Abstract
Background
Negative reinforcement theories of drug addiction posit that addicts use drugs to alleviate negative mood states. In a preclinical model developed in our laboratory, rats exhibit negative affect to a normally rewarding taste cue when it predicts impending but delayed cocaine. The emergence of this state is accompanied by a reduction in dopamine concentration in the rostral nucleus accumbens (NAc) shell. However, the rostral and caudal regions of the shell have been implicated in promoting opposing appetitive and aversive states, respectively. Here, we tested whether dopamine transmission along the rostral-caudal axis of the shell plays differential roles in the emergence of drug-induced negative affect.
Methods
In TH::Cre rats, the dopaminergic pathways from the ventral tegmental area to the rostral and caudal regions of the shell were optogenetically stimulated during intraoral delivery of a taste cue signaling delayed cocaine. Affective responses to the taste cue were measured using taste-reactivity, and optical self-stimulation of the rostral and caudal shell was also examined.
Results
Optical stimulation of the rostral shell during tastant infusion prevented the emergence of negative affect, but activation of the caudal shell exacerbated aversive responses. These effects endured in the absence of optical stimulation, and the degree of negative affect in our model predicted self-stimulation responding.
Conclusions
These findings reveal unprecedented, pronounced and opposing roles of rapid dopamine signaling across the rostral-caudal axis of the NAc in the control of drug-induced negative affect, a hallmark of continued drug seeking and use in human addicts.
Keywords: Addiction, Affect, Cocaine, Dopamine, Behavior, Reward
Introduction
Drug addiction is a chronic, relapsing disorder characterized by an uncontrollable compulsion to seek out and take drug, particularly in response to drug cues, even following extended periods of abstinence (1, 2). Negative reinforcement plays a key role in the addiction cycle, such that addicts relapse and continue to use drug, in part, to mitigate negative affective states (e.g., dysphoria) associated with withdrawal (1, 3). In addicts, negative affective states are also linked to other maladaptive conditions such as the devaluation of non-drug rewards (1, 3–6). Therefore, understanding the neural circuitry that promotes drug-induced negative affect is critical for developing effective strategies to treat substance use disorders.
Preclinical rodent models provide a useful approach to examine the physiological basis of negative affect. Specifically, mood states can be assessed using the taste reactivity model where rats exhibit objective hedonic reactions to palatable (e.g., saccharin) and unpalatable (e.g., quinine) taste stimuli when infused directly into the oral cavity (7, 8). Taste reactivity consists of species-specific stereotyped oromotor behaviors (e.g., licking, gaping) that reflect the hedonic valence of tastants (7, 9). Beyond assessing hedonic value, taste reactivity also captures the affective state of the animal (6, 7, 10) and is affected by conditioned stimuli that engage sensory modalities independent of taste (11, 12).
Our laboratory developed a preclinical model to assess negative affect associated with impending, but delayed cocaine (6, 10). In this model, discrete (3.5 s) intraoral infusions of a sweet saccharin solution are delivered at regular intervals (~1 per minute) over a 35–45 minute period. At the conclusion of infusions, rats receive either experimenter-delivered or self-administered cocaine. Over successive taste-drug pairings, a dysphoric state develops as the sweet comes to predict impending but delayed drug. That is, rats initially display appetitive taste reactivity (e.g., licking) to the sweet, but rats come to devalue saccharin and exhibit aversive responses (e.g., gaping) as testing continues. When allowed to then self-administer drug, aversive taste reactivity is the best predictor of subsequent rapid drug loading (6, 13), which is interpreted as a behavioral correction for an anhedonic state (14). Importantly, we have also shown that this shift in the hedonic valence of the sweet is reflected in alterations in population responses of NAc neurons (6, 15).
The NAc shell is a functionally heterogeneous structure that exhibits contrasting control over mood states along its rostral-caudal axis (8, 16, 17). The rostral shell promotes positive mood while the caudal shell elicits aversive states. Using fast-scan cyclic voltammetry (FSCV) we have previously reported that dopamine (DA) release in the rostral nucleus accumbens (NAc) shell captures the emergence of the negative affective state associated with delayed cocaine availability in our model (10). Specifically, in naïve rats, intraoral infusion of a sweet results in increases in rapid DA release in the rostral shell (10, 18). However, after successive pairings of the same sweet with impending cocaine, DA release switches from an increase to a significant decrease in concentration. This decrease in DA is similar to that observed in naïve rats during intraoral infusion of the bitter, aversive tastant quinine (10, 18), indicating that decreases in DA in our model are associated with a drug-related negative affective state. Of note, our prior voltammetry studies measured DA in the rostral portion of the NAc shell since caudal regions contain norepinephrine that cannot be separated from DA using FSCV. As such, it is unclear if DA in the rostral and caudal shell plays differential roles in cocaine-induced negative affect.
