Abstract
Introduction
Since we recently showed in behavioural tasks that the top-down cognitive control was specifically altered in tinnitus sufferers, here we wanted to establish the link between this impaired executive function and brain alterations in the frontal cortex in tinnitus patients.
Method
Using functional magnetic resonance imaging (fMRI), we monitored the brain activity changes in sixteen tinnitus patients (TP) and their control subjects (CS) while they were performing a spatial Stroop task, both in audition and vision.
Results
We observed that TP differed from CS in their functional recruitment of the dorsolateral prefrontal cortex (dlPFC, BA46), the cingulate gyrus and the ventromedial prefrontal cortex (vmPFC, BA10). This recruitment was higher during interference conditions in tinnitus participants than in controls, whatever the sensory modality. Furthermore, the brain activity level in the right dlPFC and vmPFC correlated with the performance in the Stroop task in TP.
Conclusion
Due to the direct link between poor executive functions and prefrontal cortex alterations in TP, we postulate that a lack of inhibitory modulation following an impaired top-down cognitive control may maintain tinnitus by hampering habituation mechanisms. This deficit in executive functions caused by prefrontal cortex alterations would be a key-factor in the generation and persistence of tinnitus.
Keywords: fMRI, Prefrontal cortex, Tinnitus
Highlights
-
•
Tinnitus patients show a selective impairment of their top-down cognitive control.
-
•
Tinnitus patients show altered activity in the prefrontal cortex.
-
•
The cognitive control deficit contributes to explain the maintaining of tinnitus.
-
•
This finding opens new perspectives for successful rehabilitation of tinnitus.
1. Introduction
Tinnitus is the perception of sound, i.e. hearing a single frequency or a noise sound (e.g. ringing, hissing, whistling or buzzing) in the absence of a corresponding external acoustic stimulus. In industrialized countries, tinnitus is one of the most frequent auditory ailments as it affects 10–15% of the population. It becomes chronic in 5–15% of the cases (Heller, 2003) and severely affects the quality of life in 1–3% of them, causing e.g. sleep disturbances, concentration problems, psychiatric complications or even leading to suicide attempts (Langguth et al., 2013). The prevalence of anxiety and depression is particularly higher in tinnitus patients than in the general population (Reynolds et al., 2004, Zoger et al., 2001) indicating that tinnitus, anxiety and depression influence each other (Langguth et al., 2013).
Despite the growing interest from the scientific and medical communities, the pathophysiology of tinnitus, including the underlying cognitive and neural mechanisms, is still largely unknown. Overlong, tinnitus was thought to be a strict otological disorder, but advances in tinnitus research indicated that neuroplastic changes in several levels of the central auditory system, as well as in non-auditory brain areas, notably the amygdala, the anterior insula, the cingulate gyrus and the prefrontal cortex were associated with the development and persistence of conscious perception of tinnitus (De Ridder et al., 2014, Rauschecker et al., 2010, Roberts et al., 2010, Schlee et al., 2009). Recent models of tinnitus were proposed according to which frontal brain areas including the ventromedial, the ventrolateral and the dorsolateral prefrontal cortices (PFC) play a crucial role in the generation and perception of tinnitus (De Ridder et al., 2014, Elgoyhen et al., 2015, Leaver et al., 2011, Rauschecker et al., 2010). A reduction in the gray matter volume has been observed in the ventromedial PFC (vmPFC) in tinnitus patients (Leaver et al., 2012, Seydell-Greenwald et al., 2012). This anatomical modification was associated with a higher reactivity of the nucleus accumbens during auditory stimulation at the tinnitus frequency, as shown using functional magnetic resonance imaging (fMRI) (Leaver et al., 2011), whereas the activity in the dorsolateral PFC (dlPFC) was strongly and specifically correlated with tinnitus loudness (Seydell-Greenwald et al., 2012). In addition, transcranial magnetic stimulation (TMS) applied to the dlPFC of one brain hemisphere reduced the tinnitus perception in the contralateral side in some cases (De Ridder et al., 2011) whereas TMS applied to the left ventrolateral PFC (vlPFC) modulated the loudness of tinnitus (Vanneste and De Ridder, 2012).
Using two different behavioural tasks, we recently brought evidences that the top-down cognitive control was specifically altered in tinnitus patients (Araneda et al., 2015b, Heeren et al., 2014). We also observed that their inhibitory control was altered in a go-no go task: tinnitus patients were abnormally sensitive to cross-modal interference, revealing further their difficulties to ignore irrelevant stimuli (Araneda et al., 2015a). This led us to propose that a deficit in executive functions in the prefrontal cortex could be a key factor for tinnitus to develop and become chronic. Since earlier studies most commonly ascribed the top-down cognitive control to several functionally related PFC regions (Dosenbach et al., 2008), among which the dlPFC, we wanted to establish the direct link between a deficit of the top-down cognitive control and neuroplastic alterations in the dorsolateral and/or ventromedial PFC in tinnitus patients. To this aim we used fMRI and a spatial Stroop paradigm in both the auditory and the visual modality in the same participants.
2. Materials and methods
2.1. Ethics statement
Before the study, all participants provided their written informed consent according to the Declaration of Helsinki (BMJ 1991; 302: 1194). The experimental protocol of the study was approved by the Biomedical Ethics Committee of the school of Medicine of the Université catholique de Louvain.
2.2. Subjects
Tinnitus participants (TP) were recruited among patients who consulted the ear, nose and throat department in reason of tinnitus. Strict inclusion criteria were used in order to homogenize the best possible the group of tinnitus patients since there may be different types of tinnitus involving slightly different pathophysiological mechanisms (Landgrebe et al., 2010, Langguth et al., 2013, Roberts et al., 2010). A special attention was paid to isolate the tinnitus from frequently associated confounding factors such as hearing loss, depression, anxiety or hyperacusis (Langguth et al., 2013). We only included patients who suffered from a subjective tinnitus (1), non-pulsatile (2), permanently present (3), for at least 6 months (chronic) (4), in both ears (5), and who had either a normal hearing acuity or a slight sensorineural hearing loss (i.e. an average hearing loss for tonal stimuli minor than 35 dB in each ear) (6), no hyperacusis (7), no neurologic record or diagnosed psychiatric disorder (including major depression) at the time of testing (8), and no psychotropic medication consumption (9). In total, 16 TP were included in the study (see Table 1 for details). Control subjects (CS) were recruited via flyers posted on the university campus. All of them were healthy, without self-reported neurologic or psychiatric problem or hearing impairment. All TP and CS were French speakers and underwent a brief audiometric evaluation of each ear (for 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz frequencies) using a staircase ascending method (bottom-up approach) and an Electronica™ 600 M-Audica audiometer. The hearing loss was determined by comparing these results with the normal audiometric curves according to the age. Sixteen CS were selected and individually matched to a TP by gender, age, hearing acuity (each subject being categorized as having either (1) a normal hearing (i.e. hearing loss < 20 dB), or (2) slight hearing loss (between 21 and 35 dB) and educational level (see Table 2). Fifteen subjects were right-handed in each group (1 left-handed).
Table 1.
