Abstract
Objective
Disruption in the neural activation of the prefrontal cortex (PFC) in modulating arousal was explored in children with heavy prenatal alcohol exposure (PAE), who have known neurobehavioral impairment.
Methods
During a task that elicits frustration, functional near-infrared spectroscopy (fNIRS) was used to measure PFC activation, specifically levels of oxygenated (HBO) and deoxygenated (HBR) hemoglobin, in children with PAE (n=18) relative to typically developing Controls (n=12) and a Clinical Contrast group with other neurodevelopmental or behavioral problems (n=14).
Results
Children with PAE had less activation during conditions with positive emotional arousal, as indicated by lower levels of HBO in the medial areas of the PFC and higher levels of HBR in all areas of the PFC sampled relative to both other groups. Children in the Control group demonstrated greater differentiation of PFC activity than did children with PAE. Children in the Clinical Contrast group demonstrated the greatest differences in PFC activity between valences of task conditions.
Conclusions: Specific patterns of PFC activation differentiated children with PAE from typically developing children and children with other clinical problems.
Significance
FNIRS assessments of PFC activity provide new insights regarding the mechanisms of commonly seen neurobehavioral dysfunction in children with PAE.
Keywords: Prenatal alcohol, FNIRS, children, FASD
1. Introduction
Fetal Alcohol Spectrum Disorders (FASDS) is a term that refers to a cluster of physical and neurobehavioral abnormalities, including facial dysmorphology, growth retardation, and disruption to brain development, that have been investigated for over four decades in human and animal model studies of prenatal alcohol exposure (PAE) (Riley et al., 2011). Although public health awareness and prevention efforts have increased as a result of these findings, recent estimates of the prevalence of Fetal Alcohol Syndrome (FAS), the most severe FASD condition, have ranged from .6 to .9 percent of live births with the full range of FASDs estimated to fall between 2.4 to 4.8 percent (May et al., 2014). Despite the fairly high prevalence rates, most children with FASDs are misdiagnosed (Chasnoff et al., 2015). Improved methods of identifying alcohol-affected children are needed, including the development of biomarkers of the neural damage caused by PAE.
Although much attention was initially devoted to understanding the deficits in general cognitive functioning associated with a history of PAE (Mattson et al., 1997), many individuals who are exposed do not meet criteria for an intellectual disability (Mattson et al., 1998) but still demonstrate a complex array of neurobehavioral problems (Kable et al., 2016). Among the most significant problems are the persistent and wide-ranging difficulties in a set of cognitive skills known as executive functions (EF), particularly in planning, organization, and problem-solving (Mattson et al., 1999, Kodituwakku et al., 2001, Kodituwakku et al., 2006, Schonfeld et al., 2006, Vaurio et al., 2008, Green et al., 2009, Mattson et al., 2010). Evidence from the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) neurobehavioral study of children, 8 to 17 years, indicated that assessment of executive functioning differentiated individuals with FASD from other clinical groups (i.e. ADHD) (Mattson et al., 2013).
EF problems are expressed in multiple ways in everyday functioning of children with FASD. Difficulties with incorporating environmental feedback to correct a response are suggested by deficits in overall performance on progressive planning tasks (Kodituwakku et al., 1995, Green et al., 2009) and by more repetition errors on learning tasks (McGee et al., 2008). Making shifts in cognitive sets while learning has also been found to be impaired in individuals with PAE, specifically reversal shifts (Coles et al., 1997, Green et al., 2009, Chasnoff et al., 2010). Impairments in verbal fluency (Kodituwakku et al., 2006, Vaurio et al., 2008) and working memory skills (Rasmussen, 2005, Rasmussen et al., 2011) have also been reported. Children with PAE have been found to have problems in sustaining attention and the appropriate mental effort (Brown et al., 1991, Kodituwakku et al., 2006, Aragon et al., 2008) needed to complete tasks while inhibiting impulses (Herman et al., 1980, Streissguth et al., 1986, Streissguth et al., 1993, Streissguth et al., 1994, Kodituwakku et al., 2006). Finally, behavioral and emotional control problems are commonly reported by caregivers of children with FASDs (Kodituwakku et al., 1995, Kopera-Frye et al., 1997, Oesterheld et al., 1997, O’Connor, 2001, Kable et al., 2004, Haley et al., 2006, O’Connor et al., 2009).
Evidence suggests that the prefrontal cortex (PFC) (Malisza et al., 2005, Fryer et al., 2007, Sowell et al., 2007, Burke et al., 2009, O’Hare et al., 2009, Zhou et al., 2011) and the connectivity of the PFC to other brain regions (Wozniak et al., 2013) is adversely impacted by PAE and may be the neural substrate from which the deficits in EF skills arise. Frontal lobe deficits are thought to underlie difficulties with response inhibition, poor behavioral regulation, and other EF skill deficits noted in alcohol-exposed individuals (Niccols, 2007). Further, deficits in both EF and social functioning have been related to the metabolic composition of the frontal lobes (Nash et al., 2006), leading many to theorize that disruption to PFC activity may also help to explain impairments in interpersonal relationships and social skills commonly seen among individuals affected by PAE (Thomas et al., 1998, McGee et al., 2006, Schonfeld et al., 2006, 2009).
Evidence suggests identification of EF skills (Mattson et al., 2010) and PFC activity that supports these skills may be effective in diagnosing individuals affected by PAE and in need of intervention services. An alternative to traditional neuroimaging methods (functional magnetic resonance imaging (fMRI) and positron emission tomography (PET)) is functional near-infrared spectroscopy (fNIRS), which has been used to assess local hemodynamic changes in PFC activity (Ferrari et al., 2012, Boas et al., 2014) and has the advantage of reducing movement-artifact errors and anxiety, particularly in younger children, related to the equipment used with traditional neuroimaging assessments. The fNIRS technology has advanced so that the equipment used is now portable, noninvasive, and commercially available at relatively low cost when compared to fMRI and PET scanning equipment (Ferrari et al., 2012, Boas et al., 2014).
FNIRS assesses levels of oxygenated (HBO) and deoxygenated (HBR) hemoglobin by emitting infrared light, ranging from 650 to 1000 uv, through human biologic tissue, which is nearly transparent to light in this range (Ferrari et al., 2012, Scholkmann et al., 2014). The infrared light is then differentially absorbed by chromophores, which are light-absorbing molecules (specifically, HBO or HBR), or diffusely scattered by other human tissue. Estimates of relative changes in blood oxygenation in the PFC may be obtained by placing sensors on the scalp strategically located from the light emission and using the modified Beer-Lambert Law (MBLL) (Kocsis et al., 2006) to make adjustments needed for diffusion of the light through human tissue. The placement of the sensors directly on the scalp minimizes the impact of movement artifacts often found in other neuroimaging techniques. This method has been validated by documenting changes in blood oxygenation levels relative to blood oxygenation levels obtained in fMRI (Amyot et al., 2012, Heinzel et al., 2013) and PET studies (Obrig et al., 1995). Changes in brain activation levels using fNIRS have been found to differentiate known clinical groups from typically developing children (Ishii-Takahashi et al., 2014, Wiley et al., 2014) and to be related to parent-reported measures of behavioral functioning (Perlman et al., 2014). Changes in activation obtained from fNIRS have already been used to assess the impact of pharmacological interventions for children with Attention Deficit-Hyperactivity Disorder (ADHD) (Oner et al., 2011, Schecklmann et al., 2011, Monden et al., 2012, Matsuura et al., 2014, Araki et al., 2015) but have not yet been applied to understanding individual differences in children with FASDs.
To evaluate prenatal alcohol-related differences in PFC activity as measured by fNIRS, we used three groups of children: (1) children with a history of PAE (PAE), (2) typically developing children without a history of PAE (Controls), and (3) children with clinically significant developmental, learning, or behavioral problems (Clinical Contrast) who do not have a history of PAE. PFC activity was assessed during a task designed to elicit arousal and the associated PFC inhibitory responses needed to modulate the arousal during the course of the task. Indices of PFC activity (levels of oxygenated (HBO) and deoxygenated hemoglobin (HBR)) were anticipated to vary as a function of group status.
