Abstract
Purpose
Inpatient palliative care integrated with transplant care improves patients’ quality of life (QOL) and symptom burden during hematopoietic stem-cell transplant (HCT). We assessed patients’ mood, post-traumatic stress disorder (PTSD) symptoms, and QOL 6 months post-transplant.
Methods
We randomly assigned 160 patients with hematologic malignancies who underwent autologous or allogeneic HCT to inpatient palliative care integrated with transplant care (n = 81) or transplant care alone (n = 79). At baseline and 6 months post-transplant, we assessed mood, PTSD symptoms, and QOL with the Hospital Anxiety and Depression Scale and Patient Health Questionnaire, PTSD checklist, and Functional Assessment of Cancer Therapy-Bone Marrow Transplant. To assess symptom burden during HCT, we used the Edmonton Symptom Assessment Scale. We used analysis of covariance while controlling for baseline values to examine intervention effects and conducted causal mediation analyses to examine whether symptom burden or mood during HCT mediated the effect of the intervention on 6-month outcomes.
Results
We enrolled 160 (86%) of 186 potentially eligible patients between August 2014 and January 2016. At 6 months post-transplant, intervention participants reported lower depression symptoms on the Hospital Anxiety and Depression Scale and Patient Health Questionnaire (adjusted mean difference, −1.21 [95% CI, −2.26 to −0.16; P = .024] and −1.63 [95% CI, −3.08 to −0.19; P = .027], respectively) and lower PTSD symptoms (adjusted mean difference, −4.02; 95% CI, −7.18 to −0.86; P = .013), but no difference in QOL or anxiety. Symptom burden and anxiety during HCT hospitalization partially mediated the effect of the intervention on depression and PTSD at 6 months post-transplant.
Conclusion
Inpatient palliative care integrated with transplant care leads to improvements in depression and PTSD symptoms at 6 months post-transplant. Reduction in symptom burden and anxiety during HCT partially accounts for the effect of the intervention on these outcomes.
INTRODUCTION
High-dose chemotherapy followed by hematopoietic stem-cell transplant (HCT) is an intensive and potentially curative treatment for many patients with hematologic malignancies.1-4 The majority of transplants in the United States are performed in the inpatient setting, with patients receiving chemotherapy followed by stem-cell transplant during a 3- to 4-week hospitalization.5 Patients who undergo HCT endure substantial physical symptoms as a result of chemotherapy-induced toxicities and early post-transplant complications.6-11 These physical symptoms, along with the physical isolation patients experience during the prolonged hospitalization, contribute to a rapid deterioration in quality of life (QOL) and mood during HCT.10
Patients’ physical and psychological symptoms during HCT also have negative consequences on their long-term well-being.7,12,13 Studies demonstrate that patients’ transplant experience predicts their psychological morbidity and risk of transplant-related complications months after hospitalization.12-14 Many HCT survivors develop depression and post-traumatic stress disorder (PTSD) symptoms as a consequence of this experience.7,12,13,15 Of note, as many as 41% of HCT survivors experience PTSD symptoms up to 10 years post-transplant.16-21 These long-term psychological complications further compound the morbidity of HCT. Thus, improving patients’ experience during their hospitalization for HCT may positively affect their psychological distress and QOL post-transplant.22
Despite the short- and long-term burden that HCT causes patients, interventions to improve their QOL and reduce their psychological distress are lacking.6,10,23-25 We recently completed a single-center randomized trial of inpatient palliative care integrated with transplant care versus transplant care alone in patients with hematologic malignancies who underwent HCT.26 Patients who received the inpatient palliative care intervention reported improvement in their QOL, symptom burden, and depression and anxiety symptoms during their hospitalization; however, the impact of the intervention on their long-term psychological distress and QOL is unknown.
In this analysis, we investigated the effect of inpatient palliative care integrated with transplant care on patient-reported psychological distress and QOL at 6 months post-transplant. We hypothesized that patients who receive the intervention have fewer depression and PTSD symptoms and better QOL at 6 months than those who receive standard transplant care alone. We also explored whether improvement in symptom burden or mood during HCT hospitalization mediates the effect of the intervention on patient-reported outcomes at 6 months post-transplant.
METHODS
Study Procedure
From August 12, 2014, to January 26, 2016, we enrolled 160 patients with hematologic malignancies admitted for HCT at Massachusetts General Hospital in a nonblinded randomized controlled trial of inpatient palliative care integrated with standard transplant care compared with standard transplant care alone.26 We identified consecutively eligible patients with a planned HCT admission and enrolled 80 scheduled to undergo autologous transplant and 80 to undergo allogeneic transplant. A research assistant obtained permission by e-mail from the treating oncologist to approach eligible patients within 72 hours of admission. Willing patients provided written informed consent and completed baseline study questionnaires within the 72 hours. We then registered the participants through the Quality Assurance Office for Clinical Trials, which randomly assigned them to either inpatient palliative care integrated with transplant care (intervention group) or standard transplant care alone (control group). We used computer-generated 1:1 randomization stratified by type of HCT. Participants completed subsequent study questionnaires during the second week of hospitalization and at 3 and 6 months post-transplant. Two-week and 3-month outcomes are previously reported.26 In this article, we report the 6-month outcomes. The study was approved by the Dana-Farber Harvard Cancer Center Institutional Review Board.
Participants
Patients (age ≥ 18 years) with the ability to speak English or complete questionnaires with minimal assistance were eligible to participate. We excluded patients with a prior history of HCT and significant psychiatric or comorbid disease that prohibited adherence to study procedures.
Palliative Care Intervention
Participant assigned to inpatient palliative care integrated with transplant care met with the inpatient palliative care physician or advanced practice nurse within 72 hours of random assignment. The palliative care clinician followed patients throughout their hospitalization by seeing them at least twice per week. Participants and palliative care clinicians were permitted to initiate additional visits as needed. Participants did not have outpatient palliative care follow-up after discharge.
