Graphical abstract
Covalent, reversible, post-translational modification of cellular proteins with the small modifier, ubiquitin (Ub), regulates virtually every known cellular process in eukaryotes. A protein may be modified with a single Ub (mono-ubiquitination), several single Ubs or by multiple Ubs that are covalently linked together via any of seven lysine (Lys) residues (poly-ubiquitination) or the N-terminus of Ub (linear chains). The nature of the modification specifies the biological outcome. Regardless of what type of protein ubiquitination ensues, the process is carried out by a trio of enzymes: a Ub-activating (E1) enzyme, a Ub-conjugating (E2) enzyme, and a Ub ligase (E3) enzyme. E3 ligases orchestrate the final Ub transfer step to a substrate by binding the latter and an E2~Ub intermediate known as a conjugate. E3 ligases are categorized into three classes based the type of transfer mechanism utilized: 1) Really Interesting New Gene (RING)-type E3s, 2) Homologous to the E6AP Carboxyl Terminus (HECT)-type E3, and 3) RING-in-Between-RING (RBR) E3s. (Please refer to Table 1 for a list of abbreviations used in this article.) RING-type E3s promote Ub transfer from E2~Ub onto substrate directly, whereas HECT-type and RBR-type E3s require a covalent E3~Ub thioester intermediate to transfer Ub to substrate. While RING and HECT domains are structurally and mechanistically distinct, the more recently discovered RBR E3s contain a RING domain and an active-site cysteine (Cys), leading to their designation as RING-HECT hybrids. This review focuses on emerging mechanistic and structural understanding in the still young field of RBR E3 ligases.
Table 1.
Abbreviations Used.
| CDC34, E2 conjugating enzyme, |
| C-term, C-terminus |
| CRLs, Cullin RING ligases |
| CUL, Cullin |
| Cys, Cysteine |
| E2-25K, human E2 Ube2k |
| E2~Ub, E2 ubiquitin conjugate (thioester) |
| E3~Ub, E3 ubiquitin conjugate (thioester) |
| HECT, Homologous to the E6AP Carboxyl Terminus |
| HHARI, Human Homolog of Ariadne |
| His, Histidine |
| HOIL-1L, Haem-Oxidized IRP2 Ubiquitin Ligase1L |
| HOIP, HOIL1-Interacting protein |
| IBR, In-Between-RING |
| K48, Lys48 of ubiquitin |
| K63, Lys63 of ubiquitin |
| LDD, Linear ubiquitin chain-determining domain |
| LUBAC, Linear Ubiquitin Chain Assembly Complex |
| Lys, Lysine |
| N-term, N-terminus |
| NMR, Nuclear Magnetic Resonance |
| NZF, NPL4 Zinc Finger domain |
| PINK1, PTEN-induced kinase |
| PUB, PNGase/Ubiquitin-associated domain |
| RBR, RING-in-Between-RING |
| REP, repressor element (Parkin) |
| RING, Really Interesting New Gene |
| SHARPIN, Shank-associated RH domain-interacting protein |
| TMD, Transmembrane Domain |
| TRIAD1, Two RING fingers And DRIL |
| Ub, Ubiquitin |
| UBA, Ubiquitin Associated domain |
| UBA-L, Ubiquitin Associated-Like domain |
| UbcH5, human E2, Ube2d1, 2, or 3 |
| UbcH7, human E2, Ube2l3 |
| UBL, Ubiquitin Like Domain |
| UPD, Unique Parkin Domain |
| ZF, Zinc Finger domain |
| Zn2+, Zinc |
Meet the RBR E3s
The 14 RBR E3s [1] in humans regulate diverse cellular processes that are still being defined. The most noted member is Parkin, a highly-studied enzyme whose E3 ligase activity is associated with mechanisms that clear damaged mitochondria via a process called mitophagy [2, 3]. Early studies linked mutations in the gene that encodes Parkin (PARK2) to autosomal-recessive juvenile Parkinson’s disease [4, 5]. Linear Ubiquitin Chain Assembly Complex (LUBAC), the only E3 enzyme in any class known to generate linear Ub chains, contains two RBR family members, Haem-Oxidized IRP2 Ubiquitin Ligase1L (HOIL-1L) and HOIL1-Interacting protein (HOIP) [6]. Originally LUBAC was found to regulate inflammation by activating NF-κB pathways [7, 8], but later studies have established a broader biological role. Malfunction of the LUBAC complex is now associated with B-cell function, regulation of apoptosis, oncogenesis and diverse autoimmune diseases [9–14]. HHARI (Human Homolog of Ariadne) and its homologues in Drosophila and C. elegans are implicated in the regulation of translation, cellular proliferation, and developmental processes [15–18]. In humans, TRIAD1 (Two RING fingers And DRIL) has been associated with regulating the proliferation of myeloid progenitors, NF-κB signaling, and membrane trafficking [19–21]. HHARI and TRIAD1 have both been shown to associate physically with Cullin RING ligases (CRLs), potentially extending their known biological roles [22–24]. Though currently understudied, the RBR E3s RNF144A and RNF19A are thought to promote apoptosis in a p53-dependent manner and to confer neuronal protection, respectively [25, 26]. In this article we focus mainly on the non-Parkin members of the family. A useful list of all human RBR gene names and nicknames is available [1, 27].
A Brief History of RBR E3s
RBR E3s were originally defined based on sequence alignments that predicted a tripartite motif of three Zn2+-binding domains: two RING domains (RING1 and RING2) connected via an In-Between-RING (IBR) domain [28, 29]. The prediction together with initial observations of ubiquitination activity led to the belief that RBR E3s constitute a sub-class of the largest E3 class, the RING-type E3s [30–33]. However, more than ten years after they were defined, RBR E3s were shown to differ fundamentally from their eponymous RING E3 cousins by virtue of their possessing an active site—a feature lacking in all RING-type E3s [34]. Similar to canonical RINGs, the RING1 domain of the RBR module binds E2s loaded with Ub (E2~Ubs). But RING2s were found to contain an essential active-site Cys that receives Ub from an E2~Ub to generate a covalent E3~Ub intermediate, otherwise seen only in HECT-type E3s [34]. Based on those two features, RBR E3s are referred to as RING-HECT-hybrids. The hybrid mechanism was initially revealed for HHARI and Parkin [34], but the functional significance of an active-site Cys for catalytic activity has since been confirmed for HOIP, HOIL-1L, TRIAD1, and RNF144A and is now considered a defining feature of RBR E3s [23, 25, 35, 36].
While a useful concept, the description of RBR E3s as RING-HECT hybrids does not fully embrace emerging understanding of their mechanisms. Here, we present current status of the molecular details of how RBR E3s function. Molecular processes that an RBR E3 must undergo during its catalytic cycle include 1) E2~Ub binding, 2) E3~Ub generation, 3) substrate binding and 4) transfer of Ub to the substrate. While these steps must follow a logical sequence, the precise order of events is still unknown. Furthermore, early biochemical studies of Parkin uncovered a mode of auto-inhibition [37]. Subsequently, several other human RBR E3s were shown to be auto-inhibited, though through different mechanisms [23, 35, 36, 38, 39]. Each RBR E3 has its own set of protein targets and each RBR E3 dictates the type of modification (mono-, linear, or poly-ubiquitin chains) that it generates on its targets. As is the case for the other classes of E3s, identification of substrates is challenging and under-developed. At present, it is not even clear where a protein substrate binds on an RBR E3. How an RBR E3 determines the type of modification is known for only one RBR E3, HOIP (discussed below, [40]) and remains to be defined for all others. As presented in subsequent sections of this article, more progress has been made to define the structural and biochemical underpinnings of steps in the catalytic cycle prior to the ultimate attachment of Ub to substrate.
