Abstract
Relationships between total soil or bioaccessible lead (Pb), measured using an in vitro bioaccessibility assay, and children’s blood lead levels (BLL) were investigated in an urban neighborhood in Philadelphia, Pennsylvania, USA, with a history of soil Pb contamination. Soil samples from 38 homes were analyzed to determine whether accounting for the bioaccessible Pb fraction improves statistical relationships with children’s BLLs. Total soil Pb ranged from 58 to 2,821 mg/kg; the bioaccessible Pb fraction ranged from 47 to 2,567 mg/kg. Children’s BLLs ranged from 0.3 to 9.8 μg/dL. Hierarchical models were used to compare relationships between total or bioaccessible Pb in soil and children’s BLLs. Total soil Pb as the predictor accounted for 25% of the variability in child BLL; bioaccessible soil Pb as the predictor accounted for 28% of BLL variability. A bootstrapping analysis confirmed a significant increase in R2 for the model using bioaccessible soil Pb as the predictor with 99.3% of bootstraps showing a positive increase. Estimated increases of 1.4 μg/dL and 1.6 μg/dL in BLL per 1,000 mg/kg Pb in soil were observed for this study area using total and bioaccessible Pb, respectively. Children’s age did not contribute significantly to the prediction of BLLs.
Graphical Abstract
INTRODUCTION
Lead (Pb) is a potent developmental neurotoxin; even low levels of exposure in early life have the potential for profound and long-lasting health effects (Surkan et al., 2007; Bellinger, 2008). Lead occurs naturally in soils typically at concentrations that range from 10 to 50 mg/kg. Human activity has dispersed Pb in the environment, resulting in extensive and persistent contamination of soil and dust (Datko-Williams et al., 2014). Widespread use of leaded paint until the mid-1970s and leaded gasoline until the mid-1980s, and contamination from various industrial sources, resulted in extensive contamination of urban soils with Pb. Urban soils often have Pb concentrations much greater than normal background levels. These concentrations frequently range from 150 mg/kg to as high as 10,000 mg/kg at the base of a home painted with lead-based paint (U.S. EPA, 1986). Because Pb does not biodegrade or dissipate over time, soil Pb levels remain elevated for many years. Elevated Pb levels in soil and dust represent significant sources of exposure for children (Laidlaw et al., 2014, von Lindern et al., 2016).
Earlier studies have evaluated children’s blood lead levels (BLL) in relation to soil Pb concentrations. A report released by the U.S. Environmental Protection Agency (1983) estimated blood Pb increases of 0.6 to 6.8 μg/dL per 1000 mg/kg of soil Pb. Other published studies have reported similar observations (Duggan, 1980; Lanphear et al., 1998; Madhavan et al., 1989; Mielke et al., 1999; Mielke et al., 2007). However, the relationship between childhood blood Pb and soil Pb is highly dependent on site-specific variables including soil vegetative cover, frequency and duration of soil contact, and behavior, hygiene, and nutritional status of exposed children (Ryan et al., 2004; U.S. EPA, 2007a).
Although earlier studies evaluated relationships between total soil Pb concentrations and BLLs in children, few have considered bioavailability or bioaccessibility of soil Pb when evaluating relationships with blood Pb concentrations in urban environments. Soil Pb bioavailability is a measure of the fraction of Pb in soil that upon ingestion is released from the soil and becomes available for absorption across the gastrointestinal barrier. Accounting for bioavailability can improve the accuracy of exposure and risk calculations when assessing human exposures to Pb in soil in urban environments. Bioaccessibility is the fraction of the total amount of Pb in soil that is soluble in a gastric-like (i.e., low pH) extraction medium (U.S. EPA, 2007a) and can be used as an estimate of bioavailability. Ren et al. (2006) have reported statistically significant relationships between bioaccessible Pb in soil, as measured by the physiologically based extraction test (Ruby et al., 1996), and children’s BLLs. However, this study was limited in that it did not directly compare total versus bioaccessible Pb with respect to BLLs and measured only eight data points per regression analysis. Zahran et al’s results from New Orleans indicate that about 67% of the variation in children’s BLL was explained by independent soil Pb sample location variables for samples collected near houses, residential and busy side streets, or open spaces (Laidlaw et al., 2017).
A blood lead study was conducted during June 2014 in several urban neighborhoods in Philadelphia, Pennsylvania with a history of soil and household lead contamination. A total of 122 households and 163 children less than 8 years were enrolled. The current study is a randomly selected subset of the original study evaluating the relationship between total or bioaccessible Pb and children’s BLLs across 49 data points using an in vitro bioaccessibility assay (IVBA) to estimate soil Pb bioavailability. We hypothesized that accounting for the bioaccessible fraction of Pb would improve statistical relationships with children’s BLLs. We also assessed the possible effect of a child’s age on the relationship between soil Pb and children’s BLLs.
