Abstract
Background/Purpose
Phosphoglucomutase-1 deficiency is a subtype of congenital disorders of glycosylation (PGM1-CDG). Previous case-reports in PGM1-CDG patients receiving oral D-galactose (D-gal) showed clinical improvement. So far no systematic in vitro and clinical studies assessed safety and benefits of D-gal supplementation. In a prospective pilot study, we evaluated the effects of oral D-gal in nine patients.
Methods/Results
D-gal supplementation was increased to 1.5 g/kg/day (maximum 50 g/day) in three increments over 18 weeks. Laboratory studies were performed before and during treatment to monitor safety and effect on serum transferrin-glycosylation, coagulation, liver and endocrine function. Additionally, the effect of D-gal on cellular glycosylation was characterized in vitro.
Eight patients were compliant with D-gal supplementation. No adverse effects were reported. Abnormal baseline results (ALT/AST/aPTT) improved or normalized already using 1g/kg/day D-gal. Antithrombin-III levels and Transferrin-glycosylation showed significant improvement, and increase in galactosylation and whole glycan content. In vitro studies before treatment showed N-glycan hyposialylation, altered O-linked glycans, abnormal LLO-profile, and abnormal nucleotide-sugars in patient fibroblasts. Most cellular abnormalities improved or normalized following D-gal treatment.
Conclusion
D-gal increased both UDP-Glc and UDP-gal levels and improved LLO fractions in concert with improved glycosylation in PGM1-CDG. Oral D-gal supplementation is a safe and effective treatment for PGM1-CDG in this pilot study. Transferrin glycosylation and ATIII levels were useful trial end points. Larger, longer duration trials are ongoing.
Keywords: phosphoglucomutase 1, D-galactose, liver function, coagulation, endocrine, transferrin glycoforms, Antithrombin III, LLO, N-glycosylation, O-glycosylation, glycomics
INTRODUCTION
Congenital disorders of glycosylation (CDGs) are defects in glycoprotein and glycolipid synthesis1. Phosphoglucomutase 1 deficiency (PGM1-CDG; MIM 612941) is a recently characterized CDG with oro-facial malformations and multisystem involvement including cardiomyopathy, coagulopathy, endocrinopathies, and hepatopathy2–4. These clinical features have been attributed to abnormal protein glycosylation. Additionally, patients suffer from recurrent hypoglycemia and myopathy, which is thought to occur by insufficient glycogen mobilization due to PGM1 deficiency5,6.
PGM1 is required for the interconversion of glucose 1-phosphate and glucose 6-phosphate, and is a key enzyme in glycolysis, glycogenesis, and glycogenolysis7,8. Although the exact mechanism remains unclear, PGM1 deficiency has been associated with abnormal intracellular levels of glucose and galactose metabolites, as well as reduced assembly and remodeling of N-linked glycans. These metabolic disturbances are thought to be responsible for both the missing and the truncated glycans detected in serum by transferrin isoform analysis. There is a lack of whole glycans and reduction of galactose units and terminal sialic acids in truncated glycans in PGM1-CDG, demonstrated by QTOF mass spectrometry3.
In a landmark study, we reported on improved glycosylation with galactose supplementation in PGM1-CDG3. In vitro studies in skin fibroblast cell lines derived from three patients showed improvement in protein glycosylation following D-galactose (D-gal) treatment. Additionally, in six patients who received dietary supplementation of D-gal, both transferrin glycosylation and total serum N-glycome showed improvement within two weeks. Oral lactose supplements had minimal effect on transferrin-IEF, and high dietary lactose content didn’t affect clinical outcome. While improvements were documented in a few patients ingesting D-gal, a systematic clinical study has yet to assess the effect of oral galactose supplementation on other features of PGM1-CDG and establish the extent of clinical improvement.
PATIENTS AND METHODS
Study design
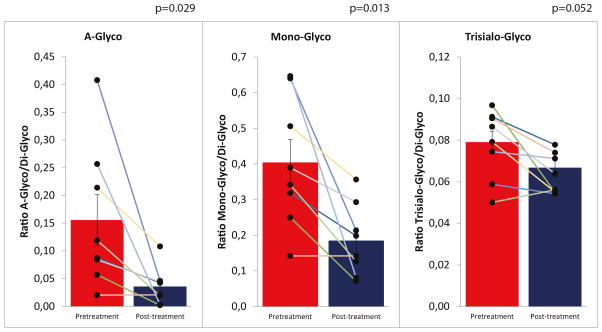
Nine patients with PGM1 deficiency were enrolled in a prospective pilot-study (Supplementary files, clinicaltrials.gov NCT02955264). The primary endpoint was short-term safety and tolerability of oral D-gal supplementation and a major goal was to identify physiological biomarkers that are responsive to D-gal supplementation in a heterogeneous genetic background. The secondary endpoints were the restoration of the plasma glycan sub-fractions, monitored via glycomics (Figure 3, Supplementary Table 3), and normalization of antithrombin III activity and serum alanine transaminase (ALT) levels.
Figure 3.
High-resolution transferrin glycosylation analysis in PGM1-CDG patients before and after D-gal supplement. Ratios of a-, mono- and tri-sialo over tetra-sialo transferrin were calculated and compared with references ranges (Repeated measures of ANOVA).
A-Glyco: <= 0,01040, Mono-glyco: <=0,02700 Trisyalo-Glyco: <=0,031900.
Patient data, mutations, and PGM1 enzyme activity are reported in Table 1, clinical features in Supplementary Table 1.
