Abstract
Objective
To compare efficacy, survival outcome and prognostic factors of conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE) and 90Yttrium-radioembolization (Y90) for the treatment of liver metastases from gastro-entero-pancreatic (GEP) neuroendocrine tumors (NELM).
Methods
This retrospective analysis included 192 patients (58.6years mean age, 56%men) with NELM treated with cTACE(N=122), DEB-TACE(N=26), or Y90(N=44) between 2000 and 2014. Radiologic response to therapy was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) and World Health Organization (WHO) using peri-procedural MR imaging. Survival analysis included Propensity score analysis (PSA), median overall survival (MOS), hepatic progression-free survival, Kaplan-Meier using log-rank test and the uni-and multivariate Cox proportional hazards model (MVA).
Results
MOS of the entire study population was 28.8months. As for cTACE, DEB-TACE and Y90, MOS was 33.8months, 21.7months and 23.6months, respectively. According to the MVA, cTACE demonstrated a significantly longer MOS as compared to DEB-TACE(p=.04) or Y90(p=.032). The five-year survival rate after initial cTACE, DEB-TACE and Y90 was 28.2%, 10.3% and 18.5%, respectively.
Conclusions
Upon PSA, our study suggests significant survival benefits for patients treated with cTACE as compared to DEB-TACE and Y90. This data supports the therapeutic decision for cTACE as the primary intra-arterial therapy option in patients with unresectable NELM until proven otherwise.
Keywords: neuroendocrine tumors, Chemoembolization, Drug-eluting Beads, DEB, 90Yttrium Radioembolization, Y90, Propensity Score
2. Introduction
Neuroendocrine tumors (NETs) comprise a heterogeneous group of relatively rare malignant tumor entities that usually appear as slow-growing neoplasms. NET originating from gastro-entero-pancreatic (GEP) tissue demonstrates a predisposition to liver metastases (NET-liver metastases [NELM]) associated with symptom-related severe impairment of the quality of life and reduced life expectancy.
In a range of 46–93% of cases, patients with NET present with NELM at initial diagnosis [1–3]. At this stage, curative treatment is only possible in 10–20% of cases [3]. Gastro-entero-pancreatic NELM can originate from pancreatic islet cells (pancreatic (p)NET), or from neuroendocrine cells (carcinoid) [4]. As secreted hormones are mainly inactivated by the liver, NELM often remain asymptomatic until the primary tumor has metastasized to the liver with impairment of liver function [5]. In this setting, based on the arterial hyper-vascularity and the preferentially arterial blood supply of NELM, palliative options include image-guided intra-arterial therapies (IAT) such as bland transarterial embolization (TAE), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), and 90Yttrium-radioembolization (Y90) [6], which improve the five-year survival rate up to 50%–80% [7, 8].
Multiple studies report evidence in support of the beneficial effects of TAE, cTACE, DEB-TACE or Y90 in patients with unresectable NELM. However, no comparative analysis of the efficacy of the three commonly used IAT options (cTACE vs. DEB-TACE vs. Y90) exists. Thus, no conclusive recommendation can be drawn for a favorable choice of IAT. To date, trials that compare the efficacy of IAT modalities have not revealed significant differences in overall survival (OS) [3].
The objective of our study was to compare efficacy, survival outcome and prognostic factors of cTACE, DEB-TACE and Y90 for the treatment of gastro-entero-pancreatic NELM. Specifically, this was done with regard to radiologic response (RR), hepatic progression-free survival (HPFS) and OS in order to identify favorable treatment options and prognostic factors in this setting.
3. Materials and Methods
a. Study cohort
This retrospective single-institution study was compliant with the Health Insurance Portability and Accountability Act (HIPAA) and approved by the Institutional Review Board. Informed consent was waived.
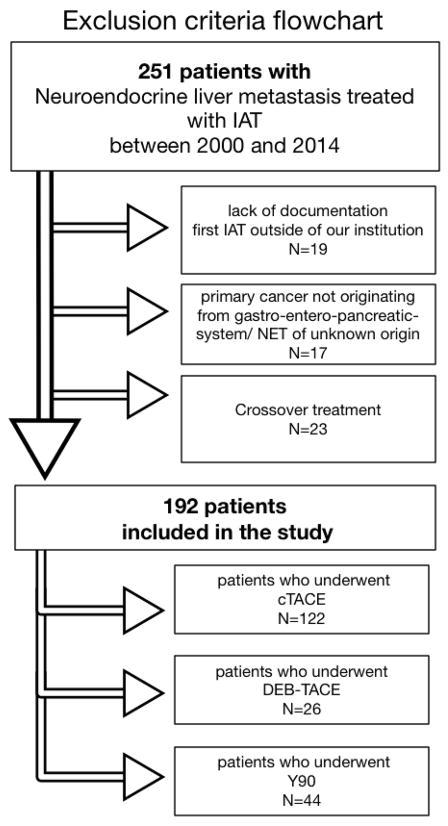
Electronic medical records, pathology reports, retrospective pathological review and grading of samples, and imaging studies were used to collect demographic, pathological, treatment, and outcome data of the patients. Between 2000 and 2014 a total of 251 patients who received cTACE (2000–2014), DEB-TACE (2009–2013) or Y90 (2003–2013) for the therapy of NELM between 2000 and 2014 were retrospectively identified and included in the database (end-of-observation date May 18th, 2014). The criteria for inclusion and exclusion of patients are itemized within the flowchart (Figure 1).
Figure 1. Exclusion criteria flow chart.
Abbreviations: conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium radioembolization (Y90)
b. Intra-arterial therapy
Histopathological NET type (pNET, carcinoid) determined by the World Health Organization (WHO) classification system was obtained from the in-house pathology service records [9, 10]. Patient cases with missing grading records were retrieved and re-graded according to the WHO standard. Samples originating from resected primary tumors or – for cases with unknown primary or unresectable disease – from biopsy specimen originating from the liver before the initial cycle of intra-arterial therapy [11]. Both functional as well as nonfunctional pancreatic NET were labeled as pNET. The choice of IAT (cTACE, DEB-TACE or Y90) was made in concordance with the National Comprehensive Cancer Network guidelines for NETs during a multidisciplinary liver tumor board which included hepatologists, medical oncologists, liver surgeons, pathologists and interventional radiologists, decision criteria are further described in the supplementary section [12, 13].
In general, cTACE, DEB-TACE or Y90 were performed on NELM patients with unresectable hepatic-dominant disease that was symptomatic or progressive in patients that qualified with an Eastern Cooperative Oncology Group score (ECOG) 0–2, and adequate hepatic, renal and hematological function.