2. Methods
2.1 Participants
Children who had enrolled in a larger multisite collaborative project designed to identify neurobehavioral characteristics that differentiate children with FASD from other groups of children (Mattson et al., 2013) were recruited for this study. Participants were from the Atlanta metropolitan area who agreed to undergo neurobehavioral testing, a physical exam and allowed a 3-D photograph of their face to be obtained for the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) study. After being pre-screened for enrollment criteria, guardians were asked to sign an informed consent form for the various components of the study, including a separate consenting procedure for the fNIRS assessment. Consenting procedures and documents were approved by the Human Subjects Committee of the Emory University School of Medicine. Financial incentives were given to participants for each aspect of the multisite study assessment procedures, including a $20 reimbursement for participation in the fNIRS assessment. Exclusionary criteria for the CIFASD neurobehavioral study included being non-fluent in English, having a history of traumatic head injury or a loss of consciousness lasting over 30 minutes, being adopted from abroad after 5 years of age or within 2 years before enrollment, having other known causes of abnormal brain development (e.g., chromosomal abnormalities, neurofibromatosis), or having a mental health or physical limitations that prevented completion of the study’s procedures. Participant recruitment and the study’s procedures were carried out in accordance with the guidelines established by the Declaration of Helsinki.
Forty-four children between the ages of 6 and 16 were evaluated. Participants were included in the prenatal alcohol-exposed (PAE) group if they had a history of heavy PAE, which was defined as greater than 13 drinks per week or greater than four drinks per drinking occasion during pregnancy, or when such exposure was suspected in a child with a clinical diagnosis of FAS. Participants were recruited from a multidisciplinary team clinic established to evaluate children with a history of alcohol and drug exposure or from the community when there was a confirmed positive history of PAE. Participants recruited from the clinic were identified from medical records under a HIPAA partial waiver. The clinic performs neurobehavioral testing and a physical examination where growth and dysmorphology are assessed using a standardized checklist (Fernhoff et al., 1980). Dysmorphology was assessed by either a pediatric geneticist or developmental pediatrician with specialized training in assessing alcohol-related dysmorphology. A total score is computed from 30 items that are weighted relative to each features’ predictive validity for FAS symptomatology. As part of a series of prospective studies on prenatal alcohol exposure carried out from infancy to young adulthood, the checklist differentiated individuals with and without PAE (Coles et al., 1991, Coles et al., 1997, Lynch et al., 2015). Additional details regarding the FASD diagnostic procedures of the clinic are published elsewhere (Coles et al., 2016).
PAE histories were documented either directly from the biological mother’s report or from existing medical, social or legal records. In many cases seen through the clinic, accurate exposure history was not available. Offspring of mothers who were known to be “alcoholic” or alcohol abusing during pregnancy were considered to have heavy PAE. Recruitment of participants from the community included those identified at public health fairs and other community programs (i.e. PTA events at local schools). A total of 18 children were recruited for the PAE group. Nine had an FASD clinical diagnosis and nine were recruited from the community and identified as having heavy PAE. Of the 18 enrolled, 12 were identified by their parents as having a significant behavioral problem and 7 were identified as having a significant learning or developmental problem.
A comparison sample of 12 typically developing children (Control Group) was recruited from the Atlanta community. Typically developing children were recruited from the non-alcohol exposed siblings of the children who were receiving services at the clinic and from community recruitments by targeting community programs and health fairs. Subjects were included in the Control group if there was a reliable history of minimal or no exposure in pregnancy and the parent had no clinical significant emotional or behavioral concerns about the child. Minimal PAE exposure was defined as less than one drink per week and never more than 2 drinks per occasion). None of the children assigned to the group was identified by their parents as having significant learning or developmental problems but one was identified as having a behavioral problem. The parent was concerned about the child’s hyperactivity but the concern had not reached the level of seeking professional consultation regarding the behavior.
Finally, an additional comparison sample of 14 children with identified developmental, learning or emotional/behavioral problems (Clinical Contrast Group) was recruited from siblings of those seen in the clinic, from community recruitments as described for the Control Group, and from the waiting room of a child psychiatry clinic. Clinically significant levels of concern regarding behavioral and emotional functioning were defined as seeking assistance from a primary doctor or mental health professional regarding a developmental, learning, or emotional/behavioral problem or having a specific neurodevelopmental or mental health diagnosis. Subjects were included if there was a reliable history of minimal or no PAE in pregnancy. Of the 14 enrolled, 13 were identified by their parents as having a significant behavioral problem and 4 were identified as having a significant learning or developmental problem. The Clinical Contrast group provides an important contrast to the PAE group in that this group has similar neurobehavioral dysfunction but with heterogeneous etiologies.
2.1 FNIRS Procedures
To collect hemodynamic changes in the brain, FNIR Devices’ wearable neuroimaging device (distributed by Biopac as Model# FNIRS 100B) was placed on the forehead of the participant and Cognitive Optical Brain Imaging (COBI) Studio (Ayaz et al., 2011) software was used to encode changes in light absorption during the computer game play. The FNIRS device enables continuous and non-invasive monitoring of blood oxygenation and blood volume changes associated with human brain functioning. The system consisted of a pad containing the LEDs and sensors that were placed on the participant’s forehead, a control box and power supply for acquisition of the infrared-light signal, and a laptop for the digital data acquisition and analysis software. The hardware box was connected to a computer via a standard universal serial bus (USB) cable for data acquisition and controlled using COBI Studio. The flexible sensor pad consisting of 4 light emitting diodes (LEDs) sources and 10 detectors, dividing the forehead into 16 optodes. Light sources were controlled by the software and wavelengths of 730 nm and 850 nm were used to estimate simultaneously HBO and HBR levels and were presented with a time sequence of 50 msec. The system recorded the two wavelengths and dark current for each of the optodes, totaling 48 measurements for each sampling period, and was collected at a rate of 2Hz. The center of the pad was placed at the nasion point of the participant and secured to the head using Velcro wraps. FNIRS does not allow for the absolute level of HBO and HBR at any one time point but instead relies on a comparison to baseline level of HBO and HBR to obtain an estimate of the changes in concentration. A 10 second baseline period collected prior to initiating the computer game was used as a comparison to obtain estimates of the relative levels of change in HBO and HBR as a function of task performance.
The professional version of FNIRSoft@ from Drexel University (Ayaz et al., 2010) was used to process, analyze, and visualize the fNIRS signals. The software used MBLL to estimate the concentration of the chromophores (light absorbing molecules (HBO and HBR) from the intensity of the light detected by the sensors. Raw fNIRS and event marker data were uploaded into the software and then underwent preprocessing to remove artifacts in the data. A linear phase, low pass filter was applied to attenuate high frequency components of the signal followed by a median filtering procedure. Next, to control for motion artifacts a Sliding-window Motion Artifact Rejection (SMAR) algorithm was applied (Ayaz et al., 2010, Ayaz et al., 2011). Oxygenation levels were then computed from the cleaned data using MBLL, resulting in 16 optode estimates of HBO and HBR. A detrending procedure was then applied to the data to eliminate measurement errors associated with prolonged sampling procedure used in the study.
2.2 FNIRS Task
The Frustration Emotion Task for Children (FETCH) is a computer-administered task that assesses individual differences in tolerance to frustrating events. Using fNIRS, children were found to have increased activity in the middle PFC in response to successful performance on the task and lateral PFC activity in response to frustration trials (Perlman et al., 2014). The game requires the child to compete with a dog to retrieve a bone. Three blocks are Win blocks (1, 3, & 5), defined by the child being able to be successful on 5 of the 6 trials, and three are Loss blocks (2, 4, & 6), defined as the child being unable to be successful on 5 of 6 trials. Each of the first 6 trials lasts approximately 10,000 ms and has a delay, catch, feedback, and rest component. The delay and rest phases combined last approximately 6000 ms. The catch phase and individual trial feedback each last 2000 ms. The total task performance time analyzed was 60,000 ms. Data were analyzed by aggregating across three blocks for each of the conditions (Win or Loss) and were then exported from the FNIRSoft software for further data analysis. The HBO and HBR levels during the entire sampling period were analyzed to assess changes in neural activation as opposed to phasic changes associated with making a given response.
After completing the respective trials for each block, participants were given feedback regarding their overall block performance and then asked to rate their emotional state by pointing to a series of faces with different expressions. Their responses were coded using a likert scale ranging from −3 to +3 with 0 reflecting a neutral face, −3 reflecting a very unhappy face, and +3 a very happy face. The stimuli were those used previously (Perlman et al., 2014) but the scaling was altered from the previous 1–7 to more accurately reflect the valence of the emotion.
During game play, increased neural activation was anticipated for all of the children. Increased neural activation is indicated by increased levels of HBO as either increased concentrations of oxygenated blood relative to HBR are carried to the area activated and/or cerebral blood flow level increases. HBR levels were anticipated to decrease relative to baseline levels, resulting in negative values, as a functioning of relative increases in HBO as compared to HBR.
2.3 Analytic Plan
SPSS 24.0 was used for statistical analysis. Descriptive statistics and frequency distributions to describe the characteristics of the individual and their families as well as the outcome measures were computed. To examine group differences in PFC activity, a mixed model analysis of variance was used to compare group differences in indices of PFC activity across the task conditions, Win vs. Loss. Two separate analyses were conducted, which included separate analyses for HBO and HBR. Condition (Win or Loss) was the between subjects factor and time was the repeated measure for each of the analyses with 120 samples. To minimize the number of comparisons carried out in the study, data were aggregated across optodes. The 4 optodes placed on both the right and left side were aggregated to obtain an estimate of lateral PFC activity. Data obtained from the 8 optodes in the center were aggregated to estimate medial PFC activity.