Three palliative care clinicians (two nurse practitioners and one physician) conducted all palliative care intervention visits. They underwent a half-day training focused on addressing the main topics covered by the intervention, which are previously reported.26 After each encounter, the palliative care clinicians documented the following topics covered during the visit: establishing rapport, addressing patients’ physical and psychological symptoms, enhancing coping efforts, and addressing patients’ illness understanding. The median duration of transplant hospitalization was 20 days (range, 13 to 102 days). The median number of palliative care visits was eight (range, four to 40).
Standard Transplant Care
Control participants received standard transplant care, with the transplant team instituting supportive care measures. Participants and transplant clinicians were permitted to request consultation with palliative care. Of note, only two participants randomly assigned to the standard transplant care group received a palliative care consultation.
Study Measures
Participants completed study questionnaires prior to random assignment (baseline) during the second week of hospitalization for HCT (ie, at the blood count nadir when they experience the lowest blood counts and highest symptom burden during HCT) and at 3 and 6 months post-transplant.
Patient-Reported Measures
We measured anxiety and depression symptoms with the 14-item Hospital Anxiety and Depression Scale (HADS). The HADS comprises two subscales that assess anxiety (HADS-A) and depression (HADS-D), with scores ranging from 0 (no distress) to 21 (maximum distress).27 A score > 7 on the HADS-A or HADS-D represents clinically significant anxiety or depression, respectively.27 We also assessed mood by using the Patient Health Questionnaire 9 (PHQ-9), a nine-item measure that detects symptoms of major depressive disorder according to the criteria of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders.28 A major or other depressive syndrome is diagnosed if a patient reports at least two of the nine symptoms of depression on the PHQ-9, with one of the symptoms being anhedonia or depressed mood.28
We used the Post-Traumatic Stress Disorder Checklist-Civilian Version (PCL) to measure PTSD symptoms.16 The PCL is a 17-item measure that evaluates the severity of PTSD symptoms, with higher scores indicating worse symptoms. A PCL score ≥ 32 represents clinically significant PTSD symptoms.16
We used the 47-item Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) to assess QOL.29 The FACT-BMT comprises five subscales that assess physical, functional, emotional, and social well-being as well as BMT-specific concerns. A five-point change in the FACT-BMT is considered clinically significant.10,29 We measured fatigue by using the 13-item FACT-Fatigue subscale.30
To assess symptom burden during HCT hospitalization, we administered the revised Edmonton Symptom Assessment Scale (ESAS),31 which measures 10 symptoms on a scale of 0 to 10, with higher scores indicating greater symptom burden. As a result of a clerical error, the first 38 study participants did not complete the nausea item, which was thus omitted from the composite ESAS score analyses.
Demographic and Clinical Factors
Participants also completed a baseline demographic questionnaire to indicate their race, sex, relationship status, education, and income. We reviewed their electronic health records to obtain cancer diagnosis, comorbidities, and date of transplant. For each participant, we calculated the HCT Comorbidity Index32 at the time of their transplant consultation.
Statistical Analysis
We performed statistical analyses with STATA 9.3 software (StataCorp, College Station, TX). The study was powered for the primary outcome of change in patient-reported QOL from baseline to week 2 of hospitalization (reported in a separate article).26 For all analyses, we considered a two-sided P < .05 to be statistically significant. Given that these secondary and exploratory analyses are meant to be hypothesis generating, we did not adjust P values for multiple testing.
We used analysis of covariance (ANCOVA) models that controlled for baseline criterion scores to assess the effect of the intervention on patient-reported outcomes at 6 months post-transplant on the basis of available patients without accounting for missing data. Although ANCOVA was not a prespecified per-protocol analysis, it is the preferred approach to analyzing the effect of the intervention on 6-month outcomes.33 By using standard cutoffs, we also compared the rates of clinically significant depression, anxiety, and PTSD symptoms between the two groups at 6-months post-transplant using Fisher’s exact test.
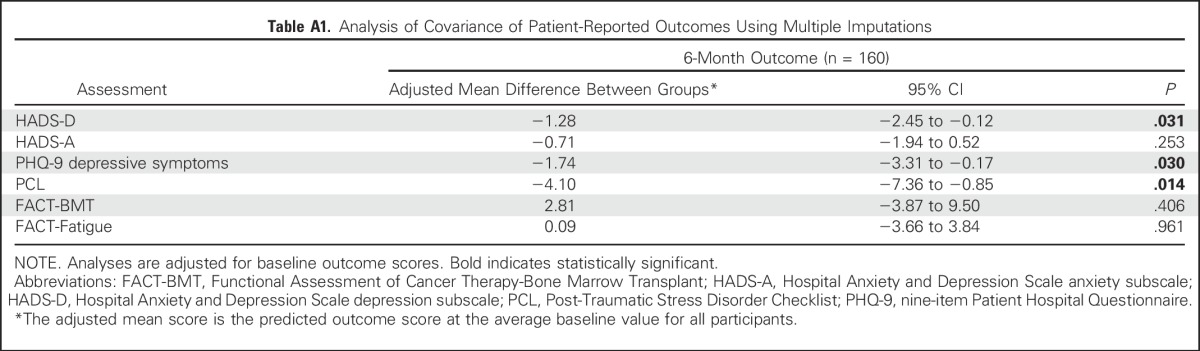
We used multiple imputations for missing observations to account for missing data. Specifically, the multiple imputation approach used baseline characteristics (age, sex, transplant type, HCT Comorbidity Index, and performance status) to build a regression model to impute missing outcomes data with 50 imputations. We then used ANCOVA models that controlled for baseline scores on the imputed data set to assess the intervention effects on patient-reported outcomes at 6 months post-transplant. To account for missing outcome as a result of death, we also used the partly conditional modeling framework34,35 to analyze patient-reported outcomes conditional on participants being alive.
