Abstract
Objectives
We assessed prevalence and determinants of high blood pressure, and barriers to diagnosis and treatment, in Dar es Salaam, Tanzania.
Methods
We surveyed and screened 2,174 community-dwelling adults aged ≥40 years in 2014 and conducted a follow-up after one year.
Results
Median blood pressure was 131/81 mmHg and hypertension prevalence was 37%. Mean adjusted difference in SBP was 4.0 mmHg for overweight, 6.3 mmHg for obese class I, and 10.5 mmHg for obese class II/III compared with normal weight participants. Those who were physically inactive had 4.8 mmHg higher SBP compared to those with more than 24 hours of moderate or vigorous activity per week. Drinkers of at least 10 grams of alcohol per day had 4.5 mmHg higher SBP than did non-drinkers.
Among hypertensives, 48% were diagnosed, 22% were treated, and 10% were controlled. Hypertensives without health insurance were 12% less likely to be diagnosed than insured hypertensives. Of referred participants, 68% sought care, but only 27% were on treatment and 8% had controlled blood pressure at follow-up. Reasons for not seeking care included lack of symptoms, cost of visit, and lack of time. Reasons for not being on treatment included lack of symptoms, not being prescribed treatment, and having finished one course of treatment.
Conclusions
Major risk factors for hypertension in Dar es Salaam are overweight, obesity, inadequate physical activity, and limited access to quality medical care. Increased insurance coverage and community-based screening, along with quality medical care and patient education, may help control this burgeoning epidemic.
Keywords: hypertension, obesity, epidemiology, lifestyle, physical activity, Tanzania, sub-Saharan Africa
Introduction
Cardiovascular diseases are a rising public health concern in sub-Saharan Africa (SSA). The Global Burden of Disease Study estimates that age-standardized stroke incidence in Tanzania has increased 21% from 1990 to 2010 [1]. The mean number of annual stroke admissions at a tertiary hospital in Moshi, Tanzania has increased sevenfold from the 1980s to the 2000s [2] and stroke death rates observed in Dar es Salaam between 2003 and 2006 were higher than those observed among African-Americans in Manhattan [3].
High blood pressure is the leading risk factor for stroke [4] and levels have been increasing substantially in eastern SSA, mirroring the stroke epidemic. A global pooling analysis of population surveys estimated that, from 1980 to 2008, mean age-standardized systolic blood pressure (SBP) increased by 6 mmHg to 131 mmHg in men and by 8 mmHg to 134 mmHg in women in this region, the largest regional increases worldwide in men and second largest in women [5]. Hypertension prevalence and blood pressure levels have risen particularly quickly in Tanzania, especially in urban areas. Two repeated cross-sectional population surveys conducted in Dar es Salaam in 1987 and 1998 showed an increase in hypertension prevalence from 33% to 63% in men and from 48% to 62% in women [6]. Other cross-sectional surveys in the same city have found varying prevalences, from 29–30% in 1996 to 19–43% around 2010 [7–10].
Across SSA, medical care for hypertensive individuals has lagged behind the rise in prevalence. A 2015 meta-analysis of hypertension studies in SSA found that slightly less than one in three hypertensives were diagnosed, less than one in five were treated, and less than one in ten had controlled blood pressure [11]. In Dar es Salaam, a one-year follow-up study in 1999–2000 found that only 34% of hypertensive participants visited a health professional, 29% took anti-hypertensives, and 5% were still taking drugs after one-year [12]. Reported barriers to care were lack of symptoms and the cost of healthcare and treatment [12]. However, much is still unknown about barriers to hypertension care and treatment. Previous studies have been underpowered and have not had the in-depth questionnaires needed to elicit details on a wide variety of potential barriers in fully adjusted regression models.
The aim of this study was to determine the current prevalence, risk factors, and barriers to diagnosis and treatment for hypertension in Dar es Salaam, Tanzania. Few previous studies in Tanzania have analyzed the modifiable determinants of high blood pressure as well as barriers to diagnosis and treatment of hypertension.
Methods
Sampling design and study population
The Dar es Salaam Urban Cohort Hypertension Study (DUCS-HTN) is a cohort of adults living in the Ukonga ward of Dar es Salaam who had been registered in the Dar es Salaam Health and Demographic Surveillance System (HDSS) in 2011. The HDSS is in a peri-urban area on the outskirts of Dar es Salaam. Due to the large size of Ukonga, we chose to randomly sample two of the seven neighborhoods that compose Ukonga and then conducted a census of these two randomly selected neighborhoods, Mwembe Madafu and Markazi. We attempted to contact all 4,896 HDSS participants who were at least 40 years of age and lived in one of two randomly selected neighborhoods (Figure 1). We excluded pregnant women and physically or mentally disabled individuals. Trained interviewers conducted face-to-face interviews and physical examinations in participants’ homes from March to June 2014. Follow-up visits were conducted from April to June 2015.
Figure 1.
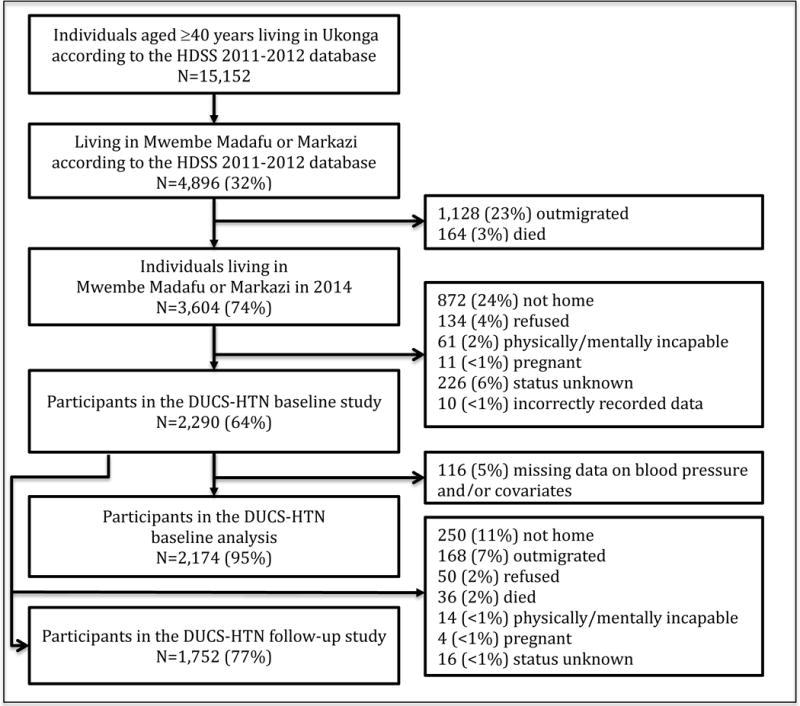
Process for selecting participants into the Dar es Salaam Urban Cohort Hypertension Study.
Among 4,896 potentially eligible participants from the HDSS baseline survey, 3,604 (74%) were still living at the same address in 2014. Of these, 2,290 (64%) enrolled in the DUCS-HTN study (Figure 1). Of the participants enrolled at baseline, 1,752 (77%) participated in the follow-up study.
The Institutional Review Board of the Harvard T.H. Chan School of Public Health and the Muhimbili University of Health and Allied Sciences Ethical Committee approved the study protocol. Written informed consent was obtained from all participants (or, if the participant was unable to sign, a witness signed on behalf of the participant).
Blood pressure measurements
Trained interviewers measured blood pressure with digital blood pressure monitors (15 Omron M2 and 5 Beurer BM 40 monitors) [13,14]. Standard, large, and extra-large cuff sizes were used according to the size of a participant’s arm. Blood pressure was measured three times with at least a 5-minute rest before the first measurement and 3-minute rests between each subsequent measurement. Blood pressure was taken on the left arm with the participant seated and the arm straight at heart level. Usual blood pressure values were calculated as the mean of the second and third readings. If a second visit was conducted, usual blood pressure values were defined as the mean of the second and third readings at both the first and second visits.
