Abstract
A method based on capillary electrophoresis (CE) with electrophoretic injection and absorbance detection was developed for the direct analysis of AGP glycoforms in human serum. Electrophoretic injection of AGP was performed in the reversed-polarity mode of CE with a capillary coated with poly(ethylene oxide) and that had minimal electroosmotic flow. This situation created an essentially stationary interface between the sample and running buffer during injection and sample stacking. This approach allowed an 11,000-fold increase in sample loading for a 5 min injection versus hydrodynamic injection and without introducing any significant levels of extra band-broadening. This method was used with sample pretreatment methods based on acid precipitation and desalting to examine AGP glycoforms in only 65 μL of serum. A limit of detection of 2.1–11.3 nM was obtained for the major AGP glycoform bands in serum, and the sample pretreatment method gave a recovery of 72.3–80.9% for these glycoforms. The precision for the migration times was ± 0.08–0.13% and the precision for the peak areas was ± 0.34–1.18% when using serum samples and an internal standard. This method was used for both normal pooled serum and serum from individuals with systemic lupus erythematosus. Results were obtained in a separation time of 25 min and allowed the comparison of up to eleven glycoform bands in these samples. A similar approach may be useful in examining additional glycoproteins in serum or other types of biological samples.
Keywords: Alpha1-acid glycoprotein, Glycoform analysis, Capillary electrophoresis, Sample stacking, Electrophoretic injection, Systemic lupus erythematosus
1. Introduction
Alpha1-acid glycoprotein (AGP) is an acute phase glycoprotein with a low isoelectric point (pI, 2.8–3.8) and a high carbohydrate content (~40% w/w) [1]. The concentration of AGP ranges from 0.5–1.0 mg/ml, or 12–24 μM, in normal human serum and can increase by ten-fold during some clinical conditions [1]. There are around 12–20 major glycoforms of AGP that have been previously observed for human serum, which result from the various complex-type glycans that can occur at the five glycosylation sites of this protein [2]; however, more than 150 human AGP isoforms (including both glycoforms and genetic variants) have been characterized by CE-MS [3] and an even larger number of glycoforms may be present if linkage isomers are also considered [4]. The glycosylation of AGP can be altered in some disease states, which has made AGP glycoforms and their carbohydrate chains potential biomarkers for disease diagnosis [5–7]. For instance, a change in the degree of branching for the carbohydrate chains on AGP has been used to detect infections in systemic lupus erythematosus (SLE) [5]. In addition, an increase in α1–3 fucosylation for AGP has been noted in pancreatic cancer [6], and hypersialylation of AGP has been reported in ovarian cancer and lymphoma [7,8].
Several methods based on capillary electrophoresis (CE) [9] have been developed for the separation and analysis of AGP glycoforms, as based on the use of buffer additives such as putrescine and urea or the use of coated capillaries [7,10–16]. For instance, a relatively fast method for the analysis of AGP glycoforms in aqueous solutions has recently been reported that used both static and dynamic coatings of poly(ethylene oxide) (PEO) in a silica capillary and a pH 4.2 running buffer that contained 0.1% Brij 35. This resulted in a separation of nine glycoform bands within 20 min for standard samples of AGP and with detection limits for the individual glycoform bands that were in the range of 0.09–0.38 μM when using absorbance detection [17]. However, this approach has not yet been adapted for use with more complex biological samples, such as human serum. A highly sensitivity method would ideally be needed for such work to analyze samples that contain abnormally low levels of AGP and/or any minor glycoforms that may be present in these samples [1,17].
A number of schemes have been developed to isolate AGP from serum and related samples prior to the analysis of this glycoprotein by liquid chromatography or CE [12,13,18–20]. Previous methods for the purification of AGP have often involved multiple chromatographic steps (e.g., ion-exchange and dye-ligand affinity chromatography), which can be time-consuming and generally require relatively large sample volumes (e.g., 1–2 mL of serum or plasma) [18,19]. A method based on acidic precipitation has been described for the isolation of AGP from 50 μL of plasma [20]. However, significant interferences in the resulting samples have still been noted with this approach, which has resulted in the further use of anti-AGP antibodies in columns or on magnetic beads to purify AGP [12,13].
Other approaches that have been used to examine AGP glycoforms at levels found in serum have made use of labeling or detection based on mass spectrometry [21–24]. For instance, CE and laser-induced fluorescence (CE-LIF) has been used to analyze AGP glycoforms in serum after labeling the AGP with a fluorescent tag [21]. CE-MS has been used with immunoaffinity extraction to look directly at AGP glycoforms in serum [22–24]. However, the use of sample labeling adds extra steps and time to the analysis of AGP and modifies the structure of this glycoprotein [21], while the high cost of instrumentation and level of training that is needed in CE-MS can limit the use of such a method in routine clinical testing [25].
Another possible option to obtain lower detection limits in CE is to use sample stacking, although this technique has not been previously used for AGP or AGP glycoforms. Field-enhanced sample injection (FESI) is a stacking technique that uses samples that have been prepared in a low-conductivity solution [26]. The placement of a small water plug in the capillary before sample injection has been used in this method with anionic solutes to minimize effects due to retreating of the stacking interface, but this method has only allowed injection times of up to 10 s [27,28]. A longer water plug can be used with suppressed electroosmotic flow in FESI to provide longer injection times and higher loading capacities [29]. In addition, large volume sample stacking has been reported in which a low conductivity sample zone has been injected along the entire length of a capillary [30–32]. Unfortunately, the amount of sample that can be stacked by this last technique is limited by the capillary volume (i.e., typically around 2 μL) [30]. External pressure has been used to increase sample loading by providing resistance to retreating of the stacking interface during FESI, but this approach requires that a delicate balance be maintained between electroosmotic flow and the applied pressure [33].
In this study, a CE approach with electrophoretic injection/sample stacking and absorbance detection will be developed and tested for use in separating and analyzing AGP glycoforms in serum. Electrophoretic injection will be performed in the presence of negligible electroosmotic flow by using a capillary with a neutral coating [17], thus providing a stable stacking interface that can provide large loading capacities and long injection times for AGP samples. In addition, both acid precipitation and desalting will be explored as pretreatment methods to allow this CE approach to be used with serum samples. The final method will be characterized by using it to analyze the glycoforms in standard samples of normal, purified AGP. The same method will be tested for use in examining and comparing AGP glycoforms from normal pooled serum and serum from individuals with SLE.
2. Experimental section
2.1 Materials
The normal, purified AGP (from pooled human plasma, ≥ 99% pure; product number G9885; lot numbers, SLBJ6840V and SLBG6410V; purified from Cohn Fraction VI by the manufacturer through several chromatographic steps), pooled normal human serum (from human male AB plasma, USA origin, sterile-filtered; product number H4522), PEO (viscosity-average molar mass, 8,000 kDa), Brij 35 (number-average molar mass, 1.198 kDa), and Lucifer yellow CH (LyCH, dilithium salt) were purchased from Sigma-Aldrich (St Louis, MO, USA). The SLE serum samples were de-identified and pre-existing samples from individuals known to have this disease, as provided by Johns Hopkins University School of Medicine (Baltimore, MD, USA; this work was determined to be exempt from IRB review by the Johns Hopkins School of Medicine, according to the Code of Federal Regulations - 45 CFR 46.101 b); a similar sample of pooled normal human serum was also obtained from this source. All aqueous solutions and samples were prepared using water obtained from a Milli-Q Advantage A10 water purification system (EMD Millipore Corporation, Billerica, MA, USA). Zeba spin desalting columns (0.5 mL, 7 kDa cutoff) were purchased from Thermo Fisher Scientific (Rockford, IL, USA).
