Abstract
Background
Pain is often a complaint that precedes total knee arthroplasty (TKA), however the procedure itself is associated with considerable post-operative pain lasting days to weeks which can predict longer-term surgical outcomes. Previously, we reported significant opioid-sparing effects of motor cortex transcranial direct current stimulation from a single-blind trial. In the present study, we used double-blind methodology to compare motor cortex tDCS and prefrontal cortex tDCS to both sham and active-control (active electrodes over non-pain modulating brain areas) tDCS.
Methods
58 patients undergoing unilateral TKA were randomly assigned to receive 4 20-minute sessions (a total of 80 minutes) of tDCS (2mA) post-surgery with electrodes placed to create 4 groups: 1) MOTOR (n=14); anode-motor/cathode-right prefrontal, 2) PREFRONTAL (n=16); anode-left-prefrontal/cathode-right-sensory, 3) ACTIVE-CONTROL (n=15); anode-left-temporal-occipital junction/cathode-medial-anterior-premotor-area, and 4) SHAM (n=13); 0mA-current stimulation using placements 1 or 2. Patient controlled analgesia (PCA; hydromorphone) use was tracked during the ∼72-hours post-surgery.
Results
Patients in the sham group and the active-control group used 15.4mg (SD=14.1) and 16.0mg (SD=9.7) of PCA hydromorphone respectively. There was no difference between the slopes of the cumulative PCA usage curves between these two groups (p=.25; ns). Patients in the prefrontal tDCS group used an average of 11.7mg (SD=5.0) of PCA hydromporhone, and the slope of the cumulative PCA usage curve was significantly lower than sham (p<.0001). However, patients in the motor tDCS group used an average of 19.6mg (SD=11.9) hydromorphone and the slope of the PCA use curve was significantly higher than sham (p<.0001).
Conclusions
Results from this double-blind cortical-target-optimization study suggest that anodal transcranial direct current stimulation (tDCS) over the left prefrontal cortex may be a reasonable approach to reducing post-TKA opioid requirements. Given the unexpected finding that motor cortex failed to produce an opioid sparing effect in this follow-up trial, further research in the area of post-operative cortical stimulation is still needed.
Introduction
Adequate postoperative pain control is an important factor in determining recovery time and hospital length of stay (1-3). Opioid medications (including the use of patient-controlled analgesia pumps post-operatively) represent a very common approach for achieving pain relief, however there are risks and problems associated with opioid use (4). Among the risks are the numerous potential side-effects including: respiratory depression, nausea and vomiting, cough suppression, mental clouding, confusion, sedation, itching of the skin and nose, and constipation (5-7). Total knee arthroplasty (TKA) procedures along with the associated intraoperative anesthesia protocols have been independently associated with increased risk for post-operative cognitive problems especially among the elderly, and for obese TKA patients, apnea is a real concern that systemic opioid use can complicate (5-7). Thus, new interventions that have the potential to reduce reliance on postoperative opioids in this patient population need to be explored.
Several brain stimulation techniques including transcranial direct current stimulation (tDCS) may offer new treatment strategies for a variety of pain conditions (8-11). These techniques permit induction of changes in cortical excitability and may, in part, be related to changes in concentrations of glutamate and GABA in the stimulated area (12,13).
Results from prior pilot feasibility studies suggest that transcranial direct current stimulation (tDCS) over motor cortex may be able to reduce post-surgical opioid requirements. (14, 15) Despite using less opioid medication in these studies, participants in the real tDCS group reported no pain exacerbation or worse mood relative to those in the sham tDCS group. One major limitation of these pilot trials was their single-blind design. Studies employing more rigorous double-blind methodology are needed. Nonetheless, the preliminary results from that trial support the need for further research in the area of adjunctive cortical stimulation in the management of post-surgical pain.
Some studies have shown that stimulation of the prefrontal cortex via TMS can reduce post-operative analgesia use and subjective pain ratings (16, 17), however, the optimal cortical targets for stimulation are not well-established and thus an exploration of optimal electrode placement is needed. The present study employed double-blind methodology to compare motor cortex tDCS and prefrontal cortex tDCS to both sham and active-control (active electrodes over non-pain modulating brain areas) tDCS.