Hypertension was defined as SBP≥140 mmHg or DBP≥90 mmHg, or self-reported use of anti-hypertensive medication. Grade I hypertension was defined as SBP of 140 to 159 or DBP of 90 to 99 mmHg; grade II hypertension was defined as SBP of 160 to 179 or DBP of 100 to 109 mmHg; and grade III hypertension was defined as SBP≥180 or DBP≥110 mmHg [15]. Hypertension control was defined as current antihypertensive use and blood pressure of less than 140/90 mmHg. If a participant was found to have grade I or II hypertension, a second visit was scheduled, at least three days later. Those with grade III hypertension at the last reading of the first visit or grade I or II hypertension at the last reading of the second visit were told that they had high blood pressure, advised to see a health professional and were given a referral letter. In addition, a second visit and blood pressure reading was scheduled for a random sample of one-fifth of participants, who were selected for additional blood, urinary, and dietary measurements.
Assessment of covariates
All participants were administered a socio-demographic and lifestyle questionnaire and had their height, weight, and waist and hip circumference measured. Some demographic information (age, sex, neighborhood, religion, and assets used to create a household wealth index) was previously recorded during the HDSS baseline. Information on household health insurance coverage was collected in 2015 as part of routine HDSS updates.
Standard protocols were used to take anthropometric measurements. Participants were weighed with minimal clothing using a digital scale (Seca, Germany) to the nearest 0.1kg and height was measured, with participants not wearing shoes, to the nearest 1cm. Body mass index (BMI) was calculated as the ratio of weight in kilograms to height in meters squared (kg/m2) and categorized according to WHO categories [16].
The Global Physical Activity Questionnaire (GPAQ) was used to assess physical activity for work, transportation, and leisure [17]. We defined physical inactivity according to WHO guidelines [18]. Number of servings of alcoholic beverages consumed was reported over the past 30 days. We assumed 14 grams of alcohol as a standard drink portion size [19]. A household wealth index was created through a principal component analysis of household characteristics and assets, and was categorized into quintiles (Table S1) [20]. In the follow-up visit, participants were asked about their health over the past year and the reasons for seeking (or not seeking) hypertension care.
Statistical analysis
Participants with missing data on baseline blood pressure or baseline covariates that were missing in less than five percent of participants were excluded from baseline analyses, leading to a baseline sample of 2,174 participants. A missing indicator was used for health insurance status, which was missing in 16% of participants [21]. Mean differences and 95% confidence intervals (CIs) of SBP by potential blood pressure determinants were estimated with multivariable linear regression. Covariates in this regression were chosen based on a-priori knowledge of lifestyle and socioeconomic determinants of high blood pressure.
This multivariable linear regression was repeated with diastolic blood pressure (DBP) as the outcome. Poisson relative risk regression with an exchangeable correlation structure was used to estimate prevalence ratios (PRs) and 95% CIs for hypertension as well as prevalence of hypertension awareness among hypertensives and treatment among those aware of their hypertension [22].
We used inverse probability of censoring weighting (IPCW), with stabilized weights, to adjust for potential selection bias due to non-random selection of participants into the study [23]. This method weights individuals included in the analysis by the inverse of the probability of being in the analysis according to measured covariates. We used logistic regression to estimate the probability of being included in the analyses based on the following covariates and their interaction with sex: age, sex, wealth index quintile, and education. The numerator for the weights is the marginal probability of being included in the analysis (among all those aged 40 years and older in the HDSS database). The denominator for the stabilized weights is the probability of being included in the analyses conditional on the covariates listed above.
All regression analyses accounted for clustering at the household level (39% of participants came from households with two participants, and 8% came from households with three to five participants). We adjusted for interviewer (n=21) in all analyses to account for differences in measurement of subjective risk factors by interviewer [24,25]. Analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
Results
We enrolled 47% (2,290/4,896) of potentially eligible participants in the HDSS 2011 database. The main reasons for not enrolling were that the participant had moved (23%) or that the participant was not home when the interviewer came for a visit, despite at least three attempts by the interviewer to visit the participant (18%) (Figure 1). Eligible participants who were not included in baseline analyses were similar to those who were included with respect to household wealth, health insurance coverage, and neighborhood. However, they tended to be younger, male, Christian (versus Muslim), and more highly educated (Table S2).
A slight majority of participants were female (56%) and the median age was 51 years (Table 1). Fifty-one percent of participants were self-employed, 17% were employed by government or private companies, and 31% were retired or unemployed. Nearly 10% of participants had no formal education. Median [interquartile range (IQR)] BMI was 24.8 [22.0, 27.9] kg/m2 in men and 28.3 [24.3, 32.7] in women and prevalence of obesity was 14% in men and 41% in women. Twenty-three percent of participants had health insurance. Seventy-five percent of men and 62% of women met the WHO physical activity guidelines. Thirteen percent were former smokers and 6% were current smokers. Alcohol use was relatively uncommon, with only 7% of participants reporting drinking at least one drink a day. Compared to women, men were more likely to be married, employed, formally educated, physically active, smokers, alcohol drinkers, and less likely to be overweight or obese.
Table 1.
Baseline characteristics of the Dar es Salaam Urban Cohort Hypertension Study participants, 2014 (n=2,174)
