Summary
Intraoperative optical coherence tomography (OCT) is a potentially transformational technology that is now commercially available to ophthalmic surgeons. Currently, the use of the technology is primarily limited to a “stop and image” approach due to the lack of OCT compatibility with surgical instrumentation. In this report, we describe multiple OCT-compatible surgical instruments that were developed for various surgical needs, based on previous evaluation of potential surgical materials for optical features and physical properties. OCT-compatible instrumentation included 2 membrane scrapers, a surgical pick, and vitreoretinal forceps. Imaging during in vitro and ex vivo simulated surgical procedures demonstrated excellent visualization of the instrument tip and of the tissue-instrument interaction. These OCT-compatible instruments may be a key component to the feasibility of real-time image-guided surgery with intraoperative OCT.
Introduction
The recent development of intraoperative optical coherence tomography (OCT) has provided new opportunities in the approach to various vitreoretinal diseases during vitreoretinal surgery.[1–7] Real-time identification of pathological conditions and anatomic configurations have enhanced surgeons’ understanding of anatomy and impacted surgical management.[1 2] Use of intraoperative OCT has been previously described in several types of retinal surgery, including full-thickness macular hole, epiretinal membrane, retinal detachment and others.[1–3 5–7] In general, most studies have focused on a “stop and scan” approach with intraoperative OCT. Real-time intraoperative OCT with visualization of tissue-instrument interaction has been limited due to the lack of OCT compatibility with conventional surgical instruments. Metallic instrumentation results in absolute shadowing of the underlying tissues and the light scattering properties limits visualization of the actual instrument tip.[2 3 8 9] Previous work in this area has demonstrated that various alternative materials (e.g., silicone, plastics) provide more optimal light scattering and shadowing properties compared to metal.[8 10] In particular, the more diffuse light scattering allows for greater visibility of the entire instrument tip compared to only a focal area of reflection in the highly-reflective metallic instruments.[8 10] In addition, the semi-transparent nature of the materials provide greater transmission of the OCT signal and reduction in shadowing compared to metallic instrumentation.[8 10] In this report, we describe novel surgical instrumentation for vitreoretinal surgery that has been designed for compatibility with intraoperative OCT for real-time surgical manipulation and demonstrate OCT visualization in simulated surgical conditions.
Materials and Methods
Surgical instruments made of semi-transparent rigid plastic material were imaged using Rescan 700 system (Carl Zeiss Meditec, Oberkochen, Germany) and EnFocus system (Leica, Wezlar, Germany). EnFocus system was implemented as an attachment to a Leica M844 (Leica) ophthalmic surgical microscope and intraoperative OCT information as visualized on an external monitor. The Rescan 700 includes a heads-up display system within the microscope ocular that allows for visualization of the instrument-tissue interaction. Prototype OCT compatible vitreoretinal surgical instruments were designed, constructed and evaluated including an OCT-compatible diamond dusted membrane scraper (DDMS), a non-diamond dusted membrane scraper, a surgical pick, and vitreoretinal forceps designed and manufactured in collaboration with Synergetics (O’Fallon, Missouri, USA) and Bausch and Lomb (Rochester, New York, USA). These instruments were machined in both 23- and 25-gauge sizes. For comparison, a standard membrane pick (DORC, Zuidland, Netherlands), asymmetrical vitreoretinal forceps (DORC) and Tano DDMS (Synergetics) were also imaged on intraoperative OCT as conventional instruments.
In vitro imaging was performed with an opened petri dish filled with water containing paper towel lining the bottom. Paper towel was placed to simulate ocular tissue. During the imaging process, the tip of each instrument was kept submerged in water. Images were evaluated for visualization of the instrument tip and the underlying materials.
Simulated surgical imaging was also performed with human cadaver eye globes obtained from human donors (supplied by the Eversight Ohio, USA). Dextran solution was used to reduce the corneal edema. Each eye was positioned under the microscope-integrated intraoperative OCT system using a suction platform to maintain fixation. A valved trocar cannula system was utilized for posterior segment surgical maneuvers in laboratory setup. Posterior segment intraoperative OCT visualization was obtained with RESIGHT wide-angle fundus viewing system (Carl Zeiss Meditec). The tip of each instrument was placed in contact with the retina and was imaged with intraoperative OCT. Intraocular maneuvers included removal of a simulated intraocular foreign body, a small fragment created from intraocular lens glide. Simulated surgery was performed by a trained vitreoretinal surgeon and the intraoperative OCT system was operated by an imaging technician.