MATERIALS AND METHODS
Soil Collection
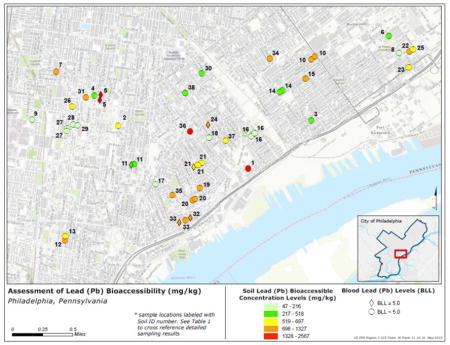
To evaluate the relationship between total or bioaccessible Pb and children’s BLLs, soil samples were collected from yards in selected urban residential homes in Philadelphia, Pennsylvania, USA. The majority of the Philadelphia single-family row-style homes included in the study were built before 1900. Soil samples were collected from neighborhoods (Kensington, Olde Richmond, Port Richmond, North Philadelphia East, and Fishtown) with a history of nearby industrial operations dating back to the mid-1800s, several of which are known to have released Pb in air emissions. The study area comprised ZIP codes, 19125, 19134, 19145, 19146, 19147 and 19148. During 2015, based on PDPH child blood lead surveillance data, the percent of children with BLLs 5–9 μg/dL ranged from <1–6% (PDPH, 2015). Of particular interest in this investigation was a large Pb paint and product manufacturer that operated from 1849 to 1996 (ATSDR, 2015) located near map locations 1, 3, 16, 18, 21, 24, 37 (TOC Map). The area adjacent to this former manufacturing facility has been the subject of various environmental and public health investigations since the 1970s. Most industries in all the neighborhoods where soil was collected had closed by the 1970s, corresponding to a nationwide decline in many types of manufacturing operations.
Many of the yards in this row-home section of Philadelphia are quite small (approximately 15–20 yd2), with the majority of yards sampled containing both bare soil and concrete. Surface soil samples were collected primarily from areas of residential yards where parents or guardians said children play. Samples were randomly selected from a subset of yards in the original study and collected from both bare and vegetative soil depending on yard conditions, although bare soil was preferred for sampling. In some cases, samples were collected from a garden area if the small yard was used solely for this purpose. Five discrete soil samples were collected from approximately 0 to 8 cm deep to make one composite sample for each location. Small rocks or pebbles and other foreign materials were removed from the discrete samples before combining the samples in a 3.78 L (1 gallon) resealable plastic bag. An aliquot of soil from this composite sample was sent to U.S. EPA’s Office of Research and Development (Research Triangle Park, North Carolina, USA) under chain of custody for further processing and analysis. Samples were dried (< 40 ºC), sieved to < 250 μm, homogenized, and riffled for mixing and splitting of samples for total and bioaccessible Pb determination. A total of 38 composite samples from independent homes were included in this study.
Total and Bioaccessible Pb Determination
Total Pb concentration in soil samples was determined using microwave-assisted acid digestion in accordance with U.S. EPA SW-846 Test Method 3051A (U.S. EPA, 2007b), with analysis by inductively coupled plasma–optical emission spectrometry in accordance with U.S. EPA SW-846 Method 6010D (U.S. EPA, 2014). The in vitro bioaccessible (IVBA) fraction of Pb in soil samples was determined using U.S. EPA SW-846 Method 1340 (U.S. EPA, 2013). Briefly, 1 g of soil sample was extracted in 100 mL of 0.4 M glycine solution acidified to a pH of 1.5 using hydrochloric acid and rotated end over end in a 37 ºC water bath for 1 hour. Extracts were filtered and analyzed for Pb concentration by inductively coupled plasma–mass spectrometry in accordance with U.S. EPA SW-846 Method 6020A (U.S. EPA, 1998). Lead bioaccessibility was reported as both a mass fraction (mg bioaccessible Pb per kg of soil) and a percentage as follows:
| (1) |
| (2) |
Children’s BLL Determination
To protect human participants, institutional review board approval was obtained from both the Centers for Disease Control and Prevention and the Philadelphia Department of Public Health (PDPH). Children’s BLLs were measured within the last two weeks of July 2014 from 49 children aged 1 to 7 years who were associated with the 38 homes where soil samples were collected (some homes had more than one child). For blood collection from participating children, a trained health care provider collected the venous blood samples. The procedure for collecting and processing blood samples and blood collecting materials is described in the Supporting Information (SI). Venous samples were stored in coolers on cold packs and delivered daily to the PDPH laboratory. Blood sample tubes remained at 10–32 ºC and were analyzed for Pb by the PDPH laboratory within 24 hours of collection using an atomic absorption method developed by PDPH on an AAnalyst 600 (PerkinElmer, Waltham, Massachusetts, USA). The PDPH laboratory is certified by the State of Pennsylvania (state laboratory ID 000255A) and the Centers for Medicare and Medicaid Services (CLIA ID 39D0657854). The lower limit of detection for BLLs was 0.1 μg/dL.
