 3B). We then used the Spectralis high resolution angiography (HRA) system to examine the eye fundus. This instrument is built on a confocal scanning laser ophthalmoscopy (cSLO) platform that is sensitive to the fluorescence of Fluoresceine or GFP, providing focused and high contrast images of the back of the eye. For the GFP construct, green fluorescence (shown in white) detected by the Spectralis HRA system showed gene expression for the 5 × 1011 dose starting at 1 month post-injection. Fluorescence increased up to 8 weeks post-injection (Figure 3A, middle) but was invisible for the low-dose injection (Figure 3A, left). Expression stabilized between 8 and 12 weeks post-injection. This in vivo observation was consistent with CatCh expression in the retina for high viral vector doses, a result that was subsequently confirmed functionally and histologically in retinas where CatCh was expressed without the GFP tag (see below).
Table 1.
Intravitreal Injections in NHPs Indicating the Primate Number, Vector Used, Dose, and Time after Injection
| NHP No. | Eye | AAV Vector | Dose (vg/eye) | Age (Years) | Euthanasia (Months) |
|---|---|---|---|---|---|
| 1 | right | AAV2-SNCG-CatCh-GFP | 1 × 1011 | 10 | 3 |
| left | AAV2-SNCG-CatCh-GFP | 5 × 1011 | |||
| 2 | right | AAV2-SNCG-CatCh | 5 × 1011 | 8 | 3 |
| left | AAV2-SNCG-CatCh | 5 × 1011 | |||
| 3 | right | AAV2-SNCG-CatCh | 5 × 1011 | 8 | 3 |
| left | AAV2-SNCG-CatCh | 5 × 1011 | |||
| 4 | right | AAV2-SNCG-CatCh | 1 × 1011 | 4 | 4 |
| left | AAV2-SNCG-CatCh | 1 × 1011 | |||
| 5 | right | AAV2-SNCG-CatCh | 1 × 1011 | 4 | 6 |
| left | AAV2-SNCG-CatCh | 1 × 1011 | |||
| 6 | right | AAV2-CMV-CatCh | 1 × 1012 | 9 | 6 |
| left | AAV2-CMV-CatCh | 1 × 1012 | |||
| 7 | right | AAV2-SNCG-CatCh | 1 × 1012 | 8 | 6 |
| left | AAV2-SNCG-CatCh | 1 × 1012 | |||
| 8 | right | AAV2-SNCG-CatCh | 5 × 1011 | 6 | 6 |
| left | AAV2-SNCG-CatCh | 5 × 1011 | |||
| 9 | right | AAV2-SNCG-CatCh | 5 × 1011 | 16 | 4.5 |
| left | AAV2-CMV-CatCh | 5 × 1011 | |||
| 10 | right | AAV2-CMV-CatCh | 5 × 1011 | 6 | 6 |
| left | AAV2-CMV-CatCh | 5 × 1011 |
Figure 3.
In Vivo Imaging, Ophthalmic Examinations, and Histopathology in AAV2-CatCh-Injected NHPs
(A) Fluorescence fundus images of NHP1 injected with low-dose (left) and high-dose (center) AAV2-SNCG-CatCh-GFP, respectively. OCT scan of the high-dose eye. Images were obtained at 2 months post-injection. Fluorescence around the fovea (white arrow) is only visible in the high-dose-injected left eye. Scale bars represent 500 μm. (B) Uveitis and the level of vitreous haze scores for eyes from all 10 NHPs that received low-dose (1011 vg), mid-dose (5 × 1011 vg), and high-dose (1012 vg) injections of AAV2 encoding CatCh under SNCG or CMV promoters. (C) Histo-pathological examination of the eye from NHP3 with a mid-dose injection of AAV2-SNCG-CatCh at 3 months post-injection [eye indicated with a white arrow in (B)]. Retinal slice across the vertical meridian of the eye imaged at a resolution of ×40. Absence of detectable lymphocytes, macrophages, or damage to ocular structures in the trabecular meshwork, ciliary body, optic nerve, iris, and retina on magnified areas.
Standardized uveitis nomenclature38, 39 was used to score anterior chamber flare, cells, corneal precipitates (Figure S4), posterior uveitis, and the level of vitreal haze (Figure 3B). Between months 1 and 3, one animal in each group showed minimal to mild vitreal haze and faint to moderate posterior uveitis. All vitreal haze resolved by 4 months post-injection. A small focal vein coating resembling periphlebitis was identified in the far periphery of four animals by indirect ophthalmoscopy at 2 months post-injection. Fluorescent angiography was performed in these animals and showed no pathological signs (Figure S4C). All of these symptoms resolved by month 3 after the injection. Although the number of macaques in each dosing group is too small to conclude, it seems there was no correlation between the viral dose and the inflammatory symptoms. This suggests that the inflammatory signs were in response to the procedure rather than to the viral vector or transgene product itself. Finally, none of the observed events of the inflammatory reaction throughout the 5- to 6-month observation period had an influence on vision.
One eye from animal NHP3, which showed moderate posterior uveitis and minimal vitreal haze in the 5 × 1011 vg group (eye indicated with a white arrow in Figure 3B), was enucleated at 3 months and examined for pathological signs related to treatment. The entire eye was fixed, sectioned, and observed using Nanozoomer technology allowing ×40 resolution anywhere within the section (Figure 3C). No structural changes indicative of inflammation (existence of lymphocytes or plasma cells in the trabecular meshwork and the irido-corneal angle, inflammatory cells in the vitreous, or perivascular lymphocytes in the retina) were noted (Figure 3C). Despite the variable levels of vitreal haze and cells observed upon indirect ophthalmoscope evaluation at 1–2 months post-injection (Figures 3B and 3C), the absence of inflammatory cells and retinal damage at 3 months indicates that any preceding immune reaction did not lead to permanent changes in retinal structure. Overall, the retina and anterior segments of the eye were void of any signs of damage or inflammation.
