Abstract
Natural selection favors individuals to act in their own interests, implying that wild animals experience a competitive psychology. Animals in the wild also express helping behaviors, presumably at their own expense and suggestive of a more compassionate psychology. This apparent paradox can be partially explained by ultimate mechanisms that include kin selection, reciprocity, and multilevel selection, yet some theorists argue such ultimate explanations may not be sufficient and that an additional “stake in others” is necessary for altruism’s evolution. We suggest this stake is the “camaraderie effect,” a by-product of two highly adaptive psychological experiences: social motivation and empathy. Rodents can derive pleasure from access to others and this appetite for social rewards motivates individuals to live together, a valuable psychology when group living is adaptive. Rodents can also experience empathy, the generation of an affective state more appropriate to the situation of another compared to one’s own. Empathy is not a compassionate feeling but it has useful predictive value. For instance, empathy allows an individual to feel an unperceived danger from social cues. Empathy of another’s stance toward one’s self would predict either social acceptance or ostracism and amplify one’s physiological sensitivity to social isolation, including impaired immune responses and delayed wound healing. By contrast, altruistic behaviors would promote well-being in others and feelings of camaraderie from others, thereby improving one’s own physiological well-being. Together, these affective states engender a stake in others necessary for the expression of altruistic behavior.
1 Introduction
Since its inception, a challenge for evolutionary theory has been widespread evidence of altruistic behavior (Darwin 1888). Natural selection works at the level of the individual, not the species. When the environment favors the survival and reproduction of one individual over another, the frequency of that individual’s alleles represents a larger proportion of the gene pool in the following generation. Inherent in this process is the requirement that individuals compete for limited resources. Yet wild animals also help one another, apparently at personal cost. Helping behaviors include biological altruism and cooperation. “Biological altruism” refers to instances when individuals seemingly pay a cost, at least in the short term, to benefit another individual. “Cooperation” refers to instances when individuals act together to acquire benefits for survival and reproduction that they might be unable to acquire by acting alone (Roberts 2005). Some altruistic behaviors are expressed by only a particular species; a vampire bat risks starvation when regurgitating a blood meal to share it with another (Wilkinson 1984). Other behaviors are widespread, such as participation in the mobbing of a predator, a risky behavior that can help protect other colony members (Graw and Manser 2007; Krams et al. 2006; Templeton et al. 2005). Efforts to reconcile altruistic behaviors within the context of natural selection have largely focused on the development of ultimate mechanisms, such as kin selection, various forms of reciprocity, and multilevel selection (Nowak 2006).
An implication of natural selection is that it favors a competitive and individualistic “natural” psychology; that individuals in the wild harbor affective experiences focused on acquiring resources and providing for progeny. But what do we actually know about animal psychology in the wild? Is a constant motivation for resource acquisition the “natural” affective state of wild non-human animals? If so, why do wild animals help each other when resources are restricted? Likewise, an implication of altruistic behavior is that it reflects an underlying psychological experience of compassion. In both of these instances, we confuse the expression of a behavior with affective experiences. This conflation of animal behavior with psychological experience is a persistent problem in behavioral science, sharing similarity with a much earlier problem regarding the psychology of an animal with its movement toward or away from an environmental stimulus.
We can dissociate behavior from the reading of intent into underlying affective states by employing operant and classical conditioning experiments that help us infer internal subjective responses to environmental rewards and punishments (Bardo and Bevins 2000). With classical conditioning experiments, we can infer affective experiences occurring within social interactions, whether a rodent can experience social reward and empathy, affective capacities that can be inherited and are thereby sensitive to natural selection.
How might social reward and empathy contribute to the expression of altruistic behavior in the wild? Social rewards can motivate individuals to shelter and forage together, behavioral strategies that can be adaptive. Empathy, the generation of an affective state more appropriate to the situation of another compared to one’s own, has useful predictive value, allowing an individual to detect a threat from the behavioral cues of others. The “camaraderie effect” emerges from the motivation for social reward and the predictive sensitivity of empathy. A socially motivated individual can detect feelings held by others that may be interpersonal (e.g., affinity, indifference, and enmity) or may pertain to changes in the environment (e.g., calm and fear). Once established, the camaraderie effect gains its own survival value because the detection of amicable, dispassionate, or hostile feelings becomes predictive of well-being within the group versus ostracism and social isolation. As the psychological experience of social isolation can compromise immune responses and impair wound healing, one possibility is that the detection of social cues predicting social isolation, or serving as a surrogate for physical ostracism, would also compromise physiology. Likewise, an individual might be motivated to express altruistic behaviors to share in the positive affective experience of others, an experience that might promote one’s physiological well-being. Components of the camaraderie effect have established neurobiological substrates (Dolen et al. 2013; Jeon et al. 2010) that are consistent with the theoretical underpinnings of affective neuroscience (Ekman and Davidson 1994; Panksepp 1998) and also meet the requirements for genetic self-interest imposed by natural selection.
Topics to be covered in this review include brief overviews of emotion and its expression in social interaction, social motivation, empathy, helping behavior, and the camaraderie effect. Rodent studies are a primary focus of this review because they underscore the widespread capacity for social reward and empathy in mammals and because rodent studies offer the most comprehensive neurobiological assessments underlying emotion expression and receptivity in non-human animals.
2 Social Interaction
There was speech in their dumbness, language in their very gestures.
William Shakespeare
A meeting between individuals is comprised of a remarkably complex array of signals transmitted and received, spoken and heard, involving language responsive to how arousal moderates the acoustic parameters of speech (Scherer 1986) or existing as mere utterances: a laugh, a sigh, or a grunt. A visual cue might feature a redirection of eye gaze or an extra bounce in a step. An eye movement, a facial expression, or a gesture, each one is a social cue that occurs by volition or by reflex, each one having the capacity to pivot the gestalt and trajectory of a social interplay, shaping its direction, and turning it aggressive, compassionate, or disengaged.
Beneath this surface of signals are the emotions, moods, interpersonal stances, attitudes, and personality traits of the interacting participants—affective experiences and behavioral expressions that can be fleeting or long-standing, influencing what each participant expects from the other and what each participant detects or feels, correctly or incorrectly, in another’s motives and emotions (Scherer 2003).
Emotion
A relatively brief episode of synchronized response to an external or internal event valued as being of major significance (anger, sadness, joy, fear, and desperation).
Mood
A diffuse affect state, most pronounced as change in subjective feeling, of low intensity but relatively long duration, often without apparent cause (cheerful, gloomy, and irritable).
Interpersonal stance
An affective stance taken toward another individual in a specific interaction, coloring the interpersonal exchange in that situation (cold, warm, supportive, and contemptuous).
Attitudes
Relatively enduring, affectively colored beliefs, references, and predispositions toward objects or persons (liking, loving, hating, and desiring).
Personality traits
Emotionally laden, stable dispositions and behavioral tendencies of an individual (nervous, anxious, hostile, and jealous).
Emotions, moods, interpersonal stances, attitudes, and personality traits are not directly measurable in humans and non-humans but these terms help us to conceptually dissociate affective states experienced during social interactions. For instance, dominant-subordinate relationships, which occur broadly in nature and in the laboratory (Blanchard et al. 1987), can be considered in the context of interpersonal stance. Adjustments in attitude might explain how Pinyon jays make transitive inferences about dominance hierarchies (Bond et al. 2004). Mice express variations in aggressive personality traits (Caramaschi et al. 2008). Such distinctions in affective states will also be useful for understanding wild animal social behavior.
Each of us might be aware of our own emotions, interpersonal stances, and attitudes, but these internal subjective experiences elude the scientific process. Science is a way-of-knowing that requires objectivity and a deliberate attempt to depart from subjective experience. As scientists, we measure physical entities, what can be poured into a beaker or measured in wavelengths, not sensory or emotional experiences. When two people sit in the same room and look at a red apple, neither one can know the color perceived by the other (Russell 1912). My daughter might see magenta while I see crimson. We both call it “red.” Our verbal report is unreliable, perhaps misleading, suggesting that our shared experiences, like the “red” in an apple, are identical. Our verbal report of affective states is even more likely misinterpreted. As a result, scientists often choose to avoid questions about subjective experience, more so when we lack a shared language with our non-human experimental subjects. Critically, to improve treatments for mental illness, which often features impaired social-emotional regulation, we are compelled to study the mechanisms underlying non-human animal affective experiences in a social context.
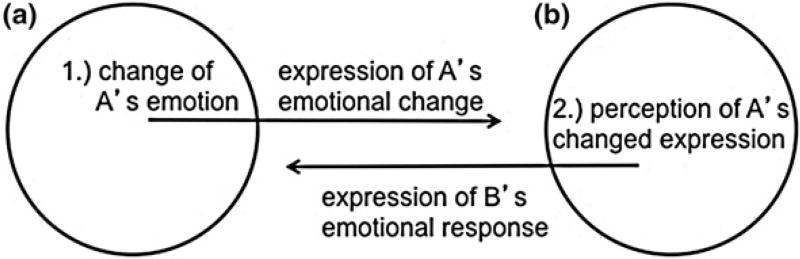
Studies of animal subjects focus on measurements of behavioral expressions that signify affective states. The Reciprocity Chain (Fig. 1) is a simple model that shows how affective experiences, in this case emotions, can be dissociated from the measurable behaviors expressed during a social interaction (Bishop and Lahvis 2011). This model is a simplified analogue of the Brunswikian lens model, a useful model of social-emotional interaction also employed in communication research (Scherer 2003). Measurable signals include the visual, auditory, tactile, and olfactory cues and their timing.
Fig. 1.
The Reciprocity Chain represents emotions felt and expressed during a dyadic social interaction between Individual A (a) and Individual B (b). In this simple model, Individual A experiences a change in emotion while interacting with Individual B. This change in affect is expressed as a behavioral cue by Individual A that is detected by Individual B and in turn provokes an emotional change in Individual B, which is again expressed, and detected by Individual A. While emotions elude direct scientific observation, their expressions can be measured
In Expression of Emotion in Man and Animal, Darwin argues that the expression of an emotional experience can occur reflexively, borrowed from a related experience (Darwin 1872). As depicted in the Reciprocity Chain, a minced facial expression (see Fig. 1b) responding to a bitter or repugnant thought or emotion (see Fig. 1a) taps into an analogous experience, perhaps the taste of a bitter berry or the smell of a putrid deposit. Even when involuntary, these expressions can engender emotional changes in those who perceive them that are, in turn, expressed. The Brunswikian lens model considers additional features, such as how a signal expressed might differ from the signal perceived; a minced look might be seen as a smile.