The class-defining elements of RBR E3s: RING1-IBR-RING2
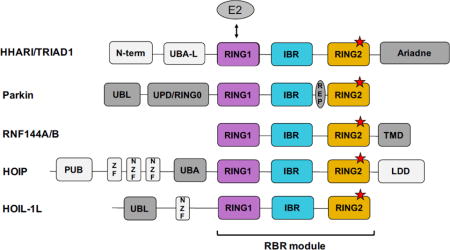
RBR E3s are complicated multi-domain enzymes that contain a variety of domains in addition to their RBR module, as shown in Fig. 1. The three domains that define the RBR module always appear in the order RING1-IBR-RING2, but the position of the module itself relative to other domains varies (Fig. 1). Structures of RBR E3s HHARI, Parkin, and HOIP provide the structural foundation on which understanding of RBR E3 ligases is currently built [38, 40–50]. A detailed review of RBR E3 structures has been published [51], so here we highlight the main common features and more recent insights gained from newer studies.
Figure 1.
RBR E3s have diverse domain architectures. All RBR E3s contain an RBR module comprised of RING1 (purple), IBR (blue), and RING2 (orange). An RBR module can appear at any position relative to other domains, but its three domains are always found in the order: RING1-IBR-RING2. RING1 binds the E2 (grey oval) and RING2 contains the conserved active-site Cys (red stars). Domains not shared among RBR family members are colored grey, with dark grey indicating domains implicated in autoinhibition/activation. (UBA-L = Ubiquitin Associated-Like domain, UPD = Unique Parkin Domain; UBL = Ubiquitin Like domain; REP = Repressor Element; TMD = Transmembrane Domain; PUB = PNGase/Ubiquitin-associated domain; ZF = Zinc Finger domain; NZF = NPL4 Zinc Finger domain; LDD = Linear chain Determining Domain). Current information available regarding chain specificity (based on rigorous biochemical evidence) and mode of activation for each RBR is summarized.
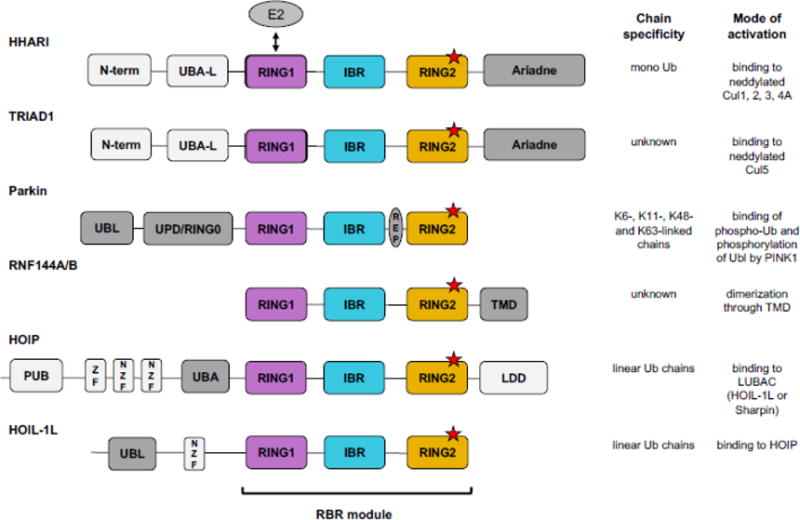
The three domains that compose an RBR, originally defined by sequence analysis, were named RING1, IBR, and RING2 [28, 29]. Each of the three domains coordinates two Zn2+ ions (Fig. 2A–C). Structures have confirmed that RING1 domains adopt a typical cross-brace fold similar to the canonical RINGs for which they were named (Fig. 2B) [38, 41–45, 47, 48, 50]. But contrary to the original thinking that led to the name “RING2” for the third RBR sub-domain, structures revealed that RING2s are dissimilar to RINGs and are instead structurally similar to the IBRs (Fig. 2C, [38, 40–50, 52]). Although the “RING2” moniker inevitably leads to some misperceptions, several suggestions to change it have failed to be adopted [40, 51]. Most importantly, all RING2 sequences contain a conserved Cys residue that is not involved in Zn2+ coordination that serves as the active site to which Ub is attached [34]. While they resemble RING2s in topology, IBR domains do not contain an active-site Cys and their sequences vary greatly among RBR E3s (Fig. 2C; [38, 41–45, 47, 48, 53]). IBRs and their linkers on either side have been implicated in binding Ub during Ub transfer reactions, but the exact function of IBRs remains enigmatic [47, 50, 54].
Figure 2.
Features of RBR E3 structures. A) Structures of auto-inhibited HHARI (top, PDB 4KBL) and Parkin (bottom, PDB 4K95) shown in cartoon representation. RING1 is in purple, IBR in cyan, and RING2 in orange. Red spheres represent the RING2 active-site Cys that is partially buried by other domains (Ariadne domain in the case of HHARI and UPD/RING0 in the case of Parkin). The relative arrangement of the sub-domains of the RBR module differs between the two RBRs. In the auto-inhibited state of HHARI the E2-binding RING1 domain is accessible to bind E2, but the active-site Cys-containing RING2 domain is far away. Compared to HHARI, Parkin’s RING1 and RING2 domains are positioned closer together, but the REP element of Parkin blocks the E2 binding site on RING1. Hence, both structures suggest that major domain rearrangements must occur prior to Ub transfer onto the active site. B) RBR RING1 domains (left, HHARI, PDB 5UDH) are structurally similar to canonical RINGs (right, BRCA1, PDB 1JM7) as demonstrated by the typical cross-brace fold. C) Structures of RBR RING2 domains (left, HHARI, PDB 5UDH) do not resemble RING domains, but are structurally similar to IBR domains (right, HHARI, PDB 5UDH). D) Crystal structure of HOIP RING2-LDD (RING2 in orange, LDD in green, PDB 4LJO) in complex with a donor Ub (blue) covalently bound to the active site Cys (orange spheres) through its C-term (blue spheres). The acceptor Ub (red) is non-covalently bound to the LDD. The N-term of the acceptor Ub (red spheres) is in proximity to the thioester of RING2~Ub donor poised for Ub transfer. The LDD (green) is tightly integrated structurally with RING2 (orange) generating a compact RING2-LDD unit.
RBR E3s carry out the final Ub transfer step and control the type of product generated
That RBR E3s contain an active site distinguishes them from canonical RING-type E3s. This distinction is more than a technicality because it is the enzyme that carries out the final Ub transfer reaction that determines the type of Ub modification (i.e., mono- vs. poly-ubiquitination and chain linkage configuration) on a given substrate. In chemical terms, the final step is the only one that is not a transthiolation reaction (i.e., transfer from one Cys to another). Thus, the E2 determines the product type when working with RING-type E3s by transferring the Ub from its active-site Cys to a non-Cys (most often, Lys) residue on the substrate. As a result, RING-type E3s can modify their substrates with different Ub products depending on which E2 it pairs with. But in the case of E3s that form an E3~Ub intermediate (i.e., HECT- and RBR E3s), the E3 dictates the final Ub product. A clear example of this switching of control between E2 and E3 enzymes is presented in a study of LUBAC (which contains HOIP) with the E2 known as E2-25K (Ube2K), which possesses intrinsic ability to build K48-linked Ub chains in the absence of an E3. This chain linkage preference is suppressed when E2-25K works with LUBAC where the E2/E3 pair robustly generates linear Ub chains [6]. In this case, the E2~Ub simply acts as a supplier of Ub to the E3, and must not modify substrates (Lys) directly so as to allow linear chain-building specificity to be conferred by the E3 HOIP. Consequently, HOIP (and presumably other RBR E3s) dictates final product formation on substrates independent of the E2 involved [6, 35, 36].