2.2 Apparatus
The CE work was carried out using a P/ACE MDQ instrument (Beckman Instruments, Fullerton, CA, USA). The capillary in this system was maintained at 25°C during the separation, which was performed at an applied potential of −30 kV. This system used 60.2 cm × 50 μm I.D. fused silica capillaries (Polymicro Technologies, Phoenix, AZ, USA) with an effective length to the detector of 50 cm. New capillaries were activated by rinsing them with 1 M sodium hydroxide for 30 min, followed by a 10 min rinse of water. Modification of the capillaries with static and dynamic coatings of PEO was carried out as described previously [17]. In this method, the capillary was first cleaned by using a 5 min rinse with 1 M sodium hydroxide, followed by a 3 min rinse with water. The coating was applied by rinsing the capillary for 5 min with 1 M hydrochloric acid and then rinsing for 5 min with a 0.2% (w/v) PEO solution that contained 0.1 M hydrochloric acid. The capillary was then rinsed for 5 min with a running buffer that was pH 4.2, 20 mM acetate buffer containing 0.05% (w/v) PEO and 0.1% (w/v) Brij 35. All of these rinses were performed at an applied pressure of 50 psi, and this rinsing procedure was repeated before each CE separation.
2.3 Electrophoretic injection of AGP
Electrophoretic injection was performed by applying a potential of −5 kV to a PEO-coated capillary for times ranging from 1 to 20 min. Hydrodynamic injection was performed at 0.5 psi for 3 s. The signal enhancement factor (SEF) that was obtained by electrophoretic injection was calculated by using Eq. (1) [34],
| (1) |
where IFESI in this study was the collective area for all the observed AGP glycoform bands, CFESI was the concentration of the AGP sample that was used for electrophoretic injection, Icontrol was the collective area for all the AGP glycoform bands during a hydrodynamic injection, and Ccontrol was the concentration of the AGP sample that was used for hydrodynamic injection. When serum was loaded by electrophoretic injection, 0.5 g/L LyCH in water was subsequently injected at 0.1 psi for 2 s for use as a mobility marker.
2.4 Serum pretreatment
All serum samples were kept in BSL-2 facilities and handled according to standard procedures for dealing with materials that may contain blood borne pathogens. The acid precipitation of AGP from serum or aqueous samples was performed according to a previously-reported procedure [20]. In this pretreatment method, a 65 μL portion of serum and 130 μL of 0.5 M perchloric acid were combined and vortexed for 20 s (Note: Use appropriate chemical hazard precautions when handling perchloric acid) [35]. The resulting sample was centrifuged at 6,600 rpm for 10 min on a Mini Centrifuge (Fisher Scientific, Pittsburgh, PA, USA) at room temperature. A 130 μL portion of the supernatant was put into a Zeba spin desalting column and spun for another 2 min at 6,600 rpm. The filtrate was diluted with water to a total volume of 5 mL for use in CE. The initial serum sample was diluted by 115.4-fold as a result of this procedure.
When using desalting alone for pretreatment, a 130 μL portion of human serum was placed into a Zeba spin desalting column and centrifuged for 2 min at 6,600 rpm. The filtrate was then diluted with water to a total volume of 15 mL for CE analysis, giving a 115.4-fold dilution (i.e., as was used for the pretreatment of serum in the previous paragraph). A control sample was prepared for comparison with each type of pretreatment method by using a 130 μL portion of serum that was diluted with water to a total volume of 15 mL without any other form of pretreatment (i.e., again giving a 115.4-fold dilution for direct comparison with the other samples that were processed in this section).
2.5 Method validation
The limit of detection, precision and separation efficiency of the final CE method with electrophoretic injection were characterized by external calibration with aqueous standards containing 0.125 to 2.0 mg/L of normal and purified AGP. These standards were stored at 4°C prior to use and were each analyzed 3–5 times within a week of their preparation. Electrophoretic injection was performed for 5 min with each sample. The concentration of the individual AGP glycoform bands was found by using the total AGP concentration and relative peak area for a given band versus the total peak area for all glycoforms.
Acid precipitation with desalting and electrophoretic injection was also examined in terms of its recovery, limit of detection, precision and separation efficiency by analyzing AGP in a commercial sample of pooled normal human serum. In this case, 32.5 μL portions of serum were used that had been combined with 32.5 μL of aqueous standards that contained 1.0–5.0 g/L of normal, purified AGP; the spiked serum samples were then treated by acidic precipitation and desalting. Similar samples from serum were prepared that were instead spiked with AGP after the pretreatment steps; the recovery of AGP during pretreatment was found by dividing the slopes of the resulting response curves for the samples that had been spiked before or after the pretreatment steps.
Each sample solution was diluted to 5 mL with an aqueous solution containing 0.33 mg/L LyCH, which was used as an internal standard to correct for variations in the peak areas during the injection process (Note: LyCH was used for this purpose because it is an easily-detectable compound that is anionic under the conditions used in this study and that is well-resolved from all of the AGP glycoform bands). The mixture of each sample with LyCH was stored in the dark within a 20 ml glass vial at 4°C prior to use and each of these samples was analyzed in triplicate by CE within two days; these conditions gave glycoform band areas, when normalized versus the area for LyCH, that had precisions of ± 1.18% or less for injections of serum spiked with AGP, as discussed in Section 3.5. The calibration curves that were generated were based on the corrected peak or band areas, as determined by dividing the observed peak or band areas for AGP by the measured peak area for LyCH in the same sample. The concentration of AGP that was originally present in a serum sample was also determined by using this approach in a standard addition format.
3. Results and discussion
3.1 Selection of conditions for electrophoretic injection
In CE, the apparent velocity of an analyte is the result of both the analyte’s electrophoretic mobility and electroosmosis. Some anionic species such as AGP glycoforms (isoelectric point, 2.8–3.8) [1] can migrate against electroosmotic flow in a bare silica capillary (e.g., when the pH is greater than 3.8 for AGP) [17]. This condition can make it difficult to use electrokinetic injection to apply these anionic solutes onto a capillary in either the normal- or reversed-polarity modes [27]. When FESI is used in the reversed-polarity mode of CE, the electric field at the injection point for a sample that is prepared in a low concentration buffer or water is much stronger than the field strength throughout a capillary that is filled with a running buffer that has a higher concentration of ions. This situation will result in amplified electrophoretic migration for analytes that are present in the region with the high electric field [26].