Results
The tip of each instrument was successfully captured with both intraoperative OCT platforms. OCT-compatible instrumentation demonstrated excellent light scattering and reflectivity properties for visualization of the instrument tip (Figures 1 and 2, Supplemental Video 1). Meanwhile, conventional metallic instruments demonstrated highly variable light scattering properties that often resulted in limited visualization. Shadowing of underlying tissues was significantly reduced with OCT-compatible instrumentation.
Figure 1. Intraoperative Optical Coherence Tomography Compatible Surgical Instruments.
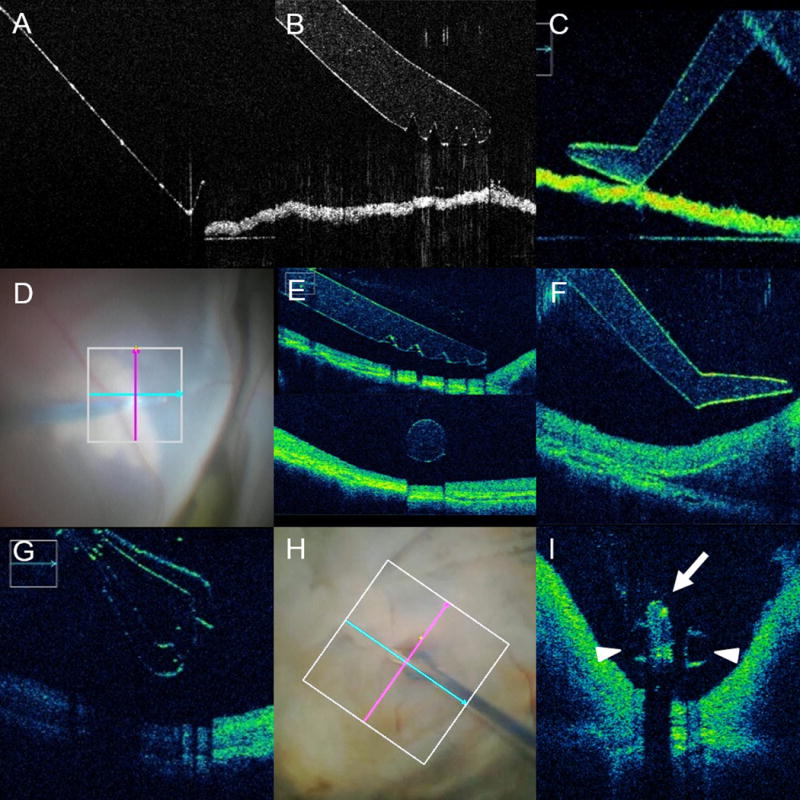
(A–C) In vitro imaging of optical coherence tomography (OCT) compatible and non-compatible conventional instrumentation. (A) OCT B-scan of conventional metallic surgical pick. Note limited visualization of the instrument contours and absolute shadowing of the underlying membrane. No visualization of actual tissue-instrument interaction is feasible. (B–C) B-scan of OCT-compatible instruments, including membrane scraper (B) and surgical pick (C). Excellent visualization of the instrument edges and angles are noted. In addition, minimal-to-no shadowing is noted of underlying membrane. (D–F) Ex vivo imaging in human cadaver eye of OCT-compatible surgical instruments, including membrane scraper (E) and surgical pick (F). Similar to the in vitro imaging, excellent visualization of the actual instrument and underlying retinal tissues was achieved. (G–I) Ex vivo simulated surgical procedure with human cadaveric eye and OCT-compatible forceps. (G) OCT B-scan parallel to the axis of the forceps revealing platform and shape of the forceps tip. Slight increased shadowing is noted of the underlying tissues given the complexity of the forceps. (H–I) OCT-compatible forceps engagement of a highly-reflective intraocular foreign body (arrow) with the tips of the forceps well visualized (arrowheads). Note the minimal shadowing of the underlying tissues from the forceps compared to the near-absolute shadowing of the foreign body.
Figure 2. Intraoperative Optical Coherence Tomography Compatible Diamond Dusted Membrane Scraper.
