Abstract
Human papillomaviruses (HPVs) cause approximately 5% of cancer cases worldwide. Fortunately, three prophylactic vaccines have been approved to protect against HPV infections. Gardasil-9, the most recent HPV vaccine, is predicted to offer protection against the HPV types that cause ~90% of cervical cancer, 86% of HPV-associated penile cancers, and ~93% of HPV-associated head & neck cancers. As an alternative to Gardasil-9, we developed and tested a novel candidate vaccine targeting conserved epitopes in the HPV minor capsid protein, L2. We displayed a tandem HPV31/16L2 peptide (amino acid 17-31) or consensus peptides from HPV L2 (amino acid 69-86 or 108-122) on the surface of bacteriophage MS2 virus-like particles (VLPs). Mice immunized with the MS2 VLPs displaying the tandem peptide or immunized with a mixture of VLPs (displaying the tandem peptide and consensus peptide 69-86) elicited high titer antibodies against individual L2 epitopes. Moreover, vaccinated mice were protected from cervicovaginal infection with HPV pseudoviruses 16, 18, 31, 33, 45, and 58 at levels similar to mice immunized with Gardasil-9. These results suggest that immunization with a tandem, L2 peptide or a low valency mixture of L2 peptide-displaying VLPs can provide broad protection against multiple HPV types.
Keywords: HPV vaccine, tandem L2 peptide, bacteriophage MS2-L2 VLPs, Gardasil-9, protection, neutralization
1. Introduction
Human papillomaviruses (HPVs) are the most common sexually transmitted infections; approximately 40 HPV types can be transmitted sexually and they cause neoplasias such as genital warts and cancers (Schwarz et al. 2005; Matsukura and Sugase 2001). Persistent infection with high-risk HPV (HR-HPV) types (HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 70, 73, 82) is associated with ~94% of anal cancers, ~63% of penile cancers, ~74% of vaginal cancers, ~5–20% of head & neck squamous cell carcinomas, and nearly all cases of cervical cancers (Crow 2012; Zhai and Tumban 2016; Boscolo-Rizzo et al. 2013). Infections with low-risk HPV types (HPV6, 11, 43, and 44) are associated with genital warts (Munoz et al. 2003; Dickens et al. 1991; Sanchez et al. 2013). HPV-associated cancers can be initiated by infection with a single HR-HPV type or by infection with multiple HR-HPV types (Schmitt et al. 2010; Schmitt et al. 2013); a situation that occurs especially in human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) patients. HIV/AIDS patients are more susceptible to HPV-associated cancers compared to non-HIV infected patients (Munoz et al. 2004; Schmitt et al. 2010; Levi et al. 2002; McKenzie et al. 2014; Beachler et al. 2012; Ortiz et al. 2014). Thus, these patients need broad protection from infections by multiple HPV types.
Three prophylactic vaccines (Cervarix, Gardasil-4 and Gardasil-9) have been approved to protect against certain types of HPV infections. The vaccines are composed of virus-like particles (VLPs) derived from HPV major capsid protein (L1). The vaccines are highly immunogenic. However, because L1 neutralizing epitopes are not highly conserved among HPV types, L1 vaccines protect mostly against the HPV types included in the vaccines, with minimal cross-protection against non-vaccine HPV types (Brown et al. 2009; Joura et al. 2015; Smith et al. 2007; Toft et al. 2014; Wheeler et al. 2009). For example, Gardasil-9 offers protection against the HR-HPV types (HPV16, 18, 31, 33, 45, 52, 58) that cause ~93% of head and neck cancers, ~90% of cervical cancers, 90–95% of anal cancers and more than 80% of vaginal, penile and vulvar cancers. The vaccine also protects against HPV6 and 11, types that cause 90% of genital warts. (Zhai and Tumban 2016). As such, women who are vaccinated with Gardasil-9 vaccine (and also the other HPV vaccines) are still advised to continue screening for other HPV types not included in the vaccine. To broaden protection, researchers have focused on developing next-generation HPV vaccines targeting the HPV minor capsid protein (L2) (Seitz et al. 2014; Jagu et al. 2009; Schellenbacher, Roden, and Kirnbauer 2009a). The minor capsid protein, especially the N-terminus, is highly conserved among diverse HPV types (Gambhira et al. 2007; Kondo et al. 2007). Nevertheless, normal infection does not induce protective anti-L2 antibody responses because L2 is only transiently exposed on the capsid. During HPV infection, the capsid binds to heparan sulfate proteoglycan (HSPG) on the basement membrane; the capsid then undergoes a series of conformational changes thus transiently exposing the N-terminal portion of L2 (Kines et al. 2009; Selinka et al. 2007). This conformational change allows the virus to bind to and infect epithelial cells. If neutralizing antibodies, following immunization with L2 antigens are available, they can bind to exposed-neutralizing L2 epitopes thus preventing the infection (Kines et al. 2009; Selinka et al. 2007). Neutralizing L2 antibodies can cross-protect against diverse HPV types (Roden et al. 2000; Alphs et al. 2008; Pastrana et al. 2005). However, L2-based immunogens generally elicit much lower antibody titers than L1 VLPs because, unlike L1, L2 cannot self-assemble into VLPs. Different approaches have been explored to enhance the immunogenicity of L2 protein; these include conjugation of an L2 peptide to thioredoxin (Rubio et al. 2009; Seitz et al. 2014), construction of L2 concatemer proteins derived from different HPV types (Jagu et al. 2009; Jagu et al. 2010), and the display of L2 peptides on different types of VLPs (Schellenbacher, Roden, and Kirnbauer 2009b; Tumban et al. 2012; Nieto et al. 2012; Tyler et al. 2014; McGrath et al. 2013). While these approaches enhance cross-protection against diverse HPV types, they do not offer complete protection against all cancer-causing HPV types. In a previous study, we displayed a conserved epitope (representing amino acid 17-31) from HPV16 L2, on the surface of bacteriophage MS2 VLPs. MS2-16L2 VLPs elicited strong antibody response in mice and offered complete protection against HPV16 pseudovirus (PsV) 16 as well as sub-optimal cross-protection against other HPV PsV types (Tumban et al. 2012). In another study, we showed that immunization with bacteriophage VLPs displaying a consensus L2 epitope (representing amino acid 65-85 derived from an alignment of different HR-HPV types), enhanced cross-neutralization, albeit at low titers, against diverse HPV PsV types (PsV 16, 31, 18, 45 and 58) compared to immunization with VLPs displaying a similar epitope derived from HPV16 (Tyler et al. 2014). These results suggested that the immunogenicity as well as the spectrum of protection against diverse HPV types could be enhanced by displaying conserved or consensus L2 epitopes on the surface of bacteriophage VLPs. In this study, we assessed the spectrum of protection against diverse HPV types following immunization with a mixture of two MS2-L2 VLPs in comparison to immunization with Gardasil-9.