HOIP’s ability to build linear Ub chains arises from a unique domain that follows directly after the RING2 domain, the linear ubiquitin chain-determining domain (LDD) (Fig. 1). Structures have revealed that the LDD is tightly integrated into RING2, creating a single RING2-LDD unit (Fig. 2D, [40, 47]). The LDD binds an acceptor Ub (i.e., the substrate) and orients its N-terminus (N-term) towards the RING2 active-site Cys, thereby ensuring linear Ub chain formation in which the C-terminus (C-term) of a donor Ub attached to the E3 active site is covalently linked to the N-term of an acceptor Ub (Fig. 2D) [40]). HOIP is the only RBR E3 that contains an LDD, consistent with the HOIP-containing LUBAC being the only E3 complex known to generate linear Ub chains. HOIP’s high specificity for the N-term of the acceptor Ub raises the question of how LUBAC transfers the initial Ub to a substrate Lys. Currently there are two proposed models: 1) attachment of the initial Ub is facilitated by other LUBAC components [55, 56] and/or 2) LUBAC substrates are first modified (by another E2/E3 pair) with K63-linked poly-Ub chains and these serve as a template onto which HOIP builds linear chains [14, 57].
Product specificities and how these are dictated are much less well defined for other RBR E3s. There is strong biochemical evidence that HHARI is a mono-ubiquitinating E3 [22, 48] but the structural basis for this preference has yet to be uncovered. Parkin is reported to be responsible for a variety of product types that include K6-, K11-, K48- and K63-linked chains [58], suggesting either that the E3 lacks chain-type specificity or that this is dictated by as yet unknown factors.
RBR E3 ubiquitin transfer mechanism
Insights into the steps of the RBR mechanism that involve the E2~Ub have been forthcoming from recent studies and structures [47, 48, 50, 54]. Several RBR E3s have been shown to be active with more than one E2 [6, 23, 32, 34–37, 54, 59–61], but a full description of the cadre of E2s (there are more than 30 in humans) that work with each human RBR E3 is not yet available. Two available structures of RBR E3s in complex with E2~Ub include the two well-characterized human E2s, UbcH5 and UbcH7. Importantly, UbcH5 (and most other E2s) can perform both transthiolation reactions and aminolysis reactions, explaining how it can be active with either RBR E3s or RING-type E3s [34–36, 59–61]. The human E2 UbcH7 is a more specialized enzyme that solely performs transthiolation reactions [34], implying that it can function with HECT- and RBR-type E3s, but not with RING-type E3s. Paradoxically, UbcH7 can bind to some canonical RINGs [62, 63], but is not active for Ub transfer with them. It is not yet known whether UbcH7 is a biologically relevant E2 for all RBR E3s, but a strong case can be made for HHARI and HOIP. The orthologues of HHARI and UbcH7 act together in a critical developmental step of the gastro-intestinal system in C.elegans [17, 64]. UbcH7 (but not UbcH5) is able to activate NF-κB reporters in cell culture and some alleles of UbcH7 have been linked to auto-immune diseases suggesting that UbcH7 is a biologically relevant E2 for HOIP in vivo [14, 65, 66].
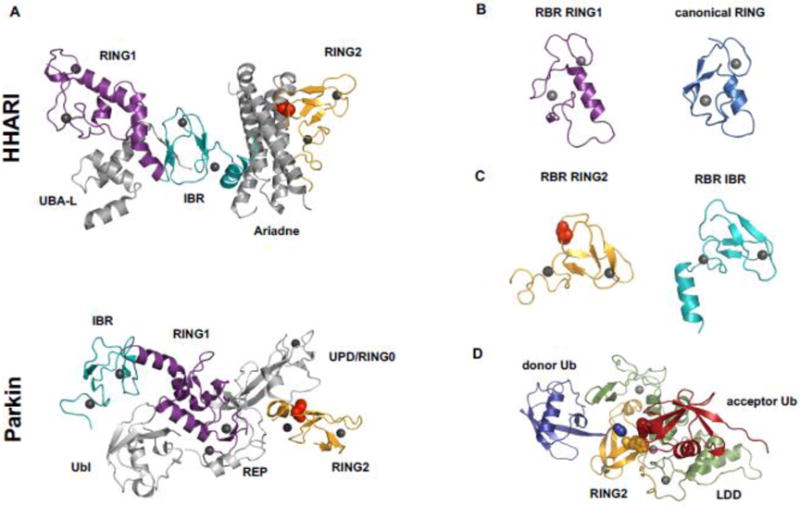
How do the structurally similar canonical RINGs and RBR RING1s (Fig. 2B) direct a bound E2~Ub to perform different reactions—aminolysis in the case of RINGs and transthiolation in the case of RING1s? On their own, E2~Ub species are highly dynamic and flexible [67]. Upon binding to canonical RING domains, E2~Ubs adopt closed states in which the hydrophobic surface of Ub is buried in a contact with the E2 (Fig. 3A, [68–72]). As closed E2~Ub states display increased reactivity towards Lys amino groups [71], canonical RINGs facilitate the direct transfer of Ub from its bound E2~Ub to substrate. In contrast, RBR RING1 domains of HHARI and RNF144A actively disfavor closed E2~Ubs (Fig. 3B, [54]). By promoting open E2~Ub, RBRs ensure that Ub transfer occurs through their E3 active site by reducing E2~Ub reactivity towards Lys residues (which would be off-pathway, [54]). The open E2~Ub conformation also reveals the hydrophobic patch of Ub that is otherwise buried in the E2/Ub interface in closed E2~Ub conformations (Fig. 3, [54]). Both ramifications of the open E2~Ub are important mechanistically, as discussed below.
Figure 3.
Comparison of E2~Ub conformations when bound to canonical RING and RBR RING1. A) Cartoon and surface representations of a canonical RING/E2~Ub complex (E3 = BIRC7; E2 = UbcH5; PDB 4AUQ). RING domains (blue cartoon) induce closed E2~Ub conformations. In RING-induced closed E2~Ub states the hydrophobic surface of Ub (teal) is buried in an interface with the E2. B) Cartoon and surface representations of a RBR RING1/E2~Ub (E3 = RING1; E2 = UbcH7; PDB 5UDH). RING1 domains (purple cartoon) promote open/extended E2~Ub conformations that expose the Ub hydrophobic surface (teal). E2s are shown in grey cartoon representations. Ub is shown in salmon-colored surface representation.
A structural explanation for how RBR RING1s handle their bound E2~Ubs differently from canonical RINGs has not been readily apparent. Only a few residue positions are strongly conserved in RING and RING1 domains, most of which are zinc-coordinating Cys and His residues. A key position in canonical RINGs is the linchpin residue which is largely responsible for the ability to promote closed E2~Ubs by forming hydrogen bonds to both E2 and Ub [71]. RBRs do not have a conserved residue that can fulfill the linchpin function. This provides a possible explanation for RING1’s failure to induce closed states of E2~Ub but it does not explain how a RING1 domain actively promotes open E2~Ubs.