Characteristics of Tinnitus participants.
| Subjects | Age [years] | Gender | Hearing loss right/left [dB] | Tinnitus frequency [Hz] | Tinnitus duration [months] | THI [score: 0–100] |
|---|---|---|---|---|---|---|
| 1 | 62 | M | 31/32 | 2000 | 120 | 22 |
| 2 | 34 | M | 10/10 | 6000 | 18 | 42 |
| 3 | 34 | M | 11/10 | 2000 | 144 | 24 |
| 4 | 33 | M | 17/18 | 4000 | 65 | 44 |
| 5 | 50 | F | 18/20 | 6000 | 60 | 48 |
| 6 | 54 | M | 15/9 | 6000 | 26 | 20 |
| 7 | 36 | F | 17/16 | 6000 | 27 | 76 |
| 8 | 44 | F | 17/16 | 6000 | 24 | 36 |
| 9 | 34 | M | 10/15 | 8000 | 26 | 36 |
| 10 | 24 | M | 12/9 | 8000 | 22 | 54 |
| 11 | 37 | M | 11/13 | 6000 | 23 | 34 |
| 12 | 56 | F | 16/15 | 6000 | 253 | 38 |
| 13 | 55 | M | 23/24 | 4000 | 84 | 58 |
| 14 | 22 | F | 9/9 | 6000 | 35 | 36 |
| 15 | 48 | M | 12/15 | 4000 | 21 | 50 |
| 16 | 44 | F | 13/9 | 6000 | 24 | 44 |
THI: Tinnitus Handicap Inventory.
Bilateral Hearing Loss: Average between 250 and 8000 Hz.
The tinnitus frequency was identified in each TP by presenting pure tones and narrow band sounds of frequencies ranging between 250 and 8000 Hz.
Table 2.
Clinical evaluation and scores to the questionnaires.
| Tinnitus |
Control |
Unpaired t-test | |
|---|---|---|---|
| (n = 16) |
(n = 16) |
||
| Mean (SD) | Mean (SD) | ||
| Age [years]ns | 41.69 (11.76) | 40.94 (12.06) | p = 0.4088 |
| Bilateral Hearing Loss [db]ns | 16.12 (5.66) | 13.14 (6.86) | p = 0.0893 |
| Educational level [years]ns | 15.56 (1.67) | 15.75 (1.39) | p = 0.3663 |
| Beck Depression Inventory [13 items]ns | 2.69 (2.33) | 2.31 (1.66) | p = 0.3020 |
| Zung Self-rating Depression Scalens | 0.47 (0.10) | 0.45 (0.09) | p = 0.1475 |
| Beck Anxiety Inventoryns | 6.13 (1.93) | 5.19 (2.61) | p = 0.1287 |
ns : no significant difference across groups
2.3. Questionnaires
The participants filled out five questionnaires recommended by the Tinnitus Research Initiative (TRI) (http://www.tinnitusresearch.org/index.php) or commonly used by clinicians and researchers in the field: the Tinnitus Sample Case History Questionnaire (TSCHQ), the Tinnitus Handicap Inventory (THI), the Beck Depression Inventory (BDI-13), the Self-Rating Depression Scale (SDS) and the Beck Anxiety Inventory (BAI). The TSCHQ allows identifying the history and characteristics of tinnitus, including tinnitus loudness, tinnitus frequency and the time of tinnitus awareness (Langguth et al., 2007) while the THI provides an evaluation (between 0 and 100) of its impact on daily living (Newman et al., 1998) (see Table 2 for the scores in TP and CS).
2.4. Equipment and stimuli
During the fMRI session, we used a spatial Stroop paradigm to evaluate the top-down cognitive control in TP and matched CS in the auditory and in the visual modality separately.
Auditory stimuli were recorded and edited using Adobe® Audition CS6. They consisted in the French words “gauche” (left) and “droite” (right) that were successively presented monaurally, either in the left or the right ear, through an MRI compatible sound delivery system (NordicNeuroLab®). Scanner noise was attenuated by the headphone (30–35 dB). In the visual condition, we used the same two words written in cursive letters using E-Prime 2 Professional® (Psychology Software Tools, Pittsburgh, PA, USA). These stimuli were projected either at the left or the right middle part of a black screen (In room Viewing Device NordicNeuroLab®) placed at the rear of the magnet and viewed through a tilted mirror mounted on the head coil. Participants responded by pressing the right or left button of an MRI compatible response pad (Response Grip NordicNeuroLab®) held in the dominant hand.
In each condition of the Stroop task, the duration of each stimulus (word) was 750 ms and each word was presented 47 times for a total of 94 trials. The word “droite” (right) appeared 21 times on the right and 26 times on the left. The same applied to the word “gauche” (left) that appeared 21 times on the left and 26 times on the right. In 55% of the trials there was a conflict between the stimulus location and its meaning (i.e. incongruent trials, e.g. the word “gauche” presented on the right side), whereas in the other 45% the stimulus characteristics were congruent. Therefore, the congruent trials served as controls to isolate the specific effect of interference on the task performance. Stimulations and response recordings were controlled using E-Prime 2 Professional® (Psychology Software Tools, Pittsburgh, PA, USA).
2.5. Experimental design, tasks and fMRI procedure
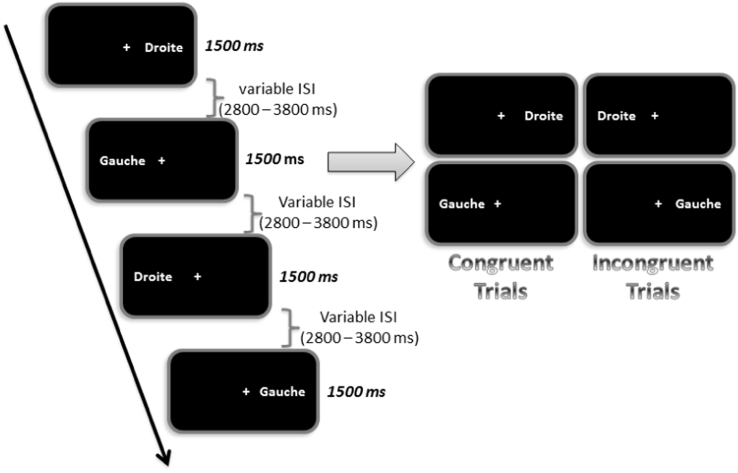
Before the fMRI session a brief familiarization with the tasks (consisting in 4 trials in each modality) was performed to ensure the understanding of the task by the participants. During the fMRI session, subjects were instructed to press as quickly as possible on the left response button when the word “gauche” (left) was presented and on the right button otherwise (Fig. 1). We used an event-related paradigm, because it is the best way to perform a Stroop task by mixing the various types of stimuli randomly during the fMRI. The event-related design included 94 experimental trials (duration: 1.5 s) alternating with resting state periods lasting from 2.8 to 3.8 s to reduce the overlapping of the hemodynamic responses. There were four runs of 463.5 s each (2 runs for each sensory modality). The presentation order of the modality was counterbalanced across subjects. Participants were instructed to maintain fixation on a central cross throughout the visual conditions and to close their eyes during the auditory conditions. The participants were interviewed after each scan and did not report any difference in tinnitus level in and outside the magnet.
Fig. 1.
Illustration of a sequence of stimulation in the visual modality.
Subjects performed a word recognition task in a sequence where the word “gauche” (left) and the word “droite” (right) appeared either at the left or the right part of a computer screen viewed through a mirror inside the magnet. The subjects were required to press the left or the right button of a response pad as quickly as possible according to the meaning of the word (e.g. left button when the word “gauche” appeared). There were two types of trials: 45% congruent trials (e.g. the word “droite” (right) appeared at the right side of the screen) and 55% incongruent trials (e.g. the word “droite” (right) appeared at the left side of the screen). During fMRI acquisition, congruent and incongruent trials alternated and were separated by variable inter-stimulus-intervals (ISI). Visual stimulations lasted 1.5 s and the ISI duration was comprised between 2.8 and 3.8 s.