Here, we used optogenetics to selectively stimulate DA projections from the ventral tegmental area (VTA) to the rostral NAc shell to determine if the shift in DA release dynamics in this region following repeated taste-drug pairings in our model is causally linked to the emergence of negative affect. Additionally, we determined if DA transmission in the caudal (compared to rostral) shell differentially affects the development and maintenance of the negative affective state observed in our model. Our findings reveal unique roles of rapid DA signaling across the rostral-caudal axis of the shell that differentially blunts or exacerbates drug-induced negative affect.
Methods and Materials
Subjects
In-house bred male Long-Evans rats (450–650 grams) were used. They were either transgenic (TG) rats that expressed Cre-recombinase in TH neurons (TH::Cre+/−, n=19) or non-transgenic (Non-TG) littermate controls (TH::Cre−/−, n=10). All rats were singly-housed and maintained on a 12h/12h reverse light-dark cycle (testing occurred during the dark phase). Rats were provided ad libitum access to water and chow (Purina Prolab Isopro RMH 3000) except during testing (water was restricted to 20–30 mls/day). All protocols were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and were approved by the University of North Carolina, Chapel Hill Institutional Animal Care and Use Committee.
Surgeries
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg, i.m.) and microinjected with Cre-dependent channel rhodopsin (ChR2; AAV5-DIO-ChR2-EYFP) into the VTA. The virus (4 µls, 4 × 1012 virus molecules/ml) was delivered across 4 injection sites (1 µl per injection site; coordinates: −5.4 AP, ±.8 ML, −6.2 DV and −6.2 AP, ±.8 ML, −7.4 DV from the skull surface). Rats were allowed at least 8 weeks of recovery for ChR2 to express in VTA DA terminals located in the NAc (19). A second surgery was performed to implant both an intraoral catheter and optical fibers (200 µcore) in the NAc shell (+1.3 to +1.5 AP, ±2.1 ML, −6.3 to −6.7 DV from skull, lowered at a 10° angle), using established procedures (20). After each surgery, rats received subcutaneous injections of meloxicam (1 mg/kg/day for 3 days). Following the second surgery, rats also received subcutaneous enrofloxacin injections (5 mg/kg, 2 injections/day for 5 days) to prevent catheter infection. An additional week of recovery occurred before testing began.
Cocaine-induced natural reward devaluation
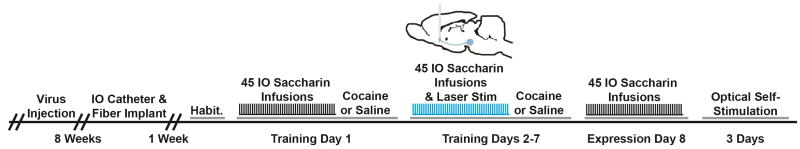
Figure 1 shows a schematic diagram of the training procedure. Rats were initially habituated to the test chamber (2 days) and to delivery of intraoral infusions. Taste reactivity and optogenetic stimulation were performed similar to previous experiments (6, 10, 21). On training day 1, rats were given 45 discrete 3.5s duration intraoral infusions of 0.15% saccharin delivered on a variable interval 45 second schedule. Immediately thereafter, rats were injected with either saline or 20 mg/kg cocaine (IP). On training days 2–7, the same procedure was completed but each intraoral infusion was paired with simultaneous optical stimulation of the rostral or caudal shell. Non-TG also received optical stimulation to control for heating artifacts that can accompany optical illumination. Specifically, a 20 mW blue wavelength (473 λ) laser was applied to the optical fibers at a frequency of 20 Hz for 5s beginning at the onset of each intraoral infusion to elevate DA release in the NAc, as previously reported (21). Optical stimulation was absent on the first day to ensure that rats across groups had comparable initial taste reactivity to the saccharin. Optical stimulation was not applied on test day 8, serving as a probe to identify whether activation of the dopaminergic VTA-NAc pathway is necessary for the expression of behavioral changes observed during training days 2–7. Rats did not receive cocaine or saline injections on day 8.
Figure 1.
The experimental timeline (see main text for details). Briefly, 8 weeks after virus surgery rats were implanted with IO catheters and optical fibers in the NAc shell, allowed to recover (1 week), and then habituated to the operant chamber and IO infusions. During training day 1, rats received 45 discrete 3.5s duration IO saccharin infusions over a ~35 minute period and then were immediately injected with saline or cocaine (IP, 20 mg/kg). The same procedure was repeated on training days 2–7, but here laser stimulation was applied to the NAc shell to stimulate DA inputs from the VTA. Stimulation was initiated with each IO infusion and lasted 5 s. On training day 8, the same procedure was completed without optical stimulation (similar to training day 1) or cocaine injection. Finally, once taste reactivity testing concluded rats were allowed to self-stimulate the dopaminergic VTA to NAc pathway for 3 days.