Table 1.
List of all study participants with their cDNA mutations, the respective amino acid changes and residual activities.
| Patient | Sex | Age* | cDNA mutation (NM_002633.2) | Amino Acid Change (NP_002624.2) | Genotype | PGM1 enzyme activity in cultured skin fibroblasts (% of controls) |
|---|---|---|---|---|---|---|
| 1# | F | 21 years | c.1264C>T c.1588C>T |
p.R422W p.Q530X |
Heterozygous compound nonsense and missense | 0 |
| 2% | M | 11 years | c.1010C>T c.1508G>A |
p.T337M p.R503Q |
Heterozygous compound missense | 5 |
| 3$ | F | 19 years | c.988G>C c.1129G>A |
p.G330R p.E377K |
Heterozygous compound missense | 1.3$ |
| 4# | M | 2 years | c.157_158delinsG c.1507C>T c.661C>T c.1258T>C |
p.Q53Gfs*15 p.R503X p.R221C p.Y420H |
Heterozygous compound nonsense and missense | 5# |
| 5$ | M | 13 years | c.787G>T c.1551C>A |
p.D263Y p.Y517X |
Heterozygous compound nonsense and missense | 2.8$ |
| 6# | F | 3 years | c.689G>A | p.G230E | Homozygous missense | NA# |
| 7 | F | 19 months | c.661C>T c.1258T>C |
p.R221C p.Y420H |
Heterozygous compound missense | 17** |
| 8$ | F | 16.5 years | 1507C>T | p.R503X | Homozygous nonsense | 7.7 |
| 9# | M | 2 years | c.112A>T | p.N38Y | Homozygous missense | 3.1# |
F, female; M, male; NA, not available.
PGM1 enzyme activity measurements are included where available. Enzyme activity was assayed in cultured skin fibroblasts derived from patients, except for patient 7, where the activity was measured in patient blood. PGM1 is present in leukocytes but absent in red blood cells, where PGM2 is the dominant PGM isoenzyme. Although PGM2 is more active as a phosphopentomutase than as a phosphoglucomutase, it has shown to exhibit about 10% phosphoglucomutase activity in vitro (Maliekal et al. 2007).
Individuals previously reported are indicated by
(Ondruskova et al. 2014), and
Age at the time of study enrollment
PGM1 enzyme activity measured in blood
Additionally, we studied the biological effect of galactose in vitro in patient skin fibroblasts.
Three escalating doses of D-gal over 18 weeks
Participants in this pilot study received oral D-gal supplementation added to the regular diet for 18 weeks. D-gal (D-GALACTOSE© or Galaxtra©; Supplementary files) intake was increased over the study period in increments to avoid gastrointestinal side effects; weeks 0–6 (T0–T1), 0.5 g/kg per day; weeks 6–12 (T1–T2), 1.0 g/kg per day; weeks 12–18 (T2–T3), 1.5 g/kg per day (Supplementary files, Figure Sa). The maximum daily oral dose of D-gal any patient received was 50.0 g, an amount that is within the recommended daily maximum intake and demonstrated safe9. Dietary assessment (lactose intake; Supplementary files), clinical evaluation, and laboratory studies were completed every 6 weeks (T0, T1, T2 and T3). Liver transaminases (AST, ALT), creatine kinase (CK), and commonly assayed glycoproteins (TSH, TBG, IGF3BP), and coagulation parameters (factors IX, aPTT, and ATIII), were measured in blood (Supplementary files). Blood galactose-1-phosphate and urine galactitol were measured to monitor safety. Glycosylation studies included transferrin-IEF and glycomics in serum by mass spectrometry. Electrocardiography/echocardiography and hepatic ultrasonography were conducted at T0 and T3.
Long term monitoring
Patient 1 remained on D-gal 1g/kg/day after the study-period as D-gal is the only currently available compassionate treatment for PGM1-CDG. The parameters monitored during the study-period, including additional coagulation factor measurements, have been followed as part of routine care (Supplementary Table 2).
Phosphoglucomutase 1 enzyme activity
Measurements in patient fibroblasts (Supplementary files) were performed in all patients, except for patient 7 (screening performed in blood).
Glycomics analysis in patient blood at T0 and T3
Based on serum glycan subfractions, ratios were calculated and compared to reference ranges10 (Supplementary files, Figure 3, Supplementary Table 3).
Statistics
Quantitative data are presented as the mean ± standard error of the mean (SEM). Repeated measures of ANOVA was used to determine significant differences between pre and post D-gal supplement use. p ≤ 0.05 was considered significant.
In vitro studies characterizing the effect of D-gal supplementation on glycosylation
Skin fibroblast culture
Fibroblasts were studied from four PGM1-CDG patients (patient 1, 2, 5 and 8) who participated in this prospective pilot study. Additionally, cell lines of two other PGM1-CDG patients were used in some of the in vitro experiments (patient cell-line 2015X and cell-line 2013Y, Table 1).
D-gal supplementation in culture
D-gal (Sigma-Aldrich) was added to culture media at concentrations 0, 0.75, 2.0, or 5 mM. The duration of D-gal feeding was one, four, five or seven days. Culture media was refreshed every two days. For LLO, and PLO measurements 10mM D-gal was added to a serum deprived culture medium 1 hour prior to the labeling procedure (Supplementary files).
PGM1 Western blotting and ICAM-1 Western blotting
Western blotting in patients’ fibroblasts in the absence and the presence of D-gal was performed in patients 1, 2, 5, 8, and cell-line 2015X in vivo (Supplementary files).