For all patients, only one lobe of the liver was subjected to embolization during each treatment session. Additional sessions of cTACE, DEB-TACE or Y90 were considered when palliation of symptoms or local tumor control were not achieved by the initial therapy cycle. All IAT procedures were performed by the same interventional radiologist (J.F.G.) with meanwhile 19 years of experience in hepatic interventions, using a standardized approach in accordance to our institutional protocols [14]. A brief summary of each protocol is described in the supplementary section.
a. Imaging Data Evaluation
Patients underwent baseline and follow-up (MR or CT) imaging within 3–4 weeks before and after the initial cTACE, DEB-TACE or Y90. Additional follow-up abdominal (MRI or CT) scans were obtained 3 to 4 months after the first embolization, then every 4 to 6 months for the first two years, and annually thereafter. A brief summary of imaging protocol (MR, CT) is described in the supplementary section.
Follow-up MRI (acquired between 30 and 40 days after IAT) was compared with the baseline imaging to determine the objective tumor radiologic response (RR) in the liver according to complete response (CR), partial response (PR), minor response (MR), stable disease (SD) and progressive disease (PD) based on the WHO and response evaluation criteria in solid tumors (RECIST). WHO and RECIST response rates were measured by two independent radiologists, Q.H. (5 years of experience) and C.L. (8 years of experience), who did not perform the IAT and were blinded to the study data. Their results were averaged.
a. Adverse events
Procedure-related adverse events were assessed within the framework of direct post-procedural care and during clinical follow-up appointments. Recorded data was assessed retrospectively and reported for patients with complete data.
a. Statistical analysis
OS was defined as the period between the initial embolization procedure and the date of death from any cause or last follow-up for patients who remained alive at the time of last follow-up. HPFS was defined as the period between the date of the initial embolization and the date of progression for patients who displayed radiologic evidence of disease progression in the liver or the date of death or last follow-up for patients who did not progress. Due to the retrospective nature of our study, randomization was not possible and so propensity score analysis (PSA) was performed in order to compensate for selection bias and cohort heterogeneities [15]. After considering different methods for PSA, such as matching, sub-classification or full matching, we chose the inverse probability of treatment weighting because it allowed using all patients available in a relatively small study cohort while minimizing cohort size differences [16–18]. A brief summary of the PSA is described in the supplementary section.
PSA-adjusted univariate Cox proportional hazards regression models (UVA) were set up to evaluate the predictive value of each covered factor. PSA-adjusted multivariate Cox models (MVA) were created to assess the prognostic effects of the inspected factors simultaneously. Only those variables showing p<.05 in the UVA and the treatment variables (cTACE vs. DEB-TACE vs. Y90) were further investigated in the MVA. The MOS was calculated using the PSA-adjusted Kaplan–Meier method. The PSA-adjusted log-rank test was used to compare cumulative survival between the groups [19]. As recommended by the literature the PSA can only be performed between two groups, assuring a statistically robust conclusion [17]. Therefore, to compare the three treatment arms, we conducted three pairwise comparison analyses: 1) cTACE cohort vs. DEB-TACE cohort, 2) cTACE cohort vs. Y90 cohort and 3) DEB-TACE cohort vs. Y90 cohort. The probability of CR, PR and MR was determined by UVA and MVA logistic regression techniques. A Hosmer and Lemeshow goodness-of-fit test was assessed for each model. Fisher’s exact test was performed to compare the differences in adverse events between the treatment modalities. Statistical significance was defined as p<.05. Statistical tests were performed using SPSS software (IBM SPSS Statistics, version 23, 2015, IBM Corp, Armonk, NY, USA). The PSA was applied using the R 3.2.3 (R Core Team, 2015, Vienna, Austria) including the add-in R package twang [20, 21].
4. Results
a. Patient characteristics
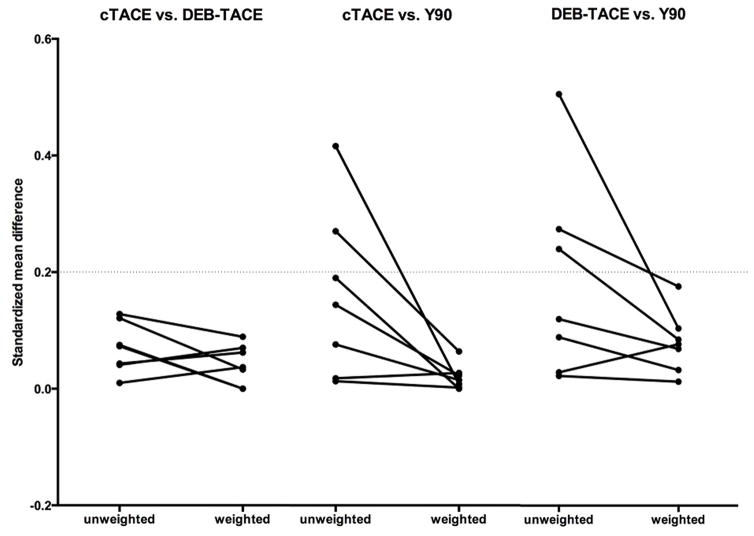
A total of 192 patients were included in the final data analysis, and consisted of N=122, N=26 and N=44 patients who underwent cTACE, DEB-TACE or Y90, respectively. The MOS of the study cohort was 28.8 months and by the end-of-observation date (May 18th, 2014), during a median follow-up time of 75.6 months a total of 74.5% (143/192) patients were deceased. Of 192 patients, 125 were identified with carcinoid (65.1%) and 67 with pNET (34.9%). Fifty-seven patients (29.7%) had a tumor burden (TB) >50%. The mean number of IAT sessions per patient was 2.9, 2.1 and 1.7 for cTACE, DEB-TACE and Y90, respectively. Patient demographics, tumor characteristics, and treatment details for patient groups are summarized in Table 1. Applying PSA served to minimize selection bias for the choice of treatment, and to achieve a balance among covariates. We have chosen those covariates, which according to the reviewed literature, have an impact on survival outcome (ECOG, liver tumor resection with curative intention/radiofrequency ablation (Rx/RFA), primary tumor resection, chemotherapy, liver metastases debulking, TB>50%, extra-hepatic metastases), balance was achieved (Figure 2) [4, 22–26].
Table 1.