Electrophysiological Recordings Reveal CatCh-Driven Responses in RGCs around the Fovea at 3 Months Post-Injection
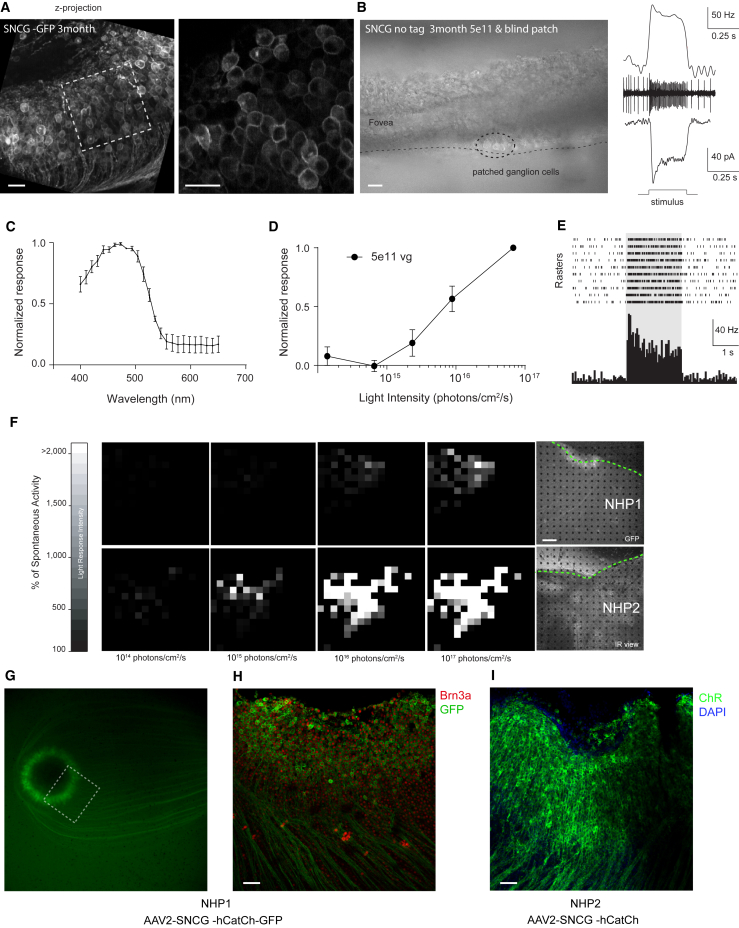
To investigate the functionality of CatCh expression, NHP1 was injected with AAV2-SNCG-CatCh-GFP and was euthanized 3 months post-injection. Retinas were dissected and foveal regions were carefully cut into two halves for MEA and patch-clamp recordings because the green fluorescence attributed to GFP was maximal in this area (Figures 4A, 4G, and 4H). In all of our experiments, endogenous ON responses were blocked using the metabotropic glutamate receptor agonist L-AP4 at 50 μM in the bath solution. We previously validated the L-AP4 blockade of all ON responses in wild-type retinas in both mouse, macaque, and human retinas.11, 15
Figure 4.
Characteristics of CatCh-Mediated Light Responses at 3 Months Post-Injection
(A) Two-photon images of the peri-foveolar region of NHP1, injected with AAV2-hCatCh-GFP displaying the high density of transfected cells with membrane-bound expression. The white dotted square on the left indicates the area displayed at higher magnification on the right. (B) Retinal slice showing the peri-foveolar region of NHP2 injected with AAV2-hCatCh (no GFP tag), where light-responsive ganglion cells were recorded. Recordings in the cell-attached mode show spikes and their increase in frequency during light stimulation. Typical spiking response and photocurrent of cells recorded in cell-attached or whole-cell patch-clamp configuration, respectively. Scale bars in (A) and (B) represent 20 μm. (C) Average spectral tuning at 1017 photons/cm2/s after application of L-AP4 in primate retinas expressing hCatCh. (D) Average normalized response to different stimulus intensities. (E) Raster plot and peri-stimulus time histogram of ganglion cell responses to full-field flashes at 480 nm after application of L-AP4 in primate retina injected with AAV2-SNCG-hCatCh-GFP. (F) Grayscale maps based on firing rates of responding neurons (expressed as a percentage of their spontaneous activity) at increasing light intensities in primate retinas injected with AAV2-SNCG-hCatCh-GFP (top) and AAV2-SNCG-hCatCh (bottom) at a 5 × 1011 vg per eye dose. The macular area is indicated by dashed green lines and the black dots represent the locations of the MEA recording electrodes. The scale bar represents 200 μm. (G) The macular region of NHP1 prior to dissection, showing CatCh expression in the peri-foveolar ring. (H) Half of the foveal ring as indicated by the white rectangle in (G), after MEA recordings and RGC immunolabeling. The retinal flat mount was stained with Brn3a (red) and GFP antibodies (green). (I) The foveal region of retina from NHP2 [shown in the lower right panel of (F)] after labeling with antibodies against channelrhodopsin (green). Nuclei were stained with DAPI. Scale bars in (H) and (I) represent 50 μm. Error bars represent SEMs.
At the single-cell level, cell-attached recordings revealed ON light responses under 1.46 1016 photons/cm2/s at 470 nm in RGCs from the AAV2-SNCG-CatCh-GFP retina and in the retinas from NHP2 infected with AAV2-SNCG-CatCh without GFP, which were recorded without assistance by fluorescent labeling (Figure 4B, left and center/top right). In all patched cells under whole-cell configuration, we observed typical channelrhodopsin-evoked photocurrents consisting of a fast transient current followed by a steady-state current at a holding potential of −60 mV (Figure 4B, bottom right). To define how these photocurrents control RGC activity at the population level, retinal flat mounts were recorded with the MEA technique. Spectral tuning of the firing frequency was calculated and showed the highest frequency responses to 480 nm light (Figure 4C), which corresponds to the excitation peak of ChR2.40 The firing rate frequency of responsive cells was intensity dependent, with the maximum frequency reached at the maximum light intensity applied (1017 photons/cm2/s) (Figure 4D). Figure 4E illustrates raster plots from a single-unit recording of left eye of NHP1 (5 × 1011 vg). Data in Figure 4F show the distribution of light responses represented as a percentage of spontaneous activity over light intensities ranging from 1014 to 3 × 1017 photons/cm2/s for NHP1 and NHP2. Similar light responses were obtained for the right eye of NHP2 injected with 5 × 1011 vg and both eyes from NHP3. 45%–90% of electrodes detected light responses in the four retinas injected with the dose of 5 × 1011 vg. Our results by patch clamp and MEA indicate that the GFP tag is not required to obtain functional CatCh expression in RGCs. Eyes injected with the 1011 vg dose showed either no responses or only a few cells were responsive, suggesting that 1011 particles are at the threshold where we can expect reliable optogenetic activation of RGCs through expression of CatCh.