We performed a series of causal mediation analyses to explore whether symptom burden or mood during hospitalization for HCT mediated the effect of the intervention on 6-month outcomes that were significantly different between the two groups (PTSD symptoms and depression).36,37 For each outcome, mediation regression models were constructed with group assignment (intervention versus control) as the independent variable and symptom burden (ESAS), depression (HAD-D), and anxiety (HADS-A) during hospitalization for HCT (week 2) as the mediator variables. All models controlled for baseline values of patient-reported outcomes of interest. Mediator variables were examined in separate regression analyses given potential overlap between symptom burden and mood. Bootstrap estimates of the average causal mediation effect, direct effect, and total effect were interpreted with 95% CIs. By definition, a CI that does not contain zero indicates that the effect is statistically significant. Because depression during hospitalization is highly correlated with depression outcomes after transplant, examination of depression symptoms during hospitalization as a mediator of the effect of the intervention on depression outcomes at 6 months post-transplant would not be appropriate. Given their exploratory nature, mediation analyses were performed on all available data without imputations.
RESULTS
Participant Characteristics
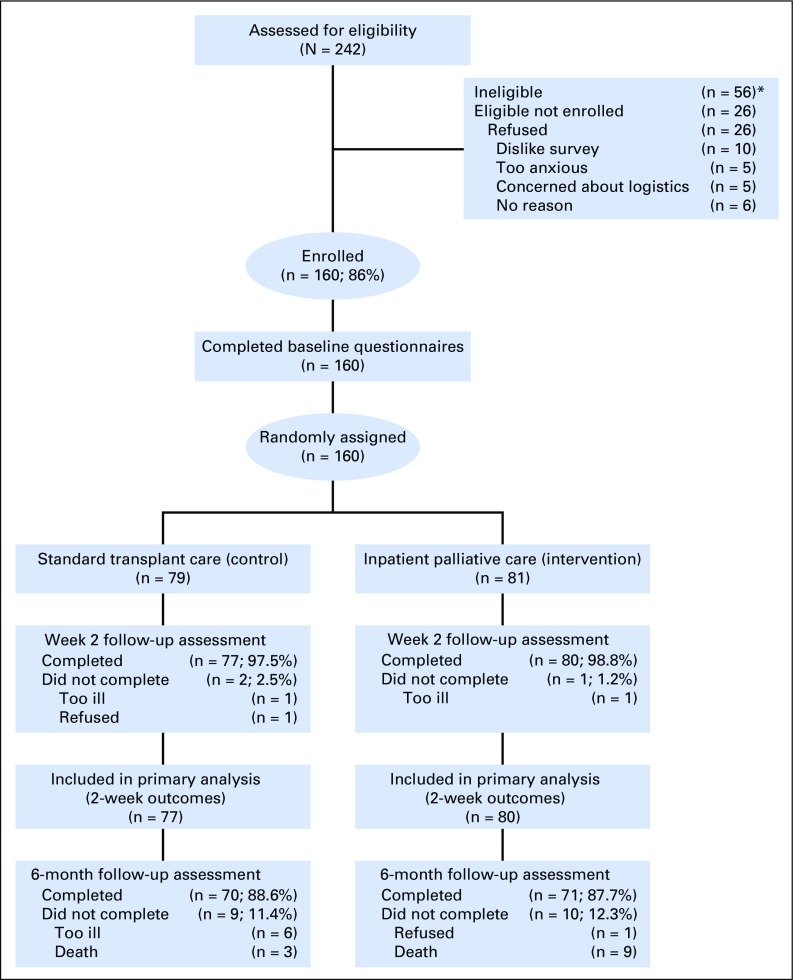
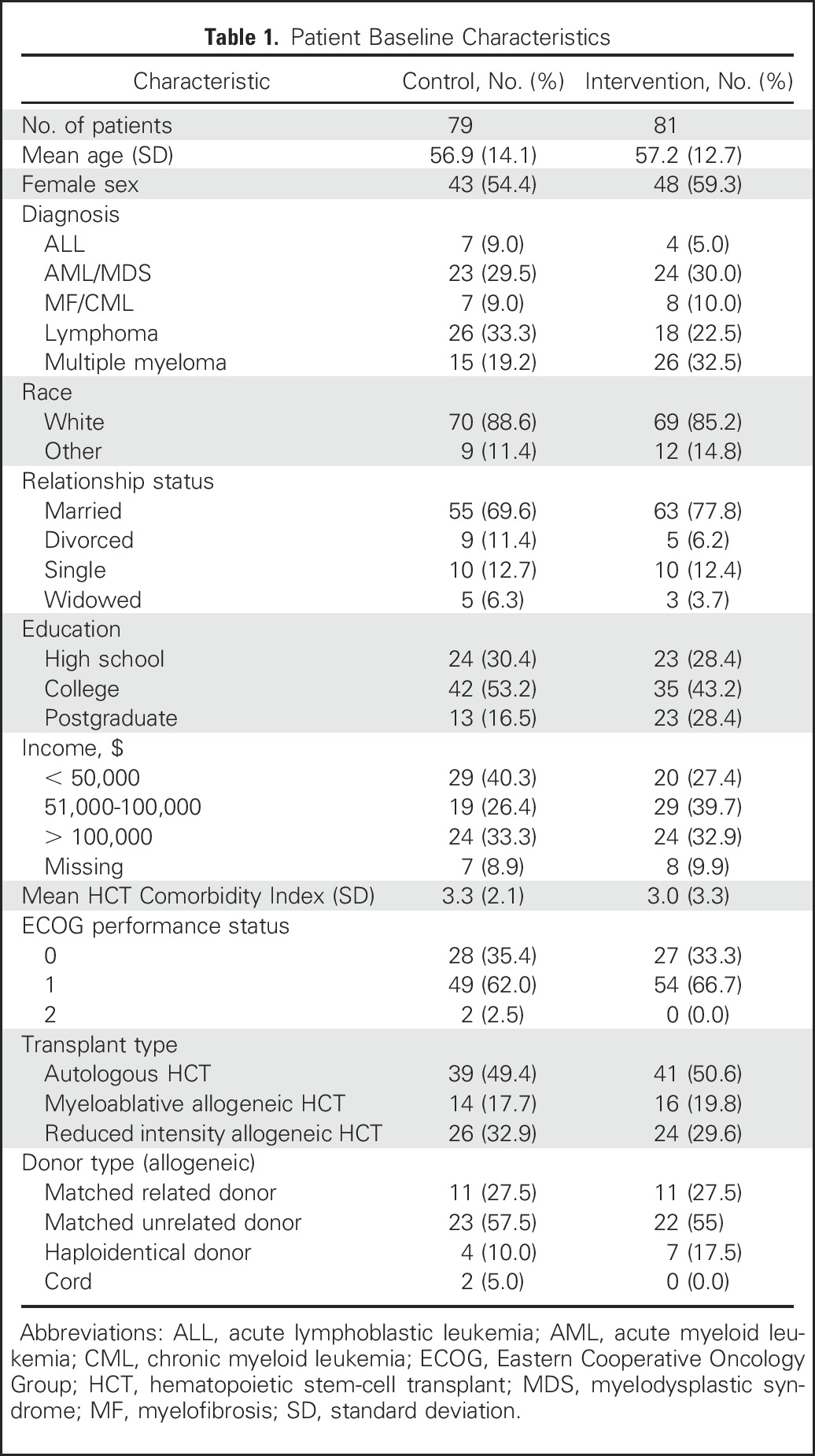
We approached 186 eligible patients and enrolled 160 (86%; Fig 1). Enrolled participants were mostly white (86.9%) with a mean age of 57.1 years (standard deviation, 13.3 years); 56.9% were female (Table 1). Clinical characteristics and baseline self-report measures did not differ meaningfully between study groups. At 6 months post-transplant, 11.3% (nine of 81) and 11.4% (nine of 79) of participants randomly assigned to palliative care intervention and standard transplant care had missing data, respectively.