Missing fNIRS data can result from large head motions and sensor saturation or noise from the sensor pad pulling away from the skull. To deal with the missing data, estimation was obtained using restricted maximum likelihood (REML), which imputes missing values and then generates parameter estimates and repeats this procedure using the estimates until there are minimal differences between iterations (McKnight, 2007). REML has advantages over the maximum likelihood (ML) in that the REML method adjusts the degrees of freedom used for estimating fixed effects when estimating variance components (Evans et al., 2001) and has been used previously in fNIRS studies (Perlman et al., 2014, Tak et al., 2014).
Post hoc comparisons were then carried out to contrast significant group effects using least square means and adjustments for multiple comparisons were made using a false discovery rate correction (Benjamini et al., 1995). Both the actual p-value and the adjusted p-value or q* are reported for the comparisons. We hypothesized that children in the PAE group and in the Clinical Contrast group would differ from Controls as both groups have a pattern of behavioral or developmental dysfunction. Contrasts between the PAE and the Clinical Contrast group were also carried out to differentiate features of PFC activity that could be used to identify specific alcohol-related neurodevelopmental impairment of PFC activity relative to other disruptions to PFC activity that result in developmental and behavioral disruption.
3. Results
3.1 Group Characteristics
Demographic characteristics of the sample are outlined by group status in Table 1. The groups did not differ in age, gender, racial identity, ethnicity, or child protective service involvement. Children’s current placement or caregiver varied as a function of group status (X (4) = 11.020, p < .026). All of the children in the Control group were living with a biological parent while only 9 (50%) of the children with PAE were with a biological parent. The other children with a history of PAE were living with a relative (n=6, 33.3%) or either a legal guardian or adoptive parent (n=3, 16.7%). For the Clinical Contrast Group, 12 (85.7%) of the children were living with a biological parent, 1 (7.1%) was in kinship care, and 1 (7.1%) was living with a legal guardian or adoptive parent. Overall level of intellectual functioning as estimated using the Differential Ability Scales, 2nd edition’s General Conceptual Ability score (Elliot, 2007) also varied as a function of group status (F (2, 41) = 4.94, p < .012) with the Clinical Contrast group (x=78.3 (std = 12.7)) performing significantly lower than did the Controls (x = 92.8 (std = 9.1)) and those in the PAE group (x=87.8 (std = 13.2)).
Table 1.
Sample Characteristics by Group Status
| Measure | Controls n=12 |
Prenatal Alcohol-Exposed n=18 |
Clinical Contrast n=14 |
Statistic and p-Value |
|---|---|---|---|---|
| Child’s Age [M (SD)] | 11.4 (3.1) | 10.9 (2.6) | 10.7 (2.8) | ns |
| Child’s Gender [n (% male)] | 6 (50) | 10 (55.6) | 7 (50.0) | ns |
| Race [n (% African American or Mixed Race with African American)] | 12 (100) | 15 (83.3) | 14 (100) | ns |
| Ethnicity [n (% Non-Hispanic)] | 12 (100) | 15 (83.3) | 13 (92.9) | ns |
| Parental Behavioral Concern [n (% yes)] | 1 (8.3) | 12 (66.7) | 13 (92.9) | X (2) = 19.820, p < .000 |
| Developmental or Learning Problem [n (% yes)] | 0 (0) | 7 (38.9) | 4 (28.6) | X (2) = 5.947, p < .051 |
| Child Protective Service Involvement [n (% yes)] | 1 (8.3) | 5 (27.8) | 2 (14.3) | ns |
| Placement | X (4) = 11.020, p < .026 | |||
| Biological Parent [n (% yes)] | 12 (100.0) | 9 (50.0) | 12 (85.7) | |
| Kinship Care [n (% yes)] | 0 (0.0) | 6 (33.3) | 1 (7.1) | |
| Legal Guardian/Adoptive Parent [n (% yes)] | 0 (0.0) | 3 (16.7) | 1 (7.1) | |
| DAS GCA1 | 92.8 (9.1) | 87.8 (13.2) | 78.3 (12.7) | F (2, 41) = 4.94, p < .012 |
DAS GCA refers to the Differential Ability Scale’s General Conceptual Ability (Elliot, 2007) score, which is measure of overall intelligence with a Mean of 100 and standard deviation of 15.
3.2 Missing PFC Activity Data
Incomplete data for HBO occurred across the entire sampling period was 9.1% for the Medial area, 6.3% for the Lateral Left side, and 3.9% for the Lateral Right side. For HBR, incomplete data occurred in 3.6% for the Medial area, 8.7% for the Lateral Left side, and 5.9 % for the Lateral Right side.
3.3 FETCH Task Validity
Ratings of emotional reactions differed by condition with Win blocks associated with significantly higher (t (1,43) = 9.35, p < .000) positive emotional ratings (x = 2.11 (1.17)) as compared to the Loss Blocks (x = −0.65 (1.77). For each of the comparison groups this was true as well (Controls: t (1, 11) = 4.407, p < .001; PAE : t (1, 17) = 6.75, p < .000; Clinical Contrast: t (1,13) = 4.641, p < .000) and the magnitude of the difference between the conditions did not differ by group status. There was, however, a significant relationship between the child’s age and their ratings in the Win condition (r = −0.38, p < .011) and the Loss condition (r = 0.43, p < .004) as well as the discrepancy in ratings between the two conditions (r = −0.61, p < .000).
3.4 Child’s Age and PFC Activation
The relationship between participant age and levels of HBO and HBR during performance on the Loss and Win conditions were examined but only one significant relationship was obtained. The child’s age was negatively related to activation, or levels of HBO, in the Right Lateral PFC during the Loss blocks (r = −0.421, p < .004).
3.5 Group Differences in Changes in Oxygenation Levels During Game Play
In comparing baseline relative to game play, all groups across both Win and Loss conditions demonstrated an increase in HBO, reflecting an increase in neural activation. The exception was the performance of controls in the area of the Lateral Right PFC. Although there was a minimal increase in HBO in this area during the Win condition for this group, during the Loss condition the level of HBO was reduced, reflecting an inhibition of this area relative to the baseline period. All groups demonstrated a reduction in HBR levels as a function of task perfomance relative to baseline levels, indicating an increased utilization of oxygen and increased levels of neural activation during task performance relative to baseline levels. Figures 1 (HBO) and 2 (HBR) display the overall means across the Win and Loss conditions by group status and scatter plots of the dispersion of HBO and HBR levels are presented by group, condition, and PFC area in Supplemental Figures 1 and 2. The following describes the relative group and condition differences obtained in the study.
Figure 1.
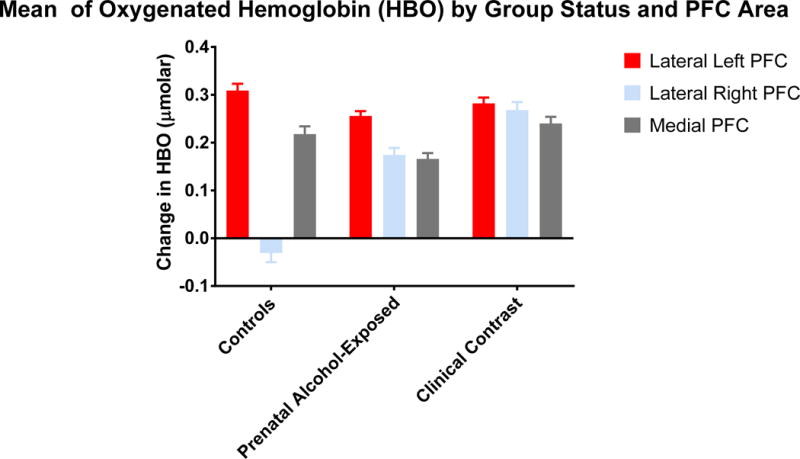
This figure depicts the levels of oxygenated hemoglobin (μmolar) as a function of group status and prefrontal corical area.
3.5.1 HBO During Game Play
During game play, a significant Condition effect was obtained for each of the aggregated measures (Lateral Left: F (1, 8433.739) = 106.635, p < .000; Lateral Right: F (1, 9140.677) = 49.336, p < .000; and Medial: F (1, 9056.906) = 217.143, p < .000) with each area associated with a reduction in HBO levels in the Loss relative to the Win condition.