2. Materials and methods
2.1. Cloning, expression and purification of MS2-L2 VLPs
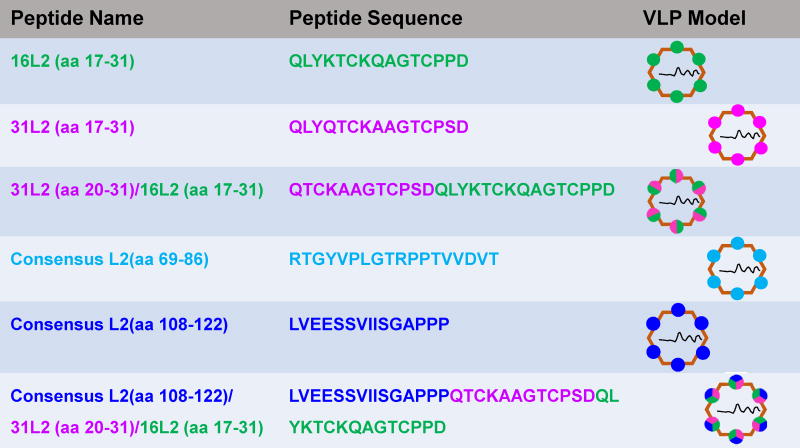
Polymerase chain reaction (PCR) was used, as previously described (Tumban et al. 2011), to insert different HPV L2 epitopes on the N-terminus of the single-chain dimer of MS2 bacteriophage coat protein. Plasmid pDSP62 (which expresses the single-chain dimer of MS2 coat protein) was used as a PCR template. The following HPV L2 peptides (Fig. 1A and Table 1) representing amino acids (aa) sequences were inserted: a tandem peptide (aa 20-31 from HPV31 L2 & aa 17-31 from HPV16 L2), aa 69-86 from a consensus (cons)L2 sequence, aa 108-122 from a consensus (cons)L2 sequence, and a multivalent epitope representing consL2(108-122)/HPV31L2(20-31)/HPV16L2(17-31). These L2 sequences were engineered into forward PCR primers. A reverse primer, E3.2 (5′ CGGGCTTTGTTAGCAGCCGG 3′), which anneals downstream of a unique BamHI site in the pDSP62 plasmid was used for all PCR amplifications above. Amplified MS2-L2 PCR fragments were cloned into pDSP62 plasmid using NcoI and BamHI restriction sites and the plasmids were then transformed into C41 E. coli bacteria. All constructs were sequenced to confirm insertions. To express recombinant MS2-L2 proteins, transformed C41 cells were grown at 37°C until the cells reached an optical density (OD)600 of 0.6. Protein expression was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside for 4 hours or overnight. Cell pellets were collected and lysed using 0.2% lysozyme solution. Soluble MS2-L2 VLPs were purified by gel filtration on Sepharose CL-4B column.
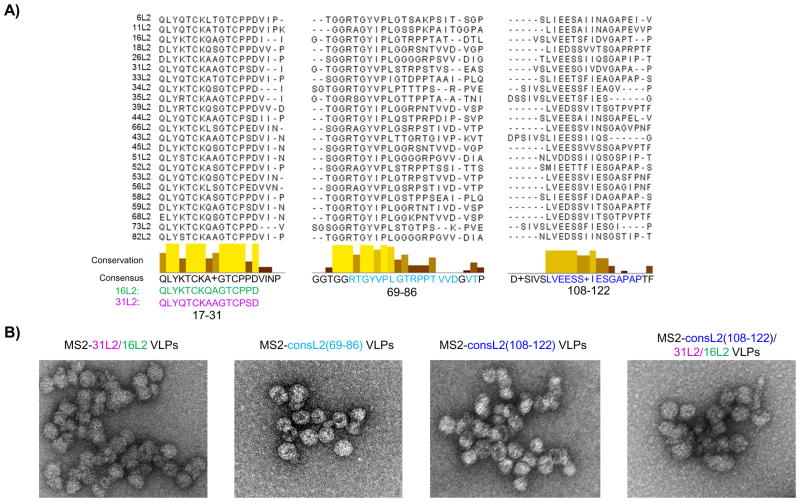
Fig. 1. Consensus sequence and Transmission electron microscopy (TEM) images of MS2-L2 VLPs.