After MEA experiments, retinal flat mounts were stained with antibodies against channelrhodopsin41 and Brn3a. Tissue from NHP1 was labeled with anti-GFP antibodies in green and anti-Brn3a antibodies in red (Figure 4H). This is the only retina where we could use the RGC-specific marker (Brn3a) in conjunction with an antibody indicating localization of CatCh, as both Brn3a and anti-channelrhodopsin antibodies are produced in the same species. In this tissue spanning an ∼1-mm square from the center of the fovea, we counted Brn3a(+) and CatCh-GFP(+) cells in half a circle with a 600-μm radius around the fovea (Figures 4G and 4H). In this area, around 37% of Brn3a(+) cells were also positive for GFP (523 GFP-positive cells for 1,413 Brn3a-positive cells). In the tissue from NHP2, we counted 1,351 CatCh-positive cells for one-half of a retina and 455 in the contralateral retina in the region spanning 600 μm from the center of the fovea (Figure 4I). One of the retinas from the 5 × 1011 dose group was damaged following the MEA recording process and could not be used for immunofluorescence labeling. Our results indicate that at least one-fourth of peri-foveolar RGCs were labeled with CatCh after an injection with the 5 × 1011 vg dose.
CatCh-Driven Light Responses at 6 Months Post-Injection
The electrophysiological experiments done at 3–4 months post-injection showed that SNCG-driven CatCh expression is safe and functional at a viral dose of 5 × 1011 vg. Next, we aimed to extend the dosing range and compare SNCG to the CMV promoter at a 6-month time point. This extended time point was chosen as previous studies established that after intravitreal delivery of AAV2, the transgene expression levels are stable after 6 months.29 Based on this, we first wanted to examine whether the low dose (1 × 1011 vg) would lead to better functional results at 6 months. NHP5 was euthanized at 6 months post-injection and MEA was used to measure light responses as described previously. A few light-responsive cells were present in the right eye of this animal injected with 1 × 1011 vg. The contralateral eye was unresponsive, confirming that the 1011 vg dose is too low to obtain reliable optogenetic activation.
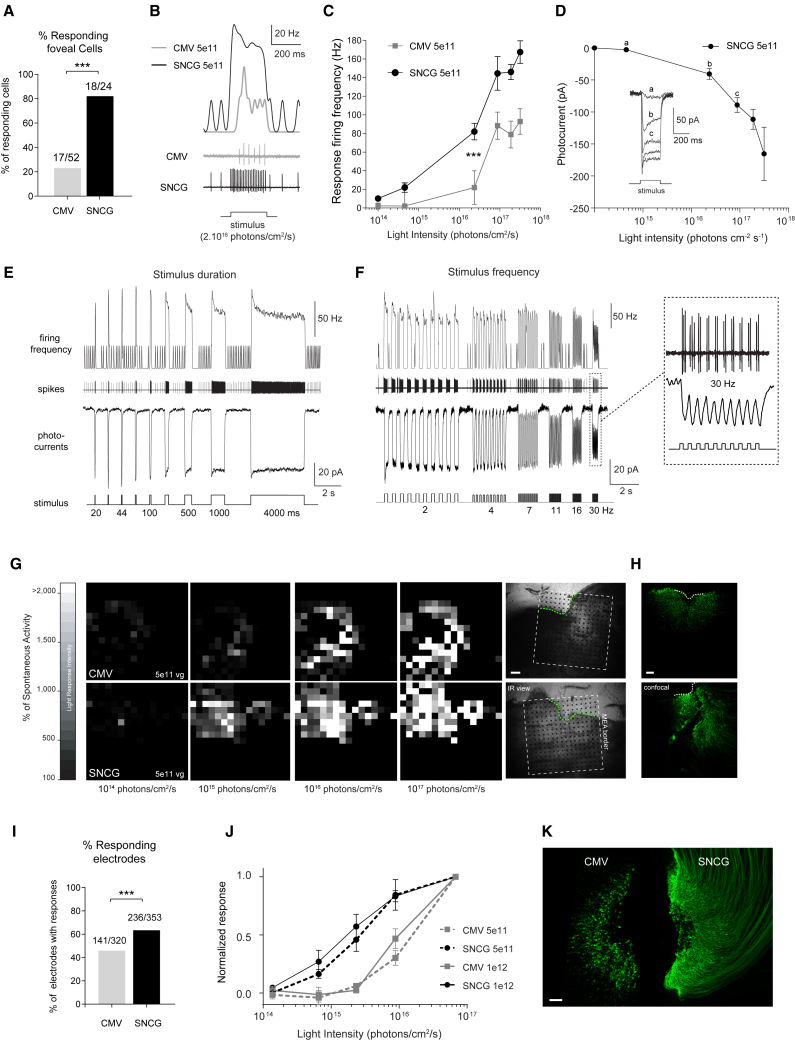
For the rest of the study, we focused on comparing CatCh-mediated responses under CMV or SNCG at the two highest doses (5 × 1011 vg and 1012 vg). Recordings were first performed on macaques injected with 5 × 1011 vg of either AAV2-CMV-CatCh (n = 3 retinas) or AAV2-SNCG-CatCh (n = 3 retinas). First, we compared light responses with CMV-CatCh to SNCG-CatCh using single-cell recordings. Of the 52 peri-foveolar RGCs recorded in the CMV-CatCh group, 17 were responsive to light. This ratio was as high as 18 of 24 cells for SNCG-CatCh (Figure 5A; p < 0.001, chi-square). RGCs recorded in the cell-attached configuration showed significantly higher spiking frequencies for SNCG-CatCh compared to CatCh expressed under the control of CMV (Figure 5B; see averaged response in Figure 5, p < 0.001 by the t test at 2 × 1016 photons/cm2/s, unpaired t test). Response latencies were also significantly shorter with SNCG than CMV (see Figure 5B; first spike latency of 12.5 ms and 32.5 ms at 1016 photons/cm2/s, respectively; p < 0.01 at 2 × 1016 photons/cm2/s, unpaired t test). Next, we recorded cells expressing CatCh under SNCG in the whole-cell patch-clamp configuration in order to measure CatCh-elicited inward currents at different light intensities. We found that photocurrents can be elicited at just over 1014 photons/cm2/s and response amplitudes increase non-linearly with light intensity (Figure 5D). We observed photocurrents (with average tau on (τOn) ∼8 ms and tau off (τOff) ∼16 ms at 2 × 1016 photons/cm2/s) characterized by a transient phase followed by a robust steady-state phase under whole-cell configuration. These currents were converted into robust spiking activities under the cell-attached configuration, lasting for the duration of the stimuli ranging from 20 ms to 4 s in our conditions (Figure 5E). These photocurrents and spiking activity were fast enough to follow up to 30-Hz light pulses (Figure 5F). Next, the light-intensity requirements at the population level were investigated over the entire macular region using MEA recordings. Light responses originated from the peri-foveolar area where GFP expression was observed on the retinal flat mount and their amplitude increased with stimulation intensity (Figure 5G). Figure 5H represents confocal stack projections of the same retinas labeled with ChR2 (green) antibodies. In agreement with our single-cell recordings, the number of light-responsive cells over the total number of cells displaying spontaneous activity was significantly higher in retinas transduced with SNCG-CatCh compared to CMV-CatCh (Figure 5I; p < 0.001, chi-square). Normalized spiking frequencies across all eyes treated with SNCG (n = 3) and CMV-CatCh (n = 3) at a 5 × 1011 vg dose are shown in Figure 5J. MEA recordings over a larger population of RGCs in the macular region show the higher light sensitivity of RGCs expressing CatCh under the SNCG promoter compared to CMV. Firing frequencies recorded across three retinas for each condition indicate a clear separation in the light sensitivity of the responses obtained using the two promoters.