Baseline Characteristics by intra-arterial therapy option before propensity score weighting
| cTACE (N=122) | DEB-TACE (N=26) | Y90 (N=44) | cTACE vs. DEB TACE | cTACE vs. Y90 | DEB-TACE vs. Y90 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | p value | |||
| Age | |||||||||
| ≤ 70 years | 108 | (88.5) | 20 | (76.9) | 35 | (79.5) | |||
| > 70 years | 14 | (11.5) | 6 | (23.1) | 9 | (20.5) | |||
| mean age (CI95%) | 57.3 | (55.2–59.4) | 61.4 | (56.1–66.6) | 60.8 | (57.4–64.2) | 0.12 | 0.20 | 1.00 |
|
| |||||||||
| Sex | |||||||||
| male | 65 | (53.3) | 16 | (61.5) | 27 | (61.4) | |||
| female | 57 | (46.7) | 10 | (38.5) | 17 | (38.6) | 0.51 | 0.38 | 1.00 |
|
| |||||||||
| ECOG | |||||||||
| ECOG 0 | 62 | (50.8) | 11 | (42.3) | 19 | (43.2) | |||
| ECOG ≥ 1 | 60 | (49.2) | 15 | (57.7) | 25 | (56.8) | 0.52 | 0.48 | 0.9 |
|
| |||||||||
| Prior Rx/RFA | |||||||||
| no | 105 | (86.1) | 22 | (84.6) | 39 | (88.6) | |||
| yes | 17 | (13.9) | 4 | (15.4) | 5 | (11.4) | 0.76 | 0.79 | 0.71 |
|
| |||||||||
| Prior Primary tumor resection | |||||||||
| intact | 71 | (58.2) | 15 | (57.7) | 26 | (59.1) | |||
| resected | 51 | (41.8) | 11 | (42.3) | 18 | (40.9) | 1.00 | 1.00 | 1.00 |
|
| |||||||||
| Prior Chemotherapy | |||||||||
| no | 97 | (79.5) | 22 | (84.6) | 27 | (61.4) | |||
| yes | 25 | (20.5) | 4 | (15.4) | 17 | (38.6) | 0.79 | 0.03 | 0.06 |
|
| |||||||||
| Prior Octreotide | |||||||||
| no | 63 | (51.6) | 13 | (50.0) | 15 | (34.1) | |||
| yes | 59 | (48.4) | 13 | (50.0) | 29 | (65.9) | 1.00 | 0.05 | 0.22 |
|
| |||||||||
| Prior Metastases debulking | |||||||||
| no | 93 | (76.2) | 19 | (73.1) | 37 | (84.1) | |||
| yes | 29 | (23.8) | 7 | (26.9) | 7 | (15.9) | 0.80 | 0.39 | 0.35 |
|
| |||||||||
| Tumor type | |||||||||
| pNET | 44 | (36.1) | 10 | (38.5) | 13 | (29.5) | |||
| Carcinoid | 78 | (63.9) | 16 | (61.5) | 31 | (70.5) | 0.83 | 0.46 | 0.59 |
|
| |||||||||
| Tumor burden | |||||||||
| ≤ 50 % | 82 | (67.2) | 18 | (69.2) | 35 | (79.5) | |||
| > 50% | 40 | (32.8) | 8 | (30.8) | 9 | (20.5) | 1.00 | 0.18 | 0.39 |
|
| |||||||||
| Dominant lesion size | |||||||||
| ≤ 5 cm | 52 | (42.6) | 8 | (30.8) | 28 | (63.6) | |||
| > 5 cm | 70 | (57.4) | 18 | (69.2) | 16 | (36.4) | 0.28 | 0.02 | 0.01 |
|
| |||||||||
| Multiplicity | |||||||||
| Unilobular | 13 | (10.7) | 2 | (7.7) | 2 | (4.5) | |||
| Bilobular | 109 | (89.3) | 24 | (92.3) | 42 | (95.5) | 0.30 | 0.36 | 0.63 |
|
| |||||||||
| Extrahepatic metastases* | |||||||||
| no | 91 | (74.6) | 18 | (69.2) | 30 | (68.2) | |||
| yes | 31 | (25.4) | 8 | (30.8) | 14 | (31.8) | 0.63 | 0.43 | 1.00 |
|
| |||||||||
| Hormonal status | |||||||||
| inactive | 50 | (41.0) | 13 | (50.0) | 19 | (43.2) | |||
| active | 72 | (59.0) | 13 | (50.0) | 25 | (56.8) | 0.51 | 0.86 | 0.63 |
|
| |||||||||
| Tumor grade | |||||||||
| G1 | 102 | (83.6) | 21 | (80.8) | 37 | (84.1) | |||
| G2 | 17 | (13.9) | 3 | (11.5) | 6 | (13.6) | 1.0 | 1.00 | 1.00 |
| G3 | 3 | (2.5) | 2 | (7.7) | 1 | (2.3) | 0.21 | 1.00 | 0.55 |
Extrahepatic metastases (lung, bone, soft tissue, retro peritoneal, gastro intestinal system, lymph node)
Radio frequency ablation (RFA), Tumor resection with curative intention (Rx), pancreatic neuroendocrine tumor (pNET), Intra-arterial therapy (IAT), Confidence interval (CI), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90),
Figure 2. Graphical assessment of balance after propensity score weighting.
To assess the balance in baseline covariates (ECOG, liver tumor resection with curative intention/radiofrequency ablation (Rx/RFA), primary tumor resection, chemotherapy, liver metastases debulking, tumor burden > 50%, extra-hepatic metastases) the standardized mean difference (SMD) has been used. Figure 2 shows line plots of SMD before and after weighting. These plots display the effect of weights on the magnitude of differences between the treatment cohorts on each pretreatment covariate. A recommended threshold value for balance in the covariates is 0.2. As in these plots for all included covariates SMD <0.2 balance has been achieved by means of PSA.
Abbreviations: Eastern Cooperative Oncology Group score (ECOG), standardized mean difference (SMD), propensity score analysis (PSA)
b. Overall survival
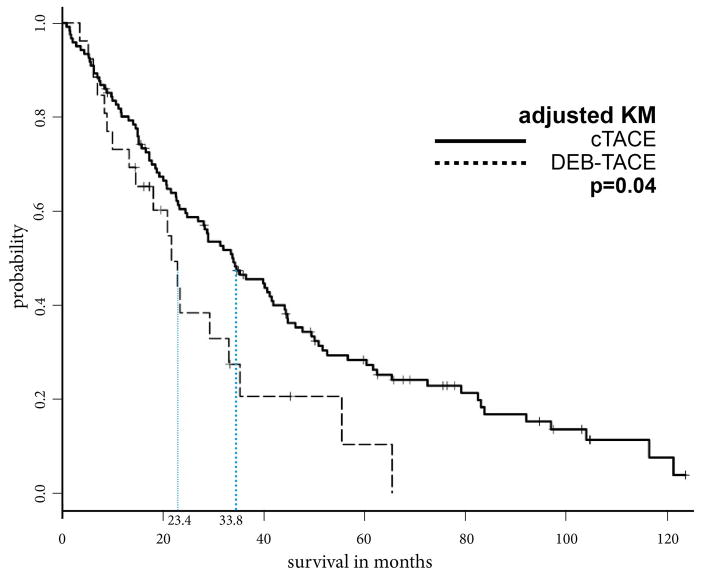
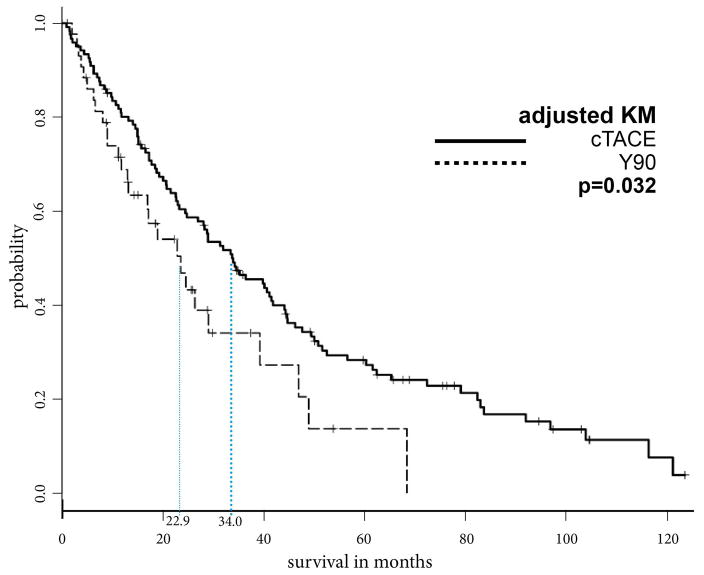
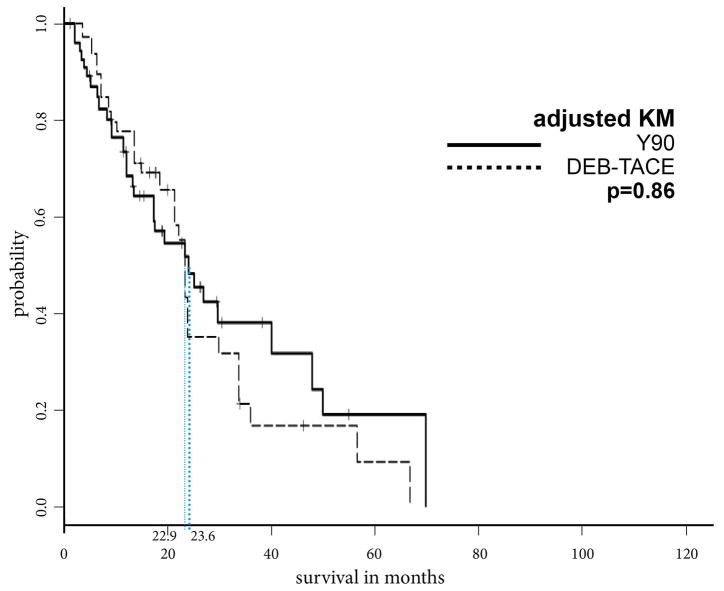
Prior to PSA, the MOS after cTACE, DEB-TACE and Y90 were 33.8, 21.7, and 23.6 months, respectively. Additionally, the overall cumulative 1-year, 2-year, and 5-year OS rates were 80.9%, 60.4% and 28.2% after cTACE, respectively, 73.0%, 38.3% and 10.3% after DEB-TACE, respectively, and 71.2%, 49.4% and 18.5% after Y90, respectively. After PSA, patients who underwent cTACE lived significantly longer than patients who received DEB-TACE or Y90 (cTACE vs. DEB-TACE, 33.8 vs. 23.4 months, p=.04; cTACE vs. Y90, 34.0 vs. 22.9 months, p=.032). A comparison of DEB-TACE vs. Y90 did not reveal statistically significant differences in MOS (p=.86) (Figures 3a–c).
