Capsule Summary
Because IL-13 induces expression of the anti-apoptotic Bcl-2 and the pro-apoptotic Bik, blocking Bcl-2 causes a Bik-dependent death of hyperplastic mucous cells. Therefore, the Bcl-2-inhibitor, ABT-263, may be highly efficient in reducing mucous hypersecretion.
Keywords: ABT-263, airway epithelial cells, apoptosis, Bcl-2 family of proteins, IL-13, mucous cell hyperplasia, cystic fibrosis, bronchitic asthma, chronic bronchitis
To the Editor
Bcl-2 protects cells against a wide range of apoptotic stimuli by preventing mitochondrial permeabilization caused by pro-apoptotic Bcl-2 family members (1). Although Bcl-2 levels are regulated by various cytokines (2), inflammatory mediators that affect Bcl-2 expression in non-hematopoietic primary cells have been poorly studied. Bcl-2 expression is upregulated in airway mucous cells of patients with chronic bronchitis, bronchitic asthma, and cystic fibrosis (CF), and in the respective animal models to sustain hyperplastic mucous cells (3, 4). The purpose of this study was to determine whether identifying and understanding the mechanisms by which cytokines regulate Bcl-2 expression can be used to reduce mucous cell hyperplasia (MCH). Because we repeatedly have observed that Bcl-2 and MUC5AC are co-expressed in airway epithelial cells (AECs) (4, 5), we investigated whether IL-13 also induces Bcl-2 expression. We found that IL-13 induced Bcl-2 and MUC5AC but did not affect Bcl-xL mRNA levels in differentiated primary human AECs (HAECs) from 10 individuals (Fig. 1A) and induction was in a dose-dependent manner (Fig. 1B). Inhibitors of both EGFR (AG1478) and ERK1/2 (U0126) suppressed IL-13-induced MUC5AC (Fig. 1C), Bcl-2 mRNA (Fig. 1D) and protein (Fig. 1E) levels, suggesting that Bcl-2 and MUC5AC may be regulated by identical pathways. Interestingly, blocking Bcl-2 expression using a retroviral shRNA construct (shBcl-2) (Fig. 1F) reduced IL-13–induced MUC5AC mRNA levels (Fig. 1F), suggesting Bcl-2 may be upstream of MUC5AC expression. However, when Bcl-2 was constitutively overexpressed (Bcl-2 O/E), MUC5AC mRNA levels remained unchanged (Fig. 1G). Rather, in IL-13-treated cells the cell numbers of untransfected (UT) and control shRNA with random DNA sequence (shCTRL)-transfected controls increased, while suppression of the anti-apoptotic Bcl-2 using a shBcl-2 construct caused reduction of cell numbers (Fig. 1H) by inducing apoptosis (Fig. 1I).
Figure 1. IL-13 increases Bcl-2 and Bik expression and suppressing Bcl-2 induces apoptosis.
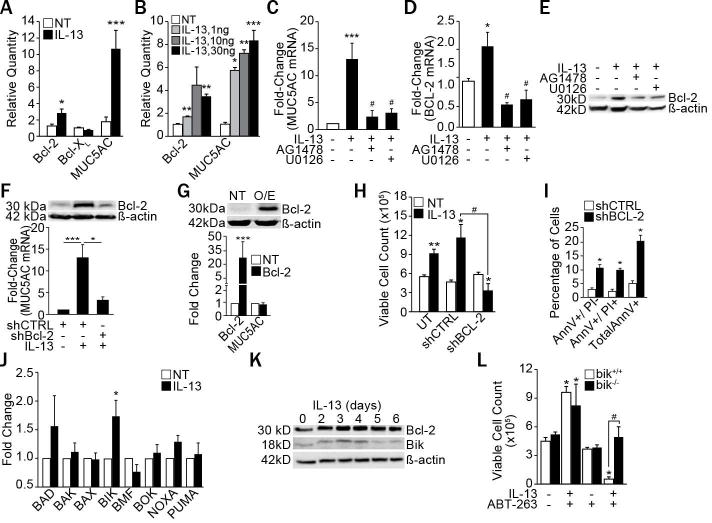
(A) Bcl-2, Bcl-XL and MUC5AC mRNA levels in differentiated primary HAECs from 10 different subjects treated with IL-13 (10 ng/ml) for 5 d and analyzed by qRT-PCR. (B) Primary HAECs treated with the indicated concentrations of IL-13 were analyzed for Bcl-2 and MUC5AC mRNA levels. Quantification of MUC5AC mRNA (C), Bcl-2 mRNA (D), and Bcl-2 protein (E) in HAECs treated with IL-13 (10 ng/ml) and with either EGFR inhibitor, AG1478 (1 μM) or with ERK1/2 inhibitor, U0126 (1 μM). (F) MUC5AC mRNA and Bcl-2 protein levels in HAECs transfected with shBcl-2 or shCTRL, and treated with IL-13. (G) Bcl-2 protein and MUC5AC mRNA