Optical Self-Stimulation
Following day 8, rats were trained to optically self-stimulate the VTA-NAc pathway in 30 minute sessions (over 3 days). A lever was extended into the chamber, and a cue light above it was illuminated. Each lever depression terminated the cue light and elicited a 5 second pulse of 20 Hz laser stimulation to the optical fiber and a 20 second timeout accompanied by illumination of the house light and presentation of a tone.
Groups
Rats were divided into 4 groups: 1) Non-TG rats that received cocaine (Non-TG Cocaine, n = 10), 2) TG rats that were administered cocaine but had optical fiber placements in the rostral NAc shell (TG Cocaine Rostral, n = 8), 3) TG rats that were administered cocaine but had optical fiber placements in the caudal NAc shell (TG Cocaine Caudal, n = 8), and 4) TG rats that were injected with saline (TG Saline, n = 10, optical fiber placements were distributed across the rostral-caudal axis of the NAc shell). The TG Saline group served as a control to ensure that effects of DA terminal stimulation were specific to drug-induced negative affect.
Taste Reactivity
A GoPro camera was positioned to face a mirror below the chamber to record oromotor responses to intraoral infusions. Responses were categorized as appetitive or aversive based upon previous work (7, 9). Briefly, rhythmic tongue protrusions, lateral tongue protrusions, and paw licking were classified as appetitive. Chin rubs, gaping, and mouth-to-floor-wiping were evaluated as aversive (each serving to expel fluid from the mouth). To control for differences in the duration of these taste reactivity responses, data were analyzed as the percent of trials where an appetitive or aversive response was performed.
Histology
Histology procedures and results are described in detail in the Supplemental Information.
Statistics
Taste reactivity and self-stimulation data were analyzed via a 2-way repeated measures ANOVAs followed by Newman-Keuls post hoc tests. Correlations were performed using Pearson’s correlation coefficient. Statistical tests were completed using commercially available software, with an alpha of 0.05 (GraphPad Prism ver. 7.00, San Diego, CA).
Results
Taste cues that predict impending but delayed cocaine elicit aversive taste reactivity in non-transgenic (control) rats
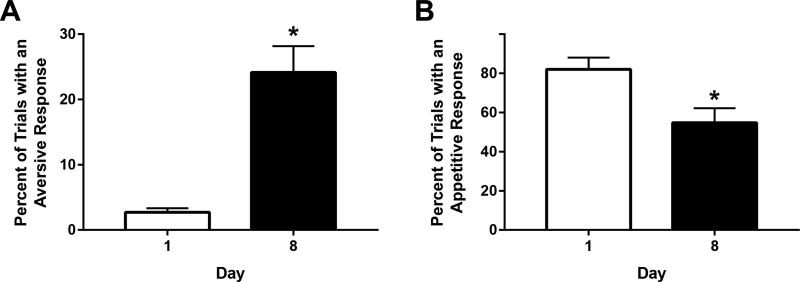
On day 1 of training, Non-TG rats exhibited classic appetitive taste reactivity during intraoral infusion of saccharin with minimal aversive responses (% trials aversive = 2.7%; percent of trials appetitive = 82%; Figure 2). However, repeated pairings of the sweet with delayed cocaine resulted in an elevation of aversive taste reactivity. A paired t-test revealed a significant increase in the percentage of trials with aversive responses on day 8 (versus day 1) of saccharin-cocaine pairings (t(8) = 5.298; p < 0.001; Figure 2A). A corresponding decrease in appetitive taste reactivity was observed across days 1 and 8 (t(8) = 4.766; p < 0.01; Figure 2B). These findings show that a palatable saccharin solution is devalued as it comes to predict impending but delayed cocaine, consistent with our prior reports (6, 10, 13, 22).
Figure 2.
Shift in behavioral responses to sweet that predicts impending, but delayed cocaine. An increase in aversive taste reactivity (A) and a decrease in appetitive responses (B) was observed over training days 1 through 8 in Non-TG rats that received cocaine. *p<0.01.