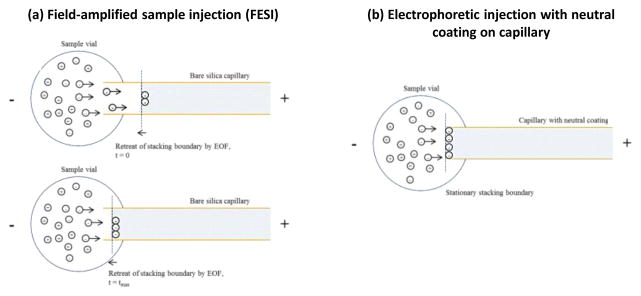
The overall electroosmotic velocity in this situation will be a weighted average for the high and low concentration regions, and the change in electroosmotic velocity will be insignificant from that of the running buffer due to the small amount of the low concentration buffer that will be present in the capillary [36]. As a result, the amplified electrophoretic velocity of the anionic species will outperform the bulk electroosmotic velocity and these negative ions will rapidly migrate through the high field region and decelerate (or become stacked) once they cross the boundary between the low and high concentration regions of the buffer. This process is illustrated in Figure 1(a). When this effect occurs in FESI, it is accompanied by the retreat of this boundary towards the capillary inlet due to the presence of electroosmotic flow. The retreat of this boundary tends to limit the usable injection time in this method to an interval on the order of a few seconds [27,28].
Figure 1.
Injection mechanism for anionic compounds when using (a) field-enhanced sample injection (FESI) for an anionic compound at time 0 (when a water plug is initially injected by pressure) and time tmax (when the stacking boundary has retreated to the sample vessel) or (b) electrophoretic injection with a capillary containing a neutral coating, as used in this current study. The shaded regions on the right of each figure represent the region that contains the running buffer; the light regions to the left represent the sample solution and a plug of water that has been previously placed in the capillary, both of which have a much lower concentration of ions than the running buffer. The term EOF refers to the electroosmotic flow of the system.
In a previous study it was found that electroosmotic flow could be greatly reduced (i.e., by almost 99%) through the use of static and dynamic coatings of PEO in a silica capillary [17]. It was hypothesized in this current study that these conditions, which result in minimal electroosmotic flow, should allow electrophoretic injection to be performed for AGP without significant retreating of the stacking boundary, as is illustrated in Figure 1(b). This feature was tested for use in extending the injection time and amount of AGP that could be loaded into a capillary through the inherent electrophoretic mobility of this glycoprotein alone.
The length of time that could be obtained for sample injection/stacking and the level of signal enhancement that could be obtained with this approach were initially characterized by using a 4.0 mg/L standard solution of normal and purified AGP. The signal enhancement factor of this technique, as based on the collective peak area for all of the observed AGP glycoform bands in the electropherogram, was measured at an injection voltage of −5 kV at various sample injection times. These results were compared to those that were obtained by hydrodynamic injection, as performed at 0.5 psi for 3 s for an aqueous standard containing 4.0 g/L of normal and purified AGP (see Supplementary Materials for electropherograms).
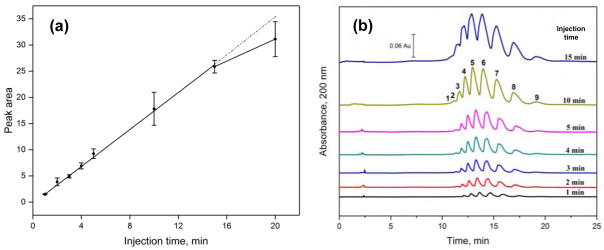
The data and electropherograms that were acquired during this comparison are illustrated in Figure 2. As is shown in Figure 2(a), a linear relationship with a correlation coefficient of 0.9987 (n = 7) was present between the peak (or band) area and injection time as this time was increased from 1 min up to 15 min. This type of linear relationship indicated that a stable stacking interface was present, as has been observed in previous work that instead used external pressure to help create such a situation [33]. An additional, non-linear increase in peak area of 20.4% was observed when the injection time was further increased from 15 min to 20 min. When the results were compared to those seen with hydrodynamic injection, a signal enhancement factor (and corresponding increase in sample loading) of 11,000-fold was measured for an injection time of 5 min; 30,000-fold was obtained for an injection time of 15 min; and 36,000-fold was acquired for an injection time of 20 min. These periods of time were much longer than the typical duration of electrokinetic injection (i.e., several seconds) [27,28] and could be reached without the need for a water plug, as has been used in FESI techniques for anionic compounds [27–29].
Figure 2.
(a) Relationship between the collective peak area for AGP glycoform bands and the time used for electrophoretic injection, and (b) electropherograms acquired for AGP glycoforms when using electrophoretic injection for various lengths of time. Conditions: separation voltage, −30 kV; capillary temperature, 25 °C; running buffer, 20 mM acetate buffer (pH 4.2) containing 0.05% PEO and 0.1% Brij 35; injection voltage, −5 kV; injection time, 1, 2, 3, 4, 5, 10, 15, or 20 min. The capillary coating procedure is described in Section 2.2.
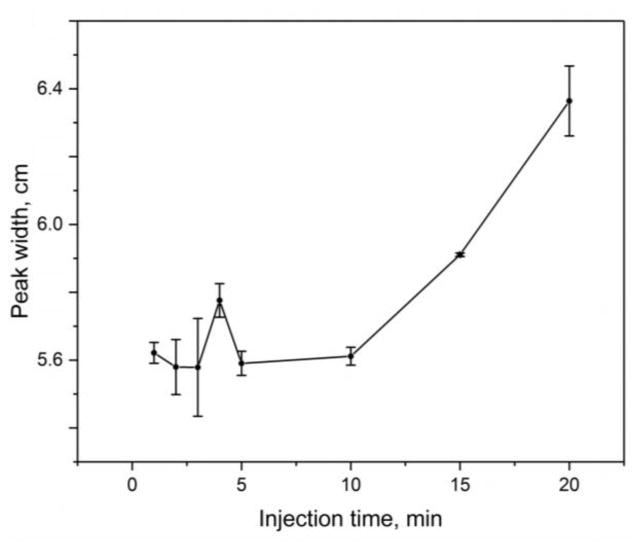
Some additional band-broadening was noted as the injection time was increased during the electrophoretic injection of AGP. This was examined by determining the peak width of the ninth band in the cluster of AGP glycoform bands in Figure 2(b) (i.e., with the ninth band having the highest resolution from its neighboring bands). Because both the speed and the actual width of this band were contributing to its observed width in terms of time, the peak widths were instead expressed in terms of distance by using the migration time and electrophoretic mobility of the glycoform band [37]. As is shown in Figure 3, it was found that the change in band-broadening was negligible in this sample application method and showed only random variations when using an injection time of up to 10 min. A small increase in band-broadening of 5.3% was seen when going from an injection time of 10 min to 15 min, and an increase of 13.4% was seen between 10 min and 20 min.
Figure 3.
Relationship between the width for the ninth glycoform band for AGP (see electropherograms in Figure 2), when expressed in distance units, versus the time used for electrophoretic injection of the AGP sample. The conditions were the same as used in Figure 2. The error bars represent a range of ± 1 S.D. of the mean (n = 3); the small apparent increase in the peak width between 3 min and 4 min is not significant at the 95% confidence level.