Relative to group differences, a significant group effect was found in the Lateral Left (F (2, 8352.206) = 5.086, p < .006), Lateral Right (F (2, 9114.632) = 75.292, p < .000) and Medial (F (2, 9107.268) = 8.699, p < .000) areas but the group differences were not uniform. Table 2 displays the means by group status. For the Lateral Left, the Control group demonstrated greater levels of HBO than did the PAE group (t = 3.089, p < .0020, q* < .006) but was not significantly different from the Clinical Contrast group who also did not differ from the PAE group. For the Lateral Right mean, the Controls had the least amount of HBO throughout the measurement period, followed by the PAE group, and then the Clinical Contrast group with each group significantly differing from each other (Controls vs. PAE: t = 8.477, p < .0000, q* < .0000; Controls vs. Clinical Contrast: t = 11.729, p < .0000, q* < .0000; PAE vs. Clinical Contrast: t = 4.148, p < .0000, q* < .0000). For the Medial mean, the PAE group displayed less HBO relative to both other groups who did not differ from one another (PAE vs. Controls: t = 2.601, p < .0093, q* < .01395; PAE vs. Clinical Contrast: t = 4.013, p < .0001, q* < .0003).
Table 2.
Mean and Standard Error of Change in Oxygenated Hemoglobin (HBO) by Group Status (μmolar)
| Controls | Prenatal Alcohol-Exposed | Clinical Contrast | Statistical effect | |
|---|---|---|---|---|
| Lateral Left | .309 (.014) | .256 (.010) | .282 (.012) | F (2, 8352.206) = 5.086, p < .006 PAE < Controls; Clinical Contrast= Controls, PAE |
| Lateral Right | −.031 (.019) | .174 (.015) | .268 (.017) | F (2, 9114.632) = 75.292, p < .000 Controls < PAE < Clinical Contrast |
| Medial Mean | .218 (.016) | .166 (.012) | .240 (.014) | F (2, 9107.268) = 8.699, p < .000 PAE < Controls, Clinical Contrast |
In addition to the main effects for group, significant interaction effects were obtained on the Lateral Left (F (2, 8352.307) = 65.799, p < .000) and the Medial (F (2, 9107.666) = 20.604, p < .000) areas. Figure 3 displays the mean levels of HBO as a function of group status and condition for each of the aggregated measures. In the Win condition, HBO levels in the Lateral Left of the Clinical Contrast group were higher than both other groups (Clincal Contrat vs. PAE: t = 6.643, p < .0001, q* < .00015; Clinical Contrast vs. Controls: t = 7.394, p < .0001, q* = .00015) who did not differ from each other. In the Medial PFC during the Win condition, the PAE group had significantly lower HBO than than did both other groups (Controls vs PAE: t = 3.641, p < .0003, q* < .00054; PAE vs Clinical Contrast: t = 7.500, p < .0001, q* = .00023) who also differed from each other (Controls < Clinical Contrast: t = 3.287, p < .001, q* = .0015). In the Loss condition, the Medial PFC levels did not differ by group status but the PAE demonstrated lower levels of HBO in the Lateral Left relative to the Controls (t = 5.646, p < .0001, q* < .00015) but greater levels relative to the Clinical Contrast group (t = 3.700, p < .0002, q* = .00026) who also differed from Controls (t=8.6609, p < .0001, q* < .00015).
Figure 3.
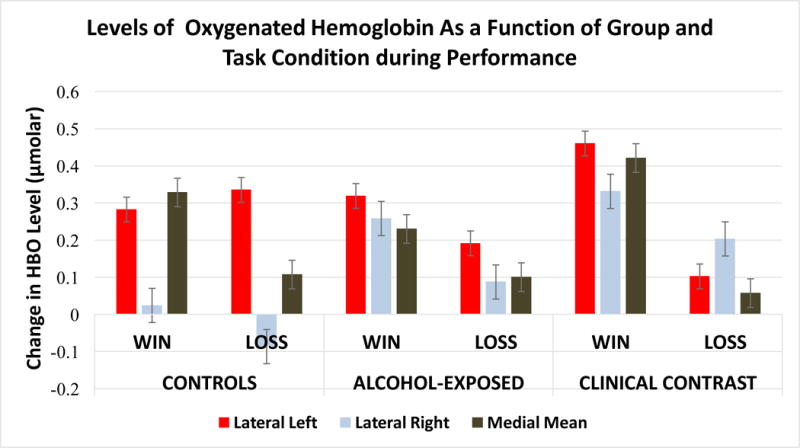
This figure depicts the levels of oxygenated hemoglobin (μmolar) as a function of task condition during performance by group status and prefrontal cortical area.
Contrasts of within group differences relative to their levels of HBO between the two conditions were also done. For the Lateral Left, the Controls demonstrated increased HBO in the Loss condition (t = 1.970, p < .0489, q* < .0550) but both the PAE (t = 6.022, p < .0001, q* < .00015) and Clinical Contrast group (t = 14.864, p < .0001, q* < .00015) demonstrated a reduction in HBO in this condition with the Clinical Contrast group having a greater reduction in HBO than did the PAE group. Finally, in the Medial area, each of the groups demonstrated a reduction in HBO in the Loss relative to the Win condition but the magnitude of the change was greatest among the Clinical Contrast group (t = 13.181, p < .0001, q* < .00023), followed by the Controls (t = 7.081, p < .0001, q* < .00023), and then the PAE group (t = 5.248, p < .0001, q* < .00023).
3.5.2 HBR During Game Play
Condition effects were found for each of the indices of PFC activation (Lateral Left: (F (1, 8495.145) = 90.775, p < .000; Lateral Right: (F (1, 8905.423) = 125.019, p < .000; and Medial (F (1, 8741.428) = 105.563, p < .000) with greater levels of HBR present in Loss relative to the Win condition for each measure.
Significant group and group by condition effects were also found for each sampled area of the PFC (Lateral Left: Group-F (2, 8009.136) = 17.465, p < .000; Interaction-F (2, 8009.217) = 44.215, p < .000; Lateral Right: Group-F (2, 8865.029) = 13.736, p < .000; Interaction-F (2, 8864.881) = 35.864, p < .000; and Medial: Group- F (2, 8516.959) = 8.003, p < .000; Interaction-F (2, 8517.283) = 49.737, p < .000). Relative to the main effects of group, for all of the areas of PFC, the PAE group demonstrated greater levels of HBR than did the Controls (Lateral Left t = 3.690, p < .0002, q* < .0003; Lateral Right t = 4.434, p < .0001, q* < .00015; Medial t = 3.796, p < .0001, q* < .0003) and the Clinical Contrast group (Lateral Left t= 5.629, p < .0001, q* < .0003; Lateral Right t = 4.3352, p < .0001, q* < .00015; Medial t = 2.704, p < .0069, q* < .01035). Table 3 displays the mean level of HBR for each of the three groups. When examining group means within a condition, the main effect of greater levels of HBR in the PAE group was true in all of the PFC areas for the Win condition (Lateral Left: PAE vs. Controls t = 6.130, p < .0001, q* < .00016; PAE vs. Clinical Contrast t = 11.335, p < .0001, q* < .00016; Lateral Right: PAE vs. Controls t = 2.065, p < .0390, q* < .0501; PAE vs. Clinical Contrast t = 8.316, p < .0001, q* < .00015; Medial: PAE vs. Controls t = 6.225, p < .0001, q* < .00013; PAE vs. Clinical Contrast t = 9.936, p < .0001, q* < .00013) and the HBR levels of Controls were higher than the Clinical Contrast group in all of the PFC areas in the Win condition (Lateral Left t = 4.281, p < .0001, q* < .00016; Lateral Right t = 5.462, p < .0001, q* < .00015; Medial t = 3.039, p < .0024, q* < .0027). In the Loss condition, the PAE group continued to have higher levels of HBR than did Controls in the Lateral Right area of the PFC (t = 4.045, p < .0001, q* .00015) but did not differ in the other areas in comparison to this group. Relative to the Clinical Contrast group, the PAE group had lower levels of HBR in the Lateral Left and Medial areas of the PFC (Lateral Left t = 2.426, p < .0153, q* < .0204; Medial t = 4.673, p < .0001, q* < .00013) as did the Controls in the Lateral Right (t = 5.342, p < .0001, q* < .00015) and Medial (t = 3.988, p < .0001, q* < .00013) areas of the PFC.
Table 3.