Optical stimulation of the VTA-NAc rostral shell pathway blunts the development of aversive taste reactivity
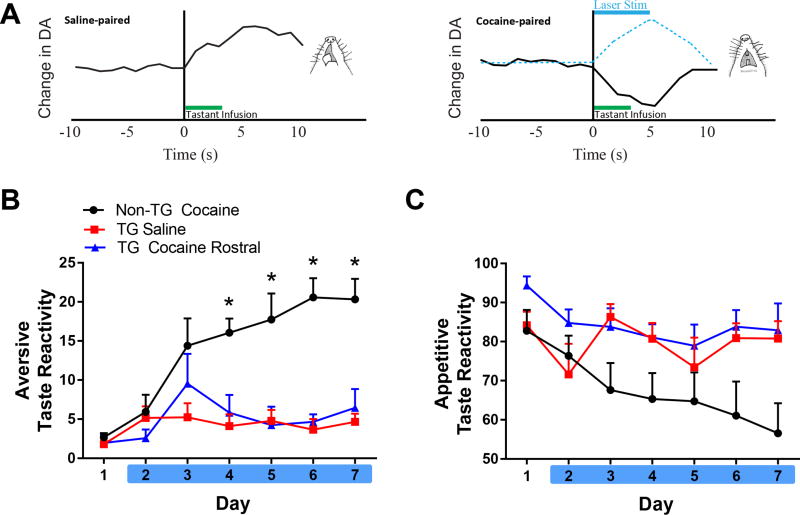
We previously reported that intraoral infusion of a sweet in naïve or saline-paired rats elicits appetitive taste reactivity and concomitant increases in rapid DA release in the NAc rostral shell, as measured by FSCV (10, 18), and illustrated in the schematic in Figure 3A. However, when animals learn that the same sweet signals impending, but delayed cocaine, a decrease in rapid DA signaling is observed during intraoral infusion simultaneous with the emergence of a negative affective state (Figure 3A, right). Here, we paired each infusion of the sweet with laser stimulation of the NAc shell (indicated by blue dashed line in Figure 3A, right) to prevent this decrease in DA release and to determine if it is causally linked to the emergence of negative affect observed in our model.
Figure 3.
Optical activation of the VTA to rostral NAc pathway prevents negative affect associated with impending cocaine. A. Schematic illustrating DA release in the rostral-medial NAc shell relative to saline-paired or cocaine-paired tastant, derived from (10). Left: IO infusions of saccharin elicit appetitive taste reactivity responses (licking) concomitant with an increase in DA concentration in this region. Right: However, the emergence of aversive responses (e.g., gaping) along with shift to a decrease in DA concentration occurs when saccharin is paired with impending, but delayed cocaine. Here, optogenetics was used to elevate DA release during IO infusions (indicated by dashed blue line) to prevent the decrease normally observed following repeated taste-drug pairings. B. Non-TG Cocaine rats exhibited an elevation in aversive taste reactivity on days 4–7 (*p<0.05 vs. TG Saline and TG Cocaine Rostral) and optical stimulation prevented this increase. No significant differences were observed between TG Saline and TG Cocaine Rostral groups. C. Percent of appetitive responses over days across groups.
Optical stimulation of the VTA DA input to the rostral NAc shell prevented the emergence of the negative affective state as shown in Figure 3B. Specifically, a 2-way ANOVA revealed a significant main effect of time [F(6,150) = 7.584; p < 0.0001], group [F(2,25) = 27.78, p < 0.0001], and interaction of time × group [F(12,150) = 3.775; p < 0.0001)] on aversive taste reactivity. Post hoc tests showed that rats in the Non-TG Cocaine group exhibited a greater amount of aversion on days 4–7 compared to both TG Saline and TG Cocaine rats (p < 0.0001). Importantly, the TG Cocaine and TG Saline groups did not significantly differ from one another.
We also examined if optical stimulation of the DA pathway to the rostral shell altered appetitive taste reactivity across days (Figure 3C). A 2-way ANOVA on the percent of trials with an appetitive response revealed a significant main effect of time [F(6,150) = 3.8; p < 0.01] and of group [F(2,25) = 4.0; p < 0.05)], but no significant interaction [F(12,150) = 1.681; p = 0.0761]. Non-TG Cocaine exhibited less appetitive taste reactivity across days, compared to the TG Saline and TG Cocaine rostral groups.