Figure 5.
Characteristics of CatCh-Mediated Light Responses at 6 Months Post-Injection
(A–F) Results from single-cell recordings (using cell-attached and whole-cell patch-clamp configurations) of ganglion cells recorded in the peri-foveolar region of retinas injected with either CMV-CatCh or SNCG-CatCh at a dose of 5 × 1011 vg, after application of L-AP4. (A) Comparison of the proportions of responsive cells recorded either from CMV-CatCh or SNCG-CatCh. (B) Representative recordings showing the spiking frequency obtained for SNCG-CatCh compared to CMV-CatCh (bottom, raw data; top, rate evolution curves) during light stimulation (intensity of 2 × 1016 photons/cm2/s, wavelength of 480 nm). (C) Response firing frequency of RGCs as a function of light stimulation intensity for CMV-CatCh and SNCG-CatCh at the 5 × 1011 vg viral dose. (D) CatCh photocurrents (inward currents recorded at −60 mV in whole-cell configuration) of RGCs as a function of light stimulation intensity for SNCG-CatCh at 5× 1011 vg per eye. Photocurrents of a representative cell are shown under the intensity curve. (E) Response characteristics to varying stimulus duration. The middle and top rows show raw data (cell-attached) of the spiking activity and firing frequency in response to stimuli of increasing duration (20 ms to 4 s). The bottom row shows the photocurrent recorded in the whole-cell configuration from another representative cell using the same pattern of stimulation. (F) Response characteristics to varying stimulation frequencies. The middle and top rows show raw data (cell-attached) showing the spiking activity of a cell and its firing frequency in response to stimuli of increasing frequencies (2–30 Hz). The bottom row shows photocurrents recorded in another representative cell using the same pattern of stimulation. A magnified trace is shown at 30 Hz displaying the robust photocurrents and spiking activity obtained for each of the 10 successive stimulations. (G–K) Results from MEA recordings and histology. (G) Grayscale maps based on firing rates of responding neurons (expressed as a percentage of their spontaneous activity) at increasing light intensities in primate retinas injected with AAV2-SNCG-hCatCh-GFP (top) and AAV2-SNCG-hCatCh (bottom) at 5 × 1011 vg per eye, after application of L-AP4. On the right, the MEA chip perimeter is represented by a dotted white square, and the putative fovea area is indicated by a dashed green curves in the pictures. The black dots represent the locations of the MEA recording electrodes. The scale bar represents 200 μm. (H) The same foveal regions after MEA recordings and RGC immunolabeling. The retinal flat mount was stained with antibodies against channelrhodopsin (green). Note that the SNCG retina was partially damaged while handling the tissue after MEA procedures. The scale bar represents 100 μm. (I) Percentage of responsive MEA electrodes for each promoter at 5 × 1011 vg per eye. (J) Normalized responses of RGCs to light at increasing intensities at 6 months post-injection. Each line represents discharge frequencies normalized across retinas for the high-dose (n = 2 retinas) and low-dose (n = 3 retinas) groups. (K) Foveal regions of two representative NHP retinas that received a 1012 vg dose of AAV-CatCh expressed under the CMV (left) or SNCG (right) promoter, after MEA recordings and immunolabeling. The retinal flat mount was stained with antibodies against channelrhodopsin (green). The scale bar represents 100 μm. Error bars represent SEMs. ***p < 0.001, statistically significant.
Finally, we investigated the utility of further increasing the viral dose to 1012 vg using both CMV and SNCG promoters. MEA recordings of the retina expressing CatCh under either the CMV (n = 2) or the SNCG (n = 2) promoter at the 1012 particle dose corroborate previous conclusions on the better efficacy of SNCG compared to CMV. This superiority was confirmed at both the functional (Figure 5J) and cell density (Figure 5K) levels. Using the highest dose (1012 vg) slightly increased the maximum multi-unit spiking frequencies obtained with SNCG. The firing frequencies with SNCG were already very high (276.3 ± 17.9 Hz compared to 199.6 ± 40.6 Hz at 5 × 1011 vg under 8 × 1015 photons/cm2/s) at 5 × 1011 vg. Moreover, the light-sensitivity curves were similar for the two doses with the SNCG promoter as well as with the CMV promoter (Figure 5J). These results clearly demonstrate the superiority of the SNCG promoter, which cannot be compensated for by increasing the viral dose while using the CMV promoter.
Discussion
There is great interest in developing optogenetics as a therapeutic modality that can be applied in humans with photoreceptor degeneration. To predict the safety and feasibility of optogenetics in humans, gene delivery-related parameters need to be examined in NHPs, which most closely represent the human eye and immune system. There are two primate models that can be used for this type of experiment: macaques and marmosets. The marmoset has a small eye with an axial length of 11 mm, and AAV transduction patterns in this animal model differ from macaques in terms of cellular selectivity, spatial pattern, and depth of transduction through the retina.28 Immune responses in the marmoset are weaker than macaques, although marmosets have an advantage over macaques for functional studies because genetic neurodegeneration models can be generated in this species.42 As there is no NHP model of retinal degeneration available today, we decided to use macaques for our study. The success of optogenetics in vision restoration depends on our ability to express a functional amount of the microbial opsin without eliciting immune responses to the gene delivery vector and the opsin itself. Finally, the light-intensity requirements for functional activation need to fall in the range of radiation safety limits for the human eye. In view of these requirements, we explored the safety and feasibility of RGC activation with CatCh in the macaque retina. Several AAVs have been tested for retinal gene delivery via the intravitreal route, and some have evoked safety concerns that have not been fully addressed at this stage.25, 43 For example, a recent study suggested that the engineered AAV variant AAV2-7m8 might be more immunogenic when used at high doses via intravitreal injections.25 Therefore, we chose AAV2 for its known safety and efficacy in targeting RGCs in NHP studies27, 28, 29 and the large body of work using this vector in human clinical trials.20, 21, 22, 23, 44 High-level expression with a limited viral dose being a major parameter in obtaining functional expression, we designed a new RGC-specific promoter, driving strong transgene expression in RGCs. We show that this promoter, named SNCG, drives expression in twice as many RGCs compared to the ubiquitous CMV promoter. We also found that the strength of transgene expression was qualitatively stronger with the SNCG promoter compared to CMV. This observation was corroborated with single-cell patch experiments where higher amplitude photocurrents were recorded from cells expressing CatCh under the SNCG promoter. In macaque retinas, CatCh without the GFP tag localizes to the membrane in peri-foveolar RGCs and is able to mediate light-driven action potential firing in a population of RGCs with light intensities above 1015 photons/cm2/s. As expected, RGCs responding to light are concentrated on the foveal center in a 500- to 600-μm disc where AAV2 affords efficient transduction.28 This transduction pattern is expected to lead to a narrow visual field, albeit with good acuity. New AAV vectors and surgical procedures could help expand this limited area of transduction. Finally, we show (to our knowledge, for the first time) that it is possible to stimulate CatCh expressing RGCs above 30 Hz, ensuring high temporal resolution.