Fig 1.
CONSORT diagram. (*)Reasons for ineligibility: language barrier (n = 10), benign disease (n = 6), previous HCT (n = 15), clinician refusal (n = 2), participation in another supportive care trial (n = 8), transplant aborted within 24 h of admission (n = 6), combined solid organ and hematopoietic stem-cell transplant (n = 3), primarily outpatient transplant (n = 6).
Table 1.
Patient Baseline Characteristics
Patient-Reported Outcomes at 6 Months Post-Transplant
Table 2 lists the results of available analyses of patient-reported outcomes at 6 months post-transplant. After controlling for baseline scores, participants assigned to the palliative care intervention reported lower depression symptoms as measured by the HADS-D (adjusted mean difference, −1.21; 95% CI, −2.26 to −0.16; P = .024) and PHQ-9 (adjusted mean difference, −1.63; 95% CI, −3.08 to −0.19; P = .027] than those who received standard transplant care alone. In addition, patients assigned to the palliative care intervention compared with standard transplant care reported lower PTSD symptoms (adjusted mean difference, −4.02; 95% CI, −7.18 to −0.86; P = .013]. Anxiety, QOL, and fatigue did not differ significantly between groups (Table 2). Similar results were obtained with multiple imputations (Appendix Table A1, online only) as well as with partly conditional models to account for missing data (data not shown). Of note, no significant intervention effects on patient-reported outcomes at 6 months post-transplant were found after adjusting for outcomes at 3 months post-transplant.
Table 2.
Effect of the Inpatient Palliative Care Intervention on Patient-Reported Outcomes at 6 Months Post-Transplant
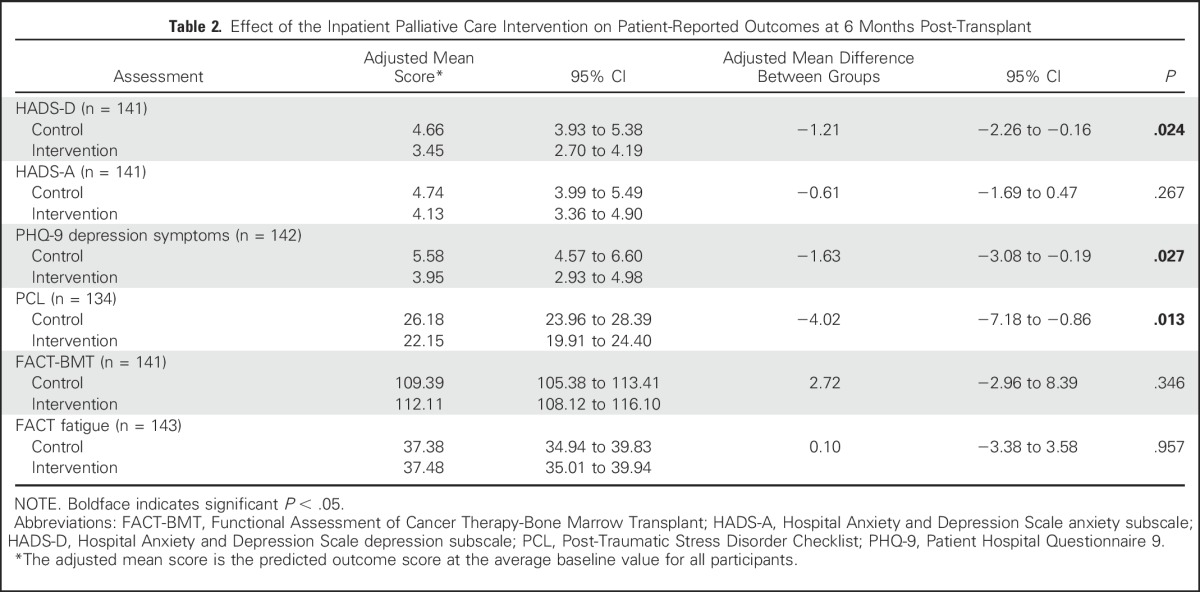
At 6 months post-transplant, palliative care intervention participants had a lower rate of clinically significant depression symptoms (HADS-D, 10.14% v 26.40% [P = .017]; PHQ-9, 14.29% v 33.33% [P = .010]) and lower rates of clinically significant PTSD symptoms (PCL, 7.35% v 21.13%; P = .029). The rates of clinically significant anxiety at 6 months post-transplant did not differ between groups (HADS-A, 15.94% v 22.2%; P = .396).