2.6. 3D-MRI and fMRI acquisition
Structural brain imaging was obtained in all subjects in the bicommissural (AC-PC) orientation (Talairach and Tournoux, 1988) on a 3 Tesla MRI unit (Achieva, Philips Healthcare®, Best, The Netherlands) using a 3D fast T1-weighted gradient echo sequence with an inversion pre-pulse (Turbo field echo (TFE), TR [repetition time] = 9 ms, TE [echo time] = 4.6 ms, flip angle = 85°, 150 slices, 1 mm thickness, in plane resolution = 0.764 × 0.764 mm). The field of view was 220 × 197 mm, and the SENSE factor (parallel imaging) was 1.5. We used a thirty-two channels phased array head coil.
Blood oxygen level dependent (BOLD) fMRI data were acquired using a 2D single shot T2*-weighted gradient echo-planar imaging (EPI) sequence (TR = 2250 ms, TE = 27 ms) with 41 axial slices (thickness = 3 mm), in the AC-PC orientation. The field of view was 220 mm2. The in-plane resolution was 2.75 mm2. The fMRI paradigm consisted in 4 runs (2 in each sensory modality) of events and baseline (1.5 s per event with a variable inter-stimulus interval (ISI) lasting between 2.8 and 3.8 s).
2.7. Data analysis
2.7.1. In-scan behavioural data
Response times and response accuracy were recorded. Data reduction was first applied to deal with errors and outliers in the response time data: (1) trials with incorrect responses (1.76% of the trials) were excluded from the response time analyses and (2) response times beyond 2 standard deviations below or above each participant's mean for each experimental condition were discarded as outliers (0.05% of the trials).
Analyses of variance (ANOVA) were performed on the mean response times for both congruent and incongruent trials, and on the subtraction of individual mean response times between incongruent and congruent trials. An additional ANOVA was performed on the percentages of correct answers in all conditions. Post-hoc comparisons were performed using Holm-Sidak. Statistical analyses were performed using STATA/SE 12.0 for Windows (Stata Corp LP).
2.7.2. fMRI data
Data analysis was performed using BrainVoyager QX 2.8 software package (Brain Innovation™, Maastricht, The Netherlands) with standard pre-processing procedures. Functional MRI data pre-processing included slice scan time correction, head motion correction and high-pass filtering (cut-off frequency: 2 cycles/run) in the frequency domain. Functional and anatomical data sets for each subject were co-registered and the resulting matching brain images were fit to the standardized Talairach space (Talairach and Tournoux, 1988) and resliced (voxel size: 3 × 3 × 3 mm). Single-subject functional data were spatially smoothed (5 mm FWHM) in order to reduce inter-subject anatomical variability. Functional data were further analyzed using a multiple regression model (General Linear Model (GLM) (Friston et al., 1994)) that consisted in the two experimental conditions (congruent and incongruent trials) in both modalities and in both groups. In this model, the beta weights quantified the potential contribution of each predictor in each voxel time course. The predictor time courses of the regression model were computed on the basis of a linear model of the relation between neural activity and hemodynamic response, assuming a rectangular neural answer convolved with a standard hemodynamic response function (HRF) during phases of active conditions (Boynton et al., 1996, Friston et al., 1994).
Random-effects (RFX) group analyses (Friston et al., 1999) were performed at the whole-brain level. All the analyses were performed using a threshold of p < 0.001 with a correction for multiple comparisons using a cluster size threshold adjustment (minimum cluster size: 184 voxels) to achieve a corrected p < 0.05. This was performed based on the Forman et al. (Forman et al., 1995) Monte Carlo simulation approach, extended to 3D data sets using the threshold size plug-in Brain Voyager QX (Goebel et al., 2006).
To isolate the brain areas specifically involved in the interference conditions of the spatial Stroop task (i.e. the top-down cognitive control), we contrasted the incongruent trials with the congruent ones in the two modalities plotted together and in each group separately. To identify the brain regions altered in tinnitus patients during the task in each modality, we used the contrast [(TP congruent + TP incongruent) > (CS congruent + CS incongruent)]. To isolate the brain areas specifically activated in the incongruent trials (interference conditions with cognitive conflict) in TP, we used the contrast [TP incongruent > (TP congruent + CS congruent + CS incongruent)] in each modality. This contrast allowed us to investigate the Stroop effect specifically in TP.
To evaluate the link between the performance in the Stroop task (in incongruent trials minus congruent trials) and the brain activity level (beta values) in each modality, correlation analyses were performed in all the brain areas that had been identified as more recruited in tinnitus patients than in controls, i.e. in the differential contrast between tinnitus group and control group ((TP congruent + TP incongruent) > (CS congruent + CS incongruent)). Additional correlations between the beta values and the THI scores were performed to test the relationship between brain activity changes and the impact of tinnitus on quality of life.
3. Results
3.1. Clinical scores
The analysis of clinical scores did not reveal any group difference for depression nor for anxiety (see Table 2).
3.2. Behavioural results
3.2.1. Word recognition task
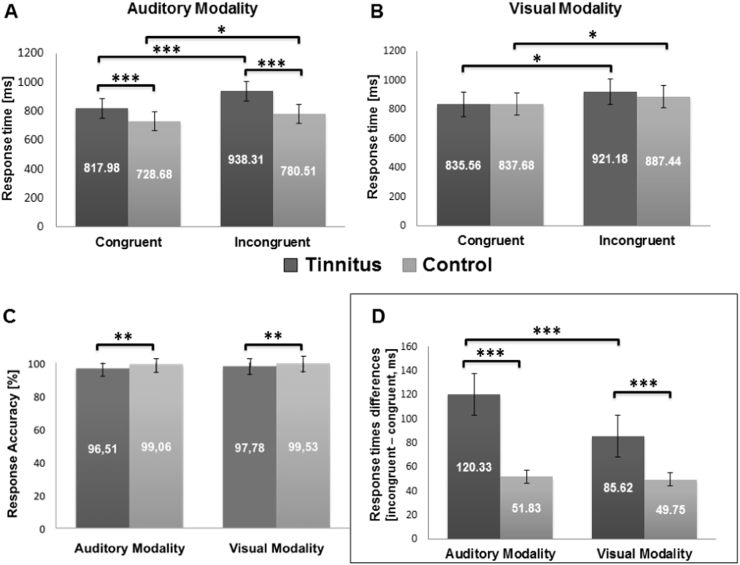
In each group and condition, the response times to incongruent trials were longer than the response times to congruent trials (all p's < 0.05, Fig. 2). The 2 (group: TP vs CS) × 2 (trial: congruent vs incongruent) ANOVA on the response times in each modality considered separately revealed an effect of the group in the auditory modality only, TP being slower than CS [F(3, 60) = 55.14, p < 0.001] in audition. In addition, the 2 (group: TP vs CS) × 2 (sensory modality: auditory vs visual) ANOVA performed on the response accuracy rate (to incongruent and congruent trials considered together) revealed an effect of the group, TP being less accurate than CS [F(3, 60) = 33.63, p < 0.001], both in the auditory and the visual modalities (all p's < 0.005, post-hoc comparisons).
Fig. 2.
Response times, response accuracy and Stroop effect during the word recognition task in tinnitus patients and control subjects.