An expression might be intentionally directed toward another individual (Tomasello et al. 2005). Feeling frustrated after seeing a full-bellied raccoon waddle from my grape trellis, I might keep this feeling to myself or point him out to my wife. I experience frustration either way but I’m intentionally sharing my feeling when I direct my wife’s attention toward the raccoon. In this shared affective experience, she needs to “get it” and respond in turn, acknowledging my attempt to communicate. Perhaps she will look downward, sigh, or laugh. If her response is not perceptible to me, I’ll remain unsure whether our experience was shared. As with reflexive signals, intentional signals connote emotion. A concerned facial expression and a softer voice help relate a sad story. A widening of one’s eyes and staccato in one’s voice help express surprise and excitement (see Markova and Legerstee 2006). Emotions signaled by intention can be different from emotions portrayed by actors (see Scherer 2003). The Reciprocity Chain and the Brunswikian lens models help us differentiate affective states from their expressions, but they may neglect the embodied cognition experienced in the first or second person, an emergent property experienced when one is engaged within a social interaction rather than just observing it (Schilbach et al. 2013).
Neuroscientists often study laboratory rodents to model social-emotional challenges: how adolescent social motivation responds to substance abuse and addiction, how vocalizations and social behaviors are integrated in a rodent model of autism, or how empathy can be re-instilled in an individual crippled by traumatic memories. We ask how social relationships form, what allows individuals to bond, how these relationships are maintained, where motivations for social bonding are orchestrated in the brain, and how these relationships fade. Laboratory rodents, such as mice and rats, are useful because their wild conspecifics live at a variety of densities (Fitzgerald et al. 1981) that require multiple layers of social competence: navigating complex and dynamic social hierarchies (Butler 1980; Pocock et al. (2005); assessing, accepting, and rejecting mating opportunities (Drickamer et al. 2000; Krackow and Matuschak 1991; Wolff 1985); escalating and resolving territorial disputes (Chambers et al. 2000); acquiring food preferences based upon social cues (Valsecchi et al. 1996); and even avoiding parasitized conspecifics (Kavaliers and Colwell 1995). Some rodent species, such as prairie voles, engage in social monogamy (McGraw and Young 2010). Others, such as members of the squirrel family, emit alarm calls (Baack and Switzer 2000; Blumstein et al. 1997; Mateo and Holmes 1999). By studying small rodents, we can collect large sample sizes that help us to elucidate the anatomy, physiology, and genetics underlying social bonding, social learning, empathy, cooperation, and altruistic behaviors.
In the laboratory, investigators often measure social approach (Lahvis and Black 2011). The two most common tests are the three-chambered social approach test (Nadler et al. 2004) and the social investigation (SI) test (Winslow and Camacho 1995). Both tests measure levels of subject approach toward a stimulus rodent, also called the object. In the SI test, both the test subject and the object are free to move inside a test structure. In the social approach test, the subject moves freely, while the object is confined to a small cage within the test structure. Social recognition tests (Ferguson et al. 2001) and social preference tests (Moy et al. 2004) are variants of these social approach measures. Social approach tests have been used to compare the responses of rodent sociability to genetic background (Moy et al. 2009; Panksepp et al. 2007; Sankoorikal et al. 2006), variations in brain anatomy (Fairless et al. 2008), targeted alleles including knockout mice (Spencer et al. 2008), brain lesions (Yang et al. 2009), exposures to modulators of opiate and dopamine pathways (Benton et al. 1984; Gariepy et al. 1998; Kennedy et al. 2011), toxic chemicals (Belloni et al. 2011), and candidates for pharmacological treatments (Calamandrei et al. 2000; and for reviews, see Halladay et al. 2009; Moy et al. 2009; Silverman et al. 2010). Measures of social approach often tally how often the subject rodent brings itself into close proximity or physical contact with the object rodent. Strains that maintain close proximity are typically classified as more “social,” whereas strains that approach less often or withdraw from the object rodent are often classified as “asocial.”
Classifications of “sociality” based solely upon body movement imply that social interactions within a test structure are restricted to proximity, ignoring other dimensions of communication including vocal and olfactory signals. Rodents emit audible and ultrasonic vocalizations (USVs) that exceed the upper limit of human hearing, emitted at frequencies above 20 kHz (20,000 cycles per second). USVs can be transmitted across cage structures, can be associated with affiliative or aggressive social interactions, and can engender behavioral responses from others. Infant rodents emit wriggling calls (~35 kHz) to solicit maternal care (D’Amato et al. 2005; Ehret and Bernecker 1986) and distress calls (~90 kHz) soliciting return to the nest (Branchi et al. 1998). Vocalizations can also be associated with social approach. Adolescent mice that engage in more robust social approach vocalize more often (Panksepp et al. 2007). Laboratory rats emit 22- and 50-kHz calls to signal negative and positive affect (Burgdorf et al. 2005; Carden et al. 1993; Harmon et al. 2008) and female mice emit a 38-kHz calls to coordinate paternal pup retrieval (Liu et al. 2013). Like vocalizations, scent marking is used in social interactions, establishing territorial dominance among males (Hurst 1990) and attracting females (Roberts et al. 2014; Thonhauser et al. 2013). Assessments of scent marking, and of subject responses to maternal scent, have been used to phenotype mouse models of autism (Kane et al. 2012; Wöhr et al. 2011a, b).
Approach behaviors should not to be confused with the desire for a reward (Schneirla 1959). An amoeba, single-celled and brain-free, can move up a chemical gradient. While assessments of social approach, vocal, and scent marking behaviors give us a sense of the level of social interaction between experimental subjects, by themselves they offer no measurable insight to underlying affective experiences. Social approach behaviors are not necessarily an expressed indication of the affective experience of social seeking, a desire for a social reward. Similarly, social withdrawal behaviors do not necessarily indicate that the subject feels the social interaction is an aversive experience.
To elucidate a few of the affective experiences underlying social interaction, we can make use of classical conditioning. With conditioned place preference testing, we can infer that rodents find some experiences pleasurable, including both natural rewards, such as social interactions, and drug rewards, such as methamphetamine exposure. How these subjective experiences can be inferred is the topic of the next section.
3 Social Reward
Seeking and avoidance are of a higher evolutionary and developmental order than approach and withdrawal, and these terms should not be mismated.
Theodore C. Schneirla
Children and adults with developmental disabilities can be challenged by social interactions (Lord et al. 2001). For some, these social interactions may not feel rewarding or be sufficiently desirable for maintaining and enhancing relationships (Chevallier et al. 2012). Studying rodent models of autism, we can use Pavlovian conditioning approaches to determine whether subjects derive pleasure from social access. If a test subject finds a stimulus rewarding, it will return to an environment associated with that stimulus (Glickman and Schiff 1967; Schneirla 1959). The social conditioned place preference (social CPP) test is used to determine whether a mouse prefers housing with its peers versus housing in social isolation. Prior to testing, each subject is “conditioned,” alternately placed within one of two housing environments specifically paired with the presence or absence of other mice. We hypothesized that social interactions would be most rewarding for mice under comfortable conditions because social interactions are generally most rewarding for humans in comfort. Thus, each environment contains novel bedding, such as wood chips or paper bedding, rather than the traditional steel bars and metal floors used for CPP tests in drug abuse experiments. PVC couplers are also added, either threaded or smooth, to add environmental complexity to the conditioning experience and testing conditions (Panksepp and Lahvis 2007). Each day, mice are transferred from one housing environment to the other, paired with the same social condition, in a group or alone. After conditioning, subjects are tested in a social CPP test structure with separate chambers offering rival beddings and their associated couplers. If a subject derives a rewarding experience from social housing, this feeling becomes paired with the bedding environment, and on test day, the subject chooses to spend more time in the bedding associated with access to that positive feeling (Panksepp and Lahvis 2007). Social CPP experiments allow us to determine whether the test subject seeks social reward, avoids social isolation, or is indifferent to social context.
Social CPP tests demonstrate that positive affective experiences occur during juvenile social interactions (Calcagnetti and Schechter 1992; Douglas et al. 2004), mating opportunities (Camacho et al. 2004; Jenkins and Becker 2003), access to offspring (Mattson et al. 2001), and even in response to aggressive social interactions (Martinez et al. 1995; Tzschentke 2007). CPP has demonstrated that laboratory rodents typically prefer environments associated with social access, a behavior driven by anticipation of a social reward (Calcagnetti and Schechter 1992; Douglas et al. 2004; Panksepp and Lahvis 2007). In a limited number of experiments, mouse strains expressing low levels of social CPP, or social indifference, also express minimal social approach (Panksepp and Lahvis 2007).
Social affect
The feeling experienced during a social encounter, such as pleasure from a social reward or fear from a social threat.
Social motivation
What drives an individual to engage in a social interaction, irrespective of the kind of affective experience or even the presence of affect.
Social reward
The positive affective experience associated with access to others can be inferred from a subject’s behavioral response to the social conditioned place preference (CPP) test. Spending of more time exploring bedding experimentally paired with social housing versus bedding paired with isolation indicates that the subject derives pleasure from social access.
Social CPP reveals that social reward is mediated by coordinated activities of oxytocin and serotonin in the nucleus accumbens (Dolen et al. 2013). These neurological circuits, recruited during social interactions, also play critical roles in the rewarding experiences mediated by drugs of abuse. These findings confirm the prediction that natural reward systems of wanting and liking (Berridge 2004, 2007; Berridge and Robinson 2003; Smith and Berridge 2007) are engaged, systems that are co-opted by drugs of abuse (Kelley et al. 2005; Kelley and Berridge 2002; Schroeder et al. 2001) and serve as specific neurobiological substrates for the anticipating and rewarding experiences that motivate social interaction.
The design of the social CPP provides insight to how social reward contributes to group living in the wild. Social CPP identifies a motivation for individuals to interact with others and environments where they would encounter conspecifics. Outside the cage, individuals have opportunity to make choices about where to move based upon various features across a heterogeneous environment, including those stimuli associated with social access, such as shelters and common foraging areas. For many species, living within a group confers survival and reproductive benefits, either throughout their lives or during specific developmental life stages. Group living is not always adaptive, especially when local resources are depleted, when colony parasitism is high, or during adolescent maturation (Hoogland 1979), so we might expect that social reward is also adaptive only under specific environmental conditions.