Mean and Standard Error of Change in Deoxygenated Hemoglobin (HBR) by Group Status (μmolar)
| Controls | Prenatal Alcohol-Exposed | Clinical Contrast | Statistical effect | |
|---|---|---|---|---|
| Lateral Left | −.701 (.028) | −.572 (.021) | −.760 (.026) | Group: F (2, 8009.136) = 17.465, p < .000 PAE > Clinical Contrast, Controls |
| Lateral Right | −.792 (.032) | −.612 (.025) | −.778 (.029) | Group: F (2, 8865.029) = 13.736, p < .000 PAE > Clinical Contrast, Controls |
| Medial Mean | −.673 (.028) | −.538 (.022) | −.628 (.025) | Group: F (2, 8516.959) = 8.003, p < .000 PAE > Clinical Contrast, Controls |
Contrast in HBR levels between conditions for each group were also examined. In the Lateral Left and Medial areas, both the Controls (Lateral Left t =5.136, p < .0001, q* < .00016; Medial t = 4.922, p < .0001, q* < .00016) and the Clinical Contrast (Lateral Left t =11.617, p < .00018, q* < .00016; Medial t = 12.995, p < .0001, q* < .00013) group showed greater levels of HBR in the Loss condition relative to the Win condition but the PAE group did not show a significant difference in HBR relative to condition. Interaction effects in the Lateral Right indicated that all of the groups had greater levels of HBR in the Loss relative to the Win condition but the magnitude of the difference was greatest in the Clinical Contrast group (t = 13.336, p < .0001; q* < .00015) followed by the PAE group (t = 4.833, p < .0001; q* < .00015) and then the Controls who only demonstrated a marginal effect of condition (t= 1.715, p < .0816, q* < .0908). Figure 4 displays the means as a function of group status and condition for each of the areas.
Figure 4.
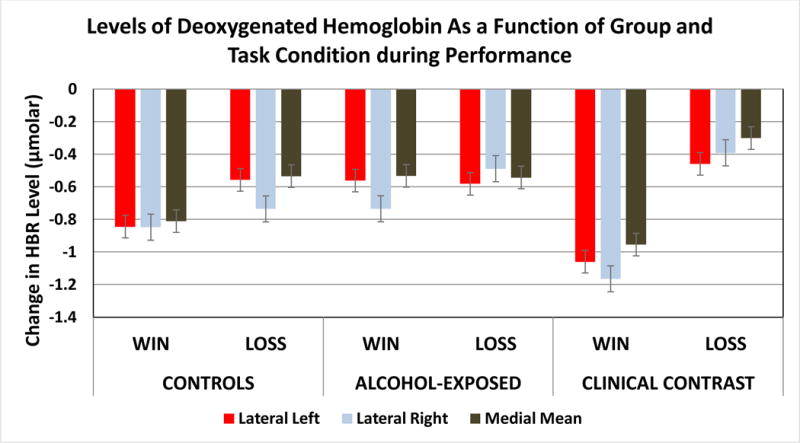
This figure depicts the levels of deoxygenated hemoglobin (μmolar) as a function of task condition during performance by group status and prefrontal cortical area.
4. Discussion
PFC activity as assessed by fNIRS was used to compare participants with a history of PAE to typically developing children and those in a clinical contrast group with other neurobehavioral problems. A computerized game designed to elicit activity in the PFC assessed the differential effects of winning and losing in a competitive situation. Ratings of emotional responses to the game play suggested that winning was associated with positive emotions and losing was associated with negative emotions with participant age attenuating the magnitude of the ratings. When examining the game effects across all of the participants enrolled in the study, losing blocks were, on average, associated with lower levels of HBO and higher levels of HBR relative to the winning blocks in both the task performance. These findings suggest that on average, when dealing with negative emotions, there was less PFC neural activation in contrast to conditions associated with positive emotions. Although there were significant age effects on the emotional ratings associated with the task conditions, maturational effects on levels of HBO and HBR were only present on the Loss condition in the Right Lateral area, which indexes activity of the right dorsal lateral PFC (R-DLPFC). A decrease in HBO level in the Loss condition was associated with older participants, suggesting that less neural activity in the R-DLPFC was needed to cope with negative emotions as individuals matured.
For the R-DLPFC, only a main effect of group status was found with typically developing children showing the least amount of HBO followed by the PAE group and then the Clinical Contrast group, suggesting the emotional valence of the condition was irrelevant to group differences in activation of the R-DLPFC during game play. This was the only PFC area where condition of the task did not impact group differences. Relative to group differences in the Win condition, children with PAE had less activation, as indicated by lower levels of HBO in the medial areas of the PFC and higher levels of HBR in all areas of the PFC relative to both contrast groups. Medial areas of the PFC have been linked to important functional skills, including reward processing (May et al., 2004) and self-monitoring (Devinsky et al., 1995, Davidson et al., 2007), and are known to be disrupted by PAE (Zimmerberg et al., 1988, Nguyen et al., 2014, Graham et al., 2016, Rai et al., 2017). In the Loss condition, the clinical contrast group demonstrated lower levels of medial PFC HBO as compared to the PAE group and typically developing controls who did not differ from each other, suggesting that neural activation in the context of negative emotions was better at discriminating the clinical contrast group. In the L-DLPFC, the PAE group had lower levels of HBO during the winning condition when compared to the clinical contrast group but did not differ from controls. While losing, activation of the L-DLPFC was intermediate for the PAE group who had lower levels of HBO than the typically developing children but higher levels than that of the clinical contrast group. These findings suggest that oxygenation levels of children with PAE may be better differentiated from both typically developing children and other children with clinical problems using tasks that elicit positive emotions.
Relative to changes in level of response in the Win and Loss conditions within each group, the PAE group had no significant differences in HBR levels as a function of the condition (Win vs. Loss) in either the L-DLPFC or the medial area during game play but both other groups demonstrated increased HBR levels in response to the Loss condition. These findings suggest that the valence of the emotional experience during game play did not trigger different levels of PFC activity in these areas in children with PAE in contrast to the reaction of the other groups of children. The only area that demonstrated a condition effect on HBR levels in children with PAE was the R-DLPFC with the magnitude of the difference falling between those of the other two groups. The diminished differences in PFC activity associated with losing relative to winning in the PAE children may be related to perceived effectiveness in playing the game. Although there were no group differences in emotional ratings, these were not made until after the children received feedback regarding their performance for an entire block of six trials. As problems with both number perception (Jacobson et al., 2011) and self-monitoring (Nguyen et al., 2014) are associated with PAE, it is possible that children with PAE were not monitoring their Win and Loss experiences or overall success rate within the context of the game as closely as were the other children. This lack of monitoring may have diminished differential oxygen utilization associated with conditions. Magnitude estimation, which is a skill needed for estimating on-going performance levels during game play, was associated with a reduction in medial PFC activation in children with PAE relative to typically developing children during a proximity judgement task (Meintjes et al., 2010).
The most distinguishing feature of the responses of the typically developing children was their pattern of differential responsiveness relative to specific areas of the PFC. When contrasting the change in level of response in the Win and Loss conditions, increased activation as indicated by increased levels of HBO in the L-DLPFC in response to losing during game performance was found in typically developing children relative to both other groups who demonstrated reductions in HBO levels when contrasting the Win and Loss conditions. L-DLPFC is an area of the brain linked to dealing with negative emotions (Perlman et al., 2014), planning (Ruocco et al., 2014) and inhibitory control (Rodrigo et al., 2016). Typically developing controls also seemed to rely less on activation of the R-DLPFC to cope with the emotional arousal associated with the task regardless of the valence of arousal. The age effect found in the R-DLPFC, which was a decrease in HBO level in the Loss condition being associated with increased age, is suggestive that the hemispheric specialization found in the typically developing children may be a positive developmental maturational process but it will be important to explore this by relating this pattern of response to neurobehavioral outcomes and replicating the finding in another sample.
Although differentiating children with PAE from typically developing children often is not a difficult task, it is also important to differentiate patterns of responses of these children from other children with clinical problems not caused by PAE. Children in the Clinical Contrast group demonstrated the greatest activation, as indexed by increased HBO levels, of the R-DLPFC during both conditions of game play and had the greatest contrast in PFC activation between the Win and Loss conditions. Although children with PAE demonstrated a similar pattern to the children in the clinical contrast group, the magnitude of the decline in HBO in the Loss relative to the Win condition in game play was greater for the Clinical Contrast group, suggesting that children in the Clinical Contrast group were more responsive to the valence of the emotional arousal. This pattern of differential responsiveness to the valence of the emotional arousal was also seen in all areas of the PFC that showed increased levels of HBR in response to losing, which is the key distinguishing difference between those in the PAE and Clinical Contrast groups. This suggests that the Clinical Contrast group had a specific alteration in coping with negative emotions as compared to children with PAE who were better differentiated by the positive emotional condition.
Although the use of FNIRS provides an opportunity to explore functional brain activation in populations that could not be appropriately sampled using traditional neuroimaging techniques, the methodology has limitations in that it can only sample a limited window of brain activation. The infrared light is only capable of being absorbed and appropriately reflecting back within a range of about 2–3 mm into the cortex and therefore can only provide estimates of changes in blood oxygenation observed at the cortical surface level (Vanderwert et al., 2014). Subcortical regions that are involved in inhibitory control, problem-solving, reward processing and emotional regulation cannot be sampled using this methodology.