In (A) and (B), response times are displayed as a function of the group and of the trial type (congruent vs incongruent) in audition and in vision. In (C), the response accuracy (percentage of correct responses to congruent and incongruent trials considered together) is displayed as a function of the group and the sensory modality. In (D), the “response times differences” (i.e. the differences in response times between incongruent and congruent trials, to be considered as the time needed to resolve the conflicts and reflecting the Stroop effect) are displayed as a function of the group and the sensory modality. Error bars are standard errors of the mean (SEM). *p < 0.05; **p < 0.005; ***p < 0.001.
3.2.2. Stroop effect
A 2 (group: TP vs CS) × 2 (sensory modality: auditory vs visual) ANOVA was performed on “response times differences” (i.e. response times for the incongruent trials minus response times for the congruent trials) (Fig. 2). For this variable that could be considered as an indicator of a Stroop effect (independent from any group difference in processing speed and lower level sensory processing), we observed an effect of the group [F(3, 60) = 90.41, p < 0.001], an effect of the modality [F(3, 60) = 73.35, p < 0.001] and an interaction between group and modality [F(3, 60) = 57.70, p < 0.001]. Post-hoc comparisons showed an effect of the group in the auditory (p < 0.001) and the visual modalities (p < 0.001). The differences in response times between incongruent and congruent trials were higher in TP than in CS. There was also an effect of the modality in TP only (p < 0.001), with larger differences in response times (in [incongruent minus congruent trials]) in audition than in vision in this group.
3.3. Functional imaging results
3.3.1. Brain areas recruited during conflict resolution in each group
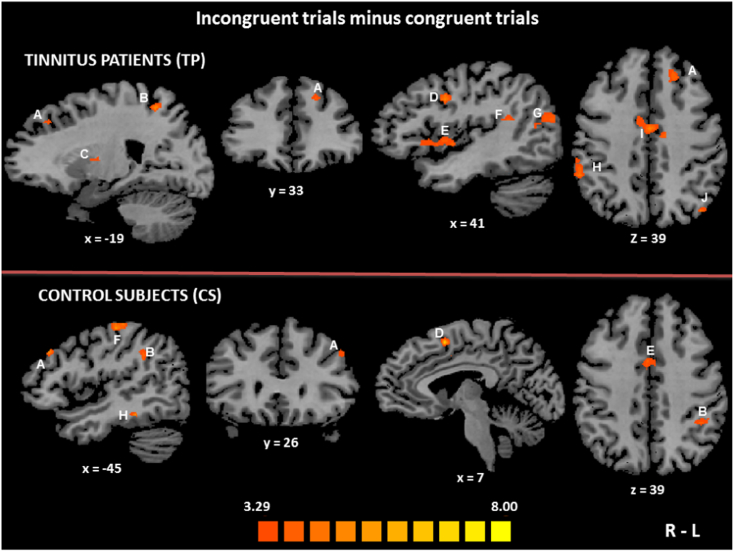
To identify in each group the neural network specifically involved in the conflict resolution of the spatial Stroop task (i.e. the top-down cognitive control), we contrasted the incongruent trials conditions to the congruent ones. In TP, using the contrast [(Auditory incongruent + Visual incongruent) > (Auditory congruent + Visual congruent)], six distinct brain areas were found to be bilaterally active: the inferior parietal lobule (IPL), the middle temporal gyrus (MiTG), middle frontal gyrus (MiFG, dlPFC), the insula, the superior parietal lobule (SPL) and cingulate gyrus. In the right hemisphere, the superior temporal gyrus (STG) and the superior frontal gyrus (SFG) were activated. There was also an activation focus in the left precuneus (Table 3, Fig. 3).
Table 3.
Brain activation foci (positive values) related to conflict resolution (incongruent trials minus congruent trials) in each group.
| Brain region | Brodmann area | Cluster size | t-Value | p-Value | Coordinate (x, y, z) | ||
|---|---|---|---|---|---|---|---|
| TP group (p < 0.001 corrected) | |||||||
| R inferior parietal lobule | BA 40 | 1005 | 6.59 | 0.000009 | 62 | − 29 | 18 |
| R middle temporal gyrus | BA 21 | 199 | 4.27 | 0.000075 | 56 | − 53 | 3 |
| R insula | BA 13 | 1228 | 5.14 | 0.000012 | 47 | 1 | 0 |
| R middle temporal gyrus | BA 39 | 1004 | 5.22 | 0.000014 | 50 | − 59 | 12 |
| R middle frontal gyrus | BA 9 | 271 | 5.02 | 0.000015 | 41 | 15 | 33 |
| R superior temporal gyrus | BA 22 | 215 | 4.29 | 0.000047 | 38 | − 47 | 18 |
| R putamen | 340 | 5.56 | 0.000055 | 26 | − 14 | 3 | |
| R superior parietal lobule | BA 7 | 305 | 4.67 | 0.000031 | 32 | − 53 | 51 |
| R superior frontal gyrus | BA 6 | 325 | 4.16 | 0.000045 | 23 | − 5 | 67 |
| L/R cingulate gyrus | BA 24 | 541 | 5.34 | 0.000083 | − 1 | − 8 | 39 |
| L precuneus | BA 7 | 1396 | 7.43 | 0.000002 | − 19 | − 53 | 48 |
| L thalamus | 240 | 5.39 | 0.000075 | − 16 | − 8 | 12 | |
| L cerebellum | 195 | 4.18 | 0.000081 | − 22 | − 80 | − 18 | |
| L middle frontal gyrusa | BA 8 | 190 | 4.24 | 0.000071 | − 19 | 31 | 39 |
| L middle frontal gyrusa | BA 9 | – | 3.92 | 0.000785 | − 27 | 32 | 31 |
| L insula | BA 13 | 189 | 5.16 | 0.000016 | − 34 | 19 | 3 |
| L superior parietal lobule | BA 7 | 218 | 4.42 | 0.000049 | − 40 | − 65 | 45 |
| L inferior parietal lobule | BA 40 | 432 | 4.27 | 0.000071 | − 49 | − 35 | 21 |
| L middle temporal gyrus | BA 21 | 238 | 5.45 | 0.000067 | − 52 | − 2 | − 18 |
| L middle temporal gyrus | BA 22 | 386 | 5.17 | 0.000013 | − 61 | − 38 | 3 |
| CS group (p < 0.001 corrected) | |||||||
| R insula | BA 13 | 126 | 4.22 | 0.000074 | 44 | 7 | 3 |
| R superior frontal gyrus | BA 6 | 388 | 4.43 | 0.000048 | 15 | 4 | 64 |
| R thalamus | 223 | 4.16 | 0.000084 | 26 | − 35 | 3 | |
| R superior parietal lobule | BA 7 | 186 | 4.98 | 0.000012 | 8 | − 68 | 54 |
| R superior frontal gyrus | BA 8 | 402 | 8.13 | 0.000001 | 9 | 11 | 51 |
| R/L cingulate gyrus | BA 24 | 344 | 4.35 | 0.000057 | 2 | 4 | 36 |
| L cingulate gyrus | BA 33 | 225 | 4.48 | 0.000044 | − 1 | 22 | 18 |
| L cingulate gyrusb | BA 24 | 90 | 4.35 | 0.000181 | − 2 | 3 | 39 |
| L superior parietal lobule | BA 7 | 571 | 6.45 | 0.000011 | − 22 | − 62 | 57 |
| L thalamus | 185 | 4.81 | 0.000017 | − 28 | − 32 | 3 | |
| L insula | BA 13 | 604 | 4.07 | 0.000099 | − 34 | 7 | 18 |
| L insula | BA 13 | 266 | 4.84 | 0.000021 | − 43 | 16 | 9 |
| L inferior parietal lobule | BA 40 | 1422 | 5.52 | 0.000058 | − 40 | − 41 | 33 |
| L inferior parietal lobule | BA 40 | 459 | 4.17 | 0.000081 | − 58 | − 29 | 33 |
| L middle frontal gyrus | BA 9 | 185 | 4.13 | 0.000088 | − 46 | 22 | 34 |
| L inferior frontal gyrus | BA 44 | 273 | 4.48 | 0.000044 | − 55 | − 2 | 33 |
Belonging to the same voxels cluster.