Lipid-linked and Protein-linked Oligosaccharide Analysis
Fibroblasts of patients 1, 2, 8, cell-line 2013Y and two controls were analyzed with or without D-gal supplementation11.
Nucleotide sugar analysis
Nucleotide sugar analysis for UDP-galactose (UDP-Gal) and UDP-glucose (UDP-Glc) was performed in 1 million skin fibroblast cells derived from controls, patients 1, 2 and 8, and 2013Y. Either 0.75 or 2.0 mM D-gal was added to the culture medium for one day or four days prior to harvesting by scraping12 (Supplementary files).
MALDI-TOF Mass Spectroscopy
Fibroblasts from patient 2 were used for N- and O-linked glycan analysis with and without D-gal feeding in vitro13 (Supplementary files).
RESULTS
Escalating dosing of D-gal up to 50 g/day is safe and tolerated
Of the nine patients, eight patients were compliant with daily oral D-gal supplementation. Protocol violations were reported in two patients (Supplementary data). Patient 8 was non-compliant, and we excluded her from clinical analysis. Her glycomics analysis was evaluated, however, since her serum was collected throughout the study.
Dietary evaluation reported variable daily dairy intake. No patients consumed more than 0.4g/kg/day dietary lactose (equivalent to 0.2g/kg/day D-gal).
Adverse events (AEs) were monitored weekly. Aside from gastroenteritis in patient 2, no clinical or metabolic AEs were reported to be associated with incremental increase in D-gal intake. No reported serious AE were reported.
Safety parameters were normal and remained stable during the trial, with no patients experiencing increased galactitol excretion in urine. Galactose-1-phosphate levels were successfully monitored in patient 1, 2, 3, 5, 7 and 9, and no abnormalities were found at T2 and T3 (data not shown). No structural cardiac or liver changes were noted at study endpoint (data not shown).
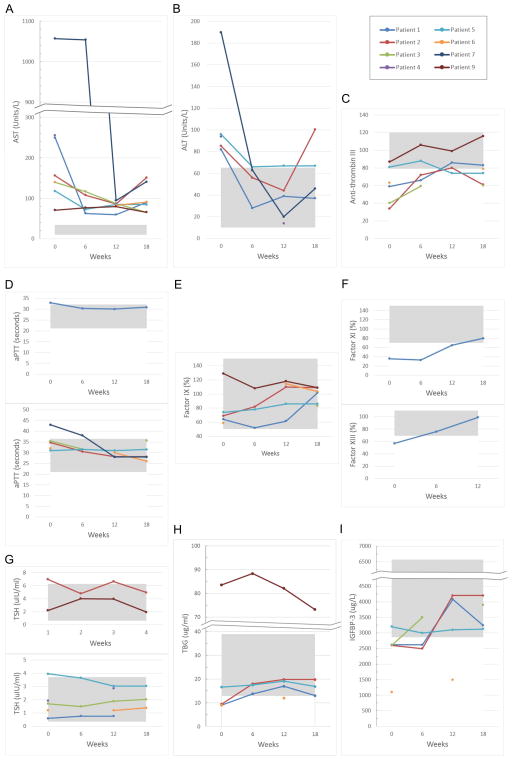
Liver function: improvement in AST and normalization of ALT
Baseline AST of all patients was 3- to 30-fold higher than the upper limit of normal (Figure 1a). AST moderately declined at 6 weeks, and at 12 weeks, AST declined by 23% to more than 10-fold. With the exception of patient 2, AST remained relatively stable, and while it did not normalize at 18 weeks, it fell below baseline. patient 2 developed an intercurrent illness and discontinued galactose supplementation for 2 weeks between the 12- and 18-week time-point. His AST spiked upwards, almost reaching baseline at 18 weeks.
Figure 1.
Effect of D-gal supplementation on (a) aspartate transaminase (AST) and (b) alanine transaminase (ALT), on (c) anti-coagulation and (d) (e) (f) coagulation, on (g) TSH, (h) TBG, and (i) IGFBP-3. The shadowed area represents the reference range.
Unlike AST, ALT normalized in several patients (Figure 1b). Baseline ALT was abnormal in 5 patients (patient 1, 2, 4, 5, and 7); the rest exhibited normal ALT throughout the study. Among the 5 patients with abnormal baseline ALT, patient 1, 2, 4, and 7 normalized at 6 or 12 weeks. Except for patient 2, ALT remained normalized and stable at 12 and 18 weeks. While ALT in patient 2 normalized at 6 and 12 weeks, at 18 weeks it spiked upwards and exceeded baseline by 18 %. Patient 2 had an inter-current illness (see above). Patient 5 ALT decreased by 43 % at 6 weeks and remained stable at 12 and 18 weeks, at 3 % above the upper limit of normal.
ALT levels were found to be significantly decreased from T0 to T2 during trial (p=0.05). A dosage of 1.5 g/kg/day in the last 6 weeks of the trial did not lead to significant improvement of the laboratory parameters compared to those at 1 g/kg/day.
Improvement and normalization of coagulation parameters
Anticoagulation data was available for patients 1, 2, 3, 5, 6, and 9 (Figure 1c). Only patient 9 had a normal baseline. ATIII normalized in patients 1 and 2 at 12 weeks. Patient 1 remained within normal range at 18 weeks. While patient 2’s ATIII dropped 30 % following illness and cessation of galactose intake, ATIII was maintained at two-fold higher than baseline at 18 weeks. Patients 3 and 6 improved by 25 % to 50 % at 18 weeks, but did not normalize. Patient 5 transiently normalized at 6 weeks, but fell 8 % below the lower limit of normal at 12 and 18 weeks.