While social CPP experiments show that laboratory rats and mice can derive pleasure from a social interaction, a possible explanation for social reward is that it emerged from breeding under highly constrained housing conditions that force individuals to live together. Domestication also relieves tame animals of the natural selective pressures to compete for limited resources, rendering them more docile (Nelson and Chiavegatto 2001). For instance, tame animals are less aggressive toward conspecifics (Boreman and Price 1972; Ebert 1976) and more readily engage in mating opportunities without requiring mate choice (Drickamer et al. 2000; Manning et al. 1992; Penn and Potts 1999).
To determine whether rodent social reward is an artifact of domestication, a social CPP test was conducted on undomesticated thirteen-lined ground squirrels (Ictidomys tridecemlineatus), a species considered asocial among ground squirrels (Armitage 1981) because they appear to form colonies not out of attraction to one another but because they prefer living in a specific environment (McCarley 1966). However, in the social CPP test, captive juveniles that were second- and third-generation descendants of wild ground squirrels expressed a robust preference for environments paired with social access, indicating that social interactions can be rewarding for rodents with undomesticated genetic backgrounds (Lahvis et al. 2015). This finding suggests that wild squirrels, known for their diminished sociality, can derive pleasure from a social interaction. Additional comparisons of laboratory experiments with concurrent field experiments showed that while maturing wild juveniles gradually foraged at increasing distances from one another, a behavioral pattern that predicts dispersal, captive juveniles simultaneously expressed diminished social approach and increased play fighting (Lahvis et al. 2015). Taken together, this comparison supports the idea that the adolescent thirteen-lined ground squirrel can experience social reward and that social motivation diminishes as maturing adolescents begin to disperse, an idea akin to the “ontogenetic switch” (Holekamp 1984).
Social reward propels individuals into social proximity in the wild under conditions that are more expansive and more patchy than the laboratory cage. Social proximity facilitates opportunities for individuals to learn from others through interactions between demonstrator and observer, teacher and learner. Social reward also motivates communication with others, the expression of cues and receptivity to the cues of others that distinguishes social interaction from an encounter with a physical object. In a natural environment, individuals must be sufficiently motivated to attend to the alarm calls, tail flicks, abrupt stops in eating behaviors, and upright stances of their alert conspecifics.
Social motivation may also enhance the learning process. For instance, in a door opening experiment, observer mice must learn from a demonstrator mouse to swing a door to the left to obtain a food reward (Collins 1988). Adult males more readily learn to open the door from adult females than from other males. Perhaps males more closely attend to adult females and this added attention improves the learning process. In the wild, social learning can be an efficient alternative to untutored trial-and-error learning. For instance, naïve red squirrels must to learn to consume hickory nuts in a fashion that requires minimal investment of time and effort (i.e., energy). When introduced to hickory nuts in the presence of an experienced squirrel, naïve squirrels learn more efficient techniques for consuming the nuts, suggesting that red squirrels can learn by observation (Weigl and Hanson 1980).
In summary, the conditioned place preference test shows that rodents can derive pleasure from a social interaction, preferring to spend time in an environment that predicts access to peers versus an environment that predicts isolation. Experiences of social reward and illicit drug reward share common brain circuitry. In seeking the pleasure derived from social reward, or in avoiding the adversity of social isolation, individuals would be more likely to sleep and forage together and engage in social communication.
4 Empathy
Empathy is the generation of an affective state more appropriate to the situation of another compared to one’s own (Hoffman 1975; Preston and de Waal 2002). In popular usage, empathy is jumbled with feelings of compassion or with behaviors often associated with compassion: acts of kindness, helpful acts, or displays of sorrow for a victim. Empathy is not a compassionate feeling nor is it a helping behavior. Empathy involves adopting the feelings of another: fear, joy, pain, anxiety, appreciation, or distain.
Empathy is not always a strictly affective experience and can include various levels of cognitive function. More extreme examples arise from abstractions, such as the experience you may feel upon learning that over two millennia ago, the armies of Alexander the Great conquered the city of Thebes, killed the majority of its inhabitants, sold the remaining 30,000 into slavery, and then burned the city to the ground. Even during this cognitive process, you might feel suffering, desperation, and anger.
Empathy
The generation of an affective state more appropriate to the situation of another compared to one’s own. This change in affective state is not compassion and does not necessarily result in the expression of an altruistic act.
A term often associated with empathy is emotional contagion, which is not an affective experience. Rather, emotional contagion refers to an individual’s spontaneous expression of a behavior that resembles the behavior expressed by another individual. Jointly expressed behaviors might include two people yawning together or several babies crying in unison. A classic rodent experiment that demonstrates emotional contagion involves different concentrations of acetic acid injected into two mice. When isolated, a mouse injected with a lower concentration of the irritant acetic acid writhes at a subdued level relative to an isolated mouse injected with a higher concentration of acetic acid. When these two mice are placed together, their responses change. The mouse exposed to the higher concentration of irritant writhes less. The mouse exposed to the lower concentration of irritant writhes more (Langford et al. 2006). This convergence of behavior also occurs when the paws of mice are injected with different concentrations of formalin. The frequency of paw licking is greater for a solitary mouse exposed to high concentrations of formalin, and diminishes if that mouse is placed next to a mouse exposed to lower concentrations of formalin (Langford et al. 2006).
As mentioned, emotional contagion suggests an affective experience but the term actually refers to a behavior, a tendency to automatically mimic and synchronize expressions, vocalizations, postures, and movements with those of another individual (Hatfield et al. 1994). The behavior suggests affective convergence but may include alternative explanations. For instance, in the Langford experiments, the mouse experiencing greater levels of abdominal pain may suppress its writhing frequency to match the behavior of its more comfortable partner, thereby masking its own expression of weakness. Likewise, social facilitation might serve as an alternative explanation for the increased writhing behavior of the more comfortable individual.
Emotional contagion
A reflexive behavioral change within the context of a motivationally salient event in which an individual spontaneously expresses a behavior that resembles the behavior expressed by another individual.
Irrespective of the emotions experienced by the two writhing mice, they appear to adjust their converging behaviors based upon visual cues expressed in the writhing of the nearby conspecific. Visual cues of emotions among rodents might also be signaled by facial expressions, which are responsive to painful stimuli (Langford et al. 2010). Facial expressions in rodents can indicate positive and negative affective states (Kelley and Berridge 2002). Olfactory cues, such as urine odors, and vocalizations also serve to signal emotional changes in rodents. When a mouse is offered two cotton balls, one soaked with urine from a recently shocked conspecific and the other soaked with the urine of an undisturbed conspecific, the mouse avoids the cotton ball soaked in urine of its shocked conspecific (Rottman and Snowdon 1972). More recent work shows that the urine of an alarmed mouse releases volatile molecules that evoke in others increased systemic corticosterone levels (Brechbühl et al. 2013).
Social CPP experiments help us to determine if a rodent derives pleasure from a social interaction by assessing whether its access to a social reward in a particular environment changes the affective salience of that paired environment. Vicarious fear learning experiments are used to infer empathy in a rodent, whether observation of a conspecific in pain in a particular environment changes the affective salience of the paired environment. In context-dependent vicarious fear learning experiments, demonstrator rodents are repeatedly exposed to a distressing stimulus (an electrical shock) within a chamber and they begin to freeze for short periods, nearly motionless and often trembling in place, and this response becomes more frequent as they are delivered more shocks. These responses reflect a change in the rodent’s affective state because the rodent expresses freezing behavior primarily in the chamber where it experiences the shock, but not in other environments. In context-dependent vicarious fear learning experiments, rodents hear the vocalizations of conspecifics undergoing repeated shocks in a nearby chamber. When these rodents are in turn placed within the chamber, they express increased freezing behavior. The subjects acquire the affective fear experience of their distressed conspecifics to the context, the chamber, during the conditioning phase, which is subsequently expressed during the test phase of the experiment (Jeon and Shin 2011).
In cue-conditioned vicarious fear learning experiments, subjects become fearful of a repeated temporal cue, such as a tone, paired with an aversive stimulus, usually a shock, applied to the rodent in an adjacent chamber. In our experiments, a 30-s tone co-terminated with a 2-s electrical shock is applied to demonstrator mice inside the shock chamber, followed by 90s of silence. With each shock, the mice emit audible vocalizations that sound to the human ear like a squeak. These mouse vocalizations lack nuance, appearing on a spectrogram as undefined broadband noise (Chen et al. 2009), suggesting an expulsion of air, and lacking the audible nuance and precise overtone frequency modulation of referential alarm calls, such as those emitted by prairie dogs to reference predators (Kiriazis and Slobodchikoff 2006) or the 22-kHz vocalizations emitted by rats when they are shocked (Atsak et al. 2011). With repeated tone-shock pairings, mice inside the shock chamber freeze when they hear the tone. This change in behavior represents an affective experience analogous to a fear response in anticipation of an imminent shock.
After demonstrators are conditioned, subjects are placed inside the shock chamber. When they hear the tone, they freeze. This behavioral response suggests that the subject mice have learned from the conditioned demonstrators that the tone predicts an aversive experience. In other words, the affective experiences of the subject mice, garnered while observing the demonstrator mice undergo fear conditioning, alter their subsequent responses to the tone inside the shock chamber. The vocalizations emitted by the demonstrator mice when they are shocked are sufficient to engender fear in the subject mice. When experimenters replace demonstrators with playbacks of 2-second recordings of squeaks, these vocalizations alone are sufficient to induce a freezing response in mice (Chen et al. 2009). While playbacks of mouse vocalizations are sufficient for vicarious fear conditioning, playbacks of 22-kHz vocalizations emitted by rats during shock do not necessarily engender freezing behaviors by observer rats (Atsak et al. 2011), suggesting a role among rats for other signaling modalities during vicarious fear experiments.