| Characteristic | All* (n=2,174) | Men* (n=961) | Women* (n=1,213) |
|---|---|---|---|
| Age, years | 51 [44, 59] | 52 [45, 61] | 50 [44, 57] |
| 40–44 | 570 (26%) | 225 (24%) | 345 (28%) |
| 45–49 | 419 (19%) | 165 (17%) | 254 (21%) |
| 50–54 | 384 (18%) | 157 (16%) | 227 (19%) |
| 55–59 | 318 (15%) | 158 (16%) | 160 (13%) |
| 60–64 | 198 (9%) | 104 (11%) | 94 (8%) |
| 65–69 | 128 (6%) | 78 (8%) | 49 (4%) |
| ≥ 70 | 157 (7%) | 74 (8%) | 83 (7%) |
| Male | 961 (44%) | ||
| Neighborhood | |||
| Mwembe Madafu | 1723 (79%) | 756 (79%) | 967 (80%) |
| Markazi | 448 (21%) | 205 (21%) | 246 (20%) |
| Religion | |||
| Muslim | 1084 (50%) | 489 (51%) | 595 (49%) |
| Christian | 1090 (50%) | 472 (49%) | 618 (51%) |
| Marital status | |||
| Married | 1587 (73%) | 826 (86%) | 761 (63%) |
| Single | 89 (4%) | 29 (3%) | 60 (5%) |
| Separated or divorced | 155 (7%) | 45 (5%) | 110 (9%) |
| Widowed | 343 (16%) | 61 (6%) | 282 (23%) |
| Employment | |||
| Retired or unemployed | 679 (31%) | 213 (22%) | 466 (38%) |
| Self-employed | 1115 (51%) | 488 (51%) | 627 (52%) |
| Employed | 380 (17%) | 260 (27%) | 120 (10%) |
| Household wealth index quintile† | |||
| Richest | 430 (20%) | 188 (20%) | 242 (20%) |
| Richer | 432 (20%) | 173 (18%) | 259 (21%) |
| Average | 439 (20%) | 203 (21%) | 236 (19%) |
| Poorer | 419 (19%) | 194 (20%) | 225 (19%) |
| Poorest | 454 (21%) | 203 (21%) | 251 (21%) |
| Education | |||
| None | 197 (9%) | 39 (4%) | 158 (13%) |
| At least some primary | 1313 (60%) | 564 (59%) | 749 (62%) |
| At least some secondary | 663 (31%) | 358 (37%) | 306 (25%) |
| MET-hours per week | 18.8 [4.5, 81.0] | 27.0 [7.0, 126.0] | 13.5 [3.0, 60.0] |
| Physical activity quintile | |||
| Highest (144.0–1008.0 MET-hrs/wk) | 439 (20%) | 262 (27%) | 177 (15%) |
| Higher (47.0–141.3) | 430 (20%) | 183 (19%) | 247 (21%) |
| Average (14.7–46.7) | 411 (19%) | 192 (20%) | 219 (18%) |
| Lower (4.4–14.0) | 425 (20%) | 178 (19%) | 247 (20%) |
| Lowest (0.0–4.0) | 469 (22%) | 146 (15%) | 323 (27%) |
| Television watching, hours per day | |||
| 0 | 523 (24%) | 203 (17%) | 320 (26%) |
| >0 – <2 | 1000 (46%) | 469 (49%) | 531 (44%) |
| ≥ 2 | 651 (30%) | 289 (30%) | 362 (30%) |
| Smoking status | |||
| Never | 1747 (80%) | 578 (60%) | 1169 (96%) |
| Former | 279 (13%) | 247 (26%) | 32 (3%) |
| Current, 0–9 cigarettes per day | 118 (5%) | 106 (11%) | 12 (1%) |
| Current, ≥10 cigarettes per day | 30 (1%) | 30 (3%) | 0 (0%) |
| Alcohol use‡ | |||
| 0 grams/day | 1752 (81%) | 685 (71%) | 1067 (88%) |
| >0–<10 grams/day | 280 (13%) | 163 (17%) | 117 (10%) |
| ≥10 grams/day | 142 (7%) | 113 (12%) | 29 (2%) |
| BMI, kg/m2 | 26.3 [22.9, 30.9] | 24.8 [22.0, 27.9] | 28.3 [24.3, 32.7] |
| Underweight (<18.5) | 93 (4%) | 53 (5%) | 40 (3%) |
| Normal weight (18.5–<25) | 764 (35%) | 447 (47%) | 317 (26%) |
| Overweight (25–<30) | 689 (32%) | 329 (34%) | 360 (30%) |
| Obese, class I (30–<35) | 407 (19%) | 97 (10%) | 310 (25%) |
| Obese, class II or III (≥35) | 221 (10%) | 35 (4%) | 186 (16%) |
| Household health insurance§ | |||
| Uninsured | 1419 (77%) | 630 (78%) | 788 (77%) |
| Insured | 340 (23%) | 176 (22%) | 239 (23%) |
| Systolic blood pressure, mmHg | 131 [119, 143] | 134 [122, 145] | 128 [116, 142] |
| Diastolic blood pressure, mmHg | 81 [73, 88] | 81 [74, 89] | 80 [73, 87] |
| Hypertensive‖ | 803 (37%) | 377 (39%) | 426 (35%) |
| Aware | 382 (48%) | 137 (36%) | 245 (58%) |
| Using antihypertensive drugs | 180 (22%) | 59 (16%) | 121 (28%) |
| Controlled | 84 (10%) | 25 (7%) | 59 (14%) |
| High cholesterol history | 78 (4%) | 27 (3%) | 51 (4%) |
| Using lipid-lowering drugs | 29 (17%) | 6 (22%) | 22 (20%) |
| Diabetes history# | 136 (6%) | 64 (7%) | 72 (6%) |
| Using oral hypoglycemic agents | 54 (40%) | 21 (33%) | 33 (46%) |
| Using insulin | 20 (15%) | 9 (14%) | 11 (15%) |
| Coronary heart disease history** | 99 (5%) | 31 (3%) | 68 (6%) |
| Stroke history*** | 28 (1%) | 11 (1%) | 17 (1%) |
N (%) for binary/categorical variables and median [IQR] for continuous variables.
Household wealth index created by principal component analysis of household characteristics and household asset ownership
Defined based on number of days consumed alcohol over the past 30 days and the average number of drinks per drinking session
n=1,834, missing 340
SBP≥140 or DBP≥90 or on antihypertensive medication
n=2,145, missing 29
n=2,170, missing 4
n=2,159, missing 15
Prevalence of hypertension was 37% overall, 39% in men and 35% in women. Median [IQR] of SBP was 131 [119,143] and that of DBP was 81 [73, 88] mmHg. A sensitivity analysis in which we used IPCW to adjust for selection bias found similar results (Table S3). Similar to previous studies [26], we observed lower SBP in second and third measurements in the first visit as well as lower measurements in the second visit compared with the first visit especially among participants who had grade III hypertension or were on antihypertensives (Figure S1).
Modifiable lifestyle factors associated independently with higher SBP were overweight or obesity, inadequate physical activity, and alcohol use of at least 10 grams per day. After adjustment for potential confounders, overweight participants had 4.0 (95% CI: 1.9, 6.1) mmHg higher SBP compared with those who had normal weight (i.e. BMI<25). Obese class I participants (BMI between 30 and 35) had 6.3 (3.8, 8.8) mmHg higher SBP and obese class II or III participants (BMI≥35) had 10.5 (7.2, 13.8) mmHg higher SBP than normal weight participants. Those who were physically inactive had 4.8 (1.4, 8.1) mmHg higher SBP compared to those with more than 24 hours of moderate or vigorous activity per week. Those who drank at least 10 grams of alcohol per day had 4.5 (1.2, 7.8) mmHg higher SBP than did non-drinkers.
Men, on average, had a higher SBP than women, as did older participants. Lack of formal education and being unemployed or retired were also associated with higher SBP. Wealth quintiles showed no independent association with SBP, but there was a qualitative interaction (p=0.03) between wealth quintiles and gender. Increasing wealth was associated with lower SBP in women but higher SBP in men, but neither of the sex-specific relationships with wealth index quintile were statistically significant (not shown). No other potential blood pressure determinants showed effect modification by gender. A sensitivity analysis in which we used IPCW to adjust for selection bias found similar results (Table S4). Risk factors for higher DBP (Table S5) and hypertension (Table 3) were similar to those for higher SBP.
Table 3.