Gene therapeutic approaches that can provide treatments beyond the degeneration of photoreceptors are particularly important, as most patients do not come to the clinic until the degenerative process has gone beyond the loss of rods and the start of cone degeneration.45 In this regard, the results presented here provide basis for further clinical development of optogenetic reactivation of RGCs. Our study investigated the feasibility of optogenetic activation of RGCs in macaques and constitutes the basis for further investment in this direction toward improved application in patients. Because optogenetic activation relies on the resolution of the optical stimulation and the cell size, it is very likely to provide greater visual acuity than current electrical stimulations with retinal prostheses.6 The peri-foveolar expression pattern obtained in the NHP retina indicates that it will be possible to optogenetically stimulate these retinal ganglion cells, making use of the human retina’s innate high-acuity RGC circuit. However, it is not yet possible to achieve peripheral vision with the current technologies.
We found that reliable CatCh activity is seen while stimulating at an intensity threshold around 1015 photons/cm2/s, with response amplitudes increasing in a light-intensity-dependent manner when using the SNCG promoter, and that significant responses can be obtained up to ∼500 nm. This activation threshold is just below the radiation safety limits for the human eye.46 By contrast, the one-log-unit shift in light sensitivity for the CMV promoter suggests that it could fail to elicit light responses in a safe dosing range. Moreover, potential long-term effects of expressing a light-driven channel (CatCh) with increased calcium permeability need to be taken into account when using this approach. To avoid potential blue light toxicity while stimulating the retina at high light intensities, developments in opsin engineering47 and discovery48, 49 will help further refine optogenetic vision restoration strategies. In the future, we anticipate that developments in both viral technologies and surgical techniques will aid in obtaining better distribution of the optogenetic protein across the primate retina. Finally, for patients who still have an intact bipolar cell layer, new viral technologies might also grant access to the bipolar cells of the primate retina for circuit-specific insertion of G protein-coupled vertebrate opsins.50, 51, 52
Our study provides evidence that the choice of an appropriate promoter is essential in obtaining high-level microbial opsin expression compatible with illumination safety. A promoter that can drive strong gene expression in designated cells is crucial in the clinical development of a strategy using a protein of foreign origin, which requires high-level expression for functional activation.
Materials and Methods
Animals
All experiments were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the local animal ethics committees and was conducted in accordance with Directive 2010/63/EU of the European Parliament. All animals used in this study were C3H/HeN (rd1) or C57Bl6J (wild-type) mice from Janvier Laboratories or cynomolgus macaques (Macaca fascicularis) of foreign origin.
AAV Production
SNCG and CMV promoters were cloned into an AAV backbone plasmid containing a human codon-optimized CatCh sequence in fusion with or without GFP. The constructs all included woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) and bovine growth hormone poly(A). Recombinant AAVs were produced by the plasmid co-transfection method,53 and the resulting lysates were purified via iodixanol gradient ultracentrifugation as previously described. Briefly, a 40% iodixanol fraction was concentrated and buffer was exchanged using Amicon Ultra-15 centrifugal filter units. Vector stocks were then titered for DNase-resistant vector genomes by real-time PCR relative to a standard.54
Injections
Mice were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg; Rompum, Ferguson and Associates Equine Hospital). 1 μL stock containing 107–109 particles of AAV was injected, with direct observation of the needle in the center of the vitreous cavity. Primates were anesthetized with 10:1 mg/kg ketamine/xylazine. 100 μL of viral vector containing either 1–5 × 1011 or 1012 viral particles was injected into the vitreous. An ophthalmic steroid and antibiotic ointment was applied to the cornea post-injection.
Immunohistochemistry
Transduced mouse retinas were dissected and fixed in 4% paraformaldehyde for 30 min at room temperature. Retinas were incubated in PBS with 1% Triton X-100, 0.5% Tween 20, and 5% BSA blocking buffer for 1 hr at room temperature. Retinas were incubated overnight at 4°C with polyclonal antibodies directed against GFP (1:2,000; Life Technologies) and ChAT (1:400; Millipore) and with monoclonal anti-Brn3a antibody (1:100; Millipore Chemicon) in half diluted blocking buffer. Secondary anti-rabbit IgG, anti-mouse IgG, and anti-goat IgG conjugated with Alexa TM594, Alexa TM488, and Alexa TM647, respectively (1:500; Molecular Probes), were applied for 1 hr at room temperature. Primate retinas were labeled with antibodies directed against Brn3a, GFP, and channelrhodopsin in similar conditions. Cell nuclei were revealed with 4′,6-diamidino-2-phenylindole (10 μg/mL; Sigma-Aldrich). Retinas were rinsed and flat mounted in mounting medium. Cryosections of the labeled flat mounts were obtained by unmounting, cryopreserving, and embedding in optical coherence tomography (OCT) before cryosectioning (15 μm).
Confocal Microscopy
An Olympus FV1000 laser-scanning confocal microscope was used to acquire images sequentially, line by line. Step size was defined according to the Nyquist-Shannon sampling theorem. Twelve-bit images were then processed with FIJI (BSD), and Z sections were projected on a single plane using maximum intensity under the Z-project function and finally converted to 8-bit RGB color mode. Transduction efficiency was assessed by counting Brn3a(+) cells transduced with CatCh-GFP in mice and in the foveal area in primate retinas. Confocal stacks through the RGC layer were acquired using the ×20 objective in four adjacent regions (above, below, to the right, and to the left of the optic nerve).