Shown here for reference though below the voxels cluster size threshold adjustment (184 voxels to achieve a corrected p < 0.05, corrected for multiple comparisons).
Fig. 3.
Brain activation foci observed during interference conditions as contrasted to congruent trials (Stroop effect) in each group.
In each group, the brain regions activated in the contrast [(Auditory incongruent + Visual incongruent) > (Auditory congruent + Visual congruent)] are displayed according to the colour scale that codes for the activation level based on the t-values. The activation maps are superimposed on the sagittal, coronal and transverse sections of an individual normalized brain MRI. In the tinnitus patients (TP) group (upper part of the figure), the brain activation foci included the left middle frontal gyrus (Brodmann area (BA) 8–9) (A), the left precuneus (BA 7) (B) and the left thalamus (C), as well as the right middle frontal gyrus (BA 9) (D), the right insula (BA 13) (E), the right superior temporal gyrus (BA 22) (F), the right middle temporal gyrus (BA 39) (G) and the right inferior parietal lobule (BA 40) (H). There was a bilateral activation of the cingulate gyrus (BA 24) (I) and the left superior parietal lobule (BA7) (J) was also activated. In the control subjects (CS) group (lower part of the figure), the brain activation foci included the left middle frontal gyrus (BA 9) (A), the left inferior parietal lobule (BA 40) (B), the right superior frontal gyrus (BA 6–8) (D), the cingulate gyrus bilaterally (BA 24) (E) and the left superior parietal lobule (BA 7)/postcentral gyrus (BA 2) (F). In addition there was a small voxels cluster (not significant) in the left inferior temporal gyrus (BA 20) (H). R: right, L: left.
In CS, using the same contrast we observed that the insula, the CG and the SPL were bilaterally activated. There were also activation foci in the right SFG, the left IPL, the left MiFG (dlPFC) and the left inferior frontal gyrus (Table 3, Fig. 3).
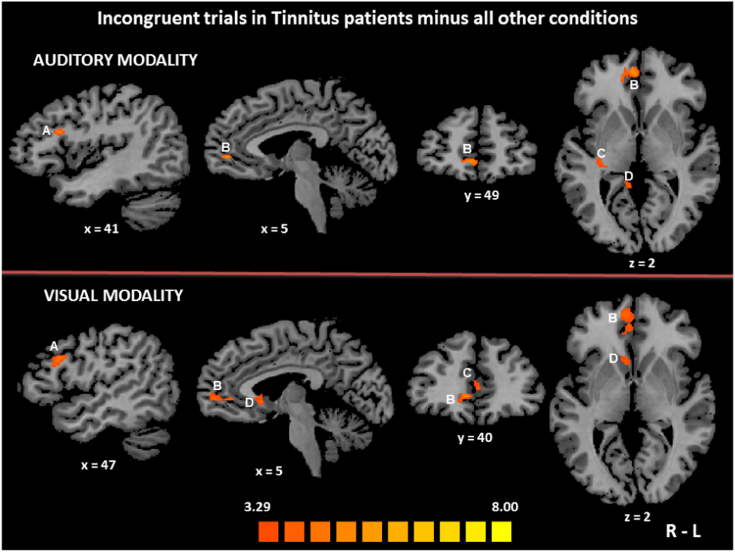
3.3.2. Brain areas selectively recruited in tinnitus patients during incongruent trials
To isolate the brain areas specifically activated by conflict effects in TP (i.e. while TP processed incongruent stimuli as compared to all other conditions), we used the contrast [TP incongruent > (TP congruent + CS congruent + CS incongruent)] in each sensory modality separately. In the auditory modality, the resulting activation map involved regions in the right hemisphere, including the MiFG (dlPFC), the SPL, the caudate nucleus, the posterior CG and the MeFG (vmPFC), as well as the CG bilaterally, although the activation of its anterior part was below the voxels cluster size threshold adjustment (Table 4). In the visual modality, we observed activation foci in the right hemisphere in the MiFG (dlPFC), the caudate nucleus and the MeFG (vmPFC), as well as a bilateral activation of the CG (Fig. 4).
Table 4.
Brain activation foci (positive values) that were selectively activated in tinnitus patients during interference conditions with cognitive conflict [TP incongruent > (TP congruent + CS congruent + CS incongruent)].
| Brain region | Brodmann area | Cluster size | t-Value | p-Value | Coordinate (x, y, z) | ||
|---|---|---|---|---|---|---|---|
| Auditory modality (p < 0.001 corrected) | |||||||
| R middle frontal gyrus (dlPFC) | BA 46 | 222 | 5.28 | 0.000001 | 41 | 20 | 24 |
| R superior parietal lobule | BA 7 | 364 | 5.25 | 0.000001 | 33 | − 62 | 51 |
| Tail of R caudate | 321 | 5.90 | 0.000001 | 32 | − 26 | 0 | |
| R medial frontal gyrus (vmPFC) | BA 10 | 293 | 5.38 | 0.000001 | 5 | 49 | 2 |
| R cingulate gyrus | BA 30 | 331 | 5.83 | 0.000001 | 11 | − 41 | 0 |
| L/R cingulate gyrusa | BA 24 | 82 | 4.07 | 0.00086 | − 1 | 19 | 24 |
| Visual modality (p < 0.001 corrected) | |||||||
| R middle frontal gyrus (dlPFC) | BA 46 | 930 | 7.10 | 0.000001 | 47 | 28 | 23 |
| R caudate | 563 | 5.56 | 0.000001 | 8 | 19 | 3 | |
| R medial frontal gyrus (vmPFC) | BA 10 | 1142 | 5.90 | 0.000001 | 5 | 55 | 3 |
| R superior frontal gyrus, medial part - medial frontal gyrus | BA 8 | 345 | 4.50 | 0.000020 | 2 | 43 | 39 |
| R/L cingulate gyrus | BA 29 | 303 | 5.36 | 0.000001 | 2 | − 53 | 12 |
| L/R cingulate gyrus | BA 24 | 304 | 4.17 | 0.000068 | − 4 | 40 | 12 |
Shown here for reference though below the voxels cluster size threshold adjustment (184 voxels to achieve a corrected p < 0.05, corrected for multiple comparisons).
Fig. 4.
Brain regions that were specifically activated by conflict effects in tinnitus patients.
The activation maps obtained using the contrast [TP incongruent > (TP congruent + CS congruent + CS incongruent)] are displayed according to the colour scale that codes for the activation level based on the t-values. The activation maps are superimposed on the sagittal, coronal and transverse sections of an individual normalized brain MRI. In the auditory modality (upper part of the figure), the right middle frontal gyrus (dlPFC, BA 46) (A) and the right medial frontal gyrus (vmPFC, BA 10) (B) were specifically activated in tinnitus patients while they processed incongruent stimuli, as compared to all other conditions. Brain activation foci also included the tail of the right caudate (C) and the right posterior cingulate gyrus (BA 30) (D). In the visual modality (lower part of the figure), the right middle frontal gyrus (BA 46) (A), the right medial frontal gyrus (BA 10) (B), the cingulate gyrus (BA 24) (C) and the right caudate (D) were specifically activated in tinnitus patients during interference conditions, as compared to all other conditions. R: right, L: left.