Another methodological limitation of fNIRS is that the MBLL formula that is used to estimate the changes in HBO and HBR from baseline to task performance assumes the extracranial blood flow remains constant. It is possible that differential changes in skin blood flow during game play may have contributed to the obtained group differences but these differences would have had to vary as a function of group status and be uncorrelated with the cerebral changes in HBO and HBR in order to threaten the internal validity of the study. Recent research has indicated that changes in skin blood flow were highly positively related to changes in HBO and HBR levels (r = 0.737) (Hirasawa et al., 2015). To obtain an estimate of changes in skin blood flow, one study formulated a correction factor used in the MBLL formula that was derived for each individual by monitoring simultaneously changes in cerebral oxygenation levels and changes in skin blood flow (Hirasawa et al., 2016) and then applied the individual correction factor to the analysis of task performance changes. Future fNIRS studies with children with FASDs may benefit from using such a protocol to rule out any such biases that differential levels of skin blood flow may have in group comparison studies.
The fNIRS assessment provided a method of quantifying individual differences in PFC activation in both children with a history of PAE and those with other clinical problems not related to PAE that resulted in group differences relative to typically developing children and from each other. With both of these populations replications and extensions of these findings are needed but the initial results suggests that changes in oxygenation levels as a function of coping with emotional arousal may serve as potential biomarker of alcohol-related neurodevelopmental damage. Of course, additional studies are needed where estimates of the sensitivity and specificity of the fNIR outcomes would need to be assessed relative to their ability to differentiate children with PAE from both typically developing children and other children with neurobehavioral disorders.
For individuals with a history of PAE, considerable effort has been devoted to understanding the neural bases of the learning and behavioral deficits seen in these children with considerable evidence supporting the adverse impact on the size, cytoarchitecture, function, and connectivity of the brain (Ashwell et al., 1996, Coles et al., 2011, Lebel et al., 2011, Moore et al., 2014, Wilhelm et al., 2015). The results of this study indicated that utilization of oxygen in the PFC to modulate arousal was altered as a function of PAE, which may indicate damage to neural tissue of the PFC or disruption to the connectivty of the neural tissue in this area resulting in disruption to oxygen utilization. The results, however, may also indicate another source of disruption to neural activation that is not as well understood, the neurovascular system that provides the oxygen supply to the brain tissue.
The results of this study suggest that there is a disruption to cerebral blood flow during the task in children with a history of PAE that is most salient when dealing with positive emotional arousal. Across all areas of the PFC in the Win condition, these children showed less neural activation as indicated by increased levels of HBR relative to children in the other two groups but the results are not consistent with the estimates of neural activation obtained from changes in levels of HBO. In the Win condition, the PAE group had lower levels of L-DLPFC HBO relative to the clinical contrast group, which is consistent with the higher levels of HBR but did not differ in HBO levels from the typically developing group of children despite showing differences in HBR levels. Although the childern in the PAE group demonstrated higher levels of HBR in the R-DLPFC and lower levels of HBO in this area relative to the clinical contrast group, the PAE group demonstrated an increased level of HBO relative to the typically developing group despite showing an increased level of HBR. These findings suggest that there was an increase of cerebral blood flow as indicated by an increase in HBO but not an associated decrease in HBR. This pattern of change in relative levels of HBO and HBR is suggestive of disruption to the perfusion of oxygen to the activated neural tissue between the groups (Villringer, 1995), resulting in changes in the ratio of HBO and HBR.
Increased prevalence of cardiac malformations and disruptions to cardiac function in the form of heart murmurs, which are mostly considered benign, are commonly found in children with FASDs (Jones et al., 2013). Yet, little is known about the neurovascular system that connects the heart and the brain and to what extent problems with this system may alter the perfusion of oxygen to the brain needed for neural activation in children with FASDs. In animal models, PAE has been found to alter reproductive vasculature of the mother’s placenta, including alterations to blood flow, the structural resistance of vessels, and angiogenesis (Ramadoss et al., 2012) and in offspring, to alter the cortical vascular density, disrupt spatial orientation of microvessels, and alter the receptor expression of vascular endothelial growth factors (Jegou et al., 2012). Using brains of deceased human fetuses, those with FASD had alterations to the radial organizaton of the vasculature and disorganization of microvessels, particularly among older fetuses (Jegou et al., 2012) and alterations in the overall amount of vessel area (Solonskii et al., 2008, Jegou et al., 2012). The results of this study suggest that the delivery of oxygen to neural tissue during activation may be altered as a function of a history of PAE.
The association between the fNIRS indices of PFC activation during the FETCH task and the negative behavioral outcomes often found in children with PAE has not yet been explored but will be important to assist with our understanding of the implications of the obtained group differences found in this study. Future work should evaluate the relationships between the various indices of HBO and HBR and parental ratings of behavioral functioning and the child’s performance on standardized measures of neurocognitive functioning. Previous research has indicated that fNIRS outcomes during the FETCH task were related to parental measures of frustration tolerance (Perlman et al., 2014). Evaluating the differential predictive validity of these outcomes in children with PAE relative to children with other neurobehavioral problems will be aid in our understanding of the specificity of the teratogenic effects of PAE.
5. Conclusion
Children with Fetal Alcohol Spectrum Disorders have multiple neurobehavioral symptoms of which deficits in executive functioning skills seem to underlie. Assessment of PFC activity using fNIRS resulted in group differences between children with PAE and typically developing children and a clinical contrast group, suggesting such assessments may be a potential useful biomarker for improving identification of alcohol-affected children. In dealing with positive emotional arousal, children with PAE had less activation in the medial PFC areas, higher levels of HBR in all areas of the PFC, and less specialization of areas of PFC activity in response to a task known to elicit emotional arousal. The relationship between changes in PFC activation associated with emotional arousal should be further explored in children with a history of PAE to provide insight into the mechanisms by which learning, problem-solving, and arousal regulation are disrupted in these children. Specifically, the relationship between PFC activation should be related to behavioral measures of EF and behavioral adjustment to determine if differential responsiveness in brain activation and oxygen depletion predicts the real-world behavioral deficits that are often reported in these children (Kable et al., 2016). In addition, changes in PFC activation may provide an important tool in evaluating intervention programs for these children that frequently target behavioral and cognitive inhibition (Kable et al., 2007, Kerns et al., 2010, Wells et al., 2012, Kable et al., 2014, Nash et al., 2015).
Supplementary Material
Supplemental Figure 1. This figure depicts the scatter plots of individual’s mean level of HBO across Lateral Left (top), Lateral Right (middle), and Medial PFC areas (bottom) by group status with Controls on the left, PAE in the middle, and Clinical Contrast on the right. Error bars indicate the 95% confidence level for each individual’s mean. Within each scatter plot, the same subject is indicated by a consistent color.
Supplemental Figure 2. This figure depicts the scatter plots of individual’s mean level of HBR across Lateral Left (top), Lateral Right (middle), and Medial PFC areas (bottom) by group status with Controls on the left, PAE in the middle, and Clinical Contrast on the right. Error bars indicate the 95% confidence level for each individual’s mean. Within each scatter plot, the same subject is indicated by a consistent color.
Figure 2.
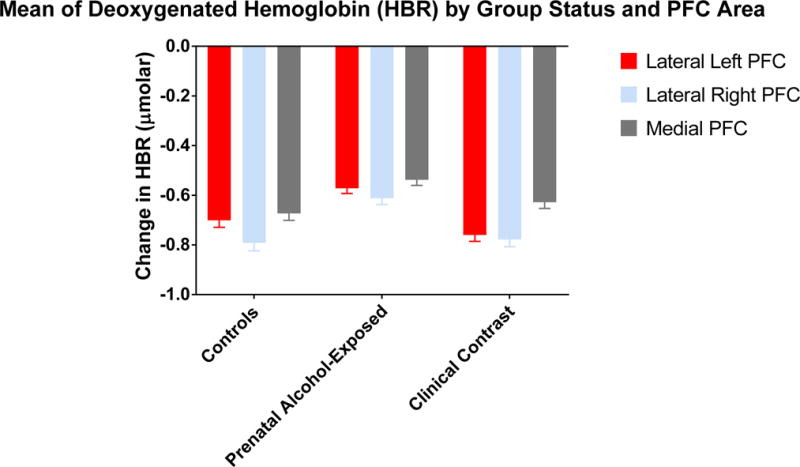
This figure depicts the levels of deoxygenated hemoglobin (μmolar) as a function of group status and prefrontal corical area.
Highlights.