MEA Recordings of Isolated Retinas
Mice were anesthetized and euthanized by a quick cervical dislocation. Eyeballs were removed and placed in Ames’ medium (A1420; Sigma-Aldrich) bubbled with 95% O2 and 5% CO2 at room temperature. Eyecups were obtained under red light using binoculars by cutting around the sclera, below the ora serrata. The cornea and lenses were then removed, and the retinas were isolated and flattened. Primate eyeballs were transferred to CO2-independent medium (Life Technologies) after the primates were euthanized with a lethal dose of pentobarbital. Retinas were isolated upon arrival, and the perifoveal area was cut into square-shaped pieces (approximately 4–5 mm) and cultured in Neurobasal medium complemented with B27 serum-free supplement (Life Technologies) on Transwell permeable culture support (Corning) as previously described.15 Primate retinal explants were left to rest in an incubator 1–2 days prior to MEA recordings. Isolated retinas were placed on a cellulose membrane and gently pressed against an MEA (MEA256 100/30 iR-ITO; Multichannel Systems), with the RGCs facing the electrodes. The retina was continuously perfused with bubbled Ames’ medium at 34°C at a rate of 1–2 mL/min during experiments. Metabotropic glutamate receptor agonist L-AP4 (catalog no. 0103; Tocris Bioscience) and glutamate antagonists 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX disodium salt, catalog no. 1045; Tocris Bioscience) and 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) (catalog no. 0247; Tocris Bioscience) were freshly diluted to concentrations of 50 μM, 25 μM, and 10 μM, respectively, and were bath-applied through the perfusion system 30 min prior to recordings. Retinas were dark-adapted 1 hr prior to recordings. Full-field light stimuli were applied with a Polychrome V monochromator (Olympus) driven by a STG2008 stimulus generator (MCS). Output light intensities were calibrated to range from 1 × 1014 photons/cm2/s to 1 × 1017 photons/cm2/s. Stimuli were presented for 2 s, with 10-s intervals. The wavelength sensitivity of responses was determined by stimulating 10 times, from 400 nm to 650 nm, with 10-nm steps. The order of the tested wavelengths was randomized to prevent any adaptation of the retina.
Raw extracellular RGC activity was amplified and sampled at 20 kHz. Resulting data was stored and filtered with a 200-Hz high-pass filter for subsequent offline analysis using Spike2 software (version 7; CED). Single-unit raster plots were obtained using a combination of template matching and cluster grouping based on principal component analysis of the waveforms. In our population analysis, significant responses were determined based on a z-score analysis. We estimated the mean and SD of the activity prior to stimulus and considered that a response was detected if the activity exceeded the mean by more than four times the SD in the 2 s after the onset or offset of the stimulus (for a bin size of 50 ms). Error bars were calculated over the different experiments. For the responses to light at different wavelengths, we measured the response to each flash in a 1-s window after the stimulus. We then normalized the response of each cell by its maximum firing rate response. For the responses to light at different intensities, we estimated the error bars by bootstrapping over the set of recorded cells.
Two-Photon Imaging and Electrophysiological Recordings
A custom-made two-photon microscope equipped with a ×25 water immersion objective (XLPLN25xWMP/NA1.05; Olympus) with a pulsed femtosecond laser (InSight DeepSee; Newport Corporation) was used for imaging CatCh-GFP-positive retinal ganglion cells. AAV-treated retinas from rd1 mice were isolated in oxygenized (95% O2, 5% CO2) Ames’ medium (Sigma-Aldrich). For live two-photon imaging, retinas were placed in the recording chamber of the microscope, and z stacks were acquired using the excitation laser at a wavelength of 930 nm. Images were processed offline using ImageJ software (NIH). During imaging, the retina was superfused with oxygenized Ames’ medium.
We used an Axon Multiclamp 700B amplifier for whole-cell patch-clamp and cell-attached recordings. Patch-clamp electrodes were made from borosilicate glass (BF100-50-10; Sutter Instruments) and pulled to 8–10 MΩ. Pipettes were filled with 112.5 mM CsMeSO4, 1 mM MgSO4, 7.8 × 10−3 mM CaCl2, 0.5 mM 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 10 mM HEPES, 4 mM ATP-Na2, 0.5 mM GTP-Na3, and 5 mM lidocaine N-ethyl bromide (QX314-Br) (pH 7.2). Cells were clamped at a potential of −60 mV to isolate excitatory currents. Cell-attached recordings were obtained in current-clamp configuration (current zero) with electrodes filled with Ames’ solution. L-AP4 was added to Ames’ medium during all electrophysiological recordings. Retinas were dark-adapted 1 h prior to recordings.
In Vivo Recordings in the Visual Cortex
Mice were sedated with a low-dose injection of 100 mg/kg ketamine and 10 mg/kg xylazine and then anesthetized with urethane [1.0 g/kg, 10% (w/v) in saline]. Animals were placed in a stereotaxic holder. The temperature was maintained at 37°C and a coverslip covered with vitamin A (Allergan) was placed on both eyes to prevent corneal dehydration. A craniotomy (1 mm2) above V1 in the contralateral hemisphere to the treated eye was centered 3 mm lateral and 0.5 mm rostral from the lambda point. The dura was removed and an electrode was inserted using a three-axis micromanipulator (Sutter Instruments) with a 30° angle to the cortical surface. It was advanced 800 μm and the exposed surface was covered with agarose (1.2% in cortex buffer).
Visual stimuli were generated by a 470-nm collimated light-emitting diode (LED) (model M470L3; Thorlabs) placed at 1 cm from the eye. An isolating cone ensured that the illumination was restricted to the stimulated eye. Linear multisite silicon microprobes (16 electrodes at 50-μm intervals) were used for recordings. For each acquisition, after averaging over the 200 trials, the electrode showing the VEP with maximal peak amplitude was selected for quantification. The stimulation consisted of 200-ms pulses of blue light repeated 200 times at 1 Hz triggered by a Digidata digitizer (Axon). Signals were analyzed in MATLAB using custom scripts. For local field potentials, signals were low-pass filtered at 300 Hz and averaged over the 200 trials.
In Vivo Imaging and Ophthalmic Exams in NHPs
Fluorescent images of GFP (Fundus Autofluorescence mode: excitation wavelength of 488 nm and barrier filter of 500 nm) and infrared pictures of the eye fundus and OCT images were acquired using a Spectralis HRA+OCT system (Heidelberg Engineering) after pupil dilation. Ophthalmic examinations consisting of slit lamp biomicroscopy (portable slit lamp model SL-14; Kowa) and indirect ophthalmoscopy (indirect binocular ophthalmoscope model HK 150-1 uno; Heine) were performed on all macaques before dosing, at 2 weeks, and then on a monthly basis.