In two recent crystal structures of HOIP/UbcH5~Ub and of HHARI/UbcH7~Ub complexes, the E2~Ub bound to the RING1 domain is in an open conformation [47, 48]. When not bound to an E3, UbcH5~Ub is predominantly in open states that exhibit limited aminolysis reactivity [67]. Paradoxically, although the human E2 UbcH7 solely performs transthiolation reactions [34], unbound UbcH7~Ub exists mainly in closed conformations [54], indicating that an as yet unidentified feature of the UbcH7 active site must be responsible for its restricted chemical reactivity profile. The observation of an open UbcH7~Ub bound to HHARI implies that binding to HHARI RING1 either actively favors open states or disfavors closed states. No contacts with any HHARI domains are observed for the Ub moiety of UbcH7~Ub in the HHARI/UbcH7~Ub complex, suggesting that the open state is not stabilized by additional E3 contacts (Fig. 4A, [48, 54]). Instead, an extension of the second Zn2+-loop of HHARI RING1 is largely responsible for disfavoring the closed E2~Ub conjugate [48]. In canonical RING domains, the final two Zn2+-coordinating Cys residues are consistently separated by exactly two residues (C7th-X-X-C8th), but the same loop contains up to four residues in RING1s (C7th-X-X-X-X-C8th) [51]. Deletion of the extra residues in the HHARI Zn2+-loop II to create a two-residue loop generates a RING1 with diminished ability to disrupt closed UbcH7~Ub [48]. It is the loop length rather than a specific side chain that leads to the opening activity, consistent with the lack of conservation in four-residue loops among RBR RING1s. Thus, in the case of HHARI and other RBR E3s with extended Zn2+-loops, the open E2~Ub conformation is achieved mainly through use of a steric wedge that restricts the conjugate from adopting closed conformations [48].
Figure 4.
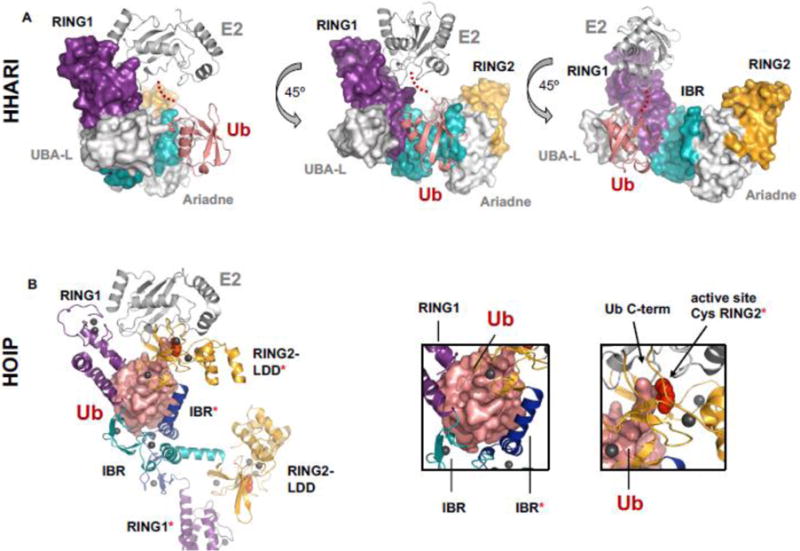
Comparison of an auto-inhibited RBR/E2~Ub complex and an “activated” RBR/E2~Ub complex. A) HHARI bound to E2~Ub (E2 = UbcH7; PDB 5UDH) is still auto-inhibited. Ub (salmon cartoon) makes no contacts with any domains of its cognate HHARI molecule. In the auto-inhibited state the active-site Cys (red spheres, but not visible in the surface representation of the auto-inhibited structure) is occluded by the Ariadne domain and therefore not visible in the surface representation shown here. RING1 (purple surface) and RING2 (orange surface) domains are far apart (as seen in apo-HHARI, Fig. 2A). IBR is shown in cyan surface representation. Density for the C-terminus of Ub is missing in the crystal structure and is instead indicated by red dots. B) HOIP RBR module bound to E2~Ub (E2 = UbcH5; PDB 5EDV). The Ub moiety (salmon surface) of E2~Ub contacts IBR and RING2 domains of two different HOIP molecules (left box, all domains belonging to the second (non-cognate) polypeptide are marked with a [*]). Domain colors are the same as A). The domain swap in the crystal affords a view of what an activated version of HOIP RBR could look like, with the C-term of ~Ub conjugated to the E2 (right box, black arrow) in proximity to the active-site Cys of HOIP (right box; black arrow and red spheres).
The steric wedge model provides a structural explanation for some RBR E3s, but not all family members have four-residue Zn2+-loops II. Parkin has a three-residue loop and two other RBRs, including HOIP, have two-residue loops. A notable difference between HHARI and Parkin is that auto-inhibited HHARI can bind E2~Ub with high affinity whereas auto-inhibited Parkin has modest E2 binding at best because its E2-binding site is partly occluded by the inhibitory element known as the REP (repressor element) (Fig. 1A and 2A; [38, 41, 48]). It may therefore be less important for Parkin to actively disfavor reactive closed E2~Ub states than it is for HHARI, as Parkin may only be able to bind an E2~Ub effectively once it is in an activated state.
HOIP contains a short two-residue Zn2+-loop II, yet in the crystal structure in complex with UbcH5~Ub, the conjugate is in an open conformation (Fig. 4B). UbcH5~Ub exists predominantly in open states on its own, so it may not require a specific mechanism to maintain the open state of UbcH5. Even so, and in contrast to HHARI/UbcH7~Ub in which no contacts to the ~Ub moiety (Ub of E2~Ub) are observed, there are non-covalent contacts from the ~Ub of UbcH5~Ub to all three domains in the HOIP RBR that may serve to position the ~Ub moiety within the complex and minimize the chances that a closed E2~Ub will form (Fig. 4B).
The multiple contacts observed for a single ~Ub in the HOIP/UbcH5~Ub structure as shown in Fig. 4B involve two chains of HOIP (due to a dimer swap in the crystal, [47]). For example, the Ub moiety of UbcH5~Ub is seen bound to IBR domains from two different HOIP polypeptides. Reported evidence for a HOIP dimer existing in solution is equivocal so it is not clear if the observed interactions occur simultaneously within a HOIP/UbcH5~Ub complex [47]. In this complex, the structure of a single HOIP polypeptide may still reflect a less active state, as the RING2 active site is far from the active site of the bound E2~Ub (Fig. 4B). Despite that caveat, it is likely that some, if not all, of the observed contacts involving Ub take place at some point along the reaction pathway, though the details remain to be fleshed out.
Among the ~Ub interactions observed in the HOIP structure is one that involves a RING2 domain (from the non-cognate HOIP) [47]. A similar binding interaction was defined between HHARI RING2 and the hydrophobic patch on Ub by NMR [54]. HHARI RING2 mutations that ablate the interaction inhibit formation of the E3~Ub intermediate and a similar loss of function occurs when the E2~Ub carries mutations in the hydrophobic surface of the attached Ub [54]. The common feature in HOIP and HHARI suggests that interaction between ~Ub and RING2 plays an integral part in the Ub transfer mechanism of RBRs by recruiting RING2 to the E2~Ub bound to RING1. Additionally, Ub contacts on IBR domains of Parkin and HOIP observed in crystal structures have been shown to be important for Ub transfer reactions, though the exact roles these interactions play remain to be defined [47, 50]. Considering the multiple domains that are involved and the large domain movements that must occur to bring the active sites of the E2 and the E3 together, a role for multiple interactions involving Ub is not surprising. Thus, while the multitude of interactions involving ~Ub in the HOIP structure may not reflect a HOIP/E2~Ub complex at a single point in time, it likely represents at least several of the possible configurations of RBR domains relative to a bound E2~Ub along the reaction pathway.