Statistical Analyses
Regression models were developed to evaluate the relationship between children’s BLL (the response variable) and soil Pb mass fraction (the predictor variable). Total Pb and bioaccessible Pb in the soil were used as alternate predictor variables in two candidate model formulas. Models were fit using R statistical software (R Core Team, 2013) and were evaluated based on the coefficient of determination (R2), which represents the proportion of the variance in the response variable that is explained by the model (Kutner, 2005). In addition to R2, the mean absolute error (MAE) is also reported to characterize the magnitude of model errors.
Before final model development, potential BLL outliers were identified by calculating their Studentized deleted residual (SDR) (Kutner, 2005). A data point with a high SDR exerts a disproportionate influence in a model and could represent an outlier individual in the sample population (e.g., a child who engages in behavior that increases Pb exposure risk). SDR values were calculated based on the residuals of simple linear regression models between BLL and soil Pb (total or bioaccessible). Outliers were identified from the resulting t distributions using the Bonferroni test procedure. The “car” package (Fox and Weisberg, 2011) in the R statistical software environment was used in this portion of the analysis. Identified outliers were excluded from the final model development but are noted in the results and discussion.
The assumption of independence among samples taken across a geographic area can only be made after accounting for inherent correlation between nearby data locations, i.e., spatial autocorrelation (Tobler, 1970). With spatial autocorrelation present, samples taken in close proximity can over represent their shared environmental conditions in a model if they are given equal influence with samples taken from a broader geographic distribution. In these cases, the spatially clustered samples represent the same environmental phenomenon and thus are not independent observations (Waller and Gotway, 2004). The extent of spatial autocorrelation in the BLL data was assessed by mapping the error (i.e., residuals) of the BLL predictions of simple linear regression models based on total and bioaccessible Pb (SI Figure 1). If spatial autocorrelation were present, similar residual values were expected to be clustered at the spatial scale of correlation.
Based on the mapping of residuals, spatial correlation of BLLs was observed to be limited to within individual households, indicating that blood samples collected from children within the same household were not independent. To address this, a hierarchical model structure (Gelman and Hill, 2007) was developed that grouped samples by households to account for the variation among households separately from the variation among individual samples:
| (3) |
The hierarchical model has a varying intercept offset for each household, αhouse, which follows a normal distribution, while the intercept (βint), predictor variable (slope) coefficient (βPb), and error (ε) are analogous to their simple linear regression counterparts. The coefficients for the intercept and predictor variable are often referred to as “fixed effects” of the hierarchical model, and the varying intercept offsets for households are known as “random effects” (Gelman and Hill, 2007). Hierarchical models for BLL with total Pb or bioaccessible Pb as the predictor variable were fit using the packages lme4 (Bates et al., 2014) and lmerTest (Kuznetsova et al., 2016) in the R statistical software environment. The R2 values reported in this paper represent the variance explained by the fixed effects of the model only and do not include the variance explained by the house-level random effects, which would be unknown for households outside the calibration data set. Similarly, the reported MAE values were based on model predictions that only took fixed effects into account.
A bootstrapping technique (Efron, 1979) was used to assess whether BLL prediction performance (R2) improved significantly when bioaccessible Pb rather than total Pb was used as the predictor variable. Bootstrapping involved resampling with replacement from the original data set to generate simulated data sets and calculating the R2 of models fit to each new data set (Ohtani, 2000). In this case, 10,000 bootstrapped data sets were generated, and the difference in R2 of the total versus bioaccessible Pb fitted models was calculated for each. The distribution of differences in R2 was compared to a null hypothesis of zero difference at a 95% confidence level.
To evaluate the influence of child age on the relationship between total or bioaccessible soil Pb levels and BLLs, hierarchical model residuals were plotted against child age to identify any patterns. The residuals, which represent the variation in data unexplained by soil Pb level, would be expected to exhibit a trend when plotted with child age if age were indeed an additional source of variation in BLLs (Kutner, 2005). Additionally, a hierarchical multiple regression model including both bioaccessible Pb and child age as predictors was explored as an alternative to the hierarchical model with only bioaccessible Pb as the predictor variable.
RESULTS AND DISCUSSION
Soil Lead Totals and Bioaccessible Fractions
Table 1 summarizes total and bioaccessible Pb levels in soils. Total soil Pb values for the 38 soil samples ranged from 58 to 2,821 mg/kg (mean = 751 mg/kg; median = 722 mg/kg). In 27 of these samples, Pb concentration exceeded 400 mg/kg. Notably, Pb is considered a hazard in children’s play areas when its concentration equals or exceeds 400 mg/kg in bare soil (U.S. EPA, 2001).
Table 1.