A) Consensus sequences were obtained by aligning HR-HPV types (HPVs 16, 18, 26, 31, 33, 34, 35, 39, 45, 51–53, 56, 58, 59, 66, 68, 73 and 82) and low-risk HPV types (HPVs 6, 11, 43, and 44) using Jalview software and T-coffee program. Highly conserved amino acids (conservation) are shown in yellow/gold tall bars whereas less conserved amino acids are shown in tan/chocolate short bars. Glycine (G) at position 85 was not included in designing consensus (69–86) peptide because only HPV 11 had G at this position. “+” at position 114 was replaced with valine (V) since most HPVs have V at this site. Glutamic acid (E) at 116 and Alanine (A) at 121 were replaced with isoleucine (I) and proline (P), respectively. B) TEM images of purified MS2 VLPs displaying L2 peptides (70,000X).
Table 1.
HPV L2 peptides inserted on MS2 VLPs
2.2. Characterization of MS2-L2 VLPs
Transmission electron microscopy (TEM) was done as previously published (Tumban et al. 2011) to assess whether all constructs assembled into VLPs. To assess whether the L2 epitopes were displayed on the MS2 bacteriophage VLPs, enzyme-linked immunosorbent assay (ELISA) and Western blot were done. Western blotting was conducted as follows: 100 ng of MS2-L2 VLPs or control MS2 VLPs were run on an SDS-PAGE gel and transferred to polyvinylidene fluoride membranes. The membranes were blocked and 1:5,000 dilution of anti-HPV16 L2 (aa 1-88) serum (Tumban et al. 2012) or MS2 serum was added and incubated for 2 hours. The membranes were washed and 1:10,000 dilution of horseradish peroxide (HRP)-conjugated goat anti-mouse IgG antibodies added for 1 hour. The membranes were then washed and developed using a mixture of SuperSignal West Pico (Lumino/Enhance and Stable Peroxide) solutions. To confirm the Western blot results, ELISA was conducted as follows: Briefly, ELISA plates were coated with 500 ng of purified MS2-L2 VLPs. The wells were blocked and serial dilutions of anti-HPV16 L2 (aa 1-88) serum was added. The plates were incubated for 2 hours and 1:5,000 dilution of HRP-conjugated goat anti-mouse IgG antibodies was added for 1 hour. The plates were developed by adding 3, 3′, 5, 5′-tetramethylbenzidine (TMB) and the reactivity was determined at OD450.
2.3. Immunization of mice and characterization of antibody responses
All animal work was conducted in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines. Four Balb/c mice (per group) were immunized intramuscularly (i.m.) with 5μg each of MS2-16L2 VLPs (displaying aa 17-31 from HPV16) (Tumban et al. 2012), MS2-31L2 VLPs (displaying aa 17-31 from HPV31) (Tyler et al. 2014), MS2-31/16L2 VLPs (displaying tandem L2 peptide 20-31 and 17-31 from HPV31 and HPV16, respectively), MS2-consL2(69-86), MS2-consL2(108-122), MS2-consL2(108-122)/31L2/16L2, or immunized with a mixture of VLPs [Mixed MS2-L2 VLPs: 5μg MS2-consL2(108-122)/31L2/16L2 plus 5μg of MS2-consL2(69-86)]. All VLPs were mixed with alum hydroxide adjuvant prior to immunizations and immunizations were done twice at two-week intervals. Anti-L2 IgG antibody responses in sera were tested by L2 peptide-ELISA using synthetic HPV16L2 (17-31), HPV31L2 (17-31), consL2(69-86), and consL2(108-122) as target antigens. The peptides were conjugated to streptavidin using biotin or SMPH [Succinimidyl 6-((beta-maleimidopropionamido)hexanoate)] and were then used to coat ELISA plates. ELISAs were conducted as described above and the titers of antibodies were determined as the reciprocal of the highest sera dilution with an OD450 greater than 2-fold compared to that of control sera at the same dilution.
2.4. Pseudovirus production and purification
HPV PsV types (PsV: 16, 33, 45, 58) encapsidating a reporter plasmid [pClucf: encodes both green fluorescence protein (GFP) and luciferase] were produced in 293TT cells as previously described (Buck et al. 2005). Briefly, 293TT cells were transfected with a mixture of two plasmids (each HPV shell and a pCluf). Forty-eight hours after transfection, cells were lysed and mature PsV was purified on a cesium chloride gradient by ultracentrifugation at 20,000 rpm for 17 hours or 40,000 rpm for 4 hours.
2.5. Cervicovaginal infection with HPV PsVs
Mice were intramuscularly immunized twice each with 5 μg of MS2 control VLPs, MS2 VLPs displaying L2 peptides or with Mixed MS2-L2 VLPs at two-week intervals. In addition to these, another group of mice was immunized with 5 μg of Gardasil-9. All immunizations were done with alum hydroxide adjuvant. Two weeks after the last immunization, mice were subcutaneously treated with 3mg of Depo-Provera. After five days, mice were vaginally challenged with ~6.4 × 106 infectious unit (IU) of PsV. Forty-eight hours post-PsV challenge, mice were vaginally instilled with 0.4 mg of luciferin and imaged with a Caliper IVIS Lumina II as previously described (Tumban et al. 2011).
2.6. In vitro neutralization assays
Neutralization assays were performed as previously described (Day et al. 2012). Briefly, HPV PsV18 and PsV33 encapsidating a reporter plasmid (pfwB: expresses GFP) were made in 293TT cells as above. HPV PsV18 and PsV33 were titered in pgsa-745 cells and virus titers that lead to 20% and 75% infectivity (expression of GFP relative to control cells), respectively, were used in the neutralization assay as followings: extracellular matrix (ECM) from MCF10A cells was deposited in 96-well plates followed by the addition of the PsVs. The ECM-PsVs were incubated overnight followed by the addition of serial-dilutions of sera collected from immunized mice or addition of growth media as control. The plates were incubated for 6 hours and pgsa-745 cells were added. Forty-eight hours later, PsV infectivity/neutralizations were assessed by flow cytometry based on the expression levels of GFP in infected cells. The reciprocal of the highest sera dilution that inhibited 50% of PsV infection relative to control sera was considered the neutralization titer.