Materials and Methods
The present study is a randomized, double-blind, sham-controlled trial of tDCS in the management of post-operative opioid use among patients undergoing total knee arthroplasty (TKA). Participants were randomized to receive four sessions of tDCS using one of four possible tDCS montages/conditions (described below). Following Institutional Review Board approval and written informed consent, 58 non-smoking participants aged 18 to 80 years old and American Society of Anesthesiologists (ASA) physical status 1 through 3 who were scheduled for an elective, primary, unilateral TKA were enrolled in our study and asked to fill out a baseline pain questionnaire. Participants were not pregnant, and did not have any of the following: diabetic neuropathy or any other neurologic or neuromuscular disease, rheumatoid arthritis, current coagulopathy, skin infection at needle insertion site for the femoral or sciatic nerve blocks, significant renal or hepatic impairment, unsuccessful femoral or sciatic block or femoral catheter placement, femoral catheter dislodgement after placement, inability to understand VAS pain scales, or the inability to use an IV-PCA pump.
Surgical Anesthesia Protocol
Preoperatively, after IV access was obtained, standard monitors were applied and sedation was provided for patient comfort during placement of the regional nerve blocks (up to midazolam 2 mg IV and fentanyl 100 μg IV). The femoral nerve block was performed using a combined ultrasound-guided and neurostimulation approach with a 17 gauge × 4 cm insulated stimulating Tuohy needle (Arrow International, Inc., Reading, PA). The femoral nerve catheter was placed with ultrasound guidance and confirmed by eliciting a patella snap at a voltage of 0.5 MA or lower by nerve stimulation. Stimulating the patella to “twitch” at a low voltage of 0.5 ma indicates a locale close enough to the nerve to place a catheter and inject local anesthetic to block nerve conduction. After patellar snap was elicited at less than 0.5 mA (2 Hz, 0.1 msec) of current, 20 mL of 0.5% ropivacaine was slowly injected with incremental aspirations for blood. A 19 gauge × 60 cm catheter (Arrow International, Inc., Reading, PA) was then threaded 5 cm beyond the needle tip and secured in place. A combined ultrasound-guided and neurostimulation mid femoral anterior approach to locate the sciatic nerve was utilized with a 21 gauge × 15 cm insulated stimulating needle (Arrow International, Inc., Reading, PA). Proximity to the sciatic nerve was confirmed with calf, foot or toe twitching at a current of less than 0.5 mA (2 Hz, 0.1 msec). A total of 20 mL of 1% ropivacaine was slowly injected, with incremental aspirations for blood. Intraoperative management was standardized to a general anesthetic with either endotracheal intubation or a laryngeal mask airway per the anesthesiologist's choice. Anesthesia induction was limited to the use of fentanyl, propofol, and a muscle relaxant, if needed. Maintenance of anesthesia consisted of sevoflurane and IV fentanyl boluses. No more than 250 micrograms of fentanyl was utilized for perioperative narcotic management. Intra-articular injection of local anesthetics or opioids was not allowed and considered an exclusion criterion if performed by the surgeon.
Post-Operative Pain Control
Following surgery, after arriving to the post-anesthesia care unit (PACU), a femoral nerve catheter infusion was initiated with a continuous infusion rate of 10 mL/hr of 0.2% ropivacaine. In addition, after the first tDCS session in the PACU, patients were placed on patient-controlled anesthesia (PCA) pumps for ∼48 hours consisting of hydromorphone starting at 0.2 mg with a 8-minute lockout.
tDCS Groups and Cortical Targets
Following confirmation that both the femoral and sciatic nerve blocks were functioning, patients were randomly assigned to receive a total of 80 minutes (two 20-minute sessions on post-op day-0 separated by 4 hours, and two 20-minute sessions on post-op day-1 also separated by 4 hours) of tDCS. 4 groups were created based on electrode configuration: Group-A (PREFRONTAL; n=16) received anodal stimulation (activating) over the left dorsolateral prefrontal cortex (F3) and the cathode (deactivating) over the knee representation of the sensory cortex (FPz) as was done in a previous perioperative tDCS trial (18). Group-B (MOTOR; n=14) received anodal stimulation of the knee representation of the motor cortex (C1 or C2 from the EEG-10-20 system corresponding with the sugery-side knee) and cathodal stimulation of the right dorsolateral prefrontal cortex (F4) as was done in a recent perioperative trial of tDCS in total knee arthroplasty patients (14). We chose C1/C2 instead of Cz in order to avoid stimulating directly over the longitudinal fissure (see figure 1 for a representation of the intended trajectory associated with employment of C2).
Figure 1.