Optical stimulation of the dopamine VTA-NAc caudal shell pathway exacerbates aversive taste reactivity
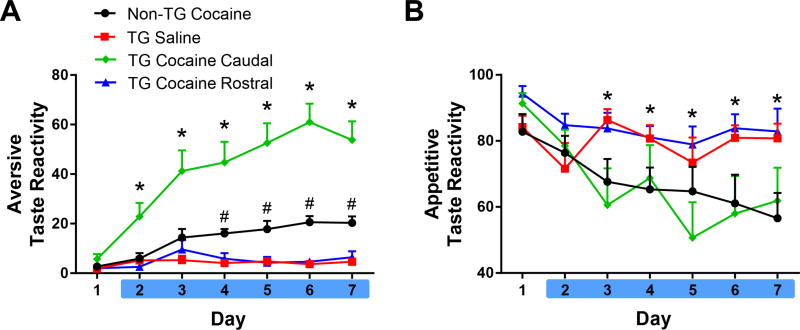
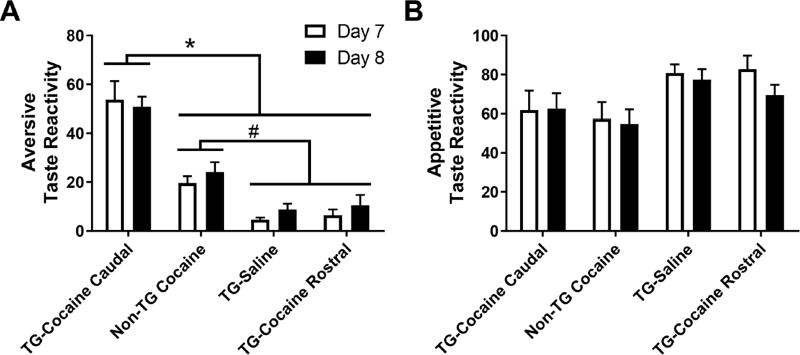
In contrast to the effects of optical stimulation in the rostral NAc shell, identical stimulation parameters in the caudal shell profoundly intensified aversive taste reactivity in our task. To highlight this finding and compare it to those observed in the rostral shell, Figure 4 shows aversive (A) and appetitive (B) taste reactivity for all groups of rats (note the differences in the y-axis between Figures 3 and 4). A 2-way ANOVA that examined aversive taste reactivity across all four treatment groups revealed a significant main effect of time [F(6,192) = 21.74; p < 0.0001], group [F(3,32) = 48.76, p < 0.0001], and an interaction of time × group [F(18,192) = 7.844; p < 0.0001). Results show that rats in the TG Cocaine Caudal group exhibited a robust enhancement in aversive taste reactivity on days 2–7 above all other groups (Figure 4A; p < 0.01). Consistent with results reported above, rats in the Non-TG Cocaine group displayed a greater amount of aversion compared to TG Saline and TG Cocaine Rostral rats on days 4–7 (p < 0.05). The TG Saline and TG Cocaine Rostral groups were not significantly different from each other. Further, no differences were observed between rostral and caudal shell stimulation in TG Saline rats (Supplemental Figure 2 A, B), suggesting that the effects observed here were specific to the drug-induced negative affective state.
Figure 4.
Optical activation of the VTA to caudal NAc shell pathway during IO infusions exacerbates negative affect associated with impending cocaine. A. Rats in the TG Cocaine Caudal group showed a marked elevation in aversive responses on days 2–7 compared to all other groups (*p<0.05). Non-TG Cocaine rats showed an increase in aversive responses above TG Saline and TG Rostral rats on days 4–7 (#p<0.05). B. Appetitive taste reactivity. Both the Non-TG Cocaine and TG Cocaine Caudal groups exhibited a decrease in appetitive responses on days 3–7, compared to day 1 (*p<0.05).
A similar analysis was completed on appetitive taste reactivity (Figure 4B). Here we found that optical stimulation of the rostral shell pathway prevented decreases in appetitive licking. Specifically, a 2-way ANOVA on the percent of trials with an appetitive response revealed no significant effect of group [F(3,32) = 2.734, p = 0.0598), but a significant effect of time [F(6,192) = 8.637, p < 0.001], and a significant interaction of time × group [F(18,192) = 2.734, p < 0.01]. Post hoc tests revealed that rats in the TG Cocaine Caudal group and Non-TG Cocaine group showed a significant decrease in appetitive behaviors on days 3–7 relative to day 1 (p < 0.05). TG Saline and TG Cocaine Rostral groups did not significantly differ from day 1.
Relationship between aversive taste reactivity and optical stimulation across the rostral-caudal shell
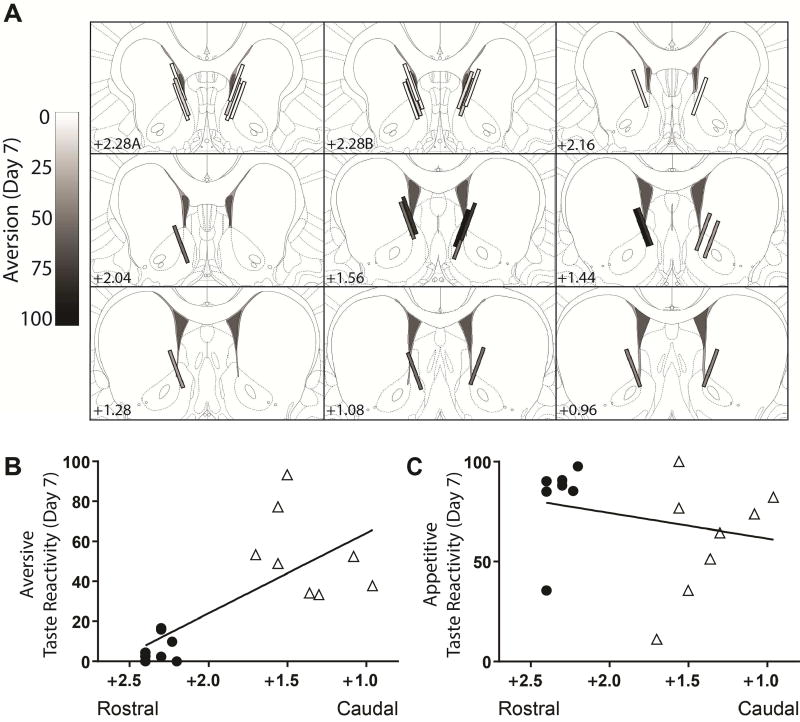
Figure 5A shows optical fiber placements in the rostral-caudal extent of the NAc shell in cocaine-treated TG rats, coded from light to dark according the degree of aversive taste reactivity on day 7 of testing. Note that aversive taste reactivity was stronger when placements were situated in the caudal shell. Pearson product moment correlations revealed an inverse correlation between the rostral-caudal placement and aversion exhibited on day 7 in TG rats that received cocaine [r(15) = −0.72; p < 0.01; Figure 5B]. No significant correlation was observed between rostral-caudal placement and aversion in TG saline rats (Supplemental Figure 2B). Likewise, no significant correlations were observed between rostral-caudal placement and appetitive behavior in either TG Cocaine (Figure 5C) or TG saline rats (all p’s > 0.05).