levels in Bcl-2 overexpressing (Bcl-2 O/E) compared with untransfected (UT) cells. (H) Number of viable HAECs recovered after IL-13 treatment of untransfected, shCTRL-, or shBcl-2-transfected cells. (I) Annexin V (AnnV) and propidium iodide (PI) positivity of shCTRL- or shBcl-2-transfected HAECs as analyzed by flow cytometry following IL-13 treatment. (J) Expression levels of pro-apoptotic Bcl-2 family members in differentiated primary HAECs treated with IL-13 compared to non-treated (NT) controls and analyzed by qRT-PCR (n=8 HAECs from different subjects). (K) Time-course of Bcl-2 and Bik protein levels in HAECs treated with IL-13. (L) Number of viable bik−/− and bik+/+ MAECs 48 h after IL-13 and ABT-263 treatment. Data shown as mean±SEM with n≥3 unless otherwise indicated; #p<0.05 between cells treated with IL-13 and ABT-263. *p<0.05; **p<0.01, ***p<0.001.
To determine the mechanism by which IL-13 induces cell death when Bcl-2 expression is suppressed, we screened for all pro-apoptotic Bcl-2 family proteins and identified Bik mRNA to consistently be induced by IL-13 in primary differentiated HAECs (Fig. 1J) and even further increased in shBcl-2 cells (Fig. E1A). Bik protein levels were upregulated on days 3 and 4 of IL-13 treatment, whereas Bcl-2 levels remained induced over 6 d (Fig. 1K). Because these findings suggested that IL-13 can be switched from a proliferative to a cell death-inducing cytokine when Bcl-2 is suppressed, we examined the role of a small molecule BH3 mimetic, ABT-263, that blocks Bcl-2 function (6). As a percentage of non-treated controls, IL-13 treated HAECs were reduced when pre-incubated with 1 μM ABT-263 for 2 h (Fig. E1B). While bik+/+ primary murine airway epithelial cells (MAECs) showed significant cell death, bik−/− MAECs were unaffected by the combination treatment of IL-13 and ABT-263 (Fig. 1L). Similar results were observed in HAECs when Bik was suppressed using shBik (Fig. E1C), confirming that Bik is required for IL-13–induced cell death when Bcl-2 is blocked.
To investigate the potential therapeutic value of these findings, we first investigated the efficacy of ABT-263 in suppressing IL-13–induced MCH in differentiated primary HAEC cultures. In IL-13-treated cultures, treatment with ABT-263 compared to vehicle suppressed MUC5AC mRNA levels (Fig. E2A) and the percentage of MUC5AC- and Bcl-2-positive cells (Fig. 2A). ABT-263 treatment had no effect on the ciliated cell population but significantly reduced MUC5AC-positive cells (Fig. 2A) without affecting the trans-epithelial electrical resistance (Fig. E2B). Because IL-13 is a Th2 cytokine central in causing bronchitic asthma (7), we investigated the effect of ABT-263 in a mouse model of allergic asthma (as illustrated in Fig. 2B). MCH was significantly reduced by intranasally administered ABT-263 (2mg/kg body weight) compared to vehicle-treated controls (Fig. 2C) as were Muc5ac-positive cells (Fig. E2C). The number of AECs positive for cleaved caspase 3 (Ac-Casp3) (Fig. 2D) and TUNEL (Fig. E2D) were increased among Scgb1A1+ secretory cells in ABT-263-treated compared to vehicle-treated mice indicating reduction of OVA-induced MCH by apoptosis.