Prefrontal cortex (PFC) activity, using fNIRS, discriminated children with prenatal alcohol exposure (PAE) from contrast groups.
Children with PAE had less activation in the medial PFC areas than did the contrast groups.
Children with PAE had greater oxygen depletion in all areas of PFC relative to the contrast groups.
Acknowledgments
The authors are deeply appreciative of the children and their families who gave of their time to participate in this research.
Funding
This research was funded by supported by a grant from the Spray Foundation and by a NIH Research Grant #U25AA014811 funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Amyot F, Zimmermann T, Riley J, Kainerstorfer JM, Chernomordik V, Mooshagian E, et al. Normative database of judgment of complexity task with functional near infrared spectroscopy–application for TBI. Neuroimage. 2012;60:879–83. doi: 10.1016/j.neuroimage.2012.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon AS, Coriale G, Fiorentino D, Kalberg WO, Buckley D, Gossage JP, et al. Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2008;32:1909–19. doi: 10.1111/j.1530-0277.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki A, Ikegami M, Okayama A, Matsumoto N, Takahashi S, Azuma H, et al. Improved prefrontal activity in AD/HD children treated with atomoxetine: a NIRS study. Brain Dev. 2015;37:76–87. doi: 10.1016/j.braindev.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Zhang LL. Forebrain hypoplasia following acute prenatal ethanol exposure: quantitative analysis of effects on specific forebrain nuclei. Pathology. 1996;28:161–6. doi: 10.1080/00313029600169803. [DOI] [PubMed] [Google Scholar]
- Ayaz H, Izzetoglu M, Shewokis PA, Onaral B. Sliding-window motion artifact rejection for functional nearinfrared spectroscopy. Conf Proc IEEE Eng Med Biol Soc. 2010:6567–70. doi: 10.1109/IEMBS.2010.5627113. [DOI] [PubMed] [Google Scholar]
- Ayaz H, Shewokis PA, Curtin A, Izzetoglu M, Izzetoglu K, Onaral B. Using MazeSuite and functional near infrared spectroscopy to study learning in spatial navigation. J Vis Exp. 2011;56:e3443. doi: 10.3791/3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Boas DA, Elwell CE, Ferrari M, Taga G. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage. 2014;85:1–5. doi: 10.1016/j.neuroimage.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, et al. Effects of prenatal alcohol exposure at school age: II Attention and behavior. Neurotoxicol Teratol. 1991;13:369–76. doi: 10.1016/0892-0362(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Burke MW, Palmour RM, Ervin FR, Ptito M. Neuronal reduction in frontal cortex of primates after prenatal alcohol exposure. Neuroreport. 2009;20:13–7. doi: 10.1097/WNR.0b013e32831b449c. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics. 2015;135:264–70. doi: 10.1542/peds.2014-2171. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, Telford E, Schmidt C, Messer G. Neurodevelopmental functioning in children with FAS, pFAS, and ARND. J Dev Behav Pediatr. 2010;31:192–201. doi: 10.1097/DBP.0b013e3181d5a4e2. [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13:357–67. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Coles CD, Gailey AR, Mulle JG, Kable JA, Lynch ME, Jones KL. A comparison among 5 methods for the clinical diagnosis of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2016;40:1000–9. doi: 10.1111/acer.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Li Z. Functional neuroimaging in the examination of effects of prenatal alcohol exposure. Neuropsychol Rev. 2011;21:119–32. doi: 10.1007/s11065-011-9165-y. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–61. [PubMed] [Google Scholar]
- Davidson RJ, Fox A, Kalin NH. Neural bases of emotion regulation in nonhuman primates and humans In: Gross JJ, editor Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 47–68. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Elliot CD. Differential Ability Scales. Second. San Antonio, TX: PsychCorp; 2007. [Google Scholar]
- Evans BA, Feng Z, Peterson AV. A comparison of generalized linear mixed model procedures with estimating equations for variance and covariance parameter estimation in longitudinal studies and group randomized trials. Stat Med. 2001;20:3353–73. doi: 10.1002/sim.991. [DOI] [PubMed] [Google Scholar]
- Fernhoff PM, Smith IE, Falek A. Document available through the Maternal Substance Abuse and Child Development Project. Division of Psychiatry, Emory University School of Medicine; Atlanta, GA: 1980. Dysmorphia Checklist. [Google Scholar]
- Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage. 2012;63:921–35. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontalstriatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–24. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Graham DM, Glass L, Mattson SN. The Influence of Extrinsic Reinforcement on Children with Heavy Prenatal Alcohol Exposure. Alcohol Clin Exp Res. 2016;40:348–58. doi: 10.1111/acer.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, et al. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) J Child Psychol Psychiatry. 2009;50:688–97. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Haeussinger FB, Hahn T, Ehlis AC, Plichta MM, Fallgatter AJ. Variability of (functional) hemodynamics as measured with simultaneous fNIRS and fMRI during intertemporal ch oice. Neuroimage. 2013;71:125–34. doi: 10.1016/j.neuroimage.2012.12.074. [DOI] [PubMed] [Google Scholar]
- Herman CS, Kirchner GL, Streissguth AP, Little RE. Vigilance paradigm for preschool children used to relate vigilance behavior to IQ and prenatal exposure to alcohol. Percept Mot Skills. 1980;50:863–7. doi: 10.2466/pms.1980.50.3.863. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Kaneko T, Tanaka N, Funane T, Kiguchi M, Sorensen H, et al. Near-infrared spectroscopy determined cerebral oxygenation with eliminated skin blood flow in young males. J Clin Monit Comput. 2016;30:243–50. doi: 10.1007/s10877-015-9709-4. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Yanagisawa S, Tanaka N, Funane T, Kiguchi M, Sorensen H, et al. Influence of skin blood flow and source-detector distance on near-infrared spectroscopy-determined cerebral oxygenation in humans. Clin Physiol Funct Imaging. 2015;35:237–44. doi: 10.1111/cpf.12156. [DOI] [PubMed] [Google Scholar]
- Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Kuwabara H, Matsubayashi J, et al. Prefrontal activation during inhibitory control measured by near-infrared spectroscopy for differentiating between autism spectrum disorders and attention deficit hyperactivity disorder in adults. NeuroImage Clinical. 2014;4:53–63. doi: 10.1016/j.nicl.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW. Number processing in adolescents with prenatal alcohol exposure and ADHD: differences in the neurobehavioral phenotype. Alcohol Clin Exp Res. 2011;35:431–42. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegou S, El Ghazi F, de Lendeu PK, Marret S, Laudenbach V, Uguen A, et al. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann Neurol. 2012;72:952–60. doi: 10.1002/ana.23699. [DOI] [PubMed] [Google Scholar]
- Jones KL, Jones MC, Del Campo M. Smith’s Recognizable Patterns of Human Malformation 7th Edition ed. Philadelphia, PA: Elsevier; 2013. [Google Scholar]
- Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. 2004;28:489–96. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Taddeo E. Socio-cognitive habilitation using the math interactive learning experience program for alcohol-affected children. Alcohol Clin Exp Res. 2007;31:1425–34. doi: 10.1111/j.1530-0277.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Taddeo E. Parent training to improve self regulation and adaptive living skills of children with FASD. Alcohol Clin Exp Res. 2014;38:258A. [Google Scholar]
- Kable JA, O’Connor MJ, Olson HC, Paley B, Mattson SN, Anderson SM, et al. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Proposed DSM-5 diagnosis. Child Psychiatry Hum Dev. 2016;47:335–46. doi: 10.1007/s10578-015-0566-7. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Macsween J, Vander Wekken S, Gruppuso V. Investigating the efficacy of an attention training programme in children with foetal alcohol spectrum disorder. Developmental neurorehabilitation. 2010;13:413–22. doi: 10.3109/17518423.2010.511421. [DOI] [PubMed] [Google Scholar]
- Kocsis L, Herman P, Eke A. The modified Beer-Lambert law revisited. Phys Med Biol. 2006;51:N91–8. doi: 10.1088/0031-9155/51/5/N02. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Adnams CM, Hay A, Kitching AE, Burger E, Kalberg WO, et al. Letter and category fluency in children with fetal alcohol syndrome from a community in South Africa. J Stud Alcohol. 2006;67:502–9. doi: 10.15288/jsa.2006.67.502. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in selfregulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19:1558–64. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kalberg W, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol Res Health. 2001;25:192–8. [PMC free article] [PubMed] [Google Scholar]