The differential activation between tinnitus patients and controls during the task is provided in supplementary material (Table S1 and Fig. S1).
3.3.3. Correlation analyses: relationship between behavioural performance and brain activation in tinnitus patients
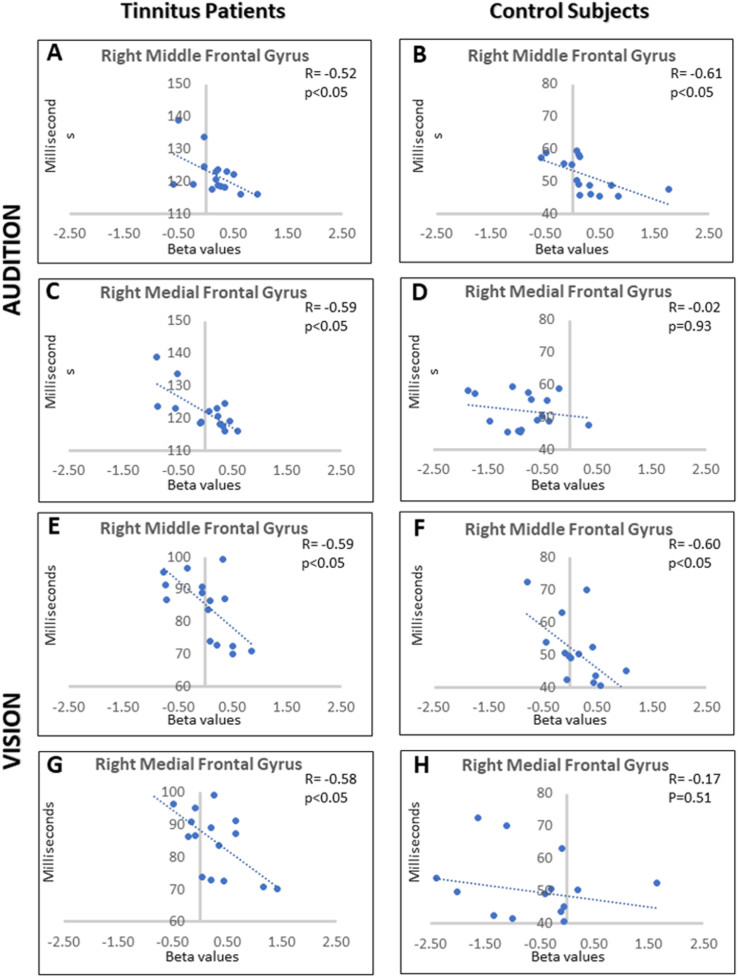
Correlation analyses were performed in each modality between the performance in the spatial Stroop task (i.e., contrasting the response times between incongruent and congruent trials, a smaller difference indicating a better performance) and the beta weights related to incongruent trials in all the brain areas that had been identified as more recruited in tinnitus patients than in controls (using the contrast [(TP congruent + TP incongruent) > (CS congruent + CS incongruent)]). As illustrated in Fig. 5, results were consistent across modalities and significant correlations were observed between individual performance and brain activity level in two distinct brain areas in the right hemisphere, the MiFG (dlPFC) and the MeFG (vmPFC), which were the same in audition (right MiFG-dlPFC (r = − 0.52, p < 0.05, coordinates of focus peak: x = 41, y = 22, z = 24); right MeFG-vmPFC (r = − 0.59, p < 0.05, coordinates of focus peak: x = 5, y = 49, z = 3)) and in vision (right MiFG-dlPFC (r = − 0.59, p < 0.05, coordinates of focus peak: x = 47, y = 19, z = 27); right MeFG-vmPFC (r = − 0.58, p < 0.05, coordinates of focus peak: x = 8, y = 65, z = 12)). When performing the same correlation analyses in the CS group, a correlation between individual performance and brain activity level was observed in the right MiFG (dlPFC) only (in audition, r = − 0.61, p < 0.05, coordinates of focus peak: x = 42, y = 25, z = 32; in vision, r = − 0.60, p < 0.05, coordinates of focus peak: x = 51, y = 12, z = 27). There were no significant correlations between the beta values and the THI scores in any brain region.
Fig. 5.
Correlation between the performance in the spatial Stroop task and the beta weights related to incongruent trials in activated prefrontal brain areas. Charts depict increases in brain activation level (individual beta values) with subtracted response times between incongruent and congruent trials (a smaller difference indicating a better performance) in the auditory modality in tinnitus patients (A, C) and controls (B, D) and in the visual modality in tinnitus patients (E, G) and controls (F, H).
4. Discussion
Using fMRI, we observed that tinnitus patients (TP) differed from control subjects (CS) for the brain activity changes related to the Stroop effect in the dorsolateral prefrontal cortex (dlPFC, in the lateral part of MiFG, BA9 and BA46), the ventromedial prefrontal cortex (vmPFC, in MeFG, BA10) and the cingulate gyrus. We observed a correlation between the performance (efficiency of the top-down cognitive control) and the brain activity level in the right dlPFC (MiFG) and vmPFC (MeFG) in tinnitus patients, both in audition and in vision. This correlation was only observed in the right dlPFC (MiFG) in control subjects.
4.1. Altered responses to the Stroop effect in the cingulate cortex in tinnitus patients
In the present study, the cingulate cortex (CG) was more activated in TP during the processing of incongruent trials as compared to all other conditions, both in audition and in vision. Brain activity changes in several parts of the CG were previously observed in tinnitus patients (Maudoux et al., 2012). It has been reported that the posterior CG was activated during the perception of tinnitus (Plewnia et al., 2007). Applying tDCS or TMS on the anterior CG modulated tinnitus intensity and tinnitus-related distress (Vanneste and De Ridder, 2011). It is noteworthy that the subgenual cingulate cortex and the vmPFC are parts of the so-called “noise-cancellation” system (Leaver et al., 2011, Rauschecker et al., 2010). Given the role of the anterior CG in the allocation of attention resources during a Stroop task, we may hypothesize that the larger recruitment of this brain area observed in tinnitus patients, despite their poor behavioural performance, was related, at least partly, to a chronic altered response of this brain area involved in a persisting salience attribution to the tinnitus sound (Shore et al., 2016).
4.2. Altered responses in the dorsolateral prefrontal cortex in tinnitus patients
There was a selective recruitment of the right dlPFC (MiFG, BA 46) in TP during interference conditions with cognitive conflict (i.e. while TP processed incongruent stimuli as compared to all other conditions). According to previous studies (Egner and Hirsch, 2005a, Egner and Hirsch, 2005b), the dlPFC specifically supports the resolution of conflicts in general. This leads us to postulate that the higher level of brain activity observed in the dlPFC (MiFG) in TP was due, at least partly, to attempts of the brain to neutralize the tinnitus sensations (i.e. to cancel the tinnitus noise) during the Stroop task. It is worth noting that the level of brain activity in the right dlPFC (MiFG) correlated with the individual performance in both groups: the higher the brain activity, the better was the efficiency of cognitive control. However, despite a higher level of brain activity in this region in TP, the performance was still worse in TP than in CS, indicating that this higher level of recruitment of the dlPFC (MiFG) was not sufficient to compensate for tinnitus-related interference in the spatial Stroop task. This hypothesis is in line with previous observations. In a categorization task of auditory stimuli, the fMRI hemodynamic signal observed in the lateral prefrontal cortex (BA46, i.e. the dlPFC-MiFG) and in the right medial prefrontal gyrus (BA10, i.e., the vmPFC-MeFG) in trials with auditory stimulation at the tinnitus frequency correlated with tinnitus loudness ratings on the day of testing (Seydell-Greenwald et al., 2012). This is consistent with the hypothesis according to which the dlPFC was involved in the neutralization of tinnitus that acted as an additional internal distractor during the experimental task (Seydell-Greenwald et al., 2012). We assume that the dlPFC (MiFG) activation observed in the present study reflected the effort made to focus on performing the spatial Stroop task while ignoring distractors, among which tinnitus. This idea is also supported by the fact that stimulation of the dlPFC in TP using tDCS or TMS modulated the intensity of tinnitus perception and the amount of tinnitus-related distress (De Ridder et al., 2012, Vanneste et al., 2013).