Figure 2. ABT-263 reduces IL-13-induced MCH in vivo.
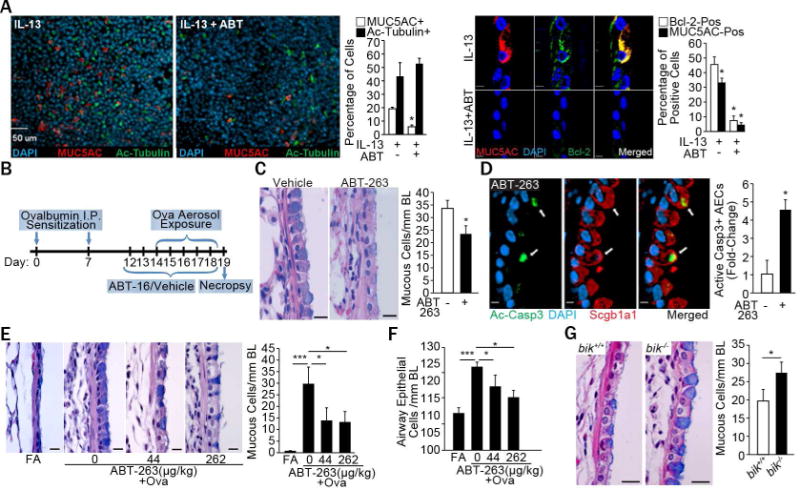
(A) Micrographs and quantification of mucous (MUC5AC+, red) and ciliated (acetylated-tubulin+, green) cells among differentiated HAECs treatedwith 10 ng/ml IL-13 or IL-13 and 1 M ABT-263. Bcl-2 (green) and MUC5AC (red) expression of cross-sections with DAPI (blue) denoting nuclei and quantification. Triplicate transwells from each treatment were analyzed. (B) Experimental outline for mice exposed to OVA and treated with ABT-263. (C) Micrographs of axial airways from allergic mice treated with vehicle or ABT-263 (2 mg/Kg) and stained with Alcian blue (AB) and hematoxylin and eosin (H&E) and quantification. (D) Micrographs showing Ac-Casp3 (green)-positive Scgb1a1-secretory cells (red) in mouse axial airways treated with ABT-263 and DAPI-stained nuclei (blue) and relative fold-change in ac-Casp3+ secretory cells. (E) Testing the therapeutic efficacy of aerosolized ABT-263 in OVA-induced allergic mice model (see details in supplemental methods). Groups of mice challenged with OVA received 0 (vehicle), 44, or 262 g/kg lung deposited dose of ABT-263 and lung tissues harvested on d 6 were analyzed for airway mucous cells per mm BL in AB-H&E stained sections and representative micrographs. (F) Numbers of total AECs/mm BL in mice treated with 0 (vehicle), 44 or 262 g/kg ABT-263. (G) Micrographs of airways stained with AB-H&E from allergic bik+/+ and bik−/− mice treated with aerosolized ABT-263 (46 μg/kg) and quantification of mucous cells/mm BL. Scale = 20 μM; Data shown as mean±SEM (n=5–10/group); *p<0.05; **p<0.01; ***p<0.001.