The fact that little change in band-broadening was seen up to an injection time of 10 min can be explained by the conservation of mass for the analyte before and after it has crossed the stacking interface [28]. For instance, if a certain amount of the analyte crosses the stacking boundary, the mass conservation relationship for this situation can be described by Eqs. (2–4),
| (2) |
| (3) |
| (4) |
where C1 is the initial sample concentration, C2 is the concentration of analyte after crossing the stacking boundary, L1 is the zone width of the sample ions that travel towards the boundary, and L2 is the zone width of the sample ions after they cross the boundary. Other terms in these equations include t as the time interval for this comparison, μep as the electrophoretic mobility of the analyte, E0 as the electric field throughout the capillary (i.e., during electrophoretic injection), and γ as the resistivity ratio between the sample and running buffer.
It is possible to show by combining Eqs. (3) and (4) that the zone width of the sample band during electrophoretic injection and stacking should be reduced by the factor γ, where γ = L1/L2. If Eqs. (3) and (4) are inserted into Eq. (2), it can also be shown that the injected sample should be concentrated by the value of γ, because C2 = γ C1. In addition, the loading capacity (which is reflected by the observed total peak area) of this injection technique can be described by Eq. (5) [28],
| (5) |
where Ni(t) is the moles of analyte i that are applied to the system over injection time t, and the A is the cross-sectional area of the capillary. This means the sample loading capacity can be increased substantially during electrophoretic injection through the presence of a high resistivity ratio (γ) and the use of a long injection time (t), as shown by Eq. (5), while the separation efficiency is maintained, as indicated by Eqs. (2–4). In this study, an injection time of 5 min was used in all further experiments as a compromise between the change in sensitivity, the total analysis time, and the effects on band-broadening.
3.2 Effects of injection bias and speed bias during electrophoretic injection
The possible effects of injection bias and speed bias were also considered when using electrophoretic injection. Injection bias can result from the differential mobility of analytes during electrokinetic injection [38], and is a key reason as to why hydrodynamic injection is often the preferred injection mode in CE [39]. To consider this effect, the quantity Q (or Ni, when expressed in moles) of an analyte in a physical zone within a CE system can be described by Eq. (6),
| (6) |
where C is the concentration of the analyte in this zone, V is the volume of the zone, A is the cross-sectional area of capillary, and dL is the length of capillary that contains this zone. This relationship can also be written in the terms of time (e.g., dt), as shown in Eq. (7) by using the analyte’s electrophoretic mobility (μ), the applied electric field during the separation (E) and the fact that the inherent migration velocity of the analyte is equal to the product μE [37].
| (7) |
The observed peak area when using absorbance detection in CE will be the sum of the absorbance values measured over a range of time, as described by using the Beer-Lambert law [40],
| (8) |
where Pobserved is the observed peak or band area, Abs is the signal due to absorbance by the analyte at time t, is the molar absorptivity of the analyte, and b is the path length for the light in the capillary. Eq. (9) can then be obtained by combining Eq. (7) and (8).
| (9) |
This new equation indicates that the quantity Q of an analyte in its observed peak can be found by using the product of the observed peak area and the term ( ), which will be constant for a given analyte.
If a sample is injected through electrophoretic injection and in the absence of any appreciable electroosmotic flow (i.e., as was the case in this study), the only means for analytes to enter the capillary will be through their inherent mobilities. This means Eq. (9) will be equal to Eq. (5), which gives rise to Eq. (10).
| (10) |
This combined relationship suggests that when a constant time is used in such a situation for electrophoretic injection, the observed peak area will not depend on either injection bias or speed bias. This occurs because the electrophoretic mobility μ is cancelled out in Eq. (10) when both injection bias and speed bias are considered simultaneously (and a correction is made for their effects) during the use of these injection conditions with absorbance detection in CE.
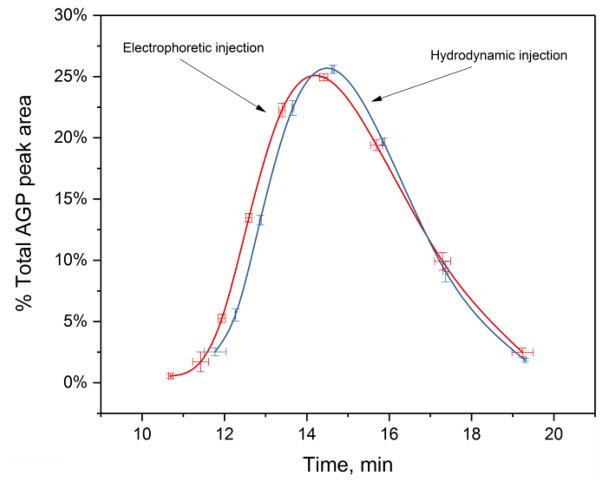
This predicted relationship was tested by comparing the relative composition of glycoform bands that were measured for normal and purified AGP when using electrophoretic injection versus the composition for the same AGP glycoform bands that were obtained when using hydrodynamic injection. The composition when using hydrodynamic injection was obtained by using the speed-corrected peak areas of the AGP glycoform bands, while the peak areas were used directly to find the AGP glycoform composition after electrophoretic injection. The results are shown in Figure 4 (Note: The corresponding electropherograms are given in the Supplementary Materials). The relative composition of the AGP glycoform bands, as represented by the y-axis in Figure 4, was the same at the 95% confidence level for both injection techniques. This confirmed the prediction made by Eq. (10) that the observed peak areas resulting from true electrophoretic injection with absorbance detection should be essentially free from both mobility-induced injection bias and speed-induced detection bias. This feature meant that these peak areas could be used directly for the quantitative analysis of AGP glycoform bands, as was employed in the remainder of this study.
Figure 4.
Comparison of glycoform pattern obtained for a standard sample of normal AGP when using electrophoretic injection versus hydrodynamic injection. Speed-corrected peak areas were used to obtain the results shown for hydrodynamic injection, while measured peak areas were used directly for the results given for electrophoretic injection. Conditions: sample, 4.0 mg/L (electrophoretic injection) or 4.0 g/L (hydrodynamic injection) normal AGP in water; electrophoretic injection, −5 kV for 5 min; hydrodynamic injection, 0.5 psi for 3 s. Other conditions are the same as described in Section 2.2. The error bars represent a range of ± 1 S.D. (n = 3).
3.3 CE analysis and electrophoretic injection of AGP standards
The CE and electrophoretic injection method was next characterized in terms of its analytical performance when using 5 min for sample injection. External calibration was first performed by using aqueous standards that contained 0–2.0 mg/L of normal and purified AGP. The results are summarized in Table 1. An average correlation coefficient of 0.9991 (range, 0.9971–0.9997) was obtained for the glycoforms of AGP. The limit of detection for the AGP glycoform bands under these injection conditions was 0.05–0.2 nM when using absorbance detection, or a range that was 0.6–4.8 × 105-fold smaller than the total normal concentration of AGP in human serum [1]. This detection limit was 1,800- to 1,900-fold lower than what has previously been reported under similar CE conditions when using hydrodynamic injection at 0.5 psi for 15 s and for aqueous samples of normal, purified AGP [17] (Note: This latter method corresponded to an injected zone volume equal to approximately 1% of the capillary volume, which was within the maximum range of 1–3% that is generally recommended to avoid overloading effects when using hydrodynamic injection in CE) [39]. The detection level obtained by electrophoretic injection, and as determined for the highest band in the electropherograms for AGP, was also 2- to 10-fold lower than what has been reported for the highest observed bands when employing CE-LIF and hydrodynamic injection for the analysis of labeled AGP and its glycoforms [21].