2.7. Statistical analysis
Statistical analyses for ELISA and PsV challenges were determined by unpaired two-tailed t-test and unpaired one-tailed t-test, respectively.
3. Results
3.1. Insertion of multiple L2 epitopes on MS2 coat protein does not affect VLP assembly
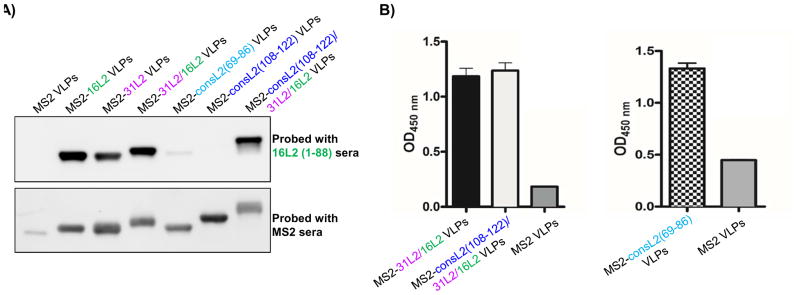
We had previously shown that immunization with MS2 bacteriophage VLPs displaying HPV16L2 (17-31) offered complete protection against HPV PsV16 but suboptimal cross-protection against other high-risk HPV PsV types. To enhance protection against these other HPV types, we explored several strategies. First, we constructed recombinant coat proteins displaying a tandem L2 peptide (aa 20-31 from HPV31 and aa 17-31 HPV16) or consensus L2 sequences (representing aa 69-86 or aa 108-122) at the N-terminus of the MS2 coat protein. The consensus sequence was obtained by aligning amino acid sequences from 19 HR-HPV types plus 4 low-risk types using Jalview software (version 2.9.0b2) and T-coffee program (Fig. 1A). In a second strategy, we inserted multiple epitopes [consL2(108-122) and the tandem 31L2/16L2 peptide] on the MS2 coat protein. TEM analysis indicated that all these recombinant constructs assembled into VLPs (Fig. 1B and Table 1). To confirm that the coat proteins contained L2, VLPs were analyzed by Western blot using anti-MS2 and anti-HPV16 L2 (aa 1-88) serum. As shown in Fig. 2A, anti-HPV 16L2 serum reacted with all VLPs except with MS2-consL2(108-122) and control MS2 VLPs as expected. As expected, anti-MS2 serum reacted with each VLP. The varying sizes of recombinant MS2-L2 protein bands reflect the differences in the sizes of inserted L2 epitopes.
Fig. 2. Characterization of L2 peptides on MS2 VLP.
A) Reactivity of HPV16 L2 (aa 1-88) serum and MS2 serum with MS2-L2 VLPs or MS2 VLPs in Western blots. One hundred ng of VLPs were loaded to 10% SDS-PAGE gel and 16L2 (1-88) serum (top) and MS2 serum (bottom) were used as primary antibodies at a dilution of 1:5,000. B) Reactivity of HPV16 L2 (aa 1-88) serum with MS2-L2 VLPs or MS2 VLPs. ELISA plates were coated with 500 ng of VLPs and reacted with the serum at a dilution of 1:10,240 (left) and 1:640 (right). The average ODs450 for 4 mice are shown. Error bars stand for standard error of the mean (SEM).
To assess if the L2 peptides were actually displayed on intact VLPs, an ELISA was conducted using MS2-L2 VLPs or control MS2 VLPs as target antigens. As shown in Fig. 2B, anti-HPV16 L2 serum (at a 1:10,240 dilution) reacted with MS2-31/16L2 VLPs and MS2-consL2(108-122)/31L2/16L2 VLPs, and, to a lesser extent (at a 1:640 dilution) with MS2-consL2(69-86) VLPs. These data suggest that the peptides are displayed on the surface of VLPs.
3.2. MS2 VLPs displaying multiple L2 epitopes are immunogenic
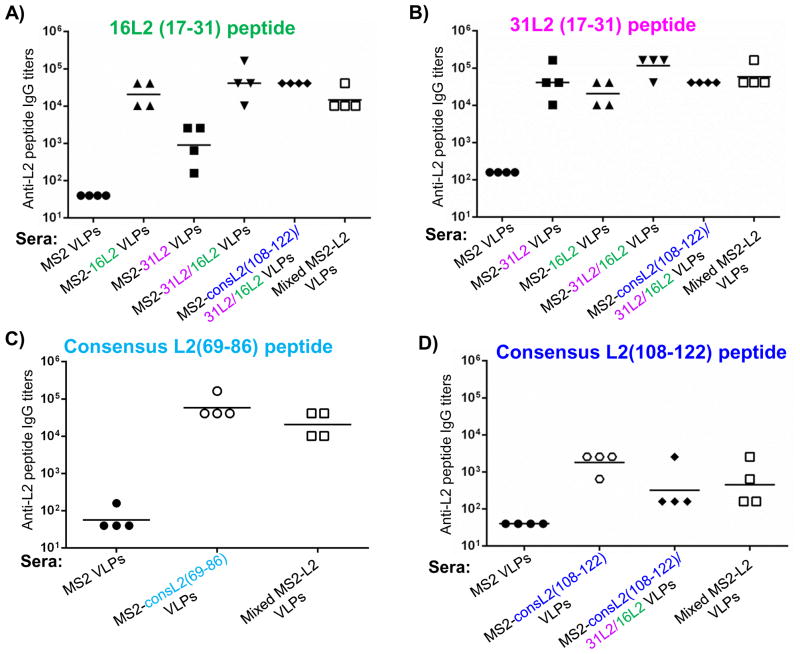
To assess the immunogenicity of MS2 VLPs displaying the L2 peptides, mice were immunized with two doses (5 μg/dose) of purified MS2-L2 VLPs or control MS2 VLPs. Another group of mice was immunized twice with a mixture of VLPs [Mixed MS2-L2 VLPs; MS2-consL2(108-122)/31L2/16L2 plus MS2-consL2(69-86)] in order to assess whether immunizing with multiple L2 VLPs could enhance the spectrum of protection. In general, all of the VLPs elicited high-titer antibody responses. Sera collected from mice immunized with MS2-16L2, MS2-31L2, MS2-31/16L2, and MS2-consL2(108-122)/31L2/16L2 VLPs had high antibody titers (>104) against HPV16 and HPV31 L2 peptides (Figs. 3A–B). Moreover, mice immunized with MS2-consL2(69-86) VLPs had high IgG titers against consL2(69-86) peptide (Fig. 3C). In contrast, VLPs displaying the consL2(108-122) peptide were less immunogenic and induced lower antibody titers (<103) (Fig. 3D). Mice immunized with Mixed MS2-L2 VLPs had similar antibody titers (>104) against HPV16 L2, HPV31 L2 and consL2(69-86) peptides as mice immunized with individual VLPs (Figs. 3A, B and C), suggesting that mixing the VLPs did not compromise the immunogenicity of individual L2 epitopes.