With the inclusion of all patient data for ATIII there was a significant difference between T0 and T3 data point for ATIII (P=0.020)
The aPTT baseline was normal in patients 2, 3, 5, 6, and remained stable during the study (Figure 1d). aPTT baseline was abnormal in patients 1 and 7 and normalized at 6 and 12 weeks, respectively.
Factor IX data was available for patients 1, 2, 5, 6 and 9, and the baseline was normal (Figure 1e). Factor IX levels remained normal following 18 weeks of galactose supplementation.
Other coagulation parameters, factor XI and factor XIII, normalized in patient 1 after 12–18 weeks of galactose supplementation (Figure 1f, Figure Sb).
Improvement in glycoproteins TSH, TBG, and IGFBP-3
TSH data is available for all patients except patient 7 (Figure 1g). Patient 2 and 5 had abnormal baseline. Patient 2 normalized at 18 weeks while patient 5 normalized at 6 weeks and remained stable at 12 and 18 weeks.
TBG data is available for patients 1, 2, 5, 6, and 9 (Figure 1h). All patients had abnormal baseline, except for patient 5. Patients 1 and 2 normalized at 6 weeks and remained stable at 12 and 18 weeks. Patient 6 improved by 30 % at 12 weeks, only 8 % below the lower limit of normal. Patient 9’s baseline was two-fold the upper limit of normal. Although his TBG level declined by 12 % at 18 weeks, it was still 80 % higher than the upper limit of normal.
IGFBP-3 data is available for patient 1, 2, 3, 5, and 6 (Figure 1i). Except for patient 5, the baselines were all abnormal, ranging from 8 % to 60 % below the lower limit of normal. Patients 1 and 2 normalized at 12 weeks and remained stable at 18 weeks. The 12-week time point data was missing for patient 3; nevertheless it normalized at 18 weeks. Patient 6 showed slight improvement at 12 weeks but remained about 50 % below the lower limit of normal (Figure Sb).
Fluctuating and abnormal levels of serum creatine kinase and glucose
CK and glucose levels fluctuated and remained abnormal during the study. Despite severe elevation of CK, none of the patients experienced clinical rhabdomyolysis while on galactose supplementation. The frequency of hypoglycemic episodes decreased significantly in four patients. Patient 1 and 6 had recurrent hypoglycemic episodes upon fasting. Patient 5 remained on Diazoxide treatment due to hyperinsulinism (data not shown).
Improvement of serum transferrin glycosylation in 8 patients
IEF and high-resolution mass spectrometry of intact transferrin confirmed the characteristic mixed type I/II pattern with increased fractions of both truncated glycans and lack of whole glycans in all patients’ sera. Clear improvement of transferrin glycosylation was seen in 8 patients, with patterns shifting from a combined type 1/2 pattern to a mild type 1 pattern. No change was observed in patient 5. None of the patients showed a fully normalized pattern at T3. (Supplementary files, Supplementary Table 3, Figure 3).
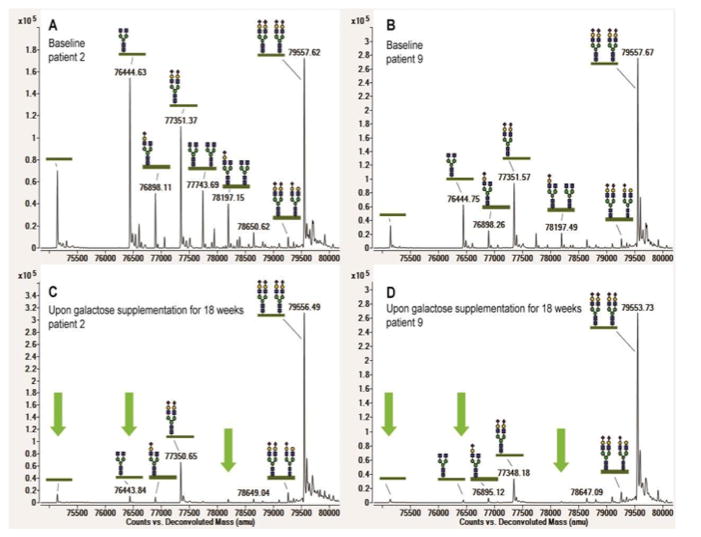
The most abundant peaks in the transferrin mass spectrometry profiles were annotated, and ratios were calculated for the non-glycosylated (A-Glyco), mono-glycosylated (Mono-Glyco), and trisialo-transferrin (Trisialo-Glyco) over the normal transferrin peak (Di-Glyco). Ratios were compared in patient samples before D-gal supplementation and 18 weeks after starting oral supplements according to protocol. An example is shown in Figure 2 for a severely (patient 2) and a mildly abnormal profile (patient 9). A-Glyco- and Mono-Glyco ratios were abnormal in all patients. All patients except patient 5 showed significant improvement on D-gal supplementation (Figure 3). The most pronounced improvement was measured in patient 4. In patients 3 and 8, the A-Glyco peak normalized. (Supplementary Table 3, Figure 3).
Figure 2.
High-resolution mass spectrometry of intact serum transferrin. Baseline profiles of patient 2 (a) and patient 9 (b) show characteristic PGM1 glycoforms with truncated glycans and lack of whole glycans. Patient 9 shows a milder profile than patient 2; Spectra of patient 2 (c) and patient 9 (d) show large improvement through the reduction of abnormal glycosylation peaks upon 18 weeks of galactose treatment, as is highlighted by the green arrows.