Figure 5.
The relationship between rostral-caudal optical fiber placement and taste reactivity. A. Placements of optical fibers are coded from light (few to no aversive responses) to dark (many aversive responses). Note that rats with placements in the rostral NAc shell tended to exhibit few aversive responses. In contrast, placements in the caudal shell predominantly resulted in increased aversive responses. Bottom: Correlations between rostral-caudal placement and aversive responses (B) and appetitive responses (C). Closed circles represent rats with placement in the caudal shell and open triangles indicate rats with placements in the rostral shell.
Inhibition and potentiation of aversive taste reactivity remain when optical stimulation is removed
Next, we determined if stimulation of the VTA to NAc DA pathway is necessary for the expression (not just acquisition) of aversive taste reactivity after repeated taste-drug pairings. Intraoral infusions were delivered on the final day of taste reactivity testing (day 8) in the absence of optical stimulation, and day 8 responses were compared to those measured the previous day when optical stimulation was applied (Figure 6). We found that the negative affective state that was inhibited (TG Cocaine Rostral) or exacerbated (TG Cocaine Caudal) by optical stimulation of the VTA DA input was seemingly a learned response that was maintained even in the absence of laser stimulation on day 8. That is, a two-way repeated measures ANOVA on aversive responses on days 7 and 8 revealed a significant main effect of group [F(3,33) = 46.91; p < 0.0001], but no significant main effect of time [F(1,33) = 1.433; p > 0.05] or a time × group interaction [F(3,33) = 0.6867; p > 0.05]. Post hoc tests on the main effect of group found that rats in the Non-TG Cocaine group exhibited significantly more aversive responses than rats in the TG Saline and TG Cocaine Rostral groups, independent of day (p < 0.01; Figure 6A). Furthermore, the TG Cocaine Caudal group displayed elevated aversive responses above all other groups (p < 0.0001). These findings were unique to the aversive response since no significant effects were found in the analysis of appetitive behavior [main effect of time: F(1,31) = 2.7; p > 0.05 or an interaction of group × time: F(3,31) = 1.081; p > 0.05. A main effect of treatment group was found [F(3,31) = 3.016; p < 0.05], however post-hoc tests revealed no significant differences between groups.
Figure 6.
Aversive (A) and appetitive (B) responses during day 7 (laser stimulation present) and day 8 (laser stimulation absent) of taste reactivity testing. The TG Cocaine Caudal group exhibited a greater level of aversive responses than all other groups (*p<0.01). Rats in the Non-TG Cocaine group exhibited significantly more aversive responses than the TG Saline and TG Cocaine Rostral groups (#p<0.01). No significant differences in appetitive responses were observed.
Optical Self-Stimulation of the VTA-NAc shell (rostral vs caudal) pathways
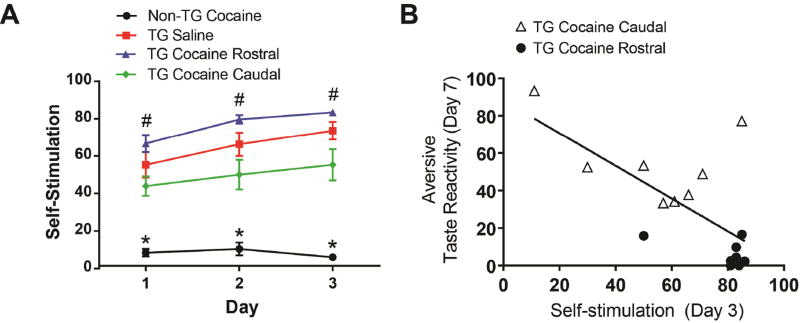
Lastly, we examined if the same animals tested above would self-stimulate for optical activation of the VTA-NAc shell (rostral and caudal) pathways over the course of 3 days. A two-way ANOVA of self-stimulation rewards received revealed a significant main effect of day [F(2,60) = 24.58, p < 0.0001], group [F(3,30) = 35.06, p < 0.0001], and a day × group interaction [F(6,60) = 4.362, p < 0.01]. All TG rats exhibited a significant elevation in self-stimulation rates above the Non-TG cocaine rats (p < 0.0001; Figure 7A). However, the TG Cocaine Rostral group self-stimulated significantly more than the TG Cocaine Caudal group on days 1–3 (p < 0.05). Self-stimulation behavior in the TG Saline group was not significantly different from either the TG Cocaine Caudal or TG Cocaine Rostral groups on any day of self-stimulation testing. Nor were differences observed between rostral and caudal shell stimulation in TG Saline rats (Supplemental Figure 2 C, D). Finally, aversive behaviors exhibited on day 7 of saccharin infusions inversely correlated with self-stimulation rewards received on all self-stimulation sessions in TG rats that received cocaine [See Supplemental Information for days 1 and 2; correlation for day 3 of self-stimulation: r(15) = −0.67, p < 0.01; Figure 7B]. There was no indication of differences in self-stimulation based upon rostral-caudal placement in TG Saline rats (see Supplemental Figure 2D).