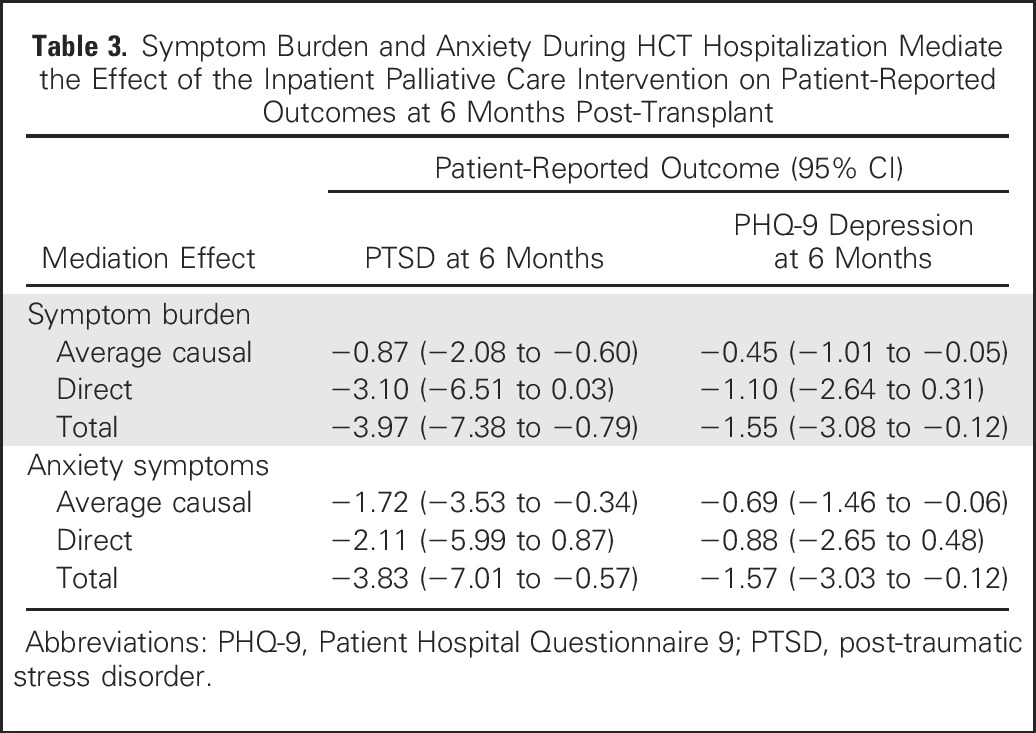
Symptom Burden and Mood During HCT Hospitalization as Potential Mediators
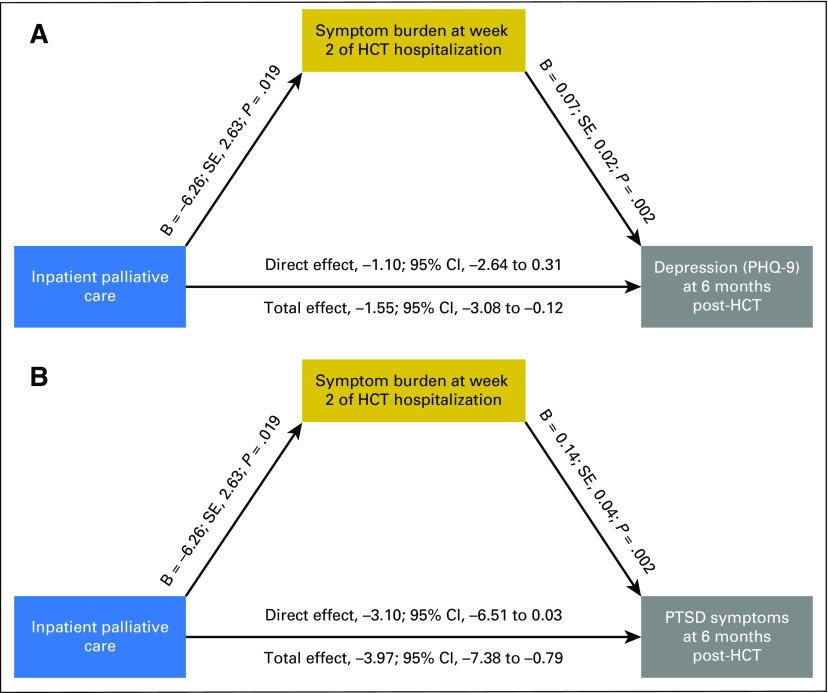
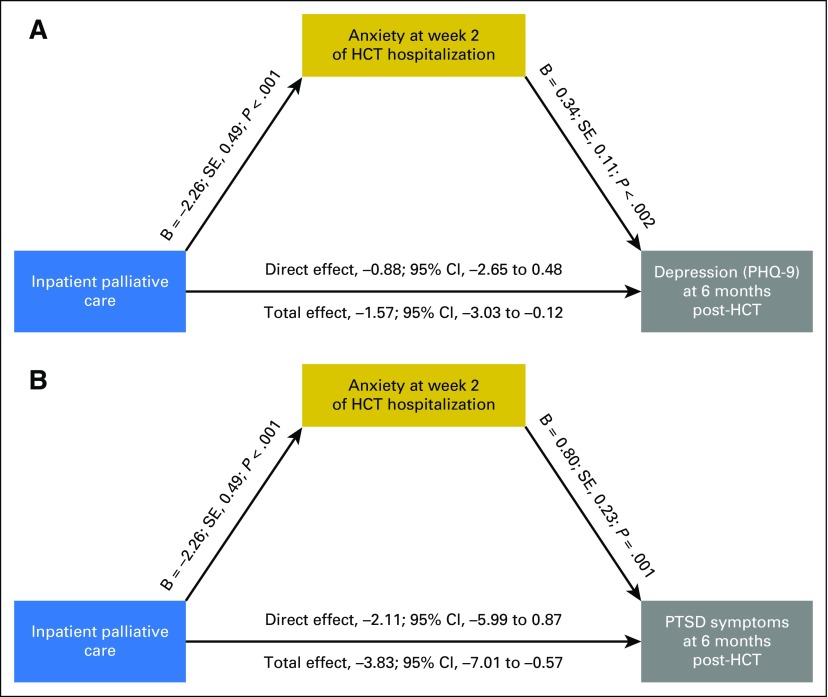
In exploratory analyses, we examined whether symptom burden, anxiety, and depression during the HCT hospitalization mediated the observed effects of the palliative care intervention on depression and PTSD symptoms at 6 months post-transplant (Figs 2 and 3; Table 3]. Symptom burden during HCT hospitalization partially mediated the effect of the intervention on depression symptoms as measured by the PHQ-9 (B = 0.06; standard error [SE], 0.02; P = .006; average causal mediation effect, −0.45 [95% CI, −1.01 to −0.05]; total effect, −1.55 [95% CI, −3.08 to −0.12]; percent mediated effect, 29%) and on PTSD symptoms (B = 0.12; SE, 0.05; P = .008; average causal mediation effect, −0.87 [95% CI, −2.08 to −0.60]; total effect, −3.97 [95% CI, −7.38 to −0.79]; percent mediated effect, 22%). Anxiety symptoms during the HCT hospitalization also partially mediated the effect of the intervention on depression symptoms as measured by the PHQ-9 (B = 0.29; SE, 0.16; P = .014; average causal mediation effect, −0.69 [95% CI, −1.46 to −0.06]; total effect, −1.57 [95% CI, −3.03 to −0.12]; percent mediated effect, 43.9%) and on PTSD symptoms (B = 0.67; SE, 0.25; P = .008; average causal mediation effect, −1.72 [95% CI, −3.53 to −0.36]; total effect, −3.83 [95% CI, −7.01 to −0.58]; percent mediated effect, 44.8%). Depression symptoms during hospitalization did not significantly mediate the effect of the intervention on PTSD symptoms at 6 months post-transplant.
Fig 2.
Symptom burden as a mediator of intervention effects on 6-month psychological outcomes. (A) Symptom burden during hematopoietic stem-cell transplant (HCT) hospitalization mediates the effect of the palliative care intervention on depression symptoms at 6 months post-transplant. Models control for baseline criterion scores. (B) Symptom burden during HCT hospitalization mediates the effect of the palliative care intervention on post-traumatic stress disorder (PTSD) symptoms at 6 months post-transplant; models control for baseline criterion scores. PHQ-9, Patient Hospital Questionnaire 9; SE, standard error.
Fig 3.
Anxiety symptoms as a mediator of intervention effects on 6-month psychological outcomes. (A) Anxiety symptoms during hematopoietic stem-cell transplant (HCT) hospitalization mediate the effect of the palliative care intervention on depression symptoms at 6 months post-transplant: models control for baseline criterion scores. (B) Anxiety symptoms during HCT hospitalization mediate the effect of the palliative care intervention on post-traumatic stress disorder (PTSD) symptoms at 6 months post-transplant: models control for baseline criterion scores. PHQ-9, Patient Hospital Questionnaire 9; SE, standard error.
Table 3.
Symptom Burden and Anxiety During HCT Hospitalization Mediate the Effect of the Inpatient Palliative Care Intervention on Patient-Reported Outcomes at 6 Months Post-Transplant
DISCUSSION