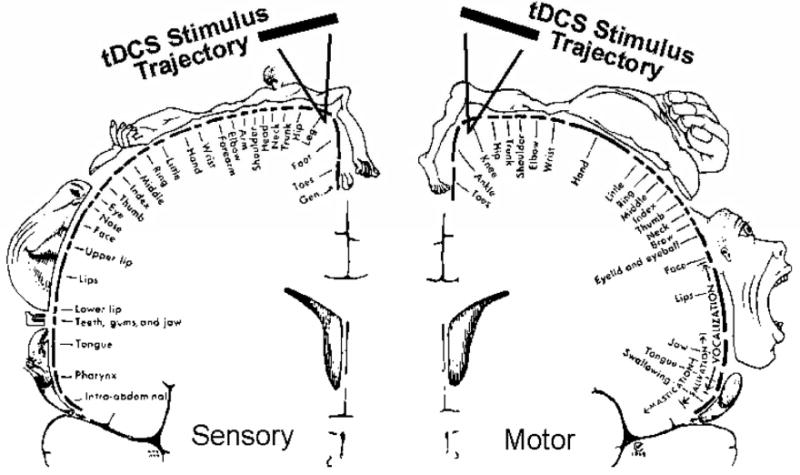
Depiction of the C1/C2 tDCS cortical targeting trajectories employed.
In order to examine the specificity of cortical targeting on pain, Group-C (ACTIVE-CONTROL; n=15) received active stimulation over areas thought not to be involved with pain including anodal stimulation of the left temporal-occipital junction (P3) and cathodal stimulation of the medial anterior pre-motor area (FCz). For the sham control group (Group-D; SHAM; n=13), one of the configurations from Group-A or Group-B was randomly selected, but the stimulator was turned-off by custom-developed blinding software after 30 seconds.
tDCS Methods
Because patients in Group C had electrode placement assignments that were unique to the active-control condition, thus compromising the experimenter blind, we elected to employ a “masking technician” for tDCS electrode placement. After patients underwent TKA, head measurements were taken and relevant EEG-coordinates were located by the masking technician. The masking technician in this study was aware of the tDCS targets for each subject, although remained blind to whether patients were in Groups-A, B or D. Since Group-C used unique coordinates, the masking technician could not be completely blinded. Thus, the masking technician placed the electrodes for all patients without other investigators in the room, and then covered patients' scalps with an occlusive hood so that other members of the study team did not know where the electrodes were placed on each patient. The masking technician had no contact with investigators and no additional contact with patients outside of placing the electrodes. The tDCS device was connected and wired into a custom-developed, software-driven blinding-interface and the occlusive hood prevented the researchers delivering treatment and collecting ratings from seeing where the electrodes were placed. The treating researcher entered a unique 6-digit ID code into the software controller which was linked to real or sham stimulation parameters. 20-minute tDCS treatments were delivered using this same methodology: 1) in the PACU immediately after patients were awake and the nursing staff attached all necessary monitors (e.g., heart rate, blood pressure, respiratory rate, pulse oximetry), 2) 4 hours later, 3) the morning of post-operative day-1, and 4) the afternoon of post-operative day-1. VAS pain ratings were collected before and after each tDCS session.
Electrodes were standard 4cm × 4cm sponge electrodes soaked in a sterile solution of 0.9% sodium chloride insulated by a latex casing. The electrodes were held in place with Velcro straps and the occlusive hood. For each session of real tDCS, the device was ramped to 2 mA and maintained this current for 20 minutes. For sham tDCS, the device was ramped to 2 mA, but after 30 seconds, was ramped back down to 0 mA automatically by the blinding software and stayed off for the remainder of the 20 minutes. tDCS was conducted with the Chatenooga Ionto (iontophoresis device) using 2.0 mA current. The current density and total charge delivered by the above parameters is consistent with those that have been used safely in previous tDCS studies (19,20).
The femoral nerve catheter was removed from the patient on postoperative day (POD) 2, at which time each subject's pain was managed by the primary orthopedic service. VAS pain and mood ratings were collected pre- and post- each tDCS session. Patient controlled analgesia (PCA; hydromorphone) use was tracked during the 72-hours post-surgery.
Statistical Analysis
One-way ANOVAs were conducted to examine differences in pain ratings, mood, anxiety, chronic opioid use and functional impairment due to pain at baseline between groups. Multi-level modeling (Mixed procedure in SPSS) was used to conduct latent growth curve analysis on cumulative PCA hydromorphone usage during the 72 hours post-op. Subject-level intercepts were entered into the model as random effects at level one.