Fig. 3. Immunogenicity of MS2-L2 VLPs in mice.
Mice were immunized twice with 5 μg of MS2-L2 VLPs or control MS2 VLPs at two-week intervals. Sera were collected two weeks after the last immunization and anti-L2 peptide IgG titers were determined by end-point dilution ELISA using: A) 16L2 (17-31) peptide, B) 31L2 (17-31) peptide, C) consensus L2(108-122) peptide, and D) consensus L2(69-86) peptide as target peptides. Titers were determined as the reciprocal of highest sera dilutions at which reactivity of experimental sera was at least twice that of control MS2 sera. “Mixed MS2-L2 VLPs” serum was collected from mice immunized with a mixture of consL2(108-122)/31L2/16L2 and consL2(69-86) VLPs. Each datum (circles, squares, rectangles, and triangles, etc.) represents titer in an individual mouse and lines stand for the geometric mean for each group.
3.3. MS2 VLPs displaying multiple L2 epitopes offered broad protection against HPV PsVs, at levels similar to Gardasil-9 vaccine
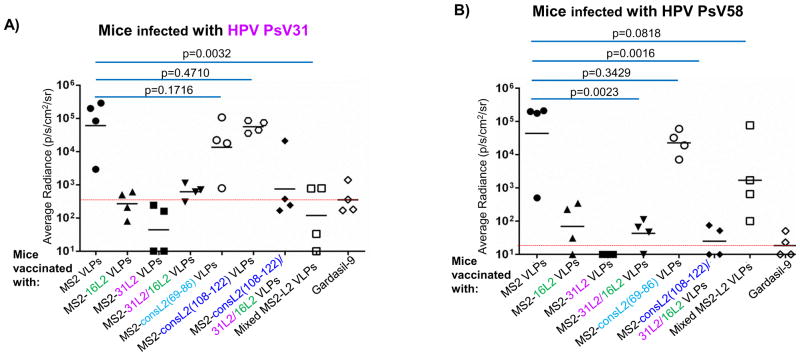
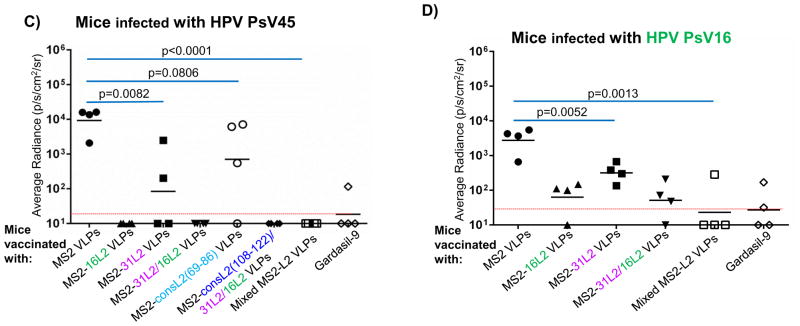
To determine whether immunization with MS2-L2 VLPs conferred protection from genital infection with various HPV types, immunized mice were vaginally infected with HPV PsV (16, 31, 45, and 58) and protection efficacies were compared to those of mice immunized with Gardasil-9 or control MS2 VLPs. We assessed cross-protection against HPV PsV31 and 58 because in a previous study (Tumban et al. 2012) we observed suboptimal cross-protection against these PsVs following immunization with MS2-16L2 VLPs. Mice immunized with MS2-16L2, MS2-31L2, MS2-31/16L2, MS2-consL2(108-122)/31L2/16L2, and Mixed MS2-L2 VLPs showed protection against HPV PsV31, at levels similar to Gardasil-9 (which contains HPV31 VLPs) vaccine-immunized mice (Fig. 4A); Moreover, almost complete protection were observed against PsV58, PsV45, and PsV16 challenge (Figs. 4B–4D). Mice immunized with MS2-consL2(69-86) VLPs showed only slight protection (not significant) against the HPV PsV types tested, and MS2-consL2(108-122) was not protective. Mixed MS2-L2 VLPs offered the best protection against all PsV types except against PsV58.
Fig. 4. Mice immunized with MS2-L2 VLPs elicit protective antibody responses against HPV PsV31, 45, 58, and 16.
Mice vaccinated with MS2-L2 VLPs or MS2 control VLPs were vaginally challenged with ~6.4 × 106 IU of HPV PsVs. Forty-eight hours post-challenge, luciferin was instilled vaginally and images were taken 3 minutes post-luciferin instillation except in 4A were images were taken 10 minutes post-luciferin instillation. The average radiance (p/s/cm2/sr) of luciferase expression at the genitals was determined using Living Image 3.2 software. Each datum represents the radiance value of an individual mouse and the lines stand for the average geometric mean for each group. Red dashed lines highlight MS2-L2 VLPs efficacy in comparison to Gardasil-9 vaccine.