Histopathological Studies on Macaque Retina
The eye from an NHP injected with the 5 × 1011 dose was enucleated at 3 months post-injection, a needle was inserted, and 0.15–0.3 mL fixative was injected, until the eyeball became turgid. The eye was immersion fixed in fixative overnight and processed to make horizontal cross-sections across the entire structure. A retinal cross-section presenting all of the desired ocular structures was then imaged on a Nanozoomer scanner (Hamamatsu).
Author Contributions
A.C., R.C., E.Br., L.D., M.D., E.D., and C.J. conducted the experiments and analyzed the data. A.C., M.D., R.C., O.M., A.-P.B, E.M., and D.D. designed the experiments. P.B., P.H., A.-P.B., E.Ba., J.D., J.-A.S., and S.P. provided expertise and feedback. J.D., J.-A.S., S.P., and D.D. supervised the study. D.D. conceptualized the study, analyzed the data, and wrote the paper.
Conflicts of Interest
J.-A.S. is a founder of Fovea Pharmaceuticals, Ophtimalia, Streetlab, Pixium Vision, GenSight Biologics, Chronocam, and Chronolife. S.P. is a founder and consultant for GenSight Biologics and Pixium Vision. D.D. is a consultant for GenSight Biologics and is co-inventor on patent #9193956 (adeno-associated virus virions with variant capsid and methods of use thereof), with royalties paid to Avalanche Biotechnologies.
Acknowledgments
The authors thank the Sanofi Optovision team, Xavier Palazzi, Claire-Maelle Fovet, Valérie Fradot, and Florian Senlaub for technical help and scientific advice. This work was supported by Sanofi, INSERM, UPMC, the CNRS, FRM (Fondation pour la Recherche Medicale), the Agence Nationale pour la Recherche Investissements d’Avenir Recherche Hospitalo-Univesritaire en santé (RHU) (Light4Deaf and ANR: OPTIMA), the E-Rare Project (OPTOREMODE), the Foundation Fighting Blindness (Wynn-Gund translational research award TA-GT-0911-0543-UPMC-WG), the Fédération des Aveugles de France, the City of Paris, the Regional Council of Ile-de-France, the French State program “Investissements d'Avenir” managed by the Agence Nationale de la Recherche (LIFESENSES; grant ANR-10-LABX-65), the European Research Council (ERC) (OptogenRet; starting grant 309776), the Deutsche Forschungsgemeinschaft (DFG) (grant SFB 807), the Max Planck Society, and the NIH (award U01NS090501).
Footnotes
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.07.011.
Contributor Information
Jens Duebel, Email: jens.duebel@gmail.com.
José-Alain Sahel, Email: j.sahel@gmail.com.
Serge Picaud, Email: serge.picaud@inserm.fr.
Deniz Dalkara, Email: deniz.dalkara@gmail.com.
Supplemental Information
References
- 1.Bessant D.A.R., Ali R.R., Bhattacharya S.S. Molecular genetics and prospects for therapy of the inherited retinal dystrophies. Curr. Opin. Genet. Dev. 2001;11:307–316. doi: 10.1016/s0959-437x(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 2.Wright A.F., Chakarova C.F., Abd El-Aziz M.M., Bhattacharya S.S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 3.Humayun M.S., Prince M., de Juan E., Jr., Barron Y., Moskowitz M., Klock I.B., Milam A.H. Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1999;40:143–148. [PubMed] [Google Scholar]
- 4.Humayun M.S., Dorn J.D., da Cruz L., Dagnelie G., Sahel J.A., Stanga P.E., Cideciyan A.V., Duncan J.L., Eliott D., Filley E., Argus II Study Group Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology. 2012;119:779–788. doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson S.G., Sumaroka A., Luo X., Cideciyan A.V. Retinal optogenetic therapies: clinical criteria for candidacy. Clin. Genet. 2013;84:175–182. doi: 10.1111/cge.12165. [DOI] [PubMed] [Google Scholar]
- 6.Lorach H., Goetz G., Smith R., Lei X., Mandel Y., Kamins T., Mathieson K., Huie P., Harris J., Sher A., Palanker D. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 2015;21:476–482. doi: 10.1038/nm.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi A., Cui J., Ma Y.-P., Olshevskaya E., Pu M., Dizhoor A.M., Pan Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagali P.S., Balya D., Awatramani G.B., Münch T.A., Kim D.S., Busskamp V., Cepko C.L., Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg K.P., Pham A., Werblin F.S. Differential targeting of optical neuromodulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism. Neuron. 2011;69:713–720. doi: 10.1016/j.neuron.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Pan Z.-H., Ganjawala T.H., Lu Q., Ivanova E., Zhang Z. ChR2 mutants at L132 and T159 with improved operational light sensitivity for vision restoration. PLoS ONE. 2014;9:e98924. doi: 10.1371/journal.pone.0098924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busskamp V., Duebel J., Balya D., Fradot M., Viney T.J., Siegert S., Groner A.C., Cabuy E., Forster V., Seeliger M. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- 12.Tomita H., Sugano E., Isago H., Hiroi T., Wang Z., Ohta E., Tamai M. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp. Eye Res. 2010;90:429–436. doi: 10.1016/j.exer.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Caporale N., Kolstad K.D., Lee T., Tochitsky I., Dalkara D., Trauner D., Kramer R., Dan Y., Isacoff E.Y., Flannery J.G. LiGluR restores visual responses in rodent models of inherited blindness. Mol. Ther. 2011;19:1212–1219. doi: 10.1038/mt.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaub B.M., Berry M.H., Holt A.E., Reiner A., Kienzler M.A., Dolgova N., Nikonov S., Aguirre G.D., Beltran W.A., Flannery J.G., Isacoff E.Y. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc. Natl. Acad. Sci. USA. 2014;111:E5574–E5583. doi: 10.1073/pnas.1414162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sengupta A., Chaffiol A., Macé E., Caplette R., Desrosiers M., Lampič M., Forster V., Marre O., Lin J.Y., Sahel J.A. Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol. Med. 2016;8:1248–1264. doi: 10.15252/emmm.201505699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doroudchi M.M., Greenberg K.P., Liu J., Silka K.A., Boyden E.S., Lockridge J.A., Arman A.C., Janani R., Boye S.E., Boye S.L. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther. 2011;19:1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita H., Sugano E., Murayama N., Ozaki T., Nishiyama F., Tabata K., Takahashi M., Saito T., Tamai M. Restoration of the majority of the visual spectrum by using modified Volvox channelrhodopsin-1. Mol. Ther. 2014;22:1434–1440. doi: 10.1038/mt.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macé E., Caplette R., Marre O., Sengupta A., Chaffiol A., Barbe P., Desrosiers M., Bamberg E., Sahel J.A., Picaud S. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV restores ON and OFF visual responses in blind mice. Mol. Ther. 2015;23:7–16. doi: 10.1038/mt.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busskamp V., Roska B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr. Opin. Neurobiol. 