Other binding sites for Ub have been identified on RBR E3s that involve domains outside the RBR module. These are idiosyncratic to a particular RBR and likely impart special functionality. They are also unlikely to involve a Ub that is conjugated to an E2. For example, the relevant Ub to bind at the HOIP LDD is actually the substrate in the linear chain building reaction (and therefore derives from the distal end of a growing chain). Ub binds to the UBA domain of HHARI [48], but the relevant source of Ub (free, part of an E2~Ub, attached to substrate, etc.) is unknown. The same binding site is used by HHARI to bind to the Ub-like Nedd8 that is required for the interaction between HHARI and CRLs [22, 23].
RBR E3s are auto-inhibited and activated by different mechanisms
First observations of an auto-inhibitory mechanism was provided by in vitro biochemical studies of Parkin, followed by HOIP and HOIL-1 [35–37]. Removal of specific domains enhances ubiquitination activity of these enzymes, indicative of an auto-inhibitory mechanism [35–37]. Additional biochemical evidence now supports auto-inhibition of HHARI, TRIAD1, and RNF144A, suggesting that auto-inhibition is a common feature among RBR E3s [23, 35, 36, 39, 47]. Therefore, the activity and biochemical study of RBR E3s requires a method to release the auto-inhibition. The diverse and complicated domain architecture of RBR E3s leads to different modes of auto-inhibition and consequently, different mechanisms by which each RBR E3 is activated. Furthermore, it has become increasingly clear that activation of RBR E3s can be brought about through the actions and interactions of other proteins, including protein kinases and even by other E3 Ub ligases.
Initial crystal structures of HHARI and Parkin revealed sources for their auto-inhibition: their active-site Cys residues are at least partially occluded by non-RBR domains and the E2-binding RING1 domain and the active-site Cys-containing RING2 domain are far apart (Fig. 2A) [38, 41 ]. However, different domains are responsible for occlusion of the active site and the relative dispositions of the RBR domains in the Parkin and HHARI structures are substantially different. Large domain movements must occur in either case to allow Ub transfer from the E2~Ub to the RING2 active site Cys [45, 73]. Consistent with this notion, the long linkers that connect RING1 to IBR and IBR to RING2 are predominantly disordered in the crystal structures, indicating conformational flexibility. Flexible linkers can allow domains to assume positions that are quite distant (as in the auto-inhibited conformations) as well as proximal to each other (as in presumed activated conformations). Part of the linker that connects the IBR and RING2 is helical in an NMR solution structure of HHARI RING2 and in crystal structures of HOIP RING2-LDD and the helix composes part of the RING2 Ub-binding site in HHARI [40, 46, 47]. The observations suggest that the linker may undergo a disorder-to-order transition as part of the structural rearrangements that must occur for RBRs to become active.
The structure of the HOIP RBR-LDD module bound to UbcH5~Ub reveals insights into an activated HOIP component [47]. Contacts between the E2~Ub and the non-cognate RBR module in the crystal suggest how a structure in which the active sites of the E2 and the E3 are in close proximity might look. In this complex, the E2 binds to RING1 and the hydrophobic surface of ~Ub exposed in the open E2~Ub contacts the IBR-RING2 linker, bringing RING2 close to the E2 (Fig. 4B). For these contacts to be made by the cognate HOIP chain, other contacts involving the IBR will have to be disrupted. Finally, comparison of the HOIP RBR-LDD/E2~Ub and the RING2-LDD~Ub/Ubacceptor complex structures reveals that prior to the formation of the RING2~Ub species, the Ub moiety of E2~Ub bound to RING1 resides at the same RING2 site as the acceptor Ub that is required for Ub chain formation [40, 47]. This observation seems to imply that Ub transfer from the E2~Ub onto the E3 active site must occur before the substrate (acceptor Ub) can bind in a manner that poises it for the ultimate reaction. There is currently no evidence whether a similar order of events is shared by other RBR E3s.
Activation of HOIP by its binding partners in LUBAC
HOIP ligase activity is increased by binding to either of its LUBAC partners, HOIL-1L (another RBR) or SHARPIN [36]. Complex formation is mediated through interactions of HOIP’s UBA domain and UBL domains of HOIL-1L or SHARPIN [74–77]. Addition of HOIL-1L UBL or an N-terminal segment of SHARPIN containing its UBL activate HOIP ligase activity in vitro [36]. Evidence exists that additional domains may also be involved in activation and/or inhibition [36, 74–77]. HOIP is not known to exist outside the context of the LUBAC complex in vivo [76, 78, 79], suggesting either that HOIP is constitutively active within LUBAC or that other mechanisms modulate its function in a cellular context. A recent report that chain synthesis by HOIP can be allosterically activated by binding of free di-Ub to the RBR [47] supports a model in which LUBAC builds linear Ub chains onto K63-linked chains attached to its substrates [14, 57]. Although HOIP is the presumed catalytic center of LUBAC, HOIL-1L also contains an RBR module that contains a conserved active-site Cys residue in its RING2 that is required for HOIL-1L ligase activity in vitro [36]. Compared to HOIP, HOIL-1L displays much lower ubiquitination activity in vitro which may be partially explained by its lack of an LDD domain. But it remains a formal possibility that domains of HOIL-1L’s RBR module play an active role in the LUBAC mechanism of Ub transfer, perhaps carrying out some of the multiple interactions observed in the HOIP/UbcH5~Ub structure discussed above.
Activation of Parkin by PINK1 phosphorylation
Structures of auto-inhibited Parkin reveal its five domains to be engaged in a tight embrace (Fig. 2A, [41–45, 50, 80]. It is now clear that release of Parkin’s auto-inhibition is coupled to phosphorylation events carried out by the kinase PINK1 in response to mitochondrial damage that ultimately lead to mitophagy [2, 3]. PINK1 phosphorylates the Ubl domain of Parkin and this both reduces the affinity of the Ubl for other RBR domains of Parkin and increases parkin ligase activity [44, 45, 80, 81]. PINK1 also phosphorylates Ub itself and binding of phospho-Ub to Parkin can activate the ligase through an allosteric mechanism that may involve displacement of the Ubl domain, removal of the REPressor element that prevents E2 binding (Fig. 2A), and exposure of a Ub-binding site utilized by the E2-conjugated Ub moiety [44, 45, 50, 80, 81]. Notably, binding of phospho-Ub by Parkin can increase phosphorylation of its Ubl domain by PINK1, and phosphorylation of the Parkin Ubl increases Parkin’s affinity for phospho-Ub, setting up a synergistic situation [44, 45, 80, 81]. Whether a specific temporal sequence of the events is required in vivo had been unclear, but a recent study suggests that Parkin binding to phospho-Ub on mitochondria enables Parkin phosphorylation and activation [73]. However, it remains possible that both processes may occur simultaneously. Overall, it is clear that activation of Parkin is brought about by phosphorylation and, possibly, other processes and that multiple binding events enable large and coupled domain movements.