Figure 3.
Figure 3a: Kaplan Meier curves demonstrating survival of cTACE vs. DEB-TACE cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for overall survival were calculated according to IAT modality, cTACE and DEB-TACE. After PSA the MOS of patients who underwent cTACE was significantly longer compared to the MOS of patients who underwent DEB-TACE (33.8 vs. 23.4 months, respectively, p=0.04).
Abbreviations: propensity score analysis (PSA), Intra-arterial therapy (IAT), median overall survival (MOS), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB- TACE)
Figure 3b: Kaplan Meier curves demonstrating survival of cTACE vs. Y90 cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for overall survival were calculated according to IAT modality, cTACE and Y90. After PSA the MOS of patients who underwent cTACE was significantly longer compared to the MOS of those who underwent Y90 (34.0 vs. 22.9 months, respectively, p=0.032).
Abbreviations: propensity score analysis (PSA), Intra-arterial therapy (IAT), median overall survival (MOS), conventional transarterial chemoembolization (cTACE), 90Yttrium Radioembolization (Y90)
Figure 3c: Kaplan Meier curves demonstrating survival of DEB-TACE vs. Y90 cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for overall survival were calculated according to IAT modality, DEB-TACE and Y90. After PSA, there was no statistically significant difference in OS between DEB-TACE and Y90 (22.9 vs 23.6 months, respectively, p=0.86).
Abbreviations: propensity score analysis (PSA), Intra-arterial therapy (IAT), median overall survival (MOS), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90)
In the comparison of cTACE vs. DEB-TACE, cTACE corresponded with prolonged OS in the MVA (p<.01, HR, .55). Further, in the comparison of cTACE vs. Y90, cTACE was predictive for improved OS in the MVA (p=.02, HR, 0.61).
In the entire study cohort, age (p=.01, HR, 1.81), TB>50% (p<.01, HR, 1.93) and presence of extra-hepatic metastases (p<0.01, HR, 1.63) were identified as independent predictors for reduced OS (Table 2).
Table 2.
Univariate and multivariate Cox Proportional Hazard Models for Overall Survival after propensity score weighting
(only the choice of IAT and covariates which were significant in MVA are displayed)
| Entire study cohort (only covariates which were significant in MVA are displayed) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| UVA | MVA | ||||||
|
| |||||||
| Covariates | N=192 | MOS (m) | CI 95% | p-value | HR | CI 95% | p-value |
| Age | |||||||
| ≤70 years | 163 | 31.4 | 23.4–40.7 | 1 | |||
| > 70 years | 29 | 15.2 | 11.2–39.3 | 0.03* | 1.81 | 1.14–2.87 | 0.01* |
| Tumor burden | |||||||
| ≤50 % | 135 | 34.6 | 29.0–44.8 | 1 | |||
| > 50% | 57 | 17 | 13.2–22.9 | <0.01* | 1.93 | 1.27–2.94 | <0.01* |
| Extrahepatic metastases | |||||||
| no | 139 | 33.1 | 24.6–41.9 | 1 | |||
| yes | 53 | 22.9 | 15.2–34.0 | <0.01 | 1.63 | 1.15–2.32 | <0.01* |
| IAT modality (only the choice of IAT is displayed) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| UVA | MVA | ||||||
|
| |||||||
| Study cohort | MOS (m) | CI 95% | p-value | HR | CI 95% | p-value | |
| cTACE vs. DEB-TACE (N=148) | |||||||
| DEB-TACE | 26 | 23.4 | 28.1–41.6 | 1 | |||
| cTACE | 122 | 33.8 | 20.9–23.4 | 0.018* | 0.55 | 0.36–0.85 | <0.01* |
| cTACE vs. Y90 (N=166) | |||||||
| Y90 | 44 | 22.9 | 17.0–26.4 | 1 | |||
| cTACE | 122 | 34 | 28.3–41.2 | 0.02* | 0.61 | 0.39–0.93 | 0.02* |
| Y90 vs. DEB-TACE (N=70) | |||||||
| Y90 | 44 | 23.6 | 17.0–47.0 | 1 | |||
| DEB-TACE | 26 | 22.9 | 20.9–29.3 | 0.69 | 2.06 | 0.67–2.19 | 0.52 |
Indicator for statistical significance (*)
Intra-arterial therapy (IAT), Univariate analysis (UVA), Multivariate analysis (MVA), Confidence interval (CI), Hazard ratio (HR), median overall survival (MOS) in months (m), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90)
PSA-adjusted univariate Cox proportional hazards regression models (UVA) were set up to evaluate the predictive value of each covered factor. PSA-adjusted multivariate Cox models (MVA) were created to assess the prognostic effects of the inspected factors simultaneously. Only those variables showing p<.05 in the UVA and the treatment variables (cTACE vs. DEB-TACE vs. Y90) were further investigated in the MVA for each treatment group. The table displays the summary of the UVA and MVA. The covariates which were significant in the UVA and were included into MVA, subsequently, are displayed in the supplementary material.