Table 1.
Characteristics of an electrophoretic injection CE method for examining AGP glycoforms in aqueous samplesa
| Peak | Plate number, N (×103) | Migration time (min) | Electrophoretic mobilityb | Precision of migration time | Precision of peak area | Limit of detection (nM)c |
|---|---|---|---|---|---|---|
| 2 | 4.15 (± 0.30) | 11.41 (± 0.02) | −9.03 (± 0.01) | 0.17% | 7.23% | 0.079 |
| 3 | 4.78 (± 0.12) | 12.01 (± 0.01) | −8.59 (± 0.01) | 0.21% | 6.29% | 0.12 |
| 4 | 6.01 (± 0.17) | 12.67 (± 0.02) | −8.16 (± 0.01) | 0.15% | 4.52% | 0.097 |
| 5 | 5.75 (± 0.08) | 13.45 (± 0.02) | −7.69 (± 0.01) | 0.19% | 4.80% | 0.19 |
| 6 | 4.81 (± 0.05) | 14.42 (± 0.03) | −7.19 (± 0.01) | 0.21% | 4.67% | 0.20 |
| 7 | 4.28 (± 0.05) | 15.64 (± 0.03) | −6.65 (± 0.01) | 0.23% | 4.44% | 0.19 |
| 8 | 3.86 (± 0.04) | 17.16 (± 0.04) | −6.08 (± 0.02) | 0.28% | 4.28% | 0.082 |
| 9 | 3.08 (± 0.14) | 19.00 (± 0.05) | −5.52 (± 0.02) | 0.36% | 4.51% | 0.052 |
These values were measured for a standard containing 2 mg/L AGP (n = 5). The numbers in parentheses represent ± 1 S.D. The precisions for the migration times and peaks areas are given in terms of ± 1 relative standard deviation.
The units for the electrophoretic mobility are ×10−3cm2min−1V−1.
The limit of detection was determined by using a signal-to-noise ratio of three along with the standard deviation of the intercept and the slope for a calibration curve that was generated using standards that ranged in concentration from 0 to 2 mg/L AGP, with each being injected in triplicate.
The precision for the migration times of the AGP glycoform bands in this method was ± 0.15–0.36%, as measured by using a 2.0 mg/L AGP sample. The precision in the peak areas for the same glycoform bands and sample ranged from ± 4.2–7.2%. This level of precision in both the migration times and peak areas is consistent with what has been achieved in prior work using similar separation conditions with hydrodynamic injection to examine AGP glycoforms by CE [17]. The number of theoretical plates (or plate numbers) that were measured for these glycoform bands ranged from approximately 3,000 to 6,000. This relatively low efficiency was also observed in prior work using CE to examine AGP glycoforms and has been suggested to be due to the fact that each of the observed bands consists of multiple glycoforms with the same charge but slightly different masses (or structures) [2,17]. The resolution between the nine glycoform bands, as shown in Figure 2(a), ranged from 0.41 to 1.10 when using an injection time of 5 min, and the analysis time was 25 min per sample for the electrophoretic injection and separation steps.
3.4 Selection of conditions for serum pretreatment
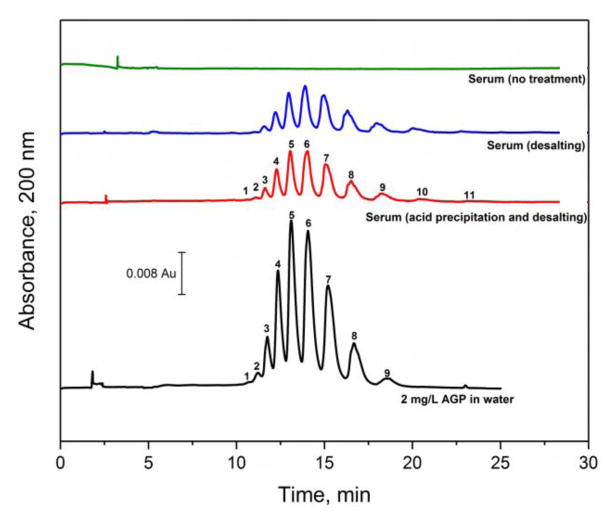
The CE and electrophoretic injection method that was developed and characterized in the previous sections was next tested for use with serum. As is shown in the top portion of Figure 5, no observable signal due to AGP or its glycoforms were obtained by this approach when using for the direct injection of human serum without the use of any sample pretreatment. This lack of signal was a result of both the reduced sample loading and broadening of the injection band that was caused by the presence of salts in the sample. For instance, Table 2 shows that the ratio of the measured resistivities (i.e., the inverse of the ratio of the conductivities) for the sample solution versus the running buffer increased from 2.6 to 18.3 when going from untreated serum to serum that had been pretreated with desalting (i.e., as discussed later in this section). According to Eqs. (2–5), the lower resistivity for the untreated serum sample would have resulted in a 7-fold lower loading capacity versus a desalted serum sample, as well as an increase in zone width for the injected sample band after it crossed the stacking boundary. This effect of salts and small ions on the electrokinetic injection of AGP from serum has also been noted previously by others when using a coated capillary [15].
Figure 5.
Electropherograms obtained for AGP glycoforms when using various samples and sample pretreatment methods, including 2.0 mg/L AGP in water (i.e., which was used as a positive control), serum that was processed by acid precipitation and desalting, serum that was processed by desalting alone, and serum that was used with no pretreatment (as a control). Conditions: separation voltage, −30 kV; capillary temperature, 25 °C; running buffer, 20 mM acetate buffer (pH 4.2) containing 0.05% PEO and 0.1% Brij 35; and injection conditions, −5 kV for 5 min. The capillary coating procedure is described in Section 2.2.
Table 2.
Conductivity and resistivity measurements for samples after various pretreatment stepsa
| Sample or solution | Conductivity (μS/cm) | Resistivity ratio (γ) vs. running buffer |
|---|---|---|
| Serum with no pretreatmentb | 147.5 (± 0.2) | 2.59 (± 0.01) |
| Serum after desaltingb | 20.95 (± 0.03) | 18.25 (± 0.04) |
| Serum after acid precipitation and desaltingb | 12.96 (± 0.05) | 29.5 (± 0.1) |
| Sample of 2 g/L AGP in water | 6.76 (± 0.04) | 56.6 (± 0.3) |
| Running bufferc | 382.4 (± 0.6) | n/a |
The numbers in the parentheses represents a range of ±1 S.D.
The serum sample was diluted by 115.4-fold after each of these pretreatment procedures.
The running buffer was pH 4.2, 20 mM acetate buffer that contained 0.05% (w/v) PEO and 0.1% (w/v) Brij 35.
Several pretreatment methods were considered for reducing the content of small ions in the serum samples prior to the analysis of AGP by the CE and electrophoretic injection method. These pretreatment methods included the use of desalting or acid precipitation (i.e., which also allowed the isolation of AGP from many other serum proteins) [20] followed by desalting. The direct injection of serum, without any pretreatment, was used as a control, along with the direct injection of an aqueous standard of normal, purified AGP. The electropherograms and glycoform patterns that were obtained with these samples and pretreatment methods are shown in Figure 5. Only AGP was observed in the electropherograms obtained with both pretreatment methods even for commercial serum samples that had originally contained a full set of serum proteins. The selectively of this method for AGP was mainly a result of the minimal electroosmotic flow that was present (i.e., suppressed by almost 99%) [17] and the low isoelectric point of AGP when compared to the majority of other serum proteins (pI > 4.2 except for AGP, which also formed the basis for the acid precipitation pretreatment method) [19,40]. As a result of this combination of factors and use of the reversed-polarity mode for CE, AGP was the only significant, negatively-charged serum protein that was injected onto and passed through capillary in the presence of a pH 4.2 running buffer.