Figure 7.
Optical self-stimulation of the VTA-NAc shell pathways. A. All TG rats self-stimulated more than the Non-TG Cocaine group (*p<0.0001). Rats in the TG Cocaine Rostral exhibited greater self-stimulation compared to TG Cocaine Caudal rats (#p<0.05). B. An inverse correlation was found between the amount of aversive taste-reactivity exhibited on day 7 and self-stimulation rewards received on day 3. Rats with placements in the rostral and caudal shell are represented by closed circles and open triangles, respectively.
Discussion
The present study reveals that DA transmission across the rostral-caudal axis of the NAc shell plays unique and opposing roles in drug-induced negative affect. Specifically, optical stimulation of DA terminals in the rostral shell during intraoral infusion prevented the emergence of negative affect while activation of terminals in the caudal shell elicited a pronounced enhancement of aversion. Interestingly, the inhibition and potentiation of negative affect endured in the absence of optical stimulation. Further, the degree of negative affect also predicted optical self-stimulation behavior; aversive taste reactivity was associated with diminished self-stimulation responding days after the last saccharin-cocaine pairing. Collectively, these findings suggest a causal role of rapid DA signaling measured electrochemically in our prior studies (10) in the development and maintenance of drug-induced negative affect.
In our model, a sweet taste predicts impending, but delayed cocaine. Over successive saccharin-cocaine pairings, the rats’ oromotor profile changes to indicate a state of dysphoria (6). Specifically, rats exhibit an increase in aversive taste reactivity and a corresponding decrease in appetitive responses during tastant infusion. Using FSCV, we previously reported that rapid DA release in the rostral NAc shell to a sweet switches from an increase to a decrease in concentration as it comes to predict impending, but delayed cocaine (10). In this study, we show that optically preventing this switch in DA concentration in this region precluded the emergence of aversive taste reactivity suggesting that DA signaling is causally linked to the development of dysphoria in our model. Critically however, the same optical stimulation parameters delivered to the caudal shell actually exacerbated the negative mood state, highlighting the unique and differing roles of the rostral versus caudal shell in dysphoria elicited when animals must wait for drug. Importantly, optical stimulation in the rostral and caudal shell of TG rats that received saline had no impact on taste reactivity, indicating that optogenetic induction of DA release specifically altered negative affect from impending but delayed cocaine.
The NAc is a heterogeneous structure that exhibits contrasting control over appetitive and aversive states along its rostral-caudal axis (16, 17, 23). Microinjections of opioids in the rostral shell promote appetitive taste reactivity, but in the caudal shell they inhibit appetitive responses (23). Additionally, the AMPA receptor antagonist DNQX elicits ingestive behavior in the rostral shell, but defensive treading (a predator defense behavior) in the caudal shell and these effects are dependent upon NAc DA (16, 24). Similarly, muscimol inactivation of the rostral shell promotes appetitive taste reactivity, but caudal shell inactivation yields aversive taste reactivity (25). The functional differences between the rostral and caudal shell may be at least partially attributable to disparities in innervation of neurotransmitter and neuromodulator systems along the rostral-caudal axis (26–29). These findings have led to the proposal that an “affective keyboard” is present along the rostral-caudal gradient of the NAc shell; the rostral shell promotes appetitive and hedonic states while the caudal shell elicits aversive states (8). Our finding that DA inhibits negative affect in the rostral shell but exacerbates aversion in the caudal shell is congruent with this view.