A relatively brief inpatient palliative care intervention during hospitalization for HCT led to a remarkable and sustained improvement in participants’ depression and PTSD symptoms at 6 months post-transplant. Symptom burden and anxiety symptoms during HCT hospitalization partially accounted for the effect of the intervention on patients’ psychological outcomes after transplant. These findings have important clinical implications and underscore the importance of addressing patients’ physical and psychological symptoms during HCT hospitalization as a means to reduce long-term psychological distress.
Despite the substantial long-term psychological burden that patients who undergo HCT face, studies to improve patient experience and outcomes are lacking.6,10,23-25 In a prior randomized trial of a telephone-based cognitive behavioral therapy intervention in HCT survivors 1 to 3 years after transplant, patients who received the intervention reported a reduction in their PTSD symptoms and general distress.21 Given the paucity of data, the National Institutes of Health Late Effects Initiative identified a critical need to address psychological distress, including depression and PTSD symptoms, in HCT survivors.38 To our knowledge, the current study is the first to demonstrate that a brief inpatient intervention during HCT hospitalization leads to a reduction in patients’ depression and PTSD symptoms after transplant. We did not, however, detect significant intervention effects on anxiety symptoms at 6 months post-transplant. Plausibly, anxiety at 6 months post-transplant is less likely to be influenced by the HCT hospitalization experience and more likely to depend on other factors, including the fear of recurrence and post-transplant complications. The effect of the palliative care intervention on patient-reported depression and PTSD symptoms at 3 months post-transplant was sustained with longer follow-up at 6 months post-transplant. The findings suggest that involvement of palliative care during the HCT hospitalization modulates patients’ traumatic experience and thereby leads to a reduction in long-term psychological distress.
These data also shed light on a potential mechanism for the effect of the palliative care intervention on patients’ psychological outcomes post-transplant. Both symptom burden and anxiety symptoms during hospitalization for HCT partially mediated the effect of the intervention on depression and PTSD symptoms after transplant. Studies have shown that the anxiety experienced during a traumatic event can play a critical role in the risk of developing PTSD in the future.39,40 Because the palliative care intervention improved anxiety symptoms during hospitalization, the intervention may mitigate the trauma and thereby lead to fewer PTSD symptoms in the future. However, patient symptom burden and anxiety during hospitalization only accounted for 30% to 45% of the effect of the intervention on post-transplant outcomes. Of note, early palliative care also has been shown to enhance patients’ adaptive coping strategies.41 The palliative care clinicians in the current study reported a focus on enhancing patients’ coping strategies in 85% of initial consultation and 63% of subsequent palliative care visits.42 Therefore, additional studies are needed to explore whether patients’ adaptive coping strategies mediate the effect of the palliative care intervention on post-transplant outcomes.