Prevalence ratios (PR) for hypertension* in the Dar es Salaam Urban Cohort Hypertension Study, 2014 (n=2,174)
| Interviewer-Adjusted† | Adjustedc | |||
|---|---|---|---|---|
|
| ||||
| Variable | PR (95% CI) | P Value§ | PR (95% CI) | P Value§ |
| Age, years | <0.0001 | <0.0001 | ||
| 40–44 | 1 | 1 | ||
| 45–49 | 1.13 (0.91, 1.41) | 1.11 (0.90, 1.38) | ||
| 50–54 | 1.65 (1.36, 2.00) | 1.53 (1.26, 1.85) | ||
| 54–59 | 1.98 (1.64, 2.39) | 1.73 (1.43, 2.10) | ||
| 60–64 | 2.30 (1.91, 2.78) | 1.90 (1.56, 2.31) | ||
| 64–69 | 2.31 (1.87, 2.85) | 1.73 (1.38, 2.17) | ||
| ≥ 70 | 2.25 (1.83, 2.77) | 1.72 (1.36, 2.18) | ||
| Sex | 0.05 | <0.0001 | ||
| Female | 1 | 1 | ||
| Male | 1.12 (1.00, 1.25) | 1.33 (1.15, 1.53) | ||
| Neighborhood | 0.97 | 0.67 | ||
| Markazi | 1 | 1 | ||
| Mwembe Madafu | 0.99 (0.77, 1.29) | 0.95 (0.74, 1.22) | ||
| Religion | 0.08 | 0.43 | ||
| Christian | 1 | 1 | ||
| Muslim | 1.10 (0.99, 1.23) | 1.05 (0.93, 1.18) | ||
| Marital status | <0.0001 | 0.08 | ||
| Married | 1 | 1 | ||
| Single | 0.73 (0.50, 1.06) | 1.01 (0.70, 1.45) | ||
| Separated or divorced | 0.97 (0.78, 1.21) | 1.08 (0.87, 1.34) | ||
| Widowed | 1.41 (1.25, 1.60) | 1.21 (1.05, 1.39) | ||
| Employment | <0.0001 | 0.001 | ||
| Retired or unemployed | 1 | 1 | ||
| Self-employed | 0.63 (0.56, 0.70) | 0.78 (0.69, 0.89) | ||
| Employed | 0.70 (0.60, 0.82) | 0.81 (0.69, 0.96) | ||
| Household wealth index quintile‖ | 0.29 | 0.10 | ||
| Richest | 1 | 1 | ||
| Richer | 1.14 (0.96, 1.36) | 1.16 (0.98, 1.38) | ||
| Average | 1.07 (0.90, 1.27) | 1.08 (0.90, 1.28) | ||
| Poorer | 1.04 (0.87, 1.25) | 1.06 (0.87, 1.29) | ||
| Poorest | 0.95 (0.79, 1.14) | 0.92 (0.74, 1.13) | ||
| Education | 0.001 | 0.01 | ||
| None | 1 | 1 | ||
| At least some primary | 0.70 (0.60, 0.82) | 0.77 (0.65, 0.91) | ||
| At least some secondary | 0.70 (0.59, 0.84) | 0.74 (0.59, 0.92) | ||
| Physical activity quintile | <0.0001# | 0.18# | ||
| Highest (144.0–1008.0 MET-hrs/wk) | 1 | 1 | ||
| Higher (47.0–141.3) | 1.10 (0.92, 1.32) | 1.03 (0.86, 1.24) | ||
| Average (14.7–46.7) | 1.28 (1.06, 1.54) | 1.09 (0.90, 1.30) | ||
| Lower (4.4–14.0) | 1.37 (1.14, 1.65) | 1.12 (0.93, 1.35) | ||
| Lowest (0.0–4.0) | 1.58 (1.31, 1.90) | 1.16 (0.95, 1.42) | ||
| Television watching, hours per day | 0.48 | 0.60 | ||
| 0 | 1 | 1 | ||
| >0 – <2 | 0.92 (0.81, 1.05) | 0.93 (0.82, 1.07) | ||
| ≥ 2 | 0.95 (0.82, 1.10) | 0.94 (0.81, 1.09) | ||
| Smoking | 0.0002 | 0.18 | ||
| Never | 1 | 1 | ||
| Former | 1.28 (1.12, 1.47) | 1.10 (0.95, 1.27) | ||
| Current, 0–9 cigarettes per day | 0.80 (0.60, 1.08) | 0.83 (0.62, 1.11) | ||
| Current, ≥10 cigarettes per day | 1.16 (0.73, 1.85) | 1.27 (0.79, 2.03) | ||
| Alcohol use** | 0.09 | 0.27 | ||
| 0 grams/day | 1 | 1 | ||
| >0–<10 grams/day | 0.84 (0.71, 1.00) | 0.92 (0.77, 1.10) | ||
| ≥10 grams/day | 1.05 (0.86, 1.29) | 1.13 (0.92, 1.39) | ||
| BMI, kg/m2 | <0.0001# | <0.0001# | ||
| Normal weight (18.5–<25) | 1 | 1 | ||
| Underweight (<18.5) | 0.69 (0.47, 1.01) | 0.59 (0.41, 0.85) | ||
| Overweight (25–<30) | 1.15 (1.00, 1.32) | 1.19 (1.04, 1.36) | ||
| Obese, class I (30–<35) | 1.25 (1.08, 1.45) | 1.41 (1.21, 1.65) | ||
| Obese, class II or III (≥35) | 1.42 (1.20, 1.68) | 1.59 (1.33, 1.89) | ||
Hypertension defined as SBP ≥140 mmHg, DBP ≥90 mmHg, and/or self-reported anti-hypertensive drug use
Adjusted for interviewer (n=21)
Adjusted for covariates included in the table and interviewer
χ2 test unless otherwise specified
A household wealth index was created by principal component analysis of household characteristics and household asset ownership.
Test of trend based on median value within each category
Defined based on number of days consumed alcohol over the past 30 days and the average number of drinks per drinking session
Slightly more than a third (36% (95% CI: 31%, 41%)) of hypertensive men and 58% (53%, 62%) of hypertensive women were aware of their hypertension (Figure 2). After adjustment for other covariates, men were 33% less likely to be aware of their disease (prevalence ratio (PR): 0.69 (0.56, 0.85)); as were participants without health insurance (PR: 0.88 (0.77, 1.01), those that were poorer (PR: 0.57 (0.43, 0.76) comparing the lowest wealth index quintile to the highest), those with no formal education compared to those with secondary education (PR: 0.69 (0.52, 0.93)), and those who drank alcohol (PRs ranging from 0.60 to 0.68 depending on amount of alcohol intake). Participants who were overweight or obese were more likely to be aware that they were hypertensive (PRs ranging from 1.33 to 1.45 depending on level of overweight and obesity) (Table 4).
Figure 2.
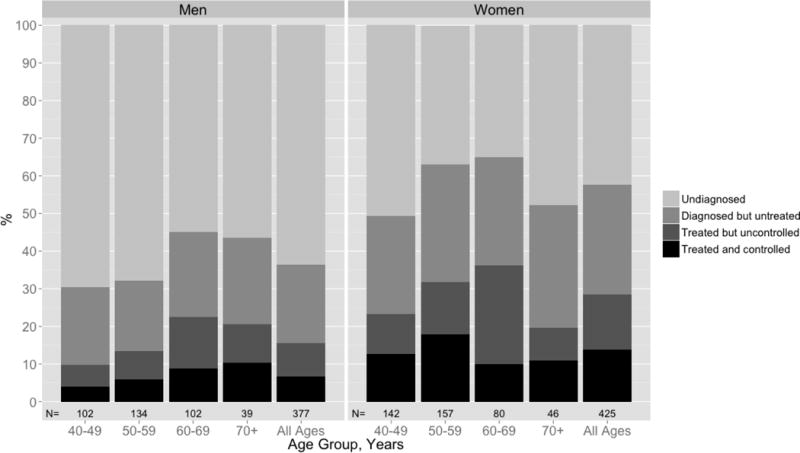
Hypertension awareness, treatment, and control by sex and age group among participants in the Dar es Salaam Urban Cohort Hypertension Study (n=796).*
Table 4.