Total and Bioaccessible Lead in Test Soils and Paired Children’s Blood Lead Levels
| Soil ID | Total Soil Pb (mg/kg) | ± SDa | Bioaccessible Pb (mg/kg) | ± SDa | BLL (μg/dL)b | Age (years) |
|---|---|---|---|---|---|---|
| 1 | 2821 | 93 | 2567 | 28 | 2.9 | 3.6 |
| 2 | 867 | 11 | 676 | 17 | 1.1 | 5.3 |
| 3 | 529 | 27 | 460 | 26 | 1.1 | 2.8 |
| 4 | 553 | 37 | 437 | 11 | 1.6 | 5.9 |
| 5 | 2100 | 145 | 1932 | 63 | 6.6, 7.6 | 2.2, 1.3 |
| 6 | 505 | 26 | 434 | 20 | 1 | 3.8 |
| 7 | 1018 | 23 | 1018 | – | 4 | 5.0 |
| 8 | 132 | 1 | 106 | 3 | 2 | 6.2 |
| 9 | 209 | 1 | 188 | 10 | 2.2 | 2.6 |
| 10 | 1031 | 43 | 1031 | – | 1.6, 2.9 | 6.0, 1.7 |
| 11 | 518 | 12 | 518 | – | 4.2, 9.8c | 5.9, 3.8c |
| 12 | 977 | 13 | 977 | – | 1.7 | 4.8 |
| 13 | 569 | 3 | 569 | – | 1 | 1.7 |
| 14 | 378 | 11 | 378 | – | 1.5, 1.8 | 3.9, 4.8 |
| 15 | 834 | 34 | 834 | 8 | 3.4 | 5.2 |
| 16 | 175 | 7 | 158 | 2 | 0.3, 0.5, 0.8 | 4.1, 4.1, 1.9 |
| 17 | 230 | 8 | 216 | 9 | 0.38 | 1.9 |
| 18 | 58 | 2 | 47 | 1 | 0.96 | 1.3 |
| 19 | 945 | 155 | 813 | 47 | 1.6 | 3.6 |
| 20 | 831 | 142 | 831 | – | 1.5, 1.9 | 3.6, 3.6 |
| 21 | 659 | 23 | 659 | – | 1.0, 2.3, 5.9 | 6.4, 4.0, 2.3 |
| 22 | 814 | 41 | 814 | – | 1.5 | 6.2 |
| 23 | 538 | 6 | 538 | – | 3.7 | 2.6 |
| 24 | 769 | 25 | 738 | 46 | 5.3 | 5.0 |
| 25 | 747 | 1 | 627 | 22 | 0.5 | 1.1 |
| 26 | 697 | 30 | 697 | – | 1.5 | 2.5 |
| 27 | 167 | 19 | 167 | – | 1.3, 2.4 | 6.8, 2.4 |
| 28 | 94 | 10 | 94 | – | 1.1 | 3.6 |
| 29 | 132 | 3 | 87 | 1 | 2.1 | 4.2 |
| 30 | 295 | 25 | 295 | – | 1 | 5.2 |
| 31 | 1382 | 26 | 1327 | 14 | 2.8 | 1.2 |
| 32 | 873 | 1 | 864 | 44 | 5 | 3.1 |
| 33 | 831 | 52 | 831 | – | 4.3, 5.5 | 6.3, 3.8 |
| 34 | 862 | 90 | 862 | – | 4.5 | 4.1 |
| 35 | 1386 | 48 | 1192 | 14 | 1.8 | 2.3 |
| 36 | 1868 | 61 | 1775 | 3 | 2.9 | 2.5 |
| 37 | 765 | 65 | 681 | 2 | 0.39 | 1.8 |
| 38 | 396 | 73 | 396 | – | 1.9 | 3.9 |
Standard deviation of two sample replicates from a single, homogenized soil sample.
More than one BLL indicates multiple children in a single household.
BLL removed as outlier in regression analysis.
The bioaccessible fraction of Pb in soil ranged from 47 to 2,567 mg/kg (mean = 706 mg/kg; median = 668 mg/kg). These bioaccessibility values corresponded to a range of 66% to 100% of total Pb in soil (mean = 93%; median = 98%).
Children’s Blood Lead Levels
BLLs for 49 children living in homes paired with the 38 soil samples were included in this study (Table 1). Observed BLLs ranged from 0.3 to 9.8 μg/dL (mean = 3 μg/dL; median = 1.8 μg/dL). Analysis for potential outlier BLLs resulted in the removal of one value (BLL = 9.8 μg/dL). This outlier value was singularly high, as it was more than three standard deviations above the mean BLL and more than twice the value of the other child sampled in the same household. The high value could be due to behavioral differences exhibited by the child (e.g., mouthing activity). The Bonferroni p-values for this sample were 0.00041 for the total Pb model SDR and 0.00032 for the bioaccessible Pb model SDR. These results strongly suggested that this sample lay outside of the trends of the remaining data and could not be included in the modeling data without unduly influencing the model (Kutner, 2005).