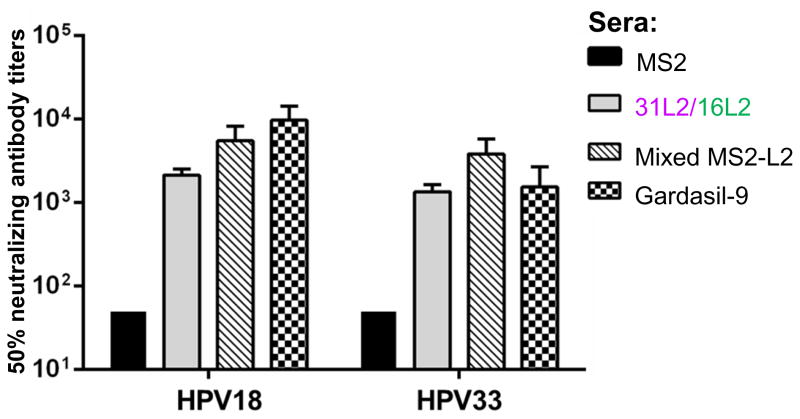
The in vitro neutralization assay is an alternative method that can be used to test the neutralization efficacy (i.e. predict protection potentials) of candidate HPV vaccines. To test the neutralizing potential of our MS2-L2 VLPs with other HPV types, sera from mice immunized with VLPs (MS2-31/16L2 and Mixed MS2-L2) that offered the best protection against vaginal infection were used in an in vitro neutralization assay against PsV18 and PsV33. Compared to control MS2 sera, sera from mice immunized with MS2-31/16L2 or Mixed MS2-L2 VLPs neutralized PsV18 and PsV33 at levels similar to sera from mice immunized with Gardasil-9 (Fig. 5). These results show that MS2-31/16L2 and Mixed MS2-L2 VLPs sera are protective in vivo and in vitro against infection by diverse HPV types.
Fig. 5. MS2-L2 sera neutralize HPV PsV18 and PsV33.
Titers of HPV PsV18 and PsV33 that led to 20% and 75% infectivity, respectively, of pgsa-745 cells were separately incubated with serial-dilutions of sera collected from mice immunized with MS2-L2 VLPs or controls. Incubated PsV and sera were then used to infect pgsa-745 cells. Forty-eight hours after infection, PsV neutralization (based on GFP expression levels) was determined by flow cytometry. Bars indicate the reciprocal of the highest sera dilution at which at least 50% HPV PsV was neutralized compared to controls with no sera. MS2 control sera neutralizing titers were <50.
4. Discussion
Current HPV vaccines protect mostly against the HPV types included in the vaccines (Zhai and Tumban 2016). Given the fact that protection from HPV infections is not 100%, patients vaccinated with any of the HPV vaccines are still recommended to continue cervical cancer screening. The recommended screening is very crucial for HIV/AIDS patients given the immunocompromised nature of their immune system, their propensity to be co-infected with up to 10 HR-HPV types (Levi et al. 2002; McKenzie et al. 2014), and the risk for their HPV infections to progress to invasive cancer 6 to 15 years earlier than non-HIV patients (Moodley et al. 2006; Moodley, Moodley, and Kleinschmidt 2001). Thus, there is a need to develop an HPV vaccine with the potential to offer complete protection against HPV-associated cancers, especially in HIV/AIDS patients. We had previously reported that a thermostable bacteriophage MS2 VLPs displaying peptide 17-31 from HPV16 offered complete protection against HPV PsV16 and other HPV PsVs, but suboptimal protection against two important heterologous HPV types (HPV31 and HPV58) (Tumban et al. 2012; Tumban et al. 2015). To enhance protection against some of these heterologous HPV types, we generated for the first time, VLPs [MS2-31/16L2 and MS2-consL2(108-122)/31L2/16L2] displaying concatemers of two or three L2 epitopes, respectively. In addition to these, we developed VLPs [MS2-consL2(69-86), MS2-consL2(108-122)] displaying consensus sequences. Anti-HPV16 L2 serum reacted with all the MS2-L2 VLPs (confirming display of peptides on VLPs) except for VLPs displaying consL2(108-122) (Figs. 2A and 2B). Lack of reactivity with MS2-consL2(108-122) VLPs was expected because the HPV16 L2 (aa 1-88) serum does not include consL2(108-122) epitope. The reactivity of anti-HPV16 L2 serum with MS2-consL2(69-86) VLPs (compared with other L2-VLPs) was low. This could be explained by the fact that HPV16 L2, from which the serum was generated is 80% identical to 31L2 (at aa 17-31) unlike peptide 69-86 of HPV16 L2, which is only 72% identical to consL2(69-86).
Mice immunized with VLPs displaying concatemers, consensus sequences [except MS2-consL2(108-122)], and Mixed MS2-L2 VLPs [MS2-consL2(108-122)/31L2/16L2 plus MS2-consL2(69-86)] elicited high anti-L2 antibody titers (Fig. 3), which protected/neutralized six HPV PsV types (16, 18, 31, 33, 45, 58) at levels similar to Gardasil-9 vaccine (Figs. 4 and 5). Mixed MS2-L2-immunized mice offered the best protection except against PsV58; we are not sure why partial protection was observed given the fact that 31L2 and 16L2 (17-31) share ~87% and 80% amino acid identity, respectively, with 58L2 at the same epitope. MS2-16L2 VLPs in this study offered good protection (although a little less compared to MS2-31L2 VLPs) against PsV31 and PsV58, whereas we had observed less protection in a previous study (Tumban et al. 2012). It is likely that mice in our previous study were challenge with higher PsV dose compared to this study. For example, control MS2 mice challenged with PsV31 in our previous study had a higher geometric mean average radiance (GMAR 105.5) compared to MS2 control mice in this study with GMAR of 104.8. Irrespective of these differences, these results suggest that immunization with a tandem L2 peptide or a mixture of L2 peptides does not compromise the immunogenicity of individual epitopes.