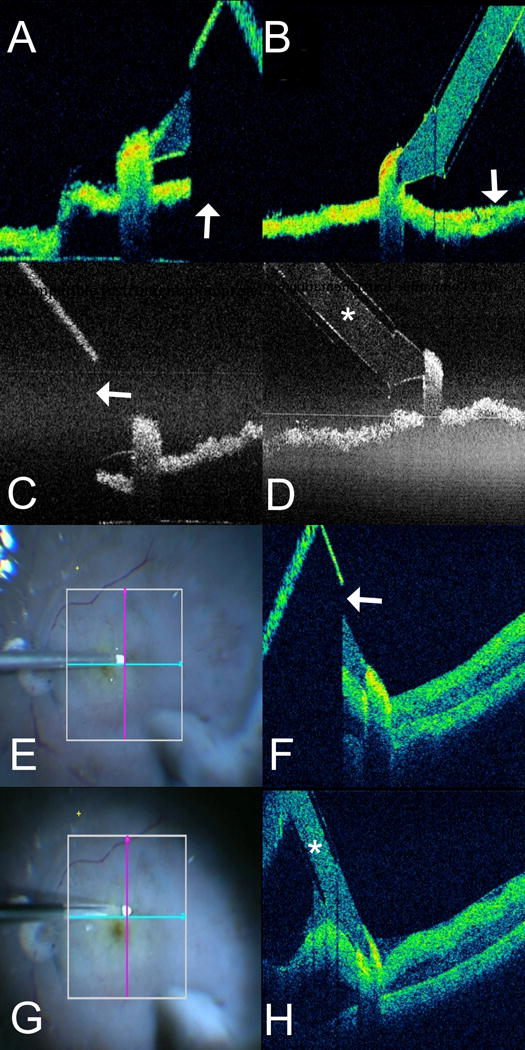
(A–D) In vitro imaging of optical coherence tomography (OCT) compatible and conventional diamond dusted membrane scraper (DDMS). (A) OCT B-scan with the Rescan 700 of conventional DDMS. Note absolute shadowing of the underlying membrane in the area beneath the shaft of the instrument (arrow). (B) B-scan of OCT-compatible DDMS with the Rescan 700 demonstrates excellent visualization of the membrane below the shaft (arrow). (C) OCT B-scan with the EnFocus OCT system of conventional DDMS. Note absolute shadowing of the underlying membrane in the area beneath the shaft of the instrument (arrow). (D) B-scan of OCT-compatible DDMS with the EnFocus OCT demonstrates excellent visualization of both instrument (asterisk) and the membrane below. (E-H) Ex vivo imaging in human cadaver eye of both conventional and OCT-compatible DDMS. (E) En face microscope view of conventional DDMS on retinal surface with OCT aiming overlay. (F) Similar to the in vitro imaging, OCT-B scan of conventional DDMS demonstrates absolute shadowing of the retinal tissue beneath the shaft of the instrument (arrow), preventing visualization of tissues in the path of the membrane scraper. (G) En face microscope view of OCT-compatible DDMS on retinal surface with OCT aiming overlay. (H) OCT B-scan of OCT-compatible DDMS demonstrates significantly improved visualization of the actual instrument (asterisk) and underlying retinal tissues, providing excellent visualization of the path of membrane peeling.
In simulated surgery, OCT-compatible instruments and underlying whole retinal tissue were successfully visualized on intraoperative OCT (Figure 1). Surgical changes in underlying retinal tissue morphology were observed on B-scans. Instrument depth information was clearly visualized and relative tissue–instrument position was easily assessed. Moreover, OCT-compatible vitreoretinal forceps and its interaction with an intraocular foreign body was successfully visualized. The subretinal foreign body was removed without difficulty using prototype vitreoretinal forceps. The tactile feedback of the non-metallic forceps was perceived to be less sensitive than conventional forceps. The complex geometry of the forceps resulted in more varied light scattering and shadowing compared to the surgical pick.
Conventional DDMS demonstrated absolute shadowing below the shaft of the instrument preventing visualization of the future path of peeling, whereas the OCT-compatible DDMS resulted in minimal to no shadowing of the underlying tissues and provided the surgeon with excellent visualization of the leading edge of tissue for membrane peeling (Figure 2).