4.3. Differential activation of ventromedial prefrontal cortex between patients and controls
The vmPFC (MeFG), which is considered as a core region of the emotional brain, was specifically activated by conflict effects in TP only (i.e. while TP processed incongruent stimuli as compared to all other conditions). However, on the one hand, this brain area was not included in the brain activation pattern related to conflict resolution (i.e. when contrasting incongruent to congruent trials). On the other hand, its recruitment during the processing of incongruent trials correlated with the individual performance in the spatial Stroop task in TP (and in TP only), in audition as well as in vision. It was previously shown that the activation of the vmPFC was associated with successful suppression of emotional responses to a negative emotional signal (Hansel and von Kanel, 2008). This is in line with the brain imaging studies that indicated a specific alteration of this brain region in people with chronic tinnitus. The first morphometric study in tinnitus patients, using MRI and voxel-based morphometry, revealed significant gray matter volume decreases within the subcallosal region, which contained the nucleus accumbens but also part of the vmPFC (Muhlau et al., 2006). Although in other studies no structural difference was observed in a subcallosal brain area that included the vmPFC (Melcher et al., 2013) when TP were compared to controls in whom reductions in subcallosal gray matter correlated with supra-clinical hearing loss above 8000 Hz, the main subsequent studies reported structural MRI differences in this brain area between tinnitus and control groups (Adjamian et al., 2014, Boyen et al., 2013, Elgoyhen et al., 2015, Leaver et al., 2011). The reported reductions in vmPFC gray matter were consistent with reduced functional output of vmPFC in tinnitus patients, as observed when using magnetoencephalography to investigate the resting-state network in these patients (Schlee et al., 2009). Interestingly, in the study of Leaver et al. (Leaver et al., 2011), those patients with greater amounts of gray matter in vmPFC exhibited less hyperactivity in the auditory cortex, reflecting a relatively better ability of the vmPFC to exert an inhibitory influence on the auditory system. Accordingly, the vmPFC (MeFG) would play a key role in tinnitus perception as postulated in the “noise-cancellation” model proposed by Rauschecker and collaborators (Rauschecker et al., 2010). This gating model postulates that chronic perception of tinnitus occurs as a consequence of a dysfunctional noise suppressing mechanism due to irregularities in a limbic-cortico-striatal-thalamic circuit that determines which sensations are important. Contributions from both cortical and subcortical brain areas were proposed in this model, including changes in the vmPFC and other regions (cingulate cortex, nucleus accumbens and reticular thalamic nucleus) that are parts of an evaluation network of positive/negative valency of sensory stimuli (Kable and Glimcher, 2009, Leaver et al., 2011) and involved in long-term habituation to continuous unpleasant sounds. The model postulates that irregularities within this circuit lead to generate a deficient sensory attentional gating mechanism, responsible for abnormal evaluation of the perceptual relevance of tinnitus sound (Adjamian et al., 2014, Leaver et al., 2011). In the present study, we excluded or controlled most potential confounding factors (e.g. hearing loss, depression, anxiety), so that the functional changes observed in the vmPFC (MeFG) could only be attributed to chronic tinnitus per se. This leads us to hypothesize that this brain area was involved in noise cancellation and emotion regulation during the spatial Stroop task as well, which in some way improved the performance in TP (the higher the activity in the right vmPFC (MeFG), the better the performance), whatever the sensory modality used in the task. However, considering the evidence and our results based on correlation analyses, we cannot conclude whether tinnitus produced deficits in the cognitive control or whether the pre-existence of an alteration in the cognitive control is needed to produce tinnitus.
4.4. Altered responses in dorsolateral and ventromedial prefrontal cortex in tinnitus patients
The level of brain activity in the right vmPFC (MeFG) and in the right dlPFC (MiFG) during the Stroop task (interference conditions) strongly correlated with the efficiency of top-down cognitive control in TP, while in CS this correlation was observed in the dlPFC (MiFG) only. This leads us to suggest that the vmPFC (MeFG) and the dlPFC (MiFG) in the right hemisphere influenced the activity of each other in tinnitus patients. The implication of the vmPFC and the dlPFC in the regulation of emotions has been previously demonstrated; the dlPFC is connected to the limbic system through the vmPFC (Ochsner and Gross, 2005). Additionally, the dlPFC, the anterior CG, the insula and the amygdala constitute part of the network that is activated during auditory stimulation in TP (Shore et al., 2016). Accordingly, the altered dlPFC could contribute to the perception of tinnitus, probably through interactions with the limbic-corticostriatal thalamic circuit and, in particular, with the vmPFC that is part of the “noise-cancellation” pathway (Adjamian et al., 2014, Muhlau et al., 2006, Rauschecker et al., 2010, Shore et al., 2016). Recently, Elgoyhen and collaborators (Elgoyhen et al., 2015) noted in a critical review that a system that would work with the vmPFC and that would underlie the mechanisms for the detection and selective suppression of the unwanted tinnitus sound was still missing to support the sound-inhibitory system in the “noise-cancellation” model (Rauschecker et al., 2010). Here, the dlPFC (MiFG) appears as a plausible candidate for such a system. We hypothesize that a specific alteration of the vmPFC (MeFG) is a key component in the pathophysiology of tinnitus, while the activity increase observed in the dlPFC (MiFG) during the Stroop task in both modalities would be a consequence of tinnitus interference, i.e., it would be related to the (inefficient) attempt to neutralize tinnitus perception during the Stroop task.
5. Conclusion
Here we identified brain activity alterations in the vmPFC (MeFG) and in the dlPFC (MiFG) in tinnitus patients during a spatial Stroop paradigm in audition and vision. The brain activity level in these two regions was correlated with the efficiency of the top-down cognitive control in tinnitus patients, although this efficiency was poor in these patients when compared to controls. Such a deficit of top-down cognitive control, underlain by an altered response in the vmPFC (MeFG) and dlPFC (MiFG), could partly explain why the suppression of tinnitus sensation did not occur in these patients. Although further studies are clearly needed, identifying the cognitive and neural mechanisms involved in tinnitus perception is an innovative approach that could contribute to develop effective treatments based on neuropsychological training, with the hope to finally offer a solution to tinnitus patients.
Acknowledgments
Acknowledgements
We wish to thank Professors Ph. Rombaux and Y. Vandermeeren for comments and support to R.A. A.G. De Volder is Senior Research Associate at the Belgian National Fund for Scientific Research. R. Araneda was a PhD student supported by the Conicyt Becas Chile scholarship program. L. Renier was a Postdoctoral researcher supported by the Brussels Institute for Research and Innovation (INNOVIRIS, Belgium). We gratefully thank all the volunteers for their participation to the study. This study was supported by FNRS grant #J.0205.17 (Belgium) and the Brussels Institute for Research and Innovation (#BB2B 2010-1-09) (INNOVIRIS, Belgium). The authors declare no competing financial interests.