c. Hepatic progression-free survival
A total of 149 patients with available baseline and follow-up MR imaging were considered for HPFS analysis comprising 90, 23, and 36 patients who underwent cTACE, DEB-TACE or Y90, respectively. In the PSA, patients who received cTACE showed a prolonged HPFS compared to patients who received Y90 (21.6 vs. 11.2 months, respectively, p=.03), whereas in the comparison of cTACE vs. DEB-TACE (20.1 vs. 14.6 months, respectively, p=.14) and Y90 vs. DEB-TACE (11.2 vs. 13.3 months, respectively, p=.72) there was no significant difference in HPFS. In the comparison of cTACE and Y90, cTACE was predictive for improved HPFS in the PS-adjusted MVA (p=.03, HR, 0.57) (Supplementary Figures 1a–c).
d. Radiologic response
The same cohort for the HPFS analysis was considered for RR analysis. In applying to the WHO classification, none of these patients demonstrated CR whereas PR was observed in 26, MR in 44, SD in 76 and PD in 3 patients (Table 3). Across all groups, statistically significant difference between responder and non-responder was not achieved according to WHO criteria. As for RECIST, 0, 4, 136 and 9 patients were identified with CR, PR, SD and PD, respectively. DEB-TACE treatment was significantly correlated with 1.33 times higher RR higher than Y90 (MVA: p=.03) (Supplementary Table 4).
Table 3.
Radiologic response after 1 month according to RECIST and WHO classification
| cTACE (N=90) | DEB-TACE (N=23) | Y90 (N=36) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| WHO | N | % | N | % | N | % |
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 14 | 15.6 | 7 | 30.4 | 5 | 13.9 |
| MR | 30 | 33.3 | 7 | 30.4 | 7 | 19.4 |
| SD | 45 | 50.0 | 8 | 34.8 | 23 | 63.9 |
| PD | 1 | 1.1 | 1 | 4.3 | 1 | 2.8 |
|
| ||||||
| (CR+PR+MR) vs. (SD+PD) | p value | |||||
|
| ||||||
| cTACE vs. DEB TACE | 0.36 | |||||
| cTACE vs. Y90 | 0.16 | |||||
| Y90 vs. DEB TACE | 0.06 | |||||
| RECIST | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 3 | 3.3 | 1 | 4.3 | 0 | 0 |
| SD | 83 | 92.2 | 21 | 91.3 | 32 | 88.9 |
| PD | 4 | 4.4 | 1 | 4.3 | 4 | 11.1 |
|
| ||||||
| (CR+PR) vs. (SD+PD) | p value | |||||
|
| ||||||
| cTACE vs. DEB TACE | 1.00 | |||||
| cTACE vs. Y90 | 0.56 | |||||
| Y90 vs. DEB TACE | 0.39 | |||||
Intra-arterial therapy (IAT), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB- TACE), 90Yttrium Radioembolization (Y90), World Health Organization criteria (WHO), response evaluation criteria in solid tumors (RECIST), objective tumor radiologic response (RR), complete response (CR), partial response (PR), minor response (MR), stable disease (SD), progress disease (PD)
In the present study, follow-up MRI was compared with the baseline imaging to determine the objective tumor radiologic response (RR) in the liver according to complete response (CR), partial response (PR), minor response (MR), stable disease (SD) and progress disease (PD) based on the World Health Organization criteria (WHO) and response evaluation criteria in solid tumors (RECIST)
e. Adverse events
Overall, adverse events were recorded in 164 (85.4%) patients with rates of 85.2%, 88.5% and 84.1% after cTACE, DEB-TACE and Y90, respectively. Common adverse events in all treatment cohorts included diarrhea (33.3%), abdominal pain (29.6%) and flushing (26.5%) (Table 4).
Table 4.
Summary from the most common clinical adverse events
| DEB-TACE (n=26) | cTACE (n=122) | Y90 (n=44) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | (%) | N | (%) | N | (%) | |
| no symptoms | 3 | 11.5 | 18 | 14.8 | 7 | 15.9 |
| Presence of symptoms | 23 | 88.5 | 104 | 85.2 | 37 | 84.1 |
|
| ||||||
| Listed symptoms: | N | (%) | N | (%) | N | (%) |
|
| ||||||
| Abdominal pain/discomfort | 9 | 34.6 | 28 | 23.0 | 20 | 45.5 |
| Flushing | 5 | 19.2 | 35 | 28.7 | 11 | 25.0 |
| Fatigue | 4 | 15.4 | 22 | 18.0 | 4 | 9.1 |
| Nausea | 3 | 11.5 | 15 | 12.3 | 4 | 9.1 |
| Weight loss | 3 | 11.5 | 16 | 13.1 | 9 | 20.5 |
| Diarrhea | 3 | 11.5 | 44 | 36.1 | 17 | 38.6 |
| Vomiting | 2 | 7.7 | 9 | 7.4 | 0 | 0.0 |
| Anorexia | 1 | 3.8 | 2 | 1.6 | 0 | 0.0 |
| Hypoglycemia | 1 | 3.8 | 2 | 1.6 | 1 | 2.3 |
| Fever | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 |
| Reflux | 0 | 0.0 | 2 | 1.6 | 1 | 2.3 |
| no symptoms vs. presence of symptoms | |||
|---|---|---|---|
|
| |||
| Comparison cohort | DEB-TACE vs. cTACE | cTACE vs. Y90 | Y90 vs. DEB-TACE |
| p-value | 1.00 | 0.81 | 0.73 |
conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90)
5. Discussion
Our main finding is that patients with NELM treated with cTACE showed clear OS benefits as compared to DEB-TACE (33.8 vs. 23.4 months, p=.04) and Y90 (34.0 vs. 22.9 months, p=.032). Importantly, after adjusting for confounders in the MVA using the propensity score methodology, the difference in survival remained statistically significant. Our study is among very few published datasets that compare clinical outcomes between different IAT modalities in NELMs and the first to specifically compare the three commonly used loco-regional therapeutic modalities (cTACE vs. DEB-TACE vs. Y90) in the largest ever reported cohort of such patients. Although retrospective in nature, our study uses a comprehensive statistical analysis to correct for potential selection bias, thus providing for relatively homogeneous groups of patients. Currently, no clinical trial is completed that would address the questions raised and answered by our data analysis and only one clinical trial is currently ongoing (NCT02724540) that may potentially answer some of the challenging IAT-related issues with respect to decision making in NELMs. As such, our results evidently support the preferential use of Lipiodol-based cTACE over other intra-arterial therapies not only by a statistically significant but rather by a substantial margin with respect to OS benefits.