Prevalence ratio (PR) for baseline hypertension awareness among those with hypertension* and PR for hypertension treatment among those aware in the Dar es Salaam Urban Cohort Hypertension Study, 2014
| Hypertension awareness (among 803 hypertensives) | Hypertension treatment (among 382 aware) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Interviewer-Adjusted† | Fully adjusted‡ | Interviewer-Adjusted† | Fully adjusted‡ | |||||
|
| ||||||||
| Characteristic | PR (95% CI) | P Value§ | PR (95% CI) | P Value§ | PR (95% CI) | P Value§ | PR (95% CI) | P Value§ |
| Age, years | 0.03 | 0.02 | 0.29 | 0.36 | ||||
| 40–44 | 1 | 1 | 1 | 1 | ||||
| 45–49 | 1.46 (1.08, 1.98) | 1.44 (1.10, 1.90) | 0.85 (0.54, 1.33) | 0.83 (0.54, 1.28) | ||||
| 50–54 | 1.40 (1.05, 1.86) | 1.26 (0.97, 1.64) | 0.89 (0.59, 1.36) | 0.79 (0.53, 1.18) | ||||
| 54–59 | 1.44 (1.08, 1.92) | 1.43 (1.10, 1.87) | 1.15 (0.79, 1.69) | 1.08 (0.74, 1.57) | ||||
| 60–64 | 1.68 (1.26, 2.23) | 1.64 (1.25, 2.16) | 1.29 (0.88, 1.88) | 1.19 (0.78, 1.81) | ||||
| 64–69 | 1.44 (1.03, 2.02) | 1.48 (1.05, 2.10) | 0.99 (0.62, 1.61) | 0.91 (0.55, 1.53) | ||||
| ≥ 70 | 1.41 (1.03, 1.94) | 1.61 (1.18, 2.20) | 0.89 (0.56, 1.40) | 0.86 (0.51, 1.46) | ||||
| Sex | <0.0001 | 0.0002 | 0.15 | 0.04 | ||||
| Female | 1 | 1 | 1 | 1 | ||||
| Male | 0.65 (0.55, 0.76) | 0.69 (0.56, 0.85) | 0.85 (0.67, 1.06) | 0.72 (0.52, 0.98) | ||||
| Neighborhood | 0.38 | 0.25 | 0.08 | 0.03 | ||||
| Markazi | 1 | 1 | 1 | 1 | ||||
| Mwembe Madafu | 1.15 (0.84, 1.58) | 1.19 (0.88, 1.62) | 0.63 (0.38, 1.06) | 0.55 (0.32, 0.96) | ||||
| Religion | 0.13 | 0.93 | 0.03 | 0.06 | ||||
| Christian | 1 | 1 | 1 | 1 | ||||
| Muslim | 0.89 (0.77, 1.03) | 0.99 (0.86, 1.15) | 0.79 (0.64, 0.98) | 0.80 (0.63, 1.01) | ||||
| Marital status | 0.12 | 0.76 | 0.07 | 0.10 | ||||
| Married | 1 | 1 | 1 | 1 | ||||
| Single | 0.83 (0.48, 1.46) | 0.89 (0.56, 1.42) | 2.11 (1.43, 3.10) | 1.87 (1.23, 2.83) | ||||
| Separated or divorced | 0.89 (0.65, 1.23) | 0.94 (0.72, 1.24) | 1.27 (0.83, 1.94) | 1.27 (0.81, 1.98) | ||||
| Widowed | 1.19 (1.02, 1.40) | 1.08 (0.91, 1.28) | 1.10 (0.87, 1.39) | 1.06 (0.82, 1.39) | ||||
| Employment | 0.03 | 0.61 | 0.03 | 0.10 | ||||
| Retired or unemployed | 1 | 1 | 1 | 1 | ||||
| Self-employed | 0.83 (0.71, 0.97) | 0.98 (0.83, 1.16) | 0.77 (0.61, 0.99) | 0.79 (0.59, 1.07) | ||||
| Employed | 0.79 (0.63, 0.98) | 0.89 (0.70, 1.13) | 1.14 (0.86, 1.49) | 1.13 (0.81, 1.57) | ||||
| Household health insurance | <0.0001 | 0.07 | 0.02 | 0.15 | ||||
| Insured | 1 | 1 | 1 | 1 | ||||
| Uninsured | 0.68 (0.58, 0.80) | 0.88 (0.77, 1.01) | 0.76 (0.60, 0.96) | 0.84 (0.65, 1.10) | ||||
| Household wealth index quintile‖ | <0.0001# | 0.0008# | 0.13# | 0.74# | ||||
| Richest | 1 | 1 | 1 | 1 | ||||
| Richer | 0.96 (0.81, 1.14) | 1.15 (0.97, 1.36) | 1.02 (0.78, 1.33) | 1.03 (0.78, 1.35) | ||||
| Average | 0.74 (0.60, 0.91) | 0.71 (0.57, 0.89) | 0.90 (0.65, 1.24) | 0.98 (0.69, 1.39) | ||||
| Poorer | 0.66 (0.52, 0.82) | 0.71 (0.56, 0.90) | 0.93 (0.67, 1.30) | 1.12 (0.76, 1.65) | ||||
| Poorest | 0.58 (0.45, 0.74) | 0.57 (0.43, 0.76) | 0.73 (0.48, 1.11) | 0.83 (0.53, 1.31) | ||||
| Education | 0.004 | 0.04 | 0.15 | 0.40 | ||||
| None | 1 | 1 | 1 | 1 | ||||
| At least some primary | 1.20 (0.93, 1.55) | 1.24 (0.97, 1.59) | 1.27 (0.82, 1.96) | 1.21 (0.72, 2.03) | ||||
| At least some secondary | 1.44 (1.11, 1.88) | 1.44 (1.08, 1.92) | 1.47 (0.94, 2.30) | 1.39 (0.79, 2.43) | ||||
| Physical activity quintile | 0.48 | 0.81 | 0.55 | 0.43 | ||||
| Highest (144–1008 MET-hrs/wk) | 1 | 1 | 1 | 1 | ||||
| Higher (47.0–141.3) | 1.08 (0.83, 1.40) | 0.96 (0.75, 1.22) | 1.00 (0.68, 1.46) | 1.11 (0.76, 1.63) | ||||
| Average (14.7–46.7) | 1.20 (0.93, 1.54) | 1.01 (0.79, 1.29) | 1.05 (0.73, 1.51) | 1.10 (0.74, 1.64) | ||||
| Lower (4.4–14.0) | 1.18 (0.91, 1.52) | 0.89 (0.68, 1.16) | 0.86 (0.59, 1.26) | 0.85 (0.58, 1.25) | ||||
| Lowest (0.0–4.0) | 1.24 (0.97, 1.60) | 1.00 (0.76, 1.30) | 1.13 (0.80, 1.60) | 1.15 (0.77, 1.72) | ||||
| Television watching, hours per day | 0.29 | 0.03 | 0.83 | 0.94 | ||||
| 0 | 1 | 1 | 1 | 1 | ||||
| >0 – <2 | 1.01 (0.84, 1.22) | 0.77 (0.63, 0.94) | 1.08 (0.82, 1.41) | 0.98 (0.73, 1.32) | ||||
| ≥ 2 | 1.15 (0.95, 1.40) | 0.86 (0.70, 1.07) | 1.01 (0.73, 1.39) | 0.94 (0.64, 1.38) | ||||
| Smoking | 0.006 | 0.73 | 0.37 | 0.25 | ||||
| Never | 1 | 1 | 1 | 1 | ||||
| Former | 0.87 (0.71, 1.07) | 1.07 (0.86, 1.33) | 1.10 (0.84, 1.45) | 1.29 (0.92, 1.80) | ||||
| Current, 0–9 cigarettes per day | 0.57 (0.33, 0.96) | 0.97 (0.66, 1.43) | 0.72 (0.27, 1.88) | 1.11 (0.36, 3.45) | ||||
| Current, ≥10 cigarettes per day | 0.39 (0.12, 1.21) | 0.66 (0.22, 1.93) | 2.45 (1.81, 3.32) | 3.58 (1.98, 6.50) | ||||
| Alcohol use** | <0.0001 | 0.008 | 0.49 | 0.59 | ||||
| 0 grams/day | 1 | 1 | 1 | 1 | ||||
| >0–<10 grams/day | 0.64 (0.47, 0.87) | 0.68 (0.51, 0.91) | 0.86 (0.56, 1.32) | 0.88 (0.57, 1.35) | ||||
| ≥10 grams/day | 0.51 (0.33, 0.79) | 0.60 (0.40, 0.90) | 0.70 (0.34, 1.46) | 0.70 (0.31, 1.58) | ||||
| BMI, kg/m2 | <0.0001 | 0.0008 | 0.67 | 0.98 | ||||
| Normal weight (18.5–<25) | 1 | 1 | 1 | 1 | ||||
| Underweight (<18.5) | 0.74 (0.36, 1.54) | 0.66 (0.30, 1.44) | 1.13 (0.49, 2.62) | 1.33 (0.51, 3.59) | ||||
| Overweight (25–<30) | 1.50 (1.21, 1.84) | 1.33 (1.08, 1.63) | 1.19 (1.89, 1.59) | 1.05 (0.77, 1.42) | ||||
| Obese, class I (30–<35) | 1.60 (1.29, 2.00) | 1.24 (0.98, 1.57) | 1.14 (0.83, 1.55) | 1.06 (0.77, 1.46) | ||||
| Obese, class II or III (≥35) | 1.61 (1.26, 2.05) | 1.45 (1.14, 1.84) | 1.29 (0.91, 1.82) | 1.07 (0.73, 1.54) | ||||
Hypertension defined as SBP ≥140 mmHg, DBP ≥90 mmHg, and/or self-reported anti-hypertensive drug use
Adjusted for interviewer (n=21)
Adjusted for covariates included in the table and interviewer
χ2-test unless otherwise specified
Household wealth index was created by principal component analysis of household characteristics and household asset ownership.