Correlating Blood Lead Level with Total and Bioaccessible Lead
Visual inspection of the spatial distribution of the residuals from simple linear regression models of BLL against Pb content (SI Figure 1) indicated that BLL values were correlated at the household scale. Correlation at this scale could be expected due to the likelihood of shared behavioral and environmental factors affecting individuals from the same household. Therefore, hierarchical linear regression models were fit to BLL against total and bioaccessible Pb, grouping samples by their household of origin and the corresponding soil sample.
All models indicated a significant positive relationship between BLL and soil Pb level (total or bioaccessible). The hierarchical BLL model based on total soil Pb had an intercept of 1.3 μg/dL BLL and a slope of 1.4 μg/dL BLL per 1000 mg/kg increase in total Pb in soil (Table 2a). The fitted hierarchical model with bioaccessible Pb as the predictor had an intercept of 1.2 μg/dL BLL and a slope of 1.6 μg/dL BLL per 1000 mg/kg increase of bioaccessible Pb in soil (Table 2b and Figure 1). Total Pb was a significant predictor of BLL at the 99% confidence interval (p = 0.0021), and bioaccessible Pb was significant at the 99.9% confidence level (p = 0.00092). These results are consistent with earlier reports of BLL increases ranging from 0.6 to 6.8 μg/dL for every 1000 mg/kg increase in soil Pb mass fraction (Lanphear et al., 1998; U.S. EPA, 1983; Duggan, 1980).
Table 2.
Hierarchical Model Variances and Linear and Multiple Regressions. Fitted BLL model intercepts, slope coefficients and coefficient standard errors (SE), variance of household random effects ( ), variance of residuals ( ), coefficient of determination (R2), and mean absolute error (MAE) for (a) a hierarchical linear regression against total Pb in soil, (b) a hierarchical linear regression against bioaccessible Pb in soil, and (c) a hierarchical multiple linear regression against bioaccessible Pb and child age. In the hierarchical multiple linear regression, child age was not significant at the 95% confidence level.
| Hierarchical model | Intercepta | Coef. (SE)a |
|
|
R2 | MAEb | ||
|---|---|---|---|---|---|---|---|---|
| (a) Total Pb | 1.3 | 1.4 (0.42) | 0.92 | 1.4 | 0.25 | 1.22 | ||
|
| ||||||||
| (b) Bioaccessible Pb | 1.2 | 1.6 (0.44) | 0.84 | 1.4 | 0.28 | 1.19 | ||
|
| ||||||||
| (c) Multiple regression | 1.6 | 1.03 | 1.3 | 0.27 | 1.21 | |||
| Bioaccessible Pb | 1.5 (0.46) | |||||||
| Child age | −0.076 (0.13)c | |||||||
Units are 1000 mg Pb per kg soil.
Mean Absolute Error (MAE); units are μg/dL.
Child age was not significant in the multiple linear regression model.
Figure 1.
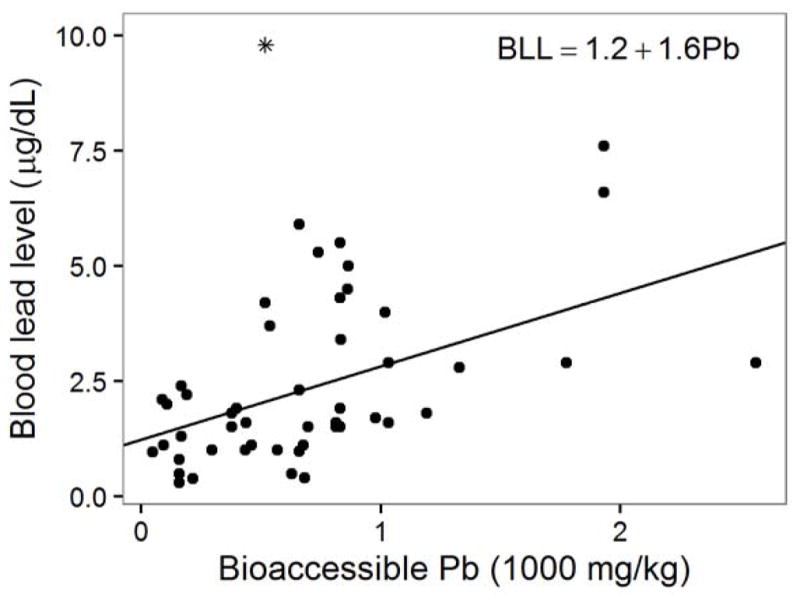
Bioaccessible Pb (in units of 1000 mg Pb per kg soil) plotted against child BLL and regression line of the bioaccessible Pb hierarchical model, along with the model formula. This model had the best fit among those developed in this study. The excluded outlier BLL value is shown as an asterisk (*) on the plot.