When desalting alone was used for serum pretreatment, ten glycoform bands for AGP could be detected (i.e., corresponding to peaks 2–11 in the other samples). A similar pattern and signal intensity for the AGP glycoform bands was observed for serum that had undergone both acid precipitation and desalting, with up to eleven bands being noted for some of these samples, as demonstrated in Figure 5. This number of AGP glycoform bands was consistent with what has been observed by using CE and anti-AGP antibody supports for sample pretreatment [12,13]. The relative composition of the glycoform bands after both pretreatment methods did differ some from the pattern seen for commercially-prepared normal and purified AGP by giving a slight shift in intensity to bands with higher migration times (see Supplementary Materials). For instance, these two pretreatment methods gave a 1.7–6.5% decrease in composition for the bands at migration times below 15 min when compared to the purified AGP and a corresponding increase of 1.2–6.4% for bands migrating after 15 min. This shift is probably related to the different approach (i.e., multiple chromatographic methods) that had been used by the manufacturer to pretreat and purify the commercial AGP standard. However, the AGP glycoform band composition for each pretreatment method was reproducible for peaks that made up more than 3% of the total area, with a relative standard deviation in the band area ranging from ± 0.5–8.4% when using acid precipitation plus desalting and from ± 0.4–7.1% when using desalting alone.
The pretreatment approach that was selected for the final assay was based on both acid precipitation and desalting. This method could be used with only 65 μL of serum and could be carried out within 15 min. The overall process provided a 5 mL solution from the pretreated and diluted sample that could be used for CE analysis.
3.5 Use of CE/electrophoretic injection and pretreatment method with serum
The combined CE and sample pretreatment method was next characterized by using this approach to examine AGP glycoforms that had been obtained from human serum (see Table 3). The recovery for the major AGP glycoform bands (i.e., bands 3–9 in Figure 5) from a commercial sample of pooled normal serum that had been spiked with a known amount of AGP was found to range from 72.3–80.9% in the overall method. The detection limits for the same AGP glycoform bands was 2.1–11.4 nM when working with the treated and diluted sample of the normal and pooled human serum, which was 40- to 50-fold higher than the results that were obtained previously in this report with aqueous AGP standards. However, these adjusted levels were still more than sufficient to examine the expected levels of AGP and these glycoform bands in samples of human serum [1].
Table 3.
Characteristics of an electrophoretic injection CE method for AGP glycoform analysis in serum using acid precipitation and desalting for sample pretreatment
| Peak | Limit of detection (nM)a | Plate number (×103)b | Precision of migration timeb | Precision of peak areab | Recoveryc |
|---|---|---|---|---|---|
| 3 | 2.5 | 8.11 (± 0.37) | ± 0.08% | 0.86% | 72.3% |
| 4 | 8.2 | 7.12 (± 0.08) | ± 0.11% | 0.94% | 80.9% |
| 5 | 11.4 | 5.34 (± 0.10) | ± 0.12% | 0.71% | 80.4% |
| 6 | 8.5 | 4.32 (± 0.06) | ± 0.12% | 0.52% | 80.5% |
| 7 | 4.8 | 4.10 (± 0.03) | ± 0.12% | 0.34% | 80.3% |
| 8 | 2.4 | 3.87 (± 0.02) | ± 0.13% | 0.86% | 78.2% |
| 9 | 2.1 | 2.84 (± 0.02) | ± 0.12% | 1.18% | 73.6% |
The limit of detection was calculated by using a signal-to-noise ratio of three, as based on the standard deviation of the intercept and the slope from a calibration curve for this assay.
The precisions and plate numbers are based on triplicate measurements for normal serum that was spiked with 2.5 g/L AGP. All numbers in parentheses represent ± 1 S.D. The precisions for the migration times and peaks areas are given in terms of ± 1 relative standard deviation.
The recovery was calculated by dividing the slopes of the corresponding calibration curves that were obtained before and after sample pretreatment with pooled normal serum that had been spiked with a known amount of AGP. Each sample was analyzed by CE in triplicate.
The concentration of AGP that was measured by this CE method in the normal and pooled commercial sample of human serum was 14.2 (± 2.9) μM, or 0.60 (± 0.12) g/L. This value fell within the range of 0.5–1.0 g/L for AGP that is expected in healthy individuals [1]. For the sake of convenience, the precisions of the peak areas and migration times were also evaluated with the normal serum samples that were spiked with AGP. The precision in the peak areas for the glycoform bands, when corrected for the area of the internal standard, ranged from ± 0.34–1.18% for the normal human serum after it had been spiked with 2.5 g/L AGP; this level of precision was improved over the precision seen in Section 3.3 when using external calibration. Good precision in the migration times was also obtained; this precision ranged from ± 0.08–0.14% for the glycoform bands in the AGP-spiked serum, which was consistent with the precisions that were obtained when using external calibration. The relative difference in the migration times for the neighboring glycoform bands was 5.3–10.7%, which was 66- to 76-fold larger than the variation observed in the migration times for the individual bands. This difference in migration times and level of precision made these neighboring peaks distinguishable at the 99% confidence level after a duplicate analysis. The number of theoretical plates, as based on the glycoform bands in the spiked serum sample, ranged from 2,840 to 8,110. These values were generally comparable to the plate numbers that were obtained in Section 3.3 for an aqueous AGP standard, although the values shown in Table 3 did cover a broader range.
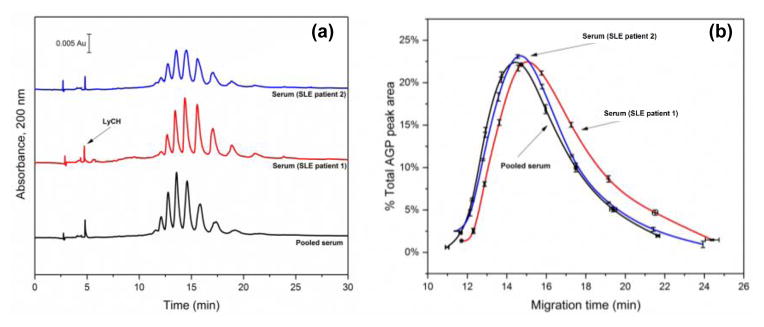
This CE method was next used to examine and compare the AGP glycoforms that were found in pooled normal serum and in serum from individuals known to have SLE. Some typical electropherograms and glycoform patterns that were obtained are provided in Figure 6. LyCH was included as a mobility marker in these serum samples to detect or correct for matrix-induced shifts in the migration times between samples. The resulting glycoform bands were consistent in their locations between samples, with normalized migration times (i.e., values adjusted versus the migration time for LyCH) that were equivalent at the 95% confidence level.
Figure 6.