Test of trend based on median value within each category
Defined based on number of days consumed alcohol over the past 30 days and the average number of drinks per drinking session
Among hypertensive men, 16% (12%, 20%) were on treatment and 7% (4%, 10%) were controlled; among hypertensive women, 28% (24%, 33%) were on treatment and 14% (11%, 18%) were controlled (Figure 2). After adjustment for other covariates, men were 72% (52%, 98%) as likely to be treated as women (Table 4). We did not have sufficient power to detect other determinants of receiving treatment among hypertensives.
Of the 1,752 participants in the follow-up study, 238 reported that they had been told to visit a health professional for high blood pressure at baseline (Figure 3). Of these, 68% reported having sought care, but only 27% were on treatment and 8% were controlled after one year. Forty-three percent sought care from a private clinic and 57% from a public clinic. About one in four patients (24%) reported also taking traditional or alternative treatment for high blood pressure. The most common reasons for not seeking care were lack of symptoms (39% of participants), the (perceived high) cost of visit (25%), and lack of time (16%). Of those who visited a health professional for hypertension care, 65% reported that they had to pay for the visit, 13% had a free visit, and 22% had a visit that was fully covered by health insurance. Major reasons for not taking medication were lack of symptoms (23%), that the health professional said participant did not need drugs (22%), that participant had finished drugs (13%), concerns about side effects (10%), and the cost of the drugs (9%).
Figure 3.
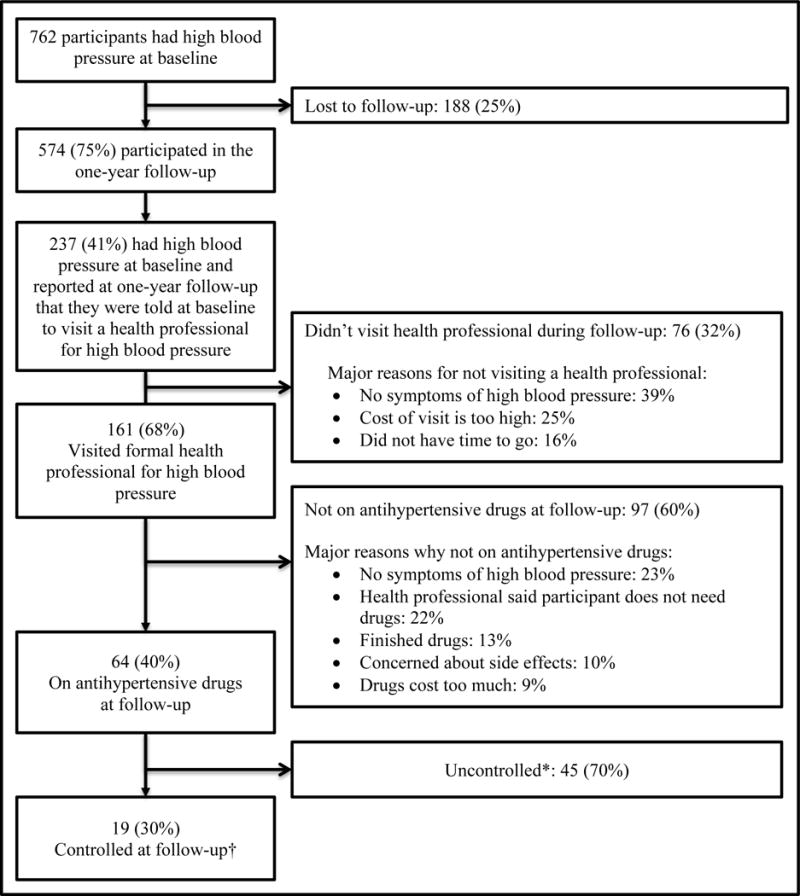
One-year follow-up of Dar es Salaam Urban Cohort Hypertension Study participants who had high blood pressure at baseline (n=764).
Discussion
We observed that 37% of adults in Ukonga were hypertensive, comparable to what has been found by other older studies in Dar es Salaam [6–8,27]. Major modifiable determinants of hypertension were overweight and obesity, inadequate physical activity, and alcohol use. At the one-year follow-up, a large fraction of hypertensives (68%) reported having visited a health professional, however, 73% remained untreated and 92% remained uncontrolled indicating that referral through a screening program is not enough to lead to substantial changes in medication use and blood pressure control. We found that lack of symptoms is a major why patients do not seek care and either do not start or discontinue treatment in this population.
The proportion of participants in DUCS-HTN that sought care, took treatment, and had their hypertension controlled, although low, is higher than what previous studies had found in Dar es Salaam [7,9,10] and other sub-Saharan African cities [11]. This may be because awareness has increased over time in Africa: from 17% in 1990 to 34% in 2010 [28]. The proportions of participants that sought medical care and took treatment in our study was much higher than those found in a similar study in Dar es Salaam in 1999–2000[12], but similar to a 2012 study in rural Uganda, perhaps indicating an improvement in hypertension understanding, care, and treatment over time [29]. The DUCS-HTN and other studies on hypertension in SSA use self-reported data, which could potentially lead to overestimates of hypertension care treatment due to social desirability bias. We instructed interviewers to be non-judgmental to limit this bias, but we acknowledge that we most likely did not eliminate it. Therefore, our estimates for visits to a health professional and use of antihypertensive drugs should be considered as upper bounds of the true proportions.
Strengths of our study include the large sample size, our standard measurement protocols, especially for blood pressure, and a detailed and culturally sensitive questionnaire that was piloted and tested in the same community. However, our study also had several limitations. The cross-sectional nature of the majority of the analyses opens the possibility of ‘reverse causation bias’ where knowledge of high blood pressure could have impacted participants’ behavior. However, in sensitivity analyses in which we restricted data to patients unaware of their hypertension, the risk factors for high blood pressure and barriers to care and treatment did not materially change (results not shown). Another limitation is that, although we attempted to contact all eligible participants three times and on evenings and weekends, we were only able to enroll 47% of those in the HDSS 2011 database. However, a sensitivity analysis in which we attempted to adjust for selection bias produced results that were similar to the main analysis (Tables S3–S4). We only collected dietary data on a subset of participants (n=441) and were unable to adjust for diet as a confounder of the association between obesity and hypertension or to examine diet as a determinant of high blood pressure. We attempted to collect a 24-hour urine sample in the same 441 participants, but measurements of total urinary creatinine showed that about two-thirds of the samples were incomplete. Among the one-third that seemed to be complete, there was no relationship between urinary sodium and SBP (P value 0.84), which is similar to findings of a previous study in Dar es Salaam [30].
Considering the predicted rise in prevalence of overweight and obesity in this region [31], and the strong associations between overweight/obesity and blood pressure reported here, it is expected that the burden of hypertension will substantially increase. Unfortunately, efforts to curb the obesity epidemic globally, including physician advice to change diet and lifestyle have so far shown limited success at the population level and increasing urbanization and caloric consumption, especially from sugar-sweetened beverages [32] may only aggravate these trends. Therefore, in the absence of a long-term solution for preventing weight gain, a first priority for low-income countries such as Tanzania may be to improve diagnosis and management of hypertension, especially among overweight and obese individuals, through increasing access to quality healthcare.