We also found that optical stimulation had relatively long-term effects on behavior in our model. Specifically, aversive taste reactivity across all groups was maintained when stimulation was not presented (day 8). This finding indicates that DA produces an enduring modulation of the negative affective state associated with delayed cocaine and is consistent with a role of DA in learning (30). Further, our study found that rats that exhibited a greater degree of aversive responses during taste reactivity testing tended to show a reduction in self-stimulation. Since the same chambers were used in the taste reactivity and self-stimulation studies, rats may have learned to associate the context (the operant chamber) with the exacerbated negative mood state induced by stimulation of the caudal shell. The context, in turn, may have evoked negative affect that inhibited self-stimulation. Importantly, rostral-caudal placement of optical fibers did not impact self-stimulation in TG Saline rats indicating that differences in self-stimulation rates between the TG Cocaine Caudal and TG Cocaine Rostral groups could not be attributed solely to optical fiber location.
Negative reinforcement is a critical component of the drug addiction cycle (1, 3). Addicts seek out and take drugs to alleviate the aversive states associated with drug withdrawal and exposure to environmental stressors (1, 3, 4). Further, a state of drug craving, typically evoked by drug-associated cues, elicits feelings of anxiety and negative affect (4, 5). Negative affect also drives natural reward devaluation, contributing to addicts limited drug-seeking focused behavioral repertoire (1). Critically, our model captures the processes of drug-related natural reward devaluation and negative affect (6, 22, 31, 32). The present findings indicate that inhibition of DA release in the rostral portion of the NAc shell leads to the emergence of negative affect (10) while DA release in the caudal shell enhances dysphoria and natural reward devaluation. Taken together, an imbalance of DA release dynamics along the rostral-caudal axis of the NAc shell may contribute to a state of dysphoria experienced in cocaine addicts that contributes to their continued drug seeking.
Supplementary Material
Acknowledgments
The work was supported by National Institute on Drug Abuse Grants, DA014339 (RMC) and DA037733 (EW). We are grateful for outstanding technical support from Xuefei Wang. We also greatly appreciate the transgenic TH::Cre rats gifted from Dr. Karl Deisseroth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the’dark side’ of drug addiction. Nature neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Rocio M, Carrera A, Gold LH, Heyser CJ, Maldonado-Irizarry C, et al. Substance dependence as a compulsive behavior. Journal of psychopharmacology. 1998;12:39–48. doi: 10.1177/026988119801200106. [DOI] [PubMed] [Google Scholar]
- 3.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological review. 2004;111:33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Sinha R, Fuse T, Aubin L-R, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of general psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience & Biobehavioral Reviews. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 8.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, et al. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learning & memory. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delamater AR, LoLordo VM, Berridge KC. Control of fluid palatability by exteroceptive Pavlovian signals. Journal of experimental psychology Animal behavior processes. 1986;12:143–152. [PubMed] [Google Scholar]
- 13.Green JL, Dykstra LA, Carelli RM. Examination of cocaine dose in a preclinical model of natural reward devaluation by cocaine. Behavioural pharmacology. 2015;26:398–402. doi: 10.1097/FBP.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed SH, Koob GF. Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 15.Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. The Journal of neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 18.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nature Neuroscience. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickham RJ, Park J, Nunes EJ, Addy NA. Examination of Rapid Dopamine Dynamics with Fast Scan Cyclic Voltammetry During Intra-oral Tastant Administration in Awake Rats. JoVE. 2015:e52468–e52468. doi: 10.3791/52468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic Dopamine Dynamically Tracks, and Is Causally Linked to, Discrete Aspects of Value-Based Decision Making. Biological psychiatry. 2015;77:903–911. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carelli RM, West EA. When a good taste turns bad: Neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology. 2014;76(Pt B):360–369. doi: 10.1016/j.neuropharm.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. The Journal of neuroscience. 2014;34:4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. The Journal of neuroscience. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PloS one. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Research. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Aragona BJ, Kile BM, Carelli RM, Wightman RM. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience. 2010;169:132–142. doi: 10.1016/j.neuroscience.2010.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowers BJ, Henry MB, Thielen RJ, McBride WJ. Serotonin 5-HT(2) receptor stimulation of dopamine release in the posterior but not anterior nucleus accumbens of the rat. Journal of neurochemistry. 2000;75:1625–1633. doi: 10.1046/j.1471-4159.2000.0751625.x. [DOI] [PubMed] [Google Scholar]
- 29.Vaccarino FJ, Rankin J. Nucleus accumbens cholecystokinin (CCK) can either attenuate or potentiate amphetamine-induced locomotor activity: evidence for rostral-caudal differences in accumbens CCK function. Behavioral neuroscience. 1989;103:831–836. [PubMed] [Google Scholar]
- 30.Wise RA. Dopamine, learning and motivation. Nature reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 31.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral neuroscience. 2002;116:321–333. [PubMed] [Google Scholar]
- 32.Colechio EM, Grigson PS. Conditioned Aversion for a Cocaine-Predictive Cue is Associated with Cocaine Seeking and Taking in Rats. International journal of comparative psychology. 2014;27:488–500. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.