The vast majority of currently available studies investigate TAE and cTACE as treatment options for patients with unresectable NELM. For example, as exhibited by Ruutiainen et al., MOS were 39 months and 44 months for patients who underwent TAE or cTACE, respectively [27]. Furthermore, a large-scale study by Dong et al. that included (N=123) patients who received cTACE for NELM reported a 5-year cumulative OS rate of 36% compared to 28.2% in our study [28]. If compared further, all of the aforementioned studies achieved slightly better OS outcomes as compared with our own cohort. This can be explained by a substantially higher rate of patients with extra-hepatic metastases and TB>50% in our cohort which reflects our institutional approach to treat patients with late-stage disease. This is in agreement with available data, which suggests a significantly higher mortality in patients with a TB>50% [29]. With respect to extra-hepatic metastases, Gupta et al. reported a substantial negative impact on survival outcomes of extra-hepatic bone and soft-tissue metastases both for carcinoids and pNET [4].
Initially, DEB-TACE seemed to emerge as an improvement over cTACE in the scope of treating patients with primary liver cancer (hepatocellular carcinoma (HCC)) [14, 30]. DEB-TACE was demonstrated to allow for a better toxicity profile primarily due to improved targeted delivery of the chemotherapy payload to the liver [31]. Thus, an auspicious impact on OS in treatment of NELM disease was also expected [14, 32–35]. Yet, initial outcome reports for DEB-TACE in patients with NELM were disappointing, and in some cases significant toxicities, such as a higher incidence of biliary injury, occurred which required a modification of protocol [36, 37]. As reported previously, the MOS for patients after receiving cTACE can range from 24 to 44 months [27, 38]. Our outcome report is among the first to provide survival data for DEB-TACE in a sizable cohort of NELM patients. When compared with cTACE, our findings unequivocally confirm the survival benefits of the Lipiodol-based protocol. When taking into account that no differences between both TACE options were observed with respect to RR, HPFS and incidence of adverse events, our results support the use of cTACE in patients with NELM when given the choice between Lipiodol-based and DEB-based protocols.
Several recent studies promote Y90 as a potential alternative to TAE/cTACE in the treatment of patients with NELM [39]. Advantages associated with Y90 treatment include lower incidence rates of adverse events, such as post-embolization syndrome, high RR and lower retreatment rates compared to cTACE, which is usually applied in repeated sessions. The majority of current studies compare Y90 to cTACE/TAE in terms of RR and symptomatic improvements with no data available with respect to survival outcomes [39]. Two recent studies, by Rhee et al. and by Kennedy et al., showed substantial disagreement with respect to MOS after Y90 in patients with NELM, ranging from 22 to 70 months [39, 40]. Our analysis demonstrates significant and substantial OS and HPFS benefits for patients who underwent cTACE as compared to those who were treated with Y90. This result remained significant upon elimination of selection bias using the PSA (MOS: 34.0 vs. 22.9 months, p=.032, HPFS: 21.6 vs. 11.2 months, p=.03) as well as after adjusting for confounders in the MVA. In addition, cTACE and Y90 showed similar incidence rates of adverse events. An important point which should be considered with respect to this outcome analysis is the number of embolization sessions applied in each group. Because of inherent and well known methodological limitations, the total number of Y90 therapy sessions is usually limited to two injections when treating in a lobar fashion. As cTACE treatment allows for additional embolization, multiple sessions can be performed in case of insufficient RR or new lesions upon completion of the initial therapy cycle [41]. In our cohort, all patients with therapy cross-over were initially excluded with the goal of reducing potential bias. Thus, a slightly higher number of sessions per patient has been recorded for cTACE than for Y90. Although clear benefits of cTACE over Y90 were evident without therapy cross-over, a true advantage might arise from sequential Y90 and cTACE therapy in patients with slow-growing NELM.
As for the comparison of Y90 and DEB-TACE, both techniques demonstrated similar effect on OS and were equally safe. However, DEB-TACE corresponded with improved RR (MVA: p=.03). As demonstrated by Whitney et al., an exceptional RR (CR+PR) of 100% was attained by Y90 and DEB-TACE treatment after three months. However, in a 12-month follow-up, RR in the Y90 group was significantly lower than in the DEB-TACE group. Thus, patients who underwent Y90 were initially salvaged with repeated DEB-TACE treatment, which again underlines the potentially tremendous opportunities of the sequential crossover therapy option [34].
There were several limitations to our study. First, due to the retrospective design and the non-randomized cohorts, there was a limited pool of patients and a correspondingly restricted statistical analysis. However, the limited number of patients with NELM mirrors the rarity of the investigated disease. Furthermore, possible confounders and selection bias were considered and minimized by PSA – as discussed previously. Most importantly, our study represents a longitudinal retrospective analysis of data collected over a period of almost 14 years. The last decade has seen an unparalleled growth and evolution of embolization and imaging technologies as well as the introduction of multiple new systemic and targeted therapies for neuroendocrine tumors. Taken together, all of these factors may have influenced PFS and OS of our patients and thus introduced a substantial bias into our analysis. Yet, given the lack of prospectively collected data, such retrospective data analyses represent the only currently available and much needed instrument to identify trends and design prospective trials.
6. Conclusion
Our propensity score analysis suggests the superiority of cTACE over DEB-TACE and Y90 with respect to overall survival among patients with unresectable NELM. In light of our findings and because of the lack of prospectively collected data from randomized controlled trials, it can be cautiously suggested that cTACE may evolve as the primary intra-arterial therapy option in patients with unresectable NELM until proven otherwise.
Supplementary Material
Table 1. Balance between IAT cohort after propensity score weighting
Standardized differences in the means (SMD), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90), Tumor resection with curative intention (Rx), Radio frequency ablation (RFA), an Eastern Cooperative Oncology Group score (ECOG)
The balance in the covariates was evaluated by the absolute standardized differences in the means before and after weighting. A recommended threshold value for balance in the covariates is 0.2.
Table 2. Univariate and multivariate Cox Proportional Hazard Models for Overall Survival after propensity score weighting (only the choice of IAT and covariates which were significant in UVA are displayed)
Table 3. Univariate and multivariate analysis for radiologic response after propensity score weighting (only choice of IAT and covariates which were significant in UVA are displayed)
Indicator for statistical significance (*)
Intra-arterial therapy (IAT), Univariate analysis (UVA), Multivariate analysis (MVA), Confidence interval (CI), Partial response (PR), Minor response (MR), Odd ratio (OR), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90), tumor grade 1–3 (G1–G3)
The probability of CR, PR and MR was determined by UVA and MVA logistic regression techniques. A Hosmer and Lemeshow goodness-of-fit test was assessed for each model. The table displays the summary of the UVA and MVA, thus the covariates which were significant in the UVA are shown in the table.