We postulated that delivery of ABT-263 by inhalation as an aerosol will directly target airway mucous cells and thereby minimize the effective dose. Therefore, we generated aerosols with respirable size particles (<1 μm) with increasing ABT-263 concentrations in a suspension formulation in 0.5% Tween 80 and water using a Pari LC Plus compressed air jet nebulizer (8) and estimated the pulmonary deposited doses (PDD) for a 30 min exposure (Table E1). Then, three groups of ovalbumin-sensitized mice were exposed daily by nose-only inhalation (as outlined in Fig. E3A) to either 0 (vehicle only – 0.5% Tween 80 in water), or the average PDD of 44 (Table E2) or 262 (Table E3) g/kg of body weight over 5 d. Aerosolized ABT-263 at both 44 and 262 μg/kg PDD suppressed the allergen-induced MCH compared to control mice (Fig. 2E) and significantly reduced the number of total AECs/mm BL (Fig. 2F). As expected, ABT-263 levels observed in plasma and lung tissues were increased in a dose-dependent manner at 6 and 24 h post the last ABT-263 dose and decreased at 24 h for the 44 and 262 g/kg doses by 4.2- and 10-fold, respectively (Fig. E3B and C). The importance of Bik in the resolution of MCH was assessed by exposing bik+/+ and bik−/− mice to ABT-263 aerosols at 46 μg/kg PDD (Table E4). Nose-only inhalation of ABT-263 suppressed MCH in bik+/+ but not in bik−/− mice (Fig. 2G), confirming that Bik is crucial in reducing MCH when Bcl-2 function is blocked.
The current studies demonstrate that the pro-inflammatory IL-13 induces Bcl-2 in airway epithelial cells. Because IL-13 also induces the pro-apoptotic Bik, targeted blocking of Bcl-2 function switches IL-13 into a cell death-inducer. Therefore, the small molecule Bcl-2 inhibitor, ABT-263, when delivered by nose-only inhalation reduced allergen-induced MCH in a Bik-dependent manner at 4,000-fold lower doses compared with the 200 mg/kg dose that causes reduction in platelets by 50% as used in clinical trials for treating lymphoma (9). Whether airway epithelial cells will be more prone to injury from infection or exposure to noxious substances will be studied in the future. However, IL-13, as a pleiotropic cytokine, effects airway epithelial cells to produce not only mucins, but also chemokines, TGF-β, eotaxin and thereby may perpetuate inflammation. Therefore, reducing the number of hyperplastic airway epithelial cells with aerosolized ABT-263 that is rapidly cleared from the body may be an effective therapy with reduced side effects for reducing inflammation and mucous hypersecretion.
Acknowledgments
The authors thank Lois Herrera and Susan Fort for assistance on the selected experiments.
This study was supported by NIH grants to YT (HL068111 and ES015482), to YM (AI115291) and to HSC (AI117560); and by American Lung Association to HSC (RG306208).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nature reviews Cancer. 2016 Feb;16(2):99–109. doi: 10.1038/nrc.2015.17. Epub 2016/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 2.Chand HS, Harris JF, Mebratu Y, Chen Y, Wright PS, Randell SH, et al. Intracellular Insulin-like Growth Factor-1 Induces Bcl-2 Expression in Airway Epithelial Cells. J Immunol. 2012 Mar 28; doi: 10.4049/jimmunol.1102673. Epub 2012/03/31. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris JF, Fischer MJ, Hotchkiss JR, Monia BP, Randell SH, Harkema JR, et al. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. American journal of respiratory and critical care medicine. 2005 Apr 1;171(7):764–72. doi: 10.1164/rccm.200408-1108OC. Epub 2004/12/25. eng. [DOI] [PubMed] [Google Scholar]
- 4.Chand HS, Montano G, Huang X, Randell SH, Mebratu Y, Petersen H, et al. A Variant of p53 Restricts the Mucus Secretory Phenotype by Regulating SPDEF and Bcl-2 Expression. Nat Commun. 2014;5:5667. doi: 10.1038/ncomms6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesfaigzi Y. Roles of Apoptosis in Airway Epithelia. American journal of respiratory cell and molecular biology. 2006 Jan 26;34(5):537–47. doi: 10.1165/rcmb.2006-0014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer research. 2008 May 1;68(9):3421–8. doi: 10.1158/0008-5472.CAN-07-5836. Epub 2008/05/03. eng. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nature reviews Immunology. 2015 May;15(5):271–82. doi: 10.1038/nri3831. Epub 2015/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 8.Kuehl PJ, Anderson TL, Candelaria G, Gershman B, Harlin K, Hesterman JY, et al. Regional particle size dependent deposition of inhaled aerosols in rats and mice. Inhalation toxicology. 2012 Jan;24(1):27–35. doi: 10.3109/08958378.2011.632787. [DOI] [PubMed] [Google Scholar]
- 9.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013 Feb;19(2):202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]