(a) Electropherograms and (b) corresponding glycoform patterns obtained for AGP from SLE serum or pooled normal serum. The mobility marker (LyCH) was introduced by hydrodynamic injection after sample injection. The error bars shown for the migration times and % peak areas represent ± 1 S.D. of the mean (n = 3). Conditions: separation voltage, −30 kV; capillary temperature, 25°C; running buffer, 20 mM acetate buffer (pH 4.2) containing 0.05% PEO and 0.1% Brij 35; and injection conditions, −5 kV for 5 min. The capillary coating procedure is described in Section 2.2.
Around ten-to-eleven AGP glycoform bands were again seen with this method for the various serum samples. For serum that was from individuals with SLE, the glycoform pattern appeared to give an additional band at a higher migration time (i.e., around 24 min) and at a lower charge than was seen in Figure 5 for the bands from normal purified AGP (see enlarged view in Supplementary Materials). There was also a general shift in the relative size of the glycoform bands to those bands that had longer migration times in the SLE samples, although the extent of this shift did vary between individual samples. This shift indicated that the composition of the AGP glycoforms had a decrease in the number of negative charges (e.g., through a smaller degree of branching and fewer sialic acid residues) for the serum samples that were from the individuals with SLE. These results are consistent with an increase in the amount of biantennary-containing glycoforms for AGP that have been reported in some individuals with SLE [5]. These results confirmed that this CE method could be used to examine the glycoform patterns for AGP in serum from individuals with diseases such as SLE and to compare these with the pattern seen for normal serum.
4. Conclusion
In this study a CE method based on electrophoretic injection and absorbance detection was developed for analyzing the glycoforms of AGP in human serum. This method was made possible by using the reversed-polarity mode of CE and a coated capillary that had minimal electroosmotic flow. This situation created a stable interface between the sample and running buffer that allowed the application of samples at an injection potential of −5 kV over the course of up to 15–20 min. With this approach it was possible to increase the loading capacity by 11,000-fold (versus hydrodynamic injection) at an injection time of 5 min and without introducing significant levels of extra band-broadening to the system.
This method was first validated by using aqueous samples of normal, purified AGP and then was further modified for use with serum. The final method made use of both acid precipitation and desalting for the pretreatment of serum. This method allowed the analysis of AGP glycoform bands from 65 μL of human serum when using a combined injection and separation time of 25 min. Up to eleven glycoform bands were observed for AGP under these conditions, with the detection limits for these individual bands ranging from 2.1 to 11.3 nM. The method had good precision in both its migration times and peak areas, and it was shown that this approach could be used to compare the glycoform profiles for AGP in pooled normal serum and serum taken from patients with SLE. Based on the results and observations that were obtained in this study, it should be possible to adapt this injection and CE analysis method to additional types of glycoproteins that are found in serum (e.g., antibodies) or for the study of glycoproteins in other types of biological samples. For instance, this could be done by using electrophoretic injection with the normal- or reversed-polarity modes of CE and altering the separation pH or sample pretreatment conditions that are used for these other glycoproteins or samples.
Supplementary Material
Highlights.
A CE method was developed to examine glycoforms of alpha1-acid glycoprotein (AGP)
Electrophoretic injection of AGP samples were carried out on a neutral-coated capillary
A pretreatment scheme was created for the use of this method with human serum
This method required only 65 μL of serum had an injection/separation time of 25 min
The method had a detection limit of 2.1–11.3 nM for the separated AGP glycoform bands
Normal AGP and AGP from various serum samples were compared by this method
Acknowledgments
This work was supported by the National Institutes of Health under grant R01 GM044931.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.FC, Pocacqua V. The acute phase protein α1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8:91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 2.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta - Protein Struct Mol Enzymol. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 3.Ongay S, Neusüβ C. Isoform differentiation of intact AGP from human serum by capillary electrophoresis–mass spectrometry. Anal Bioanal Chem. 2010;398:845–855. doi: 10.1007/s00216-010-3948-5. [DOI] [PubMed] [Google Scholar]
- 4.Koizumi K. High-performance liquid chromatographic separation of carbohydrates on graphitized carbon columns. J Chromatogr A. 1996;720:119–126. doi: 10.1016/0021-9673(94)01274-1. [DOI] [PubMed] [Google Scholar]
- 5.Mackiewicz A, Marcinkowska-Pieta R, Ballou S, Mackiewicz S, Kushner I. Microheterogeneity of alpha1-acid glycoprotein in the detection of intercurrent infection in systemic lupus erythematosus. Arthritis Rheum. 1987;30:513–518. doi: 10.1002/art.1780300505. [DOI] [PubMed] [Google Scholar]
- 6.Balmaña M, Giménez E, Puerta A, Llop E, Figueras J, Fort E, Sanz-Nebot V, de Bolós C, Rizzi A, Barrabés S, de Frutos M, Peracaula R. Increased α1–3 fucosylation of α-1-acid glycoprotein (AGP) in pancreatic cancer. J Proteomics. 2016;132:144–154. doi: 10.1016/j.jprot.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Lacunza I, Sanz J, Diez-Masa JC, de Frutos M. CZE of human alpha-1-acid glycoprotein for qualitative and quantitative comparison of samples from different pathological conditions. Electrophoresis. 2006;27:4205–4214. doi: 10.1002/elps.200600304. [DOI] [PubMed] [Google Scholar]
- 8.Lacunza I, Sanz J, Diez-Masa JC, de Frutos M. Erratum: CZE of human alpha-1-acid glycoprotein for qualitative and quantitative comparison of samples from different pathological conditions. Electrophoresis. 2007;28:492. doi: 10.1002/elps.200600304. [DOI] [PubMed] [Google Scholar]
- 9.Landers J. Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques. 3. CRC Press; Boca Raton: 2007. Introduction to capillary electrophoresis; pp. 1–73. [Google Scholar]
- 10.Puerta A, Diez-Masa JC, Martin-Alvarez PJ, Martin-Ventura JL, Barbas C, Tunon J, Egido J, de Frutos M. Study of the capillary electrophoresis profile of intact α-1-acid glycoprotein isoforms as a biomarker of atherothrombosis. Analyst. 2011;136:816–822. doi: 10.1039/c0an00320d. [DOI] [PubMed] [Google Scholar]