2011;21:942–946. doi: 10.1016/j.conb.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Bainbridge J.W.B., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 21.Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A., Wilson J.M. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., Clark K.R., During M.J., Cremers F.P., Black G.C. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenberghe L.H., Bell P., Maguire A.M., Cearley C.N., Xiao R., Calcedo R., Wang L., Castle M.J., Maguire A.C., Grant R. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran P.S., Lee V., Wei Z., Song J.Y., Casal G., Cronin T., Willett K., Huckfeldt R., Morgan J.I., Aleman T.S. Evaluation of Dose and Safety of AAV7m8 and AAV8BP2 in the Non-Human Primate Retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugano E., Isago H., Wang Z., Murayama N., Tamai M., Tomita H. Immune responses to adeno-associated virus type 2 encoding channelrhodopsin-2 in a genetically blind rat model for gene therapy. Gene Ther. 2011;18:266–274. doi: 10.1038/gt.2010.140. [DOI] [PubMed] [Google Scholar]
- 27.Ivanova E., Hwang G.-S., Pan Z.-H., Troilo D. Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Invest. Ophthalmol. Vis. Sci. 2010;51:5288–5296. doi: 10.1167/iovs.10-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin L., Greenberg K., Hunter J.J., Dalkara D., Kolstad K.D., Masella B.D., Wolfe R., Visel M., Stone D., Libby R.T. Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci. 2011;52:2775–2783. doi: 10.1167/iovs.10-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maclachlan T.K., Lukason M., Collins M., Munger R., Isenberger E., Rogers C., Malatos S., Dufresne E., Morris J., Calcedo R. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol. Ther. 2011;19:326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett J., Ashtari M., Wellman J., Marshall K.A., Cyckowski L.L., Chung D.C., McCague S., Pierce E.A., Chen Y., Bennicelli J.L. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinlogel S., Feldbauer K., Dempski R.E., Fotis H., Wood P.G., Bamann C., Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011;14:513–518. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- 32.Yin L., Masella B., Dalkara D., Zhang J., Flannery J.G., Schaffer D.V., Williams D.R., Merigan W.H. Imaging light responses of foveal ganglion cells in the living macaque eye. J. Neurosci. 2014;34:6596–6605. doi: 10.1523/JNEUROSCI.4438-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuer W.J., Schiffman J.C., Davis J.L., Porciatti V., Gonzalez P., Koilkonda R.D., Yuan H., Lalwani A., Lam B.L., Guy J. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123:558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surgucheva I., Weisman A.D., Goldberg J.L., Shnyra A., Surguchov A. Gamma-synuclein as a marker of retinal ganglion cells. Mol. Vis. 2008;14:1540–1548. [PMC free article] [PubMed] [Google Scholar]
- 35.Quina L.A., Pak W., Lanier J., Banwait P., Gratwick K., Liu Y., Velasquez T., O’Leary D.D., Goulding M., Turner E.E. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J. Neurosci. 2005;25:11595–11604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen A.E., Storchi R., Martial F.P., Bedford R.A., Lucas R.J. Melanopsin contributions to the representation of images in the early visual system. Curr. Biol. 2017;27:1623–1632. doi: 10.1016/j.cub.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotterman M.A., Yin L., Strazzeri J.M., Flannery J.G., Merigan W.H., Schaffer D.V. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussenblatt R.B., Palestine A.G., Chan C.C., Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 39.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T., Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinlogel S., Terpitz U., Legrum B., Gökbuget D., Boyden E.S., Bamann C., Wood P.G., Bamberg E. A gene-fusion strategy for stoichiometric and co-localized expression of light-gated membrane proteins. Nat. Methods. 2011;8:1083–1088. doi: 10.1038/nmeth.1766. [DOI] [PubMed] [Google Scholar]
- 42.Izpisua Belmonte J.C., Callaway E.M., Caddick S.J., Churchland P., Feng G., Homanics G.E., Lee K.F., Leopold D.A., Miller C.T., Mitchell J.F. Brains, genes, and primates. Neuron. 2015;86:617–631. doi: 10.1016/j.neuron.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalkara D., Byrne L.C., Klimczak R.R., Visel M., Yin L., Merigan W.H., Flannery J.G., Schaffer D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 44.Bennett J., Wellman J., Marshall K.A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O.U., Chung D.C., Sun J., Wright J.F. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne L.C., Dalkara D., Luna G., Fisher S.K., Clérin E., Sahel J.A., Léveillard T., Flannery J.G. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J. Clin. Invest. 2015;125:105–116. doi: 10.1172/JCI65654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J., Seregard S., Algvere P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006;51:461–481. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Lin J.Y., Knutsen P.M., Muller A., Kleinfeld D., Tsien R.Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 2013;16:1499–1508. [Google Scholar]
- 48.Klapoetke N.C., Murata Y., Kim S.S., Pulver S.R., Birdsey-Benson A., Cho Y.K., Morimoto T.K., Chuong A.S., Carpenter E.J., Tian Z. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuong A.S., Miri M.L., Busskamp V., Matthews G.A., Acker L.C., Sørensen A.T., Young A., Klapoetke N.C., Henninger M.A., Kodandaramaiah S.B. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Wyk M., Pielecka-Fortuna J., Löwel S., Kleinlogel S. Restoring the ON switch in blind retinas: Opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS Biol. 2015;13:e1002143. doi: 10.1371/journal.pbio.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cehajic-Kapetanovic J., Eleftheriou C., Allen A.E., Milosavljevic N., Pienaar A., Bedford R., Davis K.E., Bishop P.N., Lucas R.J. Restoration of vision with ectopic expression of human rod opsin. Curr. Biol. 2015;25:2111–2122. doi: 10.1016/j.cub.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaub B.M., Berry M.H., Holt A.E., Isacoff E.Y., Flannery J.G. Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol. Ther. 2015;23:1562–1571. doi: 10.1038/mt.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi V.W., Asokan A., Haberman R.A., Samulski R.J. Production of recombinant adeno-associated viral vectors. Curr. Protoc. Mol. Biol. 2007;Chapter 12 doi: 10.1002/0471142905.hg1209s53. Unit 12.9. [DOI] [PubMed] [Google Scholar]
- 54.Aurnhammer C., Haase M., Muether N., Hausl M., Rauschhuber C., Huber I., Nitschko H., Busch U., Sing A., Ehrhardt A., Baiker A. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods. 2012;23:18–28. doi: 10.1089/hgtb.2011.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.