Results
The mean age of the sample (n=58) was 61.2 (SD=8.3) years, 31 participants were female, 17 participants were African American and 41 were Caucasian. 31 patients underwent left-sided TKA, and 27 underwent right-sided TKA. 28 reported a history of chronic pain prior to TKA, and 15 were on chronic opioid therapy. Mean scores at baseline on psychosocial screening measures were as follows: Pain Numeric Rating Scale = 1.9 (SD=3.1); Beck Depression Inventory = 0.3 (SD=0.3); Beck Anxiety Inventory = 0.4 (SD=0.4); Functional Impairment Due to Pain from the Brief Pain Inventory = 4.9 (SD=2.4). Breakdown of the baseline characteristics by tDCS group are provided in table 1. One-way ANOVA indicated no differences between groups across any of the demographic, baseline medical, or baseline psychosocial measures (all p>.05). There was no difference between groups with respect to the side of TKA performed (left vs right; ChiSquare (df=5) = 4.04, ns). 17 participants were taking preoperative prescription central-nervous-system (CNS) -acting medications (including benzodiazepines, antidepressants, anticonvulsants, and narcotics). No differences were observed in tDCS group assignment with respect to the presence of CNS-acting medications (ChiSquare (df=3) = 1.27, ns). Additionally, no relationship was found between the presence or absence of CNS drugs on total PCA opioid usage at the time of discharge (r(52)=-.02, ns). In response to a forced-choice condition-guessing question, 27 (47%) participants correctly guessed whether they received real or sham stimulation. This was not significantly different from chance (50% chance of correct guessing; t(57)=0.52, ns), and correct/incorrect guessing-rates did not vary as a function of tDCS group assignment (ChiSquare (df=3) = 2.80, ns).
Table 1.
Sample characteristics at baseline by tDCS group.
| Condition | n-size | Number Female |
Number on Chronic Opioid Therapy |
Age (years) | Length of Stay (Days) |
Average Daily Pain |
Beck Depression Inventory |
Beck Anxiety Inventory |
BPI - Functional Impairment |
McGill Pain Questionnaire (Sensory) |
McGill Pain Questionnaire (Affective) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor tDCS | n=14 | 7 | 3 | Mean | 57.79 | 2.62 | 5.58 | 0.32 | 0.37 | 5.23 | 0.70 | 0.16 |
| Std. Deviation | 11.07 | 0.65 | 1.68 | 0.32 | 0.41 | 2.30 | 0.70 | 0.27 | ||||
| Prefrontal tDCS | n=16 | 8 | 5 | Mean | 60.19 | 3.07 | 4.75 | 0.29 | 0.33 | 4.87 | 0.57 | 0.17 |
| Std. Deviation | 6.72 | 1.22 | 1.66 | 0.24 | 0.32 | 2.41 | 0.76 | 0.40 | ||||
| Active Control tDCS | n=15 | 7 | 4 | Mean | 62.53 | 2.54 | 5.42 | 0.35 | 0.47 | 4.90 | 0.46 | 0.02 |
| Std. Deviation | 5.26 | 0.52 | 2.57 | 0.45 | 0.57 | 2.81 | 0.53 | 0.08 | ||||
| Sham tDCS | n=13 | 9 | 3 | Mean | 64.54 | 2.91 | 4.40 | 0.29 | 0.36 | 4.79 | 0.57 | 0.31 |
| Std. Deviation | 8.89 | 0.70 | 2.46 | 0.24 | 0.23 | 2.24 | 0.80 | 0.34 |
No tDCS sessions were stopped by participants or researchers due to reported intolerable discomfort, adverse events or tDCS-related side-effects. We gathered patient reports of sensations/discomfort at 3 time-points (during the first 30 secs, at 10 mins, and during the last 30 secs) during each tDCS session. 59% of the sample reported no sensations during any of the tDCS sessions. Descriptors of the sensations experienced by others varied considerably from participant to participant but included “cold/coolness”, “itching”, “tingling”, “stinging”, “pins and needles”, and “burning”.