Author contributions
R.A., L.R. and A.D.V. conceived, designed and conducted the experiments. R.A., L.D. and L.R. analyzed the data. R.A., L.R., M.D., D.E.-K., N.D. and A.D.V. wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.10.029.
Appendix A. Supplementary data
Supplementary material
References
- Adjamian P., Hall D.A., Palmer A.R., Allan T.W., Langers D.R. Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci. Biobehav. Rev. 2014;45:119–133. doi: 10.1016/j.neubiorev.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R., De Volder A.G., Deggouj N., Philippot P., Heeren A., Lacroix E., Decat M., Rombaux P., Renier L. Altered top-down cognitive control and auditory processing in tinnitus: evidences from auditory and visual spatial stroop. Restor. Neurol. Neurosci. 2015;33:67–80. doi: 10.3233/RNN-140433. [DOI] [PubMed] [Google Scholar]
- Araneda R., De Volder A.G., Deggouj N., Renier L. Altered inhibitory control and increased sensitivity to cross-modal interference in tinnitus during auditory and visual tasks. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyen K., Langers D.R., de Kleine E., van Dijk P. Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear. Res. 2013;295:67–78. doi: 10.1016/j.heares.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Boynton G.M., Engel S.A., Glover G.H., Heeger D.J. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Elgoyhen A.B., Romo R., Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Plazier M., Menovsky T., van de Heyning P., Kovacs S., Sunaert S. Dorsolateral prefrontal cortex transcranial magnetic stimulation and electrode implant for intractable tinnitus. World Neurosurg. 2012;77:778–784. doi: 10.1016/j.wneu.2011.09.009. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Weisz N., Londero A., Schlee W., Elgoyhen A.B., Langguth B. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 2014;44:16–32. doi: 10.1016/j.neubiorev.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T., Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Egner T., Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Elgoyhen A.B., Langguth B., De Ridder D., Vanneste S. Tinnitus: perspectives from human neuroimaging. Nat. Rev. Neurosci. 2015;16:632–642. doi: 10.1038/nrn4003. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Jezzard P., Turner R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1994;1:153–171. [Google Scholar]
- Friston K.J., Holmes A.P., Price C.J., Buchel C., Worsley K.J. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A., von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc. Med. 2008;2:21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A., Maurage P., Perrot H., De Volder A., Renier L., Araneda R., Lacroix E., Decat M., Deggouj N., Philippot P. Tinnitus specifically alters the top-down executive control sub-component of attention: evidence from the Attention Network Task. Behav. Brain Res. 2014;269:147–154. doi: 10.1016/j.bbr.2014.04.043. [DOI] [PubMed] [Google Scholar]
- Heller A.J. Classification and epidemiology of tinnitus. Otolaryngol. Clin. N. Am. 2003;36:239–248. doi: 10.1016/s0030-6665(02)00160-3. [DOI] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M., Zeman F., Koller M., Eberl Y., Mohr M., Reiter J., Staudinger S., Hajak G., Langguth B. The Tinnitus Research Initiative (TRI) database: a new approach for delineation of tinnitus subtypes and generation of predictors for treatment outcome. BMC Med. Inform. Decis. Mak. 2010;10:42. doi: 10.1186/1472-6947-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Goodey R., Azevedo A., Bjorne A., Cacace A., Crocetti A., Del Bo L., De Ridder D., Diges I., Elbert T., Flor H., Herraiz C., Ganz Sanchez T., Eichhammer P., Figueiredo R., Hajak G., Kleinjung T., Landgrebe M., Londero A., Lainez M.J., Mazzoli M., Meikle M.B., Melcher J., Rauschecker J.P., Sand P.G., Struve M., Van de Heyning P., Van Dijk P., Vergara R. Consensus for tinnitus patient assessment and treatment outcome measurement: tinnitus research initiative meeting, Regensburg, July 2006. Prog. Brain Res. 2007;166:525–536. doi: 10.1016/S0079-6123(07)66050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Kreuzer P.M., Kleinjung T., De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12:920–930. doi: 10.1016/S1474-4422(13)70160-1. [DOI] [PubMed] [Google Scholar]
- Leaver A.M., Renier L., Chevillet M.A., Morgan S., Kim H.J., Rauschecker J.P. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A.M., Seydell-Greenwald A., Turesky T.K., Morgan S., Kim H.J., Rauschecker J.P. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front. Syst. Neurosci. 2012;6:21. doi: 10.3389/fnsys.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A., Lefebvre P., Cabay J.E., Demertzi A., Vanhaudenhuyse A., Laureys S., Soddu A. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012;1485:10–21. doi: 10.1016/j.brainres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Melcher J.R., Knudson I.M., Levine R.A. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (> 8 kHz), but not with tinnitus. Hear. Res. 2013;295:79–86. doi: 10.1016/j.heares.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Muhlau M., Rauschecker J.P., Oestreicher E., Gaser C., Rottinger M., Wohlschlager A.M., Simon F., Etgen T., Conrad B., Sander D. Structural brain changes in tinnitus. Cereb. Cortex. 2006;16:1283–1288. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- Newman C.W., Sandridge S.A., Jacobson G.P. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J. Am. Acad. Audiol. 1998;9:153–160. [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Plewnia C., Reimold M., Najib A., Brehm B., Reischl G., Plontke S.K., Gerloff C. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum. Brain Mapp. 2007;28:238–246. doi: 10.1002/hbm.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J.P., Leaver A.M., Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Gardner D., Lee R. Tinnitus and psychological morbidity: a cross-sectional study to investigate psychological morbidity in tinnitus patients and its relationship with severity of symptoms and illness perceptions. Clin. Otolaryngol. Allied Sci. 2004;29:628–634. doi: 10.1111/j.1365-2273.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- Roberts L.E., Eggermont J.J., Caspary D.M., Shore S.E., Melcher J.R., Kaltenbach J.A. Ringing ears: the neuroscience of tinnitus. J. Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W., Hartmann T., Langguth B., Weisz N. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci. 2009;10:11. doi: 10.1186/1471-2202-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydell-Greenwald A., Leaver A.M., Turesky T.K., Morgan S., Kim H.J., Rauschecker J.P. Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain Res. 2012;1485:22–39. doi: 10.1016/j.brainres.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S.E., Roberts L.E., Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016;12:150–160. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Georg Thieme; Stuttgart, New York: 1988. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. [Google Scholar]
- Vanneste S., De Ridder D. Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity. Eur. J. Neurosci. 2011;34:605–614. doi: 10.1111/j.1460-9568.2011.07778.x. [DOI] [PubMed] [Google Scholar]
- Vanneste S., De Ridder D. The use of alcohol as a moderator for tinnitus-related distress. Brain Topogr. 2012;25:97–105. doi: 10.1007/s10548-011-0191-0. [DOI] [PubMed] [Google Scholar]
- Vanneste S., Walsh V., Van De Heyning P., De Ridder D. Comparing immediate transient tinnitus suppression using tACS and tDCS: a placebo-controlled study. Exp. Brain Res. 2013;226:25–31. doi: 10.1007/s00221-013-3406-7. [DOI] [PubMed] [Google Scholar]
- Zoger S., Svedlund J., Holgers K.M. Psychiatric disorders in tinnitus patients without severe hearing impairment: 24 month follow-up of patients at an audiological clinic. Audiology. 2001;40:133–140. doi: 10.3109/00206090109073108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material