Almost a quarter of hypertensive participants visited during follow-up indicated that their healthcare provider said they did not need to take antihypertensive medication, signifying a need for improved training of healthcare providers. A recent study of health facilities in Tanzania found that only 10% of healthcare professionals reported feeling very comfortable with hypertension management [33]. Developing national guidelines for hypertension diagnosis and management would be a major step in increasing knowledge and preparedness of healthcare professionals. A division of tasks between nurses, non-physician clinicians and physicians, which has recently been proposed by the WHO[34], can be clarified in such guidelines. The Pan-African Society of Cardiology is developing hypertension treatment guidelines for Africa, which could be used as a template for Tanzanian guidelines [35].
Finally, discontinuation of antihypertensive medication due to the belief that the medications must only be taken only for a short period of time must be addressed. This belief leads to expensive and inadequate care for hypertension. An 18-month community intervention in Kenya to improve hypertension control cost a hefty US$ 3,205 per participant with controlled blood pressure [36,37], partly because only 27% of those who started treatment remained treated 18 months later. Patients need to be educated on the importance of taking anti-hypertensive medications regularly and continuously. Studies in SSA on methods to improve hypertension treatment are scarce [37,38], so programs to improve adherence to antihypertensives could learn lessons from approaches developed for other long-term drug regimens such as antiretrovirals. Randomized trials could be designed to determine whether successful interventions to improve antiretroviral adherence, such as using mobile text messages [39] and financial incentives [40,41], would also improve adherence to antihypertensives.
Supplementary Material
Table 2.
Linear regression of SBP in the Dar es Salaam Urban Cohort Hypertension Study, 2014 (n=2,174)
| Interviewer-Adjusted* | Fully Adjusted†, R2=0.18 | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Mean Difference (95% CI) | P Value | Mean Difference (95% CI) | P Value |
| Intercept | 125.4 (117.3, 133.5) | <0.0001§ | ||
| Age, years | <0.0001‡ | <0.0001‡ | ||
| 40–44 | Reference | Reference | ||
| 45–49 | 3.0 (0.5, 5.3) | 3.2 (0.9, 5.6) | ||
| 50–54 | 8.0 (5.5, 10.5) | 7.1 (4.7, 9.5) | ||
| 54–59 | 11.3 (8.4, 14.1) | 9.0 (6.1, 11.9) | ||
| 60–64 | 15.8 (12.6, 19.0) | 12.9 (9.5, 16.3) | ||
| 64–69 | 16.1 (11.8, 20.4) | 11.8 (7.2, 16.4) | ||
| ≥ 70 | 17.3 (13.1, 21.5) | 13.5 (9.0, 18.0) | ||
| Male | 4.9 (3.0, 6.7) | <0.0001§ | 7.1 (4.8, 9.4) | <0.0001§ |
| Mwembe Madafu neighborhood | 3.3 (−1.4, 8.0) | 0.17§ | 2.8 (−1.7, 7.3) | 0.24§ |
| Muslim | 2.8 (1.0, 4.6) | 0.003§ | 1.9 (0.0, 3.8) | 0.05§ |
| Marital status | <0.0001‖ | 0.56‖ | ||
| Married | Reference | Reference | ||
| Single | −6.4 (−10.4, −2.5) | −0.9 (−4.8, 3.0) | ||
| Separated or divorced | −3.4 (−7.2, 0.5) | −1.5 (−5.2, 2.1) | ||
| Widowed | 4.5 (1.9, 7.2) | 1.4 (−1.3, 4.2) | ||
| Employment | <0.0001‖ | 0.04‖ | ||
| Retired or unemployed | Reference | Reference | ||
| Self-employed | −7.0 (−9.1, −5.0) | −2.4 (−4.8, −0.1) | ||
| Employed | −6.5 (−9.2, −3.8) | −3.6 (−6.5, −0.8) | ||
| Household wealth index quintilee | 0.96‖ | 0.76‖ | ||
| Richest | Reference | Reference | ||
| Richer | 0.8 (−2.0, 3.6) | 1.4 (−1.4, 4.2) | ||
| Average | 0.6 (−2.2, 3.4) | 0.9 (−1.9, 3.6) | ||
| Poorer | 1.1 (−1.8, 4.0) | 1.0 (−2.1, 4.0) | ||
| Poorest | 0.5 (−2.2, 3.3) | −0.2 (−3.4, 2.9) | ||
| Education | 0.0002‖ | 0.02‖ | ||
| None | Reference | Reference | ||
| At least some primary | −6.7 (−10.1, −3.2) | −4.3 (−7.7, −0.8) | ||
| At least some secondary | −7.5 (−11.1, −3.9) | −5.4 (−9.3, −1.5) | ||
| Physical activity quintile | <0.0001‡ | 0.005‡ | ||
| Highest (144–1008 MET-hrs/wk) | Reference | Reference | ||
| Higher (47.0–141.3) | 1.8 (−1.1, 4.7) | 1.7 (−1.0, 4.5) | ||
| Average (14.7–46.7) | 4.5 (1.4, 7.5) | 3.1 (0.3, 5.9) | ||
| Lower (4.4–14.0) | 5.3 (2.2, 8.3) | 3.6 (0.6, 6.6) | ||
| Lowest (0.0–4.0) | 8.2 (5.0, 11.5) | 4.8 (1.4, 8.1) | ||
| Television watching, hours per day | 0.06‖ | 0.15‖ | ||
| 0 | Reference | Reference | ||
| >0 – <2 | −2.4 (−4.8, −0.0) | −1.6 (−4.1, 0.8) | ||
| ≥ 2 | −3.2 (−6.0, −0.5) | −2.7 (−5.3, 0.0) | ||
| Smoking | 0.0002‖ | 0.37‖ | ||
| Never | Reference | Reference | ||
| Former | 6.4 (3.3, 9.5) | 1.8 (−1.2, 5.0) | ||
| Current, 0–9 cigarettes per day | 0.5 (−4.0, 5.0) | −0.4 (−4.7, 3.8) | ||
| Current, ≥10 cigarettes per day | 7.1 (−0.3, 14.6) | 5.4 (−2.4, 13.1) | ||
| Alcohol use** | 0.03‖ | 0.03‖ | ||
| 0 grams/day | Reference | Reference | ||
| >0–<10 grams/day | −1.0 (−3.6, 1.5) | 0.3 (−2.3, 2.8) | ||
| ≥10 grams/day | 4.2 (0.8, 7.6) | 4.5 (1.2, 7.8) | ||
| BMI, kg/m2 | <0.0001‡ | <0.0001‡ | ||
| Normal weight (18.5–<25) | Reference | Reference | ||
| Underweight (<18.5) | −6.4 (−11.5, −1.4) | −9.4 (−14.2, −4.5) | ||
| Overweight (25–<30) | 2.9 (0.7, 5.0) | 4.0 (1.9, 6.1) | ||
| Obese, class I (30–<35) | 3.1 (0.7, 5.5) | 6.3 (3.8, 8.8) | ||
| Obese, class II or III (≥35) | 6.6 (3.3, 9.9) | 10.5 (7.2, 13.8) | ||
Adjusted for interviewer (n=21)
Adjusted for covariates included in the table and interviewer
Test of trend based on median value within each category
T-test
F-test
Household wealth index was created by principal component analysis of household characteristics and household asset ownership.
Defined based on number of days consumed alcohol over the past 30 days and the average number of drinks per drinking session
Acknowledgments
We would like to acknowledge participants who generously gave their time and invited interviewers to their homes. This project would not have been possible without the hard work of many interviewers, data entry clerks, administrative staff, and drivers. Their enthusiasm and hospitality made this project a pleasure to work on.
Funding Sources: This work was supported by a pilot grant through the Center for the Global Demography of Aging. RMZ was supported by an NIH T32 training-grant (CA 09001).
Footnotes
- Oral presentation at Society for Epidemiologic Research Annual Meeting, 2015. This presentation only covered the risk factors for higher blood pressure.