Patients in the sham group and the active-control group used 15.4mg (SD=14.1) and 16.0mg (SD=9.7) of PCA hydromorphone respectively (see Figure 2). There was no difference between the slopes of the cumulative PCA usage curves between these two groups (t(1702)=1.2, p=.25; ns). Patients in the prefrontal tDCS group used an average of 11.7mg (SD=5.0) of PCA hydromporhone, and the slope of the cumulative PCA usage curve was significantly lower than sham (t(3689)=-8.8, p<.0001; opioid sparing). However, patients in the motor tDCS group used an average of 19.6mg (SD=11.9) hydromorphone and the slope of the PCA use curve was significantly higher than sham (t(3689)=8.7, p<.0001). Additional models including chronic opioid therapy, baseline pain ratings, depression and anxiety as covariates yielded the same pattern described above. Visual Analog Scale (VAS) pain ratings collected before and after each tDCS session were not different between groups at any post-operative time point.
Figure 2.
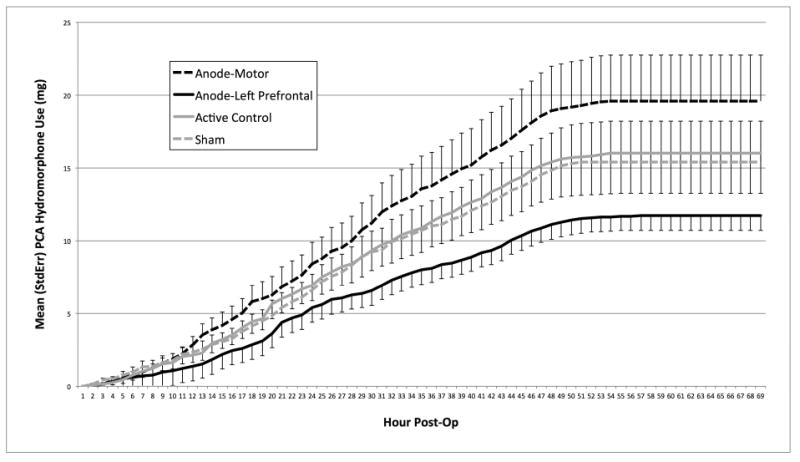
Mean (StdErr) Patient Controlled Analgesia (PCA) hydromorphone (mg) usage by tDCS group and hour post-operatively.
Discussion
Results from this double-blind, sham-controlled clinical trial investigating the analgesic effects of tDCS delivered over different cortical targets suggest that anodal stimulation of the prefrontal cortex (with cathode over the right somatosensory cortex) yielded the most promising analgeic results of the parameters examined. Participants that received anodal stimulation over the left DLPFC used approximately 37% less PCA hydromorphone than those in the control conditions during the 70 hours immediately following TKA surgery. tDCS was well-tolerated by all study patients with minimal reported side-effects. The trajectories of PCA hydromporphone use between groups began to diverge within the first 12 hours post-operatively (i.e., after only 1 or 2 sessions of tDCS; see figure 2). The separation in PCA trajectories continues to grow throughout the 2 postoperative inpatient days (the point at which patients began to stop using PCA's and began discharge procedures) without clearly observable effects for the second two tDCS sessions (which happened at approximately 24 and 28 hours post-operatively). Because the early divergence appeared to influence total hydromorphone usage at the time of discharge, timely delivery of tDCS (i.e., immediately after surgery) may be an important strategy for perioperative applications. Future studies might consider conducting more tDCS sessions as early as possible during the post-operative period to capitalize on its effects.
The fact that active-control and sham were not different supports the idea that targeting specificity may be an important consideration in this area, and the analgesic effects of tDCS are not likely due to more general, target-nonspecific electrical stimulation phenomena independent of electrode placement. In other words, despite reasonably low spatial resolution of tDCS, electrode placement appears to matter when attempting to garner specific behavioral effects. The curious finding in this trial is that those receiving anodal motor cortex stimulation used more PCA hydromorphone than those receiving anodal left prefrontal stimulation as well as sham which is inconsistent with our previous findings (14). It is possible that the more rigorous double-blind methodology employed in this trial limited demand characteristics and experimenter effects (such as subtle cues or signals from an experimenter that affect the performance or response of subjects in the experiment including but not limited to nonverbal cues, such as muscular tension or gestures, and vocal cues, such as tone of voice) that may have influenced findings in our previous study. Because of the bicephalic electrode arrangements employed using potential pain-related areas in the real stimulation conditions, the effects observed are a bit difficult to interpret. If DLPFC is indeed involved in pain control, the possibility cannot be excluded that down-regulation of the right DLPFC in the motor cortex stimulation condition antagonized the effects of stimulation of the motor cortex, although this effects was not observed in our previous trial (14). It is also possible that down-regulation of the somatosensory cortex (cathodal stimulation of somatosensory cortex in the left-DLPFC-anode condition) might have impacted on the observed analgesic prefrontal stimulation effects. For clarification of mechanisms, respective control experiments with differently placed return electrodes would have been ideal. It is also possible that the reasonably small sample-size employed in both trials resulted in enough uncaptured within- and between- group variability to push the findings in opposite directions between trials. With a larger sample-size, it might be possible to characterize differences between groups that could influence treatment response. To date, little is known about participant-level factors that might influence unique response patterns to the tDCS intervention, and future studies should consider investigating individual-level response predictors that might inform optimal electrode placement strategies from patient to patient.