The current findings also add to the growing literature of the benefits of integrating palliative care services earlier in the course of disease for patients with cancer.43-48 Specifically, although prior studies of early palliative care have shown improvement in patient-reported QOL, symptom burden, and mood,43-48 the current trial demonstrates that early integrated palliative care ameliorates the risk of PTSD symptoms as well. These findings expand our knowledge about the potential benefits of palliative care for patients with hematologic malignancies and those receiving potentially curative therapy with HCT who were excluded from prior palliative care trials in oncology.43-47 Given the highly specialized nature of HCT that can only be offered at large academic hospitals with substantial access to inpatient palliative care services, the integrated palliative and transplant care model has the potential for broad dissemination. To demonstrate definitively the efficacy and generalizability of this care model, a follow-up, large-scale, multisite randomized trial is needed.
The study has several important limitations. First, it was performed at a single tertiary care site with a specialized group of transplant and palliative care clinicians, which potentially limits the generalizability of the results to other care settings or transplant centers with different practices. Second, the sample lacked racial and ethnic diversity; thus, we were unable to assess the effect of these important factors on study outcomes. Third, although we used a randomized controlled design, the participants and clinicians could not be blinded to the intervention because blinding is impractical in palliative care studies.49 Finally, the study was not originally powered to assess the effect of the intervention on patient-reported outcomes at 6 months post-transplant or to conduct mediation analyses; thus, these findings are exploratory and warrant additional investigation.
This work demonstrates that involvement of palliative care for patients with hematologic malignancies who undergo HCT not only improves the patients’ experience during HCT but also leads to sustained improvement in their depression and PTSD symptoms post-transplant. By addressing patient symptoms during HCT hospitalization, palliative care clinicians may buffer this highly stressful and potentially traumatic experience, which may partly explain the reduction in psychological distress post-transplant.
Appendix
Table A1.
Analysis of Covariance of Patient-Reported Outcomes Using Multiple Imputations
Footnotes
Supported by funds from the National Palliative Care Research Center (to A.E.-J.) and K24 CA 181253 (to J.S.T.).
Clinical trial information: NCT02207322.
AUTHOR CONTRIBUTIONS
Conception and design: Areej El-Jawahri, Joseph A. Greer, William F. Pirl, Vicki A. Jackson, Yi-Bin A. Chen, Jennifer S. Temel
Financial support: Areej El-Jawahri, Jennifer S. Temel
Administrative support: Areej El-Jawahri, Harry VanDusen, Vicki A. Jackson, Yi-Bin A. Chen, Jennifer S. Temel
Provision of study materials or patients: Areej El-Jawahri, Thomas R. Spitzer, Steven McAfee, Yi-Bin A. Chen
Collection and assembly of data: Areej El-Jawahri. Harry VanDusen, Sarah R. Fishman, Jason Telles, Alison Rhodes, Thomas R. Spitzer, Steven McAfee, Jennifer S. Temel
Data analysis and interpretation: Areej El-Jawahri, Lara Traeger, Joseph A. Greer, Harry VanDusen, Sarah R. Fishman, Thomas W. LeBlanc, Vicki A. Jackson, Zhigang Li, Yi-Bin A. Chen, Jennifer S. Temel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Inpatient Palliative Care During Hematopoietic Stem-Cell Transplant on Psychological Distress 6 Months After Transplant: Results of a Randomized Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Areej El-Jawahri
No relationship to disclose
Lara Traeger
No relationship to disclose
Joseph A. Greer
No relationship to disclose
Harry VanDusen
No relationship to disclose
Sarah R. Fishman
No relationship to disclose
Thomas W. LeBlanc
Honoraria: Celgene
Consulting or Advisory Role: EPI-Q, Janssen Pharmaceuticals, Boehringer Ingelheim, Flatiron Health, Pfizer, Helsinn Healthcare
Research Funding: Helsinn Healthcare (Inst), Opus Science (Inst), Celgene (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Pfizer, Celgene
William F. Pirl
No relationship to disclose
Vicki A. Jackson
No relationship to disclose
Jason Telles
No relationship to disclose
Alison Rhodes
No relationship to disclose
Zhigang Li
No relationship to disclose
Thomas R. Spitzer
Consulting or Advisory Role: Bluebird Bio
Steven McAfee
No relationship to disclose
Yi-Bin A. Chen
Consulting or Advisory Role: Jazz Pharmaceuticals, REGiMMUNE, Takeda Pharmaceuticals, Seattle Genetics, INSYS Therapeutics, Magenta Therapeutics
Research Funding: Seattle Genetics, Novartis, Celgene
Jennifer S. Temel
Research Funding: Pfizer (Inst)
REFERENCES
- 1. doi: 10.1038/bmt.2011.130. Braamse AM, Gerrits MM, van Meijel B, et al: Predictors of health-related quality of life in patients treated with auto- and allo-SCT for hematological malignancies. Bone Marrow Transplant, 47:757-769, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Curtis RE, Rowlings PA, Deeg HJ, et al. : Solid cancers after bone marrow transplantation. N Engl J Med 336:897-904, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Duell T, van Lint MT, Ljungman P, et al. : Health and functional status of long-term survivors of bone marrow transplantation. Ann Intern Med 126:184-192, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A, Baldomero H, Frauendorfer K, et al. : EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone Marrow Transplant 37:1069-1085, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Majhail NS, Mau LW, Chitphakdithai P, et al. : National survey of hematopoietic cell transplantation center personnel, infrastructure, and models of care delivery. Biol Blood Marrow Transplant 21:1308-1314, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pidala J, Anasetti C, Jim H: Health-related quality of life following haematopoietic cell transplantation: Patient education, evaluation and intervention. Br J Haematol 148:373-385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pidala J, Anasetti C, Jim H: Quality of life after allogeneic hematopoietic cell transplantation. Blood 114:7-19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuellon RP, Russell GB, Rambo TD, et al. : Quality of life and psychological distress of bone marrow transplant recipients: The ‘time trajectory’ to recovery over the first year. Bone Marrow Transplant 21:477-486, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Bevans MF, Marden S, Leidy NK, et al. : Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 38:101-109, 2006 [DOI] [PubMed] [Google Scholar]
- 10.El-Jawahri AR, Traeger LN, Kuzmuk K, et al. : Quality of life and mood of patients and family caregivers during hospitalization for hematopoietic stem cell transplantation. Cancer 121:951-959, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fife BL, Huster GA, Cornetta KG, et al. : Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol 18:1539-1549, 2000 [DOI] [PubMed] [Google Scholar]