An individual human more readily feels empathy for another after having experienced a similar pain in the past (Batson et al. 1996; Eklund et al. 2009; Preis and Kroener-Herwig 2012). Likewise, rodent subjects generally require one experience with the shock prior to demonstrator conditioning (Atsak et al. 2011; Chen et al. 2009; Sanders et al. 2013; but see Jeon et al. 2010). The observing subject rodent is delivered one shock that is not paired with the tone or the unique context, prior to observing the demonstrator undergo conditioning. If the subject is temporarily deafened during its single experience with the shock, so that it cannot hear its own vocalization in response to the shock, the subject then fails to vicariously learn from the vocalizations of demonstrators that the tone predicts a shock (Jeon et al. 2010). This result is also underscored by evidence that rats learn to freeze in response to 22 kHz USVs through autoconditioning, associating their own USVs with an internal fear state (Parsana et al. 2012). Experiments showing that subjects freeze in response to the tone only in the shock chamber, not the observation chamber, suggest that subjects have a sense of place, recognizing that the distress associated with shock is dependent upon their physical position within one chamber of the two-chambered test structure.
When demonstrator vocalizations are heard by subjects, they activate dopamine and serotonin circuits (Kim et al. 2014) within the anterior cingulate cortex (ACC) (Jeon et al. 2010), activity patterns that can be lateralized in rodents (Kim et al. 2012) and share similarity with the ACC activity in human subjects, as revealed by functional MRI (Singer et al. 2004, 2006).
One important caveat should be mentioned here. Experiments that use etho- logically relevant threats may not require direct individual experience with the aversive stimulus. Mice bury themselves in cage bedding to escape from biting flies after they observe a single successful escape (Kavaliers et al. 2001). These subjects also express decreased pain sensitivity, suggesting they sense the pain experienced by the demonstrators.
Vicarious fear learning and social CPP tests share similarities. In both tests, the rodent encounters an affective experience under a specific set of temporal or contextual conditions. Both tests require classical conditioning; the subject must learn to associate a particular environmental cue or context with a specific affective experience, such as pain, reward, or fear. With repeated pairings of the cue or context with the affective experience, the subject learns that when it encounters the cue or context, it will also feel the reward, fear, or pain. The subject learns that certain affective experiences are contingent upon encountering specific environmental conditions. Since the test phase occurs at a time or place separate from the conditioning phase, the rewarding or aversive experience is carried as an affective memory, coloring the subject’s affective response to the test conditions and moderating its behavioral response. Through conditioning experiments, we infer affective experience because, when properly controlled, variability in the rodent’s behavioral response is dependent upon the retained affective experience.
Social reward brings individuals into proximity, catalyzing opportunities for interactions between demonstrator and observer. Again, social motivation likely includes attending to the behaviors of others, such as the tail flicks, standing upright, and abrupt stops in eating that might be expressed by conspecifics foraging nearby. In these situations, empathy also has a functional role in survival and reproduction. In response to the threat not directly perceived, empathy elicits arousal from these cues of others, engendering a more robust behavioral response to an urgent situation than what might be expressed with mere cognitive understanding.
In the wild, alarm signals can signify distress and referential information (Klump and Shalter 1984) and the extent that they represent differences in affective arousal versus cognitive referencing can be difficult to dissociate. For instance, under threat, chickadees emit characteristic “chicka-dee-dee” calls. More “dee” syllables correlate inversely with raptor wingspan and smaller raptors are more successful hunters, capable of pursuing their prey with a tighter turning radius (Templeton et al. 2005). It is unclear in this example whether chickadee alarm calls are proportional to their levels of arousal, but in other instances, “semantic” or referential information is communicated (Marler et al. 1992). Prairie dog alarm calls can be easily distinguished according to subtle variations in frequency modulation, differentiating raptors from humans, coyotes, and domesticated dogs, and each call elicits a predator-specific evasion technique (Fredericksen and Slobodchikoff 2007). Prairie dogs also appear to modulate their alarm calls for differences in the colored shirts worn by humans (Slobodchikoff et al. 2009), signaling differences that are less likely to be affected by variations in arousal.
Empathy might also be adaptive for juvenile play behavior, particularly rough- and-tumble play, or play fighting, which is common among juvenile and adolescent mammals. Rough-and-tumble play requires sensitivity to the moment-to-moment condition of the playmate: too hard a bite on the nape, too hard a flip, not a vigorous enough counter approach, too quick on the return from a tumble, not enough responsiveness to the moves of the playmate, and play fighting ends. Like vicarious fear learning, vocalizations play a prominent role in communicating between social participants. Emission of 50-kHz USV maintains playful contact among conspecifics in rats (Himmler et al. 2014; Kisko et al. 2015a, b). Play fighting is sensitive to opiate administration (Vanderschuren et al. 1997), suggesting hedonia is associated with this self-versus-other, back-and-forth activity (Panksepp 1998). Explanations for the energy demanding behavior among wild mammals include its role in improving emotional responsiveness to unexpected events (Nunes et al. 1999; Spinka et al. 2001), familiarizing participants with self-handicap and fair behavior (Bekoff 2004), improving abilities to cope with social challenges (van den Berg et al. 1999), establishing dominance relationships (Blumstein et al. 2013), and helping refine abilities to respond to subtle and ambiguous social signals (Pellis et al. 2010). The give-and-take of social play promotes normal brain development (Gordon et al. 2003; Pellis and Pellis 2007).
Empathy also supports nurturing behavior. USVs emitted by infant mice solicit maternal care and both pup generation of calls and maternal responses are sensitive to opiates, dopamine, and serotonin (D’Amato et al. 2005; Dastur et al. 1999; Moles et al. 2004), suggesting that USV emission and their behavioral responses require changes in affective state.
Empathy can be useful for social learning. For instance, juvenile black-tailed prairie dogs express appropriate levels of arousal and avoidance to predators after observing an experienced wild conspecific adult, and relative to unschooled controls, these captive-trained juveniles are more likely to survive in the wild after their release (Shier and Owings 2007). Empathy might also aid in social learning by helping an individual feel into the intent of the individual observed. An early model of social learning described the process as “from an act witnessed, learn to perform that act” (Thorndike 1911), emphasizing observation of an action and not explicitly dissociating it from conceptualizing the goal (Thorndike 1911 and see Galef 2013 for thorough review). This process is called imitation, learning from observations how to perform the form of a novel behavior (production imitation) or a familiar act in an unfamiliar context (contextual imitation) (Janik and Slater 1997; Galef 2013). Rats can learn to push a joystick (Heyes et al. 1994) or swing open a door (Collins 1988) in a particular direction after watching a trained conspecific. In these kinds of experiments, the action and the goal seem one and the same.
Some now question imitation as a the sole means for social learning. By simply watching others, one cannot learn Tai Chi and downhill skiing (Galef 2013). An alternative idea is that social learning may inherently require trial-and-error learning and understanding of the goal to reproduce a behavior, a concept called “emulation” (Tomasello et al. 1993; Galef 2013; Zentall and Galef 1988). Experiments with puzzle boxes can help uncouple imitation from emulation by requiring learners to reproduce the goal of the behavior (Galef 2013). Puzzle boxes are intricate devices with solutions that include multiple behavioral steps, revealing several processes involved in social learning: trial and error, social exposure, and stimulus enhancement (Valsecchi et al. 2002). For example, mice can learn from others to manipulate a puzzle box, pushing a metal tab on its side with a paw to release food into a bin and then opening a drawer in front of the box to recover the food (Carlier and Jamon 2006; Valsecchi et al. 2002). To the extent that an observer experiences the intent of the demonstrator, empathy is involved in emulation.
Imitation
Learning from observation to perform the form of a novel behavior or a familiar behavior in an unfamiliar context.
Emulation
Learning from observation that a goal, the result of an observed action, is achievable, followed by trial-and-error learning to achieve the goal.
5 Helping Behavior
Since the late 1950s, experimental psychology studies have shown that laboratory rats help their conspecifics. George Rice and Priscilla Gainer built a structure that could hoist a rat in a harness, suspended so that its paws could not quite touch the floor, hung in a pendent condition that likely causes distress. A subject rat then had the opportunity to lower the suspended rat to the floor. Given a choice, the subject was more likely to press a lever to relieve its pendent and distressed conspecific versus an alternate lever to acquire a food reward, a chocolate chip (Rice and Gainer 1962).
In more recent experiments, rat subjects were given opportunity to release a conspecific held inside a small container. Faced with the dilemma of whether to free the constrained rat or gain access to chocolate, subjects were more likely to free their distressed conspecifics and share the chocolate (Bartal et al. 2011; Bartal et al. 2014). Authors state that these helping behaviors were “empathically motivated,” a perhaps untenable inference because empathy does not by itself confer motivation to obtain a reward or to avoid an aversive experience. Consider empathy within the context of the conditioned place preference test, an experimental measure that allows us to draw inferences about the feelings that drive motivation. Empathic experience would not drive a rodent to predictably spend more time exploring one CPP-conditioned environment versus another. Rather, the direction of a rodent’s preference for a particular environment would depend entirely upon the nature of the experience felt in that shared environment, whether the shared experience was inherently pleasurable or aversive, influenced by the valence of the conspecific’s affective state.
The argument that empathy motivates the subject to free the rat distressed by confinement also requires evidence that restraint within the small container is aversive and that the subject is more likely to help if it previously experienced the same aversive condition (Greene 1969). When the experiments described above were repeated and elaborated by others (Silberberg et al. 2014), rats confined within the container and then freed and given choice to move inside and outside the container returned to move in and out. This finding is not consistent with the contention that constraint inside the container was an aversive experience (Silberberg et al. (2014) . Critically, constrained rats in the original study emitted 22-kHz USVs and the frequency of these vocalizations strongly suggests that the constrained rats experienced an aversive condition. These experiments might be reconciled if we consider that prolonged and unelected restraint is aversive but when the subject has agency to move inside and outside the container, the rat no longer finds the context unpleasant. The rat might quickly realize the container door stays open. Further, if we assume for the sake of discussion that constraint was an aversive experience, a second important condition for empathy remains unclear: whether the subject would be more likely to free the constrained rat were it to experience the same aversive condition. Subjects in this experiment were not constrained prior to testing. An alternative explanation for these results is that the subject was motivated to release the conspecific to gain access to a social reward (Silberberg et al. 2014).
In a different helping experiment, a subject rat opened a door to allow a wet rat to escape from a pool, whereupon the subject often shared its food (Sato et al. 2015). In this experiment, helper rats were more likely to help if they had been soaked themselves, a response that suggests that empathy could play a role in this helping behavior. In other experiments, rat subjects gave their conspecifics access to food without any personal benefit (Hernandez-Lallement et al. 2014; Márquez et al. 2015).