Long term monitoring of patient 1
Patient 1 remained on galactose therapy at 1g/kg/day for an additional 12 months after the study ended. The parameters that improved and normalized during the study period, including ALT, aPTT, ATIII, TSH, TBG remained stable (Supplementary Table 2). However, factor XI and factor XIII returned to before treatment levels. Surprisingly, CK, which remained high during the study (5-fold the upper limit of normal), decreased by almost 80 % over the course of one year, falling to near upper limit of normal.
In vitro studies in PGM1-CDG skin fibroblasts
PGM1 protein expression was moderately reduced in patient 2, who carries heterozygous missense mutations (T337M and R503Q). No protein was visible in patient 1 (R422W and Q530X) and cell-line 2015X (G382Vfs*2) (Figure S1a).
ICAM-1 protein expression was markedly diminished in all four patients and an up to 2-fold increase in ICAM-1 was observed in patients 1, 2, and cell-line 2015X following galactose treatment. No improvement was detected in patient 5 (Supplements and Supplementary Figure1). Glycomics showed that both asialylated and monosialylated biantennary N-glycan species were elevated (>2 STD from control mean) in N-glycan profiles of total glycoprotein isolated from the cultured skin fibroblasts of patient 2. Following galactose supplementation, asialylated biantennary glycan (Hexose5HexNAc4) at m/z 2070.06 was reduced to normal level. Monosialylated glycans(sial1hexose5HexNAc3) at m/z 2605+2431, while far from normalized, showed a slight reduction (Supplementary Table 4a).
Unexpectedly, altered O-glycosylation was detected in patient 2’s cultured skin fibroblasts, with decreased disialyl core 1 glycans at m/z 1256 and increased disialyl core 2 at 1706 m/z. These alterations improved but did not completely normalize with galactose supplementation. Interestingly, galactose supplementation increased monosialo core 1 at m/z 895 both in control and PGM1-CDG cells (Supplementary Table 4b).
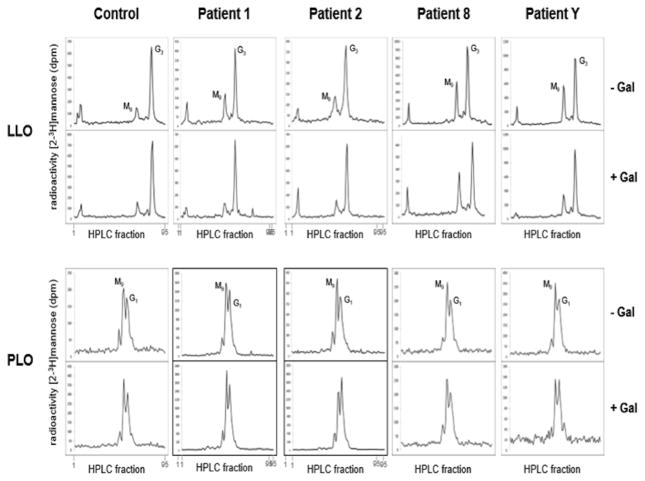
LLO analysis detected a large amount of shortened LLO in all four patient cells (Man9GlcNAc2-PP-dolichol). Galactose supplementation led to the reduction of shortened LLO (Man9GlcNAc2-PP-dolichol) in patients 1, 2, and cell-line 2013Y, resulting in a LLO profile similar to control. No improvement was observed in patient 8. In none of the patients D-gal supplementation had an effect on the PLO profile (Figure 4, LLO top and bottom row).
Figure 4.
Lipid linked oligosaccharide analysis in 4 PGM1 deficient cell lines showing reduced LLO at Baseline and Improvement Following D-gal Supplementation
To investigate whether PGM1 deficiency disrupts the formation of LLO, which is a required precursor for the synthesis of nascent N-linked glycoproteins in the ER, we performed lipid linked oligosaccharide (LLO) and protein linked oligosaccharide (PLO) analyses in the skin fibroblasts of patients 1, 2, 8, and cell-line 2013Y. The cells were deprived of glucose while the culture media was supplemented without or with 10mM D-gal (for one hour. The level of full-length LLO (Glc3Man9GlcNAc2-PP-Dol, G3) or sugar moieties bound to newly synthesized PLO (mainly Glc1Man9 and Man9), remained fairly unchanged in control cells (Figure 4, left column), indicating a high degree of metabolic fitness in these cells. In contrast, cells from all four patients showed a large amount of shortened LLO (Man9GlcNAc2-PP-dolichol) (Figure 4, LLO top row). In contrast, the PLO profile was indistinguishable from the control cells (Figure 4, PLO top row). Interestingly, galactose supplementation led to the reduction of shortened LLO (Man9GlcNAc2-PP-dolichol) in patients 1 2, and cell-line 2013Y, resulting in a LLO profile that is similar to control. No improvement was observed in patient 8 (Figure 4, LLO bottom row). D-Gal supplementation had no effect on the PLO profile in all patients (Figure 4, PLO bottom row).