- 12.El-Jawahri AR, VanDusen HB, Traeger LN, et al. : Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation. Cancer 122:806-812, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prieto JM, Atala J, Blanch J, et al. : Patient-rated emotional and physical functioning among hematologic cancer patients during hospitalization for stem-cell transplantation. Bone Marrow Transplant 35:307-314, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Prieto JM, Blanch J, Atala J, et al. : Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol 20:1907-1917, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Syrjala KL, Chapko MK, Vitaliano PP, et al. : Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant 11:319-327, 1993 [PubMed] [Google Scholar]
- 16.Smith MY, Redd W, DuHamel K, et al. : Validation of the PTSD Checklist-Civilian Version in survivors of bone marrow transplantation. J Trauma Stress 12:485-499, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen PB, Widows MR, Hann DM, et al. : Posttraumatic stress disorder symptoms after bone marrow transplantation for breast cancer. Psychosom Med 60:366-371, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen PB, Sadler IJ, Booth-Jones M, et al. : Predictors of posttraumatic stress disorder symptomatology following bone marrow transplantation for cancer. J Consult Clin Psychol 70:235-240, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Mundy EA, Blanchard EB, Cirenza E, et al. : Posttraumatic stress disorder in breast cancer patients following autologous bone marrow transplantation or conventional cancer treatments. Behav Res Ther 38:1015-1027, 2000 [DOI] [PubMed] [Google Scholar]
- 20.DuHamel KN, Ostrof J, Ashman T, et al. : Construct validity of the posttraumatic stress disorder checklist in cancer survivors: Analyses based on two samples. Psychol Assess 16:255-266, 2004 [DOI] [PubMed] [Google Scholar]
- 21.DuHamel KN, Mosher CE, Winkel G, et al. : Randomized clinical trial of telephone-administered cognitive-behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. J Clin Oncol 28:3754-3761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1002/cncr.29818. El-Jawahri AR, Vandusen HB, Traeger LN, et al: Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation. Cancer, 122:806-812, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFor TE, Burns LJ, Gold EM, et al. : A randomized trial of the effect of a walking regimen on the functional status of 100 adult allogeneic donor hematopoietic cell transplant patients. Biol Blood Marrow Transplant 13:948-955, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Syrjala KL, Donaldson GW, Davis MW, et al. : Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: A controlled clinical trial. Pain 63:189-198, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Syrjala KL, Cummings C, Donaldson GW: Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: A controlled clinical trial. Pain 48:137-146, 1992 [DOI] [PubMed] [Google Scholar]
- 26.El-Jawahri A, LeBlanc T, VanDusen H, et al. : Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: A randomized clinical trial. JAMA 316:2094-2103, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 67:361-370, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16:606-613, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McQuellon RP, Russell GB, Cella DF, et al. : Quality of life measurement in bone marrow transplantation: Development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant 19:357-368, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Santana MJ, Au HJ, Dharma-Wardene M, et al. : Health-related quality of life measures in routine clinical care: Can FACT-fatigue help to assess the management of fatigue in cancer patients? Int J Technol Assess Health Care 25:90-96, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe SM, Nekolaichuk CL, Beaumont C: The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: Development and refinement. Psychooncology 21:977-985, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106:2912-2919, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Belle GF, Heagerty LD, Lumley PJ, et al: Biostatistics: A Methodology for the Health Sciences (ed 2). Hoboken, NJ, Wiley, 2004. [Google Scholar]
- 34. doi: 10.1200/JCO.2016.69.1220. Kurland BF, Egleston BL: For health-related quality of life and other longitudinal data, analysis should distinguish between truncation by death and data missing because of nonresponse. J Clin Oncol 34:44-49, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Kurland BF, Heagerty PJ: Directly parameterized regression conditioning on being alive: Analysis of longitudinal data truncated by deaths. Biostatistics 6:241-258, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Hicks RT, Tingley D: Causal mediation analysis. Stata J 11:609-615, 2011. [Google Scholar]
- 37.Imai K, Keele L, Tingley D: A general approach to causal mediation analysis. Psychol Methods 15:309-334, 2010 [DOI] [PubMed] [Google Scholar]
- 38. doi: 10.1016/j.bbmt.2016.09.011. Bevans M, El-Jawahri A, Tierney DK, et al: National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group report. Biol Blood Marrow Transplant, 23:538-551, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordova MJ, Riba MB, Spiegel D: Post-traumatic stress disorder and cancer. Lancet Psychiatry 4:330-338, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall RD, Garakani A: Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatr Clin North Am 25:385-395, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Greer JA, El-Jawahri A, Pirl, WF, et al: Randomized trial of early integrated palliative and oncology care. J Clin Oncol 34, 2016 (suppl; abstr 104)
- 42. doi: 10.1001/jama.2016.16786. El-Jawahri A, LeBlanc T, VanDusen H, et al: Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation for hematologic malignancy: A randomized clinical trial. JAMA 316:2094-2103, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 44. doi: 10.1200/JCO.2016.70.5046. Temel JS, Greer JA, El-Jawahri A, et al: Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol 35:834-841, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 302:741-749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakitas MA, Tosteson TD, Li Z, et al. : Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 33:1438-1445, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmermann C, Swami N, Krzyzanowska M, et al. : Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 383:1721-1730, 2014 [DOI] [PubMed] [Google Scholar]
- 48. doi: 10.1001/jamaoncol.2015.5252. Grudzen CR, Richardson LD, Johnson PN, et al: Emergency department-initiated palliative care in advanced cancer: A randomized clinical trial. JAMA Oncol 10.1001/jamaoncol.2015.5252 [epub ahead of print on January 14, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kavalieratos D, Corbelli J, Zhang D, et al. : Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA 316:2104-2114, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]