- 11.Pacáková V, Hubená S, Tichá M, Maděra M, Štulík K. Effects of electrolyte modification and capillary coating on separation of glycoprotein isoforms by capillary electrophoresis. Electrophoresis. 2001;22:459–463. doi: 10.1002/1522-2683(200102)22:3<459::AID-ELPS459>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Ongay S, Lacunza I, Díez-Masa JC, Sanz J, de Frutos M. Development of a fast and simple immunochromatographic method to purify alpha1-acid glycoprotein from serum for analysis of its isoforms by capillary electrophoresis. Anal Chim Acta. 2010;663:206–212. doi: 10.1016/j.aca.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Morales-Cid G, Diez-Masa JC, de Frutos M. On-line immunoaffinity capillary electrophoresis based on magnetic beads for the determination of alpha-1 acid glycoprotein isoforms profile to facilitate its use as biomarker. Anal Chim Acta. 2013;773:89–96. doi: 10.1016/j.aca.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita M, Murakami E, Oda Y, Funakubo T, Kawakami D, Kakehi K, Kawasaki N, Morimoto K, Hayakawa T. Comparative studies on the analysis of glycosylation heterogeneity of sialic acid-containing glycoproteins using capillary electrophoresis. J Chromatogr A. 2000;866:261–271. doi: 10.1016/s0021-9673(99)01080-8. [DOI] [PubMed] [Google Scholar]
- 15.Kakehi K, Kinoshita M, Kawakami D, Tanaka J, Sei K, Endo K, Oda Y, Iwaki M, Masuko T. Capillary electrophoresis of sialic acid-containing glycoprotein. Effect of the heterogeneity of carbohydrate chains on glycoform separation using an α1-Acid glycoprotein as a model. Anal Chem. 2001;73:2640–2647. doi: 10.1021/ac001382u. [DOI] [PubMed] [Google Scholar]
- 16.Sei K, Nakano M, Kinoshita M, Masuko T, Kakehi K. Collection of α1-acid glycoprotein molecular species by capillary electrophoresis and the analysis of their molecular masses and carbohydrate chains: basic studies on the analysis of glycoprotein glycoforms. J Chromatogr A. 2002;958:273–281. doi: 10.1016/s0021-9673(02)00353-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Hage DS. Glycoform analysis of alpha1-acid glycoprotein by capillary electrophoresis. J Chromatogr A. 2016;1475:102–109. doi: 10.1016/j.chroma.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishino S, Miyazaki K. Separation methods for glycoprotein analysis and preparation. J Chromatogr B. 1997;699:371–81. doi: 10.1016/s0378-4347(97)00155-2. [DOI] [PubMed] [Google Scholar]
- 19.Kremmer T, Szöllösi É, Boldizsár M, Vincze B, Ludányi K, Imre T, Schlosser G, Vékey K. Liquid chromatographic and mass spectrometric analysis of human serum acid alpha-1-glycoprotein. Biomed Chromatogr. 2004;18:323–329. doi: 10.1002/bmc.324. [DOI] [PubMed] [Google Scholar]
- 20.Stumpe M, Miller C, Morton NS, Bell G, Watson DG. High-performance liquid chromatography determination of α1-acid glycoprotein in small volumes of plasma from neonates. J Chromatogr B. 2006;831:81–84. doi: 10.1016/j.jchromb.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 21.Garrido-Medina R, Puerta A, Rivera-Monroy Z, de Frutos M, Guttman A, Diez-Masa JC. Analysis of alpha-1-acid glycoprotein isoforms using CE-LIF with fluorescent thiol derivatization. Electrophoresis. 2012;33:1113–1119. doi: 10.1002/elps.201100473. [DOI] [PubMed] [Google Scholar]
- 22.Balaguer E, Neusüss C. Glycoprotein characterization combining intact protein and glycan analysis by capillary electrophoresis-electrospray ionization-mass spectrometry. Anal Chem. 2006;78:5384–5393. doi: 10.1021/ac060376g. [DOI] [PubMed] [Google Scholar]
- 23.Ongay S, Martín-Álvarez PJ, Neusüβ C, de Frutos M. Statistical evaluation of CZE-UV and CZE-ESI-MS data of intact α-1-acid glycoprotein isoforms for their use as potential biomarkers in bladder cancer. Electrophoresis. 2010;31:3314–3325. doi: 10.1002/elps.201000244. [DOI] [PubMed] [Google Scholar]
- 24.Ongay S, Neusüβ C, Vaas S, Díez-Masa JC, de Frutos M. Evaluation of the effect of the immunopurification-based procedures on the CZE-UV and CZE-ESI-TOF-MS determination of isoforms of intact α-1-acid glycoprotein from human serum. Electrophoresis. 2010;31:1796–1804. doi: 10.1002/elps.200900680. [DOI] [PubMed] [Google Scholar]
- 25.Marino K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 26.Simpson SL, Quirino JP, Terabe S. On-line sample preconcentration in capillary electrophoresis. J Chromatogr A. 2008;1184:504–541. doi: 10.1016/j.chroma.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Chien RL, Burgi DS. Field-amplified polarity-switching sample injection in high-performance capillary electrophoresis. J Chromatogr A. 1991;559:153–161. [Google Scholar]
- 28.Chien RL, Burgi DS. On-column sample concentration using field amplification in CZE. Anal Chem. 1992;64:489A–496A. [Google Scholar]
- 29.Zhu L, Lee HK. Field-amplified sample injection combined with water removal by electroosmotic flow pump in acidic buffer for analysis of phenoxy acid herbicides by capillary electrophoresis. Anal Chem. 2001;73:3065–3072. doi: 10.1021/ac001313f. [DOI] [PubMed] [Google Scholar]
- 30.Chien RL, Burgi DS. Sample stacking of an extremely large injection volume in high-performance capillary electrophoresis. Anal Chem. 1992;64:1046–1050. [Google Scholar]
- 31.Burgi DS. Large volume stacking of anions in capillary electrophoresis using an electroosmotic flow modifier as a pump. Anal Chem. 1993;65:3726–3729. [Google Scholar]
- 32.He Y, Lee HK. Large-volume sample stacking in acidic buffer for analysis of small organic and inorganic anions by capillary electrophoresis. Anal Chem. 1999;71:995–1001. doi: 10.1021/ac981100e. [DOI] [PubMed] [Google Scholar]
- 33.Feng YL, Zhu J. On-line enhancement technique for the analysis of nucleotides using capillary zone electrophoresis/mass spectrometry. Anal Chem. 2006;78:6608–6613. doi: 10.1021/ac0608568. [DOI] [PubMed] [Google Scholar]
- 34.Airado-Rodríguez D, Cruces-Blanco C, García-Campaña AM. Ultrasensitive analysis of lysergic acid diethylamide and its C-8 isomer in hair by capillary zone electrophoresis in combination with a stacking technique and laser induced fluorescence detection. Anal Chim Acta. 2015;866:90–98. doi: 10.1016/j.aca.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Muse LA. Safe handling of the perchloric acid in the laboratory. J Chem Educ. 1972;49:A463. [Google Scholar]
- 36.Chien RL, Helmer JC. Electroosmotic properties and peak broadening in field-amplified capillary electrophoresis. Anal Chem. 1991;63:1354–1361. [Google Scholar]
- 37.Huang X, Coleman WF, Zare RN. Analysis of factors causing peak broadening in capillary zone electrophoresis. J Chromatogr A. 1989;480:95–110. [Google Scholar]
- 38.Huang X, Gordon MJ, Zare RN. Bias in quantitative capillary zone electrophoresis caused by electrokinetic sample injection. Anal Chem. 1988;60:375–377. [Google Scholar]
- 39.Breadmore MC. Electrokinetic and hydrodynamic injection: making the right choice for capillary electrophoresis. Bioanalysis. 2009;1:889–894. doi: 10.4155/bio.09.73. [DOI] [PubMed] [Google Scholar]
- 40.Swinehart DF. The Beer-Lambert Law. J Chem Educ. 1962;39:333. [Google Scholar]
- 41.Righetti PG, Caravaggio T. Isoelectric points and molecular weights of proteins. J Chromatogr A. 1976;127:1–28. doi: 10.1016/s0021-9673(00)98537-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.