Unlike their wild conspecifics, laboratory rodents do not experience resource restriction. In nature, resources fluctuate with years of bounty and others of paucity, unpredictably driving natural selection (Grant and Grant 2002). While ultimate explanations for altruistic behaviors in the wild attempt to reconcile helping behavior with natural selection (Graw and Manser 2007; Krams et al. 2006; Templeton et al. 2005; Wilkinson 1984), laboratory animals are afforded ad libitum access to food and shelter, so they do not face similar selective pressures. Instead, the standard laboratory animal cage imposes a different set of constraints upon the maturation of brain development and behavior, including an extreme poverty of temporal and spatial variation in food availability and quality, lack of social refuge, and invariant social structure (Lahvis 2016). Therefore, we must be cognizant of possible differences in the underlying affective conditions that engender expressions of helping behavior in the laboratory versus the wild.
6 Camaradarie Effect
Grief can take care of itself, but to get the full value of joy you must have somebody to divide it with.
Mark Twain
The popular phrase “survival of the fittest” is a tautology that literally means survival of the one who survives the best and, for some, conjures notions of wild animals motivated by an ongoing desire to acquire and defend food items, territory, and mating opportunities. Despite the pressure of natural selection, wild animals also express altruistic behaviors, actions that involve payment of a personal cost, at least in the short term, to benefit other individuals (Roberts 2005). To some, these helping behaviors suggest feelings of compassion. Conditioning experiments can help us dissociate competitive and helping behaviors from their underlying feelings, but we need to first step back again and consider explanations for behaviors expressed by wild animals.
Non-human animal behaviors in nature can be explained in ultimate and proximal terms, ranging from why a behavior might be adaptive for survival and reproduction (an ultimate explanation) to how a developmental or biological mechanism, such as a neuropeptide released in brain region, supports its expression (a proximal explanation) (Tinbergen 1963). Ultimate explanations for biological altruism include, but are not limited to, kin selection (Hamilton 1964), direct reciprocity (Trivers 1971), indirect reciprocity (Nowak and Sigmund 2005), handicap models (Griffin and West 2003), multilevel selection (Wilson 1997; Traulsen and Nowak 2006), and by-product benefits (Leimar and Connor 2003). Each of these models explains how the stability of an altruistic behavior is maintained under specific social conditions (Nowak 2006; West et al. 2007). For instance, kin selection requires that the altruistic individual be genetically related to a sufficient proportion of benefiting recipients that carry alleles for the altruistic behavior. Also helpful is the capacity of the individual to discriminate kin from non-kin (Griffin and West 2003; Hamilton 1964). Direct reciprocity requires opportunities for repeated social interactions so that recipients can reciprocate the help they receive (Trivers 1971). Reciprocity also requires that the recipient can associate the identity of the helping actor with the helping behavior and also remember either the act itself or, in light of the discussion above, associate the actor with a positive interpersonal affective experience. Multilevel selection requires that the altruistic individual helps members of its own group, which competes with other groups, to indirectly gaining access to resources at another group’s expense (Traulsen and Nowak 2006). These social conditions vary with species identity, environmental harshness (Barash 1974), and social constraints and include the varied durations individuals share within a common environment, such as the temporary social arrangements of migrating birds (Wheatcroft and Price 2008).
Explanations for cooperation and altruism also include handicap and indirect reciprocity models that require a third party to witness whether the actor aids the recipient. Subsequent interactions between the actor and the witnesses are again sensitive to whether or not the actor initially provided help (Nowak and Sigmund 2005). Responses might include increased mating opportunities favoring an altruistic actor (Griffin and West 2003) and social punishment for an actor that did not help, including a decision not to reciprocate aid (Krams et al. 2008). When breeding pied flycatchers are experimentally restricted from helping in predator defense, they are not helped in return (Krams et al. 2006).
These ultimate models may not fully explain why individuals engage in altruistic behaviors—such as when they are sensitive to freeloaders that take advantage of altruistic behaviors and chose not to reciprocate (Roberts 2005). To ensure the stability of altruistic behavior, it would be ideal if an additional mechanism could buttress its adaptive value. Were the actor to have an additional “stake” in the well-being of the recipient, its long-term interests would be served as a secondary consequence even if the helping behavior is not reciprocated (Roberts 2005).
Could affective experience serve as an actor’s stake in potential recipients?
Reciprocity and handicap models require that the recipient and third-party witnesses have a memory of the actor’s identity and experience one of at least two mental processes: either a cognitive score sheet of the actor’s provision or denial of help—or an affective experience associated with the actor’s previous decision to offer or deny help. In models that require reciprocal action (help the actor that chooses to help, do not help the actor that chooses to deny help), these associated affective experiences might serve to motivate behaviors that favor or punish the actor.
If the actor were to have a capacity for empathy, would it be able to predict social threats by sensing the feelings of others? Could a difference in attitudes experienced and subtly expressed by the helped or unaided recipients and their witnesses serve as a palpable reward or punishment for the actor? CPP experiments show that laboratory rodents can express a strong preference for environments that predict future social interactions (Calcagnetti and Schechter 1992; Douglas et al. 2004) and avoid environments paired with social isolation (Panksepp and Lahvis 2007). In this regard, laboratory rodents may be similar to humans, who can have a basic need for social connections and find social separation or rejection psychologically painful (Eisenberger and Lieberman 2005). While domestication can engender prosocial behaviors that do not exist in the wild, experiments with captive ground squirrels show that undomesticated rodents can also derive pleasure from access to social interactions (Lahvis et al. 2015), indicating that social reward is a natural motivation.
For individuals living in groups, social ostracism may confer substantial costs to an outcast individual. Emmigration from a colony is associated with high mortality risks (Koopman et al. 2000; Pocock et al. 2005). Dispersal is most common for adolescents, particularly young males. Depending upon species, maturational age, and environment, adolescent dispersal may be explained both by diminished motivation of the adolescents for social interaction, indicated by an abrupt decrease in play behavior and increase in aggression (Festa-Bianchet and King 1984), and as a response to social ostracism. For instance, adult squirrels can act aggressively toward maturing adolescents, chasing them away (Festa-Bianchet and King 1984). Adults in marmot colonies can violently ostracize adolescents that fail to participate in a morning greeting (Barash 1974).
Ostracism can also extend to individuals or groups that fail to cooperate with the larger social structure. A wolf pack will reject an individual that mates with a subordinate wolf (Peterson 1979) and chimpanzees will shun individuals appearing abnormal (Goodall 1986). Social rejection is also found in rodent societies. Female Norway rats reject males that copulate with anestrous females (Galef et al. 2008).
How does an individual respond to the potential for ostracism when a motivation for social reward, for living with others, is coupled with empathy, the generation of an affective state more appropriate to the situation of another? Overt chasing behavior or violent aggression might not be required for an individual to feel social exclusion. Empathy would amplify an actor’s own feelings of fear, pain, or distress when witnesses harbor enmity or indifference to the actor for past decisions, engendering painful experiences in the actor in anticipation of social rejection. Likewise, helping decisions that cement social acceptance would increase an actor’s positive affective experience within the group. In rats, gene expression is enhanced in fear-related areas when rats hear 22-kHz vocalizations, USVs that are associated with pain and aggression (Sadananda et al. 2008). Empathy may also augment the positive affective experiences garnered when conspecifics are comforted by an altruistic act. When a rat hears affiliative 50-kHz USVs, gene expression is activated in reward-related areas of the brain (Sadananda et al. 2008) and dopamine is released in the nucleus accumbens (Willuhn et al. 2014), a physiological correlate of a reward experience (Kelley and Berridge 2002; Smith and Berridge 2007). These affective experiences might help build strong social bonds that confer a variety of reproductive benefits (Lahvis et al. 2015; Seyfarth and Cheney 2013).
Together, social motivation and empathy could be considered a camaraderie effect, a proximal explanation for biological altruism whereby an actor seeks social access and avoids social isolation, sensitive to the feelings of social acceptance or rejection that others hold for the actor (Lahvis et al. 2015). With social reward, an individual becomes susceptible to the actions of others; with empathy, these sensitivities expand to include the feelings of others, such as growing affinity or enmity, and social motivation engenders the drive to promote positive over negative feelings.
The camaraderie effect might have emerged in the evolution of mammalian psychology as a by-product of social reward and empathy. Both affective states are heritable (Chen et al. 2009; Panksepp and Lahvis 2007) and thus sensitive to natural selection. The camaraderie effect may have emerged from these related traits that offer considerable adaptive value, appearing as an evolutionary “spandrel.”
The concept of a spandrel comes from a seminal essay that uses the ceiling supports of San Marco’s Cathedral in Venice as a metaphor for the adaptive value of various biological traits (Gould and Lewontin 1979). The spandrels of San Marco’s Cathedral are marvelous tapering triangles that are demarcated by the base of the cathedral’s dome and the curves of the supporting arches beneath the dome that are perpendicular to one another (see Fig. 2). Spandrels add esthetic value to the cathedral, beautiful in their subtle shape and in the images painted on their surfaces, but they serve no primary architectural function. Similarly, while social reward and empathy are adaptive experiences, the camaraderie effect may have emerged as a spandrel, arising only in consequence as self-sustaining. Among humans, social rejection may be unhealthy, increasing salivary cortisol levels (Blackhart et al. 2007). Social isolation also confers psychological and physiological costs to rodents. Social isolation impairs brain development (Black and Greenough 1998; Champagne and Curley 2005; Wiedenmayer 2009), immune reactivity (Boissy et al. 2007; Shanks et al. 1994; Tuchscherer et al. 2010), healing from burns and wounds (Detillion et al. 2004; İşeri et al. 2010), response to ischemia (Norman et al. 2010), recovery from social defeat (Ruis et al. 1999), resiliency to metastasis (Wu et al. 2000), social-emotional health (Seffer et al. 2015), and competence in social hierarchies (van den Berg et al. 1999). With empathy, psychological rejection or indifference could serve as a psychological surrogate for social isolation, resulting in physiological impairments similar to what might be expected from ostracism.
Fig. 2.