While robust responses were observed with the VLPs displaying peptide 17-31 (single or tandem peptide), little protection was observed in mice immunized with VLPs displaying peptide consL2(69-86) and no immune responses were observed in mice immunized with VLPs displaying peptide consL2(108-122). It was surprising that no significant protection was observed in mice immunized with the MS2-consL2(69-86) given the fact that mice had high anti-L2 antibody titers against this peptide. A previous study had shown that sera (at 1:1000 dilution) from mice immunized with Qβ bacteriophage VLPs displaying a similar conjugated consensus L2 peptide neutralized HPV PsV31, 45, 58 (Tyler et al. 2014). The discrepancy in the results could be explained by the fact that the previous study used a slightly longer consensus sequence (65–85), which included 4 additional amino acids (GTGG) that are highly conserved amongst HPV types. We attempted here to insert (genetically) the same epitope on the coat protein of MS2; however, no expression of the fusion protein was detected (data not shown). With respect to consensus peptide 108-122, we think MS2-consL2(108-122) was not immunogenic because the peptide may not have been properly displayed on the VLPs; previous studies had shown that the isolated peptide contains neutralizing epitopes (Kondo et al. 2007; Tyler et al. 2014). Unfortunately, we could not confirm genetic display on MS2 due to lack of polyclonal antibodies that include peptide 108-122.
5. Conclusions
In summary, we have shown that inserting a concatemer of two or three L2 peptides on MS2 coat proteins does not affect the ability of the coat proteins to assemble into VLPs. Additionally, the L2 epitopes are displayed on the VLPs and immunizations with a mixture of MS2 VLPs displaying multiple L2 epitopes do not compromise the immunogenicity of each individual epitope on the VLPs. MS2 VLPs displaying the tandem L2 peptide, (20-31) from HPV31 and (17–31) from HPV16, cross-protected/neutralized selected HR-HPV PsV types, suggesting that this is an excellent approach to achieve broader protection against more HPV types. Moreover, mixing MS2-consL2(108-122)/31L2/16L2 with MS2-consL2(69-86) VLPs offered protection/neutralization at levels similar to Gardasil-9 vaccine, suggesting that these MS2-L2 VLPs are candidates for low valence yet broadly cross-protective HPV vaccines that should be assessed further for protection against more HPV types especially those that may be more common in HIV patients.
Highlights.
Epitopes within the HPV minor capsid protein (L2) are potentially broadly cross-type neutralizing.
We have constructed novel recombinant VLPs that display multiple HPV L2 epitopes on the N-terminus of MS2 coat protein.
MS2-31/16L2 VLPs are highly immunogenic in mice.
Immunization with mixed MS2 VLPs displaying multiple L2 epitopes does not compromise the immunogenicity of individual epitopes.
In a mouse challenge model, MS2-31/16L2 VLPs offer protection against six HPV types at levels similar to the Gardasil-9 vaccine.
Acknowledgments
This work was partially supported by a grant (1R15 DE025812-01A1) from the US National Institutes of Health (National Institute of Dental and Craniofacial Research) and by a cooperative agreement from the US National Institutes of Health (National Institute of Allergy and Infectious Diseases) establishing the Epidemiology and Prevention Interdisciplinary Center for Sexually Transmitted Diseases (U19 AI113187). We would like to thank Dr. Cosette Wheeler (University of New Mexico School of Medicine) for providing us with some of the reagents used in this study.
Footnotes
Financial interest and conflict of interest disclosures
Ebenezer Tumban and Bryce Chackerian are co-inventors of L2 bacteriophage virus-like particles-related patent applications licensed to Agilvax Biotech by the University of New Mexico. Interactions with Agilvax Biotech are managed by the University of New Mexico in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, Zeng W, Jackson DC, Roden RB. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A. 2008;105:5850–5. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachler DC, Weber KM, Margolick JB, Strickler HD, Cranston RD, Burk RD, Wiley DJ, Minkoff H, Reddy S, Stammer EE, Gillison ML, D’Souza G. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122–33. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P, Del Mistro A, Bussu F, Lupato V, Baboci L, Almadori G, Mosto DAMC, Paludetti G. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33:77–87. [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, Sings HL, James M, Hesley TM, Barr E. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- Crow JM. HPV: The global burden. Nature. 2012;488:S2–3. doi: 10.1038/488S2a. [DOI] [PubMed] [Google Scholar]
- Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol. 2012;19:1075–82. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens P, Srivastava G, Loke SL, Larkin S. Human papillomavirus 6, 11, and 16 in laryngeal papillomas. J Pathol. 1991;165:243–6. doi: 10.1002/path.1711650308. [DOI] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007;81:13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RB. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101:782–92. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagu S, Kwak K, Garcea RL, Roden RB. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine. 2010;28:4478–86. doi: 10.1016/j.vaccine.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, Pitisuttithum P, Restrepo JA, Stuart G, Woelber L, Yang YC, Cuzick J, Garland SM, Huh W, Kjaer SK, Bautista OM, Chan IS, Chen J, Gesser R, Moeller E, Ritter M, Vuocolo S, Luxembourg A H. P. V. Vaccine Study Broad Spectrum. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106:20458–63. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Ishii Y, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology. 2007;358:266–72. doi: 10.1016/j.virol.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Levi JE, Kleter B, Quint WGV, Fink MCS, Canto CLM, Matsubara R, Linhares I, Segurado A, Vanderborght B, Neto JE, van Doorn LJ. High prevalence of human papillomavirus (HPV) infections and high frequency of multiple HPV genotypes in human immunodeficiency virus-infected women in Brazil. J Clin Microbiol. 2002;40:3341–45. doi: 10.1128/JCM.40.9.3341-3345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura T, Sugase M. Relationships between 80 human papillomavirus genotypes and different grades of cervical intraepithelial neoplasia: association and causality. Virology. 2001;283:139–47. doi: 10.1006/viro.2001.0865. [DOI] [PubMed] [Google Scholar]