Activation of HHARI and TRIAD1 by Cullin RING Ligases
The presence of an Ariadne domain defines a subfamily of RBR E3s that includes HHARI, TRIAD1, ANKIB1, and PARC/CUL9 (Fig. 1). In HHARI, the RING2 active-site Cys is occluded by intramolecular contacts between the Ariadne domain and RING2 [38, 48]. While E2~Ub binding does not activate HHARI [48], removal of the Ariadne domain or mutations at the Ariadne/RING2 interface both release inhibition of auto-ubiquitination activity in vitro, consistent with a model in which the large Ariadne domain must move from its position for activation to occur [38]. Events and processes that can trigger the proposed domain movement are only beginning to be revealed. One such process involves another E3 ligase. Both HHARI and TRIAD1 can bind to neddylated cullin-type RING E3 ligases (CRLs) and complex formation stimulates the RBR ligase activity [22, 23]. Each RBR E3 binds to a non-overlapping subset of CRL scaffolds: TRIAD1 binds only to CUL5 and HHARI binds to CUL1, -2, -3, and -4A, but not CUL5. Intriguingly, the same subsets of cullins arise when sorted according to the RING subunit each uses, with RBX-2 being specific to CUL5 and all other cullins binding to RBX-1, possibly implying involvement of the cullin-specific RING subunit. Consistent with that notion, RBX-1 has been suggested to play a role in activation of HHARI ligase activity [22]. Of the eight human CRLs, only CUL4B and CUL7 do not appear to bind to either HHARI or TRIAD1 [23]. PARC/CUL9 may be a special case, as it contains both a Cullin domain and an RBR module within a single polypeptide. If the pairing of an RBR E3 and a cullin-based complex is completely generalizable (as yet, this is unknown), this would leave the remaining Ariadne-domain-containing RBR E3, ANKIB1, to work with CUL4B and CUL7 as its activating scaffold, but this remains to be tested.
In the past year, new insights into the mechanism and biological roles of HHARI/CRL complexes have emerged. Thorough biochemical studies support a mechanism in which CRL substrate ubiquitination is brought about by a collaborative effort of HHARI and either CUL1 or CUL3 [22]. Activated by binding to neddylated CRLs, HHARI carries out mono-ubiquitination of CRL substrates that are bound to the cullin scaffold. HHARI performs the initial “priming” step using the E2 UBCH7 which cannot work with the RING-associated cullin. The CRL-dedicated E2 CDC34 then extends poly-Ub chains on Ub-primed, CRL-bound substrates [22]. The observed substrate priming by HHARI has several requirements: 1) CRLs must be neddylated and must have their substrate adaptors present, 2) HHARI must be activated (by binding to neddylated CRLs), and 3) the HHARI~Ub intermediate must be formed [22]. A study performed in C. elegans revealed that the homologs of HHARI, UBCH7, CUL-1, and CDC34 together play a critical role in regulating levels of a protein involved in regulating pharyngeal development in the animal [24]. Biochemical evidence supports a coordinated mechanism similar to the one proposed for the human complexes. However, the genetic data in worms indicate that not all CUL-1-dependent processes require HHARI. Furthermore, other cullins in worm did not appear to interact with HHARI genetically [24]. Thus, how general and widespread the RBR/CRL “teamwork” mechanism is remains an open question.
Activation of RNF144A by dimerization
RNF144A consists only of an RBR module (Fig. 1) and C-terminal transmembrane domain (TMD) so it cannot be auto-inhibited by an intramolecular mechanism analogous to other RBR E3s. Instead, RNF144A ligase activity appears to be modulated by dimerization through its TMD via a specific motif (G-x-x-x-G) [39]. RNF144A, RNF19A, RNF19B, and RNF217 all have predicted TMDs that contain the proposed dimerization motif, suggesting that dimerization via transmembrane domains may emerge as another self-regulatory mechanism of RBR E3s [39]. Interestingly, Parkin self-association has recently been suggested to be important for its ligase activity [50].
CONCLUDING REMARKS
While the last to be identified as a mechanistic class of E3 ligases, research on RBR E3s has provided important insights in a relatively short timeframe. The main conceptual framework is in place:
Most, if not all, RBR E3s are auto-inhibited, likely due to the high reactivity of the E3~Ub intermediate.
Auto-inhibition of RBR E3s is usually carried out by intramolecular interactions that involve domains of the catalytic RBR module and other non-RBR domains.
Release of auto-inhibition requires large domain movements and rearrangements.
RBR RING1 domains bind an E2~Ub in an open conformation, with mechanistically important ramifications. Open E2~Ub states are associated with low aminolysis activity, so off-pathway reactions to nearby lysines are minimized, ensuring transfer of Ub from the E2~Ub to the E3 active site.
Interaction between the exposed hydrophobic surface of Ub on the E2~Ub and RING2 facilitates recruitment of the RING2 active site to the E2~Ub to generate the E3~Ub.
RBR E3s (and not the E2s with which they work) dictate the type of modification made to the substrate.
Important and fundamental questions remain:
How do RBR E3s select substrates and how and where on the E3 are substrates bound?
What are the structural determinants of product formation, for example, how is HHARI’s strict mono-ubiquitinating activity dictated?
How is auto-inhibition released and what do activated RBR E3s look like?
Are there other mechanisms in addition to auto-inhibition that modulate RBR E3 activity?
Given the high level and quality of research in this field, we fully expect that exciting new insights will be forthcoming soon.
RESEARCH HIGHLIGHTS.
Intramolecular contacts cause auto-inhibition of RBRs; release requires domain rearrangements.
Mechanism of activation differs among RBR E3s.
RBR RING1 domains bind an E2~Ub in an open conformation.
Open E2~Ubs have low aminolysis activity ensuring transfer of Ub from E2~Ubs to the E3 active site.
Contacts between the Ub of E2~Ub and RBR domains are required to generate the E3~Ub.
RBR E3s (and not their E2s) dictate the type of Ub product made to the substrate.
Acknowledgments
We thank members of the Klevit group and others for their critical reading and suggestions. This work was supported by National Institute of General Medical Sciences grant R01 GM088055 (REK), UW Hurd Fellowship Fund, and PHS NRSA 5T32 GM007270 (KKD).