Because the synthesis of the sugar donor substrate, dolichyl-phosphate-glucose (Dol-P-Glc), requires UDP-Glc, we hypothesized that PGM1 deficiency would disrupt normal glucose and galactose metabolism, leading to a dis-balance of available nucleotide sugars for Dol-P-Glc synthesis and resulting in the observed accumulation of shortened LLO (Man9GlcNAc2-PP-dolichol). In patient cell lines 1, 2, 8 and cell-line 2013Y, we found increased levels of UDP-Glc (72.2 % and 49.3 % of control, 141.3% and 90.5% of control, 60.9% and 23.0% of control and 142.1% and 87.7% of control, respectively) and in a lesser degree UDP-Gal (29.6 % and 23.4 % of control, 84.4% 69.7% of control, 9.1% and −22% of control, 48.3% and 32.7% of control, respectively). D-Gal supplementation increased the levels of both UDP-Glc and UDP-Gal. The same effect in control cells occurred on the first day of D-gal supplements but normalized at 4 days. In all patient cell lines both UDP-glc and UDP-gal remained significantly increased on 4 days of D-gal supplementation. Interestingly in Patient 1, 2 and 2013Y UDP-glc got more then twice elevated compared to control. Nucleotide sugar ratios UDP-glc/UDP-gal in controls were at a mean of 1.2, as previously reported3, both with, or without D-gal, while in patient cell lines at mean 1.63, similar to that on D-gal (1.56) (Supplementary Table 5).
DISCUSSION
Clinical and laboratory improvement in patients
There is no proven effective treatment available in most CDG types, except for MPI-CDG, where coagulopathy and hypoglycemia are successfully treated by oral mannose supplementation14–16. Based on a hypo-galactosylation glycan-pattern in PGM1-CDG, compassionate use of D-gal resolved hypogonadotropic hypogonadism in two patients3. In the present prospective pilot study, we assessed different laboratory parameters as potentially secondary end point markers in blood. Those, abnormal at baseline, improved or normalized by 12 or 18 weeks, with a few exceptions. ALT was abnormal at baseline in half of the patients and completely normalized in all but one patient. Interestingly during infection-related diarrhea in patient 2 the liver transaminases dramatically re-increased within 2 weeks. AST was abnormal in all patients. Although AST levels markedly improved in most patients on D-gal, none normalized during the study period. AST is also a biomarker for muscle involvement. Creatine kinase, another biomarker of muscle injury, remained also elevated in all patients. Persistently elevated AST might be indicative of unresolved myopathy, caused by insufficient glycogenolysis17. The observed improvement in both serum transaminases suggests that galactose supplementation ameliorates liver dysfunction, thus decreasing the risk for hepatic steatosis and fibrosis. We also demonstrated that D-gal supplementation was safe and tolerated at 1.5g/kg/day, even using 50 g/day/day (patients 1, 2 and 3). ATIII also improved and found to be significantly higher in patients on D-gal. ATIII could be a potential biomarker for future treatment trials.
Long-term use of galactose in patient 1 over one year beyond the study period showed normalization of almost all biochemical anomalies, except for transferrin glycosylation. Normalization of several coagulation factors on galactose resolved the patient’s frequent, spontaneous epistaxis episodes, and improved quality of life. Some of the coagulation factors remained abnormal, which might suggest the need for either higher D-gal dose or longer treatment and follow up. Interestingly, the frequency of hypoglycemia and fluctuation of creatine kinase levels also decreased, without episodes of rhabdomyolysis.
Improved glycosylation in patients
Serum transferrin glycosylation showed a characteristic PGM1 mixed type I/ type II profile in all patients at baseline, with truncated glycans and lack of whole glycans. Upon galactose supplementation, the spectrum shifted from a mixed profile to a mild type I profile. Only patient 5 did not shift to a type I glycosylation, but this patient was also on the mildest spectrum of all patients. The spectra did not completely normalize, mostly type 1 peaks remained present. However, most of the truncated galactose-lacking glycoforms strongly decreased during this 18 weeks of supplementation. Overall, the spectra suggest that galactose is able to directly restore the lack of galactose on the truncated glycans, while there seems to be a second, unsolved mechanism of action of galactose on the more slow improvement of CDG type I glycosylation, which could lead potentially a completely normalized glycosylation.
In vitro D-gal effects on glycosylation in patient fibroblasts
ICAM1 is a known cell-marker of glycosylation. In contrast to patient 5, patients 1 and 2, and cell-line 2015X showed an increase in ICAM1 expression upon in vitro galactose treatment. This was in concert with the improvement of glycomics results in fibroblasts of patient 2 on galactose. Interestingly, before in vitro galactose treatment the sialylation of N-glycans was found to be reduced, with increased hyposialylated subspecies. Galactose treatment improved sialylation (Supplementary).
O-linked glycosylation in PGM1-deficient cells showed a significant reduction of disialo-core1 species and increase of sialylated core2 species. Since core-2 GlcNAc-transferase, the rate-limiting step of core2 synthesis, competes with ST6GalNAc-transferase, increased sialylated core2 and core1 ratio points to a deficiency at the step of ST6GalNAc transferase that forms 2,6 sialic acid motifs on disialo-core1 T antigen. However, 2,3 sialylation in O-linked glycosylation is not significantly affected in PGM1-CDG fibroblasts. Similar to the effect on N-linked glycosylation, galactose treatment improved the level of disialo core1 species and partially corrected the ratio between disialo core2/disialo core1. The Km of CMP-sialic acid for sialyltransferase of O-linked glycosylation is at least 10 times higher than the Km for sialyltransferases of N-linked glycosylation. Thus, it is more likely that the selective difference between 2,3 sialylation and 2,6 sialylation in O-GalNAc glycosylation is related to the difference in Km of these different sialyltransferases for CMP-sialic acid. Future studies on CMP-sialic acid levels in PGM1 deficient cells is necessary to better understand the changes in O-linked glycosylation.