- McGrath M, de Villiers GK, Shephard E, Hitzeroth, Rybicki EP. Development of human papillomavirus chimaeric L1/L2 candidate vaccines. Arch Virol. 2013;158:2079–88. doi: 10.1007/s00705-013-1713-8. [DOI] [PubMed] [Google Scholar]
- McKenzie ND, Kobetz EN, Ganjei-Azar P, Rosa-Cunha I, Potter JE, Morishita A, Lucci JA, 3rd, Guettouche T, Hnatyszyn JH, Koru-Sengul T. HPV in HIV-Infected Women: Implications for Primary Prevention. Front Oncol. 2014;4:179. doi: 10.3389/fonc.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley JR, Hoffman M, Carrara H, Allan BR, Cooper DD, Rosenberg L, Denny LE, Shapiro S, Williamson AL. HIV and pre-neoplastic and neoplastic lesions of the cervix in South Africa: a case-control study. BMC Cancer. 2006;6:135. doi: 10.1186/1471-2407-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley M, Moodley J, Kleinschmidt I. Invasive cervical cancer and human immunodeficiency virus (HIV) infection: a South African perspective. Int J Gynecol Cancer. 2001;11:194–7. doi: 10.1046/j.1525-1438.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, Castellsague X, Diaz M, De Sanjose S, Hammouda D, Shah KV, Meijer CJLM. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. International Journal of Cancer. 2004;111:278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ Group International Agency for Research on Cancer Multicenter Cervical Cancer Study. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, Seitz H, Muller M, Kellner M, Horer M, Michaelis U, Roden RB, Gissmann L, Kleinschmidt JA. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One. 2012;7:e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz AP, Perez-Irizarry J, Soto-Salgado M, Suarez E, Perez N, Cruz M, Palefsky J, Tortolero-Luna G, Miranda S, Colon-Lopez V. Human papillomavirus-related cancers among people living with AIDS in Puerto Rico. Prev Chronic Dis. 2014;11:E80. doi: 10.5888/pcd11.130361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Roden RB, Yutzyth WH, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270:254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, Muller M, Ottonello S. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine. 2009;27:1949–56. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- Sanchez GI, Jaramillo R, Cuello G, Quintero K, Baena A, O’Byrne A, Reyes AJ, Santamaria C, Cuello H, Arrunategui A, Cortez A, Osorio G, Reina JC, Quint WG, Munoz N. Human papillomavirus genotype detection in recurrent respiratory papillomatosis (RRP) in Colombia. Head Neck. 2013;35:229–34. doi: 10.1002/hed.22953. [DOI] [PubMed] [Google Scholar]
- Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol. 2009a;83:10085–95. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 Virus-Like Particles as Potential Broad-Spectrum Human Papillomavirus Vaccines. J Virol. 2009b;83:10085–95. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, Pawlita M Valgent Study Group. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol. 2013;51:1458–64. doi: 10.1128/JCM.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. Abundance of Multiple High-Risk Human Papillomavirus (HPV) Infections Found in Cervical Cells Analyzed by Use of an Ultrasensitive HPV Genotyping Assay. J Clin Microbiol. 2010;48:143–49. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K, Meijerink E, Speiser DE, Tissot AC, Cielens I, Renhof R, Dishlers A, Pumpens P, Bachmann MF. Efficient homologous prime-boost strategies for T cell vaccination based on virus-like particles. Eur J Immunol. 2005;35:816–21. doi: 10.1002/eji.200425755. [DOI] [PubMed] [Google Scholar]
- Seitz H, Canali E, Ribeiro-Muller L, Palfi A, Bolchi A, Tommasino M, Ottonello S, Muller M. A three component mix of thioredoxin-L2 antigens elicits broadly neutralizing responses against oncogenic human papillomaviruses. Vaccine. 2014;32:2610–7. doi: 10.1016/j.vaccine.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Selinka HC, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J Virol. 2007;81:10970–80. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, Barr E, Brown DR, Bryan JT. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3:109–15. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- Toft L, Tolstrup M, Muller M, Sehr P, Bonde J, Storgaard M, Ostergaard L, Sogaard OS. Comparison of the immunogenicity of Cervarix(R) and Gardasil(R) human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults. Hum Vaccin Immunother. 2014;10:1147–54. doi: 10.4161/hv.27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Muttil P, Escobar CA, Peabody J, Wafula D, Peabody DS, Chackerian B. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine. 2015;33:3346–53. doi: 10.1016/j.vaccine.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One. 2011;6:e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One. 2012;7:e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler M, Tumban E, Dziduszko A, Ozbun MA, Peabody DS, Chackerian B. Immunization with a consensus epitope from human papillomavirus L2 induces antibodies that are broadly neutralizing. Vaccine. 2014;32:4267–74. doi: 10.1016/j.vaccine.2014.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, James M, Vuocolo S, Hesley TM, Barr E. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199:936–44. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- Zhai LK, Tumban E. Gardasil-9: A global survey of projected efficacy. Antiviral Res. 2016;130:101–09. doi: 10.1016/j.antiviral.2016.03.016. [DOI] [PubMed] [Google Scholar]