- Kopera-Frye K, Carmichael-Olson H, Streissguth AP. Teratogenic effects of alcohol on attention. In: Burack JA, Enns JT, editors. Attention, Development and Psychopathology. New York, NY: The Guildford Press; 1997. pp. 171–204. [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–18. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Kable JA, Coles CD. Prenatal alcohol exposure, adaptive function, and entry into adult roles in a prospective study of young adults. Neurotoxicol Teratol. 2015;51:52–60. doi: 10.1016/j.ntt.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58:1150–7. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Matsuura N, Ishitobi M, Arai S, Kawamura K, Asano M, Inohara K, et al. Effects of methylphenidate in children with attention deficit hyperactivity disorder: a near-infrared spectroscopy study with CANTAB(R) Child and adolescent psychiatry and mental health. 2014;8:273. doi: 10.1186/s13034-014-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism, clinical and experimental research. 1999;23:1808–15. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–94. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–21. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, et al. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34:1640–50. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, et al. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:517–28. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–66. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–66. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Riley EP. Brain imaging and fetal alcohol spectrum disorders. Ann Ist Super Sanita. 2006;42:46–52. [PubMed] [Google Scholar]
- McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN. Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcohol Clin Exp Res. 2008;32:1388–97. doi: 10.1111/j.1530-0277.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight PE. Missing data : a gentle introduction. New York: Guilford Press; 2007. [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, et al. An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2010;34:1450–64. doi: 10.1111/j.1530-0277.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Monden Y, Dan H, Nagashima M, Dan I, Kyutoku Y, Okamoto M, et al. Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Clin Neurophysiol. 2012;123:1147–57. doi: 10.1016/j.clinph.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Moore EM, Migliorini R, Infante MA, Riley EP. Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings. Curr Dev Disord Rep. 2014;1:161–72. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash K, Stevens S, Greenbaum R, Weiner J, Koren G, Rovet J. Improving executive functioning in children with fetal alcohol spectrum disorders. Child Neuropsychol. 2015;21:191–209. doi: 10.1080/09297049.2014.889110. [DOI] [PubMed] [Google Scholar]
- Nash K, Williamson M, Sheard ED, Desrocher M, Noseworthy M, Blaser S, et al. Oxidative stress in the metabolic functioning of children with FASD; Poster presented at the annual meeting of the Imaging Network Ontario; Toronto, Ontario. 2006. [Google Scholar]
- Nguyen TT, Glass L, Coles CD, Kable JA, May PA, Kalberg WO, et al. The clinical utility and specificity of parent report of executive function among children with prenatal alcohol exposure. J Int Neuropsychol Soc. 2014;20:704–16. doi: 10.1017/S1355617714000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccols A. Fetal alcohol syndrome and the developing socio-emotional brain. Brain Cogn. 2007;65:135–42. doi: 10.1016/j.bandc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ. Prenatal alcohol exposure and infant negative affect as precursors of depressive features in children. Inf Mental Hlth J. 2001;22:291–9. [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15:225–34. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O’Connor MJ, et al. Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Hum Brain Mapp. 2009;30:3200–8. doi: 10.1002/hbm.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Near-infrared spectroscopy in functional activation studies: Can NIRS demonstrate cortical activation? In: Villringer A, Dirnagl U, editors. International symposium on optical imaging and metabolism. Berlin, Germany: Plenum Press; 1995. pp. 113–28. [DOI] [PubMed] [Google Scholar]
- Oesterheld JR, Wilson A. ADHD and FAS. J Am Acad Child Adolesc Psychiatry. 1997;36:1163. doi: 10.1097/00004583-199709000-00004. [DOI] [PubMed] [Google Scholar]
- Oner O, Akin A, Herken H, Erdal ME, Ciftci K, Ay ME, et al. Association among SNAP-25 gene DdeI and MnlI polymorphisms and hemodynamic changes during methylphenidate use: A functional near-infrared spectroscopy study. J Atten Disord. 2011;15:628–37. doi: 10.1177/1087054710374597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Luna B, Hein TC, Huppert TJ. fNIRS evidence of prefrontal regulation of frustration in early childhood. Neuroimage. 2014;85:326–34. doi: 10.1016/j.neuroimage.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai JK, Abecassis M, Casey JE, Flaro L, Erdodi LA, Roth RM. Parent rating of executive function in fetal alcohol spectrum disorder: A review of the literature and new data on Aboriginal Canadian children. Child Neuropsychol. 2017;23:713–732. doi: 10.1080/09297049.2016.1191628. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 2012;303:H414–21. doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29:1359–67. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Soleimani M, Pei J. Executive functioning and working memory deficits on the CANTAB among children with prenatal alcohol exposure. J Popul Ther Clin Pharmacol. 2011;18:e44–53. [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo AH, Di Domenico SI, Graves B, Lam J, Ayaz H, Bagby RM, et al. Linking trait-based phenotypes to prefrontal cortex activation during inhibitory control. Soc Cogn Affect Neurosci. 2016;11:55–65. doi: 10.1093/scan/nsv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco AC, Rodrigo AH, Lam J, Di Domenico SI, Graves B, Ayaz H. A problem-solving task specialized for functional neuroimaging: validation of the Scarborough adaptation of the Tower of London (S-TOL) using near-infrared spectroscopy. Front Hum Neurosci. 2014;8:185. doi: 10.3389/fnhum.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Schaldecker M, Aucktor S, Brast J, Kirchgassner K, Muhlberger A, et al. Effects of methylphenidate on olfaction and frontal and temporal brain oxygenation in children with ADHD. J Psychiatr Res. 2011;45:1463–70. doi: 10.1016/j.jpsychires.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85:6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. 2006;12:439–52. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Behavioral regulation as a predictor of response to Children’s Friendship Training in children with fetal alcohol spectrum disorders. Clin Neuropsychol. 2009;23:428–45. doi: 10.1080/13854040802389177. [DOI] [PubMed] [Google Scholar]
- Solonskii AV, Logvinov SV, Kutepova NA. Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol. Neurosci Behav Physiol. 2008;38:373–6. doi: 10.1007/s11055-008-0053-8. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, et al. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport. 2007;18:635–9. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish-Johnson JC, Kirchner GL, Martin DC. Attention, distraction and reaction time at age 7 years and prenatal alcohol exposure. Neurobehav Toxicol Teratol. 1986;8:717–25. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. The enduring effects of prenatal alcohol exposure on child development: Birth through seven years, a Partial Least Squares Solution. Ann Arbor, MI: University of Michigan Press; 1993. [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, et al. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring–a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–18. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Tak S, Ye JC. Statistical analysis of fNIRS data: a comprehensive review. Neuroimage. 2014;85:72–91. doi: 10.1016/j.neuroimage.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–33. [PubMed] [Google Scholar]
- Vanderwert RE, Nelson CA. The use of near-infrared spectroscopy in the study of typical and atypical development. Neuroimage. 2014;85:264–71. doi: 10.1016/j.neuroimage.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2008;14:119–29. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer A. Functional neuroimaging: Optical approaches. In: Villringer A, Dirnagl U, editors. International symposium on optical imaging and metabolism. Berlin, Germany: Plenum Press; 1995. pp. 4–6. [Google Scholar]
- Wells AM, Chasnoff IJ, Schmidt CA, Telford E, Schwartz LD. Neurocognitive habilitation therapy for children with fetal alcohol spectrum disorders: an adaptation of the Alert Program(R) Am J Occup Ther. 2012;66:24–34. doi: 10.5014/ajot.2012.002691. [DOI] [PubMed] [Google Scholar]
- Wiley C, Riccio C. A review of functional near-infrared spectroscopy studies of Attention Deficit/Hyperactivity Disorder neurological activation patterns. Arch Clin Neuropsychol. 2014;29:542–3. [Google Scholar]
- Wilhelm CJ, Guizzetti M. Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective. Front Integr Neurosci. 2015;9:65. doi: 10.3389/fnint.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Hoecker HL, Boys CJ, et al. Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:748–56. doi: 10.1111/acer.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Lepage C, Rasmussen C, Evans A, Wyper K, et al. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 2011;58:16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Riley EP. Prenatal alcohol exposure alters behavioral laterality of adult offspring in rats. Alcohol Clin Exp Res. 1988;12:259–63. doi: 10.1111/j.1530-0277.1988.tb00191.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. This figure depicts the scatter plots of individual’s mean level of HBO across Lateral Left (top), Lateral Right (middle), and Medial PFC areas (bottom) by group status with Controls on the left, PAE in the middle, and Clinical Contrast on the right. Error bars indicate the 95% confidence level for each individual’s mean. Within each scatter plot, the same subject is indicated by a consistent color.
Supplemental Figure 2. This figure depicts the scatter plots of individual’s mean level of HBR across Lateral Left (top), Lateral Right (middle), and Medial PFC areas (bottom) by group status with Controls on the left, PAE in the middle, and Clinical Contrast on the right. Error bars indicate the 95% confidence level for each individual’s mean. Within each scatter plot, the same subject is indicated by a consistent color.