In vitro galactose effects on lipid-linked oligosaccharides in patient fibroblasts
LLO analysis on serum starvation revealed an accumulation of shortened LLO in cultured patient skin fibroblasts. This indicates a disruption in the synthesis of LLO, required for N-linked glycosylation in the ER. Interestingly, accumulation of Man9GlcNAc2-PP-Dol is characteristic for several type I CDG, including ALG6-CDG18, ALG8-CDG19, and MPDU1-CDG20,21. Whereas the activity of glucosyltransferases is impaired in the first two CDG subtypes, the synthesis of Dol-P-Glc is defective in the latter case. PGM1 deficiency, in the lack of normal nutrients, like in the experimental setup of serum starvation, depleted the cellular pool of UPD-Glc, available for Dol-P-Glc synthesis. Interestingly, both UDP-Gal and UDP-Glc, depleted in patient cells during serum starvation, normalized on D-gal supplements (data not shown) and also the LLO profile in patient cell-line 2013Y, strongly suggesting a link between PGM1, glucose metabolism and N-glycosylation. D-gal supplementation did not improve LLO in patient 8, who is severely PGM1-deficient. UDP-Gal and particularly UDP-Glc might have been diverted towards energy production and tnot used for Dol-P-Glc synthesis in this case.
In the presence of sufficient glucose in the culture medium (not using serum starvation), during the experiments, UDP-glc and UDP-gal pools were elevated in patients without D-Gal-therapy, compared to controls (supplementary table 5). D-gal treatment led to a further increase in nucleotide sugar levels of UDP-glc and UDP-gal in patients, but did not change the UDP-glc to UDP-gal ratio, while controls normalized after 4 days. Based on our experiments, in spite of increasing nucleotide sugar concentrations on D-gal, with or without serum starvation, the ratio of UDP-glc/UDP-gal remained abnormal, compared to control, therefore still affecting substrate availability for glycosylation. This means that the D-gal related UDP-monosaccharide pool improvement can’t be the only explanation for the improvement of glycosylation, and for the clinical improvement in patients, and we should further search for other regulatory mechanisms.
Limitations
Patients with PGM1-CDG show diverse abnormalities in endocrine, coagulation and liver function tests, therefore, our study included inconsistent baseline laboratory values. Noncompliance was observed in one patient, one patient accidentally prematurely increased D-gal dose, and a gastrointestinal infection affected treatment in another patient. Another limitation of this study was the small sample size, wide spectrum of ages at the time of enrollment (ranging from 19 months to 21 years), and the lack of adequate handling or volume of some of the blood samples at various time points.
Statistical analysis was reliable for only the serum transferrin samples, where all baseline levels were abnormal, and for ATIII, showing significant improvement on D-gal. Other laboratory values, showing frequent normal values even spontaneously during disease course were not reliable markers for end points, and for statistical analysis.
Conclusions
Here we demonstrate easy and safe administration of oral D-gal and quick, significant clinical laboratory and metabolic improvements in patients with PGM1-CDG.
The in vitro detected N-glycan hyposialylation, altered O-linked glycans and abnormal LLO-profile in patient-fibroblasts showed improvement or normalization following D-gal treatment, in concert with improvement in the glycome, liver function and coagulation in D-gal treated patients. Transferrin isoforms, ALT and ATIII could be end point markers for future clinical trials in PGM1-CDG.
We suggest using oral D-gal as medical food in PGM1-CDG. An 18-weeks escalating dose protocol up to 1.5/kg/day was proved to be safe and effective in our prospective pilot study. We should emphasize that further research is needed, with objective, placebo controlled trials, especially for optimizing long-term therapy.
Supplementary Material
Acknowledgments
We thank the patients and their families for their participation. We are also grateful to Dr. Hudson Freeze at the Burnham Institute (San Diego, CA, US) for sharing a patient cell line with us. This study is supported in part by the Hayward Foundation and by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. Additional support comes from the European Union’s Horizon 2020 research and innovation program under the ERA-Net for Research on Rare Diseases, from the Netherlands Organisation for Scientific Research (ZONMW Medium Investment grant 40-00506-98-9001 and VIDI grant 91713359 to DJL, VENI grant 016168079 to MvS) and the Prinses Beatrix Spierfonds (grant W.OR15-16 to DJL and MvS). T.H. was supported by General University Hospital in Prague, Czech Republic (RVO-VFN 64165), and the Ministry of Health of the Czech Republic (MZ CR AZV 16-31932A).
Abbreviations
- AEs
Adverse events
- ALT
Alanine Transaminase
- ATIII
Antithrombin III
- AST
Aspartate Aminotransferase
- D-gal
D-galactose
- LLO
Lipid linked oligosaccharides
- PGM1
Phosphoglucomutase 1
- PLO
Protein linked oligosaccharides
- TIEF
Transferrin isoelectric focusing
Footnotes
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest
None declared.