The camaraderie effect may have evolved as a by-product, or a spandrel, of two adaptive psychological capacities, social reward and empathy. Social reward supports a motivation for living in groups and is responsive to aggressive and affiliative social cues. The affective states included in social motivation include isolation aversion and social reward. Social reward and isolation aversion are two of four affective states that can be inferred from social CPP testing. Other states include social aversion and isolation reward and may play a role in voluntary dispersal. The survival value of social reward is that it supports group living. Empathy is useful for predicting future events from social cues, such as the presence or absence of a threat in the environment from cues of calm or fear. In turn empathy engenders an affective state of vicarious calm or vicarious fear, so one can be alert to dangers not perceived directly or one can be calm under situations felt to be safe by others. In combination, social reward and empathy can generate the camaraderie effect, a process whereby vicarious feelings of others toward the actor can be either discomforting or can engender a sense of well-being. In turn, these psychological states may affect physiological health. These ideas are presented as text over a photograph of a spandrel in San Marco Cathedral, showing social reward and empathy as adaptive psychological states, represented by the arches holding up the dome with the camaraderie effect as a spandrel between these functional and highly adaptive arches
The psychology of the witnesses and their feelings of animosity toward the unhelpful actor, perhaps in anticipation of physical ostracism, might also incur a psychological (and hence physiological) cost for the ostracizers, as these norm enforcers also deprive themselves of camaraderie. Yet ostracizers would still benefit from camaraderie with other group members, whereas the ostracized actor, experiencing more universal rejection, would bear a larger burden of the costs.
By helping others, an individual sustains a feeling of camaraderie, a sense of well-being that augments one’s own health and reproductive success. Perhaps this combination of social reward and empathy at times even obfuscates an individual’s ability to distinguish self-interest from the interests of others. For instance, when a rat witnesses another rat consuming a highly palatable food reward, dopamine is released into the ventral striatum (Kashtelyan et al. 2014), suggesting a vicarious feeling of reward.
Taken together, these findings offer a proximal mechanism for maintaining altruism as a stable behavioral phenotype. This proximal stake in others, this interdependence, or camaraderie effect, offers a necessary sustenance for altruism genes within wild communities.
Camaraderie Effect
A proximal explanation for biological altruism whereby social reward and empathy (feeling what others feel) together promote feelings of well-being that result from helping others via the positive affect of the recipient and witnesses. This sense of well-being in turn improves one’s own immunological responses to pathogens, burns, and wounds, and resiliency to ischemia, social defeat, and metastasis.
Summary
A feeling of camaraderie exists when an individual derives pleasure from social interaction and can experience what others feel, including the positive feelings others may have toward the individual. Witnesses of a single altruistic or selfish act, or perhaps repeated actions, associate a favorable or unfavorable affective experience with the actor’s identity, an affective experience that resurfaces when witnesses re-encounter the actor. With social affect, an individual becomes susceptible to the actions of others. We know that social isolation, even in rodents, can compromise immune responses and diminish wound repair. With empathy, the actor can predict future outcomes from the feelings of others, including the ability to predict social acceptance or physical rejection from the feelings of affinity or enmity harbored by group members. These changes in affective state, akin to interpersonal stances between witnesses and the actor, influence the nature of ongoing social interactions. In turn, the actor’s affective state can bolster or compromise physiological resiliency, resulting in the sustainability of the camaraderie effect as a mechanism for altruistic behavior. The camaraderie effect may thus play a substantial role as an essential stake that an actor holds in the well-being and affinity of others.
References
- Armitage KB. Sociality as a life-history tactic of ground squirrels. Oecologia. 1981;48(1):36–49. doi: 10.1007/BF00346986. [DOI] [PubMed] [Google Scholar]
- Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Keysers C. Experience modulates vicarious freezing in rats: a model for empathy. Stress Cognit. 2011;6:17. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baack JK, Switzer PV. Alarm calls affect foraging behavior in eastern chipmunks (Tamias striatus, Rodentia: Sciuridae) Ethology. 2000;106(12):1057–1066. [Google Scholar]
- Barash DP. The evolution of marmot societies: a general theory. Science. 1974;185(4149):415–420. doi: 10.1126/science.185.4149.415. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bartal IB-A, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334(6061):1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartal IB-A, Rodgers DA, Sarria MSB, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. Elife. 2014;3:e01385. doi: 10.7554/eLife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD, Sympson SC, Hindman JL, Decruz P, Todd RM, Weeks JL, Burns CT. “I’ve been there, too”: effect on empathy of prior experience with a need. Pers Soc Psychol Bull. 1996;22(5):474–482. [Google Scholar]
- Bekoff M. Wild justice and fair play: cooperation, forgiveness, and morality in animals. Biol Philos. 2004;19(4):489–520. [Google Scholar]
- Belloni V, Dessi-Fulgheri F, Zaccaroni M, Di Consiglio E, De Angelis G, Testai E, Santucci D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology. 2011;279(1–3):19–26. doi: 10.1016/j.tox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Benton D, Brain S, Brain PF. Comparison of the influence of the opiate delta receptor antagonist, ICI 154,129, and naloxone on social interaction and behaviour in an open field. Neuropharmacology. 1984;23(1):13–17. doi: 10.1016/0028-3908(84)90210-7. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Lahvis GP. The autism diagnosis in translation: shared affect in children and mouse models of ASD. Autism Res. 2011;4(5):317–335. doi: 10.1002/aur.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Greenough WT. Developmental approaches to the memory process. In: Martinez JL Jr, Kesner RP, editors. Neurobiology of learning and memory. Academic Press: San Diego; 1998. pp. 55–88. [Google Scholar]
- Blackhart GC, Eckel LA, Tice DM. Salivary cortisol in response to acute social rejection and acceptance by peers. Biol Psychol. 2007;75(3):267–276. doi: 10.1016/j.biopsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28(4):437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Steinmetz J, Armitage KB, Daniel JC. Alarm calling in yellow-bellied marmots: II. The importance of direct fitness. Anim Behav. 1997;53(1):173–184. [Google Scholar]
- Blumstein DT, Chung LK, Smith JE. Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris) Proc R Soc B: Biol Sci. 2013;280(1759):20130485. doi: 10.1098/rspb.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, Keeling LJ, Aubert A. Assessment of positive emotions in animals to improve their welfare. Physiol Behav. 2007;92(3):375–397. doi: 10.1016/j.physbeh.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430(7001):778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Boreman J, Price E. Social dominance in wild and domestic Norway rats (Rattus norvegicus) Anim Behav. 1972;20(3):534–542. [Google Scholar]
- Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33(3):249–256. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Brechbühl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, Broillet M-C. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci. 2013;110(12):4762–4767. doi: 10.1073/pnas.1214249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR. Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 2005;35(1):67–72. doi: 10.1007/s10519-004-0856-5. [DOI] [PubMed] [Google Scholar]
- Butler RG. Population size, social behaviour, and dispersal in house mice: a quantitative investigation. Anim Behav. 1980;28(1):78–85. [Google Scholar]
- Calamandrei G, Venerosi A, Branchi I, Valanzano A, Alleva E. Prenatal exposure to anti-HIV drugs. Long-term neurobehavioral effects of lamivudine (3TC) in CD-1 mice. Neurotoxicol Teratol. 2000;22(3):369–379. doi: 10.1016/s0892-0362(00)00063-5. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51(4):667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Camacho F, Sandoval C, Paredes R. Sexual experience and conditioned place preference in male rats. Pharmacol Biochem Behav. 2004;78(3):419–425. doi: 10.1016/j.pbb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, Koolhaas JM. Is hyper-aggressiveness associated with physiological hypoarousal? A comparative study on mouse lines selected for high and low aggressiveness. Physiol Behav. 2008;95(4):591–598. doi: 10.1016/j.physbeh.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Carden SE, Bortot AT, Hofer MA. Ultrasonic vocalizations are elicited from rat pups in the home cage by pentylenetetrazol and U50,488, but not naltrexone. Behav Neurosci. 1993;107(5):851–859. doi: 10.1037//0735-7044.107.5.851. [DOI] [PubMed] [Google Scholar]
- Carlier P, Jamon M. Observational learning in C57BL/6j mice. Behav Brain Res. 2006;174(1):125–131. doi: 10.1016/j.bbr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Chambers LK, Singleton GR, Krebs CJ. Movements and social organization of wild house mice (Mus domesticus) in the wheatlands of northwestern Victoria, Australia. J Mammal. 2000;81(1):59–69. [Google Scholar]
- Champagne FA, Curley JP. How social experiences influence the brain. Curr Opin Neurobiol. 2005;15(6):704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS ONE [Electronic Resource] 2009;4(2) doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cognit Sci. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL. Observational learning of a left-right behavioral asymmetry in mice (Mus musculus) J Comp Psychol. 1988;102(3):222–224. doi: 10.1037/0735-7036.102.3.222. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35(1):103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. Oxford University Press; New York: 1872. [Google Scholar]
- Darwin C. The descent of man, and selection in relation to sex. Murray; John London: 1888. [Google Scholar]
- Dastur FN, McGregor IS, Brown RE. Dopaminergic modulation of rat pup ultrasonic vocalizations. Eur J Pharmacol. 1999;382(2):53–67. doi: 10.1016/s0014-2999(99)00590-7. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29(8):1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Gowaty PA, Holmes CM. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav. 2000;59(2):371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- Ebert PD. Agonistic behavior in wild and inbred Mus musculus. Behavioral Biology. 1976;18(2):291–294. doi: 10.1016/s0091-6773(76)92214-8. [DOI] [PubMed] [Google Scholar]
- Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (mus musculus): Wriggling calls release maternal behavior. Anim Behav. 1986;34(3):821–830. [Google Scholar]
- Eisenberger NI, Lieberman MD. Why it hurts to be left out: the neurocognitive overlap between physical and social pain. The social outcast: Ostracism, social exclusion, rejection, and bullying. 2005:109–130. [Google Scholar]
- Eklund J, Andersson-Stratberg T, Hansen EM. “I’ve also experienced loss and fear”: effects of prior similar experience on empathy. Scand J Psychol. 2009;50(1):65–69. doi: 10.1111/j.1467-9450.2008.00673.x. [DOI] [PubMed] [Google Scholar]
- Ekman PE, Davidson RJ. The nature of emotion: fundamental questions. Oxford University Press; Oxford: 1994. [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, et al. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008:16. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa-Bianchet M, King WJ. Behavior and dispersal of yearling Columbian ground squirrels. Can J Zool. 1984;62(2):161–167. [Google Scholar]
- Fitzgerald B, Karl B, Moller H. Spatial organization and ecology of a sparse population of house mice (Mus musculus) in a New Zealand forest. J Anim Ecol. 1981:489–518. [Google Scholar]
- Fredericksen J, Slobodchikoff C. Referential specificity in the alarm calls of the black-tailed prairie dog. Ethol Ecol Evolut. 2007;19:87–99. [Google Scholar]
- Galef BG. Imitation and local enhancement: detrimental effects of consensus definitions on analyses of social learning in animals. Behav Process. 2013;100:123–130. doi: 10.1016/j.beproc.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Lim TC, Gilbert GS. Evidence of mate choice copying in norway rats, Rattus norvegicus. Anim Behav. 2008;75(3):1117–1123. [Google Scholar]
- Gariepy JL, Gendreau PL, Cairns RB, Lewis MH. D1 dopamine receptors and the reversal of isolation-induced behaviors in mice. Behav Brain Res. 1998;95(1):103–111. doi: 10.1016/s0166-4328(97)00215-5. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Schiff BB. A biological theory of reinforcement. Psychol Rev. 1967;74(2):81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- Goodall J. Social rejection, exclusion, and shunning among the Gombe chimpanzees. Ethol Sociobiol. 1986;7(3):227–236. [Google Scholar]
- Gordon NS, Burke S, Akil H, Watson SJ, Panksepp J. Socially-induced brain ‘fertilization’: play promotes brain derived neurotrophic factor transcription in the amygdala and dorsolateral frontal cortex in juvenile rats. Neurosci Lett. 2003;341(1):17–20. doi: 10.1016/s0304-3940(03)00158-7. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B: Biol Sci. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin’s finches. Science. 2002;296(5568):707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Graw B, Manser MB. The function of mobbing in cooperative meerkats. Anim Behav. 2007;74(3):507–517. [Google Scholar]
- Greene JT. Altruistic behavior in the albino rat. Psychon Sci. 1969;14(1):47–48. [Google Scholar]
- Griffin AS, West SA. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302(5645):634–636. doi: 10.1126/science.1089402. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Amaral D, Aschner M, Bolivar VJ, Bowman A, DiCicco-Bloom E, Threadgill DW. Animal models of autism spectrum disorders: information for neurotoxicologists. Neurotoxicology. 2009;5:811–821. doi: 10.1016/j.neuro.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behavior, I and II. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Cromwell HC, Burgdorf J, Moskal JR, Brudzynski SM, Kroes RA, Panksepp J. Rats selectively bred for low levels of 50 kHz ultrasonic vocalizations exhibit alterations in early social motivation. Dev Psychobiol. 2008;50(4):322–331. doi: 10.1002/dev.20294. [DOI] [PubMed] [Google Scholar]
- Hatfield E, Cacioppo JT, Rapson RL. Emotional contagion. Cambridge University Press; Cambridge: 1994. [Google Scholar]
- Hernandez-Lallement J, van Wingerden M, Marx C, Srejic M, Kalenscher T. Rats prefer mutual rewards in a prosocial choice task. Front Neurosci. 2014:8. doi: 10.3389/fnins.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C, Jaldow E, Nokes T, Dawson G. Imitation in rats (Rattus norvegicus): the role of demonstrator action. Behav Process. 1994;32(2):173–182. doi: 10.1016/0376-6357(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Himmler B, Kisko T, Euston D, Kolb B, Pellis S. Are 50-kHz calls used as play signals in the playful interactions of rats? I. Evidence from the timing and context of their use. Behav Process. 2014;106:60–66. doi: 10.1016/j.beproc.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Developmental synthesis of affect and cognition and its interplay for altruistic motivation. Dev Psychol. 1975;11:607–622. [Google Scholar]
- Holekamp K. Dispersal in ground-dwelling sciurids. In: Murie JO, Michener GR, editors. Biology of ground-dwelling squirrels: annual cycles, behavioral ecology, and sociality. University of Nebraska Press; Lincoln: 1984. pp. 297–320. [Google Scholar]
- Hoogland JL. Aggression, ectoparasitism, and other possible costs of prairie dog (sciurudae, cyonmys spp.) coloniality. Behaviour. 1979;69(1/2):1–35. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. I. Communication between males. Anim Behav. 1990;40(2):209–222. [Google Scholar]
- İşeri SÖ, Düşünceli F, Erzik C, Uslu B, Arbak S, Yeğen BÇ. Oxytocin or social housing alleviates local burn injury in rats. J Surg Res. 2010;162(1):122–131. doi: 10.1016/j.jss.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Janik VM, Slater PJB. Vocal learning in mammals. Adv Stud Behav. 1997;26:59–99. [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43(4):503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Jeon D, Shin HS. A mouse model for observational fear learning and the empathetic response. Curr Protoc Neurosci. 2011:8–27. doi: 10.1002/0471142301.ns0827s57. [DOI] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin S-Y, Shin H-S. Observational fear learning involves affective pain system and Cav1. 2 Ca2+ channels in ACC. Nat Neurosci. 2010;13(4):482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Peréz M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS ONE. 2012;7(11):e48975. doi: 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtelyan V, Lichtenberg NT, Chen ML, Cheer JF, Roesch MR. Observation of reward delivery to a conspecific modulates dopamine release in ventral striatum. Curr Biol. 2014;24(21):2564–2568. doi: 10.1016/j.cub.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD. Odours of parasitized males induce aversive responses in female mice. Anim Behav. 1995;50(5):1161–1169. [Google Scholar]
- Kavaliers M, Choleris E, Colwell DD. Learning from others to cope with biting flies: social learning of fear-induced conditioned analgesia and active avoidance. Behav Neurosci. 2001;115(3):661–674. [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Wong JC, Krause EJ, Lahvis GP. Age- and strain-dependent influences of morphine on mouse social investigation behavior. Behav Pharmacol. 2011;22(2):147–159. doi: 10.1097/FBP.0b013e328343d7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mátyás F, Lee S, Acsády L, Shin H-S. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci. 2012;109(38):15497–15501. doi: 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Lee J, Bang M, Am Seo B, Khalid A, Jung MW, Jeon D. Differential regulation of observational fear and neural oscillations by serotonin and dopamine in the mouse anterior cingulate cortex. Psychopharmacology. 2014;231(22):4371–4381. doi: 10.1007/s00213-014-3581-7. [DOI] [PubMed] [Google Scholar]
- Kiriazis J, Slobodchikoff C. Perceptual specificity in the alarm calls of Gunnison’s prairie dogs. Behav Process. 2006;73(1):29–35. doi: 10.1016/j.beproc.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Kisko TM, Euston DR, Pellis SM. Are 50-khz calls used as play signals in the playful interactions of rats? III. The effects of devocalization on play with unfamiliar partners as juveniles and as adults. Behav Process. 2015a;113:113–121. doi: 10.1016/j.beproc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Kisko TM, Himmler BT, Himmler SM, Euston DR, Pellis SM. Are 50-kHz calls used as play signals in the playful interactions of rats? II. Evidence from the effects of devocalization. Behav Process. 2015b;111:25–33. doi: 10.1016/j.beproc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Klump GM, Shalter MD. Acoustic behaviour of birds and mammals in the predator context; I. factors affecting the structure of alarm signals. II. The functional significance and evolution of alarm signals. Zeitschrift fur Tierpsychologie. 1984;66(3):189–226. doi: 10.1111/j.1439-0310.1984.tb01365.x. [DOI] [Google Scholar]
- Koopman ME, Cypher BL, Scrivner JH. Dispersal patterns of San Joaquin kit foxes (Vulpes macrotis mutica) J Mammal. 2000;81(1):213–222. 213. [Google Scholar]
- Krackow S, Matuschak B. Mate choice for non-siblings in wild house mice: evidence from a choice test and a reproductive test. Ethology. 1991;88(2):99–108. [Google Scholar]
- Krams I, Krama T, Iguane K. Mobbing behaviour: reciprocity-based co-operation in breeding Pied Flycatchers Ficedula hypoleuca. Ibis. 2006;148(1):50–54. [Google Scholar]
- Krams I, Krama T, Igaune K, Mand R. Experimental evidence of reciprocal altruism in the pied flycatcher. Behav Ecol Sociobiol. 2008;62(4):599–605. [Google Scholar]
- Lahvis GP. Rodent models of autism, epigenetics, and the inescapable problem of animal constraint. In: Kim Y-K, Gewirtz J, editors. Animal Models of Behavior Genetics. Springer Publishing Company; New York: 2016. pp. 265–301. 395. [Google Scholar]
- Lahvis GP, Black LM. Social interactions in the clinic and the cage: toward a more valid mouse model of autism animal models of behavioral analysis, v50. 2011:153–192. [Google Scholar]
- Lahvis GP, Panksepp JB, Kennedy BC, Wilson CR, Merriman DK. Social conditioned place preference in the captive ground squirrel (Ictidomys tridecemlineatus) social reward as a natural phenotype. J Comp Psychol. 2015;129(3):291. doi: 10.1037/a0039435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312(5782):1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7(6):447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Leimar O, Connor RC. By-product benefits, reciprocity, and pseudoreciprocity in mutualism. Genet Cult Evol Cooperation. 2003:203–222. [Google Scholar]
- Liu H-X, Lopatina O, Higashida C, Fujimoto H, Akther S, Inzhutova A, Yoshihara T. Displays of paternal mouse pup retrieval following communicative interaction with maternal mates. Nat Commun. 2013;4:1346. doi: 10.1038/ncomms2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH., Jr Quantifying the phenotype in autism spectrum disorders. Am J Med Genet. 2001;105(1):36–38. [PubMed] [Google Scholar]
- Manning CJ, Potts WK, Wakeland EK, Dewsbury DA. What’s wrong with MHC mate choice experiments? In: Doty RL, Muller-Schwarze D, editors. Chemical signals in vertebrates. Plenum; New York: 1992. pp. 229–235. [Google Scholar]
- Markova G, Legerstee M. Contingency, imitation, and affect sharing: Foundations of infants’ social awareness. Dev Psychol. 2006;42(1):132. doi: 10.1037/0012-1649.42.1.132. [DOI] [PubMed] [Google Scholar]
- Marler P, Evans CS, Hauser MD. Animal signals: motivational, referential, or both. Nonverbal vocal communication: comparative and developmental approaches. 1992:66–86. [Google Scholar]