Limitations
As briefly mentioned above, the small sample sizes employed in each of the four groups in this study may limit generalizability and potentially complicate interpretation of the findings. Also, given the fact that each active condition in this study employed both anodal and cathodal cortical targets which may have influenced the analgesic effects observed, conclusions about the mechanisms of action are difficult to make with confidence. It is possible that stimulation under the return electrodes (cathodes) was equally involved as stimulation under the anodes in producing the observed differences between groups. For a clearer understanding of tDCS cortical-targeting mechanisms, control experiments with differently placed return electrodes would have to be conducted.
We did not capture actual doses of pre- and intra-operative fentanyl in our data set. However, all patients received a medication preload in holding for the regional anesthesia procedures. The medication preload administered as standard clinical care to each patient was no more than 2mg of midazolam and no more than 100mcg of fentanyl. Additionally, patients received fentanyl intraoperatively (no more than 250 mcg) to blunt stimulation from intubation. In addition to blunting stimulation from intubation, the purpose for these relatively low-dose, pre- and intra-operative medications was to increase patient comfort during the regional anesthesia procedures, and while the investigators do not feel that these low doses significantly impacted post-operative pain and recovery, the lack of patient-level data on pre- and intra-operative medication usage represents a limitation of the current study.
Lastly, despite no observable differences between groups on the Beck Depression Inventory and the Beck Anxiety Inventory, many other psychological variables could influence outcomes. Future studies should consider assessing pain catastrophizing as well as aberrant opioid use behaviors among patients so that the potential effects of these variables on PCA opioid use can be examined.
Future Research
While the present study found opioid-sparing effects associated with left DLPFC anodal stimulation, future studies should consider cortical targeting strategies that leave less room for confusion regarding explanatory mechanisms. This can be accomplished in several ways. Return electrodes can be placed on participants' shoulders to limit competing explanations for possible brain effects under return electrodes that investigators might deem non-consequential or not-of-interest. Systematic testing of single-electrode tDCS over potential pain modulating brain areas would help clarify cortical targets of interest in pain management. Additionally, return electrode placement could be systematically varied on the scalp such that brain areas not thought to influence pain perception could be targeted in combination with hypothetical analgesic placements. Current flow could also be altered with two-electrode montages (e.g., swapping anode and cathode over established cortical targets) to determine whether there is an interaction between placement and direction of current flow.
Clinical Implications
While there is accumulating evidence that tDCS can be used in the perioperative arena as an adjunctive strategy for pain control and opioid sparing effects, much more work is needed regarding optimal cortical targeting approaches and tDCS dosing before brain stimulation can become a mainstream technique. Further, more studies are needed to clarify the clinical impact, effect-size and value of adjunctive tDCS in the perioperative arena before this approach becomes widely used.
Conclusions
Results from this double-blind, sham-controlled clinical trial suggest that anodal stimulation of the prefrontal cortex (with cathode over the right somatosensory cortex) resulted in significant opioid sparing effects. Participants that received anodal stimulation over the left DLPFC used approximately 37% less PCA hydromorphone than those in the control conditions. tDCS was well-tolerated by all study patients. The role of tDCS in pain management is not yet well-established. However, minimally invasive brain stimulation technologies such as tDCS may have an important role in the management of acute and chronic pain, and appears promising for reducing post-surgical pain; much work is still needed.
Highlights.
Transcranial direct current stimulation of the prefrontal cortex (anode left, cathode right) reduced post- surgical opioid use.
tDCS of the prefrontal cortex was better at reducing post-operative opioid use than motor cortex stimulation (anode over motor cortex, cathode over right prefrontal).
Electrode montage/placement may be important to consider when using tDCS to modulate pain and opioid use.