The authors declare no conflict of interest
References
- 1.Jaeken J. Congenital disorders of glycosylation (CDG): it’s (nearly) all in it! J Inherit Metab Dis. 2011;34(4):853–858. doi: 10.1007/s10545-011-9299-3. [DOI] [PubMed] [Google Scholar]
- 2.Timal S, Hoischen A, Lehle L, et al. Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Hum Mol Genet. 2012;21(19):4151–4161. doi: 10.1093/hmg/dds123. [DOI] [PubMed] [Google Scholar]
- 3.Tegtmeyer LC, Rust S, van Scherpenzeel M, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med. 2014;370(6):533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SY-W, Beamer LJ, Gadomski T, et al. Defining the Phenotype and Assessing Severity in Phosphoglucomutase-1 Deficiency. J Pediatr. 2016 May; doi: 10.1016/j.jpeds.2016.04.021.. [DOI] [PubMed] [Google Scholar]
- 5.Morava E. Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol Genet Metab. 2014;112(4):275–279. doi: 10.1016/j.ymgme.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojkovic T, Vissing J, Petit F, et al. Muscle glycogenosis due to phosphoglucomutase 1 deficiency. N Engl J Med. 2009;361(4):425–427. doi: 10.1056/NEJMc0901158. [DOI] [PubMed] [Google Scholar]
- 7.Beamer LJ. Mutations in hereditary phosphoglucomutase 1 deficiency map to key regions of enzyme structure and function. J Inherit Metab Dis. 2014 Aug; doi: 10.1007/s10545-014-9757-9.. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Stiers KM, Kain BN, Beamer LJ. Compromised catalysis and potential folding defects in in vitro studies of missense mutants associated with hereditary phosphoglucomutase 1 deficiency. J Biol Chem. 2014;289(46):32010–32019. doi: 10.1074/jbc.M114.597914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Smet E, Rioux J-P, Ammann H, Déziel C, Quérin S. FSGS permeability factor-associated nephrotic syndrome: remission after oral galactose therapy. Nephrol Dial Transplant. 2009;24(9):2938–2940. doi: 10.1093/ndt/gfp278. [DOI] [PubMed] [Google Scholar]
- 10.van Scherpenzeel M, Steenbergen G, Morava E, Wevers RA, Lefeber DJ. High-resolution mass spectrometry glycoprofiling of intact transferrin for diagnosis and subtype identification in the congenital disorders of glycosylation. Transl Res. 2015;166(6):639–649. e1. doi: 10.1016/j.trsl.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Thiel C, Schwarz M, Peng J, et al. A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem. 2003;278(25):22498–22505. doi: 10.1074/jbc.M302850200. [DOI] [PubMed] [Google Scholar]
- 12.Kochanowski N, Blanchard F, Cacan R, et al. Intracellular nucleotide and nucleotide sugar contents of cultured CHO cells determined by a fast, sensitive, and high-resolution ion-pair RP-HPLC. Anal Biochem. 2006;348(2):243–251. doi: 10.1016/j.ab.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Xia B, Zhang W, Li X, et al. Serum N-glycan and O-glycan analysis by mass spectrometry for diagnosis of congenital disorders of glycosylation. Anal Biochem. 2013;442(2):178–185. doi: 10.1016/j.ab.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Niehues R, Hasilik M, Alton G, et al. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest. 1998;101(7):1414–1420. doi: 10.1172/JCI2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa M, Scott DA, Losfeld M-E, Freeze HH. The metabolic origins of mannose in glycoproteins. J Biol Chem. 2014;289(10):6751–6761. doi: 10.1074/jbc.M113.544064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeze HH. Perhaps a wee bit of sugar would help. Nat Genet. 2016;48(7):705–707. doi: 10.1038/ng.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott K, Gadomski T, Kozicz T, Morava E. Congenital disorders of glycosylation: new defects and still counting. J Inherit Metab Dis. 2014;37(4):609–617. doi: 10.1007/s10545-014-9720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Körner C, Knauer R, Holzbach U, Hanefeld F, Lehle L, von Figura K. Carbohydrate-deficient glycoprotein syndrome type V: deficiency of dolichyl-P-Glc:Man9GlcNAc2-PP-dolichyl glucosyltransferase. Proc Natl Acad Sci U S A. 1998;95(22):13200–13205. doi: 10.1073/pnas.95.22.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chantret I, Dancourt J, Dupré T, et al. A deficiency in dolichyl-P-glucose:Glc1Man9GlcNAc2-PP-dolichyl alpha3-glucosyltransferase defines a new subtype of congenital disorders of glycosylation. J Biol Chem. 2003;278(11):9962–9971. doi: 10.1074/jbc.M211950200. [DOI] [PubMed] [Google Scholar]
- 20.Kranz C, Denecke J, Lehrman MA, et al. A mutation in the human MPDU1 gene causes congenital disorder of glycosylation type If (CDG-If) J Clin Invest. 2001;108(11):1613–1619. doi: 10.1172/JCI13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenk B, Imbach T, Frank CG, et al. MPDU1 mutations underlie a novel human congenital disorder of glycosylation, designated type If. J Clin Invest. 2001;108(11):1687–1695. doi: 10.1172/JCI13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Maliekal P, et al. Molecular identification of mammalian phosphopentomutase and glucose-1,6-bisphosphate synthase, two members of the alpha-D-phosphohexomutase family. The Journal of biological chemistry. 2007;282(44):31844–31851. doi: 10.1074/jbc.M706818200. [DOI] [PubMed] [Google Scholar]
- Ondruskova N, et al. Glycogen storage disease-like phenotype with central nervous system involvement in a PGM1-CDG patient. Neuro endocrinology letters. 2014;35(2):137–141. [PubMed] [Google Scholar]
- Tegtmeyer LC, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. The New England journal of medicine. 2014;370(6):533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY-W, et al. Defining the Phenotype and Assessing Severity in Phosphoglucomutase-1 Deficiency. The Journal of pediatrics. 2016 doi: 10.1016/j.jpeds.2016.04.021. Available at: http://dx.doi.org/10.1016/j.jpeds.2016.04.021. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.