Table 4. Univariate and multivariate Cox Proportional Hazard Models for hepatic progression free Survival after propensity score weighting
(only the choice of IAT and covariates which were significant in UVA are displayed)
Figure 1a. Kaplan Meier curves demonstrating hepatic progression-free survival of cTACE vs. DEB-TACE cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for hepatic progression-free survival were calculated according to IAT modality, cTACE and DEB-TACE. After PSA the MHPFS of patients who underwent cTACE was 20.1 months, as for patients who underwent DEB-TACE 14.6 months (p=.14).
Abbreviations: Kaplan Meier (KM), propensity score analysis (PSA), Intra-arterial therapy (IAT), hepatic progression-free survival (HPFS), median HPFS (MHPFS), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE)
Figure 1b. Kaplan Meier curves demonstrating hepatic progression-free survival of cTACE vs. Y90 cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for HPFS were calculated according to IAT modality, cTACE and Y90. After PSA the MHPFS of patients who underwent cTACE was 21.6 months, as for patients who underwent Y90 11.2 months (p=.03).
Abbreviations: Kaplan Meier (KM), propensity score analysis (PSA), Intra-arterial therapy (IAT), hepatic progression-free survival (HPFS), median HPFS (MHPFS), conventional transarterial chemoembolization (cTACE), 90Yttrium Radioembolization (Y90)
Figure 1c. Kaplan Meier curves demonstrating hepatic progression-free survival of DEB-TACE vs. Y90 cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for HPFS were calculated according to IAT modality, DEB-TACE and Y90. After PSA the MHPFS of patients who underwent DEB-TACE was 13.3 months, as for patients who underwent Y90 11.2 months (p=.72).
Abbreviations: Kaplan Meier (KM), propensity score analysis (PSA), Intra-arterial therapy (IAT), hepatic progression-free survival (HPFS), median HPFS (MHPFS), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90)
Key point.
cTACE achieved a significantly longer overall survival in patients with unresectable NELM.
Patients treated with cTACE showed a prolonged hepatic progression free survival.
cTACE, DEB-TACE and Y90 radioembolization demonstrated comparable safety and toxicity profiles.
Age >70years, extra-hepatic metastases and tumor burden>50% were identified as negative predictors.
Propensity score analysis suggests the superiority of cTACE over DEB-TACE and Y90.
Acknowledgments
We kindly thank Yanhong Deng, Biostatistician, School of Public Health: Yale Center for Analytical Sciences (YCAS), for the statistical review and technical support.
Funding:
This study has received funding by NIH/NCI R01 (CA160771):
Prof. Dr. Jean-Francois Geschwind
Dr. MingDe Lin
Studienstiftung des Deutschen Volkes: Duc Do Min
Rolf W. Günther Stiftung für Radiologische Wissenschaften : Duc Do Minh
List of Abbreviations
- NET
neuroendocrine tumor
- NELM
NET liver metastases
- IAT
intra-arterial therapy
- cTACE
conventional transarterial chemoembolization
- DEB-TACE
drug-eluting beads TACE
- Y90
90Yttrium-radioembolization
- carcinoid
carcinoid tumor
- pNET
pancreatic NET (islet cell tumors)
- OS
overall survival
- MOS
median overall survival
- UVA
univariate analyses
- MVA
multivariate analyses
- HR
hazard ratio
- PSA
propensity score analysis
- HPFS
hepatic progression-free survival
- Rx
liver tumor resection with curative intention
- RFA
radiofrequency ablation
- WHO
World Health Organization
- RECIST
Response Evaluation Criteria In Solid Tumors
- CR
complete response
- PR
partial response
- MR
minor response
- SD
stable disease
- PD
progressive disease
- HCC
hepatocellular carcinoma
Footnotes
Compliance with ethical standards:
Guarantor:
The scientific guarantor of this publication is Jean-Francois Geschwind, M.D.
Conflict of interest:
The authors of this manuscript declare relationships with the following companies: Jean-Francois Geschwind, M.D.:
Consultant: Biocompatibles/BTG, Bayer HealthCare, Guerbet, Nordion/BTG, Philips Healthcare and Jennerex
Founder and CEO PreScience Labs, LLC.
Statistics and biometry:
Yanhong Deng kindly provided statistical advice for this manuscript.
(Yanhong Deng, Biostatistician, School of Public Health: Yale Center for Analytical Sciences (YCAS))
Informed consent:
Written informed consent was waived by the Institutional Review Board.
Ethical approval:
Institutional Review Board approval was obtained.
- retrospective
- diagnostic or prognostic study and observational
- performed at one institution
References
- 1.Steinmuller T, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87(1):47–62. doi: 10.1159/000111037. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S. Intra-arterial liver-directed therapies for neuroendocrine hepatic metastases. Semin Intervent Radiol. 2013;30(1):28–38. doi: 10.1055/s-0033-1333651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamberlain RS, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(4):432–45. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(8):1590–602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med. 1994;120(4):302–9. doi: 10.7326/0003-4819-120-4-199402150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Ramage JK, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2012;61(1):6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madoff DC, et al. Update on the management of neuroendocrine hepatic metastases. J Vasc Interv Radiol. 2006;17(8):1235–49. doi: 10.1097/01.RVI.0000232177.57950.71. quiz 1250. [DOI] [PubMed] [Google Scholar]
- 8.Libicher M, Bovenschulte H. Arterial embolization of hepatic metastases from neuroendocrine tumors. Radiologe. 2009;49(3):233–41. doi: 10.1007/s00117-008-1787-6. [DOI] [PubMed] [Google Scholar]
- 9.Grillo F, et al. Twenty years of gastroenteropancreatic neuroendocrine tumors: is reclassification worthwhile and feasible? Endocrine. 2016;53(1):58–62. doi: 10.1007/s12020-015-0734-3. [DOI] [PubMed] [Google Scholar]
- 10.Grillo F, et al. Grade Increases in Gastro-Entero-Pancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology. 2015 doi: 10.1159/000439434. [DOI] [PubMed] [Google Scholar]
- 11.Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):3–7. doi: 10.1007/s10555-011-9292-1. [DOI] [PubMed] [Google Scholar]
- 12.Kulke MH, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10(6):724–64. doi: 10.6004/jnccn.2012.0075. [DOI] [PubMed] [Google Scholar]
- 13.Clark OH, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2006;4(2):102–38. doi: 10.6004/jnccn.2006.0013. [DOI] [PubMed] [Google Scholar]
- 14.Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol. 2011;34(1):37–49. doi: 10.1007/s00270-010-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med. 2010;29(3):337–46. doi: 10.1002/sim.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald RJ, et al. Behind the Numbers: Propensity Score Analysis—A Primer for the Diagnostic Radiologist. Radiology. 2013;269(3):640–645. doi: 10.1148/radiol.13131465. [DOI] [PubMed] [Google Scholar]
- 18.Stone CA, Tang Y. Comparing Propensity Score Methods in Balancing Covariates and Recovering Impact in Small Sample Educational Program Evaluations. Practical Assessment, Research and Evaluation. 2013;18(13):12. [Google Scholar]
- 19.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 20.Greg Ridgeway DFM, Morral Andrew R, Burgette Lane F, Griffin Beth Ann. Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG) OBJECTIVE ANALYSIS EFFECTIVE SOLUTIONS. 2014 [Google Scholar]