Discussion
OCT-compatible instruments demonstrated excellent visibility of the adjacent and underlying tissue on intraoperative OCT with high repeatability. The new instruments are designed to have semitransparent material from the tip to allow for transmission of the OCT signal. This is advantageous over conventional instruments, in which OCT signal is obstructed by metallic tip of the instruments. Compared to the other prototypes, vitreoretinal forceps demonstrated excellent visualization of the tips but slightly increased shadowing. This is likely due to the complex structure/geometry of the instrument resulting increased light scattering compared to the other prototypes.
These instruments represent significant improvements in OCT-compatibility but additional enhancements are needed to continue to maximize tissue-instrument visualization. In particular, the complex geometry of the forceps resulted in more shadowing compared to the other prototypes. In addition, the precision requirements for vitreoretinal forceps creates unique challenges for instrument reusability and comparable reliability to metallic alternatives. Ongoing refinement is needed to continue to improve functionality. Utilizing a non-metallic material will also likely require instruments to be disposable. Software and imaging hardware solutions may also enable novel approaches to minimizing instrument shadowing while improving tissue-instrument interaction visualization.[9] Potential advances that could further enable OCT-compatible instrumentation include faster OCT engines, enhancements to image processing, and tracking systems enabling more rapid processing.
Although these surgical instruments provide a major iterative step forward in intraoperative OCT, additional technical advances are needed to maximize true “real-time” image-guided surgery. Automated tracking is needed to minimize surgeon demand on OCT positioning during surgical manipulation. Advances in surgical visualization platforms are needed to better seamlessly integrate the image-guided environment into the true surgical environment. As these technologies merge, opportunities may emerge for image-guided surgery including targeted drug delivery and vascular cannulation.[8–10] More research is needed into the role of real-time intraoperative OCT and the potential implications for surgical care with OCT-compatible surgical instruments.
Supplementary Material
Supplemental video 1: Real-time imaging of optical coherence tomography (OCT) compatible forceps. Excellent visualization of the instrument tips and the overall action of the forceps is achieved.
Acknowledgments
Financial Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE); R01-EY023039-01 (JPE, SKS); Research to Prevent Blindness (Cole Eye Institutional Grant); Unrestricted travel grant from Alcon Novartis Hida Memorial Award 2015 funded by Alcon Japan Ltd (AU). Eversight Ohio (provision of tissue, JPE).
Footnotes
Financial Disclosures: JPE: Bioptigen/Leica (P, C), Synergetics (P), Zeiss (C), Thrombogenics (C, R); Regeneron (R), Genentech (R), Santen (C), Alcon (C);. AU: None. SKS: Allergan (R), Bausch and Lomb (C), Bioptigen (P), Zeiss (C), Leica (C), Santen (C), Synergetics (P);
Contributors: The authors would like to acknowledge Carmen Calabrise and Jamie L. Reese for their contributions through laboratory study support and operational support during experimental imaging. The authors would also like to acknowledge Eversight Ohio for support through provision of research tissue. The authors would also like to acknowledge Matt LaConte for design and manufacturing support.
Data Access Responsibility and Analysis: Dr. Ehlers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014;158(5):999–1007. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehlers JP, Goshe J, Dupps WJ, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015;133(10):1124–32. doi: 10.1001/jamaophthalmol.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–6. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 4.Ehlers JP, Tao YK, Srivastava SK. The value of intraoperative optical coherence tomography imaging in vitreoretinal surgery. Curr Opin Ophthalmol. 2014;25(3):221–7. doi: 10.1097/ICU.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn P, Migacz J, O’Connell R, Maldonado RS, Izatt JA, Toth CA. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging. 2011;42(Suppl):S85–94. doi: 10.3928/15428877-20110627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012;43(6 Suppl):S54–60. doi: 10.3928/15428877-20121001-08. [DOI] [PubMed] [Google Scholar]
- 7.Ray R, Baranano DE, Fortun JA, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011;118(11):2212–7. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3153–9. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013;33(1):232–6. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers JP, Srivastava SK, Feiler D, Noonan AI, Rollins AM, Tao YK. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS One. 2014;9(8):e105224. doi: 10.1371/journal.pone.0105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental video 1: Real-time imaging of optical coherence tomography (OCT) compatible forceps. Excellent visualization of the instrument tips and the overall action of the forceps is achieved.