- Márquez C, Rennie SM, Costa DF, Moita MA. Prosocial choice in rats depends on food-seeking behavior displayed by recipients. Curr Biol. 2015;25(13):1736–1745. doi: 10.1016/j.cub.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Martinez M, Guillen-Salazar F, Salvador A, Simon VM. Successful intermale aggression and conditioned place preference in mice. Physiol Behav. 1995;58(2):323–328. doi: 10.1016/0031-9384(95)00061-m. [DOI] [PubMed] [Google Scholar]
- Mateo JM, Holmes WG. Plasticity of alarm-call response development in Belding’s ground squirrels (Spermophilus beldingi, Sciuridae) Ethology. 1999;105:193–206. doi: 10.1046/j.1439-0310.1999.00389.x. [DOI] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115(3):683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- McCarley H. Annual cycle, population dynamics and adaptive behavior of Citellus tridecemlineatus. J Mammal. 1966;47:294–316. [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene.[see comment] Science. 2004;304(5679):1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Moy S, Nadler J, Perez A, Barbaro R, Johns J, Magnuson T, Crawley J. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy S, Nadler J, Young N, Nonneman R, Grossman A, Murphy D, Lauder J. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behavior. 2009;8(2):129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler J, Moy S, Dold G, Trang D, Simmons N, Perez A, Crawley J. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behavior. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24(12):713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci. 2010;107(37):16342–16347. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314(5805):1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437(7063):1291–1298. doi: 10.1038/nature04131. [DOI] [PubMed] [Google Scholar]
- Nunes S, Muecke E-M, Anthony JA, Batterbee AS. Endocrine and energetic mediation of play behavior in free-living belding’s ground squirrels. Horm Behav. 1999;36(2):153–165. doi: 10.1006/hbeh.1999.1538. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience. Oxford University Press; Oxford: 1998. [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6(7):661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Jochman K, Kim JU, Koy JJ, Wilson ED, Chen Q, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsana AJ, Moran EE, Brown TH. Rats learn to freeze to 22-kHz ultrasonic vocalizations through autoconditioning. Behav Brain Res. 2012;232(2):395–399. doi: 10.1016/j.bbr.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Rough-and-tumble play and the development of the social brain. Curr Dir Psychol Sci. 2007;16(2):95–98. [Google Scholar]
- Pellis SM, Pellis VC, Reinhart CJ. The evolution of social play. In: Wothman C, Plotsky P, Schecter D, Cummings CA, editors. Formative experiences: the interaction of caregiving, culture, and developmental psychobiology. Cambridge University Press; Cambridge: 2010. pp. 404–431. [Google Scholar]
- Penn DJ, Potts WK. The evolution of mating preferences and major histocompatibility complex genes. Am Nat. 1999;153(2):145–164. doi: 10.1086/303166. [DOI] [PubMed] [Google Scholar]
- Peterson RO. Social rejection following mating of a subordinate wolf. J Mammal. 1979;60(1):219–221. [Google Scholar]
- Pocock MJO, Hauffe HC, Searle JB. Dispersal in house mice. Linn Soc Biol J. 2005;84(3):565–583. [Google Scholar]
- Preis M, Kroener-Herwig B. Empathy for pain: the effects of prior experience and sex. Eur J Pain. 2012;16(9):1311–1319. doi: 10.1002/j.1532-2149.2012.00119.x. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25(1):1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Rice GE, Gainer P. “Altruism” in the albino rat. J Comp Physiol Psychol. 1962;55(1):123. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- Roberts G. Cooperation through interdependence. Anim Behav. 2005;70:901–908. doi: 10.1016/j.anbehav.2005.02.006. [DOI] [Google Scholar]
- Roberts SA, Davidson AJ, Beynon RJ, Hurst JL. Female attraction to male scent and associative learning: the house mouse as a mammalian model. Anim Behav. 2014;97:313–321. [Google Scholar]
- Rottman SJ, Snowdon CT. Demonstration and analysis of an alarm pheromone in mice. J Comp Physiol Psychol. 1972;81(3):483–490. doi: 10.1037/h0033703. [DOI] [PubMed] [Google Scholar]
- Ruis M, Te Brake J, Buwalda B, De Boer S, Meerlo P, Korte S, Koolhaas J. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24(3):285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Russell B. The problems of philosophy. 1997. Oxford University Press; Oxford: 1912. [Google Scholar]
- Sadananda M, Wöhr M, Schwarting RK. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett. 2008;435(1):17–23. doi: 10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Sanders J, Mayford M, Jeste D. Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS ONE. 2013;8(9):e74609. doi: 10.1371/journal.pone.0074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59(5):415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn. 2015;18(5):1039–1047. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Vocal affect expression: a review and a model for future research. Psychol Bull. 1986;99(2):143. [PubMed] [Google Scholar]
- Scherer KR. Vocal communication of emotion: a review of research paradigms. Speech Commun. 2003;40(1):227–256. [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. Toward a second-person neuroscience. Behav Brain Sci. 2013;36(04):393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. Nebraska Press; Lincoln: 1959. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Seffer D, Rippberger H, Schwarting RK, Wöhr M. Pro-social 50-kHz ultrasonic communication in rats: post-weaning but not post-adolescent social isolation leads to social impairments—phenotypic rescue by re-socialization. Front Behav Neurosci. 2015:9. doi: 10.3389/fnbeh.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Affiliation, empathy, and the origins of theory of mind. Proc Natl Acad Sci. 2013;110(Supplement 2):10349–10356. doi: 10.1073/pnas.1301223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Renton C, Zalcman S, Anisman H. Influence of change from grouped to individual housing on a T-cell-dependent immune response in mice: Antagonism by diazepam. Pharmacol Biochem Behav. 1994;47(3):497–502. doi: 10.1016/0091-3057(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Shier D, Owings D. Effects of social learning on predator training and postrelease survival in juvenile black-tailed prairie dogs, Cynomys ludovicianus. Anim Behav. 2007;73(4):567–577. [Google Scholar]
- Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H, Slotnick B. Desire for social contact, not empathy, may explain “rescue” behavior in rats. Anim Cogn. 2014;17(3):609–618. doi: 10.1007/s10071-013-0692-1. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodchikoff CN, Paseka A, Verdolin JL. Prairie dog alarm calls encode labels about predator colors. Anim Cogn. 2009;12(3):1435–9448. doi: 10.1007/s10071-008-0203-y. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Graham DF, Yuva-Paylor LA, Nelson DL, Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122(3):710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- Spinka M, Newberry RC, Bekoff M. Mammalian play: training for the unexpected. Q Rev Biol. 2001;76(2):143–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- Templeton CN, Greene E, Davis K. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science. 2005;308(5730):1934–1937. doi: 10.1126/science.1108841. [DOI] [PubMed] [Google Scholar]
- Thonhauser KE, Raveh S, Hettyey A, Beissmann H, Penn DJ. Scent marking increases male reproductive success in wild house mice. Anim Behav. 2013;86(5):1013–1021. doi: 10.1016/j.anbehav.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL. Animal Intelligence. New York: Hafner; 1911. [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Zeitschrift fur Tierpsychologie. 1963;20:410–433. [Google Scholar]
- Tomasello M, Kruger AC, Ratner HH. Cultural learning. Behav Brain Sci. 1993;16:495–552. [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci. 2005;28(05):675–691. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Traulsen A, Nowak MA. Evolution of cooperation by multilevel selection. Proc Natl Acad Sci. 2006;103(29):10952–10955. doi: 10.1073/pnas.0602530103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:189–226. [Google Scholar]
- Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A. Altered immunomodulation by glucocorticoids in neonatal pigs exposed to a psychosocial stressor. Pediatr Res. 2010;68(6):473–478. doi: 10.1203/PDR.0b013e3181f70f08. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valsecchi P, Singleton GR, Price WJ. Can social behaviour influence food preference of wild mice, Mus domesticus, in confined field populations? Austr J Zool. 1996;44(5):493–501. [Google Scholar]
- Valsecchi P, Bosellini I, Sabatini F, Mainardi M, Fiorito G. Behavioral analysis of social effects on the problem-solving ability in the house mouse. Ethology. 2002;108(12):1115–1134. [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34(2):129–138. doi: 10.1002/(sici)1098-2302(199903)34:2<129:aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Pee JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Weigl PD, Hanson EV. Observational learning and the feeding behavior of the red squirrel Tamiasciurus hudsonicus: the ontogeny of optimization. Ecology. 1980:214–218. [Google Scholar]
- West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17(16):R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wheatcroft DJ, Price TD. Reciprocal cooperation in avian mobbing: playing nice pays. Trends Ecol Evol. 2008;23(8):416–419. doi: 10.1016/j.tree.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP. Plasticity of defensive behavior and fear in early development. Neurosci Biobehav Rev. 2009;33(3):432–441. doi: 10.1016/j.neubiorev.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Reciprocal food sharing in the vampire bat. Nature. 1984;308(5955):181–184. [Google Scholar]
- Willuhn I, Tose A, Wanat MJ, Hart AS, Hollon NG, Phillips PE, Wöhr M. Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. J Neurosci. 2014;34(32):10616–10623. doi: 10.1523/JNEUROSCI.1060-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS. Altruism and organism: disentangling the themes of multilevel selection theory. Am Nat. 1997;150(S1):s122–S134. doi: 10.1086/286053. http://doi.org/10.1086/286053. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology. 1995;121(2):164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T + tf/J mouse model of autism. Genes Brain Behav. 2011a;10(1):35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE. 2011b;6(6):e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff RJ. Mating behavior and female choice: their relation to social structure in wild caught house mice (mus musculus) housed in a semi-natural environment. J Zool Ser A. 1985;207(1):43–51. [Google Scholar]
- Wu W, Yamaura T, Murakami K, Murata J, Matsumoto K, Watanabe H, Saiki I. Social isolation stress enhanced liver metastasis of murine colon 26-L5 carcinoma cells by suppressing immune responses in mice. Life Sci. 2000;66(19):1827–1838. doi: 10.1016/s0024-3205(00)00506-3. [DOI] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(8):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR, Galef BD., Jr . Social learning: psychological and biological perspectives. Lawrence Erlbaum Associates Inc; Hillsdale: 1988. [Google Scholar]