- 21.Antonio Olmos PG. A Practical Guide for Using Propensity Score Weighting in R. Practical Assessment, Research & Evaluation. 2015;20 [Google Scholar]
- 22.Yamagiwa K, et al. Survival rates according to the Cancer of the Liver Italian Program scores of 345 hepatocellular carcinoma patients after multimodality treatments during a 10-year period in a retrospective study. J Gastroenterol Hepatol. 2008;23(3):482–90. doi: 10.1111/j.1440-1746.2007.05262.x. [DOI] [PubMed] [Google Scholar]
- 23.Shen WF, et al. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg. 2011;35(9):2083–91. doi: 10.1007/s00268-011-1171-y. [DOI] [PubMed] [Google Scholar]
- 24.Bertani E, et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: possible indications for a multimodal approach. Surgery. 2014;155(4):607–14. doi: 10.1016/j.surg.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy A, et al. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford) 2015;17(1):29–37. doi: 10.1111/hpb.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memon K, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83(3):887–94. doi: 10.1016/j.ijrobp.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruutiainen AT, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18(7):847–55. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Dong XD, Carr BI. Hepatic artery chemoembolization for the treatment of liver metastases from neuroendocrine tumors: a long-term follow-up in 123 patients. Med Oncol. 2011;28(Suppl 1):S286–90. doi: 10.1007/s12032-010-9750-6. [DOI] [PubMed] [Google Scholar]
- 29.Carrasco CH, et al. The carcinoid syndrome: palliation by hepatic artery embolization. AJR Am J Roentgenol. 1986;147(1):149–54. doi: 10.2214/ajr.147.1.149. [DOI] [PubMed] [Google Scholar]
- 30.Wiggermann P, et al. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE) Med Sci Monit. 2011;17(4):CR189–95. doi: 10.12659/MSM.881714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lammer J, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown DB, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S425–34. doi: 10.1016/j.jvir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 33.de Baere T, et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol. 2008;19(6):855–61. doi: 10.1016/j.jvir.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Whitney R, et al. Transarterial chemoembolization and selective internal radiation for the treatment of patients with metastatic neuroendocrine tumors: a comparison of efficacy and cost. Oncologist. 2011;16(5):594–601. doi: 10.1634/theoncologist.2010-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon RT, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–8. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Bhagat N, et al. Phase II study of chemoembolization with drug-eluting beads in patients with hepatic neuroendocrine metastases: high incidence of biliary injury. Cardiovasc Intervent Radiol. 2013;36(2):449–59. doi: 10.1007/s00270-012-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monier A, et al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2016 doi: 10.1007/s00330-016-4488-y. [DOI] [PubMed] [Google Scholar]
- 38.Therasse E, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189(2):541–7. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy AS, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31(3):271–9. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 40.McStay MK, et al. Large-volume liver metastases from neuroendocrine tumors: hepatic intraarterial 90Y-DOTA-lanreotide as effective palliative therapy. Radiology. 2005;237(2):718–26. doi: 10.1148/radiol.2372041203. [DOI] [PubMed] [Google Scholar]
- 41.Liu DM, et al. Minimally invasive techniques in management of hepatic neuroendocrine metastatic disease. Am J Clin Oncol. 2009;32(2):200–15. doi: 10.1097/COC.0b013e318172b3b6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Balance between IAT cohort after propensity score weighting
Standardized differences in the means (SMD), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90), Tumor resection with curative intention (Rx), Radio frequency ablation (RFA), an Eastern Cooperative Oncology Group score (ECOG)
The balance in the covariates was evaluated by the absolute standardized differences in the means before and after weighting. A recommended threshold value for balance in the covariates is 0.2.
Table 2. Univariate and multivariate Cox Proportional Hazard Models for Overall Survival after propensity score weighting (only the choice of IAT and covariates which were significant in UVA are displayed)
Table 3. Univariate and multivariate analysis for radiologic response after propensity score weighting (only choice of IAT and covariates which were significant in UVA are displayed)
Indicator for statistical significance (*)
Intra-arterial therapy (IAT), Univariate analysis (UVA), Multivariate analysis (MVA), Confidence interval (CI), Partial response (PR), Minor response (MR), Odd ratio (OR), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90), tumor grade 1–3 (G1–G3)
The probability of CR, PR and MR was determined by UVA and MVA logistic regression techniques. A Hosmer and Lemeshow goodness-of-fit test was assessed for each model. The table displays the summary of the UVA and MVA, thus the covariates which were significant in the UVA are shown in the table.
Table 4. Univariate and multivariate Cox Proportional Hazard Models for hepatic progression free Survival after propensity score weighting
(only the choice of IAT and covariates which were significant in UVA are displayed)
Figure 1a. Kaplan Meier curves demonstrating hepatic progression-free survival of cTACE vs. DEB-TACE cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for hepatic progression-free survival were calculated according to IAT modality, cTACE and DEB-TACE. After PSA the MHPFS of patients who underwent cTACE was 20.1 months, as for patients who underwent DEB-TACE 14.6 months (p=.14).
Abbreviations: Kaplan Meier (KM), propensity score analysis (PSA), Intra-arterial therapy (IAT), hepatic progression-free survival (HPFS), median HPFS (MHPFS), conventional transarterial chemoembolization (cTACE), drug-eluting beads TACE (DEB-TACE)
Figure 1b. Kaplan Meier curves demonstrating hepatic progression-free survival of cTACE vs. Y90 cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for HPFS were calculated according to IAT modality, cTACE and Y90. After PSA the MHPFS of patients who underwent cTACE was 21.6 months, as for patients who underwent Y90 11.2 months (p=.03).
Abbreviations: Kaplan Meier (KM), propensity score analysis (PSA), Intra-arterial therapy (IAT), hepatic progression-free survival (HPFS), median HPFS (MHPFS), conventional transarterial chemoembolization (cTACE), 90Yttrium Radioembolization (Y90)
Figure 1c. Kaplan Meier curves demonstrating hepatic progression-free survival of DEB-TACE vs. Y90 cohort after propensity score weighting
These PSA-adjusted Kaplan-Meier curves for HPFS were calculated according to IAT modality, DEB-TACE and Y90. After PSA the MHPFS of patients who underwent DEB-TACE was 13.3 months, as for patients who underwent Y90 11.2 months (p=.72).
Abbreviations: Kaplan Meier (KM), propensity score analysis (PSA), Intra-arterial therapy (IAT), hepatic progression-free survival (HPFS), median HPFS (MHPFS), drug-eluting beads TACE (DEB-TACE), 90Yttrium Radioembolization (Y90)