Footnotes
Note Added in Proof: While this article was in its final production phase, a paper reporting another structure of a complex of HHARI with UbcH7~Ub was published (Yuan et al., "Structural insights into the mechanism and E2 specificity of the RBR E3 ubiquitin ligase HHARI." (2017) Nat. Commun. 8:211).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marin I, Lucas JI, Gradilla AC, Ferrus A. Parkin and relatives: the RBR family of ubiquitin ligases. Physiol Genomics. 2004;17:253–63. doi: 10.1152/physiolgenomics.00226.2003. [DOI] [PubMed] [Google Scholar]
- 2.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–73. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 5.Matsumine H, Saito M, Shimoda-Matsubayashi S, Tanaka H, Ishikawa A, Nakagawa-Hattori Y, et al. Localization of a gene for an autosomal recessive form of juvenile Parkinsonism to chromosome 6q25.2–27. Am J Hum Genet. 1997;60:588–96. [PMC free article] [PubMed] [Google Scholar]
- 6.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–87. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokunaga F, Iwai K. Involvement of LUBAC-mediated linear polyubiquitination of NEMO in NF-kappaB activation. Tanpakushitsu Kakusan Koso. 2009;54:635–42. [PubMed] [Google Scholar]
- 8.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–44. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Iwai K, Fujita H, Sasaki Y. Linear ubiquitin chains: NF-kappaB signalling, cell death and beyond. Nat Rev Mol Cell Biol. 2014;15:503–8. doi: 10.1038/nrm3836. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda F. Linear ubiquitination signals in adaptive immune responses. Immunol Rev. 2015;266:222–36. doi: 10.1111/imr.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elton L, Carpentier I, Verhelst K, Staal J, Beyaert R. The multifaceted role of the E3 ubiquitin ligase HOIL-1: beyond linear ubiquitination. Immunol Rev. 2015;266:208–21. doi: 10.1111/imr.12307. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, Iwai K. Roles of linear ubiquitinylation, a crucial regulator of NF-kappaB and cell death, in the immune system. Immunol Rev. 2015;266:175–89. doi: 10.1111/imr.12308. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Taraborrelli L, Walczak H. Linear ubiquitination in immunity. Immunol Rev. 2015;266:190–207. doi: 10.1111/imr.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittinger K, Ikeda F. Linear ubiquitin chains: enzymes, mechanisms and biology. Open Biol. 2017;7 doi: 10.1098/rsob.170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilera M, Oliveros M, Martinez-Padron M, Barbas JA, Ferrus A. Ariadne-1: a vital Drosophila gene is required in development and defines a new conserved family of ring-finger proteins. Genetics. 2000;155:1231–44. doi: 10.1093/genetics/155.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan NG, Ardley HC, Scott GB, Rose SA, Markham AF, Robinson PA. Human homologue of ariadne promotes the ubiquitylation of translation initiation factor 4E homologous protein, 4EHP. FEBS Lett. 2003;554:501–4. doi: 10.1016/s0014-5793(03)01235-3. [DOI] [PubMed] [Google Scholar]
- 17.Qiu X, Fay DS. ARI-1, an RBR family ubiquitin-ligase, functions with UBC-18 to regulate pharyngeal development in C. elegans. Dev Biol. 2006;291:239–52. doi: 10.1016/j.ydbio.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Elmehdawi F, Wheway G, Szymanska K, Adams M, High AS, Johnson CA, et al. Human Homolog of Drosophila Ariadne (HHARI) is a marker of cellular proliferation associated with nuclear bodies. Exp Cell Res. 2013;319:161–72. doi: 10.1016/j.yexcr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Lin AE, Ebert G, Ow Y, Preston SP, Toe JG, Cooney JP, et al. ARIH2 is essential for embryogenesis, and its hematopoietic deficiency causes lethal activation of the immune system. Nat Immunol. 2013;14:27–33. doi: 10.1038/ni.2478. [DOI] [PubMed] [Google Scholar]
- 20.Hassink G, Slotman J, Oorschot V, Van Der Reijden BA, Monteferrario D, Noordermeer SM, et al. Identification of the ubiquitin ligase Triad1 as a regulator of endosomal transport. Biol Open. 2012;1:607–14. doi: 10.1242/bio.2012778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Bei L, Shah CA, Horvath E, Eklund EA. HoxA10 influences protein ubiquitination by activating transcription of ARIH2, the gene encoding Triad1. J Biol Chem. 2011;286:16832–45. doi: 10.1074/jbc.M110.213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott DC, Rhee DY, Duda DM, Kelsall IR, Olszewski JL, Paulo JA, et al. Two Distinct Types of E3 Ligases Work in Unison to Regulate Substrate Ubiquitylation. Cell. 2016;166:1198–214 e24. doi: 10.1016/j.cell.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsall IR, Duda DM, Olszewski JL, Hofmann K, Knebel A, Langevin F, et al. TRIAD1 and HHARI bind to and are activated by distinct neddylated Cullin-RING ligase complexes. EMBO J. 2013;32:2848–60. doi: 10.1038/emboj.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dove KK, Kemp HA, Di Bona KR, Reiter KH, Milburn LJ, Camacho D, et al. Two functionally distinct E2/E3 pairs coordinate sequential ubiquitination of a common substrate in Caenorhabditis elegans development. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1705060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho SR, Mahanic CS, Lee YJ, Lin WC. RNF144A, an E3 ubiquitin ligase for DNA-PKcs, promotes apoptosis during DNA damage. Proc Natl Acad Sci U S A. 2014;111:E2646–55. doi: 10.1073/pnas.1323107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa J, Ishigaki S, Hishikawa N, Yamamoto M, Doyu M, Murata S, et al. Dorfin ubiquitylates mutant SOD1 and prevents mutant SOD1-mediated neurotoxicity. J Biol Chem. 2002;277:36793–8. doi: 10.1074/jbc.M206559200. [DOI] [PubMed] [Google Scholar]
- 27.Smit JJ, Sixma TK. RBR E3-ligases at work. EMBO Rep. 2014;15:142–54. doi: 10.1002/embr.201338166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morett E, Bork P. A novel transactivation domain in parkin. Trends Biochem Sci. 1999;24:229–31. doi: 10.1016/s0968-0004(99)01381-x. [DOI] [PubMed] [Google Scholar]
- 29.van der Reijden BA, Erpelinck-Verschueren CA, Lowenberg B, Jansen JH. TRIADs: a new class of proteins with a novel cysteine-rich signature. Protein Sci. 1999;8:1557–61. doi: 10.1110/ps.8.7.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–4. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 31.Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–5. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–9. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moynihan TP, Ardley HC, Nuber U, Rose SA, Jones PF, Markham AF, et al. The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1. J Biol Chem. 1999;274:30963–8. doi: 10.1074/jbc.274.43.30963. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel DM, Lissounov A, Brzovic PS, Klevit RE. UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature. 2011;474:105–8. doi: 10.1038/nature09966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smit JJ, Monteferrario D, Noordermeer SM, van Dijk WJ, van der Reijden BA, Sixma TK. The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension. EMBO J. 2012;31:3833–44. doi: 10.1038/emboj.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stieglitz B, Morris-Davies AC, Koliopoulos MG, Christodoulou E, Rittinger K. LUBAC synthesizes linear ubiquitin chains via a thioester intermediate. EMBO Rep. 2012;13:840–6. doi: 10.1038/embor.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaugule VK, Burchell L, Barber KR, Sidhu A, Leslie SJ, Shaw GS, et al. Autoregulation of Parkin activity through its ubiquitin-like domain. EMBO J. 2011;30:2853–67. doi: 10.1038/emboj.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duda DM, Olszewski JL, Schuermann JP, Kurinov I, Miller DJ, Nourse A, et al. Structure of HHARI, a RING-IBR-RING ubiquitin ligase: autoinhibition of an Ariadne-family E3 and insights into ligation mechanism. Structure. 2013;21:1030–41. doi: 10.1016/j.str.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho SR, Lee YJ, Lin WC. Regulation of RNF144A E3 Ubiquitin Ligase Activity by Self-association through Its Transmembrane Domain. J Biol Chem. 2015;290:23026–38. doi: 10.1074/jbc.M115.645499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stieglitz B, Rana RR, Koliopoulos MG, Morris-Davies AC, Schaeffer V, Christodoulou E, et al. Structural basis for ligase-specific conjugation of linear ubiquitin chains by HOIP. Nature. 2013;503:422–6. doi: 10.1038/nature12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trempe JF, Sauve V, Grenier K, Seirafi M, Tang MY, Menade M, et al. Structure of parkin reveals mechanisms for ubiquitin ligase activation. Science. 2013;340:1451–5. doi: 10.1126/science.1237908. [DOI] [PubMed] [Google Scholar]