The BLL hierarchical model with total soil Pb as the predictor accounted for 25% of the variability in child BLL, and the model with bioaccessible soil Pb as the predictor accounted for 28% of BLL variability. There was also a small decrease in MAE from 1.22 to 1.19 μg/dL when predicting BLL using bioaccessible Pb rather than total Pb in soil. These results indicate an improvement in prediction accuracy (based on fixed effects) when predicting BLL with bioaccessible Pb relative to total Pb.
The residual variances of the two models (Table 2a–b) were similar ( ) and greater than the variances of the household random effects ( and 0.84 for models based on total Pb and bioaccessible Pb, respectively). The household random effect variances are consistent with the observation of spatial correlation at the household level (SI Figure 1). Comparing the household random effect variances for the two models (Table 2a–b) suggests that the use of bioaccessible Pb as a predictor reduced the unexplained variability at the household level relative to the model using total Pb.
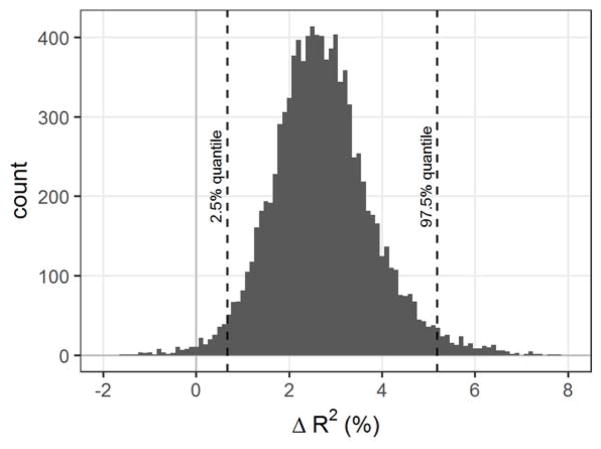
The small difference in R2 between the two models is not surprising given the high average bioaccessibility of Pb in the soils, such that bioaccessible and total Pb measurements were highly correlated (r = 0.996). Nevertheless, the results suggest that accounting for the bioaccessible fraction of Pb in soils can explain significantly more of the variation in children’s BLL than total Pb in soil. Using the total Pb concentration in soil may overestimate risk associated with soil ingestion. Bioaccessibility data are used to improve the accuracy of exposure and risk calculations (U.S. EPA, 2007a). A bootstrapping analysis comparing the total Pb and bioaccessible Pb hierarchical single-predictor models (Figure 2) showed that the model using bioaccessible Pb explained significantly more variance in BLL by a mean R2 margin of 2.7%, with a 95% confidence interval of 0.7% to 5.2%. For 99.2% of the sample bootstraps, this model explained more variance than the model using total soil Pb as the predictor.
Figure 2.
Sampling distribution of difference in R2 value (shown as change in % variance explained) between hierarchical regressions with bioaccessible Pb and total Pb in soil as alternate predictors. A positive difference represents better performance by the model using bioaccessible Pb in soil. Dotted lines show the 2.5% and 97.5% quantiles that define the 95% confidence interval.
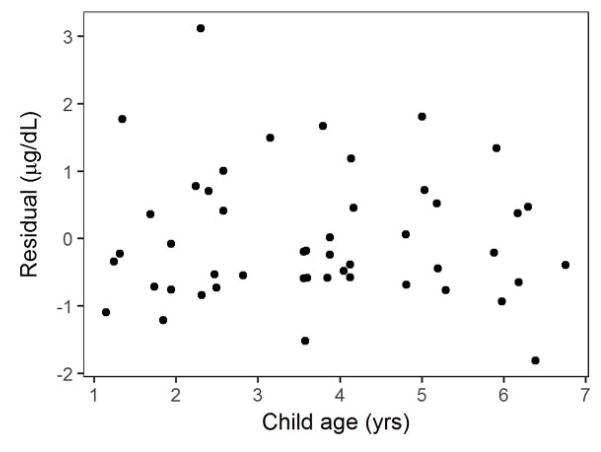
Effect of Age on Relationship between Soil Lead and Children’s Blood Lead Level
Ren et al. (2006) reported that correlation coefficients of linear relationships between bioaccessible Pb in soil and children’s BLLs increased as children aged from 3 to 5 years old. In the present study, which includes children between the ages of 1 and 7, child age did not contribute significantly to the prediction of BLL. Figure 3 shows that there is no clear relationship between child age and the single-predictor model residuals (SI Figure 2) for the hierarchical BLL model using bioaccessible soil Pb (Kutner, 2005). The absence of an observable correlation between child age and the unexplained variance in these models suggests that child age did not have a substantial effect on BLL. Furthermore, a hierarchical multiple regression model including both bioaccessible Pb and child age did not assign a significant coefficient (p = 0.56) to child age (Table 2c). Interestingly, the hierarchical multiple regression model including child age explained less of the variance in BLL (R2 = 0.27) than the hierarchical regression using bioaccessible Pb only (R2 = 0.28). One would normally expect R2 to increase with the inclusion of an additional predictor. In hierarchical modeling, however, this is not necessarily the case. For the hierarchical multiple regression developed here (Table 2c), more of the variance is partitioned to the household random effect component of the model ( ), whereas the R2 values were determined based on the fixed effect model components only. Additional variables (e.g., age of home), which may influence BLL may be further evaluated at a later time.