- Poster at student poster day at Harvard T.H. Chan School of Public Health
- Talk at the Harvard T.H. Chan School of Public Health annual Friday Forum for Cardiovascular Epidemiology Fellows and Trainees
Conflict of Interest Disclosures: None.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Lond Engl. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker RW, Viney R, Green L, Mawanswila M, Maro VP, Gjertsen C, et al. Trends in stroke admissions to a Tanzanian hospital over four decades: a retrospective audit. Trop Med Int Health TM IH. 2015;20:1290–1296. doi: 10.1111/tmi.12547. [DOI] [PubMed] [Google Scholar]
- 3.Walker R, Whiting D, Unwin N, Mugusi F, Swai M, Aris E, et al. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol. 2010;9:786–792. doi: 10.1016/S1474-4422(10)70144-7. [DOI] [PubMed] [Google Scholar]
- 4.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PloS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 6.Njelekela M, Negishi H, Nara Y, Tomohiro M, Kuga S, Noguchi T, et al. Cardiovascular risk factors in Tanzania: a revisit. Acta Trop. 2001;79:231–239. doi: 10.1016/s0001-706x(01)00134-6. [DOI] [PubMed] [Google Scholar]
- 7.Edwards R, Unwin N, Mugusi F, Whiting D, Rashid S, Kissima J, et al. Hypertension prevalence and care in an urban and rural area of Tanzania. J Hypertens. 2000;18:145–152. doi: 10.1097/00004872-200018020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bovet P, Ross AG, Gervasoni J-P, Mkamba M, Mtasiwa DM, Lengeler C, et al. Distribution of blood pressure, body mass index and smoking habits in the urban population of Dar es Salaam, Tanzania, and associations with socioeconomic status. Int J Epidemiol. 2002;31:240–247. doi: 10.1093/ije/31.1.240. [DOI] [PubMed] [Google Scholar]
- 9.Hendriks ME, Wit FWNM, Roos MTL, Brewster LM, Akande TM, de Beer IH, et al. Hypertension in sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PloS One. 2012;7:e32638. doi: 10.1371/journal.pone.0032638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, Laurence C, Adebamowo C, Ajayi I, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health. 2015;15:1211. doi: 10.1186/s12889-015-2546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension. 2015;65:291–298. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
- 12.Bovet P, Gervasoni J-P, Mkamba M, Balampama M, Lengeler C, Paccaud F. Low utilization of health care services following screening for hypertension in Dar es Salaam (Tanzania): a prospective population-based study. BMC Public Health. 2008;8:407. doi: 10.1186/1471-2458-8-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topouchian J, Agnoletti D, Blacher J, Youssef A, Ibanez I, Khabouth J, et al. Validation of four automatic devices for self-measurement of blood pressure according to the international protocol of the European Society of Hypertension. Vasc Health Risk Manag. 2011;7:709–717. doi: 10.2147/VHRM.S27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lüders S, Krüger R, Zemmrich C, Forstner K, Sturm C-D, Bramlage P. Validation of the Beurer BM 44 upper arm blood pressure monitor for home measurement, according to the European Society of Hypertension International Protocol 2002. Blood Press Monit. 2012;17:248–252. doi: 10.1097/MBP.0b013e32835b9e8e. [DOI] [PubMed] [Google Scholar]
- 15.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 16.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 17.Global Physical Activity Questionnaire (GPAQ) Analysis Guide. http://www.who.int/chp/steps/resources/GPAQ_Analysis_Guide.pdf.
- 18.WHO. Global recommendations on physical activity for health. WHO; http://www.who.int/dietphysicalactivity/publications/9789241599979/en/ (accessed 1 Dec2015) [PubMed] [Google Scholar]
- 19.Glovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, et al. The Assessment of Alcohol Consumption by a Simple Self-administered Questionnaire. Am J Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 20.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen OS. Theoretical Epidemiology: Principles of Occurrence Research in Medicine. Delmar Pub; 1985. [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis RE, Couper MP, Janz NK, Caldwell CH, Resnicow K. Interviewer effects in public health surveys. Health Educ Res. 2010;25:14–26. doi: 10.1093/her/cyp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himelein K. Interviewer Effects in Subjective Survey Questions: Evidence From Timor-Leste. Int J Public Opin Res. 2015:edv031. [Google Scholar]
- 26.Bovet P, Gervasoni J-P, Ross AG, Mkamba M, Mtasiwa DM, Lengeler C, et al. Assessing the prevalence of hypertension in populations: are we doing it right? J Hypertens. 2003;21:509–517. doi: 10.1097/00004872-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Njelekela MA, Mpembeni R, Muhihi A, Mligiliche NL, Spiegelman D, Hertzmark E, et al. Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc Disord. 2009;9:30. doi: 10.1186/1471-2261-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adeloye D, Basquill C. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PloS One. 2014;9:e104300. doi: 10.1371/journal.pone.0104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotwani P, Balzer L, Kwarisiima D, Clark TD, Kabami J, Byonanebye D, et al. Evaluating linkage to care for hypertension after community-based screening in rural Uganda. Trop Med Int Health TM IH. 2014;19:459–468. doi: 10.1111/tmi.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njelekela M, Sato T, Nara Y, Miki T, Kuga S, Noguchi T, et al. Nutritional variation and cardiovascular risk factors in Tanzania–rural-urban difference. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2003;93:295–299. [PubMed] [Google Scholar]
- 31.NCD Risk Factor Collaboration (NCD-RisC) Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet Lond Engl. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talyor AL, Jacobson MF. Carbonating the World: The Marketing and Health Impact of Sugar Drinks in Low- and Middle-income Countries. Washington, D.C.: Center for Science in the Public Interest; http://www.cspinet.org/carbonatingreport.pdf. [Google Scholar]
- 33.Peck R, Mghamba J, Vanobberghen F, Kavishe B, Rugarabamu V, Smeeth L, et al. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: a cross-sectional survey. Lancet Glob Health. 2014;2:e285–292. doi: 10.1016/S2214-109X(14)70033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyal N, Cancedda C, Kyamanywa P, Hurst SA. Non-physician Clinicians in Sub-Saharan Africa and the Evolving Role of Physicians. Int J Health Policy Manag. 2015;5:149–153. doi: 10.15171/ijhpm.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dzudie A, Ojji D, Anisiuba BC, Abdou BA, Cornick R, Damasceno A, et al. Development of the roadmap and guidelines for the prevention and management of high blood pressure in Africa: Proceedings of the PASCAR Hypertension Task Force meeting: Nairobi, Kenya, 27 October 2014. Cardiovasc J Afr. 2015;26:82–85. [PubMed] [Google Scholar]
- 36.Oji Oti S, van de Vijver S, Gomez GB, Agyemang C, Egondi T, Kyobutungi C, et al. Outcomes and costs of implementing a community-based intervention for hypertension in an urban slum in Kenya. Bull World Health Organ. 2016:94. doi: 10.2471/BLT.15.156513. http://dx.doi.org/10.2471/BLT.15.156513. [DOI] [PMC free article] [PubMed]
- 37.van de Vijver S, Oti SO, Gomez GB, Agyemang C, Egondi T, Moll van Charante E, et al. Impact evaluation of a community-based intervention for prevention of cardiovascular diseases in the slums of Nairobi: the SCALE-UP study. Glob Health Action. 2016;9:30922. doi: 10.3402/gha.v9.30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobrow K, Farmer AJ, Springer D, Shanyinde M, Yu L-M, Brennan T, et al. Mobile Phone Text Messages to Support Treatment Adherence in Adults With High Blood Pressure (SMS-Text Adherence Support [StAR]) A Single-Blind, Randomized Trial. Circulation. 2016;133:592–600. doi: 10.1161/CIRCULATIONAHA.115.017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;3:CD009756. doi: 10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell M-L. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis. 2011;11:942–951. doi: 10.1016/S1473-3099(11)70181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills EJ, Lester R, Thorlund K, Lorenzi M, Muldoon K, Kanters S, et al. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. Lancet HIV. 2014;1:e104–111. doi: 10.1016/S2352-3018(14)00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