Acknowledgments
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Disorders at NIH; Grant number: R21AR061755
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey J. Borckardt, Medical University of South Carolina, Charleston, South Carolina, USA
Scott T. Reeves, Medical University of South Carolina, Charleston, South Carolina, USA
Cole Milliken, Medical University of South Carolina, Charleston, South Carolina, USA
Brittan Carter, Medical University of South Carolina, Charleston, South Carolina, USA
Thomas I. Epperson, Medical University of South Carolina, Charleston, South Carolina, USA
Ryan J. Gunselman, Medical University of South Carolina, Charleston, South Carolina, USA
Alok Madan, Menninger Clinic, Houston, Texas, USA
H. Del Schutte, Medical University of South Carolina, Charleston, South Carolina, USA
Harry A. Demos, Medical University of South Carolina, Charleston, South Carolina, USA
Mark S. George, Medical University of South Carolina, Charleston, South Carolina, USA
References
- 1.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, et al. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty. 2001;16(4):436–45. doi: 10.1054/arth.2001.23622. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Boctor B, Verner J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Reg Anesth Pain Med. 2002;27(2):139–44. doi: 10.1053/rapm.2002.29253. [DOI] [PubMed] [Google Scholar]
- 4.Morgan D, Forst-Pineda K, Gold M. Medical and nonmedical use of prescription opioids: Epidemiology and Prevalence. Psychiatr Ann. 2006;36(6):404–9. [Google Scholar]
- 5.Talmo CT, Robbins CE, Bono JV. Total joint replacement in the elderly patient. Clin Geriatr Med. 26(3):517–29. doi: 10.1016/j.cger.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Deo H, West G, Butcher C, Lewis P. The prevalence of cognitive dysfunction after conventional and computer-assisted total knee replacement. Knee. doi: 10.1016/j.knee.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 80(9):595–9. doi: 10.1111/j.1445-2197.2010.05396.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosen AC, Ramkumar M, Nguyen T, Hoeft F. Noninvasive transcranial brain stimulation and pain. Curr Pain Headache Rep. 2009;13(1):12–7. doi: 10.1007/s11916-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6(2):188–91. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- 10.Williams JA, Imamura M, Fregni F. Updates on the use of non-invasive brain stimulation in physical and rehabilitation medicine. J Rehabil Med. 2009;41(5):305–11. doi: 10.2340/16501977-0356. [DOI] [PubMed] [Google Scholar]
- 11.OConnell N, Wand B, Marston L, Spencer S, DeSouza L. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews. 2010;9:1–123. doi: 10.1002/14651858.CD008208.pub2. [DOI] [PubMed] [Google Scholar]
- 12.George MS, Nahas Z, Kozol FA, Li X, Yamanaka K, Mishory A, et al. Mechanisms and the current state of transcranial magnetic stimulation. CNS Spectr. 2003;8(7):496–514. doi: 10.1017/s1092852900018976. [DOI] [PubMed] [Google Scholar]
- 13.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 14.Borckardt JJ, Reeves ST, Robinson SM, May JT, Epperson TI, Gunselman RJ, et al. Transcranial direct current stimulation (tDCS) reduces postsurgical opioid consumption in total knee arthroplasty (TKA) Clin J Pain. 2013;29(11):925–8. doi: 10.1097/AJP.0b013e31827e32be. [DOI] [PubMed] [Google Scholar]
- 15.Glaser J, Reeves ST, Stoll WD, Epperson TI, Hilbert M, Madan A, et al. Motor/Prefrontal Transcranial Direct Current Stimulation (tDCS) Following Lumbar Surgery Reduces Postoperative Analgesia Use. Spine. 2016;41(10):835–9. doi: 10.1097/BRS.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 16.Borckardt JJ, Reeves ST, Weinstein M, Smith AR, Shelley N, Kozel FA, et al. Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: A replication study. Brain Stimul. 2008;1(2):122–7. doi: 10.1016/j.brs.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, et al. Post-Operative Left Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS) Reduces Patient-Controlled Analgesia Use. Anesthesiology. 2006;105:557–62. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Borckardt JJ, Romagnuolo J, Reeves ST, Madan A, Frohman H, Beam W, et al. Feasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot study. Gastrointest Endosc. 2011;73(6):1158–64. doi: 10.1016/j.gie.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 19.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72(4-6):208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Iyer M, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann E. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]