Figure 3.
BLL residuals (observed – predicted) plotted against child age for the hierarchical BLL model with bioaccessible Pb as the predictor.
Our study compared relationships between total versus bioaccessible Pb in soil and children’s BLLs. The observed statistically significant correlations between both total and bioaccessible Pb in soil and children’s BLLs confirm that soil Pb is an exposure route of concern in this study area, accounting for 25% and 28% of the observed variability in BLLs, respectively. Contributions of soil Pb levels to variability in children’s BLLs were within the range of previously published values. While our study focused on soil Pb values at the local yard scale, a recent study evaluating Pb exposure at a Superfund site in Idaho reported that soil Pb at the neighborhood and community scales contribute independently to total Pb uptake by children (von Lindern et al., 2016). Additionally, other potential sources of Pb exposure not considered in this study, including Pb in house dust or paint, would also contribute to Pb exposure.
Unlike findings reported by Ren at al. (2006), however, our findings did not find age to be a factor in the relationship between soil Pb levels and children’s BLLs. Although accounting for child age did not contribute to improved BLL prediction in our study, recent studies that evaluated Pb intake rates from both soil and dust have reported differences in Pb intake rates from house dust based on age with a general trend of higher dust ingestion rates for younger children (von Lindern et al., 2016; Wilson et al., 2013). These findings suggest that differences in Pb exposure by age might be more attributable to Pb levels in house dust than soil.
The significant improvement in model R2 values indicate that the bioaccessible portion of Pb in soil might better account for variance in observed children BLLs than total soil Pb level. This finding underscores the importance of considering soil metal bioavailability, either measured directly or estimated using an IVBA assay in exposure and risk assessments of metal-impacted soils.
When available, environmental public health practitioners may improve their child lead-risk assessments by using the bioaccessible measure of soil Pb instead of total soil Pb content. We found the bioaccessible fraction of Pb explained significantly more of the variation in children’s BLL than total Pb in soil. Additionally, using total soil Pb concentration may overestimate risk associated with soil ingestion. Bioaccessibility data, a measure of bioavailability, can improve the accuracy of exposure and risk calculations. Even small changes in bioavailability can result in substantial cost savings in remediation. It may be helpful to follow EPA decision making guidance regarding assessment and incorporation of site-specific bioavailability information into risk-assessment decision-making. (U.S. EPA, 2007c).
Supplementary Material
Acknowledgments
The authors are grateful to Jonathan V. Cruz and Matt Frank, Penbay Media Contractors for U.S. EPA Region 3, for creating the map showing the soil and blood Pb concentrations for the study site. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency or the Centers for Disease Control and Prevention and the Agency for Toxic Substances and Disease Registry.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) [last accessed October 27, 2016];Childhood Blood Lead Research Study. 2014 http://www.atsdr.cdc.gov/sites/jtlewis/docs/In_Your_Neighborhood.pdf.
- Agency for Toxic Substances and Disease Registry (ATSDR) [last accessed December 22, 2016];John T. Lewis Community Childhood Blood Lead Study. 2015 https://www.atsdr.cdc.gov/sites/jtlewis/index.html.
- Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4, 2014. [last accessed October 28, 2016]; ArXiv Prepr. ArXiv1406.5823; https://arxiv.org/abs/1406.5823.
- Bellinger DC. Very low lead exposures and children’s neurodevelopment. Pediatrics. 2008;20(2):172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Datko-Williams L, Wilkie A, Richmond-Bryant J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci Total Environ. 2014;468–469:854–863. doi: 10.1016/j.scitotenv.2013.08.089. [DOI] [PubMed] [Google Scholar]
- Duggan MJ. Lead in urban dust: an assessment. Water Air Soil Pollut. 1980;14(1):309–321. [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7(1):1–26. [Google Scholar]
- Fox J, Weisberg S. An R Companion to Applied Regression. SAGE Publications, Inc; Thousand Oaks, CA: 2011. [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; New York, NY: 2007. [Google Scholar]
- Kutner MH. McGraw-Hill/Irwin Series: Operations and Decision Sciences. McGraw-Hill Irwin; Boston: 2005. Applied Linear Statistical Models. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models. 2016 [Google Scholar]
- Laidlaw MAS, Zahran S, Pingitore N, Clague J, Devlin G, Taylor MP. Identification of lead sources in residential environments: Sydney Australia. Environ Pollut. 2014;184:238–246. doi: 10.1016/j.envpol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Laidlaw MAS, Pilippelli GM, Brown S, Paz-Ferreiro J, Reichman SM, Netherway P, Truskewycz A, Ball AS, Mielke HW. Case studies and evidence-based approaches to addressing urban soil lead contamination. Appl Geochem. 2017 https://dx.doi.org/10.1016/j.apgeochem.2017.02.015.