- 42.Wauer T, Komander D. Structure of the human Parkin ligase domain in an autoinhibited state. EMBO J. 2013;32:2099–112. doi: 10.1038/emboj.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley BE, Lougheed JC, Callaway K, Velasquez M, Brecht E, Nguyen L, et al. Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases. Nat Commun. 2013;4:1982. doi: 10.1038/ncomms2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Aguirre JD, Condos TE, Martinez-Torres RJ, Chaugule VK, Toth R, et al. Disruption of the autoinhibited state primes the E3 ligase parkin for activation and catalysis. EMBO J. 2015;34:2506–21. doi: 10.15252/embj.201592337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauve V, Lilov A, Seirafi M, Vranas M, Rasool S, Kozlov G, et al. A Ubl/ubiquitin switch in the activation of Parkin. EMBO J. 2015;34:2492–505. doi: 10.15252/embj.201592237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spratt DE, Mercier P, Shaw GS. Structure of the HHARI catalytic domain shows glimpses of a HECT E3 ligase. PLoS One. 2013;8:e74047. doi: 10.1371/journal.pone.0074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechtenberg BC, Rajput A, Sanishvili R, Dobaczewska MK, Ware CF, Mace PD, et al. Structure of a HOIP/E2~ubiquitin complex reveals RBR E3 ligase mechanism and regulation. Nature. 2016;529:546–50. doi: 10.1038/nature16511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dove KK, Olszewski JL, Martino L, Duda DM, Wu XS, Miller DJ, et al. Structural Studies of HHARI/UbcH7 approximately Ub Reveal Unique E2 approximately Ub Conformational Restriction by RBR RING1. Structure. 2017 doi: 10.1016/j.str.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spratt DE, Martinez-Torres RJ, Noh YJ, Mercier P, Manczyk N, Barber KR, et al. A molecular explanation for the recessive nature of parkin-linked Parkinson’s disease. Nat Commun. 2013;4:1983. doi: 10.1038/ncomms2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar A, Chaugule VK, Condos TEC, Barber KR, Johnson C, Toth R, et al. Parkin-phosphoubiquitin complex reveals cryptic ubiquitin-binding site required for RBR ligase activity. Nat Struct Mol Biol. 2017;24:475–83. doi: 10.1038/nsmb.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spratt DE, Walden H, Shaw GS. RBR E3 ubiquitin ligases: new structures, new insights, new questions. Biochem J. 2014;458:421–37. doi: 10.1042/BJ20140006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beasley SA, Hristova VA, Shaw GS. Structure of the Parkin in-between-ring domain provides insights for E3-ligase dysfunction in autosomal recessive Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:3095–100. doi: 10.1073/pnas.0610548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wenzel DM, Klevit RE. Following Ariadne’s thread: a new perspective on RBR ubiquitin ligases. BMC Biol. 2012;10:24. doi: 10.1186/1741-7007-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dove KK, Stieglitz B, Duncan ED, Rittinger K, Klevit RE. Molecular insights into RBR E3 ligase ubiquitin transfer mechanisms. EMBO Rep. 2016;17:1221–35. doi: 10.15252/embr.201642641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smit JJ, van Dijk WJ, El Atmioui D, Merkx R, Ovaa H, Sixma TK. Target specificity of the E3 ligase LUBAC for ubiquitin and NEMO relies on different minimal requirements. J Biol Chem. 2013;288:31728–37. doi: 10.1074/jbc.M113.495846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita H, Rahighi S, Akita M, Kato R, Sasaki Y, Wakatsuki S, et al. Mechanism underlying IkappaB kinase activation mediated by the linear ubiquitin chain assembly complex. Mol Cell Biol. 2014;34:1322–35. doi: 10.1128/MCB.01538-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A. 2013;110:15247–52. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014;56:360–75. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiesel FC, Moussaud-Lamodiere EL, Ando M, Springer W. A specific subset of E2 ubiquitin-conjugating enzymes regulate Parkin activation and mitophagy differently. J Cell Sci. 2014;127:3488–504. doi: 10.1242/jcs.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim GG, Chew KC, Ng XH, Henry-Basil A, Sim RW, Tan JM, et al. Proteasome inhibition promotes Parkin-Ubc13 interaction and lysine 63-linked ubiquitination. PLoS One. 2013;8:e73235. doi: 10.1371/journal.pone.0073235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haddad DM, Vilain S, Vos M, Esposito G, Matta S, Kalscheuer VM, et al. Mutations in the intellectual disability gene Ube2a cause neuronal dysfunction and impair parkin-dependent mitophagy. Mol Cell. 2013;50:831–43. doi: 10.1016/j.molcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Zheng N, Wang P, Jeffrey PD, Pavletich NP. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–9. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 63.Brzovic PS, Keeffe JR, Nishikawa H, Miyamoto K, Fox D, 3rd, Fukuda M, et al. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc Natl Acad Sci U S A. 2003;100:5646–51. doi: 10.1073/pnas.0836054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mani K, Fay DS. A mechanistic basis for the coordinated regulation of pharyngeal morphogenesis in Caenorhabditis elegans by LIN-35/Rb and UBC-18-ARI-1. PLoS Genet. 2009;5:e1000510. doi: 10.1371/journal.pgen.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis M, Vyse S, Shields A, Boeltz S, Gordon P, Spector T, et al. Effect of UBE2L3 genotype on regulation of the linear ubiquitin chain assembly complex in systemic lupus erythematosus. Lancet. 2015;385(Suppl 1):S9. doi: 10.1016/S0140-6736(15)60324-5. [DOI] [PubMed] [Google Scholar]
- 66.Fu B, Li S, Wang L, Berman MA, Dorf ME. The ubiquitin conjugating enzyme UBE2L3 regulates TNFalpha-induced linear ubiquitination. Cell Res. 2014;24:376–9. doi: 10.1038/cr.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pruneda JN, Stoll KE, Bolton LJ, Brzovic PS, Klevit RE. Ubiquitin in motion: structural studies of the ubiquitin-conjugating enzyme approximately ubiquitin conjugate. Biochemistry. 2011;50:1624–33. doi: 10.1021/bi101913m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Branigan E, Plechanovova A, Jaffray EG, Naismith JH, Hay RT. Structural basis for the RING-catalyzed synthesis of K63-linked ubiquitin chains. Nat Struct Mol Biol. 2015;22:597–602. doi: 10.1038/nsmb.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plechanovova A, Jaffray EG, Tatham MH, Naismith JH, Hay RT. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature. 2012;489:115–20. doi: 10.1038/nature11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saha A, Lewis S, Kleiger G, Kuhlman B, Deshaies RJ. Essential role for ubiquitin-ubiquitin-conjugating enzyme interaction in ubiquitin discharge from Cdc34 to substrate. Mol Cell. 2011;42:75–83. doi: 10.1016/j.molcel.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pruneda JN, Littlefield PJ, Soss SE, Nordquist KA, Chazin WJ, Brzovic PS, et al. Structure of an E3:E2~Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol Cell. 2012;47:933–42. doi: 10.1016/j.molcel.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dou H, Buetow L, Sibbet GJ, Cameron K, Huang DT. BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat Struct Mol Biol. 2012;19:876–83. doi: 10.1038/nsmb.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang MY, Vranas M, Krahn AI, Pundlik S, Trempe JF, Fon EA. Structure-guided mutagenesis reveals a hierarchical mechanism of Parkin activation. Nat Commun. 2017;8:14697. doi: 10.1038/ncomms14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–41. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–6. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 76.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–6. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 77.Yagi H, Ishimoto K, Hiromoto T, Fujita H, Mizushima T, Uekusa Y, et al. A non-canonical UBA-UBL interaction forms the linear-ubiquitin-chain assembly complex. EMBO Rep. 2012;13:462–8. doi: 10.1038/embor.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivkin E, Almeida SM, Ceccarelli DF, Juang YC, MacLean TA, Srikumar T, et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature. 2013;498:318–24. doi: 10.1038/nature12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keusekotten K, Elliott PR, Glockner L, Fiil BK, Damgaard RB, Kulathu Y, et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell. 2013;153:1312–26. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wauer T, Simicek M, Schubert A, Komander D. Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature. 2015;524:370–4. doi: 10.1038/nature14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kazlauskaite A, Martinez-Torres RJ, Wilkie S, Kumar A, Peltier J, Gonzalez A, et al. Binding to serine 65-phosphorylated ubiquitin primes Parkin for optimal PINK1-dependent phosphorylation and activation. EMBO Rep. 2015;16:939–54. doi: 10.15252/embr.201540352. [DOI] [PMC free article] [PubMed] [Google Scholar]