- Lanphear BP, Burgoon DA, Rust SW, Eberly S, Galke W. Environmental exposures to lead and urban children’s blood lead levels. Environ Res. 1998;76(2):120–130. doi: 10.1006/enrs.1997.3801. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Rosenman KD, Shehata T. Lead in soil: recommended maximum permissible levels. Environ Res. 1989;49:136–142. doi: 10.1016/s0013-9351(89)80028-3. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Gonzales CR, Smith MK, Mielke PW. The urban environment and children’s health: soils as an integrator of lead, zinc, and cadmium in New Orleans, Louisiana, U.S.A. Environ Res. 1999;81(2):117–129. doi: 10.1006/enrs.1999.3966. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Gonzales CR, Powell E, Jartun M, Mielke PW., Jr Nonlinear assocation between soil lead and blood lead of children in metropolitan New Orleans Louisiana: 2000–2005. Sci Total Environ. 2007;388:43–53. doi: 10.1016/j.scitotenv.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ohtani K. Bootstrapping R2 and adjusted R2 in regression analysis. Econ Model. 2000;17(4):473–483. [Google Scholar]
- Ren HM, Wang JD, Zhang XL. Assessment of soil lead exposure in children in Shenyang, China. Environ Pollut. 2006;144(1):327–335. doi: 10.1016/j.envpol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol. 1996;30:422–430. [Google Scholar]
- Ryan JA, Scheckel KG, Berti WR, Brown SL, Casteel SW, Chaney RL, Hallfrisch J, Doolan M, Grevatt P, Maddaloni M, Mosby D. Reducing children’s risk from lead in soil: a field experiment in Joplin, Mo., demonstrates alternatives to traditional cleanups. Environ Sci Technol. 2004;38(1):18A–24A. doi: 10.1021/es040337r. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 μg/dL. NeuroToxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler WR. A computer movie simulating urban growth in the Detroit region. Econ Geogr. 1970;46:234–240. [Google Scholar]
- U.S. EPA. Air Quality Criteria for Lead. I. Office of Research and Development, National Center for Environmental Assessment; Research Triangle Park, NC: 1983. EPA/600/8-83/028B. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Air Quality Criteria for Lead. Office of Research and Development, Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office; Research Triangle Park, NC: 1986. EPA/600/8-83/028AF. [Google Scholar]
- U.S. EPA. Lead; Identification of Dangerous Levels of Lead; Final Rule. 2001 Docket number: OPPTS-62156H; FRL-6763-5. https://www.epa.gov/lead/hazard-standards-lead-paint-dust-and-soil-tsca-section-403.
- U.S. EPA. OSWER 9285.7-77. Office of Solid Waste and Emergency Response; Washington, DC: 2007a. Estimation of Relative Bioavailability of Lead in Soil and Soil-Like Materials Using In Vivo and In Vitro Methods. [Google Scholar]
- U.S. EPA. [last accessed October 31, 2016];Method 3051A, Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1. 2007b https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf.
- U.S. EPA. Guidance for Evaluating the Oral Bioavailability of Metals in Soils for Use in Human Health Risk Assessment. [last accessed April 1, 2017];OSWER 9285.7-80. 2007c https://semspub.epa.gov/work/HQ/175333.pdf.
- U.S. EPA. Method 1340, In Vitro Bioaccessibility Assay for Lead in Soil, Revision 0. [last accessed October 31, 2016];2013 https://www.epa.gov/sites/production/files/2015-12/documents/1340.pdf.
- U.S. EPA. [last accessed October 31, 2016];Method 6010D, Inductively Coupled Plasma–Optical Emission Spectrometry, Revision 4. 2014 https://www.epa.gov/sites/production/files/2015-12/documents/6010d.pdf.
- U.S. EPA. [last accessed November 8, 2016];Method 6020A, Inductively Coupled Plasma–Mass Spectrometry, Revision 1. 1998 https://www.epa.gov/sites/production/files/2015-07/documents/epa-6020a.pdf.
- von Lindern I, Spalinger S, Stifelman ML, Stanek LW, Bartrem C. Estimating children’s soil/dust ingestion rates through retrospective analyses of blood lead biomonitoring from the Bunker Hill Superfund Site in Idaho. Environ Health Perspect. 2016;124:1462–1470. doi: 10.1289/ehp.1510144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LA, Gotway CA. Applied Spatial Statistics for Public Health Data. John Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]
- Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess. 2013;19(1):158–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.